Sour Fruit Beers—Ethanol and Lactic Acid Fermentation in Beer Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Basic Physicochemical Parameters
2.2. Content of Fermentation Products and Carbohydrate Profile of Beers
2.3. Total Phenolic Content and Antioxidant Activity
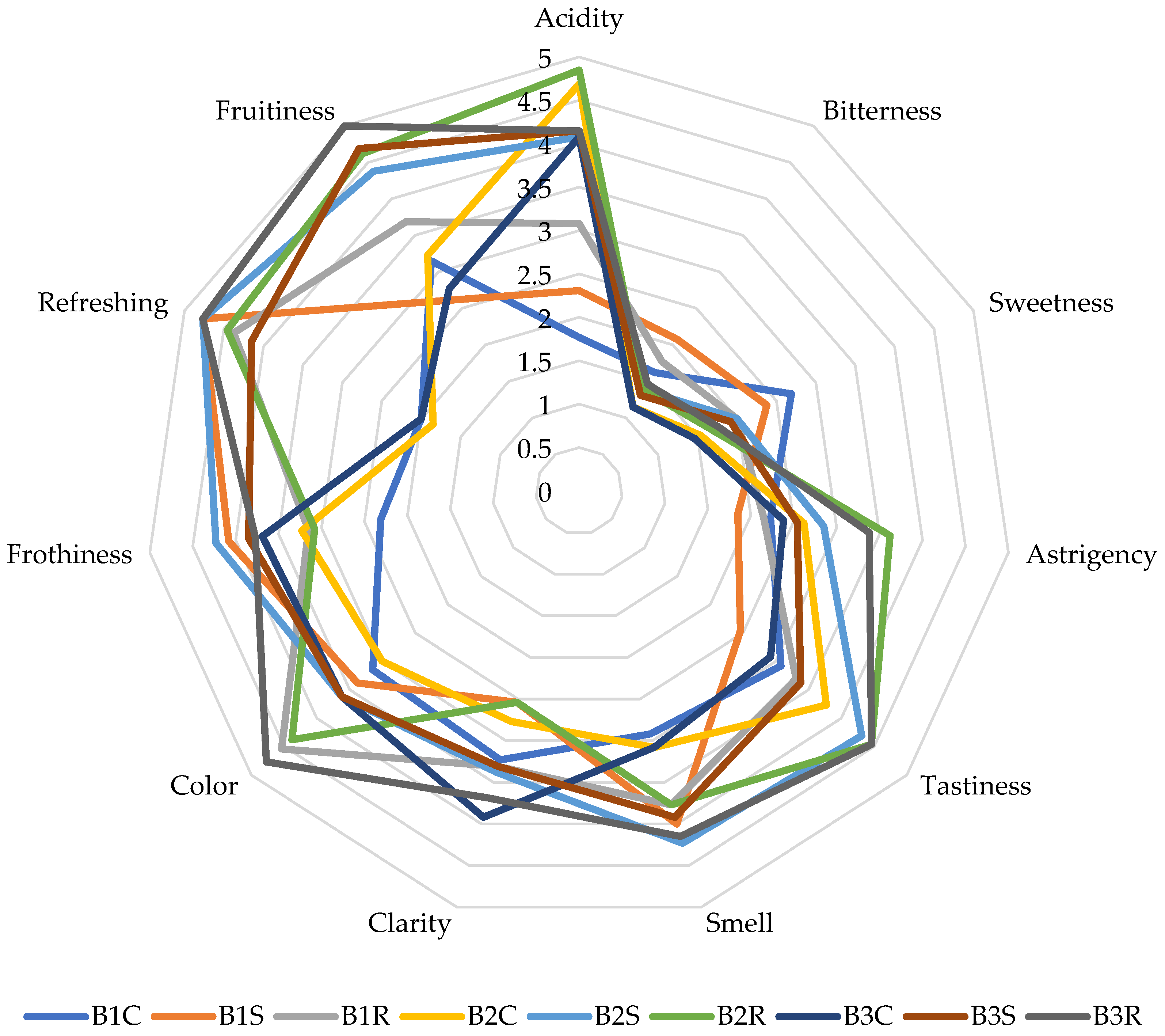
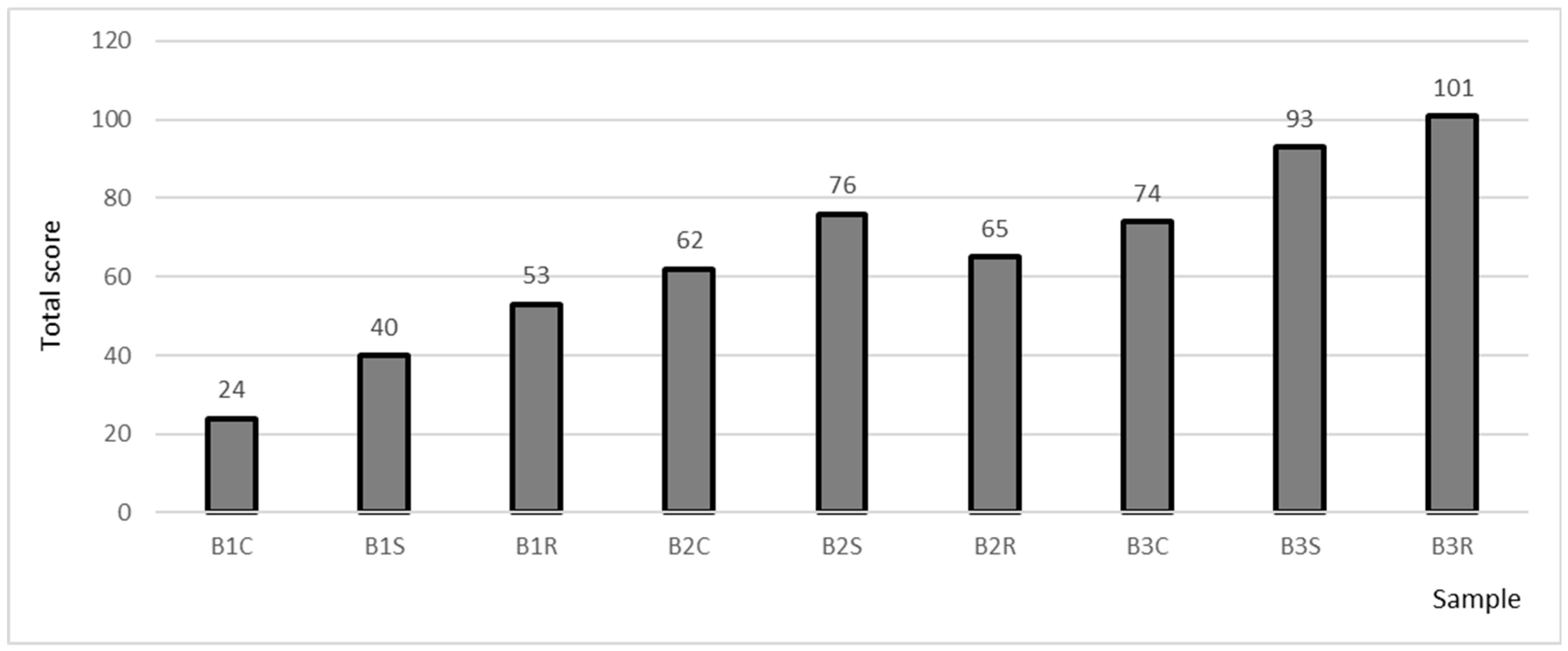
2.4. Sensory Analysis
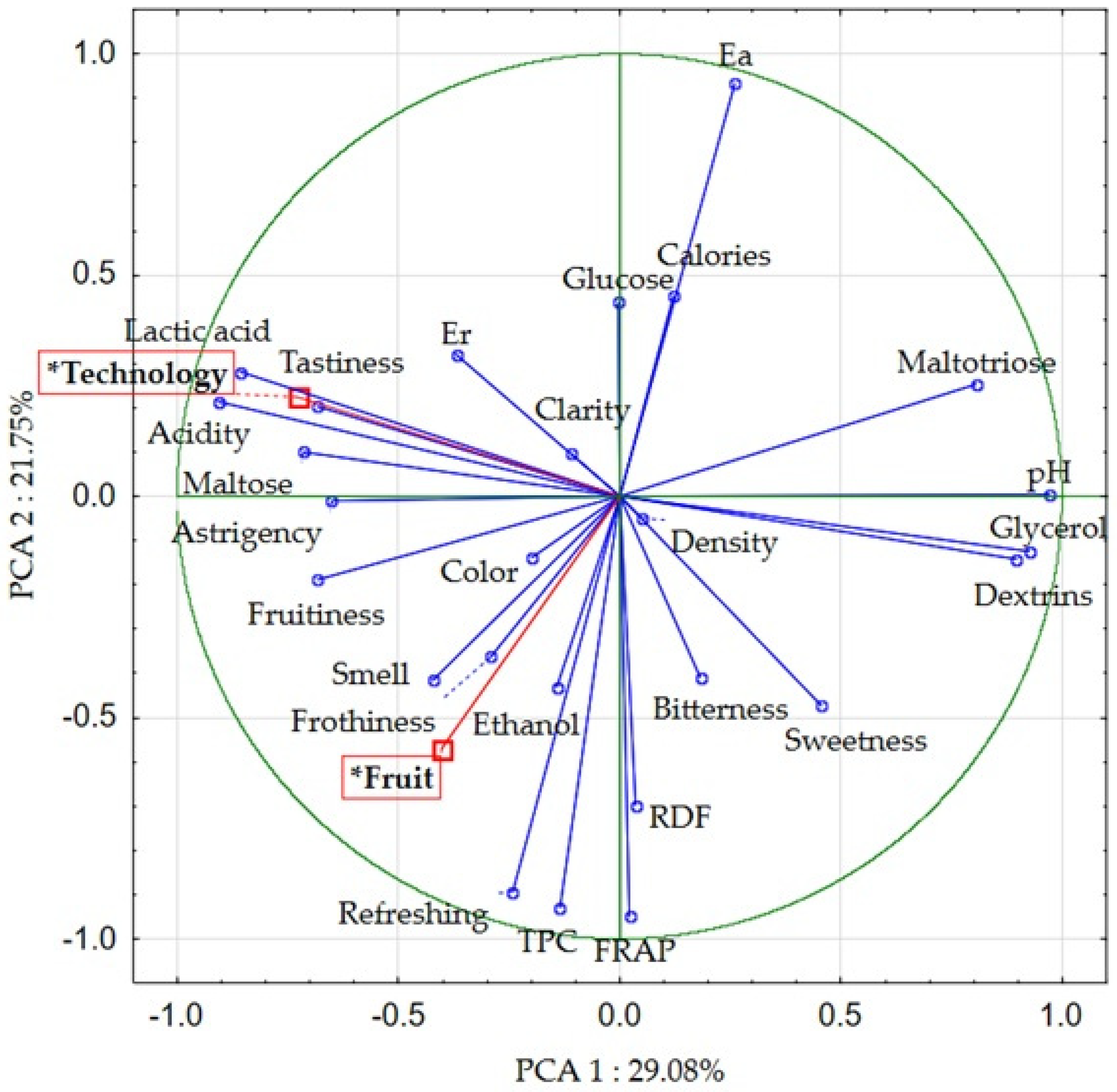
Principal Component Analysis (PCA) and Correlation Analysis of the Research Results
3. Materials and Methods
3.1. Materials and Sample Preparation
3.1.1. Raw Materials
3.1.2. Biological Material
3.1.3. Brewing Technology
3.2. Analytical Methods
3.2.1. High-Performance Liquid Chromatography (HPLC) Analysis of Carbohydrate, Glycerol, Ethanol and Lactic Acid Content
3.2.2. Total Phenolic Content
3.2.3. Ability of Iron Ion Reduction (FRAP)
3.2.4. Basic Physicochemical Parameters
3.3. Sensory Evaluation and Consumer Preferences
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Gąsior, J.; Głowacki, A. Assessment of volatiles and polyphenol content, physicochemical parameters and antioxidant activity in beers with dotted hawthorn (Crataegus punctata). Foods 2020, 9, 775. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Prorok, P.; Piórecki, N. Physicochemical and antioxidative properties of Cornelian cherry beer. Food Chem. 2019, 281, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with goji berries—The effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Cabras, I.; Bamforth, C. From reviving tradition to fostering innovation and changing marketing: The evolution of micro-brewing in the UK and US, 1980–2012. Bus. Hist. 2016, 58, 625–646. [Google Scholar] [CrossRef]
