Synergistic Antibacterial Effect of Eugenol and Biogenic Silver Nanoparticles on Staphylococcus pseudintermedius Isolated from Canine Keratoconjunctivitis Sicca
Abstract
1. Introduction
2. Results and Discussion
2.1. Antimicrobial Susceptibility Profile of Staphylococcus pseudintermedius
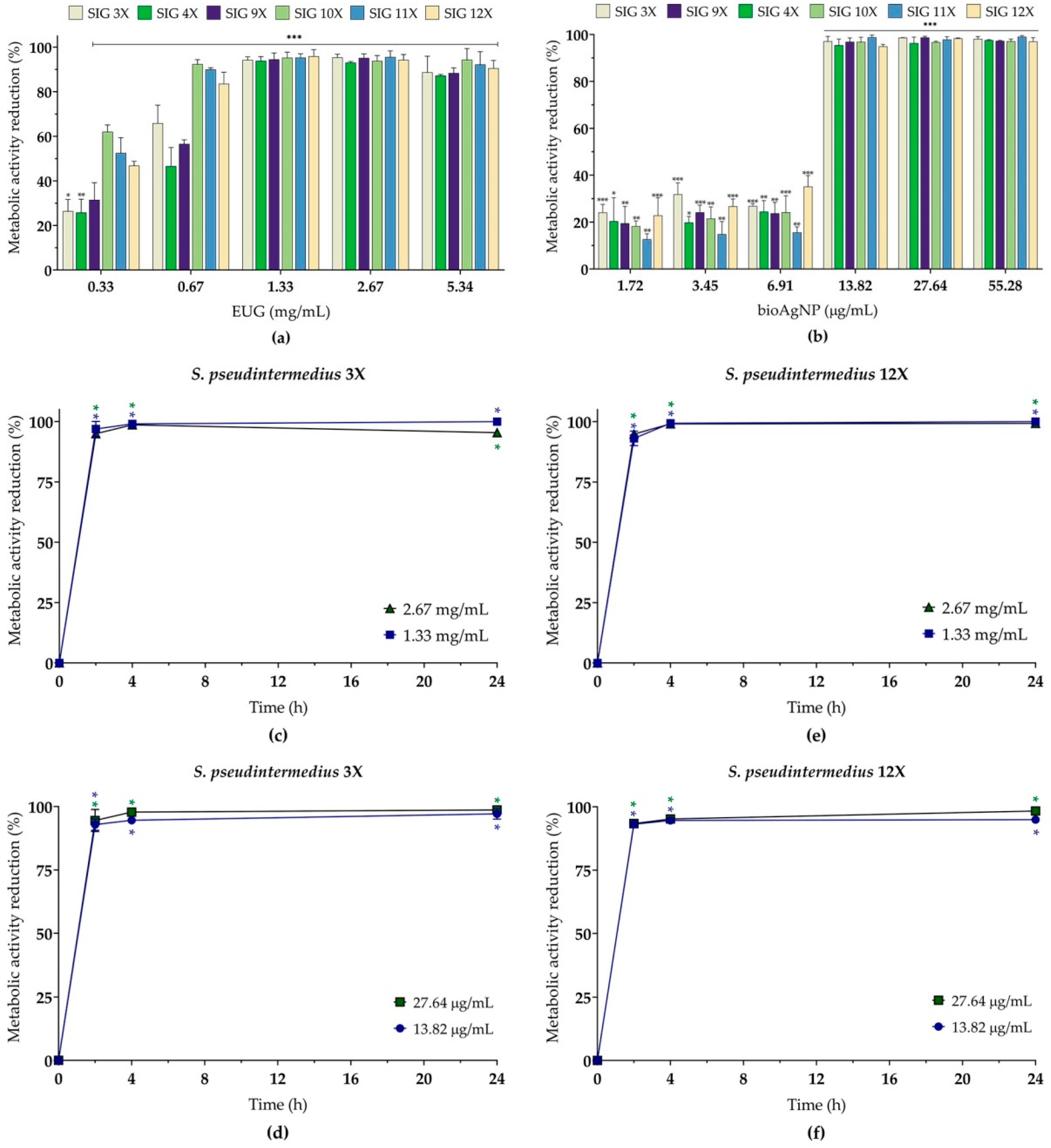
2.2. Eugenol and Biogenic Silver Nanoparticles Exhibit a Dose-Dependent Bactericidal Activity Against Planktonic Cells of MDR Staphylococcus pseudintermedius
2.3. Eugenol Displays Synergistic Antibacterial Interaction with Biogenic Silver Nanoparticles Against Planktonic Cells of MDR Staphylococcus pseudintermedius
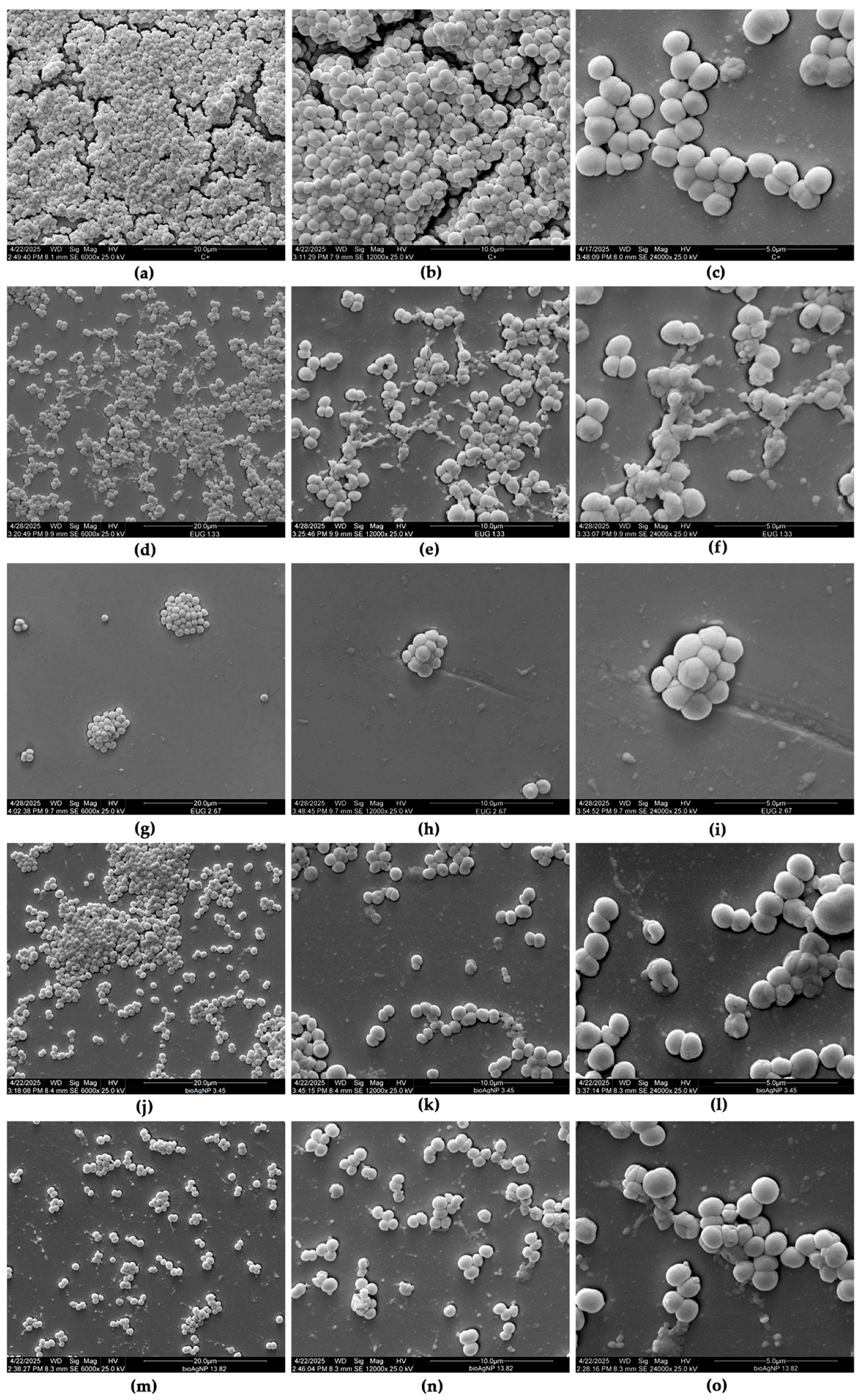
2.4. Eugenol, Alone or Combined with Biogenic Silver Nanoparticles, Inhibits the 24 h Biofilms of MDR Staphylococcus pseudintermedius on Abiotic Surfaces
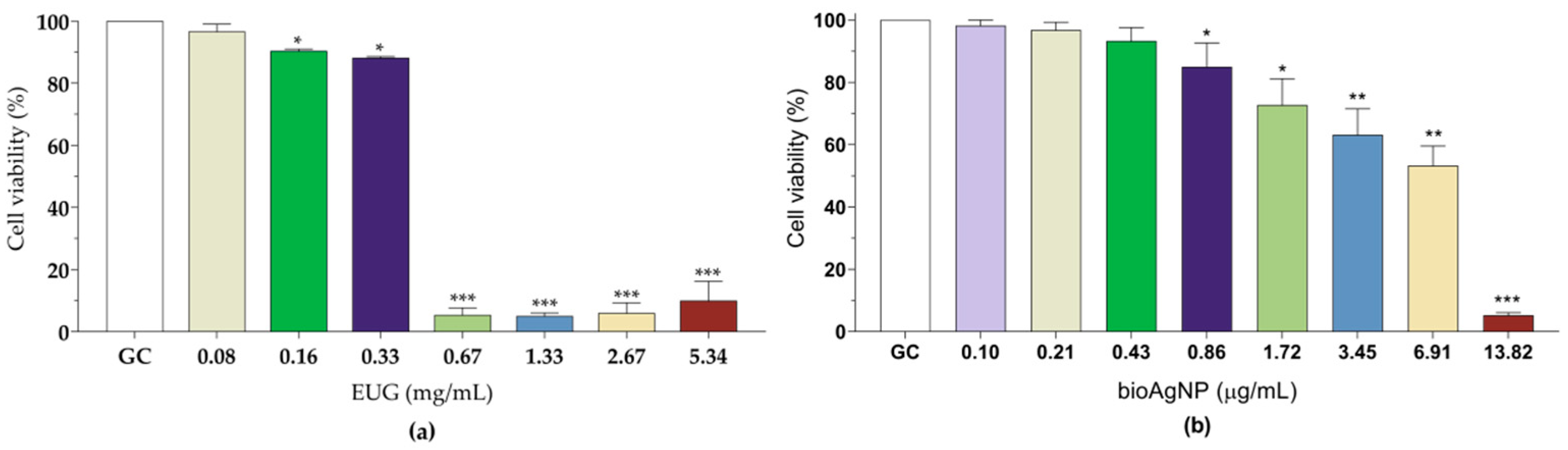
2.5. Eugenol and Biogenic Silver Nanoparticles Exhibit Reduced Toxicity Toward LLC-MK2 Cells at Concentrations Corresponding to Synergistic or Indifferent Combinations Against Planktonic and Sessile Cells of MDR Staphylococcus pseudintermedius
3. Materials and Methods
3.1. Chemicals and Culture Media
3.2. Bacteria and Culture Conditions
3.3. Antibacterial Activity Against Planktonic Cells
3.3.1. Disk Diffusion
3.3.2. Minimum Inhibitory Concentration (MIC)
3.3.3. Checkerboard Microdilution Assay
3.3.4. Time–Kill Assay
3.3.5. Cell Membrane Integrity
3.4. Antibacterial Effect on Sessile (Biofilms) Cells
3.4.1. Biofilm Production
3.4.2. Antibiofilm Activity
3.4.3. Scanning Electron Microscopy (SEM)
3.5. Effect of Eugenol and Biogenic Silver Nanoparticles on Mammalian Cells
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDR | Multidrug resistant |
| KCS | Keratoconjunctivitis sicca |
| MRSP | Methicillin-resistant Staphylococcus pseudintermedius |
| EUG | Eugenol |
| AgNPs | Silver nanoparticles |
| bioAgNPs | Biogenic silver nanoparticles |
| PCR | Polymerase chain reaction |
| MSSP | Methicillin-sensitive Staphylococcus pseudintermedius |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| CFU | Colony forming unit |
| ROS | Reactive oxygen species |
| FICI | Fractional inhibitory concentration index |
| OD | Optical density |
| SMIC | Sessile minimum inhibitory concentration |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMSO | Dimethyl sulfoxide |
| MSA | Manitol Salt Agar |
| TSB | Tryptone Soya Broth |
| CaMHB | Cation-Adjusted Müller Hinton Broth |
| MHA | Müller Hinton Agar |
| SEM | Scanning electron microscopy |
| DMEM | Dulbecco’s Modified Eagle medium |
References
- Nocera, F.P.; De Martino, L. Methicillin-resistant Staphylococcus pseudintermedius: epidemiological changes, antibiotic resistance, and alternative therapeutic strategies. Vet. Res. Commun. 2024, 48, 3505–3515. [Google Scholar] [CrossRef]
- Roberts, E.; Nuttall, T.J.; Gkekas, G.; Mellanby, R.J.; Fitzgerald, J.R.; Paterson, G.K. Not just in man’s best friend: A review of Staphylococcus pseudintermedius host range and human zoonosis. Res. Vet. Sci. 2024, 174, 105305. [Google Scholar] [CrossRef]
