Deep Eutectic Solvent Systems as Media for the Selective Extraction of Anti-Inflammatory Bioactive Agents
Abstract
1. Introduction
1.1. Anti-Inflammatory Agents: Types and Mechanisms
1.2. Bioactive Compounds: Emerging Anti-Inflammatory Agents
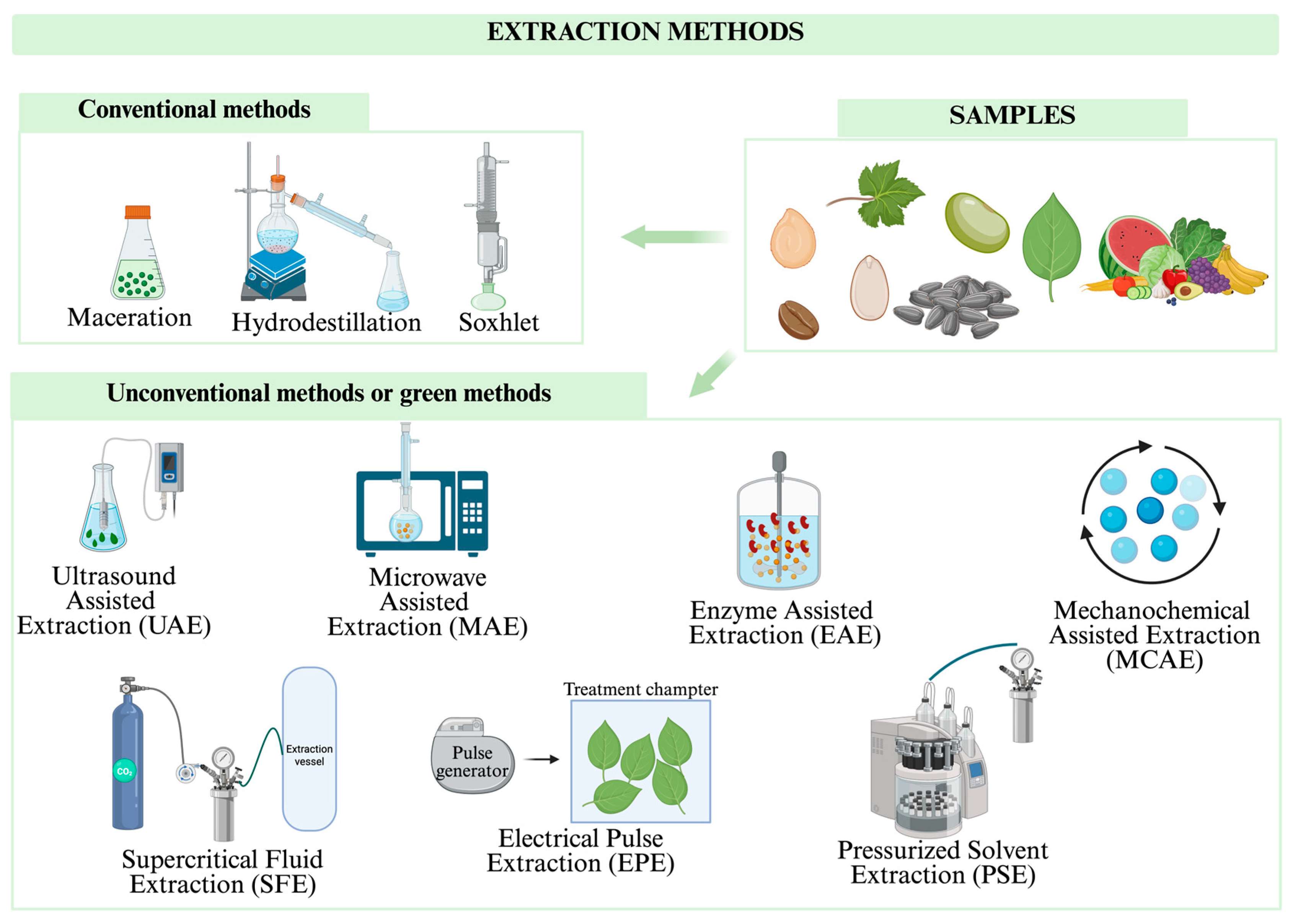
1.3. Extraction Methods for Bioactive Compounds
1.4. Deep Eutectic Solvents as Extraction Media
2. Applications of Deep Eutectic Solvents in the Extraction of Anti-Inflammatory Bioactive Compounds
| Bioactive Molecule | Type of Bioactive Molecule | Specie | DES | Extraction Method | Extraction (Yield (mg/g)) | Reference | |
|---|---|---|---|---|---|---|---|
| Components | Ratio | ||||||
| Curcumin | Phenol (curcuminoids) | Curcuma longa | ChCl–Glycerol–Water | 1–1–5 | Stirring-assisted extraction | 2.1 | [59] |
| ChCl–Fructose–Water | 1–1–5 | 0.7 | |||||
| ChCl–Glycerol–Water | 1–2–5 | 0.5 | |||||
| ChCl–Glucose–Water | 1–1–3 | 1.6 | |||||
| ChCl–Glycerol–Water | 1–1–2 | 2.0 | |||||
| ChCl–Citric acid–Water | 1–2–5 | 0.4 | |||||
| ChCl–Citric acid–Water | 2–1–5 | 0.5 | |||||
| ChCl–Glycerol | 1–1 | 0.4 | |||||
| ChCl–Glycerol | 1–2 | 0.4 | |||||
| ChCl–Glycerol | 1–3 | 0.3 | |||||
| Glycerol–Urea–Water | 1–1–2 | 0.1 | |||||
| ChCl–Xylitol–Water | 1–1–5 | 0.5 | |||||
| ChCl–Citric acid–Water | 1–2–3 | 0.4 | |||||
| ChCl–Lactic acid–Water | 1–2–5 | 1.4 | |||||
| ChCl–Citric acid–Water | 1–1–5 | 2.0 | |||||
| ChCl–Urea–Water | 1–2–5 | 0.7 | |||||
| ChCl–Glycerol–Citric acid–Water | 0.5–2–0.5–5 | 2.7 | |||||
| ChCl–1,2-propanediol–Water | 1:1:1 | 3.1 | |||||
| Ascorbic acid, citric acid, cinnamic acid, gallic acid, catechin, quercetin, coumaric acid, tartaric acid, catechol, curcumin, pyrogallic acid, caffeic acid, tannic acid, etc. | Phenolic compounds | Clematis fammula L. Leaves and Pistacia lentiscus L. Fruits | ChCl–Acetic acid | 1:2 | UAE | Not given in detail (>80%) | [60] |
| Anthocyanin-rich fraction; non-anthocyanin phenolic fraction. 42% anthocyanins: delphinidin, cyanidin, petunidin, malvidin, etc. | Polyphenols (anthocyanins) | Vaccinium corymbosum L. x Vaccinium darrowii Camp. | ChCl–Glycerol–Citric acid | 0.5:2:0.5 | UAE | 11.4 mg/g (4.57 mg of total polyphenols/mL, with the extract coming from a solution containing ~0.4 g/mL of freeze-dried powder) | [62] |
| Protocatechuic acid, chlorogenic acid isomer, chlorogenic acid, caffeine acid, p-coumaric acid, rutin | Polyphenols | Dried seeds (fruits) of Coriandrum sativum L. | ChCl–Glucose | 1–1 | UAE | Yield data obtained (mg/g) for each compound using ChCl–Urea and ChCl–glucose, respectively– - Chlorogenic acid isomer– 0.537 mg/g and 0.184 mg/g. - Chlorogenic acid– 0.453 mg/g and 0.152 mg/g. - Caffeic acid– 0.093 mg/g and 0.269 mg/g. - p-coumaric acid– 0.030 mg/g and 0.057 mg/g. - Rutin– 0.766 mg/g and 0.235 mg/g | [63] |
| ChCl–Glycerol | 1–1 | ||||||
| ChCl–Urea | 1:1 | ||||||
| ChChl–Citric acid | 1:1 | ||||||
| naringenin, naringin, hesperidin, vitexin, apigenin, luteolin, and luteolin 7-glucoside, quercetin, quercitrin, rutin, kaempferol, phloretin, phloridzin, genistein | Flavonoids | Lippia graveolens | ChCl–Ethyleneglycol–Water | 1–4–30% Water v/v | Maceration, UAE, Supercritical Fluid | 39.5 | [64] |
| ChCl–Glycerol–Water | 1–4–30% Water v/v | 39.4 | |||||
| ChCl–Lactic acid–Water | 1–4–30% Water v/v | 37.3 | |||||
| Phenolic compounds | ChCl–Ethyleneglycol–Water | 1–4–30% Water v/v | 100.1 | ||||
| ChCl–Glycerol–Water | 1–4–30% Water v/v | 123.6 | |||||
| ChCl–Lactic acid–Water | 1–4–30% Water v/v | 126.1 | |||||
| Bilberry | Anthocyanins | Vaccinium myrtillus | ChCl–Lactic acid | 1–2 | UAE | 2.48 | [65] |
| ChCl–Citric acid–Water | 1–1–2 | 1.78 | |||||
| ChCl–Malic acid–Water | 1–1–2 | 2.24 | |||||
| ChCl–Tartaric acid | 1–2 | 1.25 | |||||
| ChCl–Glycerol | 1–2 | 1.90 | |||||
| ChCl–1,2-propanediol | 1–3 | 2.00 | |||||
| ChCl–Sorbitol | 1–1 | 2.03 | |||||
| ChCl–Glucose–Water | 2–1–1 | 1.74 | |||||
| ChCl–Fructose–Water | 2–1–1 | 1.64 | |||||
| ChCl–Urea | 1–2 | 0.92 | |||||
| Comfrey | Rosmarinic acid | Symphytum officinale | ChCl–Glycerol | 1–2 | UAE | <0.5 | [69] |
| ChCl–Urea | 1–2 | ||||||
| ChCl–Sucrose | 1–2 | ||||||
| Turmeric—curcumin | Curcuminoids | Curcuma longa | ChCl–L | 1–1 | DoE-Optimized (Box–Behnken Designed) Supercritical CO2 Extraction. | 13.8 | [70] |
| ChCl–Citric acid | 1–1 | 8.22 | |||||
| ChCl–Urea | 1–2 | 12.5 | |||||
| ChCl–Propylene glycol | 1–2 | 23.1 | |||||
| Hydroxytyrosol, tyrosol, Hy-Ac, oleacein (Hy-EDA), oleocanthal, 1-acetoxypinoresinol, Hy-EA, luteolin, ty-EA, apigenin | Phenolic compounds | Olea europaea (Olive oil) | ChCl–Glycerol | 1–2 | Shaking extraction at 40 °C | 0.150 | [71] |
| ChCl–Lactic acid | 1–3 | 0.073 | |||||
