Roupala montana Aubl. Essential Oil: Chemical Composition and Emerging Biological Activities
Abstract
1. Introduction
2. Results
2.1. Isolation of Essential Oil
2.2. Chemical Constituents of Essential Oil
2.3. Enantiomeric Composition
2.4. Antifungal Activity
2.5. Antibacterial Activity
2.6. Acetylcholinesterase Inhibitory Activity
2.7. Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Materials and Chemical Reagents
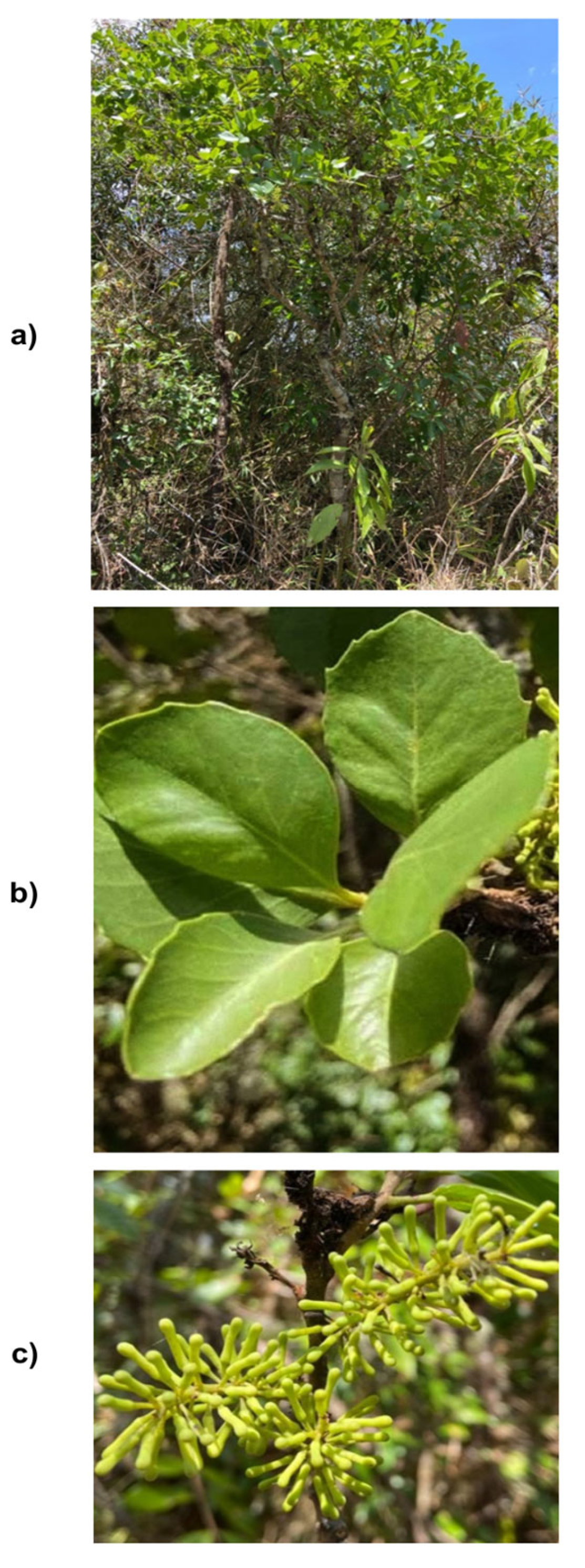
4.2. Plant Material
4.3. Post-Harvest Processing
4.4. Isolation of Essential Oil
4.5. Chemical Characterization of Essential Oil
4.5.1. Sample Preparation
4.5.2. Qualitative and Quantitative Analysis
4.5.3. Enantiomeric Analysis
4.6. Biological Activities
4.6.1. Antimicrobial Activity
4.6.2. Anticholinesterase Activity
4.6.3. Antioxidant Activity
ABTS
DPPH
4.7. Statistical Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valarezo, E.; Paucar-Costa, J.; Morales-Guamán, B.; Caraguay-Martínez, A.; Jaramillo-Fierro, X.; Cumbicus, N.; Meneses, M.A. Chemical and Biological Study of the Essential Oil Isolated from Fruits of Citrus x limonia. Plants 2025, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.L.; Pavić, V.; Jakovljević, M.; Molnar, M.; Jokić, S. Antimicrobial, Antioxidant, and Anti-Inflammatory Activities of Essential Oils from Five Selected Herbs. Antioxidants 2021, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Leal, A.C.; Palou, E.; López-Malo, A.; Bach, H. Antimicrobial, Cytotoxic, and Anti-Inflammatory Activities of Pimenta dioica and Rosmarinus officinalis Essential Oils. Biomed. Res. Int. 2019, 2019, 1639726. [Google Scholar] [CrossRef] [PubMed]
- Christenhusz, M.J.; Byng, J.W. The Number of Known Plant Species in the World and Its Annual Increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Ulloa Ulloa, C.; Moller Jorgensen, P. Árboles y arbustos de los Andes del Ecuador. Ediciones ABYA-YALA: Quito, Ecuador, 1995. [Google Scholar]
- Miranda-Melo, A.D.A.; Martins, F.R.; Santos, F.A.M.D. Estructura populacional de Xylopia aromatica (Lam.) Mart. e de Roupala montana Aubl. em fragmentos de cerrado no Estado de São Paulo. Braz. J. Bot. 2007, 30, 501–507. [Google Scholar] [CrossRef]
- Calderón de Rzedowski, G. Proteaceae. Flora Del Bajío Y De Reg. Adyac. 2006, 143, 7. [Google Scholar]