- Bannoehr, J.; Guardabassi, L. Staphylococcus pseudintermedius in the dog: taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet. Dermatol. 2012, 23, 253–266. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.; Kwon, M.; Kwon, H.; Yong, J.; Yoon, H.; Hwang, J.; Jung, J.S.; Park, K.M. Bacterial isolates and antibiotic sensitivity in canine bacterial keratitis in Korea. Vet. Ophthalmol. 2024, 1–12. [Google Scholar] [CrossRef]
- Casemiro, P.A.F.; Andrade, A.L.; Cardozo, M.V.; Rodrigues, R.A.; Silva, J.A.; Marinho, M.; Nassar, A.F.C.; Castro, V.; Braz, G.H.R.; Gujanwski, C.A.; et al. Prevalence and antibiotic resistance in bacterial isolates of dogs with ulcerative keratitis in São Paulo State, Brazil. Vet. Ophthalmol. 2025, 28, 37–47. [Google Scholar] [CrossRef]
- Engdahl, K.S.; Brodbelt, D.C.; Cameron, C.; Church, D.B.; O’Neill, D.G. English Cocker Spaniels under primary veterinary care in the UK: disorder predispositions and protections. Canine Med. Genet. 2024, 11, 1. [Google Scholar] [CrossRef]
- Lau, W.I.; Taylor, R.M. The Prevalence of Corneal Disorders in Pugs Attending Primary Care Veterinary Practices in Australia. Animals 2025, 15, 531. [Google Scholar] [CrossRef]
- Williams, D.L. Immunopathogenesis of keratoconjunctivitis sicca in the dog. Vet. Clin. North Am. Small Anim. Pract. 2008, 38, 251–268. [Google Scholar] [CrossRef]
- Dodi, P.L. Immune-mediated keratoconjunctivitis sicca in dogs: current perspectives on management. Vet. Med. 2015, 6, 341–347. [Google Scholar] [CrossRef][Green Version]
- Mauer, A.N.; Allbaugh, R.A.; Kreuder, A.J.; Sebbag, L. Impact of multi-drug resistance on clinical outcomes of dogs with corneal ulcers infected with Staphylococcus pseudintermedius. Front. Vet. Sci. 2022, 9, 1083294. [Google Scholar] [CrossRef]
- Ferran, A.A.; Liu, J.; Toutain, P.L.; Bousquet-Mélou, A. Comparison of the In vitro Activity of Five Antimicrobial Drugs against Staphylococcus pseudintermedius and Staphylococcus aureus Biofilms. Front. Microbiol. 2016, 7, 1187. [Google Scholar] [CrossRef]
- Andrade, M.; Oliveira, K.; Morais, C.; Abrantes, P.; Pomba, C.; Rosato, A.E.; Couto, I.; Costa, S.S. Virulence Potential of Biofilm-Producing Staphylococcus pseudintermedius, Staphylococcus aureus and Staphylococcus coagulans Causing Skin Infections in Companion Animals. Antibiotics. 2022, 11, 1339. [Google Scholar] [CrossRef]
- Cangui-Panchi, S.P.; Ñacato-Toapanta, A.L.; Enríquez-Martínez, L.J.; Salinas-Delgado, G.A.; Reyes, J.; Garzon-Chavez, D.; Machado, A. Battle royale: Immune response on biofilms—host-pathogen interactions. Curr. Res. Immunol. 2023, 4, 100057. [Google Scholar] [CrossRef]
- Martins, C.H.G. Special Issue Editorial: “Antibacterial Agents from Natural Sources”. Molecules 2024, 29, 644. [Google Scholar] [CrossRef]
