Doxorubicin Toxicity and Recent Approaches to Alleviating Its Adverse Effects with Focus on Oxidative Stress
Abstract
1. Introduction
2. Search Strategy and Methodology
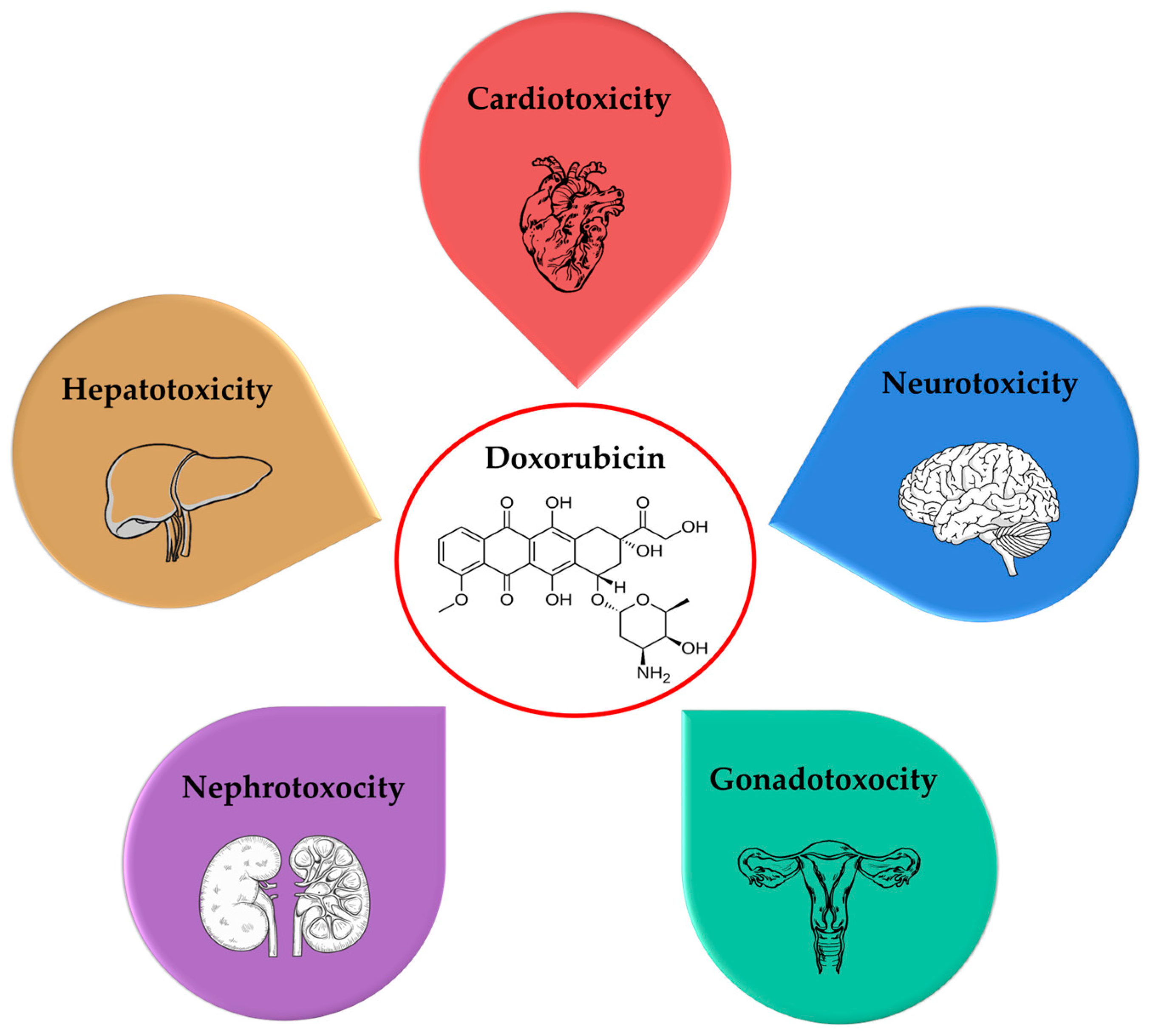
3. Toxicity of Doxorubicin
3.1. Cardiotoxicity
3.2. Neurotoxicity
3.3. Hepatotoxicity
3.4. Nephrotoxicity
3.5. Gonadotoxicity
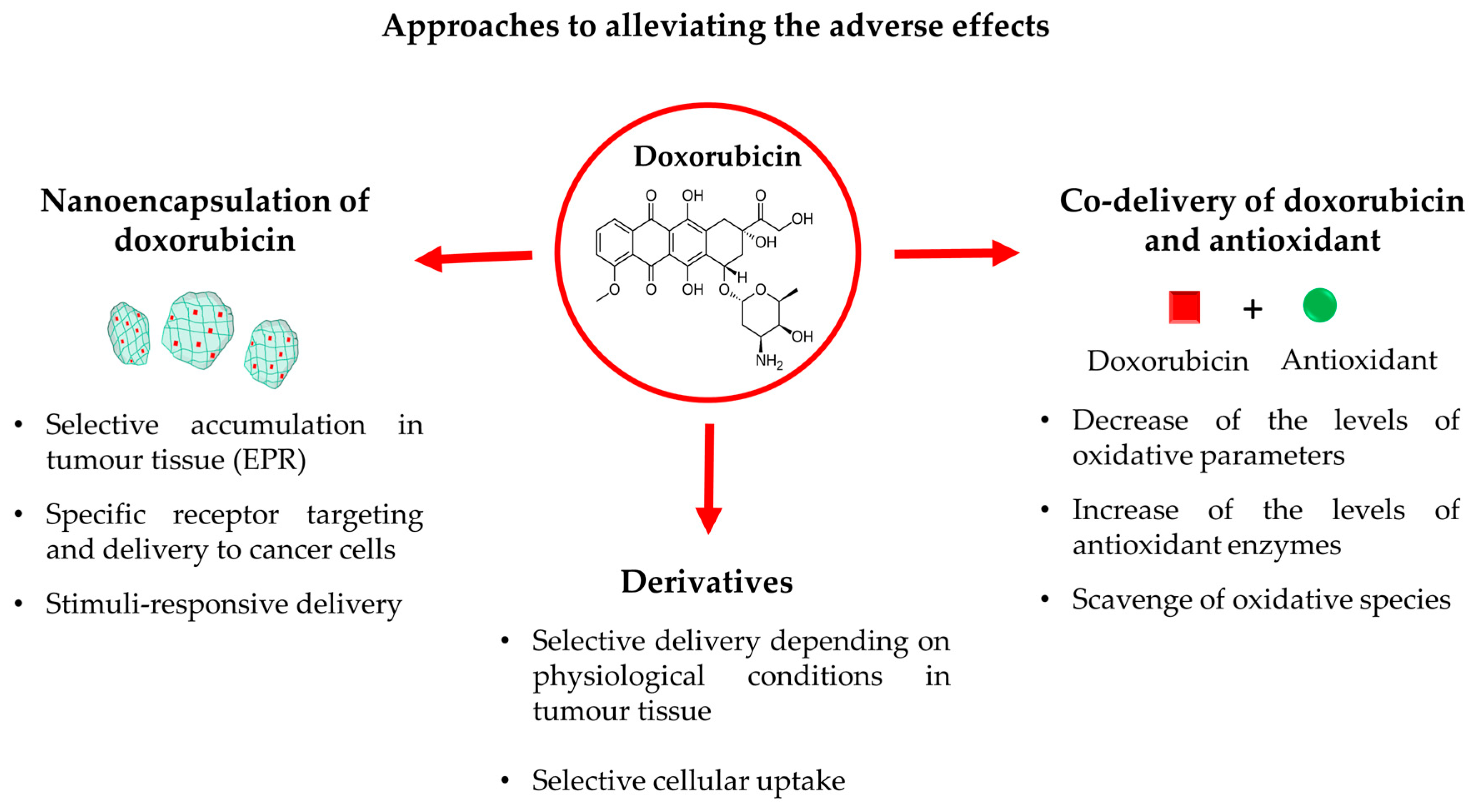
4. Novel Approaches for Alleviating the Adverse Reactions of Doxorubicin
4.1. Nanoencapsulation of Doxorubicin
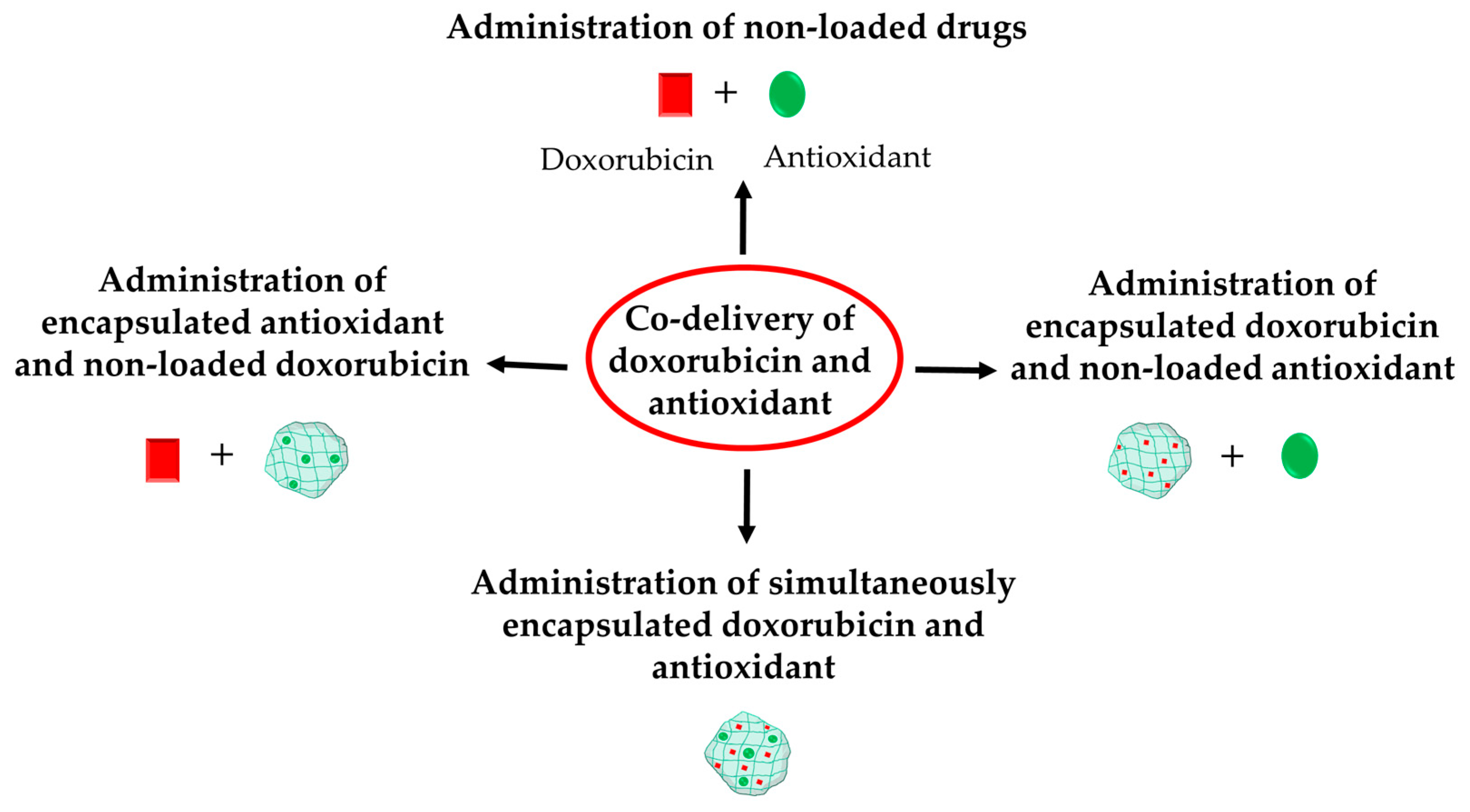
4.2. Co-Delivery of Doxorubicin and Antioxidants
4.2.1. Administration of Non-Loaded Forms of Doxorubicin and Antioxidant
| Type of Toxicity | Antioxidant | Reference |
|---|---|---|
| Cardiotoxicity | Crocin | [50] |
| Mokko lactone | [51] | |
| Ergothioneine | [52] | |
| Quercetin | [53] | |
| Minocycline | [54] | |
| Hesperidin | [55] | |
| Curcumin | [56] | |
| Sinapic acid | [57] | |
| Reduced glutathione | [58] | |
| Ethoxyquin | [60] | |
| Dihydroartemisinin | [59] | |
| Hepatotoxicity | Rutin and quercetin | [67] |
| Crocin | [61] | |
| Ginkgetin | [62] | |
| Tannic acid | [63] | |
| Curcumin | [56] | |
| Esculetin | [64,65] | |
| Luteolin | [66] | |
| Gonadotoxicity | Chlorella vulgaris | [68] |
| Quercetin | [69,70] | |
| Vitamin E | [70] | |
| Gallic acid | [71] | |
| Propolis | [72] | |
| Resveratrol | [73] | |
| Isorhamnetin | [74] | |
| Diosmin | [75] | |
| Nephrotoxicity | Naringin | [76] |
| Honey, royal jelly and propolis | [77] | |
| Ceratonia siliqua extract | [78] | |
| Hesperetin | [79] | |
| Geraniol | [80] | |
| Resveratrol | [81] | |
| Myricetin | [82] | |
| Gossypetin | [83] | |
| Neurotoxicity | Coenzyme Q10 | [84] |
| Alpha-lipoic acid | [85] | |
| Galantamine | [86,87] | |
| Curcumin | [88] | |
| Quercetin | [89,90] | |
| Juglanin | [91] | |
| Melatonin | [92] | |
| Propolis | [93] | |
| Diphenyl diselenide | [94] | |
| Luteolin | [66] |