- Francielli de Oliveira, P.; Acésio, N.O.; Leandro, L.F.; Cunha, N.L.; Uchôa, C.J.D.M.; Januário, A.H.; Tavares, D.C. Antigenotoxicity of Roupala montana Extract in the Mouse Micronucleus and Comet Assays. Drug Chem. Toxicol. 2014, 37, 93–99. [Google Scholar] [CrossRef]
- Medina, J.C.; Suárez, A.I.; Cumbicus, N.; Morocho, V. Estudio fitoquímico de Roupala montana Aubl. de la provincia de Loja. Axioma 2018, 19, 5–11. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Calva, J.; Cartuche, L.; González, S.; Montesinos, J.V.; Morocho, V. Chemical Composition, Enantiomeric Analysis and Anticholinesterase Activity of Lepechinia betonicifolia Essential Oil from Ecuador. Pharm. Biol. 2022, 60, 206–211. [Google Scholar] [CrossRef]
- Valarezo, E.; Jaramillo-Jaramillo, E.; Carrión-Campoverde, A.; Morocho, V.; Jaramillo-Fierro, X.; Cartuche, L.; Meneses, M.A. A Study of the Essential Oil Isolated from Ageratina dendroides (Spreng.) R.M. King & H. Rob.: Chemical Composition, Enantiomeric Distribution, and Antimicrobial, Antioxidant, and Anticholinesterase Activities. Plants 2023, 12, 2796. [Google Scholar] [CrossRef]
- Moncayo-Molina, L.; Pino, J.A.; Spengler, I.; Moncayo-Rivera, C.M.; Rojas-Molina, J.O. Chemical Composition and Biological Activities of Essential Oil from Oreocallis grandiflora. Chem. Nat. Compd. 2024, 60, 962–964. [Google Scholar] [CrossRef]
- Cunha, N.L.; Uchôa, C.J.D.M.; Cintra, L.S.; Souza, H.C.D.; Peixoto, J.A.; Silva, C.P.; Magalhães, L.G.; Meleiro, G.V.M.; Groppo, M.; Rodrigues, V.; et al. In Vitro Schistosomicidal Activity of Some Brazilian Cerrado Species and Their Isolated Compounds. Evid.-Based Complement. Altern. Med. 2012, 2012, 173614. [Google Scholar] [CrossRef] [PubMed]
- Haleema, S.; Sasi, P.V.; Ibnusaud, I.; Polavarapu, P.L.; Kagan, H.B. Enantiomerically Pure Compounds Related to Chiral Hydroxy Acids Derived from Renewable Resources. RSC Adv. 2012, 2, 9257–9285. [Google Scholar] [CrossRef]
- Bernés, S.; Rivadeneyra, M.S. Una Aplicación de la Técnica de Difracción de Rayos X en la Industria Farmacéutica. Rev. SPINOR 2024, 54. [Google Scholar]
- Toledo, M.V.; José, C.; Briand, L.E. Esterificación enzimática de antiinflamatorios no esteroideos con glicerol. Cienc. Apl. 2019, 2. [Google Scholar]