- Nogueira, J.O.E.; Campolina, G.A.; Batista, L.R.; Alves, E.; Caetano, A.R.S.; Brandão, R.M.; Nelson, D.L.; Cardoso, M.D.G. Mechanism of action of various terpenes and phenylpropanoids against Escherichia coli and Staphylococcus aureus. FEMS Microbiol. Lett. 2021, 368, fnab052. [Google Scholar] [CrossRef]
- Jantorn, P.; Tipmanee, V.; Wanna, W.; Prapasarakul, N.; Visutthi, M.; Sotthibandhu, D.S. Potential natural antimicrobial and antibiofilm properties of Piper betle L. against Staphylococcus pseudintermedius and methicillin-resistant strains. J. Ethnopharmacol. 2023, 317, 116820. [Google Scholar] [CrossRef]
- Phensri, P.; Thummasema, K.; Sukatta, U.; Morand, S.; Pruksakorn, C. In Vitro Antimicrobial Activity of Piper betle Leaf Extract and Some Topical Agents against Methicillin-Resistant and Methicillin-Susceptible Staphylococcus Strains from Canine Pyoderma. Animals 2022, 12, 3203. [Google Scholar] [CrossRef]
- Ribeiro, T.A.N.; Dos Santos, G.A.; Dos Santos, C.T.; Soares, D.C.F.; Saraiva, M.F.; Leal, D.H.S.; Sachs, D. Eugenol as a promising antibiofilm and anti-quorum sensing agent: A systematic review. Microb. Pathog. 2024, 196, 106937. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Mohammed, D.M.; Korma, S.A.; Alshahrani, M.Y.; Ahmed, A.E.; Ibrahim, E.H.; Salem, H.M.; Alkafaas, S.S.; Saif, A.M.; et al. Medicinal plants: bioactive compounds, biological activities, combating multidrug-resistant microorganisms, and human health benefits—a comprehensive review. Front. Immunol. 2025, 16, 1491777. [Google Scholar] [CrossRef]
- Bardaji, D.K.R.; Silva, N.B.S.; Miranda, R.R.; Martins, C.H.G.; Savka, M.A.; Hudson, A.O. Unlocking the Potential of Brazilian Plant Terpenes to Combat Antimicrobial Resistance. ACS Bio. Med. Chem. Au. 2025, 5, 365–378. [Google Scholar] [CrossRef]
- Lao, Y.; Guo, J.; Fang, J.; Geng, R.; Li, M.; Qin, Y.; Wu, J.; Kang, S.G.; Huang, K.; Tong, T. Beyond flavor: the versatile roles of eugenol in health and disease. Food Funct. 2024, 15, 10567–10581. [Google Scholar] [CrossRef]
- Perugini Biasi-Garbin, R.; Saori Otaguiri, E.; Morey, A.T.; Fernandes da Silva, M.; Belotto Morguette, A.E.; Armando Contreras Lancheros, C.; Kian, D.; Perugini, M.R.; Nakazato, G.; Durán, N.; et al. Effect of Eugenol against Streptococcus agalactiae and Synergistic Interaction with Biologically Produced Silver Nanoparticles. Evid. Based Complement. Alternat. Med. 2015, 2015, 861497. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Shahina, Z.; Dahms, T.E.S. A Comparative Review of Eugenol and Citral Anticandidal Mechanisms: Partners in Crimes Against Fungi. Molecules 2024, 29, 5536. [Google Scholar] [CrossRef]
- Nakazato, G.; Celidonio, A.P.S.; Kobayashi, R.K.T.; Panagio, L.A.; Lonni, A.A.G.S.; De Campos, A.C.L.P.; Goncalves, M.C.; Okino, G.A.K. Carta Patente—Processo de Produção de Nanopartículas Biogênicas de Prata, Nanopartículas Biogênicas de Prata e Usos das Nanopartículas Biogênicas de Prata—GRAL Bioativos LTDA. BR Patent Application No. BR1020210163755, 18 September 2021. Available online: http://www.inpi.gov.br (accessed on 10 February 2025).
