The Effects of Microencapsulation Technology on the Flavor Quality of Zanthoxylum Oil Based on E-Nose, GC–IMS, and GC–MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties Analysis
2.1.1. Sensory Analysis
2.1.2. Analysis of Colorimetric
2.1.3. E-Nose Analysis
2.2. GC–MS Analysis
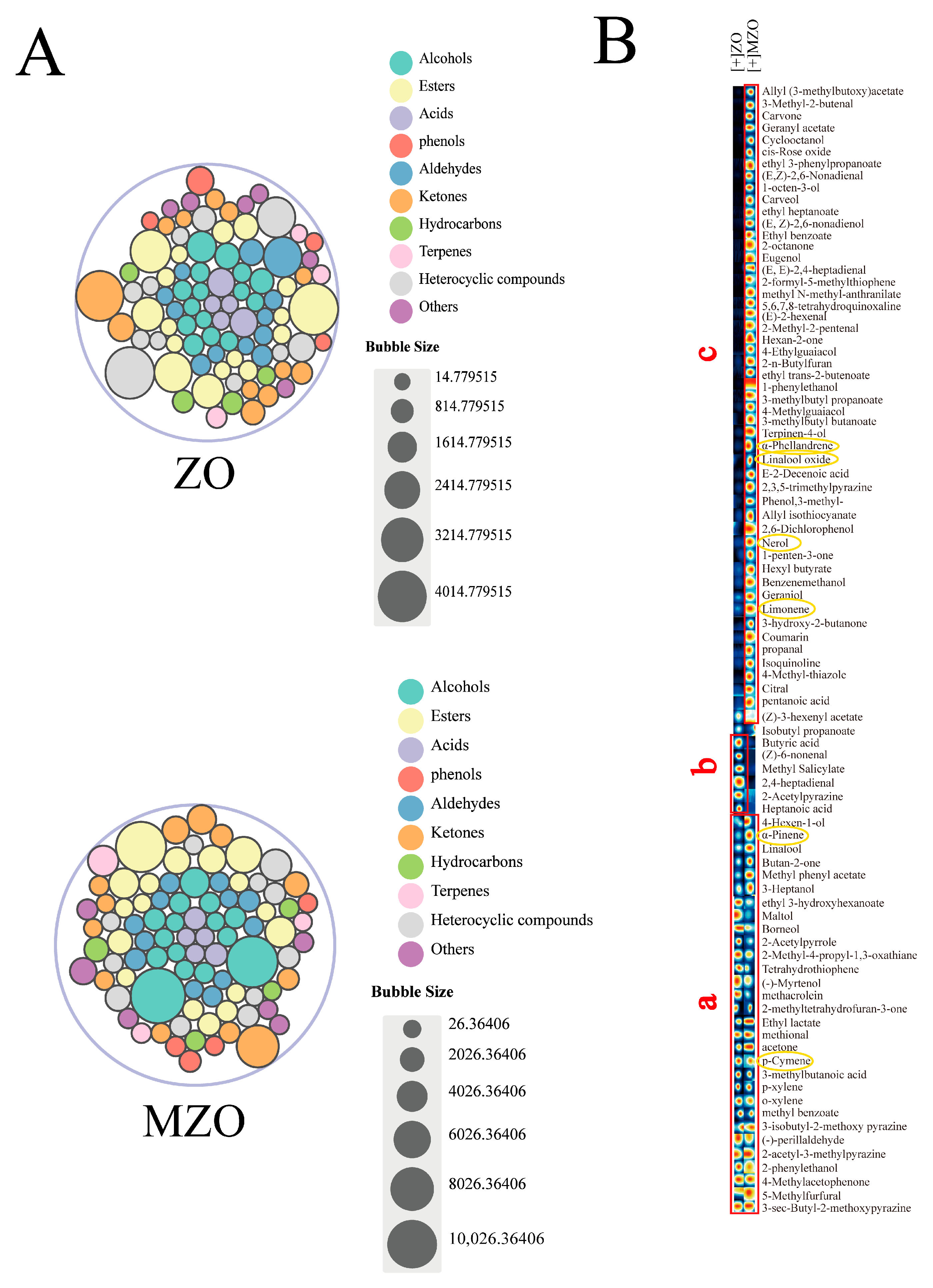
2.2.1. Analysis of VOCs Composition by GC–MS
2.2.2. Analysis of Odor Activity Values
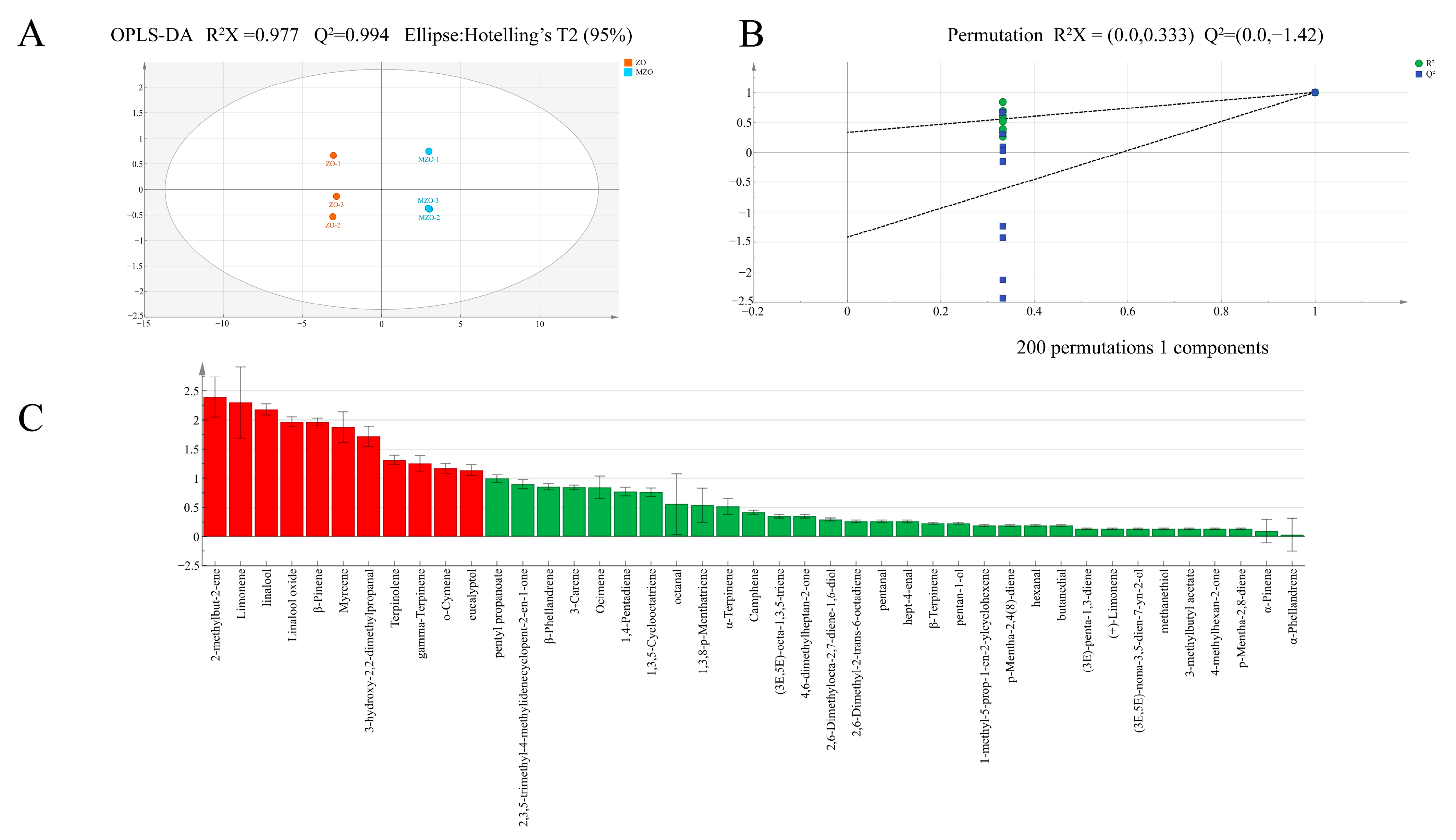
2.2.3. Analysis of Multivariate Statistical
2.3. Correlation Analysis Between E-Nose and GC–MS
2.4. GC–IMS Analysis
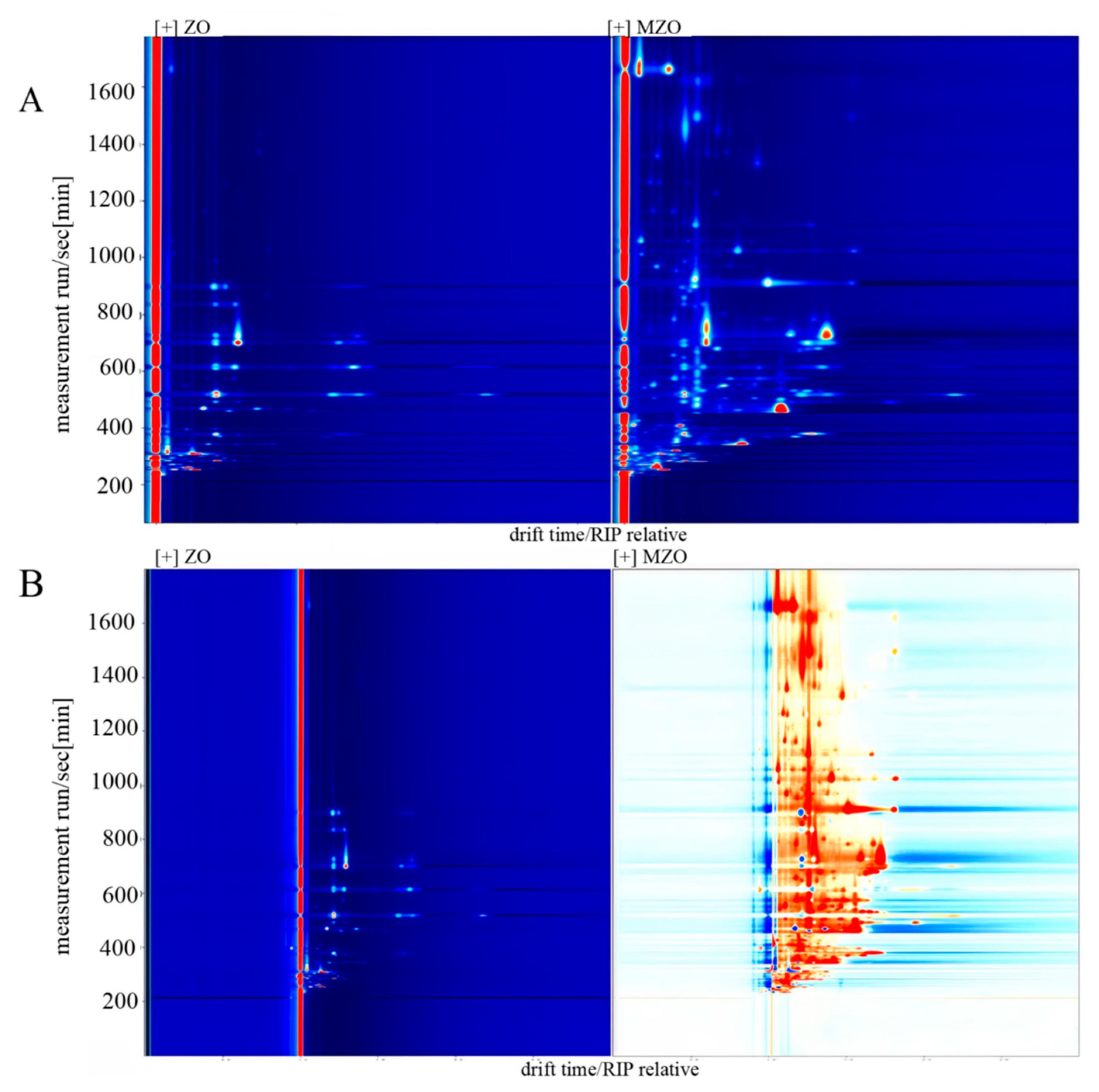
2.4.1. GC–IMS 2D Mapping Analysis
2.4.2. Analysis of VOCs by GC–IMS
3. Materials and Methods
3.1. Materials and Reagents
3.2. Microencapsulated Zanthoxylum Oil Production Process
3.3. Assessment of Physicochemical Characteristics
3.3.1. Sensory Evaluation
3.3.2. Determination of Colorimetric Analysis
3.4. Analysis of Characteristic Flavor Compounds of Zanthoxylum Oil
3.4.1. Analysis of E-Nose
3.4.2. Analysis of Volatile Compounds Using GC–MS
3.4.3. Analysis of the Odor Activity Value
3.4.4. Analysis of Volatile Compounds Using GC–IMS
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, Y.; Sun, W.-H.; Xu, T.-T.; Zhang, L.; Huang, X.-Y.; Tan, Z.-H.; Di, D.-L. Chemical constituents from the pericarps of Zanthoxylum bungeanum and their chemotaxonomic significance. Biochem. Syst. Ecol. 2021, 95, 104673. [Google Scholar] [CrossRef]
- Hu, H.; He, B.; Ma, L.; Chen, X.; Han, P.; Luo, Y.; Liu, Y.; Fei, X.; Wei, A. Physiological and transcriptome analyses reveal the photosynthetic response to drought stress in drought-sensitive (Fengjiao) and drought-tolerant (Hanjiao) Zanthoxylum bungeanum cultivars. Front. Plant Sci. 2022, 13, 968714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, L.; Han, N.; Wang, D. Characterization of phenolic chemotypes, anatomy, and histochemistry of Zanthoxylum bungeanum Maxim. Ind. Crops Prod. 2023, 193, 116149. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M.; Quilitzsch, R.; Schütze, W.; Lösing, G. Characterization of peppercorn, pepper oil, and pepper oleoresin by vibrational spectroscopy methods. J. Agric. Food Chem. 2005, 53, 3358–3363. [Google Scholar] [CrossRef]
- Yogendrarajah, P.; Samapundo, S.; Devlieghere, F.; De Saeger, S.; De Meulenaer, B. Moisture sorption isotherms and thermodynamic properties of whole black peppercorns (Piper nigrum L.). Lwt-Food Sci. Technol. 2015, 64, 177–188. [Google Scholar] [CrossRef]
- Sun, J.; Sun, B.; Ren, F.; Chen, H.; Zhang, N.; Zhang, Y.; Zhang, H. Effects of Storage Conditions on the Flavor Stability of Fried Pepper (Zanthoxylum bungeanum) Oil. Foods 2021, 10, 1292. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Veloso, C.M. Microencapsulation of natural dyes with biopolymers for application in food: A review. Food Hydrocoll. 2021, 112, 106374. [Google Scholar] [CrossRef]
- Xiao, Z.; Sun, P.; Liu, H.; Zhao, Q.; Niu, Y.; Zhao, D. Stimulus responsive microcapsules and their aromatic applications. J. Control. Release 2022, 351, 198–214. [Google Scholar] [CrossRef]
- Yuan, Y.; Kong, Z.Y.; Sun, Y.E.; Zeng, Q.Z.; Yang, X.Q. Complex coacervation of soy protein with chitosan: Constructing antioxidant microcapsule for algal oil delivery. Lwt-Food Sci. Technol. 2017, 75, 171–179. [Google Scholar] [CrossRef]
- Meng, F.B.; Li, J.J.; Zhang, Q.; Li, Y.C.; Liu, D.Y.; Chen, W.J.; Zhang, Y. Complex wall materials of polysaccharide and protein effectively protected numb-taste substance degradation of Zanthoxylum bungeanum. J. Sci. Food Agric. 2021, 101, 4605–4612. [Google Scholar] [CrossRef]