- Varón, C.S.; Ramírez, C.C.; Quintero, D.V. Síntesis orgánica de la warfarina racémica por adición de un α-β insaturado. Biociencias (UNAD) 2024, 8, 99–109. [Google Scholar] [CrossRef]
- Kurdelas, R.R.; López, S.; Lima, B.; Feresin, G.E.; Zygadlo, J.; Zacchino, S.; López, M.L.; Tapia, A.; Freile, M.L. Chemical Composition, Anti-Insect and Antimicrobial Activity of Baccharis darwinii Essential Oil from Argentina, Patagonia. Ind. Crops Prod. 2012, 40, 261–267. [Google Scholar] [CrossRef]
- Gadea, A.; Khazem, M.; Gaslonde, T. Current Knowledge on Chemistry of Proteaceae Family, and Biological Activities of Their Bis-5-Alkylresorcinol Derivatives. Phytochem. Rev. 2022, 21, 1969–2005. [Google Scholar] [CrossRef]
- Almeida-Bezerra, J.W.; Menezes, S.A.; Silva, J.T.d.C.; de Sousa, S.G.; Alves, D.S.; Alencar, G.G.; Araújo, I.M.; Rodrigues, E.Y.d.S.; Oliveira-Tintino, C.D.d.M.; da Cruz, R.P.; et al. Analysis of the Antibiotic-Potentiating Activity, Absorption, Distribution, Metabolism, and Excretion (ADME) and the Molecular Docking Properties of Phytol Against Multi-Drug-Resistant (MDR) Strains. Antibiotics 2024, 13, 1171. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A Review of Biomedical Activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Jeong, S.H. Inhibitory Effect of Phytol on Cellular Senescence. Biomed. Dermatol. 2018, 2, 13. [Google Scholar] [CrossRef]
- Fassina Brocco, V.; Gonçalves da Costa, L.; Monteiro de Castro, M.C.; Xavier Barbosa, A.V.; da Costa Lyra, P.H.; Alves Cruz da Conceição, R.C. Antifungal and Antitermitic Potential of Extracts of Industrial Wood Waste from Central Amazon, Brazil. Maderas. Cienc. Tecnol. 2025, 27, e0852. [Google Scholar] [CrossRef]
- Lima, M.; de Sousa Rodrigues, D.R.; de Oliveira Santiago, W.; dos Santos, H.C.; do Nascimento, C.C.; de Souza, J.V.B.; de Souza, A.C.A.C.; Bezerra Jensen, B.; Comandolli-Wyrepkowski, C.D.; Ramos Franco Pereira, A.M. Identification of 5-Alkylresorcinols from Roupala montana Aubl. Wood Residues and Evaluation of Their Leishmanicidal and Antifungal Activities. Figshare 2021. [Google Scholar] [CrossRef]
