Synthesis, Structure and Cytoprotective Activity of New Derivatives of 4-Aryl-3-Aminopyridin-2(1H)-One
Abstract
1. Introduction
2. Results and Discussion
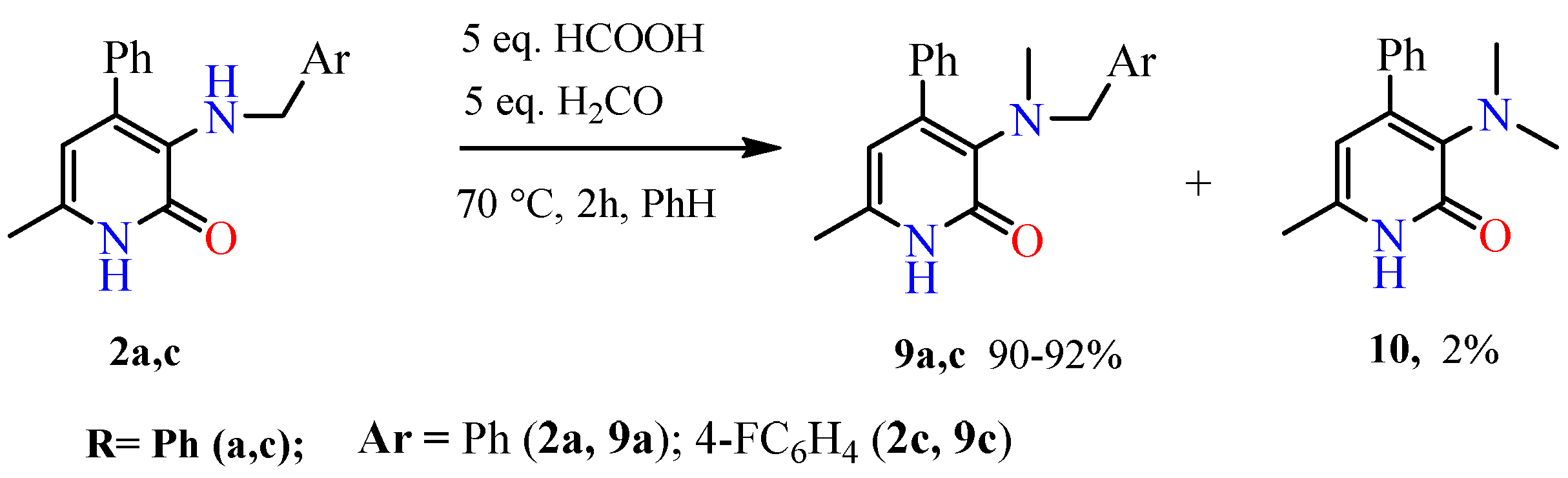
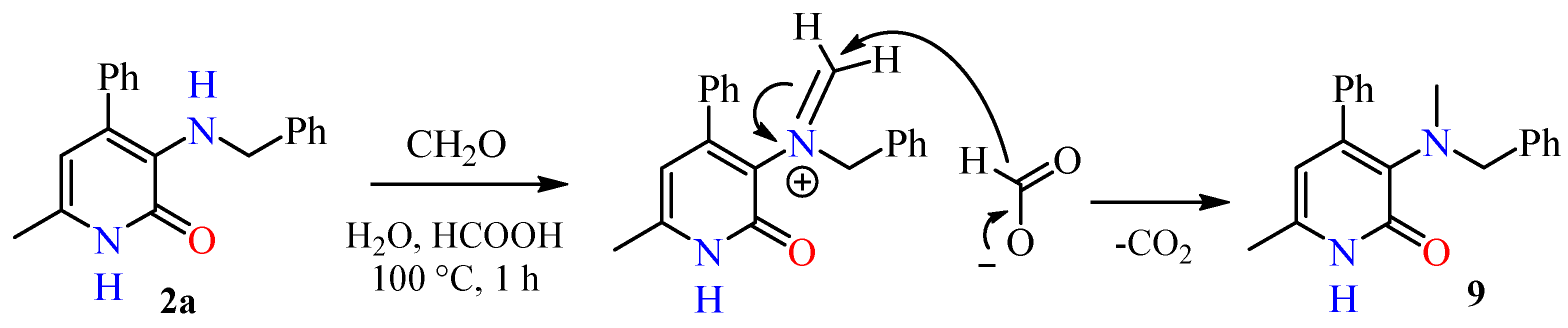
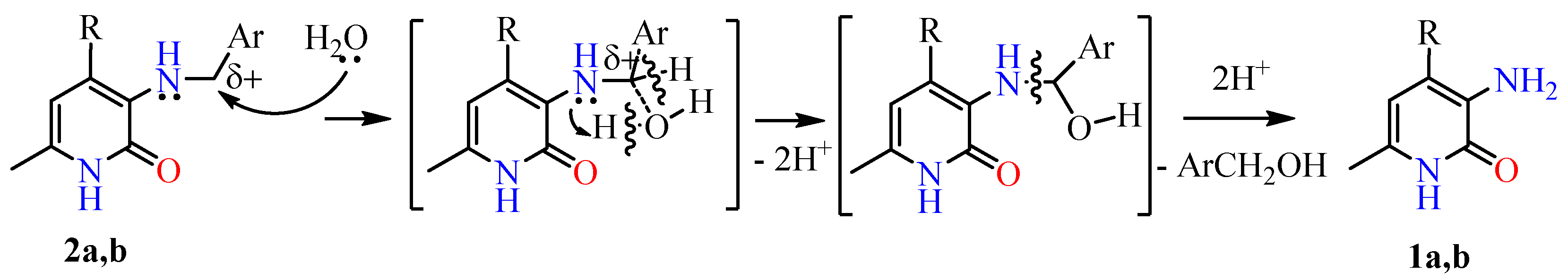
2.1. Chemical Part
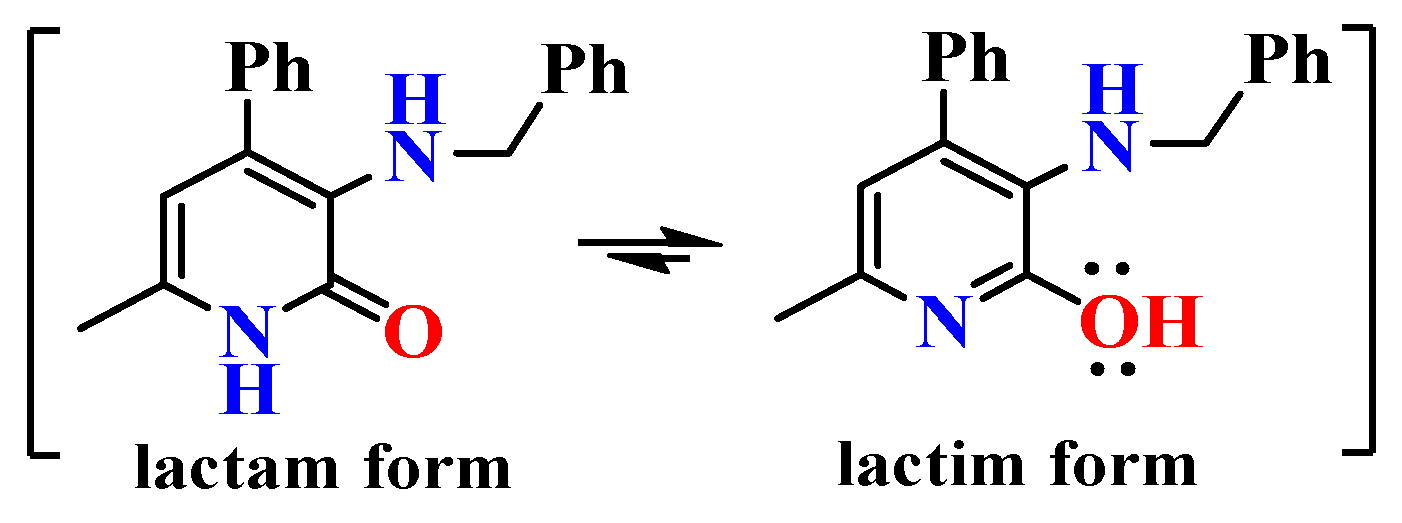
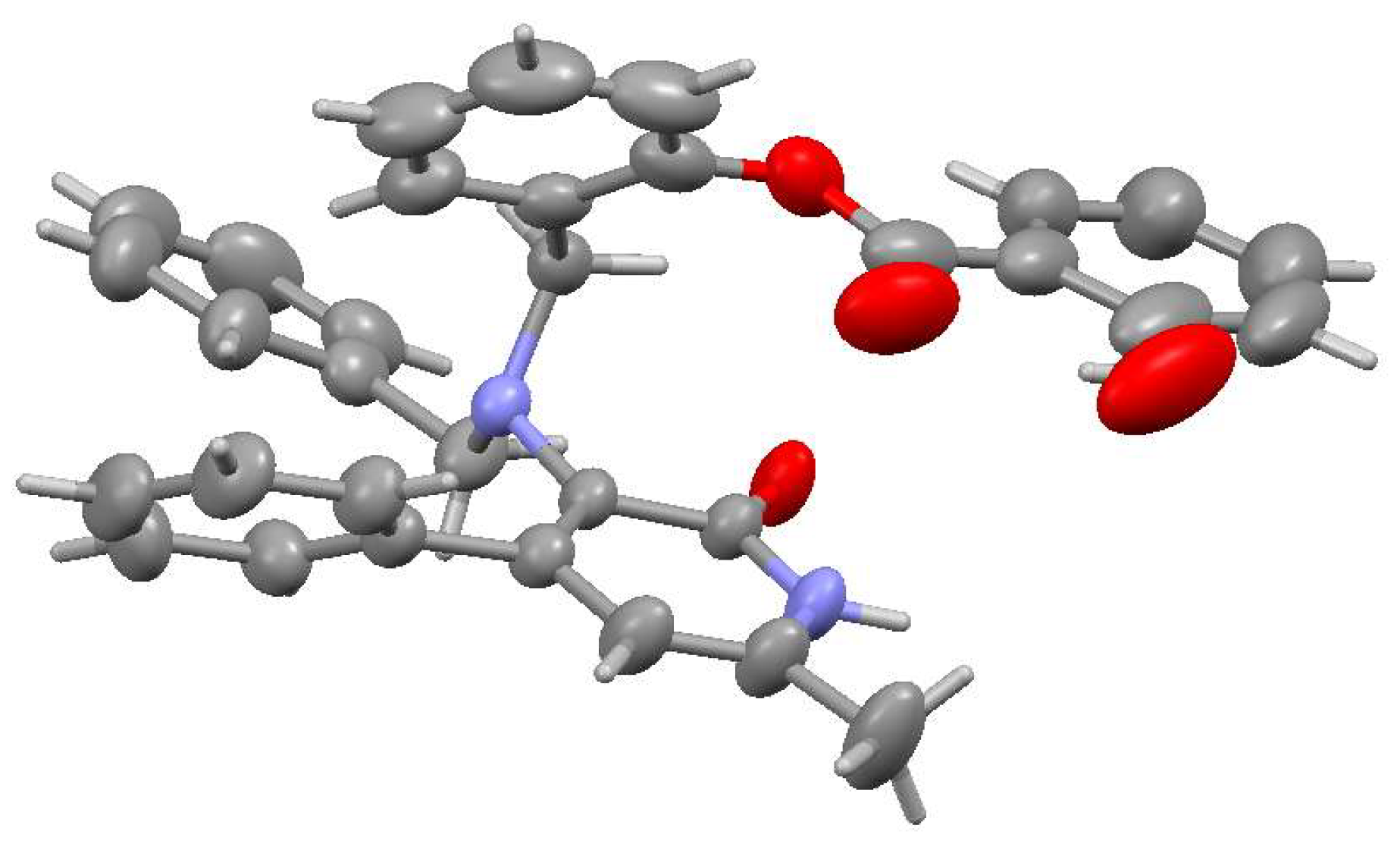
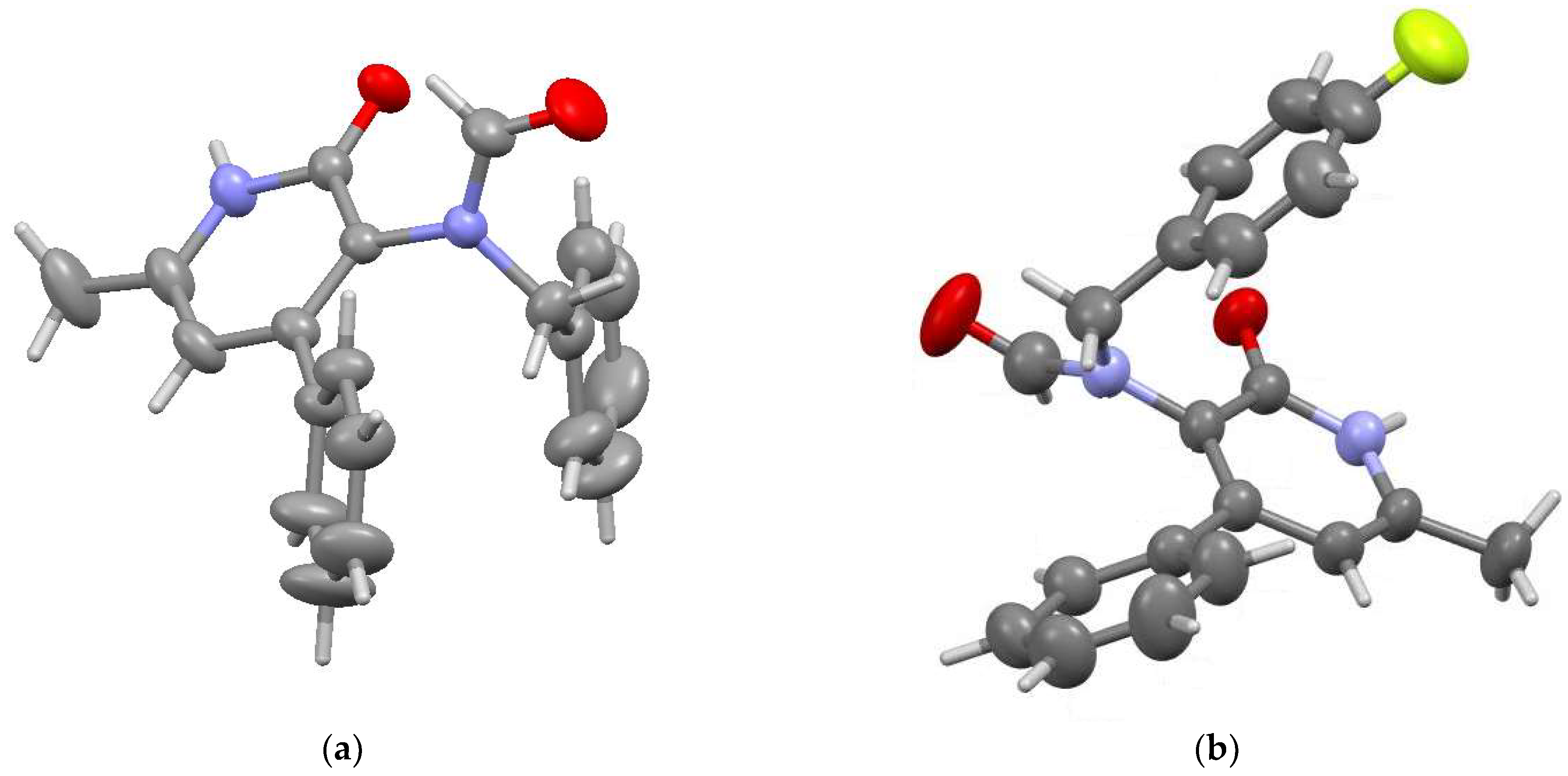
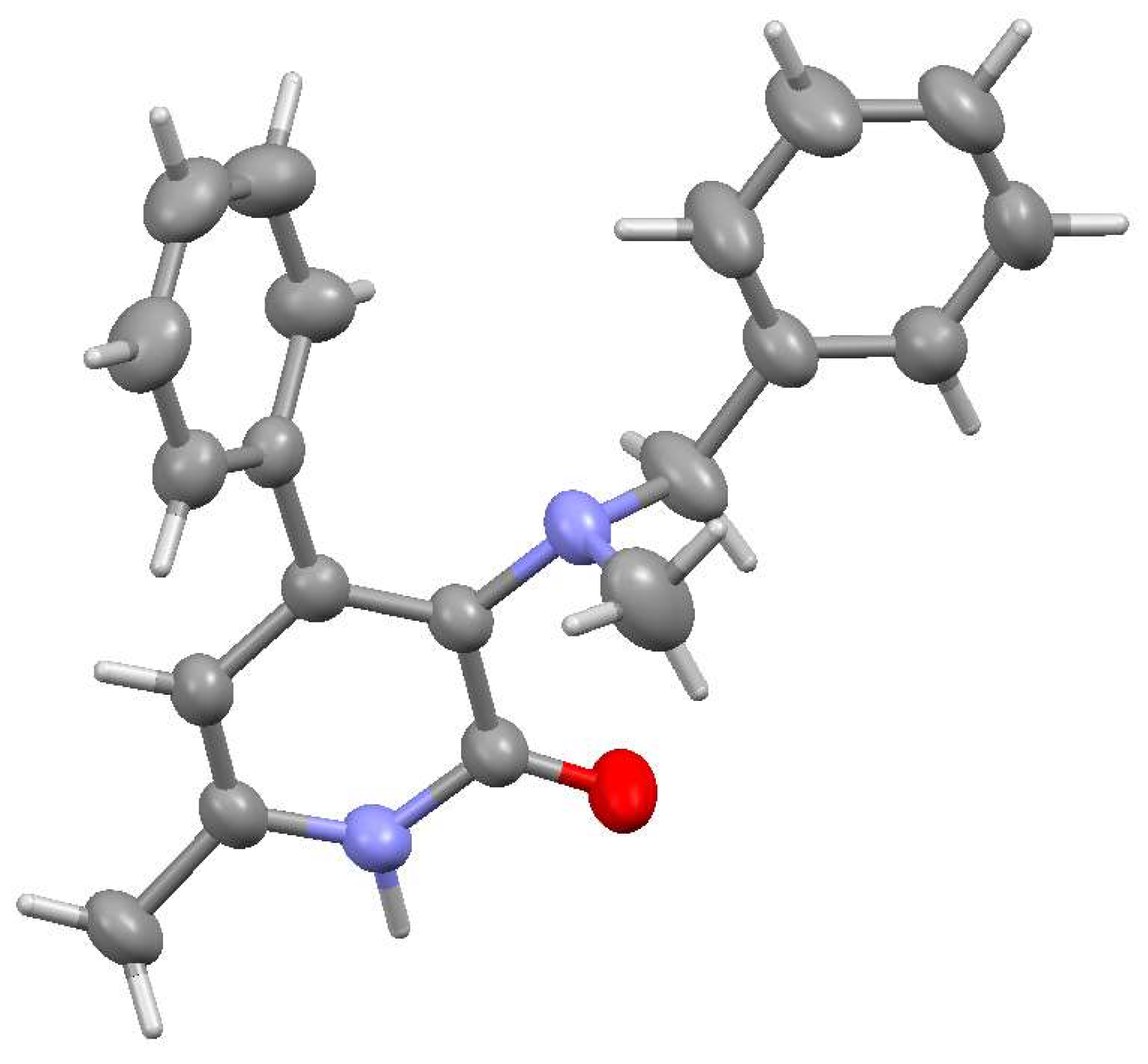
2.2. Structural Features of Compounds 6, 8a, 8c, 9a
2.3. Cytoprotective Activity Tests
3. Discussion
4. Materials and Methods
4.1. X-Ray Structural Study of Product
4.2. Synthesis of Compounds
4.2.1. 2-((Benzyl(6-methyl-2-oxo-4-phenyl-1,2-dihydropyridin-3-yl)amino)methyl)phenyl 2-Hydroxybenzoate 6
4.2.2. The General Procedure for Obtaining Formamide Derivatives 7a,b, 8a–d
4.2.3. N-(6-Methyl-2-oxo-4-phenyl-1,2-dihydropyridin-3-yl)formamide 7a
4.2.4. N-(6-Methyl-2-oxo-4-(thiophen-2-yl)-1,2-dihydropyridin-3-yl)formamide 7b
4.2.5. N-Benzyl-N-(6-methyl-2-oxo-4-phenyl-1,2-dihydropyridin-3-yl)formamide 8a
4.2.6. N-Benzyl-N-(6-methyl-2-oxo-4-(thiophen-2-yl)-1,2-dihydropyridin-3-yl)formamide 8b
4.2.7. N-(4-Fluorobenzyl)-N-(6-methyl-2-oxo-4-phenyl-1,2-dihydropyridin-3-yl)formamide 8c
4.2.8. N-(4-Fluorobenzyl)-N-(6-methyl-2-oxo-4-(thiophen-2-yl)-1,2-dihydropyridin-3-yl)formamide 8d
4.2.9. The General Procedure for the Preparation of 3-(Aryl(methyl)amino)-pyridin-2(1H)-ones 9a-d and 3-Dimethylaminopyridin-2(1H)-ones 10, 11
4.2.10. 3-(Benzyl(methyl)amino)-6-methyl-4-phenylpyridin-2(1H)-one 9a
4.2.11. 3-(Benzyl(methyl)amino)-6-methyl-4-(thiophen-2-yl)pyridin-2(1H)-one 9b
4.2.12. 3-((4-Fluorobenzyl)(methyl)amino)-6-methyl-4-phenylpyridin-2(1H)-one 9c