4.2.2. Administration of Free Doxorubicin and Encapsulated Antioxidant
| Type of Toxicity | Antioxidant | Nanoparticles | Reference |
|---|---|---|---|
| Cardiotoxicity | Quercetin | Chitosan nanoparticles | [95] |
| Quercetin and Zn2+ | Bovine serum albumin nanoparticles | [96] | |
| Xanthohumol | Poly(lactic-co-glycolic acid) (PLGA) nanoparticles, coated with an erythrocyte membrane | [97] | |
| Moringa oleifera leaf extract | Niosomes | [98] | |
| Icariin | Nanoemulsion | [99] | |
| Berberine | Vitamin E-TPGS micelles Solid lipid nanoparticles | [100,101] | |
| Hepatotoxicity | Cinammonaldehyde and naringin | Zein nanoparticles double coated with sodium caseinate and lactoferrin | [102] |
| Thymoquinone | Chitosan nanoparticles | [103] | |
| Turmeric extract | Selenium nanoparticles | [104] | |
| Gonadotoxicity | Eugenol | Methoxy-poly(ethylene glycol)-poly(lactide-co-glycolide) nanoparticles | [105] |
| Pleurotus eryngii extract | Chitosan nanoparticles | [106] | |
| Curcumin | Selenium nanoparticles | [107] | |
| Nephrotoxicity | Resveratrol | Liposomes | [108] |
| Hesperidin | Chitosan nanoparticles | [109] | |
| Neurotoxicity | Piperine | Zeolitic imidazolate metal organic frameworks | [110] |
4.2.3. Administration of Encapsulated Doxorubicin and Free Antioxidant
4.2.4. Double Encapsulation of Doxorubicin and Antioxidants in Nanoparticles
| Type of Toxicity | Antioxidant | Type of Double-Loaded Nanoparticles | Reference |
|---|---|---|---|
| Resveratrol | Pluronic P123-F127 micelles | [113] | |
| Cardiotoxicity | Sulforaphane | Liposomes | [115] |
| Epigallocatechin | Folic-acid coated polyethyleneimine (PEI) nanoparticles | [116] | |
| Epigallocatechin-3-gallocarboxylate | L-cysteine-epigallocatechin-3-gallocarboxylate-nanoparticles | [117] | |
| Hepatotoxicity | Sulforaphane | Liposomes | [115] |
| Zinc | Zinc oxide nanoparticles | [118] | |
| Nephrotoxicity | Sulforaphane | Liposomes | [115] |