- Meroni, G.; Soares Filipe, J.F.; Martino, P.A. In Vitro Antibacterial Activity of Biological-Derived Silver Nanoparticles: Preliminary Data. Vet. Sci. 2020, 7, 12. [Google Scholar] [CrossRef]
- Wongstitwilairoong, N.; Jermnark, U.; Paochoosak, N.; Limsivilai, O.; Chimnoi, W.; Yohannes, Y.B.; Ikenaka, Y.; Saengtienchai, A. Effect of Java plum (Syzygium cumini) leaves extract and a silver nanoparticles synthesis on pathogens in skin diseases of dogs. Open Vet. J. 2024, 14, 2662–2677. [Google Scholar] [CrossRef]
- Akhter, M.S.; Rahman, M.A.; Ripon, R.K.; Mubarak, M.; Akter, M.; Mahbub, S.; Al Mamun, F.; Sikder, M.T. A systematic review on green synthesis of silver nanoparticles using plants extract and their biomedical applications. Heliyon 2024, 10, e29766. [Google Scholar] [CrossRef]
- Zahran, M.; Marei, A.H. Innovative natural polymer metal nanocomposites and their antimicrobial activity. Int. J. Biol. Macromol. 2019, 136, 586–596. [Google Scholar] [CrossRef]
- Otaguiri, E.S.; Morguette, A.E.B.; Biasi-Garbin, R.P.; Morey, A.T.; Lancheros, C.A.C.; Kian, D.; de Oliveira, A.G.; Kerbauy, G.; Perugini, M.R.E.; Duran, N.; et al. Antibacterial Combination of Oleoresin from Copaifera multijuga Hayne and Biogenic Silver Nanoparticles Towards Streptococcus agalactiae. Curr. Pharm. Biotechnol. 2017, 18, 177–190. [Google Scholar] [CrossRef]
- Saeki, E.K.; Martins, H.M.; Camargo, L.C.; Anversa, L.; Tavares, E.R.; Yamada-Ogatta, S.F.; Lioni, L.M.Y.; Kobayashi, R.K.T.; Nakazato, G. Effect of Biogenic Silver Nanoparticles on the Quorum-Sensing System of Pseudomonas aeruginosa PAO1 and PA14. Microorganisms 2022, 10, 1755. [Google Scholar] [CrossRef]
- Spoladori, L.F.A.; Andriani, G.M.; Castro, I.M.; Suzukawa, H.T.; Gimenes, A.C.R.; Bartolomeu-Gonçalves, G.; Ishida, K.; Nakazato, G.; Pinge-Filho, P.; Machado, R.R.B.; et al. Synergistic Antifungal Interaction between Pseudomonas aeruginosa LV Strain Metabolites and Biogenic Silver Nanoparticles against Candida auris. Antibiotics 2023, 12, 861. [Google Scholar] [CrossRef]
- Castro, I.M.; Antunes, C.; Valentim, C.C.; Spoladori, L.F.A.; Suzukawa, H.T.; Correia, G.F.; Silva-Rodrigues, G.; Borges, P.H.G.; Bartolomeu-Gonçalves, G.; Silva, M.L.; et al. Synergistic Antibacterial Interaction of Geraniol and Biogenic Silver Nanoparticles on Methicillin-Resistant Staphylococcus aureus. Plants 2025, 14, 1059. [Google Scholar] [CrossRef]
- Samuggam, S.; Chinni, S.V.; Mutusamy, P.; Gopinath, S.C.B.; Anbu, P.; Venugopal, V.; Reddy, L.V.; Enugutti, B. Green Synthesis and Characterization of Silver Nanoparticles Using Spondias mombin Extract and Their Antimicrobial Activity against Biofilm-Producing Bacteria. Molecules 2021, 26, 2681. [Google Scholar] [CrossRef]
- Sasaki, T.; Kikuchi, K.; Tanaka, Y.; Takahashi, N.; Kamata, S.; Hiramatsu, K. Reclassification of phenotypically identified Staphylococcus intermedius strains. J. Clin. Microbiol. 2007, 45, 2770–2778. [Google Scholar] [CrossRef]