4.2.13. 3-((4-Fluorobenzyl)(methyl)amino)-6-methyl-4-(thiophen-2-yl)pyridin-2(1H)-one 9d
4.2.14. 3-(Dimethylamino)-6-methyl-4-phenylpyridin-2(1H)-one 10
4.2.15. 3-(Dimethylamino)-6-methyl-4-(thiophen-2-yl)pyridin-2(1H)-one 11
4.3. Data Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benhamú, B.; Martín-Fontecha, M.; Vázquez-Villa, H.; López-Rodríguez, M.L.; Ortega-Gutiérrez, S. New trends in aging drug discovery. Biomedicines 2022, 10, 2006. [Google Scholar] [CrossRef]
- Crimmins, E.M. Social Hallmarks of aging: Suggestions for geroscience research. Ageing Res. Rev. 2020, 63, 101136. [Google Scholar] [CrossRef]
- Quinn, C.; Mountain, G. Psychological and social factors associated with coexisting frailty and cognitive impairment: A systematic review. Res. Aging 2022, 44, 448–464. [Google Scholar] [CrossRef]
- Moskalev, A.; Guvatova, Z.; Lopes, I.D.; Beckett, C.W.; Kennedy, B.K.; De Magalhaes, J.P.; Makarov, A.A. Targeting aging mechanisms: Pharmacological perspectives. Trends Endocrinol. Metab. 2022, 33, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Juricic, P.; Lu, Y.-X.; Leech, T.; Drews, L.F.; Paulitz, J.; Lu, J.; Nespital, T.; Azami, S.; Regan, J.C.; Funk, E.; et al. Long-lasting geroprotection from brief rapamycin treatment in early adulthood by persistently increased intestinal autophagy. Nat. Aging 2022, 2, 824–836. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Anderson, R.M.; de Cabo, R. Can we make drug discovery targeting fundamental mechanisms of aging a reality? Expert Opin. Drug Discov. 2021, 17, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Kulakov, I.V.; Matsukevich, M.V.; Shulgau, Z.T.; Sergazy, S.; Seilkhanov, T.M.; Puzari, A.; Fisyuk, A.S. Synthesis and antiradical activity of 4-aryl(hetaryl)-substituted 3-aminopyridin-2(1H)-ones. Chem. Heterocycl. Compd. 2015, 51, 991–996. [Google Scholar] [CrossRef]