| ChCl–Urea | 1–4 | - | |||||
| ChCl–Sucrose | 1–1 | 0.140 | |||||
| ChCl–Sucrose | 4–1 | 0.058 | |||||
| ChCl–1,4-butanediol | 1–5 | 0.120 | |||||
| ChCl–Xylitol | 2–1 | 0.190 | |||||
| ChCl–1,2-propanediol | 1–1 | 0.170 | |||||
| ChCl–Malonic acid | 1–1 | 0.085 | |||||
| ChCl–Urea–Glycerol | 1–1–1 | - | |||||
| a-Mangostin | Polyphenol | Mangosteen (Garcinia mangostana L.) | ChCl–1,2-propanediol | 1–3 | Shaking-assisted extraction at room temp. | 5.20 | [72] |
| Rutin | Flavonoid | Sophora japonica | ChCl–1,4-butanediol–Water | 1–4–20% Water v/v | Stirring-assisted solid–liquid extraction | 170 | [68] |
| ChCl–Acetamide–Water | 1–2–20% Water v/v | 170 | |||||
| ChCl–Citric acid–Water | 1–1–20% Water v/v | 100 | |||||
| ChCl–Sorbitol–Water | 1–1–20% Water v/v | 100 | |||||
| ChCl–Ethyleneglycol–Water | 1–2–20% Water v/v | 180 | |||||
| ChCl–Fructose–Water | 5–2–20% Water v/v | 60 | |||||
| ChCl–Glucose–Water | 5–2–20% Water v/v | 100 | |||||
| ChCl–Glycerol–Water | 1–2–20% Water v/v | 150 | |||||
| ChCl–Levunillic acid–Water | 1–2–20% Water v/v | 200 | |||||
| ChCl–Malic acid–Water | 1–1–20% Water v/v | 100 | |||||
| ChCl–Malonic acid–Water | 1–1–20% Water v/v | 200 | |||||
| ChCl–Maltose–Water | 5–2–20% Water v/v | 50 | |||||
| ChCl–Oxalic acid–Water | 1–1–20% Water v/v | 100 | |||||
| ChCl–p-toluenesulfonic acid–Water | 1–1–20% Water v/v | 100 | |||||
| ChCl–Sucrose–Water | 5–2–20% Water v/v | 50 | |||||
| ChCl–Tartaric acid–Water | 2–1–20% Water v/v | 120 | |||||
| ChCl–Triethylene glycol–Water | 1–4–20% Water v/v | 194 | |||||
| ChCl–Urea–Water | 1–2–20% Water v/v | 180 | |||||
| ChCl–Xylitol–Water | 1–1–20% Water v/v | 170 | |||||
| ChCl–Xylose–Water | 1–1–20% Water v/v | 50.0 | |||||
| Tocopherols and tocotrienols | Tocols | Elaeis guineensis Crude Palm Oil | ChCl–Malonic acid | 1–3 | Liquid–liquid extraction | 0.018 | [67] |
| Apigenin | Flavonoids | Cajanus cajan (Pigeon pea) roots | 1,6-hexanediol–ChCl–Water | 7–1–30% Water v/v | MAE | 0.220 | [73] |
| Genistin | Polyphenols | 1,6-hexanediol–ChCl–Water | 7–1–30% Water v/v | 0.450 | |||
| Genistein | Polyphenols | 1,6-hexanediol–ChCl–Water | 7–1–30% Water v/v | 0.620 | |||
| Chimaphilin | Phenols | Pyrola incarnata Fisch | ChCl–1,4-butanediol–Water | 1–4–30% Water v/v | MAE | 0.350 | [74] |
| Hyperin | 1.63 | ||||||
| 20-O-galloylhyperin | 4.96 | ||||||
| Quercetin | 0.041 | ||||||
| Quercetin-Orhamnoside | 0.089 | ||||||
| Chimaphilin | 0.350 | ||||||
| Rosmarinic acid | Polyphenol | Prunella vulgaris | ChCl–Ethylene glycol–Water | 1–4–36% Water v/v | Ultrasonic | 3.66 | [75] |
| Salviaflaside | ChCl–Ethylene glycol–Water | 1–4–36% Water v/v | Ultrasonic | 1.05 | [75] | ||
| Rutin | Flavonoid | Fagopyrum esculentum (Buckwheat) | ChCl–Glycerol–Water | 1–1–20% Water v/v | UAE | 9.50 | [76] |
| Rosmarinic acid | Phenolic compounds | Salvia rosmarinus | ChCl–Urea | 1–1 | Liquid–liquid extraction and ultrasonic water bath | 15.7 | [77] |
| Satureja hortensis | ChCl–Citric acid | 1–1 | 11.5 | ||||
| Lavandula angustifolia | ChCl–1,2-propanediol | 1–1 | 1.99 | ||||
| Salvia officianalis | ChCl–1,2-propanediol | 1–1 | 6.57 | ||||
| Melissa officinalis | ChCl–Urea | 1–1 | 19.5 | ||||
| Origanum vulgare var.h | ChCl–1,2-propanediol | 1–1 | 4.69 | ||||
| Ocimum basilicum | ChCl–Urea | 1–1 | 4.50 | ||||
| Thymus serpyllum | ChCl–1,2-propanediol | 1–1 | 12.4 | ||||
| Rosavin | Flavonoids, polyphenols | Rhodiola rosea L. | ChCl–Malonic acid | 1–1 | UAE | NADESs with malonic and tartaric acids showed the highest efficiency in extracting polyphenols, outperformed reference solvents by 10–20 mg GAE/g and reached a value of 160 mg GAE/g. | [78] |
| ChCl–Malic acid | 1–1 | ||||||
| ChCl–Citric acid | 1–1 | ||||||
| ChCl–Tartaric acid | 2–1 | ||||||
| Orientin and vitexin | Flavonoids | Trollius ledebouri, (dry flowers) | ChCl–1,3 butanediol | 1–2 | UAE | Orientin 13.5 mg/g, vitexin 1.30 mg/g | [79] |
| ChCl–1,4 butanediol | 1–3 | Orientin 14.5 mg/g, vitexin 1.25 | |||||
| ChCl–Ethylenglycol | 1–2 | Orientin 13.0 mg/g, vitexin 1.20 mg/g | |||||
| ChCl–Glycerine | 1–2 | Orientin 12.5 mg/g, vitexin 1.00 mg/g | |||||
| ChCl–Oxalic acid–Ethylenglicol | 1–1–2 | Orientin 16.5 mg/g, vitexin 1.50 mg/g | |||||
| ChCl–Sorbitol | 1–1 | Orientin 12.5 mg/g, vitexin 1.40 mg/g | |||||
| ChCl–Malonic acid | 1–1 | Orientin 14.2 mg/g, vitexin 1.30 mg/g | |||||
| ChCl–Lactic acid | 1–1 | Orientin 14.5 mg/g, vitexin 1.40 mg/g | |||||
| ChCL–Citric acid | 1–1 | Orientin 16.0 mg/g, vitexin 1.6 0 mg/g | |||||
| ChCl–Malic acid | 1–1 | Orientin 14.5 mg/g, vitexin 1.25 mg/g | |||||
| ChCl–Fructose–water | 2–1–1 | Orientin 14.0 mg/g, vitexin 1.60 mg/g | |||||
| ChCl–Glucose | 2–1 | Orientin 15.0 mg/g, vitexin 1.40 mg/g | |||||