- Bossaert, S.; Crauwels, S.; De Rouck, G.; Lievens, B. The power of sour—A rewiev: Old traditions, new opportunities. Brew. Sci. 2019, 72, 78–88. [Google Scholar] [CrossRef]
- Dysvik, A.; Liland, K.H.; Myhrer, K.S.; Westereng, B.; Rukke, E.-O.; de Rouck, G.; Wicklund, T. Pre-fermentation with lactic acid bacteria in sour beer production. J. Inst. Brew. 2019, 125, 342–356. [Google Scholar] [CrossRef]
- Mastanjević, K.; Krstanović, V.; Lukinac, J.; Jukić, M.; Vulin, Z.; Mastanjević, K. Beer—The Importance of Colloidal Stability (Non-Biological Haze). Fermentation 2018, 4, 91. [Google Scholar] [CrossRef]
- Vaughan, A.; O’Sullivan, T.; van Sinderen, D. Enhancing the Microbiological Stability of Malt and Beer—A Review. J. Inst. Brew. 2005, 111, 355–371. [Google Scholar] [CrossRef]
- Gąsior, J.; Kawa-Rygielska, J.; Kucharska, A.Z. Carbohydrates profile, polyphenols content and antioxidative properties of beer worts produced with different dark malts varieties or roasted barley grains. Molecules 2020, 25, 3882. [Google Scholar] [CrossRef]
- Wiliamson, G. The Role of Polyphenols in Modern Nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, S.C. The Case of Anthocyanin Consumption to Promote Human Health: A Review. Comprevensive Rev. Food Sci. Food Saf. 2013, 12, 493–508. [Google Scholar] [CrossRef]
- Dysvik, A.; La Rosa, S.L.; De Rouck, G.; Rukke, E.-O.; Westereng, B.; Wicklund, T.; Ercolini, D. Microbial Dynamics in Traditional and Modern Sour Beer Production. Appl. Environ. Microbiol. 2020, 86, e00566-20. [Google Scholar] [CrossRef]
- Ciosek, A.; Rusiecka, I.; Poreda, A. Sour beer production: Impact of pitching sequence of yeast and lactic acid bacteria. J. Inst. Brew. 2020, 126, 53–58. [Google Scholar] [CrossRef]