- Clement, M.V.; Luo, L. Organismal aging and oxidants beyond macromolecules damage. Proteomics 2020, 20, 1800400. [Google Scholar] [CrossRef]
- Warraich, U.-e-A.; Hussain, F.; Kayani, H.U. Aging-oxidative stress, Antioxidants and computational modeling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef]
- Kusakabe, K.I.; Tada, Y.; Iso, Y.; Sakagami, M.; Morioka, Y.; Chomei, N.; Shinonome, S.; Kawamoto, K.; Takenaka, H.; Yasui, K.; et al. Design, synthesis, and binding mode prediction of 2-pyridone-based selective CB2 receptor agonists. Bioorganic Med. Chem. 2013, 21, 2045–2055. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Fan, X.; Chakaravarty, D.; Xiang, B.; Scannevin, R.H.; Huang, Z.; Ma, J.; Burke, S.L.; Karnachi, P.; Rhodes, K.J.; et al. 1-Hydroxy-2-pyridinone-based MMP inhibitors: Synthesis and biological evaluation for the treatment of ischemic stroke. Bioorganic Med. Chem. Lett. 2008, 18, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Amrinone: A Preliminary Review of its Pharmacological Properties and Therapeutic Use. Drugs 1983, 26, 468–502. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Wai, J.S.; Thomas, C.M.; Levin, R.B.; O’Brien, J.A.; Goldman, M.E. Synthesis and Evaluation of 2-Pyridinone Derivatives as HIV-1 Specific Reverse Transcriptase Inhibitors. 1. Phthalimidoalkyl and -alkylamino Analogs. J. Med. Chem. 1992, 35, 3784–3791. [Google Scholar] [CrossRef]
- Saari, W.S.; Wai, J.S.; Fisher, T.E.; Thomas, C.M.; Hoffman, J.M.; Rooney, C.S.; Smith, A.M.; Jones, J.H.; Bamberger, D.L.; Goldman, M.E.; et al. Synthesis and Evaluation of 2-Pyridinone Derivatives as HIV-1-Specific Reverse Transcriptase Inhibitors. 2. Analogues of 3-Aminopyridin-2(1H)-one. J. Med. Chem. 1992, 35, 3792–3802. [Google Scholar] [CrossRef]
