Discovery of (E)-1,3-Diphenyl-2-Propen-1-One Derivatives as Potent and Orally Active NLRP3 Inflammasome Inhibitors for Colitis
Abstract
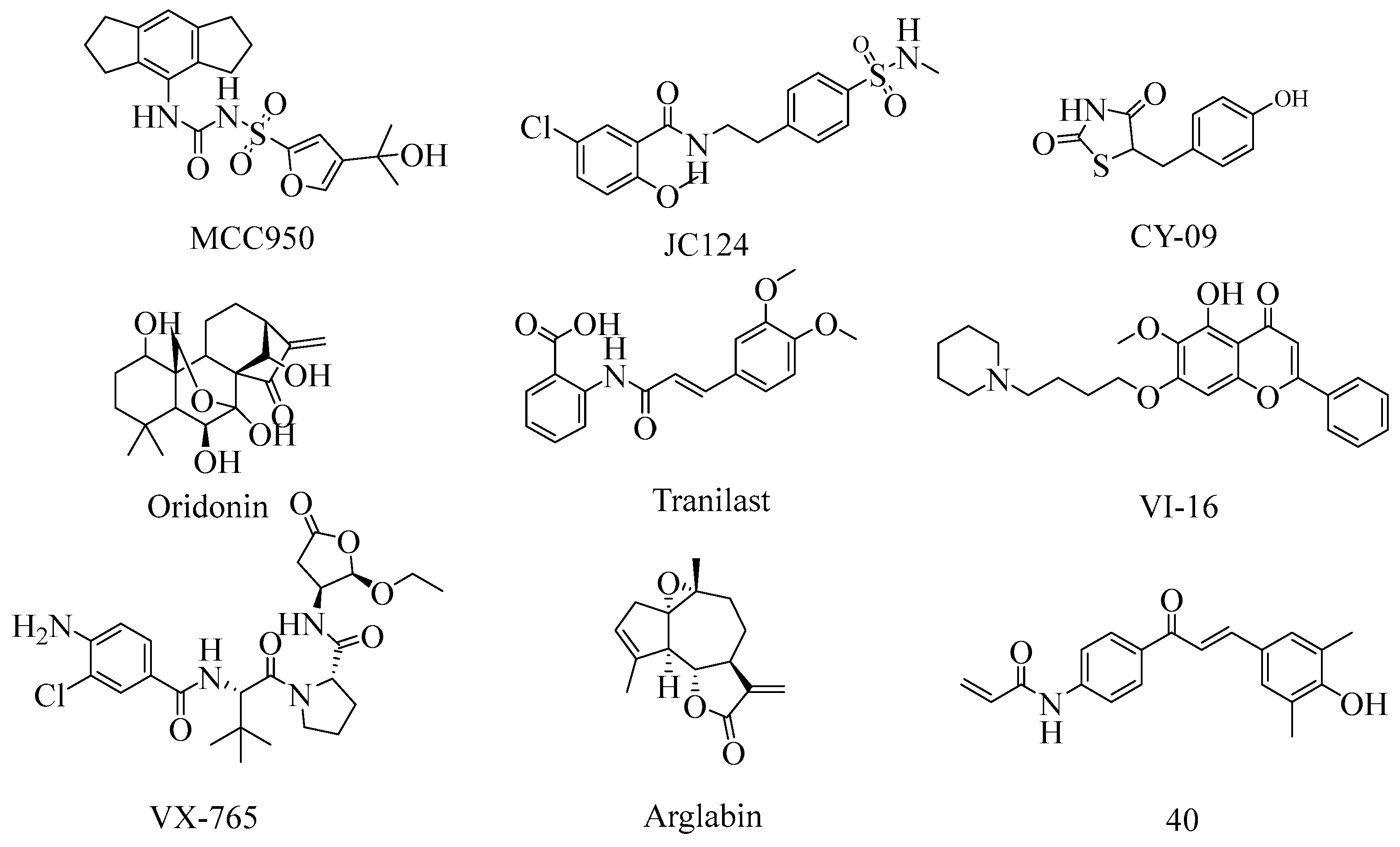
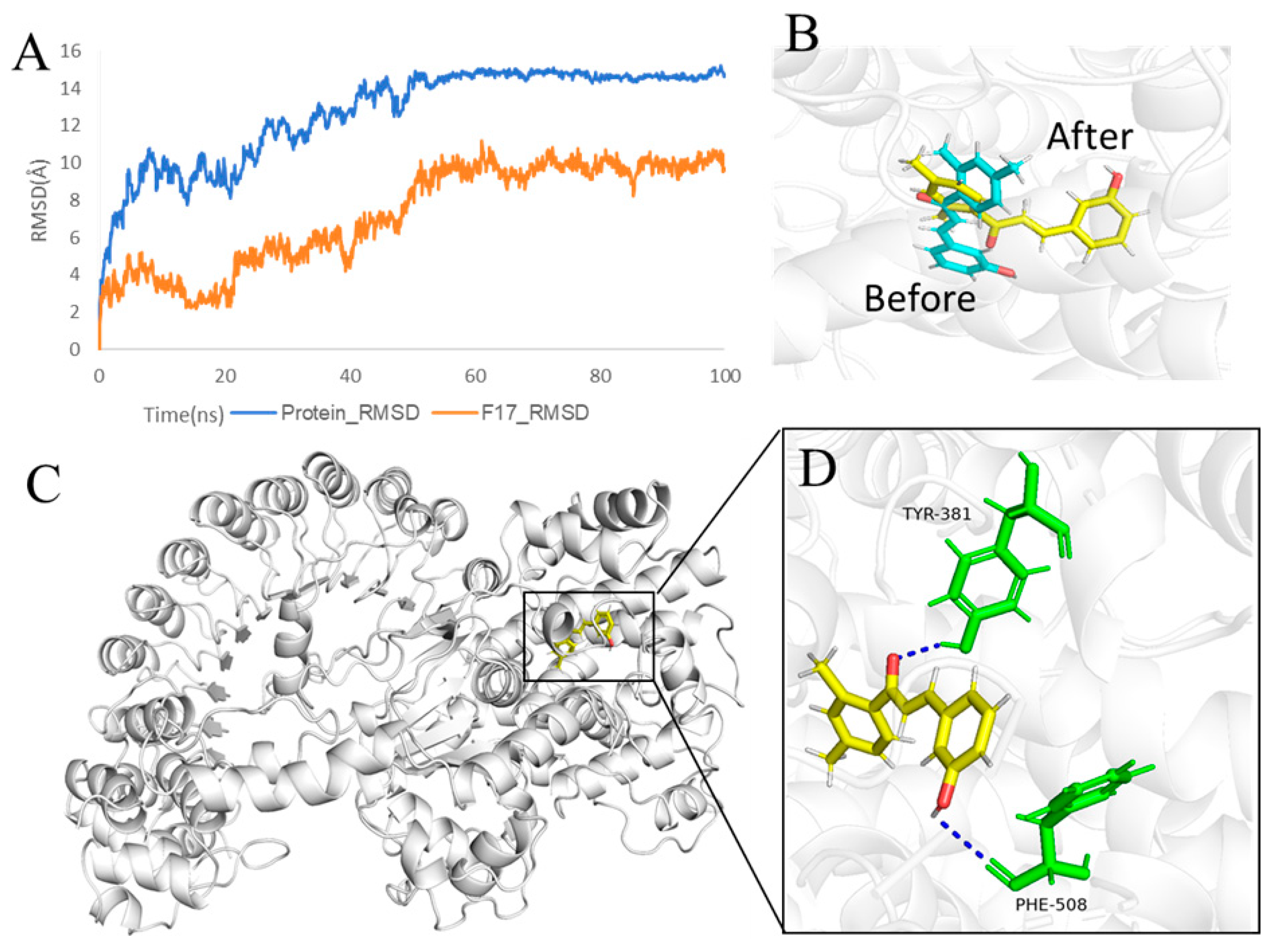
1. Introduction
2. Results and Discussion
2.1. Subsection
2.2. Synthesis of Compounds
2.3. SAR Study
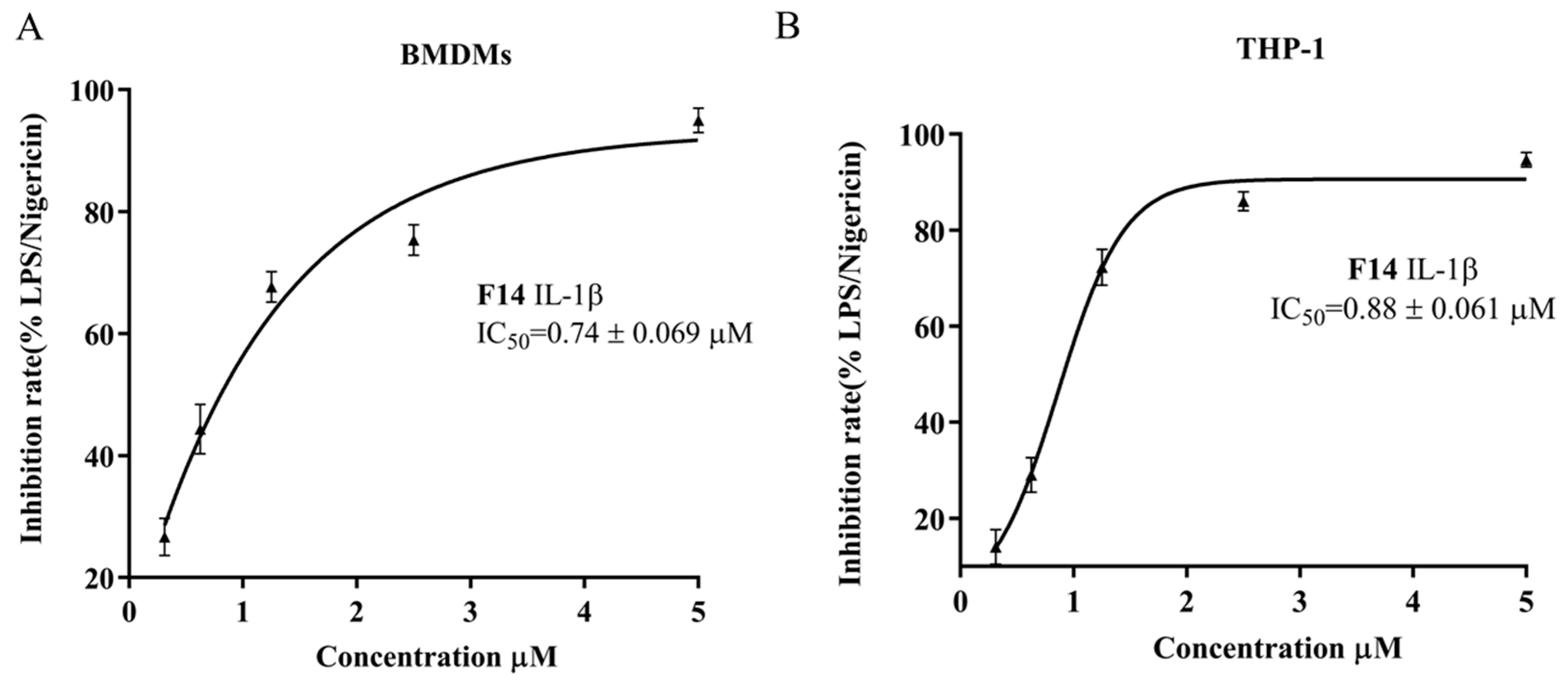
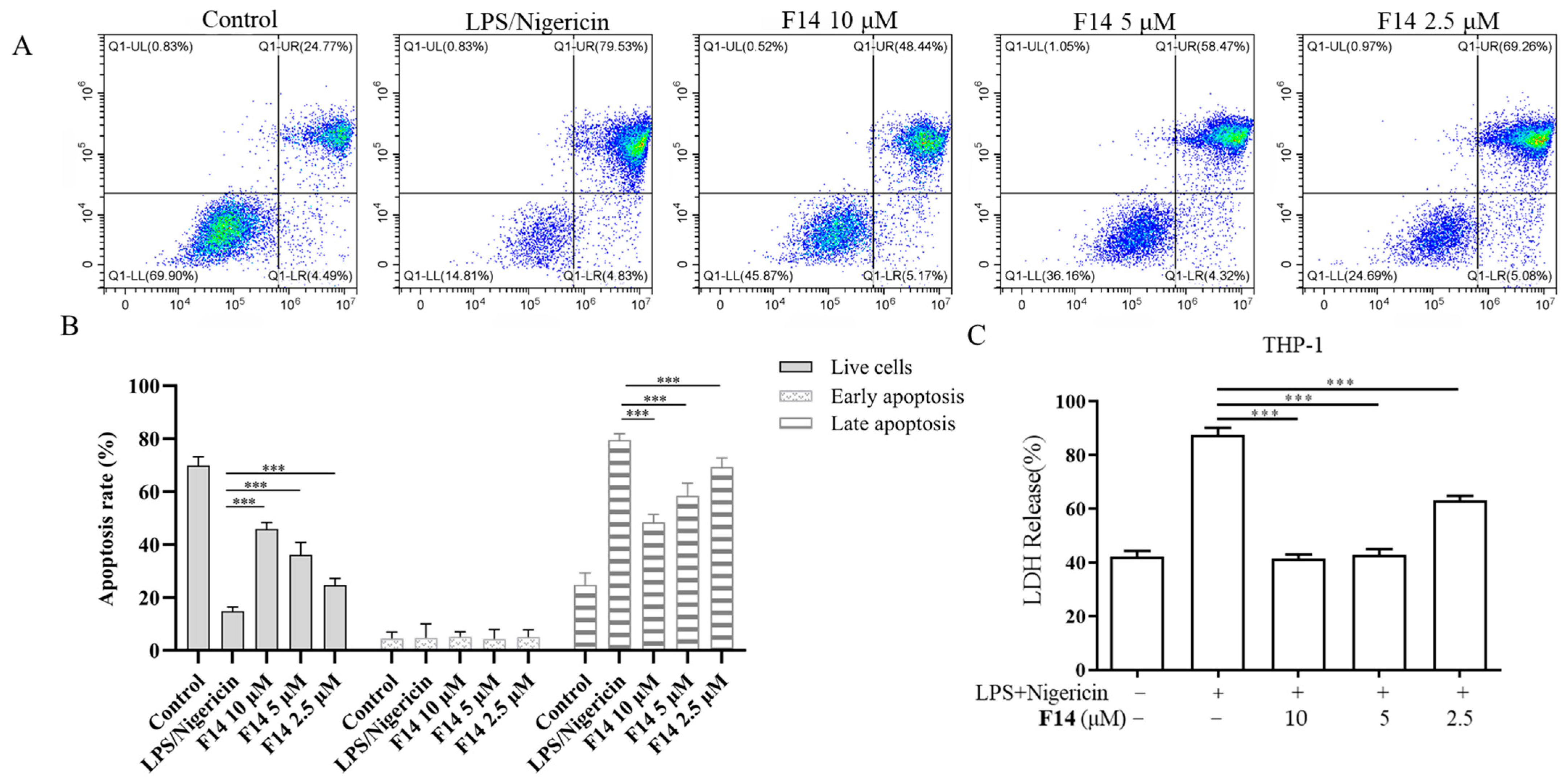
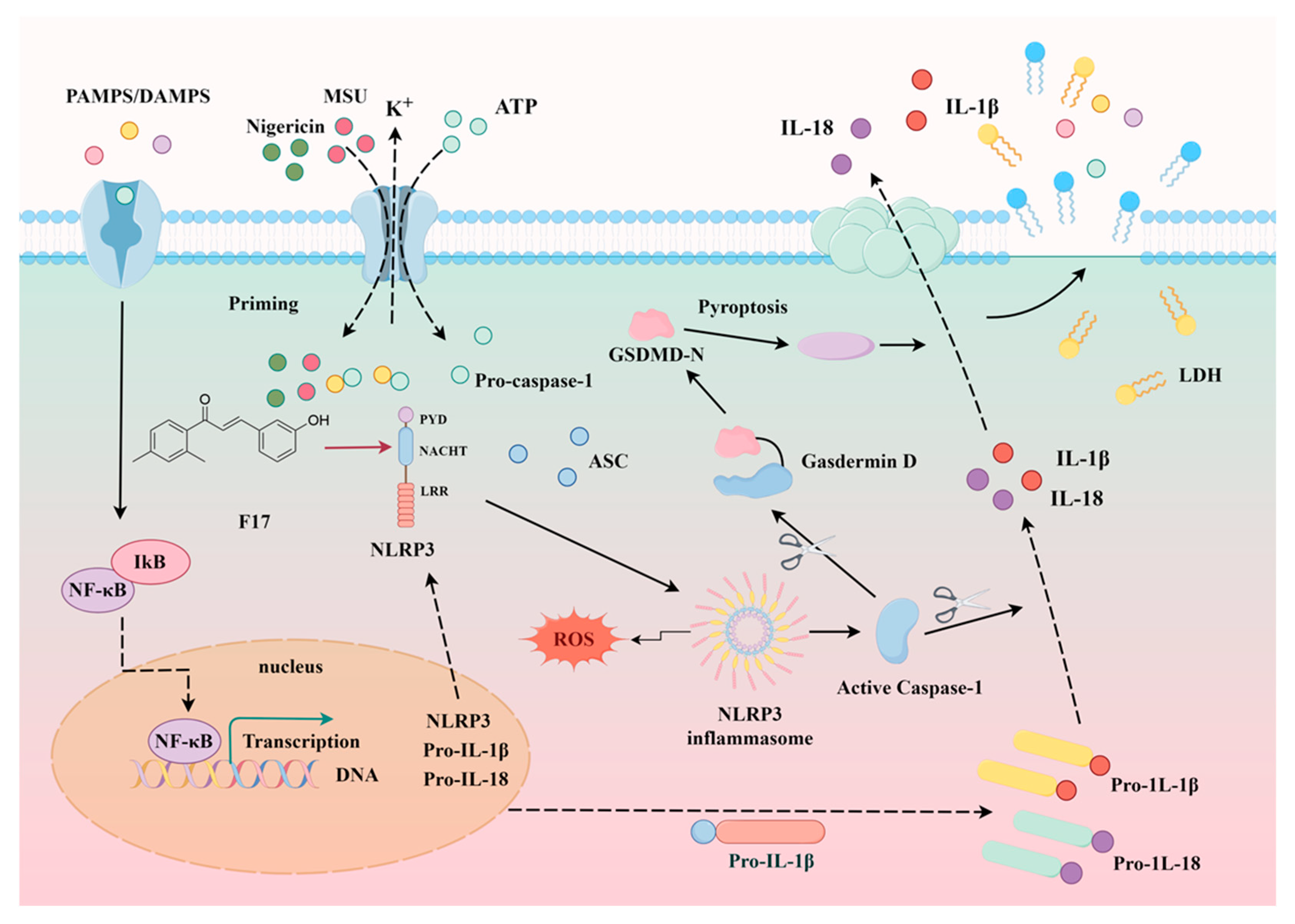
2.4. The Anti-Inflammatory Activity of F14
2.5. Compound F14 Inhibits Cell Pyroptosis Caused by NLRP3 Inflammasome Activation
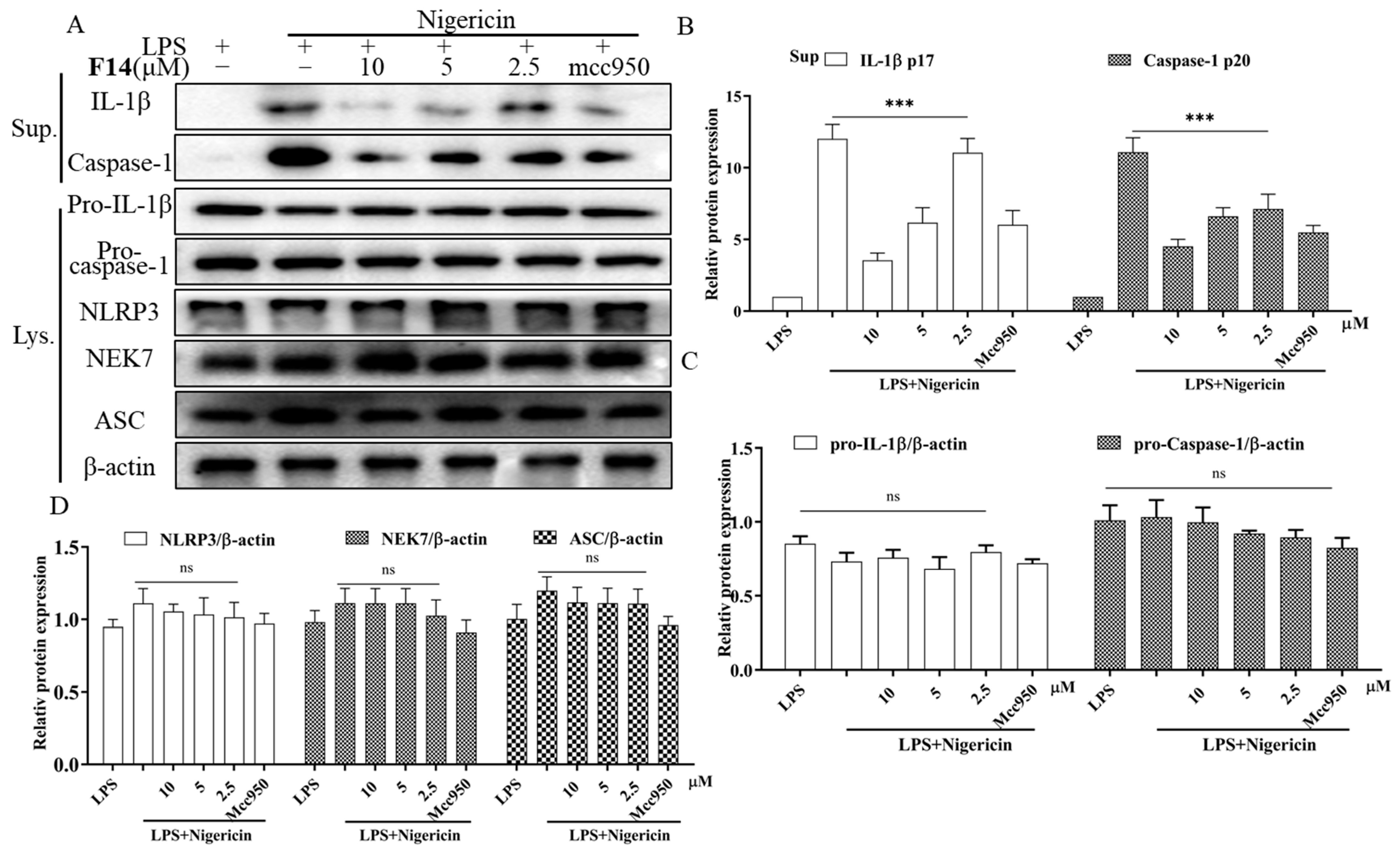
2.6. Compound F14 Inhibits NLRP3 Inflammasome Activation Induced by Multiple Agonists, Without Affecting AIM2 and NLRC4 Inflammasome Activation
2.7. The Effect of F14 on the Expression of Major Protein Components of NLRP3 Inflammasome