- Feng, X.Y.; Wang, H.W.; Wang, Z.R.; Huang, P.M.; Kan, J.Q. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2022, 375, 11. [Google Scholar] [CrossRef]
- Gao, L.; Shi, B.L.; Zhao, L.; Wang, H.Y.; Xiang, Y.K.; Zhong, K. Aroma Characteristics of Green Huajiao in Sichuan and Chongqing Area Using Sensory Analysis Combined with GC-MS. Foods 2024, 13, 836. [Google Scholar] [CrossRef]
- Niu, W.J.; Tian, H.L.; Zhan, P. The Effects of Pepper (Zanthoxylum bungeanum) from Different Production Areas on the Volatile Flavor Compounds of Fried Pepper Oils Based on HS-SPME-GC-MS and Multivariate Statistical Method. Molecules 2022, 27, 7760. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, X.; Liu, J.; Jia, M.; Blanchard, C.; Zhou, Z. Characterizing the volatile compounds of different sorghum cultivars by both GC-MS and HS-GC-IMS. Food Res. Int. 2021, 140, 109975. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, T.; Yang, D.; Xie, J. Application of gas chromatography-ion mobility spectrometry in the analysis of food volatile components. Acta Chromatogr. 2023, 35, 35–45. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, D.; Sun, J. Application of GC-IMS coupled with chemometric analysis for the classification and authentication of geographical indication agricultural products and food. Front. Nutr. 2023, 10, 1247695. [Google Scholar] [CrossRef]
- Yoshino, A.; Nakamura, H.; Okita, Y. Using the Hilbert Transform to Evaluate the Effects of Functional Foods on Autonomic Nervous System Activity: A Comparison with the Fast Fourier Transform. J. Nutr. Sci. Vitaminol. 2024, 70, 179–182. [Google Scholar] [CrossRef]