- Verissimo, E.; Berry, N.; Gibbons, P.; Cristiano, M.L.S.; Rosenthal, P.J.; Gut, J.; Ward, S.A.; O’Neill, P.M. Design and synthesis of novel 2-pyridone peptidomimetic falcipain 2/3 inhibitors. Bioorganic Med. Chem. Lett. 2008, 18, 4210–4214. [Google Scholar] [CrossRef]
- Zhu, S.; Hudson, T.H.; Kyle, D.E.; Lin, A.J. Synthesis and in vitro studies of novel pyrimidinyl peptidomimetics as potential antimalarial therapeutic agents. J. Med. Chem. 2002, 45, 3491–3496. [Google Scholar] [CrossRef]
- Sangwan, S.; Yadav, N.; Kumar, R.; Chauhan, S.; Dhanda, V.; Walia, P.; Duhan, A. A score years’ update in the synthesis and biological evaluation of medicinally important 2-pyridones. Eur. J. Med. Chem. 2022, 232, 114199. [Google Scholar] [CrossRef]
- Hurtado-Rodríguez, D.; Salinas-Torres, A.; Rojas, H.; Becerra, D.; Castillo, J.C. Bioactive 2-pyridone-containing heterocycle syntheses using multicomponent reactions. RSC Adv. 2022, 54, 34965–34983. [Google Scholar] [CrossRef] [PubMed]
- Kul’nevich, V.G.; Kaigorodova, E.A.; Arustamova, I.S.; Korobchenko, L.V.; Vladyko, G.V.; Boreko, E.I. Synthesis and anti-viral activity of N-alkyl-3-cyano-2-pyridones and 3-cyano-2-alkoxypyridines. Pharm Chem, J. 1990, 24, 132–135. [Google Scholar] [CrossRef]
- Amer, M.M.K.; Aziz, M.A.; Shehab, W.; Magda, H.; Abdellattif, M.H.; Mouneir, S.M. Recent advances in chemistry and pharmacological aspects of 2-pyridone scaffolds. J. Saudi Chem. Soc. 2011, 25, 101259. [Google Scholar] [CrossRef]
- Kulakov, I.V.; Palamarchuk, I.V.; Shulgau, Z.T.; Seilkhanov, T.M.; Gatilov, Y.V.; Fisyuk, A.S. Synthesis, structure and biological activity of 3-(arylmethyl)aminopyridine-2(1H) -ones and 1H-pyrido [2,3-b][1,4]oxazin-2(3H)-ones. J. Mol. Struct. 2018, 1166, 262–269. [Google Scholar] [CrossRef]