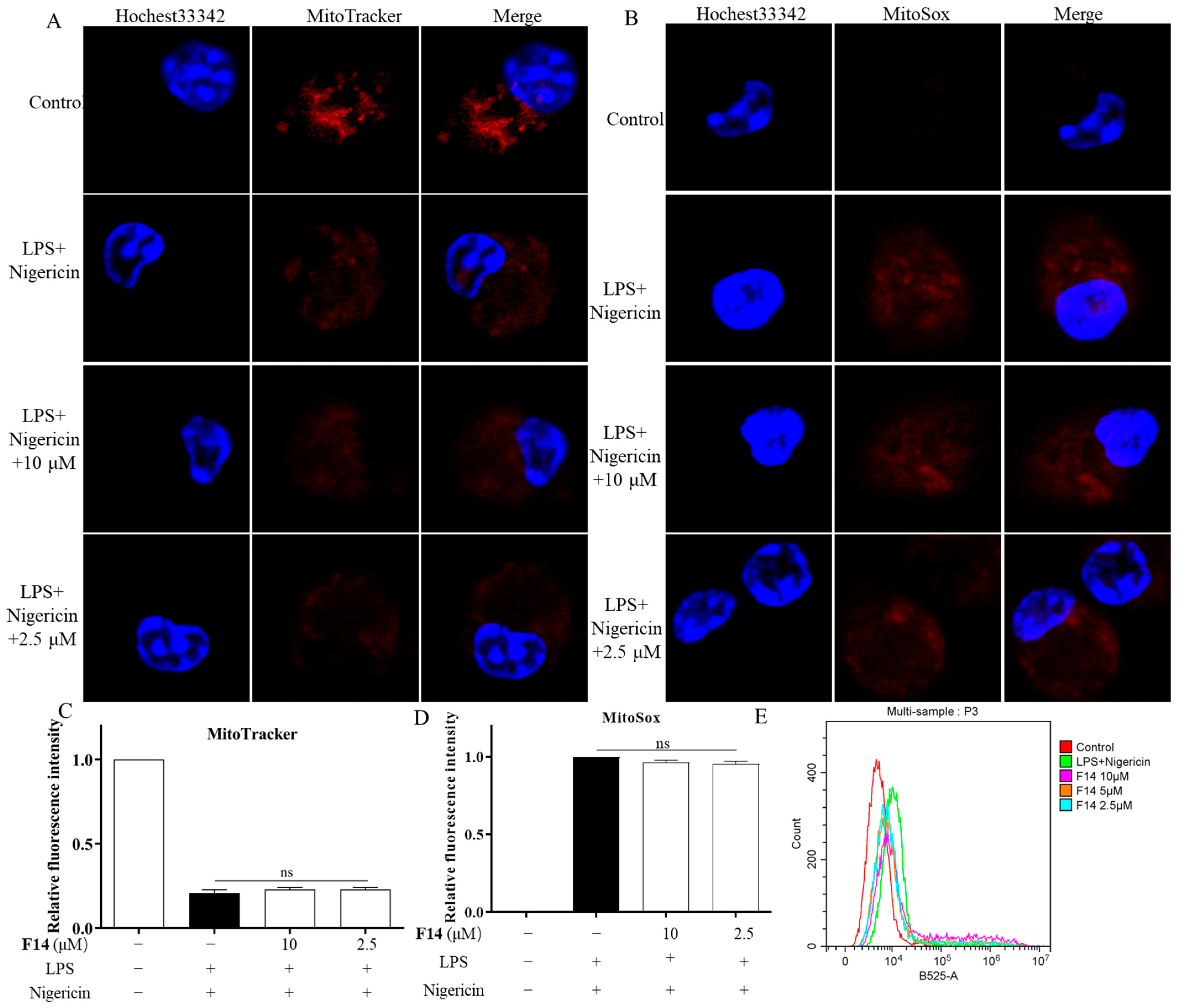
2.8. F14 Does Not Affect Mitochondrial Damage and Reactive Oxygen Species Production During the NLPR3 Inflammasome Activation Process
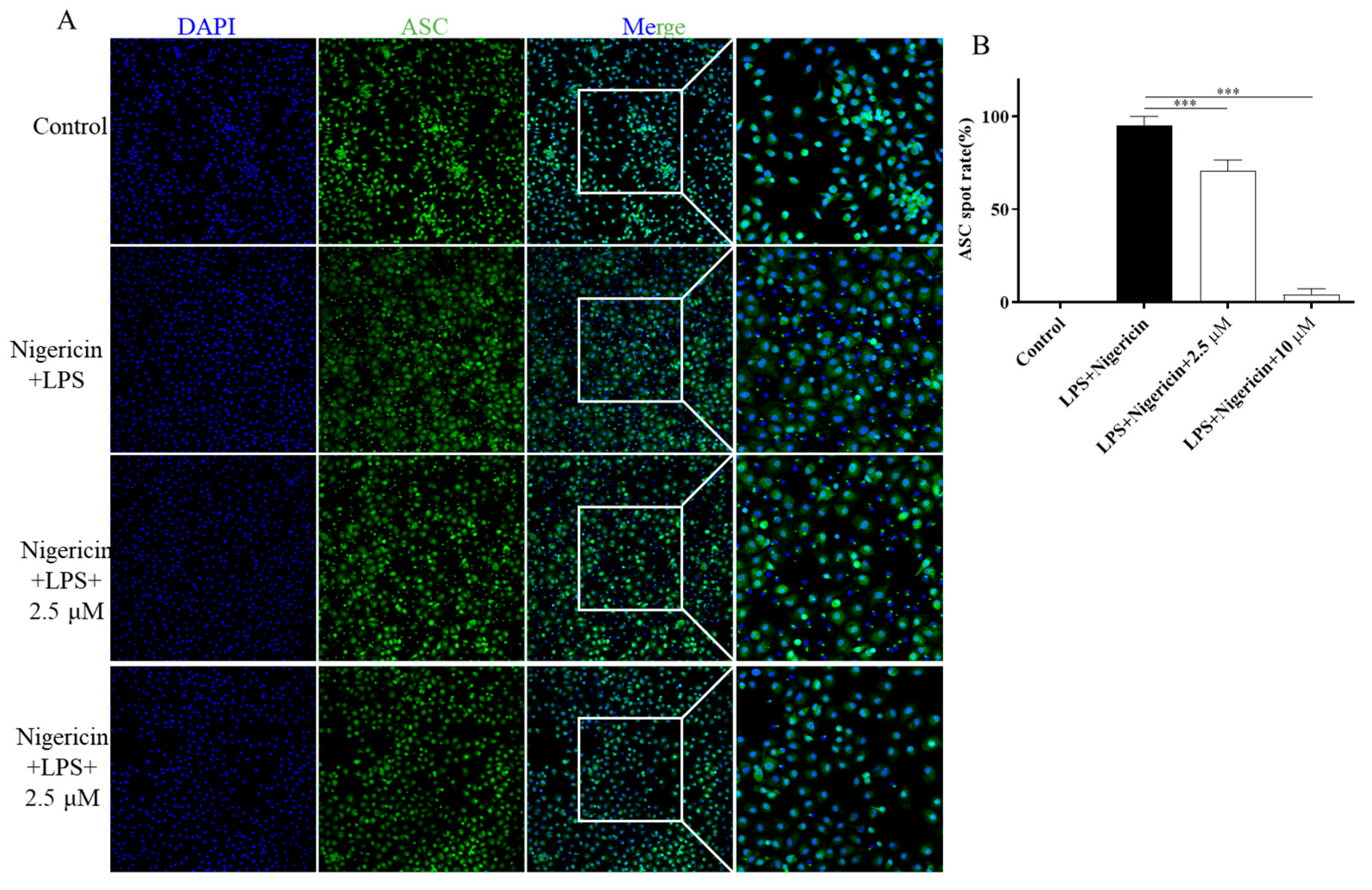
2.9. F14 Inhibits ASC Polymerization During the NLRP3 Inflammasome Activation Process
2.10. F14 Exerts Anti-Inflammatory Activity by Affecting NLRP3 Inflammasome Assembly
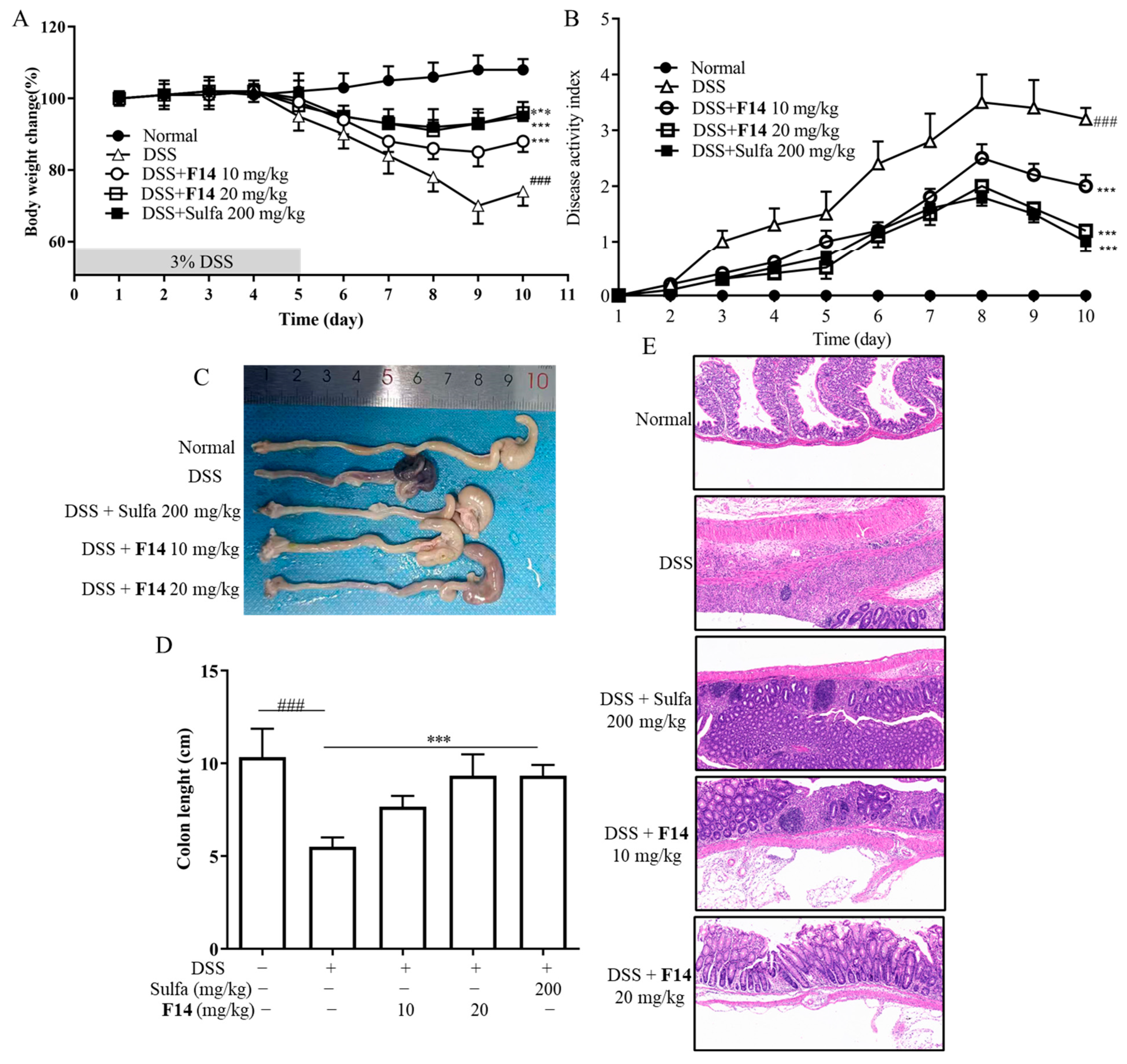
2.11. In Vivo Biological Activity of F14
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Chemical Synthesis
4.2.1. Procedure for the Synthesis of W1–W11
- (E)-3-(4-hydroxyphenyl)-1-phenylprop-2-en-1-one (W1) white solid; yield 74.1%; 1H NMR (400 MHz, DMSO-d6) δ 10.13 (s, 1H), 8.17–8.09 (m, 2H), 7.74 (dd, J = 14.2, 7.5 Hz, 4H), 7.70–7.61 (m, 1H), 7.57 (t, J = 7.6 Hz, 2H), 6.85 (d, J = 8.3 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 