| ChCl–Urea | 1–2 | Orientin 12.5 mg/g, vitexin 1.40 mg/g | |||||
| Phenolic compounds | Polyphenols | Olive leaves (OL) of the Picual variety | ChCl–oxalic acid | 1–1 | MCAE | 1.7 | [47] |
| ChCl–oxalic acid | 2–1 | 0.6 | |||||
| ChCl–urea | 1–2 | 0.8 | |||||
| ChCl–fructose–water | 5–2–5 | 0.6 | |||||
| ChCl–lactic acid | 1–2 | 0.7 | |||||
| ChCl–glucose | 3:1 | 1.3 | |||||
3. Strengths and Limitations in the Use of DESs for BCs Extraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, G.; Kuo, C. Bioactives and Inflammation. Curr. Issues Mol. Biol. 2023, 45, 5824–5829. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, V.V.; Gitaje, S.R.; Joshi, S.D.; Kharmate, S.V.; Phalle, D.R.; More, M.P.; Mali, M.A.; Shingade, P.P. Mechanisms of Inflammation Associated With Chronic Diseases: A Brief Review. J. Adv. Med. Med. Res. 2025, 37, 48–56. [Google Scholar] [CrossRef]
- Chavda, V.; Feehan, J.; Apostolopoulos, V. Inflammation: The Cause of All Diseases. Cells 2024, 13, 1906. [Google Scholar] [CrossRef] [PubMed]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); Stat Pearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Brattsand, R.; Linden, M. Cytokine modulation by glucocorticoids: Mechanisms and actions in cellular studies. Discussion. Aliment. Pharmacol. Ther. 1996, 10, 91–92. [Google Scholar] [CrossRef]
- Escoter-Torres, L.; Caratti, G.; Mechtidou, A.; Tuckermann, J.; Uhlenhaut, N.H.; Vettorazzi, S. Fighting the Fire: Mechanisms of Inflammatory Gene Regulation by the Glucocorticoid Receptor. Front. Inmunol. 2019, 10, 1859. [Google Scholar] [CrossRef]
- Lanas, A.; Chan, F. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Lanas, A.; A Garcia-Rodriguez, L.; Arroyo, M.T.; Gomollon, F.; Feu, F.; Gonzalez-Perez, A.; Zapata, E.; Bastida, G.; Rodrigo, L.; Santolaria, S.; et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. GUT 2006, 55, 1731–1738. [Google Scholar] [CrossRef]
- Brater, D. Renal effects of cyclooxygyenase-2-selective inhibitors. J. Pain. Symptom Manag. 2002, 23, S15–S20. [Google Scholar] [CrossRef]
- Sriuttha, P.; Sirichanchuen, B.; Permsuwan, U. Hepatotoxicity of Nonsteroidal Anti-Inflammatory Drugs: A Systematic Review of Randomized Controlled Trials. Int. J. Hepatol. 2018, 2018, 5253623. [Google Scholar] [CrossRef]
- Husebye, E.; Pearce, S.H.; Krone, N.P.; Kämpe, O. Adrenal insufficiency. Lancet 2021, 397, 613–629. [Google Scholar] [CrossRef]
- Auger, J.P.; Zimmermann, M.; Faas, M.; Stifel, U.; Chambers, D.; Krishnacoumar, B.; Taudte, R.V.; Grund, C.; Scholtysek, C.; Uderhardt, S.; et al. Metabolic rewiring promotes anti-inflammatory effects of glucocorticoids. Nature 2024, 629, 184–192. [Google Scholar] [CrossRef]
- Kang, H.; Kim, B. Bioactive Compounds as Inhibitors of Inflammation, Oxidative Stress and Metabolic Dysfunctions via Regulation of Cellular Redox Balance and Histone Acetylation State. Foods 2023, 12, 925. [Google Scholar] [CrossRef]
- Bernhoft, A. Bioactive Compounds in Plants-Benefits and Risks for Man and Animals; The Norwegian Academy of Science and Letters: Oslo, Norway, 2010. [Google Scholar]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Is. Super. Sanitá. 2007, 43, 348–361. [Google Scholar]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Kanwal, A.; Bilal, M.; Rasool, N.; Zubair, M.; Shah, S.A.A.; Zakaria, Z.A. Total Synthesis of Terpenes and Their Biological Significance: A Critical Review. Pharmaceuticals 2022, 15, 1392. [Google Scholar] [CrossRef]
- Manske, R.H.F.; Rodrigo, R.G.A. The Alkaloids: Chemistry and Physiology; Elsevier: Amsterdam, The Netherlands, 1981. [Google Scholar]
- Gul, W.; Hamann, M. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005, 78, 442–453. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). Rec. Adv. Nat. Products Anal. 2020, 505–567. [Google Scholar]
- Riaz, T.; Akram, M.; Laila, U.; Zainab, R.; Khalil, M.T.; Iftikhar, M.; Ozdemir, F.A.; Solowski, G.; Altable, M.; Sfera, A.; et al. Therapeutic applications of glycosides obtained from medicinal plants. Int. Arch. Integr. Med. 2023, 10, 30–38. [Google Scholar]
- Harwood, J. Polyunsaturated Fatty Acids: Conversion to Lipid Mediators, Roles in Inflammatory Diseases and Dietary Sources. Int. J. Mol. Sci. 2023, 24, 8838. [Google Scholar] [CrossRef]
- Abdel-Massih, R.M.; Debs, E.; Othman, L.; Attieh, J.; Cabrerizo, F.M. Glucosinolates, a natural chemical arsenal: More to tell than the myrosinase story. Fron. Microbiol. 2023, 14, 1130208. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Jahandideh, F.; Wu, J. Food-Derived Bioactive Peptides on Inflammation and Oxidative Stress. Biomed. Res. Int. 2014, 2014, 608979. [Google Scholar] [CrossRef] [PubMed]