- Dack, R.E.; Black, G.W.; Koutsidis, G. The effect of Maillard reaction products and yeast strain on the synthesis of key higher alcohols and esters in beer fermentations. Food Chem. 2017, 232, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Prado, R.; Gastl, M.; Becker, T. Aroma and color development during the production of specialty malts: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4816–4840. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Kim, Y.J.; Shin, Y. Influence of ripening stage and cultivar on physicochemical properties, sugar and organic acid profiles, and antioxidant compositions of strawberries. Food Sci. Biotechnol. 2019, 28, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- De Souza, V.R.; Pereira, P.A.P.; da Silva, T.L.T.; De Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Chua, J.Y.; Toh, M.; Liu, S.-Q. Survival of probiotic strain Lactobacillus paracasei L26 during co-fermentation with S. cerevisiae for the development of a novel beer beverage. Food Microbiol. 2019, 82, 541–550. [Google Scholar] [CrossRef]
- Wang, Z.; Zhuge, J.; Fang, H.; Prior, B.A. Glycerol production by microbial fermentation: A review. Biotechnol. Adv. 2001, 19, 201–223. [Google Scholar] [CrossRef]
- Zhao, X.; Procopio, S.; Becker, T. Flavor impacts of glycerol in the processing of yeast fermented beverages: A review. J. Food Sci. Technol. 2015, 52, 7588–7598. [Google Scholar] [CrossRef]
- Onilude, A.A.; Ayinla, G.S.; Eluehike, C. Properties of Alpha-amylase of Lactobacillus plantarum Isolated from Cassava Waste Samples. Biotechnol. J. Int. 2017, 19, 1–14. [Google Scholar] [CrossRef]