- Violante, I.M.P.; Hamerski, L.; Garcez, W.S.; Batista, A.L.; Chang, M.R.; Pott, V.J.; Garcez, F.R. Antimicrobial Activity of Some Medicinal Plants from the Cerrado of the Central-Western Region of Brazil. Braz. J. Microbiol. 2012, 43, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, J.K.; Rasmussen, H.B.; Willis, A.C. A New Glycoside Antimicrobial Agent from Persoonia linearis × pinifolia. J. Nat. Prod. 1997, 60, 620–622. [Google Scholar] [CrossRef]
- da Silva Sperandio, F.; da Rocha, J.R.; Spinelli, B.S.; Lisita, K.; Rodriguez, A.F.R.; Maggi, L.E. Atividade antifúngica de extratos de plantas medicinais frente a Cryptococcus neoformans: Revisão sistemática. Multidiscip. Sci. Rep. 2024, 4, 1–18. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Sathya, S.; Manogari, B.G.; Thamaraiselvi, K.; Vaidevi, S.; Ruckmani, K.; Devi, K.P. Phytol Loaded PLGA Nanoparticles Ameliorate Scopolamine-Induced Cognitive Dysfunction by Attenuating Cholinesterase Activity, Oxidative Stress and Apoptosis in Wistar Rat. Nutr. Neurosci. 2020, 25, 485–501. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Mosaddegh, M.; Naghibi, F.; Haeri, A.; Hamzeloo-Moghadam, M. Natural Sesquiterpen Lactones as Acetylcholinesterase Inhibitors. An. Acad. Bras. Cienc. 2014, 86, 801–806. [Google Scholar] [CrossRef][Green Version]
- Sülsen, V.P. Sesquiterpene Lactones and Diterpenes: Promising Therapeutic Candidates for Infectious Diseases, Neoplasms and Other Chronic Disorders. Molecules 2021, 26, 1251. [Google Scholar] [CrossRef] [PubMed]
- Calvopiña, K.; Malagón, O.; Capetti, F.; Sgorbini, B.; Verdugo, V.; Gilardoni, G. A New Sesquiterpene Essential Oil from the Native Andean Species Jungia rugosa Less (Asteraceae): Chemical Analysis, Enantiomeric Evaluation, and Cholinergic Activity. Plants 2021, 10, 2102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.-F.; Wang, Y.; Li, F.; Zhu, D. Visual Evaluation of Acetylcholinesterase Inhibition by an Easy-to-Operate Assay Based on N-Doped Carbon Nanozyme with High Stability and Oxidase-Like Activity. J. Mater. Chem. B 2023, 11, 4014–4019. [Google Scholar] [CrossRef] [PubMed]
- Vinueza, D.; Yanza, K.; Tacchini, M.; Grandini, A.; Sacchetti, G.; Chiurato, M.A.; Guerrini, A. Flavonoids in Ecuadorian Oreocallis grandiflora (Lam.) R. Br.: Perspectives of Use of This Species as a Food Supplement. Evid.-Based Complement. Altern. Med. 2018, 2018, 1353129. [Google Scholar] [CrossRef]