- Ma, R.; Shen, H.; Cheng, H.; Zhang, G.; Zheng, J. Combining e-nose and e-tongue for improved recognition of instant starch noodles seasonings. Front. Nutr. 2023, 9, 1074958. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, J.; Du, D. Assessment of high pressure processed mandarin juice in the headspace by using electronic nose and chemometric analysis. Innov. Food Sci. Emerg. Technol. 2017, 42, 33–41. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, L.; Shi, B.; Wang, H.; Zhong, K.; Yan, S. GC-MS combined with odor activity value to analyze key aroma substance characteristics of red Chinese pepper oil. Food Ferment. Ind. 2023, 49, 295–301. [Google Scholar]
- Seo, H.Y.; Shim, S.L.; Ryu, K.Y.; Jung, M.S.; Min, H.I.; Shin, D.B.; Kwon, J.H.; Schreier, P.; Kim, K.S. Analysis of Volatile Compounds and Enantiomeric Separation of Chiral Compounds of Dried Sancho (Zanthoxylum schinifolium Siebold & Zucc). Food Sci. Biotechnol. 2009, 18, 18–24. [Google Scholar]
- Xia, W.; Budge, S.M. Techniques for the Analysis of Minor Lipid Oxidation Products Derived from Triacylglycerols: Epoxides, Alcohols, and Ketones. Compr. Rev. Food Sci. Food Saf. 2017, 16, 735–756. [Google Scholar] [CrossRef]
- Ni, R.; Yan, H.; Tian, H.; Zhan, P.; Zhang, Y. Characterization of key odorants in fried red and green huajiao (Zanthoxylum bungeanum maxim. and Zanthoxylum schinifolium sieb. et Zucc.) oils. Food Chem. 2022, 377, 131984. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Peng, Y.; Li, M.; Wen, X.; Ni, Y. Research progress on extraction process of Zanthoxylum bungeanum oil and Zanthoxylum bungeanum seed oil. China Oils Fats 2024, 49, 16–21. [Google Scholar]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; Tekinay, T.; Uyar, T. Electrospinning of cyclodextrin/linalool-inclusion complex nanofibers: Fast-dissolving nanofibrous web with prolonged release and antibacterial activity. Food Chem. 2017, 231, 192–201. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, Y.; Shao, C.-Y.; Zhang, Y.; Shi, J.; Lv, H.-P.; Lin, Z. Enantiomeric analysis of linalool in teas using headspace solid-phase microextraction with chiral gas chromatography. Ind. Crops Prod. 2016, 83, 17–23. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, R.; Xu, X.; Yang, Z. Enzyme Catalytic Efficiencies and Relative Gene Expression Levels of (R)-Linalool Synthase and (S)-Linalool Synthase Determine the Proportion of Linalool Enantiomers in Camellia sinensis var. sinensis. J. Agric. Food Chem. 2020, 68, 10109–10117. [Google Scholar] [CrossRef]
- Song, S.; Zheng, F.; Tian, X.; Feng, T.; Yao, L.; Sun, M.; Shi, L. Evolution Analysis of Free Fatty Acids and Aroma-Active Compounds during Tallow Oxidation. Molecules 2022, 27, 352. [Google Scholar] [CrossRef]
- Qiao, Y.; Xie, B.J.; Zhang, Y.; Zhang, Y.; Fan, G.; Yao, X.L.; Pan, S.Y. Characterization of Aroma Active Compounds in Fruit Juice and Peel Oil of Jinchen Sweet Orange Fruit (Citrus sinensis (L.) Osbeck) by GC-MS and GC-O. Molecules 2008, 13, 1333–1344. [Google Scholar] [CrossRef]
- Gu, J.H.; Zhan, P.; Tian, H.L.; Wang, P.; He, W.Y.; Geng, J.Z.; Zhang, R.G. Study on the aroma formation pathways in huajiao (Zanthoxylum bungeanum M.) oil during the frying process: Instrumental, sensory, and correlation analysis. J. Food Compos. Anal. 2025, 146, 107968. [Google Scholar] [CrossRef]
- Xu, J.Y.; Deng, X.J.; Wu, Y.M.; Zhou, M.; Du, C.; Wang, Q.M.; Xia, Y.X.; He, J.J.; Yuan, W.X.; Wu, W.D.; et al. Characteristic Changes and Potential Markers of Flavour in Raw Pu-Erh Tea with Different Ageing Cycles Analysed by HPLC, HS-SPME-GC-MS, and OAV. Foods 2025, 14, 829. [Google Scholar] [CrossRef]