- Yang, D.; Gao, X. Research progress on the antioxidant biological activity of beer and strategy for applications. Trends Food Sci. Technol. 2021, 110, 754–764. [Google Scholar] [CrossRef]
- Nardini, M.; Garaguso, I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020, 305, 125437. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin− Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Muñoz, R.; de Las Rivas, B.; de Felipe, F.L.; Reverón, I.; Santamaría, L.; Esteban-Torres, M.; Curiel, J.A.; Rodríguez, H.; Landete, J.M. Biotransformation of phenolics by Lactobacillus plantarum in fermented foods. In Fermented Foods in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2017; pp. 63–83. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gąsior, J.; Pietrzak, W. Volatile Compounds Content, Physicochemical Parameters, and Antioxidant Activity of Beers with Addition of Mango Fruit (Mangifera Indica). Molecules 2020, 25, 3033. [Google Scholar] [CrossRef] [PubMed]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.Z. Characteristics of Cornelian cherry sour non-alcoholic beers brewed with the special yeast Saccharomycodes ludwigii. Food Chem. 2020, 312, 125968. [Google Scholar] [CrossRef]
- Ritter, S.; Dölle, K.; Bargen, M.; Piatkowski, J. Fruits in Craft Beer: A Study to Evaluate the Impact of Fruits on the pH in the Brewing Process and the Breweries Waste Water. Adv. Res. 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]

| Technology | Symbol | Alcohol [%v/v] | Apparent Extract [%w/w] | RDF [%] | Density [g/cm3] | Calories [kcal/100 mL] | pH |
|---|---|---|---|---|---|---|---|
| T1 | B1C | 2.75 ± 0.01 1 f | 3.25 ± 0.05 c | 57.83 ± 0.05 g | 0.9961 ± 0.00 ab | 26.16 ± 0.00 d | 4.79 ± 0.01 a |