- Zhang, J.; Netzel, M.E.; Pengelly, A.; Sivakumar, D.; Sultanbawa, Y. A Review of Phytochemicals and Bioactive Properties in the Proteaceae Family: A Promising Source of Functional Food. Antioxidants 2023, 12, 1952. [Google Scholar] [CrossRef]
- Delgado, M.; Zúñiga-Feest, A.; Reyes-Díaz, M.; Barra, P.J.; Ruiz, S.; Bertin-Benavides, A.; Valle, S.; Pereira, M.; Lambers, H. Ecophysiological Performance of Proteaceae Species from Southern South America Growing on Substrates Derived from Young Volcanic Materials. Front. Plant Sci. 2021, 12, 636056. [Google Scholar] [CrossRef]
- Montalván, M.; Peñafiel, M.A.; Ramírez, J.; Cumbicus, N.; Bec, N.; Larroque, C.; Bicchi, C.; Gilardoni, G. Chemical Composition, Enantiomeric Distribution, and Sensory Evaluation of the Essential Oils Distilled from the Ecuadorian Species Myrcianthes myrsinoides (Kunth) Grifo and Myrcia mollis (Kunth) DC. (Myrtaceae). Plants 2019, 8, 511. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Cartuche, L.; Calva, J.; Valarezo, E.; Chuchuca, N.; Morocho, V. Chemical and Biological Activity Profiling of Hedyosmum strigosum Todzia Essential Oil, an Aromatic Native Shrub from Southern Ecuador. Plants 2022, 11, 2832. [Google Scholar] [CrossRef]
- Andrade, J.M.; Pachar, P.; Trujillo, L.; Cartuche, L. Suillin: A Mixed-Type Acetylcholinesterase Inhibitor from Suillus luteus Which Is Used by Saraguros Indigenous, Southern Ecuador. PLoS ONE 2022, 17, e0268292. [Google Scholar] [CrossRef]
- Valarezo, E.; Ludeña, J.; Echeverria-Coronel, E.; Cartuche, L.; Meneses, M.A.; Calva, J.; Morocho, V. Enantiomeric Composition, Antioxidant Capacity and Anticholinesterase Activity of Essential Oil from Leaves of Chirimoya (Annona cherimola Mill.). Plants 2022, 11, 367. [Google Scholar] [CrossRef]
- Valarezo, E.; Gaona-Granda, G.; Morocho, V.; Cartuche, L.; Calva, J.; Meneses, M.A. Chemical Constituents of the Essential Oil from Ecuadorian Endemic Species Croton ferrugineus and Its Antimicrobial, Antioxidant and α-Glucosidase Inhibitory Activity. Molecules 2021, 26, 4608. [Google Scholar] [CrossRef]

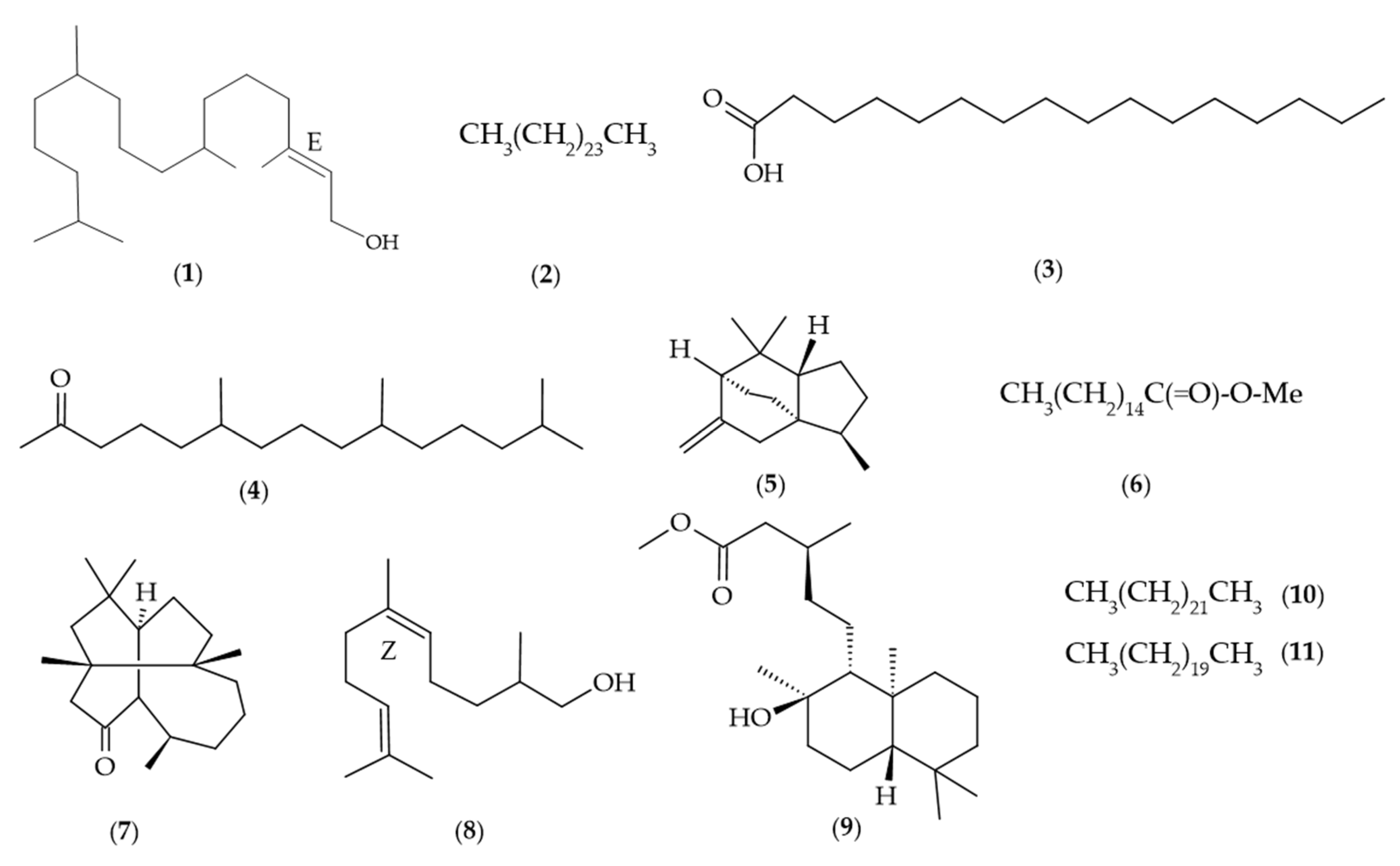


| No. | tRT | Compound | Molecular Formula | LRIa | LRIb | % ± SD |