- Sergazy, S.; Shulgau, Z.; Zhulikeyeva, A.; Ramankulov, Y.; Palamarchuk, I.V.; Kulakov, I.V. Cytoprotective activity of newly synthesized 3-(arylmethylamino)-6-methyl-4-phenylpyridin-2(1H)-ones derivatives. Molecules 2022, 27, 5362. [Google Scholar] [CrossRef]
- Palamarchuk, I.V.; Shulgau, Z.T.; Kharitonova, M.A.; Kulakov, I.V. Synthesis and neurotropic activity of new 3-(arylmethyl)aminopyridine-2(1H)-one. Chem. Pap. 2021, 75, 4729–4739. [Google Scholar] [CrossRef]
- Palamarchuk, I.V.; Shulgau, Z.T.; Dautov, A.Y.; Sergazy, S.D.; Kulakov, I.V. Design, synthesis, spectroscopic characterization, computational analysis, and in in vitro α-amylase and α -glucosidase evaluation of 3-aminopyridin-2(1H)-one based novel monothiooxamides and 1,3,4-thiadiazoles. Org. Biomol. Chem. 2022, 20, 8962–8976. [Google Scholar] [CrossRef]
- Shulgau, Z.; Palamarchuk, I.V.; Sergazy, S.; Urazbayeva, A.; Ramankulov, Y.; Kulakov, I.V. Synthesis, Computational Study, and In Vitro α-Glucosidase Inhibitory Action of 1,3,4-Thiadiazole Derivatives of 3-Aminopyridin-2(1H)-ones. Pharmaceuticals 2024, 17, 377. [Google Scholar] [CrossRef]
- Shulgau, Z.; Palamarchuk, I.; Sergazy, S.; Urazbayeva, A.; Gulyayev, A.; Ramankulov, Y.; Kulakov, I. Synthesis, Computational Study, and In Vitro α-Glucosidase Inhibitory Action of Thiourea Derivatives Based on 3-Aminopyridin-2(1H)-Ones. Molecules 2024, 29, 3627. [Google Scholar] [CrossRef] [PubMed]
- Bratulescu, G. An Excellent Procedure for the Synthesis of Oxazolidin-2-ones. Synthesis 2007, 20, 3111–3112. [Google Scholar] [CrossRef]
- Kulakov, I.V.; Shatsauskas, A.L.; Matsukevich, M.V.; Palamarchuk, I.V.; Seilkhanov, T.M.; Gatilov, Y.V.; Fisyuk, A.S. A new approach to the synthesis of benzo[c][1,7]naphthyridine-4(3H)-ones. Synthesis 2017, 49, 3700–3709. [Google Scholar]
- Bach, R.D. Preparation of tertiary N,N-dimethylamines by the Leuckart reaction. J. Org. Chem. 1968, 33, 1647–1649. [Google Scholar] [CrossRef]
- Webers, V.J.; Bruce, W.F. The Leuckart reaction: A study of the mechanism. J. Am. Chem. Soc. 1948, 70, 1422–1424. [Google Scholar] [CrossRef]
- Umar, Q.; Luo, M. A Brief Review: Advancement in the Synthesis of Amine through the Leuckart Reaction. Reactions 2023, 4, 117–147. [Google Scholar] [CrossRef]
- Afanasyev, O.I.; Kuchuk, E.; Usanov, D.L.; Chusov, D. Reductive amination in the synthesis of pharmaceuticals. Chem. Rev. 2019, 119, 11857–11911. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, S.; Tsuchida, N.; Yamazaki, S. A density functional theory study of the hydride shift in the Eschweiler–Clarke reaction. J. Phys. Org. Chem. 2021, 34, e4253. [Google Scholar] [CrossRef]
- Biriukov, K.; Podyacheva, E.; Tarabrin, I.; Afanasyev, O.; Chusov, D. Simplified Version of the Eschweiler–Clarke Reaction. J. Org. Chem. 2024, 89, 3580–3584. [Google Scholar] [CrossRef]
- Ram, S.; Spicer, L.D. Debenzylation of N-Benzylamino Derivatives by Catalytic Transfer Hydrtyation With Ammonium Formate. Synth. Commun. 1987, 17, 415–418. [Google Scholar] [CrossRef]
- Zhu, J.; Li, G.; Zhou, J.; Xu, Z.; Xu, J. Cytoprotective effects and antioxidant activities of acteoside and various extracts of Clerodendrum cyrtophyllum Turcz leaves against t-BHP induced oxidative damage. Sci. Rep. 2022, 12, 12630. [Google Scholar] [CrossRef]