- Tang, N.; Liu, J.; Chen, X.A.; Yang, Y.; Chen, S.; Zhang, Z.; Zhou, A. Analysis of Volatile Components in Essential Oil of Fingered Citrons Citrus medica L. var. sarcodactylis Swingle Harvested in Guangdong Province at Different Times by GC-MS and GC-IMS. Food Sci. 2021, 42, 193–202. [Google Scholar]
- Zheng, T.; Su, K.-X.; Gao, M.-S.; Zhang, D.-L.; Chen, X.-Y.; Liu, S.-M. Chemotaxonomic variation in volatile component contents and their correlation between climate factors in Chinese prickly ash peels (Zanthoxylum bungeanum Maxim.). Food Chem. X 2021, 12, 100176. [Google Scholar] [CrossRef]
- Zhao, M.; Li, T.; Yang, F.; Cui, X.; Zou, T.; Song, H.; Liu, Y. Characterization of key aroma-active compounds in Hanyuan Zanthoxylum bungeanum by GC-O-MS and switchable GC x GC-O-MS. Food Chem. 2022, 385, 132659. [Google Scholar] [CrossRef]
- Luo, J.J.; Ke, J.X.; Hou, X.Y.; Li, S.S.; Luo, Q.Y.; Wu, H.J.; Shen, G.H.; Zhang, Z.Q. Composition, structure and flavor mechanism of numbing substances in Chinese prickly ash in the genus Zanthoxylum: A review. Food Chem. 2022, 373, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, P.; Sun, J.; Xie, M.; Wang, H.; Shi, T.; Yu, M. Analysis of roasted peanuts based on GC-MS combined with GC-IMS. Food Sci. Nutr. 2024, 12, 1888–1901. [Google Scholar] [CrossRef]
- Maji, T.K.; Baruah, I.; Dube, S.; Hussain, M.R. Microencapsulation of Zanthoxylum limonella oil (ZLO) in glutaraldehyde crosslinked gelatin for mosquito repellent application. Bioresour. Technol. 2007, 98, 840–844. [Google Scholar] [CrossRef]
- Meng, F.-B.; Lei, Y.-T.; Zhang, Q.; Li, Y.-C.; Chen, W.-J.; Liu, D.-Y. Encapsulation of Zanthoxylum bungeanum essential oil to enhance flavor stability and inhibit lipid oxidation of Chinese-style sausage. J. Sci. Food Agric. 2022, 102, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Xiong, H.; Cai, X.; Jiang, Y.; Zhao, X.; Miao, B. Evaluation of Loquat Jam Quality at Different Cooking Times Based on Physicochemical Parameters, GC-IMS and Intelligent Senses. Foods 2024, 13, 340. [Google Scholar] [CrossRef]
- Zhao, G.; Kuang, G.; Li, J.; Hadiatullah, H.; Chen, Z.; Wang, X.; Yao, Y.; Pan, Z.-H.; Wang, Y. Characterization of aldehydes and hydroxy acids as the main contribution to the traditional Chinese rose vinegar by flavor and taste analyses. Food Res. Int. 2020, 129, 108879. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
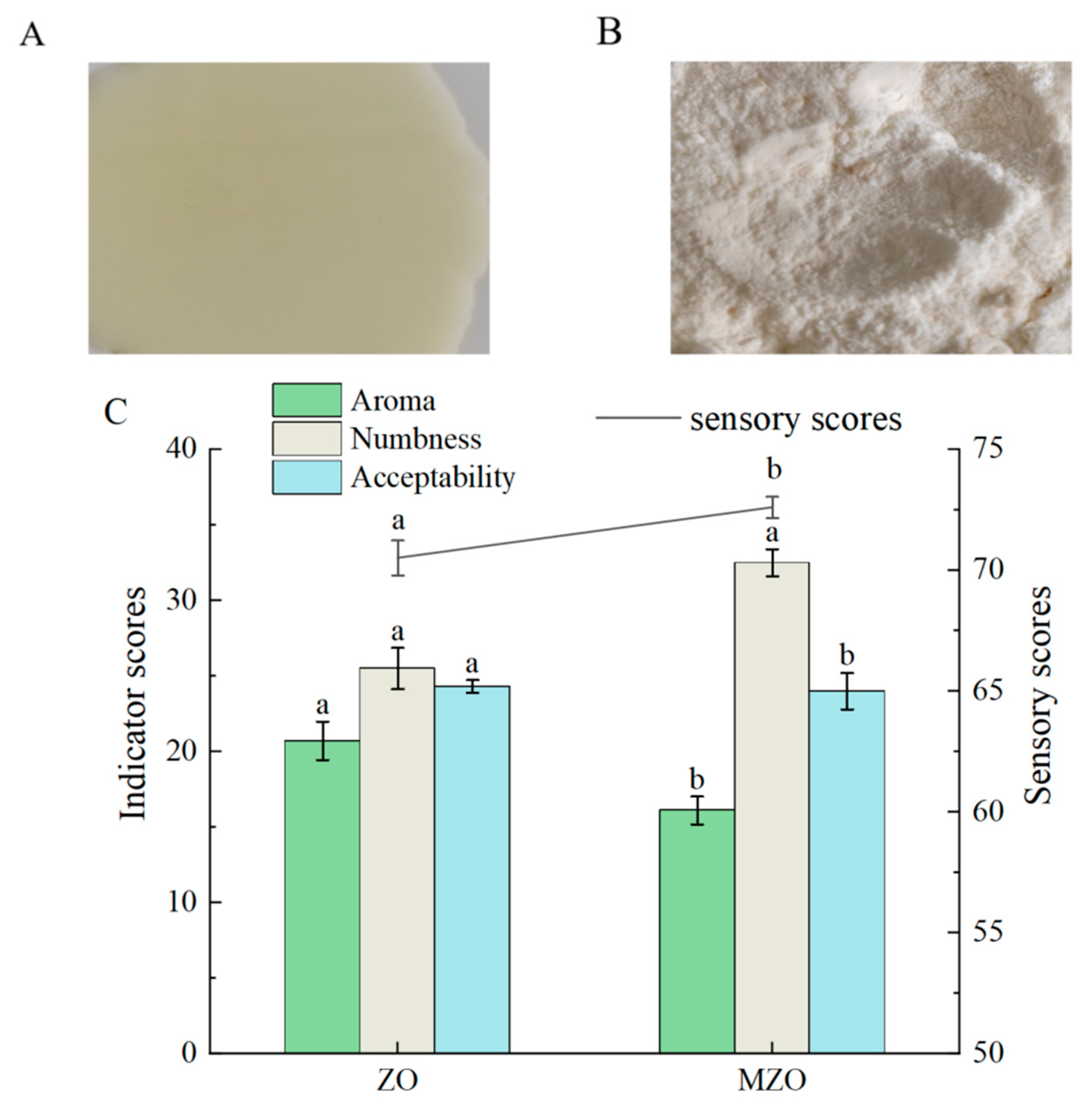



| Sample | L* | a* | b* | C* | h* | ΔE |
|---|---|---|---|---|---|---|
| ZO | 67.28 ± 0.20 b | 4.73 ± 0.06 b | 19.71 ± 0.29 b | 20.27 ± 0.27 b | 76.01 ± 0.33 a | 20.12 ± 0.10 a |
| MZO | 88.07 ± 0.11 a | 5.74 ± 0.05 a | 21.93 ± 0.26 a | 22.66 ± 0.26 a | 75.33 ± 0.05 a | 10.13 ± 0.23 b |
| Sensory Attribute | Descriptive Criteria | Score |
|---|---|---|
| Aroma Intensity | 1–10: Absent or weak aroma | 1–30 |
| 11–20: Moderate aroma without off-notes | ||
| 21–30: Strong, characteristic Zanthoxylum aroma | ||
| Numbing Sensation | 1–13: No or very slight numbness | 1–40 |
| 14–27: Noticeable and lingering numbness | ||
| 28–40: Strong trigeminal numbing effect | ||
| Acceptability | 1–10: Unacceptable | 1–30 |
| 11–20: Moderately acceptable | ||
| 21–30: Highly acceptable |
| Sensors | Performance Description | Sensors | Performance Description |
|---|---|---|---|
| LY2/LG | Sensitive to oxidizing gas | P40/1 | Sensitive to fluorine |
| LY2/G | Sensitive to ammonia, carbon monoxide | T70/2 | Sensitive to aromatic compounds |
| LY2/AA | Sensitive to ethanol | PA/2 | Sensitive to ethanol, ammonia/organic amines |