|---|---|---|---|---|---|---|
| 1 | 25.17 | n-decanol | C10H22O | 1274 | 1266 | 0.62 ± 0.03 |
| 2 | 31.86 | β-duprezianene | C15H24 | 1426 | 1421 | 3.16 ± 0.16 |
| 3 | 33.12 | (2E)-dodecenal | C12H22O | 1457 | 1464 | 0.35 ± 0.02 |
| 4 | 33.41 | α-humulene | C15H24 | 1463 | 1452 | 0.70 ± 0.04 |
| 5 | 33.58 | 9-epi-(E)-caryophyllene | C15H24 | 1467 | 1464 | 1.31 ± 0.06 |
| 6 | 34.84 | α-muurolene | C15H24 | 1498 | 1500 | 0.68 ± 0.04 |
| 7 | 35.04 | n-pentadecane | C15H32 | 1503 | 1500 | 0.22 ± 0.01 |
| 8 | 35.94 | δ-cadinene | C15H24 | 1525 | 1522 | 0.42 ± 0.02 |
| 9 | 37.62 | (E)-nerolidol | C15H26O | 1568 | 1561 | 1.67 ± 0.07 |
| 10 | 37.96 | (Z)-dihydro-apofarnesol | C14H26O | 1576 | 1571 | 2.52 ± 0.10 |
| 11 | 38.76 | caryophyllene oxide | C15H24O | 1597 | 1582 | 0.34 ± 0.02 |
| 12 | 38.87 | n-hexadecane | C16H32 | 1600 | 1600 | 1.20 ± 0.05 |
| 13 | 39.26 | 2,(7Z)-bisaboladien-4-ol | C15H26O | 1610 | 1618 | 0.90 ± 0.04 |
| 14 | 39.62 | cis-isolongifolanone | C15H24O | 1620 | 1612 | 0.47 ± 0.02 |
| 15 | 39.86 | junenol | C15H26O | 1626 | 1618 | 0.41 ± 0.02 |
| 16 | 40.38 | cubenol | C15H26O | 1640 | 1645 | 0.59 ± 0.03 |
| 17 | 41.81 | n-tetradecanol | C14H30O | 1678 | 1671 | 0.62 ± 0.03 |
| 18 | 42.01 | elemol acetate | C17H28O2 | 1683 | 1680 | tr |
| 19 | 42.12 | 4-cuprenen-1-ol | C15H24O | 1686 | 1692 | 0.40 ± 0.01 |
| 20 | 42.55 | n-heptadecane | C17H36 | 1698 | 1700 | 0.25 ± 0.01 |
| 21 | 42.84 | 2-pentadecanone | C15H30O | 1705 | 1697 | 0.79 ± 0.03 |
| 22 | 43.48 | (2Z,6E)-farnesol | C15H26O | 1724 | 1722 | 0.71 ± 0.03 |
| 23 | 43.58 | (2E,6Z)-farnesol | C15H26O | 1726 | 1714 | tr |
| 24 | 43.71 | methyl tetradecanoate | C15H30O2 | 1730 | 1722 | 0.75 ± 0.02 |
| 25 | 44.63 | (2E,6E)-farnesol | C15H26O | 1756 | 1742 | 0.55 ± 0.02 |
| 26 | 45.31 | butyl dodecanoate | C16H32O2 | 1775 | 1786 | 1.72 ± 0.09 |
| 27 | 45.93 | β-eudesmol acetate | C17H28O2 | 1792 | 1792 | 0.08 ± 0.01 |
| 28 | 46.11 | n-octadecane | C18H38 | 1797 | 1800 | 0.67 ± 0.03 |
| 29 | 47.77 | hexahydrofarnesyl acetone | C18H36O | 1843 | 1843 | 6.28 ± 0.26 |
| 30 | 48.77 | cis-thujopsenic acid | C15H22O2 | 1871 | 1863 | 0.23 ± 0.00 |
| 31 | 49.24 | n-hexadecanol | C16H34O | 1889 | 1874 | 0.80 ± 0.02 |
| 32 | 49.50 | n-nonadecane | C19H40 | 1897 | 1900 | 0.42 ± 0.02 |