189.45, 160.64, 145.01, 138.41, 133.30, 131.53, 129.20, 128.81, 126.23, 118.92, 116.29. HR-MS(ESI): calcd for C15H12O2[M + H]+, 229.03304, found, 230.0837.
- (E)-3-(4-hydroxyphenyl)-1-(3-methoxyphenyl)propyl-2-en-1-one (W2) yellow solid; yield 72.5%; 1H NMR (400 MHz, DMSO-d6) δ 10.17 (s, 1H), 7.80 (d, J = 8.5 Hz, 3H), 7.78–7.74 (m, 2H), 7.64 (t, J = 2.0 Hz, 1H), 7.52 (t, J = 7.9 Hz, 1H), 7.27 (dd, J = 8.1, 2.6 Hz, 1H), 6.89 (d, J = 8.3 Hz, 2H), 3.90 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 189.16, 160.65, 159.98, 145.09, 139.89, 131.59, 130.32, 126.23, 121.32, 119.35, 118.96, 116.31, 116.28, 113.30, 55.81. HR-MS(ESI): calcd for C16H13O3[M + H]+, 253.08702, found, 254.0943.
- (E)-1-(3-chlorophenyl)-3-(4-hydroxyphenyl)propyl-2-en-1-one (W3) light yellow solid; yield 64.5%; 1H NMR (400 MHz, DMSO-d6) δ 10.18 (s, 