- Xu, J.; Elshazly, A.M.; Gewirtz, D.A. The Cytoprotective, Cytotoxic and Nonprotective Functional Forms of Autophagy Induced by Microtubule Poisons in Tumor Cells-Implications for Autophagy Modulation as a Therapeutic Strategy. Biomedicines 2022, 10, 1632. [Google Scholar] [CrossRef]
- Burnett, C.; Valentini, S.; Cabreiro, F.; Goss, M.; Somogyvári, M.; Piper, M.D.; Hoddinott, M.; Sutphin, G.L.; Leko, V.; McElwee, J.J.; et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature 2011, 477, 482–485. [Google Scholar] [CrossRef]
- Valenzano, D.R.; Terzibasi, E.; Genade, T.; Cattaneo, A.; Domenici, L.; Cellerino, A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006, 16, 296–300. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]




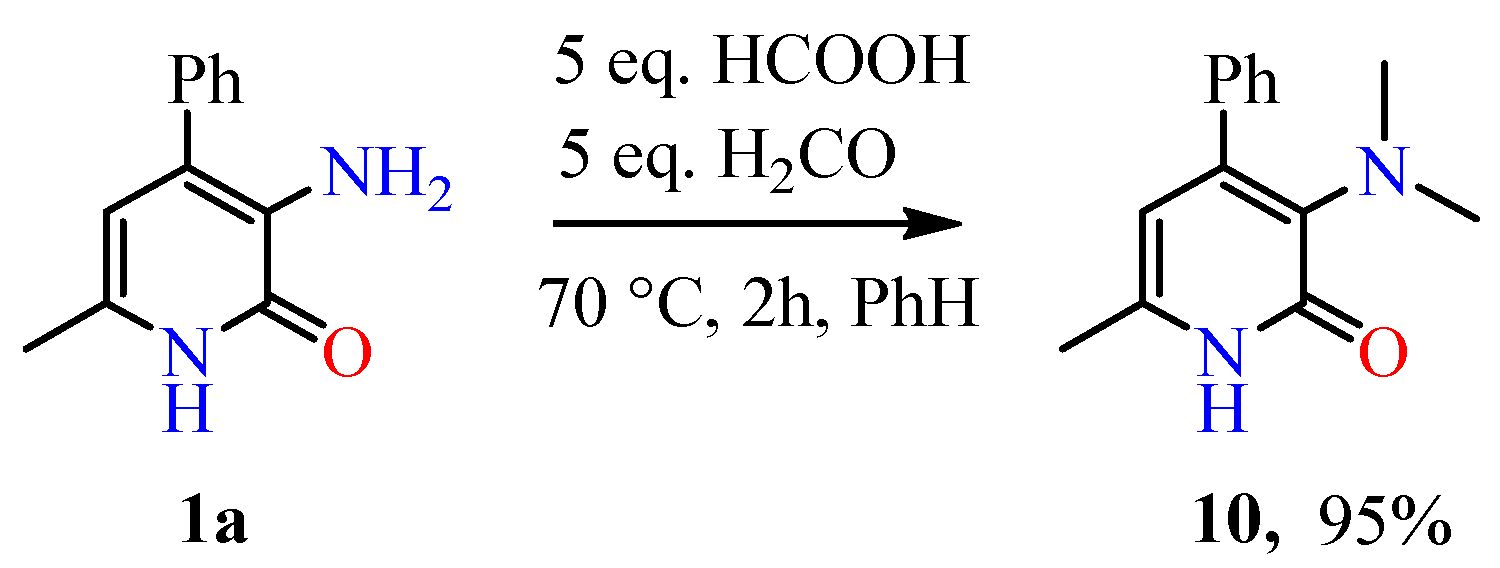


| No. | Compound | Sample Concentration, mM | The Average Values of Cell Survival (in % of Control) | |
|---|---|---|---|---|
| 1 | 7a | 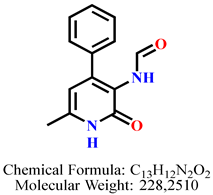 | 100 mM | 118.0 ± 3.5 |
| 50 mM | 106.8 ± 4.3 | |||
| 25 mM | 100.5 ± 1.6 | |||
| 2 | 7b | 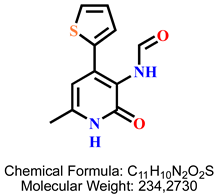 | 100 mM | 1308.6 ± 114.1 |
| 50 mM | 764.9 ± 70.7 | |||
| 25 mM | 378.4 ± 24.4 | |||
| 3 | 8a | 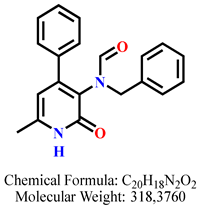 | 100 mM | 406.3 ± 55.8 |
| 50 mM | 180.6 ± 10.0 | |||
| 25 mM | 133.3 ± 3.9 | |||
| 4 | 8b | 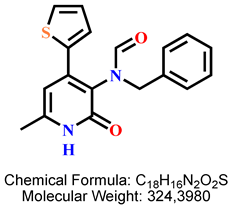 | 100 mM | 1648.6 ± 179.5 |
| 50 mM | 136.0 ± 15.3 | |||
| 25 mM | 112.2 ± 6.8 | |||
| 5 | 8c | 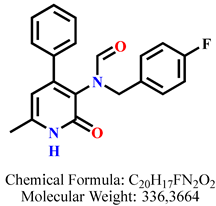 | 100 mM | 179.9 ± 20.2 |
| 50 mM | 118.5 ± 4.7 | |||
| 25 mM | 116.2 ± 9.6 | |||
| 6 | 8d | 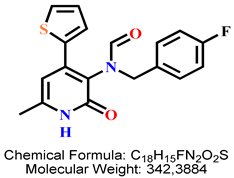 | 100 mM | 2652.3 ± 134.3 |
| 50 mM | 191.9 ± 22.1 | |||
| 25 mM | 140.5 ± 10.3 | |||
| 7 | 9a | 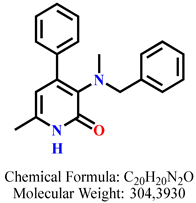 | 100 mM | 112.6 ± 16.1 |
| 50 mM | 108.1 ± 3.4 | |||