| 33 | 50.21 | (5E-9E)-farnesyl acetone | C18H30O | 1919 | 1913 | 1.25 ± 0.03 |
| 34 | 50.60 | methyl hexadecanoate | C17H34O2 | 1931 | 1921 | 2.96 ± 0.12 |
| 35 | 51.14 | isophytol | C20H40O | 1948 | 1946 | 1.29 ± 0.00 |
| 36 | 51.24 | (Z,Z)-geranyl linalool | C20H43O | 1951 | 1960 | 0.69 ± 0.01 |
| 37 | 52.12 | hexadecanoic acid | C16H32O2 | 1978 | 1959 | 8.30 ± 0.77 |
| 38 | 52.76 | ethyl hexadecanoate | C18H36O2 | 1997 | 1992 | 1.86 ± 1.41 |
| 39 | 53.57 | 1-eicosene | C20H40 | 2024 | 1988 | 0.13 ± 0.00 |
| 40 | 53.74 | (E,E)-geranyl linalool | C20H34O | 2029 | 2026 | 0.53 ± 0.02 |
| 41 | 55.93 | n-heneicosane | C21H44 | 2100 | 2100 | 2.00 ± 0.00 |
| 42 | 56.14 | laurenan-2-one | C20H32O | 2107 | 2115 | 2.68 ± 0.03 |
| 43 | 56.26 | NI | - | 2111 | - | tr |
| 44 | 56.40 | phytol | C20H40O | 2116 | 2116 | 21.17 ± 1.59 |
| 45 | 56.87 | incensole | C20H34O2 | 2132 | 2158 | tr |
| 46 | 57.35 | 1-docosene | C22H44 | 2148 | 2189 | 0.71 ± 0.40 |
| 47 | 57.47 | nezukol | C20H34O | 2152 | 2132 | 0.61 ± 0.79 |
| 48 | 57.68 | linoleic acid | C18H32O2 | 2159 | 2132 | 1.09 ± 1.20 |
| 49 | 57.91 | sandaracopimarinal | C20H30O | 2167 | 2189 | 1.09 ± 0.76 |
| 50 | 58.11 | ugandensidial (cinnamodial) | C17H24O5 | 2173 | 2198 | 1.80 ± 1.22 |
| 51 | 58.53 | incensole acetate | C22H36O3 | 2188 | 2184 | 0.40 ± 0.28 |
| 52 | 58.68 | oleic acid | C18H34O2 | 2193 | 2175 | 0.63 ± 0.12 |
| 53 | 58.80 | n-docosane | C22H46 | 2197 | 2200 | 1.25 ± 0.55 |
| 54 | 59.35 | 7-α-hydroxy-manool | C20H34O2 | 2215 | 2237 | 1.31 ± 1.50 |
| 55 | 61.63 | n-tricosane | C23H48 | 2290 | 2300 | 2.16 ± 0.00 |
| 56 | 63.46 | 3-α-acetoxy-manool | C22H36O3 | 2350 | 2359 | 0.43 ± 0.44 |
| 57 | 64.51 | methyl labdanolate | C21H38O3 | 2385 | 2381 | 2.46 ± 0.01 |
| 58 | 64.63 | n-tetracosane | C24H50 | 2389 | 2400 | 1.66 ± 0.01 |
| 59 | 66.13 | NI | - | 2438 | - | 2.26 ± 0.90 |
| 60 | 68.42 | n-pentacosane | C25H52 | 2514 | 2500 | 9.08 ± 0.04 |
| Oxygenated monoterpenes (%) | 0.62 | |||||
| Oxygenated sesquiterpenes (%) | 7.81 | |||||
| Sesquiterpenes hydrocarbons (%) | 6.49 | |||||
| Diterpene hydrocarbons | 0.13 | |||||
| Oxygenated diterpenes (%) | 29.37 | |||||
| Other compounds (%) | 55.21 | |||||
| Total (%) | 99.63 |