| Salvianolic acid A | Nanostructured lipid carriers | [119] | |
| Zinc | Zinc oxide nanoparticles | [118] | |
| Neurotoxicity | Resveratrol | Chitosan–albumin nanoparticles | [114] |
4.3. Derivatives
5. Closing Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase |
| ACP | acid phosphatase |
| ADP | adenosine diphosphate |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AMPA | amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptor |
| APG | alpha-1-acid glycoprotein |
| AST | aspartate aminotransferase |
| ATP | adenosine triphosphate |
| Bax | B-cell lymphoma-2-associated X protein |
| BBB | blood-brain barrier |
| Bcl-2 | B-cell lymphoma 2 |
| cAMP | cyclic adenosine monophosphate |
| CAT | catalase |
| CD 38 | cluster of differentiation 38 |
| CDP | cytidine diphosphate |
| CK | creatine kinase |
| CK-MB | creatine kinase myocardial band |
| COX-2 | cyclooxygenase-2 |
| DNA | deoxyribonucleic acid |
| DRP1-MFN2 | dynamin-related protein 1 |
| EPR | enhanced permeability and retention |
| FGF1 | fibroblast growth factor 1 |
| G6PD | glucose-6-phosphate dehydrogenase |
| GDP | guanosine diphosphate |
| GluA1 | glutamate receptor ionotropic, AMPA |
| GR | glutathione reductase |
| GSH | reduced glutathione |
| GSH-Px | glutathione peroxidase |
| GSSG | oxidised glutathione |
| GST | glutathione S-transferase |
| GTP | guanosine triphosphate |
| H2O2 | hydrogen peroxide |
| HCT | hematocrit |
| HGB | hemoglobin |
| HIF1-α | hypoxia-inducible factor 1 alpha |
| HO-1 | hemeoxygenase-1 |
| ICAM1 | intercellular adhesion molecule 1 |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| IP-10 | interferon gamma-inducible protein 10 |
| KIM-1 | kidney injury molecule-1 |
| (LC3)-I | microtubule-associated protein 1A/1B-light chain 3 |
| LDH | lactate dehydrogenase |
| LDL | low-density lipoptotein |
| LDL-c | low-density lipoprotein-cholesterol |