| 25 mM | 108.6 ± 3.2 | |||
| 8 | 9b | 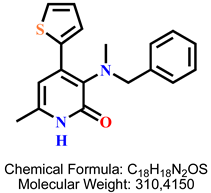 | 100 mM | 498.2 ± 45.8 |
| 50 mM | 197.3 ± 18.2 | |||
| 25 mM | 128.4 ± 2.1 | |||
| 9 | 9c | 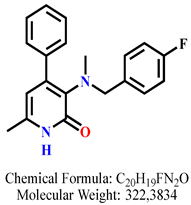 | 100 mM | 573.9 ± 58.8 |
| 50 mM | 155.9 ± 16.1 | |||
| 25 mM | 99.5 ± 2.0 | |||
| 10 | 9d | 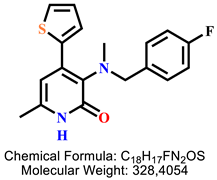 | 100 mM | 209.0 ± 8.9 |
| 50 mM | 173.0 ± 9.0 | |||
| 25 mM | 99.5 ± 5.9 | |||
| Control | 100.0 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shulgau, Z.; Palamarchuk, I.; Dezhko, E.; Sergazy, S.; Urazbayeva, A.; Safarova, Y.; Gulyayev, A.; Gatilov, Y.; Kulakov, I. Synthesis, Structure and Cytoprotective Activity of New Derivatives of 4-Aryl-3-Aminopyridin-2(1H)-One. Molecules 2025, 30, 3331. https://doi.org/10.3390/molecules30163331
Shulgau Z, Palamarchuk I, Dezhko E, Sergazy S, Urazbayeva A, Safarova Y, Gulyayev A, Gatilov Y, Kulakov I. Synthesis, Structure and Cytoprotective Activity of New Derivatives of 4-Aryl-3-Aminopyridin-2(1H)-One. Molecules. 2025; 30(16):3331. https://doi.org/10.3390/molecules30163331
Chicago/Turabian StyleShulgau, Zarina, Irina Palamarchuk, Egor Dezhko, Shynggys Sergazy, Assel Urazbayeva, Yuliya Safarova, Alexander Gulyayev, Yuri Gatilov, and Ivan Kulakov. 2025. "Synthesis, Structure and Cytoprotective Activity of New Derivatives of 4-Aryl-3-Aminopyridin-2(1H)-One" Molecules 30, no. 16: 3331. https://doi.org/10.3390/molecules30163331
APA StyleShulgau, Z., Palamarchuk, I., Dezhko, E., Sergazy, S., Urazbayeva, A., Safarova, Y., Gulyayev, A., Gatilov, Y., & Kulakov, I. (2025). Synthesis, Structure and Cytoprotective Activity of New Derivatives of 4-Aryl-3-Aminopyridin-2(1H)-One. Molecules, 30(16), 3331. https://doi.org/10.3390/molecules30163331





.JPG)