| LY2/Gh | Sensitive to ammonia/organic amines | P30/1 | Sensitive to polar compounds (ethanol) |
| LY2/gCT1 | Sensitive to hydrogen sulfide | P40/2 | Sensitive to heteroatom/chloride/aldehydes |
| LY2/gCT | Sensitive to propane/butane | P30/2 | Sensitive to alcohol |
| T30/1 | Sensitive to organic solvents | T40/2 | Sensitive to aldehydes |
| P10/1 | Sensitive to hydrocarbons | T40/1 | Sensitive to chlorinated compounds |
| P10/2 | Sensitive to methane | TA/2 | Sensitive to air quality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Chen, J.; Cai, X.; Li, D.; Zhao, X.; Fu, Y.; Huang, L.; Rao, Y.; Yi, Y.; Qiao, M.; et al. The Effects of Microencapsulation Technology on the Flavor Quality of Zanthoxylum Oil Based on E-Nose, GC–IMS, and GC–MS. Molecules 2025, 30, 3366. https://doi.org/10.3390/molecules30163366
Wang L, Chen J, Cai X, Li D, Zhao X, Fu Y, Huang L, Rao Y, Yi Y, Qiao M, et al. The Effects of Microencapsulation Technology on the Flavor Quality of Zanthoxylum Oil Based on E-Nose, GC–IMS, and GC–MS. Molecules. 2025; 30(16):3366. https://doi.org/10.3390/molecules30163366
Chicago/Turabian StyleWang, Liangyun, Jia Chen, Xuemei Cai, Dandan Li, Xinxin Zhao, Yu Fu, Lei Huang, Yi Rao, Yuwen Yi, Mingfeng Qiao, and et al. 2025. "The Effects of Microencapsulation Technology on the Flavor Quality of Zanthoxylum Oil Based on E-Nose, GC–IMS, and GC–MS" Molecules 30, no. 16: 3366. https://doi.org/10.3390/molecules30163366
APA StyleWang, L., Chen, J., Cai, X., Li, D., Zhao, X., Fu, Y., Huang, L., Rao, Y., Yi, Y., Qiao, M., & Miao, B. (2025). The Effects of Microencapsulation Technology on the Flavor Quality of Zanthoxylum Oil Based on E-Nose, GC–IMS, and GC–MS. Molecules, 30(16), 3366. https://doi.org/10.3390/molecules30163366