| LPO | lipid peroxidation |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemoattractant protein-1 |
| MDA | malondialdehyde |
| MPO | myeloperoxidase |
| mRNA | messenger ribonucleic acid |
| mTOR | mechanistic target of rapamycin |
| NAD+ | nicotinamide adenine dinucleotide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGAL | neutrophil gelatinase-associated lipocalin |
| NMDAR | N-methyl-d-aspartate receptors |
| NMNAT | nicotinamide mononucleotide adenylyl transferase |
| NO | nitric oxide |
| NOX1 | reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 1 |
| NQO1 | reduced nicotinamide adenine dinucleotide phosphate (NAD(P)H) dehydrogenase quinone 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| O2− | superoxide anion |
| •OH | hydroxyl radicals |
| ONOO− | peroxynitrite |
| p53 | tumor protein p53 |
| PARP1 | poly-ADP (adenosine diphosphate)-ribose polymerase1 |
| PPARγ | peroxisome proliferator-activated receptor γ |
| RARG | retinoic acid receptor-γ |
| RBC | red blood cells count |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| Sirt1 | Sirtuin 1 |
| Sirt3 | Sirtuin 3 |
| SOD | superoxide dismutase |
| StAR | steroidogenic acute regulatory protein |
| TGF-β1 | transforming growth factor β1 |
| TNF-α | tumor necrosis factor-α |
| UDP | uridine diphosphate |
| VCAM1 | vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
| VLDL-c | very-low-density lipoprotein-cholesterol |
| WBC | white blood cells count |
References
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New Insights into the Activities and Toxicities of the Old Anticancer Drug Doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Bisht, A.; Avinash, D.; Sahu, K.K.; Patel, P.; Das Gupta, G.; Kurmi, B.D. A Comprehensive Review on Doxorubicin: Mechanisms, Toxicity, Clinical Trials, Combination Therapies and Nanoformulations in Breast Cancer. Drug Deliv. Transl. Res. 2025, 15, 102–133. [Google Scholar] [CrossRef]