- Samiappan, K.; Chalakoth, J. Plant-Based Anti-Inflammatory Agents: A Scientific Review of Bioactive Compounds and Mechanisms. Asian J. Biol. Life Sci. 2025, 14, 34–41. [Google Scholar] [CrossRef]
- Jensen, W. The origin of the soxhlet extractor. J. Chem. Educ. 2007, 84, 1913–1914. [Google Scholar] [CrossRef]
- More, P.; Jambrak, A.; Arya, S. Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [Google Scholar] [CrossRef]
- Katekar, V.; Rao, A.; Sardeshpande, V. A hydrodistillation-based essential oils extraction: A quest for the most effective and cleaner technology. Sustain. Chem. Pharm. 2023, 36, 101270. [Google Scholar] [CrossRef]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Baglary, G.R.; Kalita, S.; Islary, A.; Kumar, S. Sustainable extraction of bioactive compounds from aromatic plants and agro-food wastes for food preservation: A review. Biocatal. Agric. Biotechnol. 2024, 61, 103399. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Safari, M.; Askari, G.; Salami, M. Microwave-assisted extraction of hempseed oil: Studying and comparing of fatty acid composition, antioxidant activity, physiochemical and thermal properties with Soxhlet extraction. J. Food. Sci. Technol. Mysore 2019, 56, 4198–4210. [Google Scholar] [CrossRef]
- Ramsey, E.; Sun, Q.; Zhang, Z.; Zhang, C.; Gou, W. Mini-Review: Green sustainable processes using supercritical fluid carbon dioxide. J. Environ. Sci. 2009, 21, 720–726. [Google Scholar] [CrossRef]
- Blikra, M.; Skipnes, D.; Skara, T. On the use of pulsed electric field technology as a pretreatment to reduce the content of potentially toxic elements in dried Saccharina latissima. LWT-Food Sci. Technol. 2022, 169, 114033. [Google Scholar] [CrossRef]
- Ranjha, M.; Sun, Q.; Zhang, Z.; Zhang, C.; Gou, W. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef] [PubMed]
- Bocker, R. and E. Silva, Pulsed electric field assisted extraction of natural food pigments and colorings from plant matrices. Food Chem. X 2022, 15, 100398. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Ma, J.; Zhang, Y.; Chen, H.; Wei, T.; Gao, D.; Li, J. Enzyme-assisted extraction, characterization, and in vitro antioxidant activity of polysaccharides from Potentilla anserina L. Front. Nutr. 2023, 10, 1216572. [Google Scholar]
- Amulya, P.; ul Islam, R. Optimization of enzyme-assisted extraction of anthocyanins from eggplant (Solanum melongena L.) peel. Food Chem. X 2023, 18, 100643. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Optimization of enzyme-assisted lipid extraction from Nannochloropsis microalgae. J. Taiwan. Ist. Chem. Eng. 2016, 67, 106–114. [Google Scholar] [CrossRef]
- Peron, G.; Ferrarese, I.; Dos Santos, N.C.; Rizzo, F.; Gargari, G.; Bertoli, N.; Gobbi, E.; Perosa, A.; Selva, M.; Dall’acqua, S. Sustainable Extraction of Bioactive Compounds and Nutrients from Agri-Food Wastes: Potential Reutilization of Berry, Honey, and Chicory Byproducts. Appl. Sci. 2024, 14, 10785. [Google Scholar] [CrossRef]
- Pawliszyn, J. 3.35—Sample Preparation for Capillary Electrophoretic Applications. In Comprehensive Sampling and Sample Preparation; Academic Press: Oxford, UK, 2012. [Google Scholar]
- Shen, D.; Jin, T.; Wang, J.; Zhu, X. Mechanochemical-assisted extraction of polysaccharides from bamboo leaves and its optimized processing parameters. Food Sci. Technol. 2022, 42, e117821. [Google Scholar] [CrossRef]
- Fan, L.; Fan, W.; Mei, Y.; Liu, L.; Li, L.; Wang, Z.; Yang, L. Mechanochemical assisted extraction as a green approach in preparation of bioactive components extraction from natural products—A review. Trends Food Sci. Technol. 2022, 129, 98–110. [Google Scholar] [CrossRef]
- Rincón, E.; Balu, A.M.; Luque, R.; Serrano, L. Mechanochemical extraction of antioxidant phenolic compounds from Mediterranean and medicinal Laurus nobilis: A comparative study with other traditional and green novel techniques. Ind. Crops Prod. 2019, 141, 111805. [Google Scholar] [CrossRef]
- Cubero-Cardoso, J.; Hernández-Escaño, M.; Trujillo-Reyes, Á.; Fermoso, F.G.; Fernández-Recamales, M.Á.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G.; Urbano, J. Mechanochemical-assisted Natural Deep Eutectic Solvent as a platform for an olive leaves biorefinery: Extraction of bioactive compounds and methane production. Sustain. Chem. Pharm. 2025, 43, 101879. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Gul, Z.; Tian, M.; Wang, J.; Zheng, K.; Zhao, C.; Li, C. Advances of responsive deep eutectic solvents and application in extraction and separation of bioactive compounds. J. Sep. Sci. 2023, 46, e2300098. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Abranches, D.; Coutinho, J. Everything You Wanted to Know about Deep Eutectic Solvents but Were Afraid to Be Told. Annu. Rev. Chem. Biomol. Eng. 2023, 14, 141–163. [Google Scholar] [CrossRef]