1H), 8.17 (t, J = 1.9 Hz, 1H), 8.09 (dt, J = 7.8, 1.4 Hz, 1H), 7.82–7.77 (m, 2H), 7.74 (d, J = 4.7 Hz, 2H), 7.73–7.69 (m, 1H), 7.60 (t, J = 7.9 Hz, 1H), 7.00–6.80 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 188.11, 160.86, 145.86, 140.26, 134.25, 133.02, 131.81, 131.17, 128.48, 127.44, 126.15, 118.48, 116.29. HR-MS(ESI): calcd for C15H10O2Cl[M]+, 257.03757, found, 257.0369.
- (E)-3-(4-hydroxyphenyl)-1-(4-methoxyphenyl)propyl-2-en-1-one (W4) yellow solid; yield 69.3%; 1H NMR (400 MHz, DMSO-d6) δ 10.07 (s, 1H), 8.21–8.11 (m, 2H), 7.78–7.71 (m, 3H), 7.64 (d, J = 15.5 Hz, 1H), 7.15–7.02 (m, 2H), 6.90–6.71 (m, 2H), 3.87 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 187.68, 163.45, 160.41, 144.09, 131.36, 131.23, 131.18, 126.38, 118.86, 116.24, 114.41, 56.00. HR-MS(ESI): calcd for C16H13O3[M + H]+, 253.08708, found, 254.0943.