- Linders, A.N.; Dias, I.B.; López Fernández, T.; Tocchetti, C.G.; Bomer, N.; Van der Meer, P. A Review of the Pathophysiological Mechanisms of Doxorubicin-Induced Cardiotoxicity and Aging. npj Aging 2024, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-Y.; Guo, Z.; Song, P.; Zhang, X.; Yuan, Y.-P.; Teng, T.; Yan, L.; Tang, Q.-Z. Underlying the Mechanisms of Doxorubicin-Induced Acute Cardiotoxicity: Oxidative Stress and Cell Death. Int. J. Biol. Sci. 2022, 18, 760. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.B.; Leung, K.T.; Poon, E.N.-Y. Mitochondrial-Targeted Therapy for Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2022, 23, 1912. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, C.; Hou, Y.; Jiang, S.; Zhang, Y.; Wang, S.; Chen, J.; Lai, J.; Wu, L.; Duan, H.; et al. Research Progress on the Role and Mechanism of Sirtuin Family in Doxorubicin Cardiotoxicity. Phytomedicine 2024, 129, 155673. [Google Scholar] [CrossRef]
- Vitale, R.; Marzocco, S.; Popolo, A. Role of Oxidative Stress and Inflammation in Doxorubicin-Induced Cardiotoxicity: A Brief Account. Int. J. Mol. Sci. 2024, 25, 7477. [Google Scholar] [CrossRef]
- Desai, V.G.; Vijay, V.; Han, T.; Moland, C.L.; Phanavanh, B.; Lee, T.; Davis, K.J.; Muskhelishvili, L.; Stine, K.C.; Fuscoe, J.C. Doxorubicin-Induced Delayed-Onset Subclinical Cardiotoxicity in Mice. J. Appl. Toxicol. 2022, 42, 778–792. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Qu, L.; Liu, S.; Qin, A.; Liu, H.; Wang, T.; Li, W.; Zou, W. Exploring the Role of Ferroptosis in the Doxorubicin-Induced Chronic Cardiotoxicity Using a Murine Model. Chem. Biol. Interact. 2022, 363, 110008. [Google Scholar] [CrossRef]
- Fan, R.; Wang, Y.; Zhang, J.; An, X.; Liu, S.; Bai, J.; Li, J.; Lin, Q.; Xie, Y.; Liao, J.; et al. Hyperhomocysteinaemia Promotes Doxorubicin-Induced Cardiotoxicity in Mice. Pharmaceuticals 2023, 16, 1212. [Google Scholar] [CrossRef] [PubMed]