- Sasaki, T.; Tsubakishita, S.; Tanaka, Y.; Sakusabe, A.; Ohtsuka, M.; Hirotaki, S.; Kawakami, T.; Fukata, T.; Hiramatsu, K. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J. Clin. Microbiol. 2010, 48, 765–769. [Google Scholar] [CrossRef]
- Milheiriço, C.; Oliveira, D.C.; de Lencastre, H. Update to the multiplex PCR strategy for assignment of mec element types in Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 3374–3377, Erratum in Antimicrob. Agents Chemother. 2007, 51, 4537. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- CLSI VET01S. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals; CLSI Press: Wayne, PA, USA, 2024. [Google Scholar]
- CLSI M26. Methods for Determining Bactericidal Activity of Antimicrobial Agents; CLSI Press: Wayne, PA, USA, 1999; Volume 19, p. 18. [Google Scholar]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 2009, 20, 791–815. [Google Scholar] [CrossRef]
- Pereira, C.S.G.; Zulim, L.F.C.; Giuffrida, R.; Cruz, A.G.; Foglia, B.T.D.; Benguella, H.; Batista, A.S.; Andrade, S.F. Antimicrobial susceptibility and minimal inhibitory concentration of bacteria isolated from the eyes of dogs with keratoconjunctivitis sicca. Pesq. Vet. Bras. 2019, 39, 757–763. [Google Scholar] [CrossRef]
- Meroni, G.; Laterza, G.; Tsikopoulos, A.; Tsikopoulos, K.; Vitalini, S.; Scaglia, B.; Iriti, M.; Bonizzi, L.; Martino, P.A.; Soggiu, A. Antibacterial Potential of Essential Oils and Silver Nanoparticles against Multidrug-Resistant Staphylococcus pseudintermedius Isolates. Pathogens 2024, 13, 156. [Google Scholar] [CrossRef]
- Silva, R.A.; da Silva, B.F.; Pereira, M.S.; Coelho, P.A.T.; Costa, R.A.; Chaves, A.C.; Silva, I.G.N.; Carneiro, V.A. Combinatorial effects between aromatic plant compounds and chlorhexidine digluconate against canine otitis-related Staphylococcus spp. Res. Vet. Sci. 2024, 170, 105182. [Google Scholar] [CrossRef]
- Thammawithan, S.; Siritongsuk, P.; Nasompag, S.; Daduang, S.; Klaynongsruang, S.; Prapasarakul, N.; Patramanon, R. A Biological Study of Anisotropic Silver Nanoparticles and Their Antimicrobial Application for Topical Use. Vet. Sci. 2021, 8, 177. [Google Scholar] [CrossRef]
- Seo, M.; Oh, T.; Bae, S. Antibiofilm activity of silver nanoparticles against biofilm forming Staphylococcus pseudintermedius isolated from dogs with otitis externa. Vet. Med. Sci. 2021, 7, 1551–1557. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef]
- González-Fernández, S.; Blanco-Agudín, N.; Rodríguez, D.; Fernández-Veja, I.; Merayo-Lloves, J.; Quirós, L.M. Silver Nanoparticles: A Versatile Tool Against Infectious and Non-Infectious Diseases. Antibiotics 2025, 14, 289. [Google Scholar] [CrossRef]
- Tripathi, N.; Goshisht, M.K. Recent Advances and Mechanistic Insights into Antibacterial Activity, Antibiofilm Activity, and Cytotoxicity of Silver Nanoparticles. ACS Appl. Bio Mater. 2022, 5, 1391–1463. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Xu, X.; Gao, P.; Xu, Z.; Zhang, Q.; Li, H.; Yan, A.; Kao, R.Y.; Sun, H. Multi-target mode of action of silver against Staphylococcus aureus endows it with capability to combat antibiotic resistance. Nat. Commun. 2021, 12, 3331, Erratum in Nat. Commun. 2021, 12, 4140. [Google Scholar] [CrossRef]
- FDA, U.S. Food and Drug Administration. Inventory of Food Contact Substances Listed in 21 CFR. Available online: https://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=IndirectAdditives&id=EUGENOL (accessed on 2 June 2025).
- WHO. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). 2021. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/3278. (accessed on 7 July 2025).
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Loeffler, A.; Cain, C.L.; Ferrer, L.; Nishifuji, K.; Varjonen, K.; Papich, M.G.; Guardabassi, L.; Frosini, S.M.; Barker, E.N.; Weese, S.J. Synopsis of the antimicrobial use guidelines for canine pyoderma by the International Society for Companion Animal Infectious Diseases (ISCAID). Vet. Dermatol. 2025, 36, 234–282. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Kawasuji, H.; Nagaoka, K.; Tsuji, Y.; Kimoto, K.; Takegoshi, Y.; Kaneda, M.; Murai, Y.; Karaushi, H.; Mitsutake, K.; Yamamoto, Y. Effectiveness and Safety of Linezolid Versus Vancomycin, Teicoplanin, or Daptomycin against Methicillin-Resistant Staphylococcus aureus Bacteremia: A Systematic Review and Meta-Analysis. Antibiotics 2023, 12, 697. [Google Scholar] [CrossRef]
- Ishak, A.; Mazonakis, N.; Spernovasilis, N.; Akinosoglou, K.; Tsioutis, C. Bactericidal versus bacteriostatic antibacterials: clinical significance, differences and synergistic potential in clinical practice. J. Antimicrob. Chemother. 2025, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Mandal, D.; Dash, S.K.; Chattopadhyay, S.; Tripathy, S.; Dolai, D.P.; Dey, S.K.; Roy, S. Eugenol Provokes ROS-Mediated Membrane Damage-Associated Antibacterial Activity Against Clinically Isolated Multidrug-Resistant Staphylococcus aureus Strains. Infect. Dis. 2016, 9, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Dharshini, K.S.; Ameen, F.; Anbazhagan, V. Mechanistic Investigation on the Antibacterial Activity of Biogenic Silver Nanoparticles Prepared Using Root Extract of Sarsaparilla and Demonstrated their In Vivo Efficacy in Zebrafish Model. Curr. Microbiol. 2024, 81, 268. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Arciola, C.R.; Baldassarri, L.; Montanaro, L. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J. Clin. Microbiol. 2001, 39, 2151–2156. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.W.; Im, G.J.; Chung, J.W.; Song, J.J. Eugenol: a phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS One 2015, 10, e0119564. [Google Scholar] [CrossRef]
- Santos, L.M.; Rodrigues, D.M.; Alves, B.V.B.; Kalil, M.A.; Azevedo, V.; Barh, D.; Meyer, R.; Duran, N.; Tasic, L.; Portela, R.W. Activity of biogenic silver nanoparticles in planktonic and biofilm-associated Corynebacterium pseudotuberculosis. PeerJ 2024, 12, e16751. [Google Scholar] [CrossRef]
- Alsirhani, A.M.; Abu-Almakarem, A.S.; Alwaili, M.A.; Aljohani, S.; Alali, I.; AlRashidi, A.A.; Abuzinadah, N.Y.; Alkhodair, S.A.; Mobasher, M.A.; Alothaim, T.; et al. Syzygium aromaticum Extract Mitigates Doxorubicin-Induced Hepatotoxicity in Male Rats. Int. J. Mol. Sci. 2024, 25, 12541. [Google Scholar] [CrossRef]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxid. Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Zhang, L.W.; Koci, J.; Jeffery, B.; Riviere, J.E.; Monteiro-Riviere, N.A. Safety assessment of potential food ingredients in canine hepatocytes. Food Chem. Toxicol. 2015, 78, 105–115. [Google Scholar] [CrossRef]
- Aboelwafa, H.R.; Ramadan, R.A.; Ibraheim, S.S.; Yousef, H.N. Modulation Effects of Eugenol on Nephrotoxicity Triggered by Silver Nanoparticles in Adult Rats. Biology 2022, 11, 1719. [Google Scholar] [CrossRef] [PubMed]
- Yousef, H.N.; Ibraheim, S.S.; Ramadan, R.A.; Aboelwafa, H.R. The Ameliorative Role of Eugenol against Silver Nanoparticles-Induced Hepatotoxicity in Male Wistar Rats. Oxid. Med. Cell Longev. 2022, 2022, 3820848. [Google Scholar] [CrossRef] [PubMed]
- Gastelum-Leyva, F.; Pena-Jasso, A.; Alvarado-Vera, M.; Plascencia-López, I.; Patrón-Romero, L.; Loera-Castañeda, V.; Gándara-Mireles, J.A.; Lares-Asseff, I.; Leal-Ávila, M.Á.; Alvelais-Palacios, J.A.; et al. Evaluation of the Efficacy and Safety of Silver Nanoparticles in the Treatment of Non-Neurological and Neurological Distemper in Dogs: A Randomized Clinical Trial. Viruses 2022, 14, 2329. [Google Scholar] [CrossRef] [PubMed]
- Deonas, A.N.; Souza, L.M.D.S.; Andrade, G.J.S.; Germiniani-Cardozo, J.; Dahmer, D.; de Oliveira, A.G.; Nakazato, G.; Torezan, J.M.D.; Kobayashi, R.K.T. Green Synthesis of Silver Nanoparticle from Anadenanthera colubrina Extract and Its Antimicrobial Action against ESKAPEE Group Bacteria. Antibiotics 2024, 13, 777. [Google Scholar] [CrossRef]
- Klepser, M.E.; Ernst, E.J.; Lewis, R.E.; Ernst, M.E.; Pfaller, M.A. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob. Agents Chemother. 1998, 42, 1207–1212. [Google Scholar] [CrossRef]
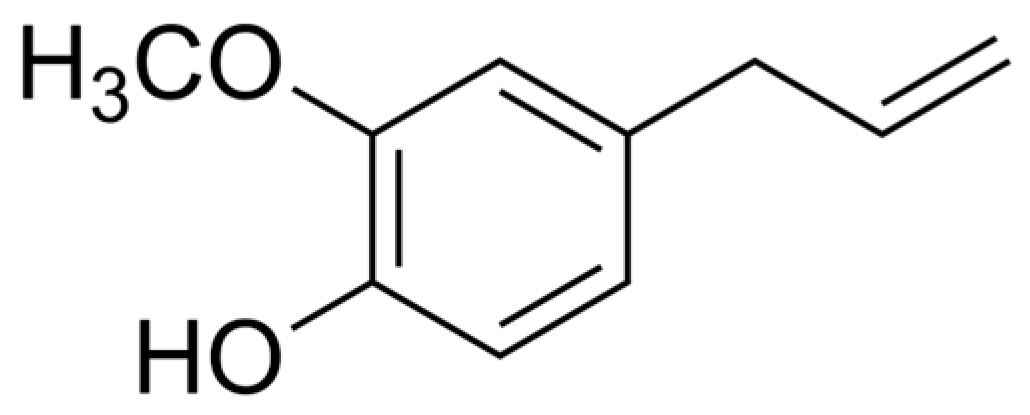
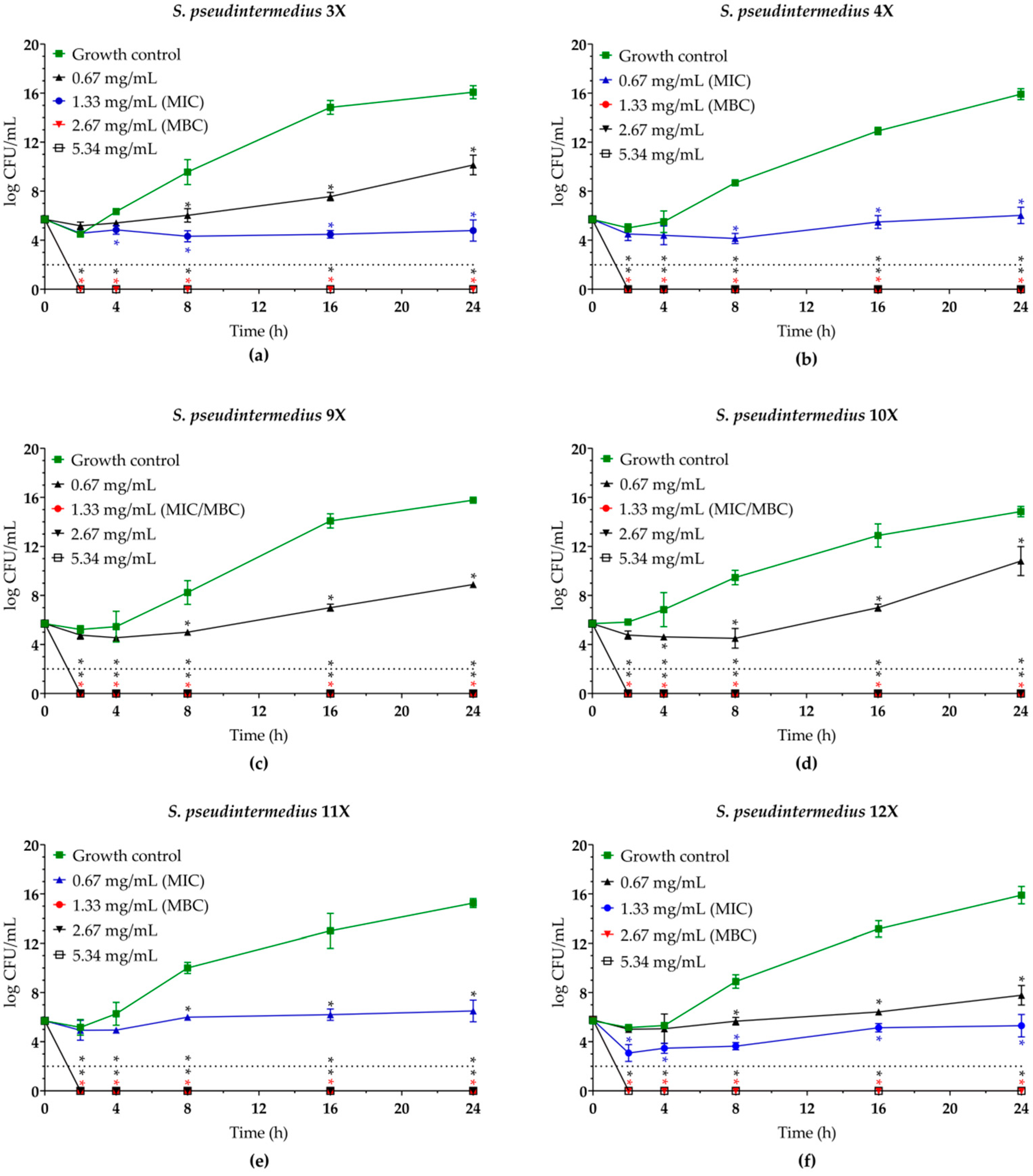
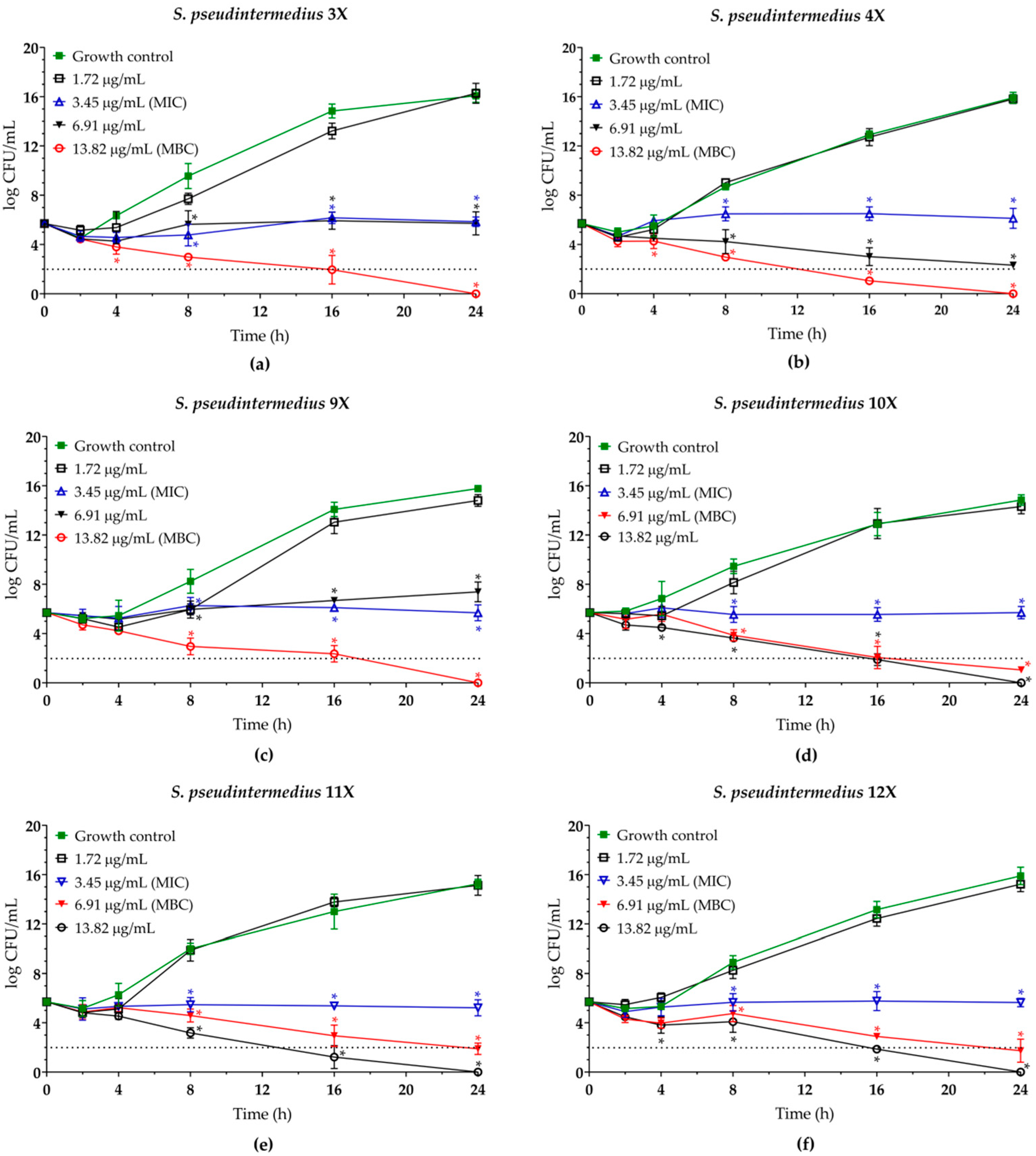
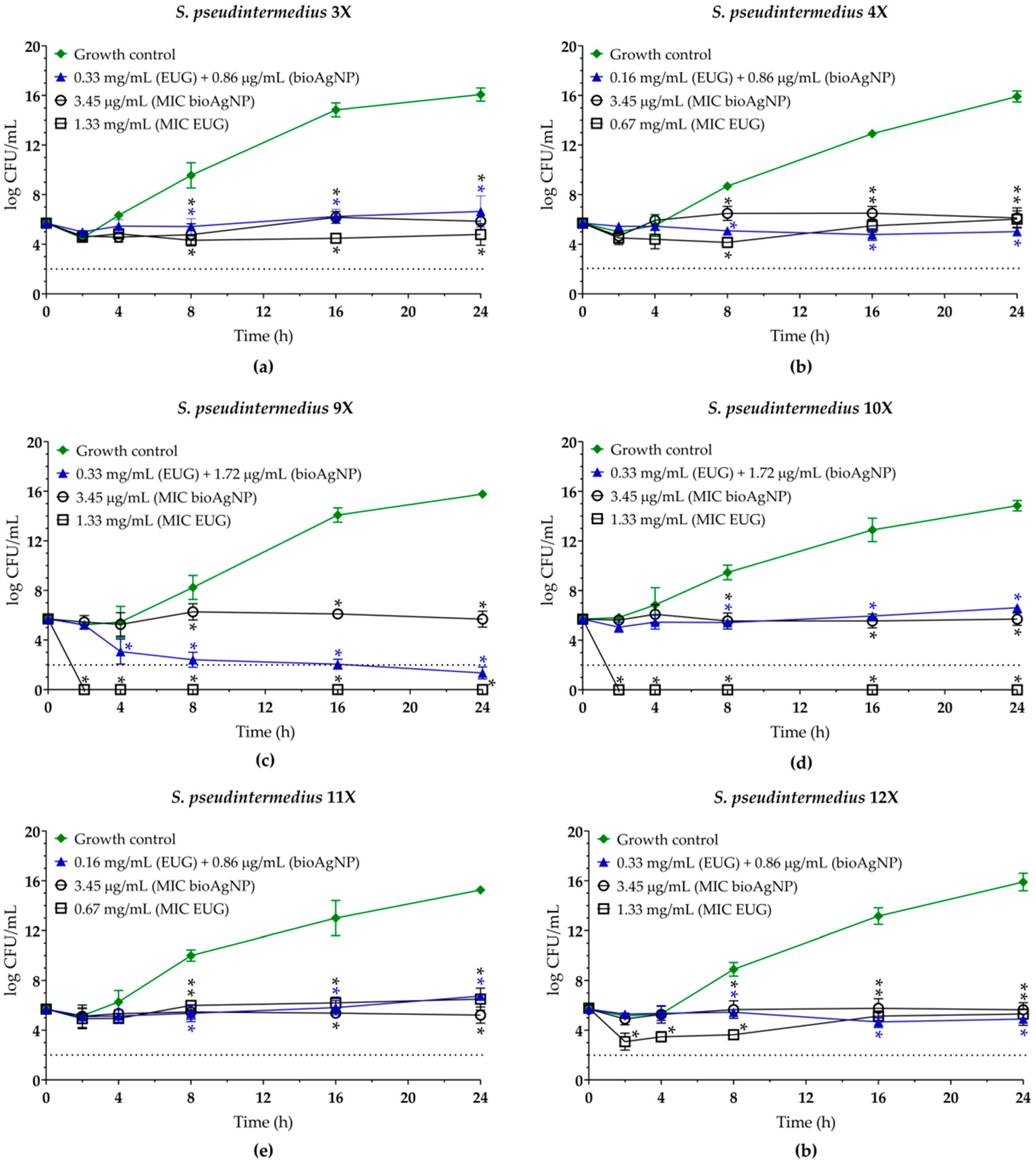
| Isolate | Resistance profilea | mecAb | EUG | BioAgNPs | MBC/MICe | |||
|---|---|---|---|---|---|---|---|---|
| MICc | MBCd | MICc | MBCd | EUG | bioAgNP | |||
| SIG 3X | OX, P, AZM, ERY, DA*, CIP, LEV, CN*, TE, CHL | positive | 1.33 | 2.67 | 3.45 | 13.82 | 2.0 | 4.0 |
| SIG 4X | OX, P, AZM, ERY, DA*, CIP, LEV, CN, CHL | positive | 0.67 | 1.33 | 3.45 | 13.82 | 2.0 | 4.0 |
| SIG 9X | OX, P, AZM, ERY, DA*, CIP, LEV, CN*, TE, CHL | positive | 1.33 | 1.33 | 3.45 | 13.82 | 1.0 | 4.0 |
| SIG 10X | OX, P, AZM, ERY, DA*, CIP, LEV, CN*, TE, CHL | positive | 1.33 | 1.33 | 3.45 | 6.91 | 1.0 | 2.0 |