- (E)-1-(2-chlorophenyl)-3-(4-hydroxyphenyl)propion-2-en-1-one (W5) white solid; yield 55.4%; 1H NMR (400 MHz, DMSO-d6) δ 10.20 (s, 1H), 7.61 (d, J = 8.6 Hz, 2H), 7.59–7.53 (m, 2H), 7.52 (s, 1H), 7.50–7.45 (m, 1H), 7.29 (d, J = 16.0 Hz, 1H), 7.04 (d, J = 16.0 Hz, 1H), 6.81 (d, J = 8.6 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 193.65, 161.05, 147.50, 139.53, 131.96, 131.60, 130.46, 130.24, 129.54, 127.80, 125.52, 123.45, 116.42. HR-MS(ESI): calcd for C15H10O2Cl[M + H]+, 257.03757, found, 258.0448.
- (E)-1-(4-bromophenyl)-3-(4-hydroxyphenyl)propyl-2-en-1-one (W6) yellow solid; yield 61.3%; 1H NMR (400 MHz, DMSO-d6) δ 10.16 (d, J = 6.0 Hz, 1H), 8.07 (t, J = 7.1 Hz, 2H), 7.77 (d, J = 6.5 Hz, 3H), 7.75–7.67 (m, 3H), 6.85 (t, J = 6.8 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 188.50, 160.78, 145.55, 137.39, 132.23, 131.66, 130.87, 127.40, 126.16, 118.55, 116.31. HR-MS(ESI): calcd for C15H10O2Br[M + H]+, 300.98709, found, 301.9942.
- (E)-1-(4-chlorophenyl)-3-(4-hydroxyphenyl)propyl-2-en-1-one (W7) white solid; yield 67.5%; 1H NMR (400 MHz, DMSO-d6) δ 10.15 (s, 1H), 8.26–8.08 (m, 2H), 7.82–7.69 (m, 4H), 7.67–7.58 (m, 2H), 6.91–6.77 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 188.30, 160.78, 145.51, 138.23, 137.07, 131.64, 130.73, 129.27, 126.17, 118.57, 116.31. HR-MS(ESI): calcd for C15H10O2Cl[M]+, 257.03763, found, 257.0369.
- (E)-1-(2,4-dimethylphenyl)-3-(4-hydroxyphenyl)propyl-2-en-1-one (W8) yellow solid; yield 77.1%; 1H NMR (400 MHz, DMSO-d6) δ 10.12 (s, 1H), 7.77 (d, J = 8.6 Hz, 1H), 7.61 (d, J = 8.3 Hz, 2H), 7.51 (d, J = 8.2 Hz, 1H), 7.38 (d, J = 15.8 Hz, 1H), 7.17 (d, J = 15.9 Hz, 1H), 6.94 (d, J = 8.3 Hz, 1H), 6.82 (d, J = 8.3 Hz, 2H), 2.34 (s, 3H), 2.32 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 194.95, 191.40, 163.79, 160.58, 145.41, 140.73, 136.97, 136.81, 132.57, 132.34, 131.24, 128.95, 128.89, 126.65, 125.94, 123.41, 116.33, 116.30, 21.36, 20.51. HR-MS(ESI): calcd for C17H15O2[M + H]+, 251.10783, found, 252.1150.
- (E)-1-(3,4-dimethylphenyl)-3-(4-hydroxyphenyl)propyl-2-en-1-one (W9) white solid; yield 64.9%; 1H NMR (400 MHz, DMSO-d6) δ 10.46–10.19 (m, 1H), 8.08 (d, J = 15.7 Hz, 1H), 7.97–7.81 (m, 4H), 7.32 (dd, J = 20.9, 7.8 Hz, 2H), 7.03–6.87 (m, 2H), 2.35 (s, 3H), 2.33 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 189.43, 157.66, 142.57, 139.38, 137.28, 136.21, 132.37, 130.26, 129.80, 129.03, 126.55, 121.95, 121.40, 119.82, 116.68, 20.06, 19.82. HR-MS(ESI): calcd for C17H15O2[M + H]+, 251.10785, found, 252.1150.
- (E)-1-(3-fluorophenyl)-3-(4-hydroxyphenyl)propyl-2-en-1-one (W10) white solid; yield 78.5%; 1H NMR (400 MHz, DMSO-d6) δ 10.19 (s, 1H), 7.99 (dt, J = 7.7, 1.2 Hz, 1H), 7.96–7.90 (m, 1H), 7.82–7.75 (m, 2H), 7.74 (d, J = 1.7 Hz, 2H), 7.61 (td, J = 8.0, 5.8 Hz, 1H), 7.50 (td, J = 8.5, 2.4 Hz, 1H), 6.90–6.83 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 188.15, 188.13, 164.02, 161.59, 160.85, 145.76, 140.72, 140.65, 131.75, 131.37, 131.29, 126.14, 124.98, 124.95, 120.28, 120.06, 118.51, 116.31, 115.46, 115.24. HR-MS(ESI): calcd for C15H12O2F[M + H]+, 241.06705, found, 242.0742.
- (E)-3-(4-hydroxyphenyl)-1-(naphthalene-1-yl)propyl-2-en-1-one (W11) light yellow solid; yield 79.2%; 1H NMR (400 MHz, DMSO-d6) δ 10.18 (s, 1H), 8.30–8.21 (m, 1H), 8.12 (d, J = 8.2 Hz, 1H), 8.06–7.99 (m, 1H), 7.89 (dd, J = 7.1, 1.2 Hz, 1H), 7.64 (dt, J = 8.4, 2.5 Hz, 3H), 7.59 (ddd, J = 7.8, 3.5, 1.9 Hz, 2H), 7.52 (d, J = 15.8 Hz, 1H), 7.34 (d, J = 15.9 Hz, 1H), 6.89–6.79 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 194.95, 160.80, 146.29, 137.33, 133.86, 131.72, 131.48, 130.35, 128.96, 127.80, 127.64, 126.88, 125.85, 125.73, 125.42, 123.91, 116.38. HR-MS(ESI): calcd for C19H13O2[M + H]+, 273.09216, found, 274.0994.
4.2.2. Procedure for the Synthesis of F1–F16
- (E)-3-(4-hydroxyphenyl)-1-phenylpropyl-2-en-1-one (F1) white solid; yield 46.8%; 1H NMR (400 MHz, DMSO-d6) δ 10.30 (s, 1H), 8.20–7.97 (m, 3H), 7.97–7.76 (m, 2H), 7.62 (dt, J = 37.2, 7.4 Hz, 3H), 7.38–7.11 (m, 1H), 7.05–6.77 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 189.95, 157.72, 139.99, 138.37, 133.39, 132.57, 129.26, 129.17, 128.82, 121.80, 121.35, 119.89, 116.69. HR-MS(ESI): calcd for C15H11O2[M + H]+, 223.07651, found,223.07651.
- (E)-3-(2-hydroxyphenyl)-1-(3-methoxyphenyl)propyl-2-en-1-one (F2) yellow solid; yield 70.2%; 1H NMR (400 MHz, DMSO-d6) δ 9.69 (s, 1H), 7.85 (d, J = 15.6 Hz, 1H), 7.78 (d, J = 7.6 Hz, 1H), 7.71–7.59 (m, 2H), 7.50 (t, J = 8.0 Hz, 1H), 7.35 (d, J = 7.7 Hz, 1H), 7.31–7.22 (m, 3H), 6.91 (dd, J = 7.8, 2.4 Hz, 1H), 3.87 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 189.42, 160.01, 158.20, 144.85, 139.51, 136.39, 130.40, 130.35, 122.39, 121.51, 120.43, 119.67, 118.33, 115.80, 113.39, 55.82. HR-MS(ESI): calcd for C16H13O3[M + H]+, 253.08705, found, 254.0943.
- (E)-3-(3-hydroxyphenyl)-1-(3-methoxyphenyl)propyl-2-en-1-one (F3) white solid; yield 68.5%; 1H NMR (400 MHz, DMSO-d6) δ 10.36 (s, 1H), 8.11 (d, J = 15.8 Hz, 1H), 7.97–7.85 (m, 2H), 7.76 (d, J = 7.6 Hz, 1H), 7.62 (t, J = 2.1 Hz, 1H), 7.54 (t, J = 7.9 Hz, 1H), 7.35–7.27 (m, 2H), 6.99 (d, J = 8.2 Hz, 1H), 6.93 (t, J = 7.5 Hz, 1H), 3.90 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 189.67, 159.99, 157.72, 140.04, 139.83, 132.59, 130.40, 129.17, 121.80, 121.36, 121.33, 119.88, 119.44, 116.67, 113.29, 55.81. HR-MS(ESI): calcd for C16H13O3[M + H]+, 253.08711, found, 254.0943.
- (E)-3-(3-hydroxyphenyl)-1-phenylpropyl-2-en-1-one (F4) white solid; yield 68.3%;1H NMR (400 MHz, DMSO-d6) δ 9.67 (s, 1H), 8.30–8.03 (m, 2H), 7.85 (d, J = 15.6 Hz, 1H), 7.78–7.54 (m, 4H), 7.41–7.12 (m, 3H), 6.89 (dd, J = 8.0, 2.4 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 189.68, 144.77, 138.04, 136.39, 133.61, 130.37, 129.27, 128.98, 122.35, 120.36, 118.31, 115.76. HR-MS(ESI): calcd for C15H11O2[M + H]+, 223.07675, found, 224.0837.
- (E)-3-(2-hydroxyphenyl)-1-(4-methoxyphenyl)propyl-2-en-1-one (F5) white solid; yield 77.4%; 1H NMR (400 MHz, DMSO-d6) δ 10.25 (s, 1H), 8.25–7.95 (m, 3H), 7.97–7.79 (m, 2H), 7.41–7.05 (m, 3H), 7.00–6.82 (m, 2H), 3.87 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 188.10, 163.51, 157.58, 139.06, 132.30, 131.19, 129.04, 121.97, 121.24, 119.85, 116.65, 114.47, 56.01. HR-MS(ESI): calcd for C16H13O3[M + H]+, 253.08720, found, 254.0943.
- (E)-3-(3-hydroxyphenyl)-1-(4-methoxyphenyl)propyl-2-en-1-one (F6) yellow solid; yield 55.3%; 1H NMR (400 MHz, DMSO-d6) δ 9.64 (s, 1H), 8.27–8.08 (m, 2H), 7.85 (d, J = 15.6 Hz, 1H), 7.62 (d, J = 15.5 Hz, 1H), 7.37–7.21 (m, 3H), 7.15–6.81 (m, 3H), 3.87 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 187.84, 163.68, 158.19, 143.89, 136.55, 131.40, 130.90, 130.33, 122.28, 120.24, 118.08, 115.68, 114.49, 56.04. HR-MS(ESI): calcd for C16H13O3S[M + H]+, 253.08720, found, 254.0943.
- (E)-1-(2-chlorophenyl)-3-(2-hydroxyphenyl)propyl-2-en-1-one (F7) white solid; yield 43.5%; 1H NMR (400 MHz, DMSO-d6) δ 10.32 (s, 1H), 7.74–7.63 (m, 2H), 7.62–7.52 (m, 4H), 7.51–7.46 (m, 1H), 7.28 (td, J = 7.8, 7.3, 1.7 Hz, 1H), 7.23 (d, J = 16.3 Hz, 1H), 6.95–6.82 (m, 2H).13C NMR (101 MHz, DMSO-d6) δ 194.21, 157.67, 142.63, 139.45, 133.09, 132.06, 130.47, 130.25, 129.58, 129.36, 127.82, 125.95, 121.13, 120.03, 116.73. HR-MS(ESI): calcd for C15H10O2Cl[M + H]+, 257.03763, found, 258.0448.
- (E)-1-(4-chlorophenyl)-3-(2-hydroxyphenyl)propyl-2-en-1-one (F8) yellow solid; yield 74.1%; 1H NMR (400 MHz, DMSO-d6) δ 10.34 (s, 1H), 8.24–8.00 (m, 3H), 7.92–7.83 (m, 2H), 7.68–7.61 (m, 2H), 7.29 (td, J = 7.8, 7.3, 1.7 Hz, 1H), 6.97–6.85 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 188.78, 157.83, 140.42, 137.01, 132.74, 130.76, 129.36, 129.20, 121.72, 120.92, 119.87, 116.71. HR-MS(ESI): calcd for C15H10O2Cl[M + H]+, 257.03769, found, 258.0448.
- (E)-1-(4-bromophenyl)-3-(2-hydroxyphenyl)propyl-2-en-1-one (F9) white solid; yield 48.9%;1H NMR (400 MHz, DMSO-d6) δ 10.34 (s, 1H), 8.27–8.02 (m, 3H), 8.02–7.71 (m, 4H), 7.30 (ddd, J = 8.6, 7.2, 1.7 Hz, 1H), 7.10–6.78 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 188.98, 157.82, 140.45, 137.33, 132.74, 132.30, 130.87, 129.21, 127.50, 121.72, 120.91, 119.88, 116.71. HR-MS(ESI): calcd for C15H10O2Br[M + H]+, 300.98712, found, 301.9942.
- (E)-1-(4-bromophenyl)-3-(3-hydroxyphenyl)propyl-2-en-1-one (F10) white solid; yield 71.2%; 1H NMR (400 MHz, DMSO-d6) δ 9.66 (s, 1H), 8.09 (d, J = 8.3 Hz, 2H), 7.91–7.74 (m, 3H), 7.67 (d, J = 15.6 Hz, 1H), 7.39–7.21 (m, 3H), 6.89 (dd, J = 7.9, 2.4 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 188.79, 158.21, 145.27, 137.02, 136.31, 132.31, 131.02, 130.36, 127.76, 122.01, 120.47, 118.45, 115.83. HR-MS(ESI): calcd for C15H10O2Br[M + H]+, 300.98727, found, 301.9942.
- (E)-1-(2,4-dimethylphenyl)-3-(2-hydroxyphenyl)propyl-2-en-1-one (F11) white solid; yield 58.4%; 1H NMR (400 MHz, DMSO-d6) δ 10.26 (s, 1H), 7.82–7.69 (m, 2H), 7.50 (d, J = 8.2 Hz, 1H), 7.39–7.21 (m, 2H), 7.12 (d, J = 5.5 Hz, 2H), 6.94 (d, J = 8.2 Hz, 1H), 6.85 (t, J = 7.5 Hz, 1H), 2.35 (s, 3H), 2.32 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 195.48, 157.51, 140.82, 140.64, 137.01, 136.74, 132.45, 132.35, 129.10, 128.96, 126.65, 125.96, 121.62, 119.93, 116.67, 21.35, 20.52. HR-MS(ESI): calcd for C17H15O2[M + H]+, 251.10788, found, 252.1150.
- (E)-1-(3,4-dimethylphenyl)-3-(3-hydroxyphenyl)propyl-2-en-1-one (F12) white solid; yield 64.5%; 1H NMR (400 MHz, DMSO-d6) δ 9.66 (s, 1H), 7.95 (d, J = 1.9 Hz, 1H), 7.92–7.78 (m, 2H), 7.63 (d, J = 15.6 Hz, 1H), 7.39–7.30 (m, 2H), 7.29–7.20 (m, 2H), 6.93–6.78 (m, 1H), 2.32 (s, 3H), 2.30 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 189.13, 158.20, 144.19, 142.87, 137.36, 136.50, 135.88, 130.33, 130.30, 129.95, 126.71, 122.38, 120.32, 118.17, 115.67, 20.09, 19.79. HR-MS(ESI): calcd for C17H15O2[M + H]+, 251.10782, found, 252.1150.
- (E)-1-(3-fluorophenyl)-3-(3-hydroxyphenyl)propyl-2-en-1-one (F13) yellow solid; yield 73.1%; 1H NMR (400 MHz, DMSO-d6) δ 10.34 (s, 1H), 8.11 (d, J = 15.8 Hz, 1H), 7.97 (d, J = 7.7 Hz, 1H), 7.95–7.82 (m, 3H), 7.63 (td, J = 7.9, 5.7 Hz, 1H), 7.52 (td, J = 8.5, 2.6 Hz, 1H), 7.35–7.26 (m, 1H), 7.00–6.85 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 188.65, 188.62, 164.02, 161.58, 157.83, 140.55, 132.82, 131.46, 131.38, 129.11, 125.02, 124.99, 121.69, 120.82, 119.87, 116.70, 115.45, 115.23. HR-MS(ESI): calcd for C15H10O2F[M + H]+, 241.06702, found, 242.0743.
- (E)-1-(2,4-dimethylphenyl)-3-(3-hydroxyphenyl)propyl-2-en-1-one (F14) white solid; yield 61.9%; 1H NMR (400 MHz, DMSO-d6) δ 9.66 (s, 1H), 7.59 (d, J = 8.3 Hz, 1H), 7.35 (q, J = 15.9 Hz, 2H), 7.22 (dt, J = 14.9, 7.8 Hz, 2H), 7.17–7.10 (m, 3H), 6.87 (dt, J = 8.0, 1.8 Hz, 1H), 2.37 (s, 3H), 2.33 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 194.58, 158.21, 144.89, 141.28, 137.44, 136.23, 136.19, 132.52, 130.42, 129.38, 126.75, 126.40, 120.18, 118.27, 115.36. HR-MS(ESI): calcd for C17H15O2[M + H]+, 251.10789, found, 252.1150.
- (E)-3-(2-hydroxyphenyl)-1-(naphthale-1-yl)propyl-2-en-1-one (F15) white solid; yield 46.5%; 1H NMR (400 MHz, DMSO-d6) δ 10.31 (s, 1H), 8.30–8.22 (m, 1H), 8.13 (d, J = 8.2 Hz, 1H), 8.08–8.00 (m, 1H), 7.92–7.83 (m, 2H), 7.76 (dd, J = 7.9, 1.7 Hz, 1H), 7.61 (qd, J = 7.5, 6.5, 2.9 Hz, 3H), 7.50 (s, 1H), 7.28 (ddd, J = 8.4, 7.3, 1.6 Hz, 1H), 6.94 (d, J = 8.2 Hz, 1H), 6.87 (t, J = 7.3 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 195.45, 157.65, 141.43, 137.28, 133.85, 132.75, 131.81, 130.34, 129.30, 128.99, 127.86, 127.68, 126.93, 126.44, 125.72, 125.40, 121.49, 119.98, 116.71. HR-MS(ESI): calcd for C19H13O2[M + H]+, 273.09229, found, 274.0994.
- (E)-3-(2-hydroxyphenyl)-1-(thiophene-2-yl)propion-2-en-1-one (F16) yellow solid; yield 61.1%; 1H NMR (400 MHz, DMSO-d6) δ 10.25 (s, 1H), 8.18 (d, J = 3.8 Hz, 1H), 8.04–7.95 (m, 2H), 7.82 (dd, J = 7.8, 1.7 Hz, 1H), 7.74 (d, J = 15.7 Hz, 1H), 7.28–7.18 (m, 2H), 6.88 (d, J = 8.2 Hz, 1H), 6.82 (t, J = 7.5 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 182.25, 157.72, 146.18, 138.95, 135.65, 133.59, 132.63, 129.37, 128.99, 121.63, 121.02, 119.86, 116.71. HR-MS(ESI): calcd for C13H9O2S[M + H]+, 229.03304, found, 230.0402.
4.3. Cell Culture
4.4. Isolation, Extraction, and Cultivation of BMDMs
4.5. MTT Assay
4.6. The Effect of ELISA Detection Compounds on IL-1β Secretion
4.7. Western Blot Operation Is Detailed in the Reference Literature [39]
4.8. ASC Spot Experiment
4.9. ROS Detection
4.10. LDH Detection
4.11. Docking
4.12. Molecular Dynamics Simulations
4.13. In Vivo Pharmacological Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Faye, A.S.; Colombel, J.F. Aging and IBD: A New Challenge for Clinicians and Researchers. Inflamm. Bowel Dis. 2022, 28, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bhardwaj, A.; Sharma, R.; Midha, V.; Sood, A. Developing IBD counsellors in low- and middle-income countries: Bridging gaps in patient care. EClinicalMedicine 2025, 83, 103218. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology 2019, 157, 647–659.e4. [Google Scholar] [CrossRef]
- Bai, R.; Jie, X.; Yao, C.; Xie, Y. Discovery of small-molecule candidates against inflammatory bowel disease. Eur. J. Med. Chem. 2020, 185, 111805. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.F.; Pires, S.; Rupert, A.; Oguntunmibi, S.; Jin, W.B.; Marderstein, A.; Funez-dePagnier, G.; Maldarelli, G.; Viladomiu, M.; Putzel, G.; et al. The gut microbiome regulates the clinical efficacy of sulfasalazine therapy for IBD-associated spondyloarthritis. Cell Rep. Med. 2024, 5, 101431. [Google Scholar] [CrossRef]
- Lucafo, M.; Bramuzzo, M.; Selvestrel, D.; Da Lozzo, P.; Decorti, G.; Stocco, G. Gender May Influence the Immunosuppressive Actions of Prednisone in Young Patients With Inflammatory Bowel Disease. Front. Immunol. 2021, 12, 673068. [Google Scholar] [CrossRef]
- Cheng, W.; Li, J.; Liu, X. 5-Aminosalicylic acid for treatment of irritable bowel syndrome: A protocol for a systematic review and meta-analysis. Medicine 2020, 99, 19351–19355. [Google Scholar] [CrossRef] [PubMed]
- Greenhill, C. IBD: Glucocorticoids revealed to augment intestinal epithelial barrier function. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 75. [Google Scholar] [CrossRef]
- Lee, J.W.J.; Plichta, D.; Hogstrom, L.; Borren, N.Z.; Lau, H.; Gregory, S.M.; Tan, W.; Khalili, H.; Clish, C.; Vlamakis, H.; et al. Multi-omics reveal microbial determinants impacting responses to biologic therapies in inflammatory bowel disease. Cell Host Microbe 2021, 29, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Niknahad, H.; Heidari, R.; Mohammadzadeh, R.; Ommati, M.M.; Khodaei, F.; Azarpira, N.; Abdoli, N.; Zarei, M.; Asadi, B.; Rasti, M.; et al. Sulfasalazine induces mitochondrial dysfunction and renal injury. Ren. Fail. 2017, 39, 745–753. [Google Scholar] [CrossRef]
- Abhishek, A.; Grainge, M.; Card, T.; Williams, H.C.; Taal, M.W.; Aithal, G.P.; Fox, C.P.; Mallen, C.D.; Stevenson, M.D.; Nakafero, G.; et al. Risk-stratified monitoring for sulfasalazine toxicity: Prognostic model development and validation. RMD Open 2024, 10, 3980–3990. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, A.; Lv, J.; Zhang, Q.; Ran, Y.; Wei, C.; Wu, J. Development of small molecule inhibitors targeting NLRP3 inflammasome pathway for inflammatory diseases. Eur. J. Med. Chem. 2020, 185, 111822. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef]
- Chen, Q.L.; Yin, H.R.; He, Q.Y.; Wang, Y. Targeting the NLRP3 inflammasome as new therapeutic avenue for inflammatory bowel disease. Biomed. Pharmacother. 2021, 138, 111442. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Nunez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Ducker, G.S.; Billingham, L.K.; Martinez, C.A.; Mainolfi, N.; Suri, V.; Friedman, A.; Manfredi, M.G.; Weinberg, S.E.; Rabinowitz, J.D.; et al. Serine Metabolism Supports Macrophage IL-1beta Production. Cell Metab. 2019, 29, 1003–1011.E4. [Google Scholar] [CrossRef]
- Zhu, Q.; Kanneganti, T.D. Cutting Edge: Distinct Regulatory Mechanisms Control Proinflammatory Cytokines IL-18 and IL-1beta. J. Immunol. 2017, 198, 4210–4215. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Apte, R.N. IL-1 in Colon Inflammation, Colon Carcinogenesis and Invasiveness of Colon Cancer. Cancer Microenviron. 2015, 8, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Liu, Z.; Pei, S.; Wu, X.; Zhao, N.; Jiang, L.; Zhang, Z.; Bai, X. Mendelian randomization study for the roles of IL-18 and IL-1 receptor antagonist in the development of inflammatory bowel disease. Int. Immunopharmacol. 2022, 110, 109020. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, Y.; Yao, J.; Li, D.F.; Wang, L.S. Role of Pyroptosis in Inflammatory Bowel Disease (IBD): From Gasdermins to DAMPs. Front. Pharmacol. 2022, 13, 833588. [Google Scholar] [CrossRef]
- Rana, N.; Privitera, G.; Kondolf, H.C.; Bulek, K.; Lechuga, S.; De Salvo, C.; Corridoni, D.; Antanaviciute, A.; Maywald, R.L.; Hurtado, A.M.; et al. GSDMB is increased in IBD and regulates epithelial restitution/repair independent of pyroptosis. Cell 2024, 187, 1011–1015. [Google Scholar] [CrossRef]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef]
- Li, N.; Zhang, R.; Tang, M.; Zhao, M.; Jiang, X.; Cai, X.; Ye, N.; Su, K.; Peng, J.; Zhang, X.; et al. Recent Progress and Prospects of Small Molecules for NLRP3 Inflammasome Inhibition. J. Med. Chem. 2023, 66, 14447–14473. [Google Scholar] [CrossRef]
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Munoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A.; et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. [Google Scholar] [CrossRef]
- Shah, F.; Leung, L.; Barton, H.A.; Will, Y.; Rodrigues, A.D.; Greene, N.; Aleo, M.D. Setting Clinical Exposure Levels of Concern for Drug-Induced Liver Injury (DILI) Using Mechanistic in vitro Assays. Toxicol. Sci. 2015, 147, 500–514. [Google Scholar] [CrossRef]
- Fulp, J.; He, L.; Toldo, S.; Jiang, Y.; Boice, A.; Guo, C.; Li, X.; Rolfe, A.; Sun, D.; Abbate, A.; et al. Structural Insights of Benzenesulfonamide Analogues as NLRP3 Inflammasome Inhibitors: Design, Synthesis, and Biological Characterization. J. Med. Chem. 2018, 61, 5412–5423. [Google Scholar] [CrossRef]
- Kuwar, R.; Rolfe, A.; Di, L.; Xu, H.; He, L.; Jiang, Y.; Zhang, S.; Sun, D. A novel small molecular NLRP3 inflammasome inhibitor alleviates neuroinflammatory response following traumatic brain injury. J. Neuroinflammation 2019, 16, 81. [Google Scholar] [CrossRef]
- Xiao, Z.; Yu, S.; Li, Y.; Ruan, S.; Kong, L.B.; Huang, Q.; Huang, Z.; Zhou, K.; Su, H.; Yao, Z.; et al. Materials development and potential applications of transparent ceramics: A review. Mater. Sci. Eng. R Rep. 2020, 139, 100518. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Q.; Zhu, Q.; Tan, R.; Bai, D.; Bu, X.; Lin, B.; Zhao, K.; Pan, C.; Chen, H.; et al. Flavonoid VI-16 protects against DSS-induced colitis by inhibiting Txnip-dependent NLRP3 inflammasome activation in macrophages via reducing oxidative stress. Mucosal Immunol. 2019, 12, 1150–1163. [Google Scholar] [CrossRef]
- Lyu, H.; Ni, H.; Huang, J.; Yu, G.; Zhang, Z.; Zhang, Q. VX-765 prevents intestinal ischemia-reperfusion injury by inhibiting NLRP3 inflammasome. Tissue Cell 2022, 75, 101718. [Google Scholar] [CrossRef]
- Shao, B.Z.; Xu, H.Y.; Zhao, Y.C.; Zheng, X.R.; Wang, F.; Zhao, G.R. NLRP3 Inflammasome in Atherosclerosis: Putting Out the Fire of Inflammation. Inflammation 2023, 46, 35–46. [Google Scholar] [CrossRef]
- He, H.; Jiang, H.; Chen, Y.; Ye, J.; Wang, A.; Wang, C.; Liu, Q.; Liang, G.; Deng, X.; Jiang, W.; et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 2018, 9, 2550. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, H.; Chen, Y.; Wang, X.; Yang, Y.; Tao, J.; Deng, X.; Liang, G.; Zhang, H.; Jiang, W.; et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 2018, 10, 8689–8704. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Kong, L.; Yuan, T.; Li, W.; Zhang, W.; Hou, B.; Lu, Y.; Du, G. The strategies and techniques of drug discovery from natural products. Pharmacol. Ther. 2020, 216, 107686. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Silva, A.M.S.; Ribeiro, D.; Silva, V.L.M.; Fernandes, E. Bis-chalcones: A review of synthetic methodologies and anti-inflammatory effects. Eur. J. Med. Chem. 2023, 252, 115280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yue, H.; Sun, P.; Hua, L.; Liang, S.; Ou, Y.; Wu, D.; Wu, X.; Chen, H.; Hao, Y.; et al. Discovery of chalcone analogues as novel NLRP3 inflammasome inhibitors with potent anti-inflammation activities. Eur. J. Med. Chem. 2021, 219, 113417. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Z.; Zhang, X.X.; Liu, M.M.; Wu, J.; Ma, D.; Diao, L.Z.; Li, Q.; Huang, Y.S.; Zhang, R.; Ruan, B.F.; et al. Discovery of Novel Pterostilbene-Based Derivatives as Potent and Orally Active NLRP3 Inflammasome Inhibitors with Inflammatory Activity for Colitis. J. Med. Chem. 2021, 64, 13633–13657. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.L.; Qiao, L.J.; Fang, H.; Fan, L.L.; Wu, D.; Xu, J.P.; Lv, Q.H.; Deng, M.Z.; Qiu, Y.H.; Zhou, Z.Y.; et al. Hydroxysafflor Yellow A Ameliorates Ischemic Stroke by Inhibiting NLRP3, AIM2, and Pyrin Inflammasome-Mediated Pyroptosis. Neurochem. Res. 2025, 50, 201. [Google Scholar] [CrossRef]
- Thapa, P.; Upadhyay, S.P.; Singh, V.; Boinpelly, V.C.; Zhou, J.; Johnson, D.K.; Gurung, P.; Lee, E.S.; Sharma, R.; Sharma, M. Chalcone: A potential scaffold for NLRP3 inflammasome inhibitors. Eur. J. Med. Chem. Rep. 2023, 7, 100100–100113. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Feng, H.; Wang, H.; Wang, Y.; Mou, W.; Xu, G.; Zhang, P.; Li, R.; Shi, W.; Wang, Z.; et al. Licochalcone B specifically inhibits the NLRP3 inflammasome by disrupting NEK7-NLRP3 interaction. EMBO Rep. 2022, 23, 53499–53517. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Schroder, K.; Pelegrin, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef] [PubMed]

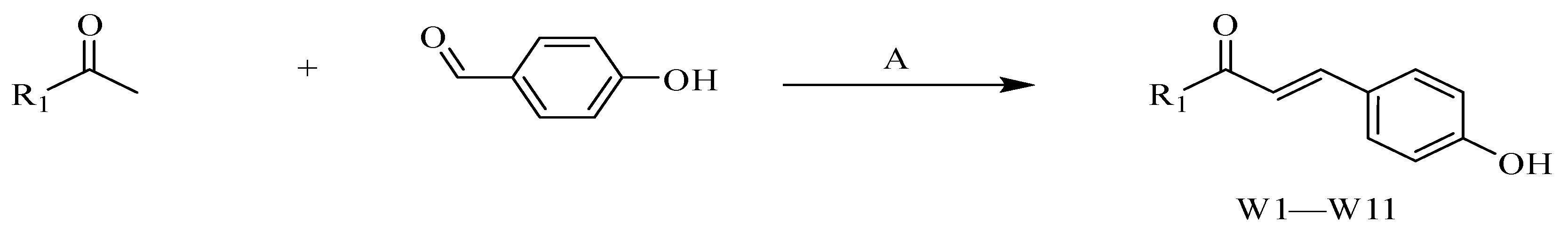
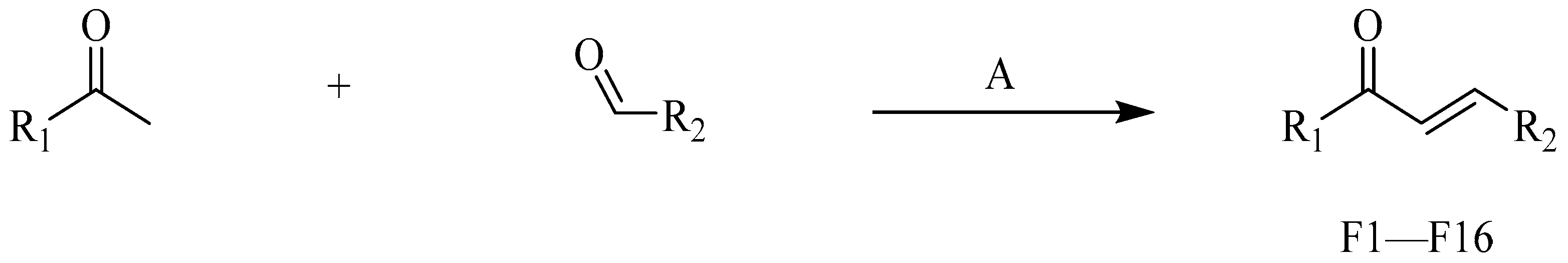

 | ||||
|---|---|---|---|---|
| Compounds | R1 | Compounds | R1 | |
| W1 |  | W7 |  | |
| W2 |  | W8 | 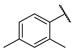 | |
| W3 |  | W9 | 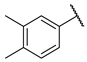 | |
| W4 | 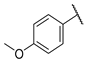 | W10 | 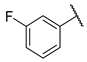 | |
| W5 | 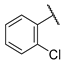 | W11 |  | |
| W6 |  | |||
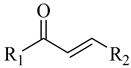 | |||||
|---|---|---|---|---|---|
| Compounds | R1 | R2 | Compounds | R1 | R2 |
| F1 |  |  | F9 | 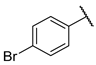 | 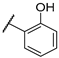 |
| F2 |  | 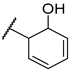 | F10 | 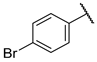 | 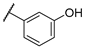 |
| F3 | 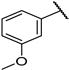 | 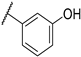 | F11 | 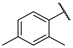 | 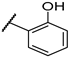 |
| F4 | 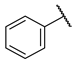 | 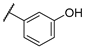 | F12 |  | 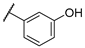 |
| F5 | 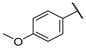 | 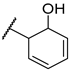 | F13 | 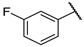 |  |
| F6 |  | 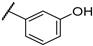 | F14 | 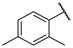 |  |
| F7 |  | 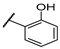 | F15 | 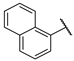 |  |
| F8 |  | 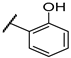 | F16 | 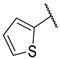 |  |
| Compound | IL-1β Inhibition Rate (%) a | HepaRG Cytotoxicity a |
|---|---|---|
| 20 µM | IC50 (µM) | |
| W1 | 17.77 ± 3.45 | >50 |
| W2 | 67.03 ± 1.23 | >50 |
| W3 | 21.84 ± 9.56 | >50 |
| W4 | 6.89 ± 4.78 | >50 |
| W5 | 51.74 ± 2.23 | >50 |
| W6 | 27.65 ± 8.12 | >50 |
| W7 | 24.31 ± 3.67 | >50 |
| W8 | 88.86 ± 5.91 | >50 |
| W9 | 10.33 ± 6.43 | >50 |
| W10 | 33.65 ± 3.21 | >50 |
| W11 | 88.31 ± 7.01 | >50 |
| MCC950 | 99.47 ± 0.12 | >50 |
| Compound | IL-1β Inhibition Rate (%) a | HepaRG Cytotoxicity a |
|---|---|---|
| 20 µM | IC50 (µM) | |
| F1 | 52.07 ± 3.72 | >50 |
| F2 | 92.01 ± 2.15 | >50 |
| F3 | 93.42 ± 2.98 | >50 |
| F4 | 25.58 ± 9.03 | >50 |
| F5 | 93.92 ± 4.61 | >50 |
| F6 | 25.08 ± 2.84 | >50 |
| F7 | 94.75 ± 3.29 | >50 |
| F8 | 45.93 ± 3.45 | >50 |
| F9 | 28.41 ± 5.76 | >50 |
| F10 | 24.16 ± 7.91 | >50 |
| F11 | 88.71 ± 9.22 | >50 |
| F12 | 15.45 ± 4.13 | >50 |
| F13 | 34.01 ± 8.74 | >50 |
| F14 | 95.44 ± 2.56 | >50 |
| F15 | 91.68 ± 5.89 | >50 |
| F16 | 92.64 ± 3.23 | >50 |
| MCC950 | 99.47 ± 0.12 | >50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zheng, X.; Li, J.; Zhou, B.; Tao, M.; Yang, Y.; Wang, Y.; Zhan, H.; Zhang, G.; Shi, J.; et al. Discovery of (E)-1,3-Diphenyl-2-Propen-1-One Derivatives as Potent and Orally Active NLRP3 Inflammasome Inhibitors for Colitis. Molecules 2025, 30, 3340. https://doi.org/10.3390/molecules30163340
Chen L, Zheng X, Li J, Zhou B, Tao M, Yang Y, Wang Y, Zhan H, Zhang G, Shi J, et al. Discovery of (E)-1,3-Diphenyl-2-Propen-1-One Derivatives as Potent and Orally Active NLRP3 Inflammasome Inhibitors for Colitis. Molecules. 2025; 30(16):3340. https://doi.org/10.3390/molecules30163340
Chicago/Turabian StyleChen, Liuzeng, Xiaoyu Zheng, Jiahui Li, Bin Zhou, Min Tao, Yuetian Yang, Yi Wang, Hao Zhan, Guoping Zhang, Jingbo Shi, and et al. 2025. "Discovery of (E)-1,3-Diphenyl-2-Propen-1-One Derivatives as Potent and Orally Active NLRP3 Inflammasome Inhibitors for Colitis" Molecules 30, no. 16: 3340. https://doi.org/10.3390/molecules30163340
APA StyleChen, L., Zheng, X., Li, J., Zhou, B., Tao, M., Yang, Y., Wang, Y., Zhan, H., Zhang, G., Shi, J., Zhang, X., & Ruan, B. (2025). Discovery of (E)-1,3-Diphenyl-2-Propen-1-One Derivatives as Potent and Orally Active NLRP3 Inflammasome Inhibitors for Colitis. Molecules, 30(16), 3340. https://doi.org/10.3390/molecules30163340