| Enantiomer | LRIa | LRIb | ED (%) | e.e. (%) |
|---|---|---|---|---|
| (S)-(+)-γ-muurolene | 1471 | 1466 | 97.53 | 95.07 |
| (R)-(−) γ-muurolene | 1474 | 1473 | 2.47 | |
| (1S,4aR,8aR)-(−)-γ -cadinene | 1529 | 1526 | 100.00 | 100.00 |
| Microorganism | Roupala montana | Positive Control * | Negative Control |
|---|---|---|---|
| MIC (µg/mL) | |||
| Yeasts | |||
| Candida albicans (ATTC 10231) | - | 0.098 | + |
| Fungi | |||
| Aspergillus niger (ATCC 6275) | 1000 | 0.098 | + |
| Microorganism | Roupala montana | Positive Control * | Negative Control |
|---|---|---|---|
| MIC (µg/mL) | |||
| Gram-positive cocci | |||
| Enterococcus faecalis (ATCC 19433) | - | 0.78 | + |
| Enterococcus faecium (ATCC 27270) | - | 0.39 | + |
| Staphylococcus aureus (ATCC 25923) | - | 0.39 | + |
| Gram-positive bacillus | |||
| Lysteria monocytogenes (ATTC 19115) | - | 1.56 | + |
| Gram-negative bacilli | |||
| Escherichia coli O157:H7 (ATCC 43888) | - | 1.5600 | + |
| Campylobacter jejuni (ATCC 33560) | 500 | 15.65 | + |
| Salmonella enterica subs enterica serovar Thypimurium WDCM 00031, derived (ATCC 14028) | - | 0.39 | + |
| EO | ABTS | DPPH |
|---|---|---|
| SC50 (µg/mL—µM *) ± SD | ||
| R. montana | >8000 | - |
| Trolox | 29.09 ± 1.05 | 35.54 ± 1.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartuche, L.; Guayllas-Avila, M.; Castillo, L.N.; Morocho, V. Roupala montana Aubl. Essential Oil: Chemical Composition and Emerging Biological Activities. Molecules 2025, 30, 3323. https://doi.org/10.3390/molecules30163323
Cartuche L, Guayllas-Avila M, Castillo LN, Morocho V. Roupala montana Aubl. Essential Oil: Chemical Composition and Emerging Biological Activities. Molecules. 2025; 30(16):3323. https://doi.org/10.3390/molecules30163323
Chicago/Turabian StyleCartuche, Luis, Mireya Guayllas-Avila, Leydy Nathaly Castillo, and Vladimir Morocho. 2025. "Roupala montana Aubl. Essential Oil: Chemical Composition and Emerging Biological Activities" Molecules 30, no. 16: 3323. https://doi.org/10.3390/molecules30163323
APA StyleCartuche, L., Guayllas-Avila, M., Castillo, L. N., & Morocho, V. (2025). Roupala montana Aubl. Essential Oil: Chemical Composition and Emerging Biological Activities. Molecules, 30(16), 3323. https://doi.org/10.3390/molecules30163323