| SIG 11X | P, AZM, ERY, DA*, CIP, LEV, CN*, TE | negative | 0.67 | 1.33 | 3.45 | 6.91 | 2.0 | 2.0 |
| SIG 12X | P, AZM, ERY, DA*, CIP, LEV, TE, CHL | negative | 1.33 | 2.67 | 3.45 | 6.91 | 2.0 | 2.0 |
| MIC | |||||
|---|---|---|---|---|---|
| Isolate | EUGa | bioAgNPsa | EUG/bioAgNPsb | FICIc | Interactiond |
| SIG 3X | 1.33 | 3.45 | 0.33/0.86 | 0.50 | Synergism |
| SIG 4X | 0.67 | 3.45 | 0.16/0.86 | 0.49 | Synergism |
| SIG 9X | 1.33 | 3.45 | 0.33/1.72 | 0.75 | Indifferent |
| SIG 10X | 1.33 | 3.45 | 0.33/1.72 | 0.75 | Indifferent |
| SIG 11X | 0.67 | 3.45 | 0.16/0.86 | 0.49 | Synergism |
| SIG 12X | 1.33 | 3.45 | 0.33/0.86 | 0.50 | Synergism |
| SMIC90 | |||||
|---|---|---|---|---|---|
| Isolate | EUGa | bioAgNPsa | EUG/bioAgNPsb | FICIc | Interactiond |
| SIG 3X | 1.33 | 13.82 | 0.33/3.45 | 0.50 | Synergism |
| SIG 4X | 1.33 | 13.82 | 0.33/3.45 | 0.50 | Synergism |
| SIG 9X | 1.33 | 13.82 | 0.33/3.45 | 0.50 | Synergism |
| SIG 10X | 0.67 | 13.82 | 0.33/3.45 | 0.74 | Indifferent |
| SIG 11X | 0.67 | 13.82 | 0.16/3.45 | 0.49 | Synergism |
| SIG 12X | 1.33 | 13.82 | 0.33/3.45 | 0.50 | Synergism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral, W.R.C.; de Oliveira, C.F.; Giuffrida, R.; de Almeida Spoladori, L.F.; de Castro, I.M.; Bartolomeu-Gonçalves, G.; Suzukawa, H.T.; Andriani, G.M.; Nakazato, G.; Tavares, E.R.; et al. Synergistic Antibacterial Effect of Eugenol and Biogenic Silver Nanoparticles on Staphylococcus pseudintermedius Isolated from Canine Keratoconjunctivitis Sicca. Molecules 2025, 30, 3353. https://doi.org/10.3390/molecules30163353
Cabral WRC, de Oliveira CF, Giuffrida R, de Almeida Spoladori LF, de Castro IM, Bartolomeu-Gonçalves G, Suzukawa HT, Andriani GM, Nakazato G, Tavares ER, et al. Synergistic Antibacterial Effect of Eugenol and Biogenic Silver Nanoparticles on Staphylococcus pseudintermedius Isolated from Canine Keratoconjunctivitis Sicca. Molecules. 2025; 30(16):3353. https://doi.org/10.3390/molecules30163353
Chicago/Turabian StyleCabral, Weslei Roberto Correia, Caio Ferreira de Oliveira, Rogerio Giuffrida, Lais Fernanda de Almeida Spoladori, Isabela Madeira de Castro, Guilherme Bartolomeu-Gonçalves, Helena Tiemi Suzukawa, Gabriella Maria Andriani, Gerson Nakazato, Eliandro Reis Tavares, and et al. 2025. "Synergistic Antibacterial Effect of Eugenol and Biogenic Silver Nanoparticles on Staphylococcus pseudintermedius Isolated from Canine Keratoconjunctivitis Sicca" Molecules 30, no. 16: 3353. https://doi.org/10.3390/molecules30163353
APA StyleCabral, W. R. C., de Oliveira, C. F., Giuffrida, R., de Almeida Spoladori, L. F., de Castro, I. M., Bartolomeu-Gonçalves, G., Suzukawa, H. T., Andriani, G. M., Nakazato, G., Tavares, E. R., Yamauchi, L. M., & Yamada-Ogatta, S. F. (2025). Synergistic Antibacterial Effect of Eugenol and Biogenic Silver Nanoparticles on Staphylococcus pseudintermedius Isolated from Canine Keratoconjunctivitis Sicca. Molecules, 30(16), 3353. https://doi.org/10.3390/molecules30163353