| B1S | 3.00 ± 0.01 c | 2.59 ± 0.01 h | 65.30 ± 0.06 b | 0.9958 ± 0.00 b | 25.36 ± 0.00 f | 4.24 ± 0.01 b | |
| B1R | 2.66 ± 0.01 g | 2.90 ± 0.01 d | 59.99 ± 0.02 d | 0.9966 ± 0.00 a | 22.92 ± 0.00 g | 4.23 ± 0.01 b | |
| T2 | B2C | 2.76 ± 0.00 f | 3.32 ± 0.01 b | 58.06 ± 0.55 f | 0.9957 ± 0.00 b | 27.81 ± 0.00 b | 3.70 ± 0.01 d |
| B2S | 3.08 ± 0.01 b | 2.59 ± 0.04 h | 65.60 ± 0.23 a | 0.9958 ± 0.00 b | 25.58 ± 0.00 e | 3.66 ± 0.01 e | |
| B2R | 2.52 ± 0.00 h | 2.80 ± 0.02 f | 63.22 ± 0.21 c | 0.9967 ± 0.00 a | 21.70 ± 0.00 h | 3.56 ± 0.01 g | |
| T3 | B3C | 2.89 ± 0.01 e | 3.38 ± 0.01 a | 57.74 ± 0.73 h | 0.9957 ± 0.00 b | 28.26 ± 0.00 a | 3.81 ± 0.01 c |
| B3S | 3.21 ± 0.01 a | 2.74 ± 0.04 g | 59.53 ± 0.00 e | 0.9956 ± 0.00 b | 26.53 ± 0.00 c | 3.60 ± 0.02 f | |
| B3R | 2.97 ± 0.00 d | 2.86 ± 0.01 e | 55.31 ± 0.59 i | 0.9959 ± 0.00 b | 25.34 ± 0.00 f | 3.53 ± 0.01 h |
| Technology | Symbol | Glucose [g/L] | Maltose [g/L] | Maltotriose [g/L] | Dextrins [g/L] | Glycerol [g/L] | Lactic Acid [g/L] |
|---|---|---|---|---|---|---|---|
| T1 | B1C | 0.28 ± 0.00 1 f | 0.00 ± 0.00 d | 2.60 ± 0.01 a | 23.13 ± 0.01 a | 1.62 ± 0.00 a | 0.00 ± 0.00 g |
| B1S | 0.00 ± 0.00 g | 0.00 ± 0.00 d | 1.08 ± 0.01 c | 19.07 ± 0.01 b | 1.36 ± 0.00 b | 0.00 ± 0.00 g | |
| B1R | 0.76 ± 0.00 a | 0.00 ± 0.00 d | 0.84 ± 0.00 e | 17.64 ± 0.04 c | 1.38 ± 0.01 b | 0.00 ± 0.00 g | |
| T2 | B2C | 0.38 ± 0.00 e | 0.32 ± 0.01 b | 0.58 ± 0.02 g | 13.26 ± 0.04 h | 1.07 ± 0.04 e | 2.08 ± 0.00 d |
| B2S | 0.00 ± 0.00 g | 0.00 ± 0.00 d | 0.58 ± 0.00 g | 16.17 ± 0.04 f | 1.11 ± 0.01 cd | 2.21 ± 0.02 b | |
| B2R | 0.53 ± 0.00 b | 0.32 ± 0.00 b | 0.46 ± 0.01 h | 10.73 ± 0.08 i | 0.83 ± 0.02 f | 1.70 ± 0.01 f | |
| T3 | B3C | 0.39 ± 0.00 d | 0.00 ± 0.00 d | 1.39 ± 0.01 b | 16.31 ± 0.06 e | 1.07 ± 0.03 e | 2.14 ± 0.00 c |
| B3S | 0.00 ± 0.00 g | 0.36 ± 0.00 a | 0.87 ± 0.00 d | 16.71 ± 0.06 d | 1.12 ± 0.01 c | 2.30 ± 0.01 a | |
| B3R | 0.44 ± 0.00 c | 0.31 ± 0.00 c | 0.68 ± 0.00 f | 13.49 ± 0.01 g | 1.08 ± 0.00 de | 2.05 ± 0.00 e |
| Technology | Symbol | Worts | Beers | |||
|---|---|---|---|---|---|---|
| Sweet | Hopped | Primary Fermented | Post Fermentated * | Maturated | ||
| T1 | B1C | 39.54 ± 1.64 1 m | 84.87 ± 0.92 m | 111.55 ± 0.81 l | 174.50 ± 1.06 gh | 153.56 ± 20.73 ij |
| B1S | 191.74 ± 2.41 de | 237.03 ± 1.96 a | ||||
| B1R | 126.44 ± 2.25 k | 207.24 ± 0.94 bc | ||||
| T2 | B2C | 58.27 ± 1.72 n | 112.91 ± 0.50 l | 176.42 ± 0.30 fgh | 150.14 ± 1.06 j | 155.24 ± 1.48 ij |
| B2S | 234.21 ± 1.91 a | 212.25 ± 0.86 b | ||||
| B2R | 163.90 ± 1.96 hij | 204.73 ± 0.24 bcd | ||||
| T3 | B3C | 188.55 ± 0.60 ef | 151.39 ± 0.26 ij | 193.80 ± 1.82 cde | 165.24 ± 0.73 hi | |
| B3S | 255.02 ± 1.21 a | 205.21 ± 0.20 bcd | ||||
| B3R | 203.63 ± 1.43 bcd | 187.79 ± 1.94 efg | ||||
| Technology | Symbol | Worts | Beers | |||
|---|---|---|---|---|---|---|
| Sweet | Hopped | Primary Fermented | Post Fermentated * | Maturated | ||
| T1 | B1C | 0.41 ± 0.01 1 jk | 0.63 ± 0.01 gh | 0.70 ± 0.01 fg | 0.56 ± 0.00 h | 0.70 ± 0.02 fg |
| B1S | 1.17 ± 0.03 a | 1.17 ± 0.02 a | ||||
| B1R | 0.90 ± 0.01 cd | 0.85 ± 0.04 de | ||||
| T2 | B2C | 0.31 ± 0.00 k | 0.46 ± 0.01 ij | 0.60 ± 0.00 h | 0.61 ± 0.01 gh | 0.54 ± 0.02 hi |
| B2S | 0.98 ± 0.00 bc | 0.82 ± 0.01 de | ||||
| B2R | 0.78 ± 0.01 ef | 0.89 ± 0.00 cd | ||||
| T3 | B3C | 0.36 ± 0.00 k | 0.63 ± 0.01 gh | 0.63 ± 0.01 gh | 0.56 ± 0.01 h | |
| B3S | 1.06 ± 0.06 bc | 1.02 ± 0.02 b | ||||
| B3R | 0.71 ± 0.02 fg | 0.83 ± 0.01 de | ||||
| Technology | T1 | T2 | T3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sweet wort | Non- acidified | Acidified | |||||||
| Hopped wort | Boiled for 60’ | Boiled for 60’ | Boiled for 5’ | ||||||
| Primary fermented beers | Non-acidified young beer | Acidified young beer | Acidified young beer | ||||||
| Post-fermented beers | Control beer | Strawberry beer | Raspberry beer | Control beer | Strawberry beer | Raspberry beer | Control beer | Strawberry beer | Raspberry beer |
| Maturated beers | Control beer (B1C) | Strawberry beer (B1S) | Raspberry beer (B1R) | Control beer (B2C) | Strawberry beer (B2S) | Raspberry beer (B2R) | Control beer (B3C) | Strawberry beer (B3S) | Raspberry beer (B3R) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głowacki, A.; Paszkot, J.; Pietrzak, W.; Kawa-Rygielska, J. Sour Fruit Beers—Ethanol and Lactic Acid Fermentation in Beer Production. Molecules 2025, 30, 3358. https://doi.org/10.3390/molecules30163358
Głowacki A, Paszkot J, Pietrzak W, Kawa-Rygielska J. Sour Fruit Beers—Ethanol and Lactic Acid Fermentation in Beer Production. Molecules. 2025; 30(16):3358. https://doi.org/10.3390/molecules30163358
Chicago/Turabian StyleGłowacki, Adam, Justyna Paszkot, Witold Pietrzak, and Joanna Kawa-Rygielska. 2025. "Sour Fruit Beers—Ethanol and Lactic Acid Fermentation in Beer Production" Molecules 30, no. 16: 3358. https://doi.org/10.3390/molecules30163358
APA StyleGłowacki, A., Paszkot, J., Pietrzak, W., & Kawa-Rygielska, J. (2025). Sour Fruit Beers—Ethanol and Lactic Acid Fermentation in Beer Production. Molecules, 30(16), 3358. https://doi.org/10.3390/molecules30163358