- Reis-Mendes, A.; Ferreira, M.; Duarte, J.A.; Duarte-Araújo, M.; Remião, F.; Carvalho, F.; Sousa, E.; Bastos, M.L.; Costa, V.M. The Role of Inflammation and Antioxidant Defenses in the Cardiotoxicity of Doxorubicin in Elderly CD-1 Male Mice. Arch. Toxicol. 2023, 97, 3163–3177. [Google Scholar] [CrossRef]
- Kamińska, K.; Cudnoch-Jędrzejewska, A. A Review on the Neurotoxic Effects of Doxorubicin. Neurotox. Res. 2023, 41, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Pureti, L.P.; Vellingiri, B.; Valsala Gopalakrishnan, A. Toxic Effects and Molecular Mechanism of Doxorubicin on Different Organs—An Update. Toxin Rev. 2022, 41, 650–674. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; Rizk, M.Z. Neurotoxicity of the Antineoplastic Drugs: “Doxorubicin” as an Example. J. Mol. Histol. 2024, 55, 1023–1050. [Google Scholar] [CrossRef]
- Bhatt, K.S.; Singh, A.; Marwaha, G.S.; Ravendranathan, N.; Sandhu, I.S.; Kim, K.; Singh, E.; Frisbee, J.C.; Singh, K.K. Different Mechanisms in Doxorubicin-Induced Neurotoxicity: Impact of BRCA Mutations. Int. J. Mol. Sci. 2025, 26, 4736. [Google Scholar] [CrossRef]
- Dias-Carvalho, A.; Ferreira, M.; Reis-Mendes, A.; Ferreira, R.; de Lourdes Bastos, M.; Fernandes, E.; Sá, S.I.; Capela, J.P.; Carvalho, F.; Costa, V.M. Doxorubicin-Induced Neurotoxicity Differently Affects the Hippocampal Formation Subregions in Adult Mice. Heliyon 2024, 10, e31608. [Google Scholar] [CrossRef]
- Moro, N.; Dokshokova, L.; Perumal Vanaja, I.; Prando, V.; Cnudde, S.J.A.; Di Bona, A.; Bariani, R.; Schirone, L.; Bauce, B.; Angelini, A.; et al. Neurotoxic Effect of Doxorubicin Treatment on Cardiac Sympathetic Neurons. Int. J. Mol. Sci. 2022, 23, 11098. [Google Scholar] [CrossRef]
- Alhowail, A.H.; Aldubayan, M.A. Doxorubicin Impairs Cognitive Function by Upregulating AMPAR and NMDAR Subunit Expression and Increasing Neuroinflammation, