Influence of Olive Oil Components on Ion Channels
Abstract
1. Olive Oil, Its Components, and Their Role in Human Health
1.1. Constituents of Olive Oil
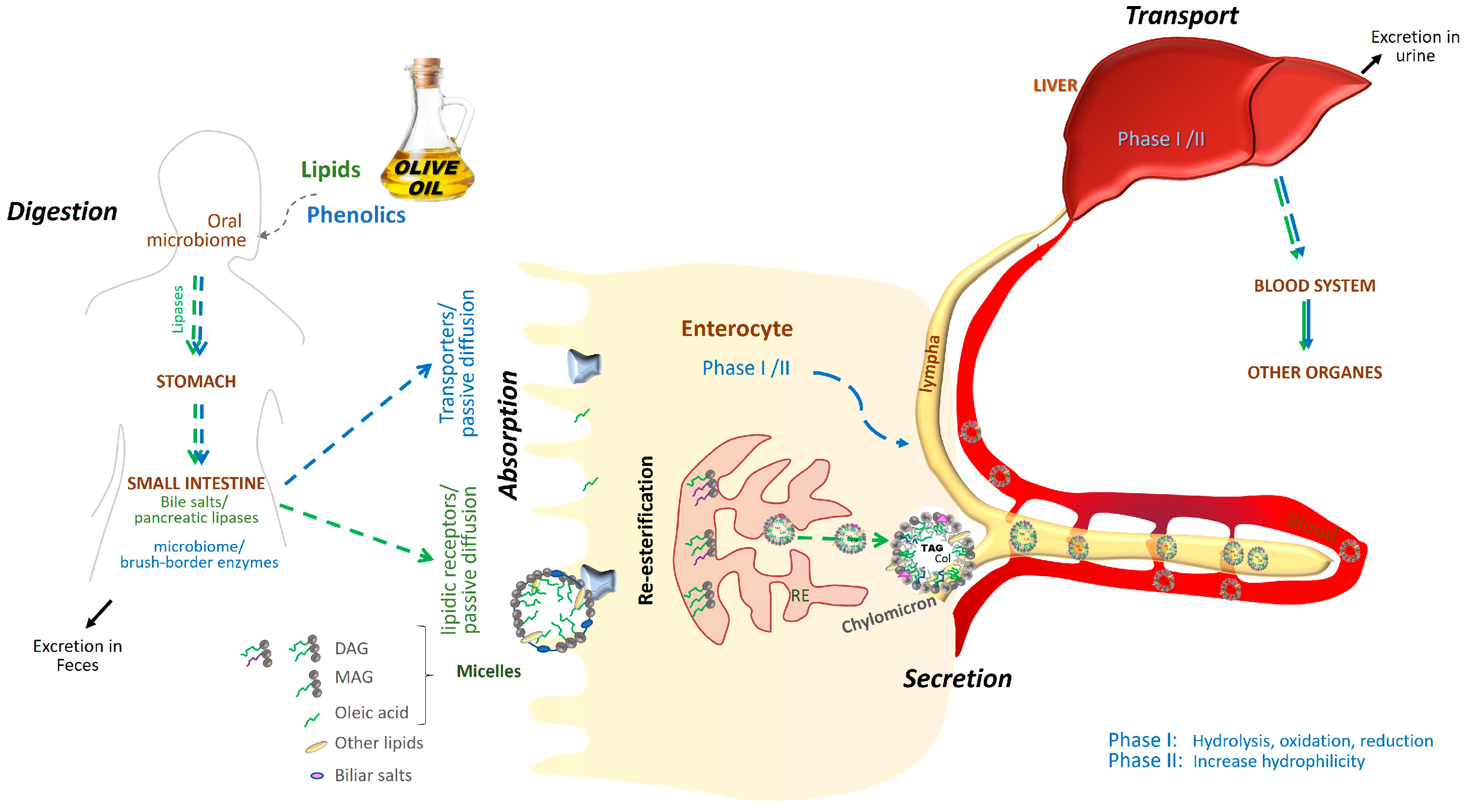
1.2. Absorption, Metabolism and Excretion of Olive Oil Components
2. Influence of Olive Oil Derivatives on Ion Channels
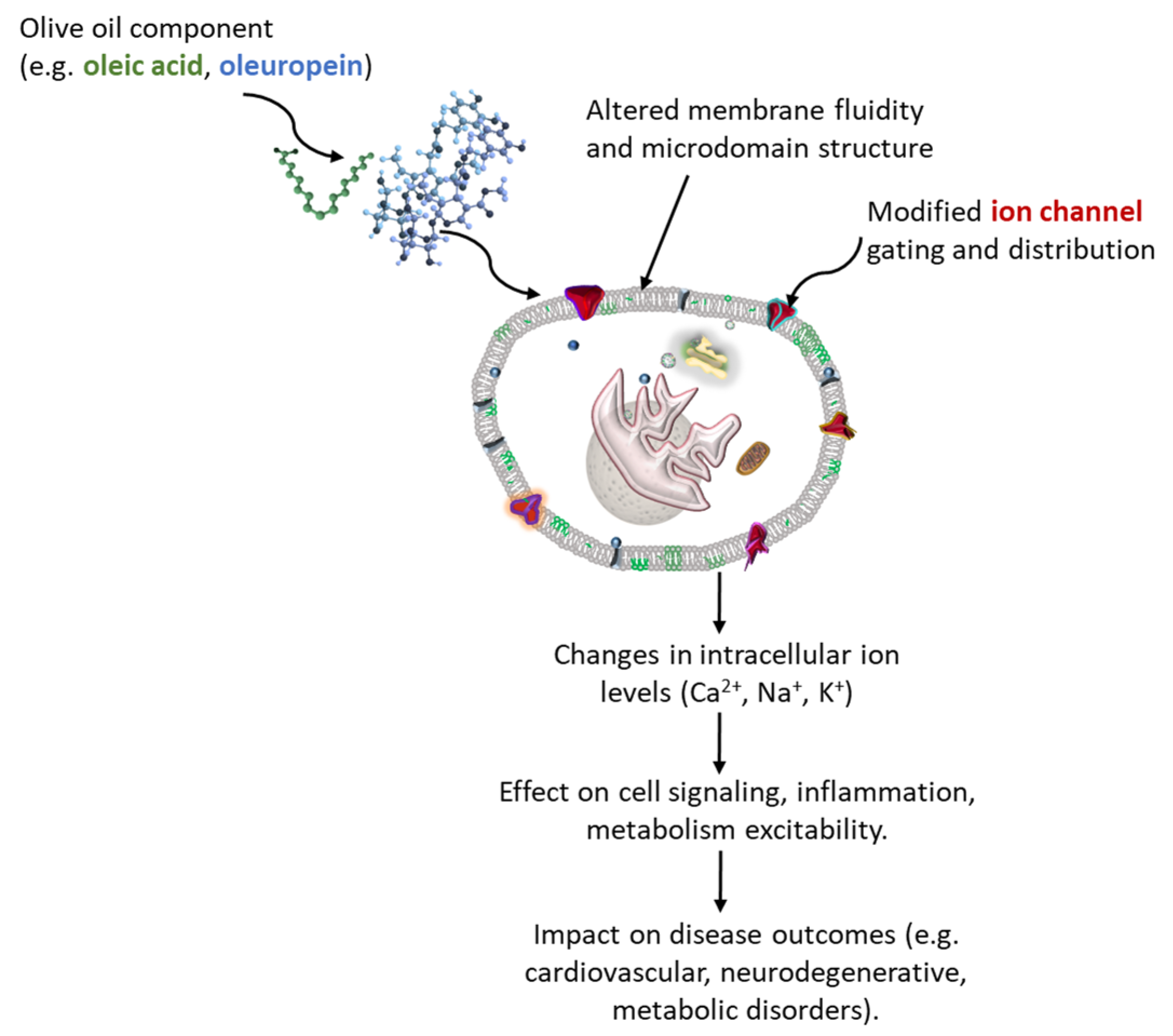
2.1. Biochemical Mechanisms Underlying the Modulatory Effects of Olive Oil Lipids on Membrane Structure and Ion Homeostasis
2.1.1. Membrane Integration and Remodeling of the Lipid Microenvironment
2.1.2. Intracellular Calcium Dynamics and Organelle Cross-Talk
2.2. Polyphenols: Antioxidant Activity and Ion Channel Interaction
2.3. Emerging Olive Oil-Derived Compounds and Their Ion-Regulating Potential
2.4. Triterpenes in Olive Oil and Their Interaction with Ion Channels
3. Beyond Classical Channels: Modulation of Ion Transport Systems by Olive Oil-Derived Compounds
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Concept |
| ACHR | Acetylcholine Receptor |
| AQP | Aquaporin |
| ATP | Adenosine Triphosphate |
| BAT | Brown Adipose Tissue |
| BKCa | Calcium Activated Potassium Channel |
| CaMKII | Ca2+/Calmodulin-Dependent Protein Kinase II |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| cGMP | Cyclic Guanosine Monophosphate |
| CNG | Cyclic Nucleotide-Gated |
| DAG | Diacylglycerols |
| ER | Endoplasmic Reticulum |
| EVOO | Extra Virgin Olive Oil |
| FFA | Free Fatty Acid |
| GLUT | Glucose Transporter |
| HEK | Human Embryonic Kidney Cells |
| hERG | Human Ether-A-Go-Go Related Gene |
| hSkM1 | Skeletal Muscle Sodium Channels |
| IR | Inward Rectifier Current |
| IOC | International Olive Council |
| KATP | Atp-Sensitive Potassium Channel |
| Kv | Voltage-Gated Potassium |
| LDL | Low-Density Lipoproteins |
| MAG | Monoacylglycerols |
| MAPK | Mitogen-Activated Protein Kinase |
| MUFA | Monounsaturated fatty acid |
| nAChRs | Nicotinic Acetylcholine Receptors |
| Nav | Voltage-Gated Sodium Channels |
| OEA | Oleoylethanolamide |
| OLEA | Oleuropein Aglycone |
| PIEZO | Mechanosensitive Ion Channel Protein |
| PKC | Protein Kinase C |
| POPC | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| POPG | 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol |
| POMC | Pro-Opiomelanocortin |
| PPAR-α | Peroxisome Proliferator-Activated Receptor Alpha |
| PUFA | Polyunsaturated fatty acid |
| ROS | Reactive Oxygen Species |
| SGLT1 | Sodium-Glucose Linked Transporter |
| SOCE | Store-Operated Calcium Entry |
| SOD | Superoxide Dismutase |
| STIM1 | Stromal Interaction Molecule 1 |
| TAG | Triacylglycerols |
| TRPA1 | Transient Receptor Potential Ankyrin 1 |
| TRPM2 | Transient Receptor Potential Melastatin 2 |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| UCP1 | Uncoupling Protein 1 |
References
- International Olive Council. Available online: https://www.internationaloliveoil.org/ (accessed on 17 May 2025).
- Avalos-Hernandez, A.; Juarez-Navarro, K.; Ruiz-Baca, E.; Meneses-Morales, I.; Espino-Saldaña, E.; Martinez-Torres, A.; Lopez-Rodriguez, A. Unlocking cellular traffic jams: Olive oil–mediated rescue of CNG mutant channels. Front. Pharmacol. 2024, 15, 1408156. [Google Scholar] [CrossRef]
- Ayadi, M.A.; Grati-Kamoun, N.; Attia, H. Physico-chemical change and heat stability of extra virgin olive oils flavored by selected Tunisian aromatic plants. Food Chem. Toxicol. 2009, 47, 261–267. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Antimicrobial, antioxidant and anti-inflammatory phenolic compounds in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Giacomino, A.; Inaudi, P.; Silletta, G.; Diana, A.; Bertinetti, S.; Gaggero, E.; Malandrino, M.; Stilo, F.; Abollino, O. Analytical methods for the characterization of vegetable oils. Molecules 2022, 28, 153. [Google Scholar] [CrossRef]
- Isaakidis, A.; Maghariki, J.E.; Carvalho-Barros, S.; Gomes, A.M.; Correia, M. Is there more to olive oil than healthy lipids? Nutrients 2023, 15, 3625. [Google Scholar] [CrossRef]
- Mirrezaie Roodaki, M.S.; Sahari, M.A.; Ghiassi Tarzi, B.; Barzegar, M.; Gharachorloo, M. Bioactive compounds of virgin olive oil extracted from Bladi and Arbequina cultivars. Curr. Nutr. Food Sci. 2017, 14, 17–27. [Google Scholar] [CrossRef]
- Revelou, P.K.; Xagoraris, M.; Alexandropoulou, A.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. Chemometric study of fatty acid composition of virgin olive oil from four widespread Greek cultivars. Molecules 2021, 26, 4151. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids and risk of cardiovascular disease: Synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 2012, 4, 1989–2007. [Google Scholar] [CrossRef]
- Ruiz-Gutiérrez, V.; Morgado, N.; Prada, J.L.; Pérez-Jiménez, F.; Muriana, F.J. Composition of human VLDL tri-acylglycerols after ingestion of olive oil and high oleic sunflower oil. J. Nutr. 1998, 128, 570–576. [Google Scholar] [CrossRef]
- Ruiz-Gutiérrez, V.; Perona, J.S.; Pacheco, Y.M.; Muriana, F.J.; Villar, J. Incorporation of dietary triacylglycerols from olive oil and high-oleic sunflower oil into VLDL triacylglycerols of hypertensive patients. Eur. J. Clin. Nutr. 1999, 53, 687–693. [Google Scholar] [CrossRef][Green Version]
- Rambold, A.S.; Cohen, S.; Lippincott-Schwartz, J. Fatty acid trafficking in starved cells: Regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 2015, 32, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Joardar, A.; Pattnaik, G.P.; Chakraborty, H. Effect of phosphatidylethanolamine and oleic acid on membrane fusion: Phosphatidylethanolamine circumvents the classical stalk model. J. Phys. Chem. B 2021, 125, 13192–13202. [Google Scholar] [CrossRef] [PubMed]
- Alemany, R.; Navarro, M.A.; Vögler, O.; Perona, J.S.; Osada, J.; Ruiz-Gutiérrez, V. Olive oils modulate fatty acid content and signaling protein expression in apolipoprotein E knockout mice brain. Lipids 2010, 45, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Funari, S.S.; Barceló, F.; Escribá, P.V. Effects of oleic acid and its congeners, elaidic and stearic acids, on the structural properties of phosphatidylethanolamine membranes. J. Lipid Res. 2003, 44, 567–575. [Google Scholar] [CrossRef]
- Lopez, S.; Bermudez, B.; Montserrat-de la Paz, S.; Jaramillo, S.; Varela, L.M.; Ortega-Gomez, A.; Abia, R.; Muriana, F.J. Membrane composition and dynamics: A target of bioactive virgin olive oil constituents. Biochim. Biophys. Acta 2014, 1838, 1638–1656. [Google Scholar] [CrossRef]
- Perona, J.S.; Cabello-Moruno, R.; Ruiz-Gutierrez, V. Modulation of the effects of chylomicron remnants on endothelial function by minor dietary lipid components. Biochem. Soc. Trans. 2007, 35, 446–450. [Google Scholar] [CrossRef][Green Version]
- Piccinin, E.; Cariello, M.; De Santis, S.; Ducheix, S.; Sabbà, C.; Ntambi, J.M.; Moschetta, A. Role of oleic acid in the gut–liver axis: From diet to the regulation of its synthesis via stearoyl-CoA desaturase 1 (SCD1). Nutrients 2019, 11, 2283. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Costa, V.; Costa, M.; Videira, R.A.; Andrade, P.B.; Paiva-Martins, F. Anti-inflammatory activity of olive oil polyphenols—The role of oleacein and its metabolites. Biomedicines 2022, 10, 2990. [Google Scholar] [CrossRef]
- Costa, M.; Costa, V.; Lopes, M.; Paiva-Martins, F. A biochemical perspective on the fate of virgin olive oil phenolic compounds in vivo. Crit. Rev. Food Sci. Nutr. 2024, 64, 1403–1428. [Google Scholar] [CrossRef]
- Darakjian, L.I.; Rigakou, A.; Brannen, A.; Qusa, M.H.; Tasiakou, N.; Diamantakos, P.; Reed, M.N.; Panizzi, P.; Boersma, M.D.; Melliou, E.; et al. Spontaneous in vitro and in vivo interaction of (−)-oleocanthal with glycine in biological fluids: Novel pharmacokinetic markers. ACS Pharmacol. Transl. Sci. 2021, 4, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Nikou, T.; Karampetsou, K.V.; Koutsoni, O.S.; Skaltsounis, A.L.; Dotsika, E.; Halabalaki, M. Pharmacokinetics and metabolism investigation of oleocanthal. J. Nat. Prod. 2024, 87, 530–543. [Google Scholar] [CrossRef]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar] [PubMed]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Tzounis, X.; Dessì, M.A.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P. The fate of olive oil polyphenols in the gastrointestinal tract: Implications of gastric and colonic microflora-dependent biotransformation. Free Radic. Res. 2006, 40, 647–658. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- López-Yerena, A.; Vallverdú-Queralt, A.; Mols, R.; Augustijns, P.; Lamuela-Raventós, R.M.; Escribano-Ferrer, E. Absorption and intestinal metabolic profile of oleocanthal in rats. Pharmaceutics 2020, 12, 134. [Google Scholar] [CrossRef]
- Edgecombe, S.C.; Stretch, G.L.; Hayball, P.J. Oleuropein, an antioxidant polyphenol from olive oil, is poorly absorbed from isolated perfused rat intestine. J. Nutr. 2000, 130, 2996–3002. [Google Scholar] [CrossRef]
- López de las Hazas, M.C.; Piñol, C.; Macià, A.; Romero, M.P.; Pedret, A.; Solà, R.; Rubió, L.; Motilva, M.J. Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Nasr, M.; Katary, S.H. From olive tree to treatment: Nano-delivery systems for enhancing oleuropein’s health benefits. Pharmaceuticals 2025, 18, 573. [Google Scholar] [CrossRef] [PubMed]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.T.; Yen, G.C. Modulation of hepatic phase II phenol sulfotransferase and antioxidant status by phenolic acids in rats. J. Nutr. Biochem. 2006, 17, 561–569. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Galmés, S.; Reynés, B.; Palou, M.; Palou-March, A.; Palou, A. Absorption, distribution, metabolism, and excretion of the main olive tree phenols and polyphenols: A literature review. J. Agric. Food Chem. 2021, 69, 5281–5296. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Galli, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Covas, M.I.; de la Torre, K.; Fitó, M. Virgin olive oil: A key food for cardiovascular risk protection. Br. J. Nutr. 2006, 96, S20–S23. [Google Scholar] [CrossRef]
- Galiano, V.; Villalaín, J. Oleuropein aglycone in lipid bilayer membranes: A molecular dynamics study. Biochim. Biophys. Acta 2015, 1848 Pt A, 2849–2858. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M.; Gimeno, E.; Fitó, M.; Castellote, A.-I.; Covas, M.; DE LA Torre-Boronat, M.C.; López-Sabater, M.C. Interaction of olive oil phenol antioxidant components with low-density lipoprotein. Biol. Res. 2004, 37, 247–252. [Google Scholar] [CrossRef][Green Version]
- Diez-Bello, R.; Jardin, I.; Lopez, J.; El Haouari, M.; Ortega-Vidal, J.; Altarejos, J.; Salido, G.; Salido, S.; Rosado, J. (−) Oleocanthal inhibits proliferation and migration by modulating Ca2+ entry through TRPC6 in breast cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 474–485. [Google Scholar] [CrossRef]
- Elinder, F.; Liin, S.I. Actions and mechanisms of polyunsaturated fatty acids on voltage-gated ion channels. Front. Physiol. 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Grubić Kezele, T.; Ćurko-Cofek, B. Neuroprotective panel of olive polyphenols: Mechanisms of action, anti-demyelination, and anti-stroke properties. Nutrients 2022, 14, 4533. [Google Scholar] [CrossRef] [PubMed]
- Vijakumaran, U.; Shanmugam, J.; Heng, J.W.; Azman, S.S.; Yazid, M.D.; Abdullah, N.A.H.; Sulaiman, N. Effects of hydroxytyrosol in endothelial functioning: A comprehensive review. Molecules 2023, 28, 1861. [Google Scholar] [CrossRef]
- Carrara, M.; Richaud, M.; Cuq, P.; Galas, S.; Margout-Jantac, D. Influence of oleacein, an olive oil and olive mill wastewater phenolic compound, on Caenorhabditis elegans longevity and stress resistance. Foods 2024, 13, 2146. [Google Scholar] [CrossRef]
- Antollini, S.S.; Barrantes, F.J. Fatty acid regulation of voltage- and ligand-gated ion channel function. Front. Physiol. 2016, 7, 573. [Google Scholar] [CrossRef]
- Wilschut, J.; Hoekstra, D. Membrane fusion: Lipid vesicles as a model system. Chem. Phys. Lipids 1986, 40, 145–166. [Google Scholar] [CrossRef]
- Carrillo, C.; Cavia, M.d.M.; Alonso-Torre, S.R. Oleic acid versus linoleic and α-linolenic acid: Different effects on Ca2+ signaling in rat thymocytes. Cell. Physiol. Biochem. 2011, 27, 373–380. [Google Scholar] [CrossRef]
- Carrillo, C.; Cavia, M.M.; Alonso-Torre, S.R. Oleic acid inhibits store-operated calcium entry in human colorectal adenocarcinoma cells. Int. J. Food Sci. Nutr. 2012, 63, 381–388. [Google Scholar] [CrossRef]
- Fu, J.; Gaetani, S.; Oveisi, F.; Lo Verme, J.; Serrano, A.; Rodríguez De Fonseca, F.; Rosengarth, A.; Luecke, H.; Di Giacomo, B.; Tarzia, G.; et al. Oleoylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature 2003, 425, 90–93. [Google Scholar] [CrossRef]
- Piomelli, D. A fatty gut feeling. Trends Endocrinol. Metab. 2013, 24, 332–341. [Google Scholar] [CrossRef]
- Escribá, P.V.; Busquets, X.; Inokuchi, J.; Balogh, G.; Török, Z.; Horváth, I.; Harwood, J.L.; Vígh, L. Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog. Lipid Res. 2015, 47, 378–410. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.; Stahl, A.; Lodish, H.F. A family of fatty acid transporters conserved from Mycobacterium to man. Proc. Natl. Acad. Sci. USA 1998, 95, 8625–8629. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.K.; Chen, Y.C.; Kao, Y.H.; Tsai, C.F.; Yeh, Y.H.; Huang, J.L.; Cheng, C.C.; Chen, S.A.; Chen, Y.J. A monounsaturated fatty acid (oleic acid) modulates electrical activity in atrial myocytes with calcium and sodium dysregulation. Int. J. Cardiol. 2014, 176, 191–198. [Google Scholar] [CrossRef]
- Moreno, C.; Macias, A.; Prieto, A.; De La Cruz, A.; Valenzuela, C. Polyunsaturated fatty acids modify the gating of Kv channels. Front. Pharmacol. 2012, 3, 163. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Juhaszova, M.; Church, P.; Blaustein, M.P. Location of calcium transporters at presynaptic terminals. Eur. J. Neurosci. 2000, 12, 839–846. [Google Scholar] [CrossRef]
- Lytton, J.; Westlin, M.; Burk, S.E.; Shull, G.E.; MacLennan, D.H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J. Biol. Chem. 1992, 267, 14483–14489. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Dolmetsch, R.E.; Xu, K.; Lewis, R.S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 1998, 392, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Wheal, A.J.; Alexander, S.P.H.; Randall, M.D. Vasorelaxation to N-oleoylethanolamine in rat isolated arteries: Mechanisms of action and modulation via cyclooxygenase activity. Br. J. Pharmacol. 2010, 160, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, G.; Weiser, A.; Bermont, F.; Migliavacca, E.; Brinon, B.; Jacot, G.E.; Hermant, A.; Sturlese, M.; Nogara, L.; Vascon, F.; et al. Mitochondrial calcium uptake declines during aging and is directly activated by oleuropein to boost energy metabolism and skeletal muscle performance. Cell Metab. 2025, 37, 477–495.e11. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Iliodromitis, E.K.; Mikros, E.; Constantinou, M.; Agalias, A.; Magiatis, P.; Skaltsounis, A.L.; Kamber, E.; Tsantili-Kakoulidou, A.; Kremastinos, D.T. The olive constituent oleuropein exhibits anti-ischemic, antioxidative, and hypolipidemic effects in anesthetized rabbits. J. Nutr. 2006, 136, 2213–2219. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Gordon, M.H.; Gameiro, P. Activity and location of olive oil phenolic antioxidants in liposomes. Chem. Phys. Lipids 2003, 124, 23–36. [Google Scholar] [CrossRef]
- Larsen, A.H. Molecular dynamics simulations of curved lipid membranes. Int. J. Mol. Sci. 2022, 23, 8098. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, S.; Huang, Y.; Wei, X.; Liu, L.; Huo, F.; Huang, P.; Wu, Y.; Tian, W. Inhibition of TRPA1 ameliorates periodontitis by reducing periodontal ligament cell oxidative stress and apoptosis via PERK/eIF2α/ATF-4/CHOP signal pathway. Oxid. Med. Cell. Longev. 2022, 4107915. [Google Scholar] [CrossRef]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK–FOXO3a pathway. Eur. J. Pharmacol. 2011, 660, 275–282. [Google Scholar] [CrossRef]
- Oi-Kano, Y.; Iwasaki, Y.; Nakamura, T.; Watanabe, T.; Goto, T.; Kawada, T.; Watanabe, K.; Iwai, K. Oleuropein aglycone enhances UCP1 expression in brown adipose tissue in high-fat-diet-induced obese rats by activating β-adrenergic signaling. J. Nutr. Biochem. 2017, 40, 209–218. [Google Scholar] [CrossRef]
- Badr, A.M.; Attia, H.A.; Al-Rasheed, N. Oleuropein reverses repeated corticosterone-induced depressive-like behavior in mice: Evidence of modulating effect on biogenic amines. Sci. Rep. 2020, 10, 3336. [Google Scholar] [CrossRef]
- Elmazoglu, Z.; Ergin, V.; Sahin, E.; Kayhan, H.; Karasu, C. Oleuropein and rutin protect against 6-OHDA-induced neurotoxicity in PC12 cells through modulation of mitochondrial function and unfolded protein response. Interdiscip. Toxicol. 2017, 10, 129–141. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.; Cui, W.; Li, G.; Yuan, S.; Xu, D.; Hoi, M.P.; Lin, Z.; Dou, J.; Han, Y.; Lee, S.M. Baicalein protects against 6-OHDA-induced neurotoxicity through activation of Keap1/Nrf2/HO-1 and involving PKCα and PI3K/AKT signaling pathways. J. Agric. Food Chem. 2012, 60, 8171–8182. [Google Scholar] [CrossRef]
- Parkinson, L.; Keast, R. Oleocanthal, a phenolic derived from virgin olive oil: A review of the beneficial effects on inflammatory disease. Int. J. Mol. Sci. 2014, 15, 12323–12334. [Google Scholar] [CrossRef] [PubMed]
- Di Risola, D.; Laurenti, D.; Ferraro, F.; Ciogli, A.; Manetto, S.; Gazzilli, Y.; Federico, R.; Francioso, A.; Mosca, L.; Mattioli, R. Spontaneous reaction of oleacein and oleocanthal with primary amines: A biochemical perspective. Molecules 2025, 30, 7. [Google Scholar] [CrossRef] [PubMed]
- Grewal, R.; Reutzel, M.; Dilberger, B.; Hein, H.; Zotzel, J.; Marx, S.; Tretzel, J.; Sarafeddinov, A.; Fuchs, C.; Eckert, G.P. Purified oleocanthal and ligstroside protect against mitochondrial dysfunction in models of early Alzheimer’s disease and brain ageing. Exp. Neurol. 2020, 328, 113248. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, T.; Ikeuchi, Y.; Matsuoka, T.; Sumikawa, K. Short-term depression and long-term enhancement of ACh-gated channel currents induced by linoleic and linolenic acid. Brain Res. 1997, 751, 253–258. [Google Scholar] [CrossRef]
- Antollini, S.S.; Barrantes, F.J. Unique effects of different fatty acid species on the physical properties of the Torpedo acetylcholine receptor membrane. J. Biol. Chem. 2002, 277, 1249–1254. [Google Scholar] [CrossRef]
- Obiol, D.J.; Amundarain, M.J.; Zamarreño, F.; Viertri, A.; Antollini, S.S.; Costabel, M.D. Oleic acid could act as a channel blocker in the inhibition of nAChR: Insights from molecular dynamics simulations. J. Phys. Chem. B 2024, 128, 2398–2411. [Google Scholar] [CrossRef]
- Esposito, M.; Gatto, M.; Cipolla, M.J.; Bernstein, I.M.; Mandalà, M. Dilation of pregnant rat uterine arteries with phenols from extra virgin olive oil is endothelium-dependent and involves calcium and potassium channels. Cells 2024, 13, 619. [Google Scholar] [CrossRef]
- Linsdell, P. Inhibition of cystic fibrosis transmembrane conductance regulator chloride channel currents by arachidonic acid. Can. J. Physiol. Pharmacol. 2000, 78, 490–499. [Google Scholar] [CrossRef]
- Tewari, K.P.; Malinowska, D.H.; Sherry, A.M.; Cuppoletti, J. PKA and arachidonic acid activation of human recombinant ClC-2 chloride channels. Am. J. Physiol. Cell Physiol. 2000, 279, C40–C50. [Google Scholar] [CrossRef]
- Cuppoletti, J.; Tewari, K.P.; Chakrabarti, J.; Malinowska, D.H. Identification of the fatty acid activation site on human ClC-2. Am. J. Physiol. Cell Physiol. 2017, 312, C707–C723. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pitsillou, E.; Liang, J.J.; Beh, R.C.; Prestedge, J.; Catak, S.; Hung, A.; Karagiannis, T.C. Identification of novel bioactive compounds from Olea europaea by evaluation of chemical compounds in the OliveNet™ library: In silico bioactivity and molecular modelling, and in vitro validation of hERG activity. Comput. Biol. Med. 2022, 142, 105247. [Google Scholar] [CrossRef] [PubMed]
- Crumb, W.J.; Munfakh, N.; Heck, H.A.; Harrison, L.H., Jr. Fatty acid block of the transient outward current in adult human atrium. J. Pharmacol. Exp. Ther. 1999, 289, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.J.; Tian, H.Y.; Wang, T.Z.; Du, Y.; Xi, Y.T.; Wu, Y.; Gao, J.; Ma, A.Q. Oleic acid inhibits the K(ATP) channel subunit Kir6.1 and the K(ATP) current in human umbilical artery smooth muscle cells. Am. J. Med. Sci. 2013, 346, 204–210. [Google Scholar] [CrossRef]
- Nkanu, E.; Owu, D.U.; Osim, E.E. Extra virgin olive oil and palm oil diets reduce blood pressure via KATP and BKCa ion channels in rats. J. Appl. Sci. 2019, 19, 537–543. [Google Scholar] [CrossRef][Green Version]
- Jowais, J.J.; Yazdi, S.; Golluscio, A.; Olivier-Meo, V.; Liin, S.I.; Larsson, H.P. Mechanistic understanding of KCNQ1 activating polyunsaturated fatty acid analogs. J. Gen. Physiol. 2023, 155, e202313339. [Google Scholar] [CrossRef]
- Székely, A.; Kitajka, K.; Panyi, G.; Márián, T.; Gáspár, R.; Krasznai, Z. Nutrition and immune system: Certain fatty acids differently modify membrane composition and consequently kinetics of KV1.3 channels of human peripheral lymphocytes. Immunobiology 2007, 212, 213–227. [Google Scholar] [CrossRef]
- Liin, S.I.; Silverå Ejneby, M.; Barro-Soria, R.; Skarsfeldt, M.A.; Larsson, J.E.; Starck Härlin, F.; Parkkari, T.; Bentzen, B.H.; Schmitt, N.; Larsson, H.P.; et al. Polyunsaturated fatty acid analogs act antiarrhythmically on the cardiac IKs channel. Proc. Natl. Acad. Sci. USA 2015, 112, 5714–5719. [Google Scholar] [CrossRef]
- Liin, S.I.; Karlsson, U.; Bentzen, B.H.; Schmitt, N.; Elinder, F. Polyunsaturated fatty acids are potent openers of human M-channels expressed in Xenopus laevis oocytes. Acta Physiol. 2016, 218, 28–37. [Google Scholar] [CrossRef]
- Scheffler, A.; Rauwald, H.W.; Kampa, B.; Mann, U.; Mohr, F.W.; Dhein, S. Olea europaea leaf extract exerts L-type Ca2+ channel antagonistic effects. J. Ethnopharmacol. 2008, 120, 233–240. [Google Scholar] [CrossRef]
- Peyrot des Gachons, C.; Uchida, K.; Bryant, B.; Shima, A.; Sperry, J.B.; Dankulich-Nagrudny, L.; Tominaga, M.; Smith, A.B., 3rd; Beauchamp, G.K.; Breslin, P.A. Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. J. Neurosci. 2011, 31, 999–1009. [Google Scholar] [CrossRef]
- Morales-Lázaro, S.L.; Llorente, I.; Sierra-Ramírez, F.; López-Romero, A.E.; Ortíz-Rentería, M.; Serrano-Flores, B.; Simon, S.A.; Islas, L.D.; Rosenbaum, T. Inhibition of TRPV1 channels by a naturally occurring omega-9 fatty acid reduces pain and itch. Nat. Commun. 2016, 7, 13092. [Google Scholar] [CrossRef]
- Leon-Aparicio, D.; Sánchez-Solano, A.; Arreola, J.; Perez-Cornejo, P. Oleic acid blocks the calcium-activated chloride channel TMEM16A/ANO1. BBA Mol. Cell Biol. Lipids 2022, 1867, 159134. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.O.; Caires, R.; Victor, K.; Ramirez, J.; Sierra-Valdez, F.J.; Walsh, P.; Truong, V.; Lee, J.; Mayor, U.; Reiter, L.T.; et al. Linoleic acid improves PIEZO2 dysfunction in a mouse model of Angelman syndrome. Nat. Commun. 2023, 14, 1167. [Google Scholar] [CrossRef] [PubMed]
- Wieland, S.J.; Gong, Q.; Poblete, H.; Fletcher, J.E.; Chen, L.Q.; Kallen, R.G. Modulation of human muscle sodium channels by intracellular fatty acids is dependent on the channel isoform. J. Biol. Chem. 1996, 271, 19037–19041. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.; Barberio, L.; Gatto, M.; Tropea, T.; De Luca, M.; Mandalà, M. Extra virgin olive oil phenols vasodilate rat mesenteric resistance artery via phospholipase C (PLC)–calcium microdomains–potassium channels (BKCa) signals. Biomolecules 2021, 11, 137. [Google Scholar] [CrossRef]
- Jo, Y.H.; Su, Y.; Gutierrez-Juarez, R.; Chua, S., Jr. Oleic acid directly regulates POMC neuron excitability in the hypothalamus. J. Neurophysiol. 2009, 101, 2305–2316. [Google Scholar] [CrossRef]
- Hu, H.Z.; Xiao, R.; Wang, C.; Gao, N.; Colton, C.K.; Wood, J.D.; Zhu, M.X. Potentiation of TRPV3 channel function by unsaturated fatty acids. J. Cell Physiol. 2006, 208, 201–212. [Google Scholar] [CrossRef]
- Smithers, N.; Bolivar, J.H.; Lee, A.G.; East, J.M. Characterizing the fatty acid binding site in the cavity of potassium channel KcsA. Biochemistry 2012, 51, 7996–8002. [Google Scholar] [CrossRef]
- Claro-Cala, C.M.; Jiménez-Altayó, F.; Zagmutt, S.; Rodríguez-Rodríguez, R. Molecular mechanisms underlying the effects of olive oil triterpenic acids in obesity and related diseases. Nutrients 2022, 14, 1606. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, E.; Tsimidou, M.Z. Edible oils from olive drupes as a source of bioactive pentacyclic triterpenes: Is there a prospect for a health claim authorization? Food Chem. 2022, 381, 132286. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Lee, D.-U. Inhibitory effect of oleanolic acid from the rhizomes of Cyperus rotundus on transient receptor potential vanilloid 1 channel. Planta Med. 2015, 81, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-de-Albuquerque, C.F.; Burth, P.; Silva, A.R.; de Moraes, I.M.; de Jesus Oliveira, F.M.; Santelli, R.E.; Freire, A.S.; Bozza, P.T.; Younes-Ibrahim, M.; de Castro-Faria-Neto, H.C.; et al. Oleic acid inhibits lung Na/K-ATPase in mice and induces injury with lipid body formation in leukocytes and eicosanoid production. J. Inflamm. 2013, 10, 34. [Google Scholar] [CrossRef]
- Carrillo, C.; Cavia, M.d.M.; Alonso-Torre, S.R. Role of oleic acid in immune system; mechanism of action; a review. Nutr. Hosp. 2012, 27, 978–990. [Google Scholar] [CrossRef]
- Cremesti, A.E.; Goñi, F.M.; Kolesnick, R. Role of sphingomyelinase and ceramide in modulating rafts: Do biophysical properties determine biologic outcome? FEBS Lett. 2002, 531, 47–53. [Google Scholar] [CrossRef]
- Gu, L.Y.; Qiu, L.W.; Chen, X.F.; Lv, L.; Mei, Z.C. Expression of aquaporin 3 and aquaporin 9 is regulated by oleic acid through the PI3K/Akt and p38 MAPK signaling pathways. Zhonghua Gan Zang Bing Za Zhi 2013, 10, 753–758. [Google Scholar] [CrossRef]
- Varela-Eirín, M.; Carpintero-Fernández, P.; Sánchez-Temprano, A.; Varela-Vázquez, A.; Paíno, C.L.; Casado-Díaz, A.; Calañas-Continente, A.; Mato, V.; Fonseca, E.; Kandouz, M.; et al. Senolytic activity of small molecular polyphenols from olive restores chondrocyte redifferentiation and promotes a pro-regenerative environment in osteoarthritis. Aging 2020, 12, 15882–15905. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Tsukahara, C.; Ikeda, N.; Sone, Y.; Ishikawa, T.; Ichi, I.; Koike, T.; Aoki, Y. Oleuropein improves insulin resistance in skeletal muscle by promoting the translocation of GLUT4. J. Clin. Biochem. Nutr. 2017, 61, 196–202. [Google Scholar] [CrossRef]
| Fraction | Category | Representative Compounds |
|---|---|---|
| Saponifiable (98–99%) | Fatty acids (as triacylglycerols) | Oleic acid (C18:1), Palmitic acid (C16:0), Linoleic acid (C18:2), Stearic acid (C18:0), Palmitoleic acid (C16:1) |
| Non-saponifiable (1–2%) | Sterols (lipidic) | β-Sitosterol, Campesterol, Stigmasterol |
| Hydrocarbons (lipidic) | Squalene | |
| Triterpenes | Oleanolic acid, Maslinic acid, Uvaol | |
| Tocopherols (Vitamin E) | α-Tocopherol | |
| Simple phenols | Hydroxytyrosol, Tyrosol | |
| Secoiridoids | Oleuropein aglycone, Ligstroside aglycone, Oleocanthal, Oleacein, Oleomissional, Oleocoronal, Oleocanthalic acid | |
| Phenolic acids | Caffeic acid, Vanillic acid, Ferulic acid | |
| Flavonoids | Luteolin, Apigenin | |
| Lignans | Pinoresinol, Acetoxypinoresinol | |
| Pigments | Chlorophylls, Carotenoids | |
| Volatile compounds | Aldehydes, ketones, esters |
| Class | Compound(s) | Ion Channel | Biophysical/Physiological Effect | Reference |
|---|---|---|---|---|
| Lipidic | Linoleic Linolenic acid Oleic acid | ACHR Acetilcholine receptor | -Linoleic and linolenic acid enhances ACh-gated currents via CaMKII activation in Xenopus oocytes. -Using the fluorescent probe suggests that there is a direct lipid-protein influence. -Acts as a channel blocker, inhibiting nicotinic acetylcholine receptor function. | [78,79,80] |
| Phenolic | EVOO | BKCa channels Large-conductance Ca2+-activated K+ channel | EVOO phenols activate BKCa channels in smooth muscle of uterine arteries of pregnant rats, mediated by Ca2+ signaling that triggers the synthesis of NO, cGMP and opening of BKCa. | [81] |
| Lipidic | Oleic and Linoleic acid | CFTR Cystic fibrosis transmembrane receptor | Using patch clamp recording from CFTR-transfected baby hamster kidney cell lines, the CFTR was inhibited by several fatty acids in the following order: linoleic ≥ arachidonic ≥ oleic. | [82] |
| Lipidic | Oleic acid | ClC2 | Patch clamp on HEK-293 cells demonstrated activation of ClC-2 by oleic acid. | [83,84] |
| Lipidic | Oleic acid | CNG Cyclic Nucleotide-Gated | Facilitates trafficking and function of mutant CNG channels related to retinopathies. | [2] |
| Phenolic | Oleuropein, Hydroxytyrosol | ERG Ether-à-go-go-Related Gene | Considered non-inhibitors of hERG channels; may enhance the action of verapamil, suggesting a favorable cardiac safety profile. | [85] |
| Lipidic | Oleic acid | IK Inward rectifying potassium channel | It blocks the transient outward current (Ito) in human atrial myocytes, while leaving the sustained current and inward rectifier current (IK1) unchanged. | [86] |
| Lipidic | Oleic acid | KATP ATP-sensitive potassium | -Inhibits protein expression and current in human umbilical artery smooth muscle cells, potentially affecting vascular tone. -Inhibits KATP currents in pro-opiomelanocortin (POMC) neurons, influencing neuronal excitability. | [87] |
| Phenolic | EVOO phenols | KATP and BKCa ATP-sensitive potassium Large-conductance Ca2+-activated K+ channel | Activation of KATP and BKCa channels in vascular smooth muscle, causing cellular hyperpolarization, vasorelaxation and reduction in mean arterial pressure. | [88] |
| Lipidic | Linoleic acid | KCNQ1 | Provide molecular models supported by experimental evidence of specific interactions between PUFA analogs and KCNQ1 channel. | [89] |
| Lipidic | Oleic acid/Linoleic acid | Kv1.3 | Whole-cell patch-clamp experiments demonstrated that the polyunsaturated linoleic acid decreased the activation and inactivation time constants of the Kv1.3 channels, but did not affect the voltage dependence of the steady-state activation and steady-state inactivation of the channels, while monounsaturated oleic acid did not result in significant changes in the biophysical parameters. | [90] |
| Lipidic | Oleic acid | Kv7.1 | Oleic acid has no effect on channel kinetic. | [91] |
| Lipidic | Oleic acid | Kv7.2/3 | Oleic acid did not facilitate opening of the human Kv 7.2/3 channel expressed in Xenopus oocytes. | [92] |
| Phenolic | Hydroxytyrosol | L-type Ca2+ channels | Direct and reversible blockade of L-type Ca2+ channels in vascular smooth muscle, in a dose-dependent manner, reducing vascular resistance and contributing to vasodilation. | [93] |
| Phenolic | Oleocanthal | TRPA1 | Selectively activates TRPA1 channels, contributing to the pungent oral sensation of extra virgin olive oil. | [94] |
| Phenolic | Oleuropein aglycone | TRPA1/TRPV1 | OA is the agonist of both TRPA1 and TRPV1 | [71] |
| Lipidic | Oleic acid | TRPV1 | Reduces open probability by stabilizing the closed state; slight antagonism to capsaicin activation. | [95] |
| Lipidic | Oleic acid | TRPC3/6 | Increases intracellular Ca2+ in T-cells via activation of TRPC3/6 channels, influencing immune cell function. | [50] |
| Lipidic | Oleic acid | TMEM16A Transmembrane member 16A | Irreversibly blocks the channel in a dose- and voltage-dependent manner at low intracellular Ca2+ concentrations. | [96] |
| Lipidic | A linoleic acid | PIEZO2 Stretch-gated ion channel | LA-enriched diet increases PIEZO2 activity, mechano-excitability, and improves gait in male mice with Angelman Syndrome. Whole-cell recordings post-mechanical stimulation confirmed increased responses and improved gait. | [97] |
| Lipidic | Oleic acid | SkM1 skeletal muscle sodium channels | Currents from SkM1 transfected into HEK293t cells were inhibited by oleic acid | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mijares-Andrade, H.; Carreño-Diaz, I.; La-Llave-Leon, O.; Meneses-Morales, I.; Ruiz-Baca, E.; Lopez-Rodriguez, A. Influence of Olive Oil Components on Ion Channels. Molecules 2025, 30, 3336. https://doi.org/10.3390/molecules30163336
Mijares-Andrade H, Carreño-Diaz I, La-Llave-Leon O, Meneses-Morales I, Ruiz-Baca E, Lopez-Rodriguez A. Influence of Olive Oil Components on Ion Channels. Molecules. 2025; 30(16):3336. https://doi.org/10.3390/molecules30163336
Chicago/Turabian StyleMijares-Andrade, Hascibe, Ismael Carreño-Diaz, Osmel La-Llave-Leon, Ivan Meneses-Morales, Estela Ruiz-Baca, and Angelica Lopez-Rodriguez. 2025. "Influence of Olive Oil Components on Ion Channels" Molecules 30, no. 16: 3336. https://doi.org/10.3390/molecules30163336
APA StyleMijares-Andrade, H., Carreño-Diaz, I., La-Llave-Leon, O., Meneses-Morales, I., Ruiz-Baca, E., & Lopez-Rodriguez, A. (2025). Influence of Olive Oil Components on Ion Channels. Molecules, 30(16), 3336. https://doi.org/10.3390/molecules30163336