- Lomba, L.; Ribate, M.P.; Sangüesa, E.; Concha, J.; Garralaga, M.P.; Errazquin, D.; García, C.B.; Giner, B. Deep Eutectic Solvents: Are They Safe? Appl. Sci. 2021, 11, 10061. [Google Scholar] [CrossRef]
- Devi, M.; Moral, R.; Thakuria, S.; Mitra, A.; Paul, S. Hydrophobic Deep Eutectic Solvents as Greener Substitutes for Conventional Extraction Media: Examples and Techniques. ACS Omega 2023, 8, 9702–9728. [Google Scholar] [CrossRef]
- Smith, E.; Abbott, A.; Ryder, K. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Ivanovic, M.; Razborsek, M.; Kolar, M. Innovative Extraction Techniques Using Deep Eutectic Solvents and Analytical Methods for the Isolation and Characterization of Natural Bioactive Compounds from Plant Material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Das, A.K.; Sharma, M.; Mondal, D.; Prasad, K. Deep eutectic solvents as efficient solvent system for the extraction of kappa-carrageenan from Kappaphycus alvarezii. Carbohydr. Polym. 2016, 136, 930–935. [Google Scholar] [CrossRef]
- Negi, T.; Kumar, A.; Sharma, S.K.; Rawat, N.; Saini, D.; Sirohi, R.; Prakash, O.; Dubey, A.; Dutta, A.; Shahi, N.C. Deep eutectic solvents: Preparation, properties, and food applications. Heliyon 2024, 10, e28784. [Google Scholar] [CrossRef]
- Rodríguez-Llorente, D.; Cañada-Barcala, A.; Álvarez-Torrellas, S.; Águeda, V.I.; García, J.; Larriba, M. A Review of the Use of Eutectic Solvents, Terpenes and Terpenoids in Liquid–liquid Extraction Processes. Processes 2020, 8, 1220. [Google Scholar] [CrossRef]
- Jovanovic, J.; Jovic, M.; Trifkovic, J.; Smilijanic, K.; Gasic, U.; Ristivojevic, M.K.; Ristivojevic, P. Green Extraction of Bioactives from Curcuma longa Using Natural Deep Eutectic Solvents: Unlocking Antioxidative, Antimicrobial, Antidiabetic, and Skin Depigmentation Potentials. Plants 2025, 14, 163. [Google Scholar] [CrossRef]
- Tebbi, S.; Trapali, M.; Letsiou, S. Exploring the Anti-Diabetic, Antioxidant and Anti-Microbial Properties of Clematis flammula L. Leaves and Pistacia lentiscus L. Fruits Using Choline Chloride-Based Deep Eutectic Solvent. Waste Biomass Valori. 2024, 15, 2869–2879. [Google Scholar] [CrossRef]
- Sitthisak, C.; Nisoa, M.; Chunglok, W.; Prasopthum, A.; Phaisan, S.; Putalun, W.; Kanchanapoom, T.; Juengwatanatrakul, T.; Yusakul, G. Efficient extraction of quassinoids and alkaloids from Eurycoma longifolia Jack roots using natural deep eutectic solvents and microwave-assisted extraction. Microchem. J. 2024, 196, 109676. [Google Scholar] [CrossRef]
- da Silva, D.; Rodrigues, R.F.; Machado, N.M.; Maurer, L.H.; Ferreira, L.F.; Somacal, S.; De Veiga, M.L.; da Rocha, M.; Vizzotto, M.; Rodrigues, E.; et al. Natural deep eutectic solvent (NADES)-based blueberry extracts protect against ethanol-induced gastric ulcer in rats. Food Res. Int. 2020, 138, 109718. [Google Scholar] [CrossRef] [PubMed]
- Ianni, F.; Scandar, S.; Mangiapelo, L.; Blasi, F.; Marcotullio, M.C.; Cossignani, L. NADES-Assisted Extraction of Polyphenols from Coriander Seeds: A Systematic Optimization Study. Antioxidants 2023, 12, 2048. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Millán, M.; Carrasco-Portugal, M.; Heredia, J.B.; Bastidas-Bastidas, P.; Gutiérrez- Grijalva, E.P.; León-Félix, J.; Angulo-Escalante, M. Green Extracts and UPLC-TQS-MS/MS Profiling of Flavonoids from Mexican Oregano (Lippia graveolens) Using Natural Deep Eutectic Solvents/Ultrasound-Assisted and Supercritical Fluids. Plants 2023, 12, 1692. [Google Scholar] [CrossRef]
- Jovanović, M.S.; Krgović, N.; Živković, J.; Stević, T.; Zdunić, G.; Bigović, D.; Šavikin, K. Ultrasound-Assisted Natural Deep Eutectic Solvents Extraction of Bilberry Anthocyanins: Optimization, Bioactivities, and Storage Stability. Plants 2022, 11, 2680. [Google Scholar] [CrossRef]
- Peng, X.; Duan, M.-H.; Yao, X.-H.; Zhang, Y.-H.; Zhao, C.-J.; Zu, Y.-G.; Fu, Y.-J. Green extraction of five target phenolic acids from Lonicerae japonicae Flos with deep eutectic solvent. Sep. Purif. Technol. 2016, 157, 249–257. [Google Scholar] [CrossRef]
- Hadi, N.A.; Ng, M.H.; Choo, Y.M.; Hashim, M.A.; Jayakumar, N.S. Performance of Choline-Based Deep Eutectic Solvents in the Extraction of Tocols from Crude Palm Oil. J. Am. Oil Chem. Soc. 2015, 92, 1709–1716. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Syarifah, A.; Suryadi, H.; Hayun, H.; Simamora, A.; Mun’iM, A. Detoxification of comfrey (Symphytum officinale L.) extract using natural deep eutectic solvent (NADES) and evaluation of its anti-inflammatory, antioxidant, and hepatoprotective properties. Front. Pharmacol. 2023, 14, 1012716. [Google Scholar] [CrossRef]
- Stasilowicz-Krzemien, A.; Wójcik, J.; Gościniak, A.; Szymański, M.; Szulc, P.; Górecki, K.; Cielecka-Piontek, J. Natural Deep Eutectic Solvents Combined with Supercritical Carbon Dioxide for the Extraction of Curcuminoids from Turmeric. Pharmaceuticals 2024, 17, 1596. [Google Scholar] [CrossRef]
- Garcia, A.; Rodriguez-Juan, E.; Rodriguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef]
- Mulia, K.; Krisanti, E.; Terahadi, F.; Putri, S. Selected Natural Deep Eutectic Solvents for the Extraction of a-Mangostin from Mangosteen (Garcinia mangostana L.) Pericarpp. Int. J. Technol. 2015, 6, 1211. [Google Scholar] [CrossRef]
- Cui, Q.; Peng, X.; Yao, X.-H.; Wei, Z.-F.; Luo, M.; Wang, W.; Zhao, C.-J.; Fu, Y.-J.; Zu, Y.-G. Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep. Purif. Technol. 2015, 150, 63–72. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, D.-Y.; Duan, M.-H.; Cui, Q.; Xu, W.-J.; Luo, M.; Li, C.-Y.; Zu, Y.-G.; Fu, Y.-J. Preparation and determination of phenolic compounds from Pyrola incarnata Fisch with a green polyols based-deep eutectic solvent. Sep. Purif. Technol. 2015, 149, 116–123. [Google Scholar] [CrossRef]
- Xia, B.; Yan, D.; Bai, Y.; Xie, J.; Cao, Y.; Liao, D.; Lin, L. Determination of phenolic acids in Prunella vulgaris L.: A safe and green extraction method using alcohol-based deep eutectic solvents. Anal. Methods 2015, 7, 9354–9364. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Juric, T.; Pavlovic, R.Z.; Uka, D.; Beara, I.; Majkiv, T.; Savic, S.; Zekic, M.; Popovic, B.M. Natural deep eutectic solvents-mediated extraction of rosmarinic acid from Lamiaceae plants: Enhanced extractability and anti-inflammatory potential. Ind. Crop. Prod. 2024, 214, 118559. [Google Scholar] [CrossRef]
- Tsvetov, N.; Paukshta, O.; Fokina, N.; Volodina, N.; Samarov, A. Application of Natural Deep Eutectic Solvents for Extraction of Bioactive Components from Rhodiola rosea (L.). Molecules 2023, 28, 912. [Google Scholar] [CrossRef]
- Zuo, J.; Ma, P.; Geng, S.; Kong, Y.; Li, X.; Fan, Z.; Zhang, Y.; Dong, A.; Zhou, Q. Optimization of the extraction process of flavonoids from Trollius ledebouri with natural deep eutectic solvents. J. Sep. Sci. 2022, 45, 717–727. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar]
- Nam, Y.; Ahn, S.M.; Seo, G.J.; Kim, N.W.; Shin, S.W.; Nuankaew, W.; Murughanantham, N.; Pandian, S.; Hwang, J.S.; Na Hong, B.; et al. Optimization of NADES-based green extraction of ellagitannins from rambutan peel with enhanced antioxidant activity. Food Chem. 2025, 475, 143308. [Google Scholar] [CrossRef]
- Solcan, M.; Vlase, A.-M.; Marc, G.; Muntean, D.; Casian, T.; Nadăș, G.C.; Novac, C.Ș.; Popa, D.-S.; Vlase, L. Antimicrobial Effectiveness of Ribes nigrum L. Leaf Extracts Prepared in Natural Deep Eutectic Solvents (NaDESs). Antibiotics 2024, 13, 1118. [Google Scholar] [CrossRef]
- Wils, L.; Leman-Loubière, C.; Bellin, N.; Clément-Larosière, B.; Pinault, M.; Chevalier, S.; Enguehard-Gueiffier, C.; Bodet, C.; Boudesocque-Delaye, L. Natural deep eutectic solvent formulations for spirulina: Preparation, intensification, and skin impact. Algal Res.-Biomass Biof. Bioprod. 2021, 56, 102317. [Google Scholar] [CrossRef]
- Kaoui, S.; Chebli, B.; Baddi, G.A.; Basaid, K.; Mir, Y. Response surface modeling and optimization of the extraction conditions using lactic acid-based deep eutectic solvents as green alternative extraction media for Mentha pulegium. Phytochem. Anal. 2022, 33, 906–914. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Li, W.; Zhan, B.; Zhu, L.; Yang, D.; Li, G.; Zhang, L.; Zhao, Z. Ultrasonic-assisted extraction of flavonoids from Amomum villosum Lour. Using natural deep eutectic solvent: Process optimization, comparison, identification, and bioactivity. Ultrason. Sonochem. 2025, 116, 107304. [Google Scholar] [CrossRef]
- Nam, M.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Paradiso, V.; Clemente, A.; Summo, C.; Pasqualone, A.; Caponio, F. Towards green analysis of virgin olive oil phenolic compounds: Extraction by a natural deep eutectic solvent and direct spectrophotometric detection. Food Chem. 2016, 212, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yang, M.; Cao, F.; Wang, J.; Su, E. Well-Designed Hydrophobic Deep Eutectic Solvents as Green and Efficient Media for the Extraction of Artemisinin from Artemisia annua Leaves. ACS Sustain. Chem. Eng. 2017, 5, 3270–3278. [Google Scholar] [CrossRef]
- Hayyan, A.; Zainal-Abidin, M.H.; Putra, S.S.S.; Alanazi, Y.M.; Saleh, J.; Nor, M.R.M.; Hashim, M.A.; Gupta, B.S. Evaluation of biodegradability, toxicity and ecotoxicity of organic acid-based deep eutectic solvents. Sci. Total Environ. 2024, 948, 174758. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Townley, G.; Martínez-Espinosa, R. Controversy on the toxic nature of deep eutectic solvents and their potential contribution to environmental pollution. Heliyon 2022, 8, e12567. [Google Scholar] [CrossRef] [PubMed]
- Shishov, A.; El-Deen, A.K.; Godunov, P.; Bulatov, A. Atypical deep eutectic solvents: New opportunities for chemical analysis. TRAC-Trends Anal. Chem. 2024, 176, 117752. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.-H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Hao, Y.; Pei, F.; Huang, J.; Li, G.; Zhong, C. Application of deep eutectic solvents on extraction of flavonoids. J. Sep. Sci. 2024, 47, e2300925. [Google Scholar] [CrossRef]
- Stanisz, M.; Stanisz, B.; Cielecka-Piontek, J. A Comprehensive Review on Deep Eutectic Solvents: Their Current Status and Potential for Extracting Active Compounds from Adaptogenic Plants. Molecules 2024, 29, 4767. [Google Scholar] [CrossRef]
- Rente, D.; Bubalo, M.C.; Panić, M.; Paiva, A.; Caprin, B.; Redovniković, I.R.; Duarte, A.R.C. Review of deep eutectic systems from laboratory to industry, taking the application in the cosmetics industry as an example. J. Clean. Prod. 2022, 380, 135147. [Google Scholar] [CrossRef]
- Ishtaweera, P.; Baker, G. Progress in the application of ionic liquids and deep eutectic solvents for the separation and quantification of per- and polyfluoroalkyl substances. J. Hazard. Mater. 2024, 465, 132959. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, M.; Guo, H.; Xu, J.; Ye, J.; Zhao, J.; Zhao, L. Ultrasound-assisted deep eutectic solvent as green and efficient media for the extraction of flavonoids from Radix scutellariae. New J. Chem. 2019, 43, 644–650. [Google Scholar] [CrossRef]

| Bioactive Molecule | Type of Bioactive Molecule | Specie | DES | Extraction Method | Extraction (Yield (mg/g)) | Reference | |
|---|---|---|---|---|---|---|---|
| Components | Ratio | ||||||
| Geraniin, corilagin, ellagic acid, gallic acid | Ellaginannis | Rambutan (Nephelium lappaceum L.) peel | Betaine–1,2-propanediol | 1–2 | Liquid–liquid extraction | 54.29 | [81] |
| p-coumaric acid, caffeic acid, chlorogenic acid, 4-o-caffeylquinic acid, gallic acid, protocatechuic acid, gentisic acid, vanillic acid | Phenolic acids and flavonoids | Ribes nigrum L. Leaf | L-proline–Propileneglycol–Water | 1–2–30% Water v/v | Ultra-turrax extraction (UTE), (different minutes) | 10 min– 169.5 mg GAE/g and 7.49 mg QE/g | [82] |
| L-proline–Propileneglycol–Water | 1–1–50% Water v/v | 10 min– 221.9 mg GAE/g and 7.64 mg QE/g | |||||
| L-proline–Propileneglycol–Water | 1–1–40% Water v/v | 5 min– 218 mg GAE/g and 7.79 mg QE/g | |||||
| L-proline–Propileneglycol–Water | 2–3–30% Water v/v | 5 min– 239 mg GAE/g and 9.10 mg QE/g | |||||
| L-proline– Lactic acid–Water | 1–2–50% Water v/v | 5 min– 74.4 mg GAE/g and 2.90 mg QE/g | |||||
| L-proline–Lactic acid–Water | 1–2–50% Water v/v | 10 min– 218 mg GAE/g and 6.92 mg QE/g | |||||
| L-proline–Lactic acid–Water | 1–2–30% Water v/v | 7.5 min– 256 mg GAE/g and 6.93 mg QE/g | |||||
| L-proline–Glucose–Water | 1–2–30% Water v/v | 5 min– 227 mg GAE/g and 6.89 mg QE/g | |||||
| L-proline–Glucose–Water | 1–1–50% Water v/v | 5 min– 192 mg GAE/g and 7.40 mg QE/g | |||||
| L-proline–Glucose–Water | 1–1–30% Water v/v | 10 min– 255 mg GAE/g and 7.73 mg QE/g | |||||
| L-proline–Propileneglycol–Water | 1–2–50% Water v/v | UAE, (different minutes) | 5 min– 205 mg GAE/g and 8.38 mg QE/g | ||||
| L-proline–Propileneglycol–Water | 1–1–30% Water v/v | 10 min– 224 mg GAE/g and 8.66 mg QE/g | |||||
| L-proline–Lactic acid–Water | 1–2–30% Water v/v | 5 min– 456 mg GAE/g and 14.3 mg QE/g | |||||
| L-proline–Lactic acid–Water | 1–1–50% Water v/v | 5 min– 408.0 mg GAE/g and 12.9 mg QE/g | |||||
| L-proline–Lactic acid–Water | 1–2–30% Water v/v | 10 min– 415 mg GAE/g and 10.9 mg QE/g | |||||
| L-proline–Lactic acid–Water | 1–1–50% Water v/v | 10 min– 365 mg GAE/g and 10.9 mg QE/g | |||||
| L-proline–Glucose–Water | 1–1–30% Water v/v | 5 min– 425 mg GAE/g and 14.6 mg QE/g | |||||
| L-proline–Glucose–Water | 1–2–30% Water v/v | 10 min– 319 mg GAE/g and 11.6 mg QE/g | |||||
| Chlorophylls, carotenoids and Phycocyanin | Biological molecules | Arthrospira platensis (Spirulina) | Betaine–Glycerol | 1–2 | Freeze dried biomasses using UAE | Chlorophyll– 0.01 mg/g, carotenoid– 0 mg/g, phycocyanin– 0.2 mg/g | [83] |
| Betaine–Glycerol | 1–4 | - | |||||
| Betaine–Glycerol | 1–8 | Chlorophyll– 0.02 mg/g, carotenoid– 0.01 mg/g, phycocyanin– 0.2 mg/g | |||||
| Glucose–Glycerol | 1–2 | - | |||||
| Glucose–Glycerol | 1–3 | - | |||||
| Glucose–Glycerol | 1–4 | - | |||||
| Glucose–Glycerol | 1–5 | - | |||||
| Glucose–Glycerol–Water | 1–2–2 | - | |||||
| Glucose–Glycerol–Water | 1–2–4 | Chlorophyll– 0.1 mg/g, carotenoid– 0.02 mg/g, phycocyanin– 1.2 mg/g | |||||
| Glucose–Glycerol–Betaine | 1–2–4 | - | |||||
| Lactic acid–Betaine | 2–1 | - | |||||
| Lactic acid–Glycerol | 1–1 | - | |||||
| Menthol–Lactic acid | 1–2 | - | |||||
| Menthol–Levulinic acid | 1–2 | Chlorophyll– 0.1 mg/g, carotenoid– 0.02 mg/g, phycocyanin– nd | |||||
| Menthol–Octanoic acid | 1–1 | - | |||||
| Octanoic acid–Lauric acid | 3–1 | Chlorophyll 0.1 mg/g, carotenoid– 0.03 mg/g, phycocyanin– nd | |||||
| Nonanoic acid–Lauric acid | 3–1 | - | |||||
| Nonanoic acid–Decanoic acid– Lauric acid | 3–2–1 | Chlorophyll– 0.1 mg/g, carotenoide– 0.02 mg/g, phycocyanin– nd | |||||
| Phenolic compounds | Polyphenols | Mentha pulegium (Lamiaceae) | Lactic acid–Glucose | 5–1 | UAE | 164 | [84] |
| Lactic acid–Sodium acetate | 3–1 | 173 | |||||
| Lactic acid–Glycine | 3–1 | 165 | |||||
| Total flavonoids | Flavonoids | Amomum villosum Lour. (A. villosum) | Betaine–Propylenglycol | 1–1 | UAE | 65.1 | [85] |
| Betaine–Glycerol | 1–4 | 66.2 | |||||
| Betaine–Urea | 1–2 | 60.2 | |||||
| Betaine–Lactic acid | 1–1 | 56.0 | |||||
| Betaine–Malic acid | 1–2 | 58.7 | |||||
| Betaine–Citric acid | 1–1 | 50.6 | |||||
| Betaine–Glucose | 1–1 | 40.6 | |||||
| Betaine–Fructose | 1–1 | 59.1 | |||||
| Betaine–Sorbitol | 1–2 | 58.7 | |||||
| Betaine–Xylitol | 1–1 | 53.0 | |||||
| Proline–Propylenglycol | 1–4 | 58.8 | |||||
| Proline–Glycerol | 1–2 | 54.6 | |||||
| Proline–Urea | 1–1 | 46.1 | |||||
| Proline–Lactic acid | 1–1 | 45.8 | |||||
| Proline–Malic acid | 1–1 | 51.1 | |||||
| Proline–Citric acid | 1–1 | 49.6 | |||||
| Proline–Glucose | 1–1 | 39.0 | |||||
| Proline–Fructose | 1–1 | 60.8 | |||||
| Proline–Sorbitol | 1–2 | 46.4 | |||||
| Proline–Xylitol | 1–1 | 61.1 | |||||
| Quercetin | Flavonoide | Flos sophorae | L-proline–Glycerol–Water | 2–5–10% Water v/v | UAE (room temp., 45 min) | 100 | [86] |
| Isorhamnetin | Flavonoide | Flos sophorae | L-proline–Glycerol–Water | 2–510% Water v/v | UAE (room temp., 45 min) | 12.0 | |
| Phenolic acid | Phenolic compounds | Olea europaea (Virgin olive oil) | Lactic acid–Glucose–Water | 6–1–6 | Agitation + centrifugation + filtration | Not given | [87] |
| Artemisin | Lactona | Artemisa annua | Methyl trioctyl ammonium chloride–1-butanol | 1–4 | UAE (temp. 45 °C, 70 min) | 7.99 | [88] |
| Hydroxisafflor, cartomin, carthamin | Polyphenols | Carthamus tinctorius L. (Safflower) | Lactic acid–Glucose–Water | 1–1–25% Water v/v | Heat- and stirring-assisted solid–liquid extraction | N/A in mg/g (only relative extraction yield per area–2244) | [80] |
| Proline–Malic acid–Water | 1–1–25% Water v/v | N/A in mg/g (only relative extraction yield per area–2813) | |||||
| Sucrose–ChCl–Water | 5–5–25% Water v/v | N/A in mg/g (only relative extraction yield per area–2680) | |||||
| cartomin | Lacticacid–Glucose–Water | 1–1–25% Water v/v | N/A in mg/g (only relative extraction yield per area–2229) | ||||
| Proline–Malic acid–Water | 1–1–25% Water v/v | N/A in mg/g (only relative extraction yield per area–2925) | |||||
| Sucrose–ChCl–Water | 5–5–25% Water v/v | N/A in mg/g (only relative extraction yield per area–2591) | |||||
| Carthamin | Lactic acid–Glucose–Water | 1–1–25% Water v/v | N/A in mg/g (only relative extraction yield per area–235) | ||||
| Proline–Malic acid–Water | 1–1–25% Water v/v | N/A in mg/g (only relative extraction yield per area–134) | |||||
| Sucrose–ChCl–Water | 5:5:25% Water v/v | N/A in mg/g (only relative extraction yield per area:152) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giner, B.; Sangüesa, E.; Zuriaga, E.; Culleré, L.; Lomba, L. Deep Eutectic Solvent Systems as Media for the Selective Extraction of Anti-Inflammatory Bioactive Agents. Molecules 2025, 30, 3357. https://doi.org/10.3390/molecules30163357
Giner B, Sangüesa E, Zuriaga E, Culleré L, Lomba L. Deep Eutectic Solvent Systems as Media for the Selective Extraction of Anti-Inflammatory Bioactive Agents. Molecules. 2025; 30(16):3357. https://doi.org/10.3390/molecules30163357
Chicago/Turabian StyleGiner, Beatriz, Estela Sangüesa, Estefania Zuriaga, Laura Culleré, and Laura Lomba. 2025. "Deep Eutectic Solvent Systems as Media for the Selective Extraction of Anti-Inflammatory Bioactive Agents" Molecules 30, no. 16: 3357. https://doi.org/10.3390/molecules30163357
APA StyleGiner, B., Sangüesa, E., Zuriaga, E., Culleré, L., & Lomba, L. (2025). Deep Eutectic Solvent Systems as Media for the Selective Extraction of Anti-Inflammatory Bioactive Agents. Molecules, 30(16), 3357. https://doi.org/10.3390/molecules30163357











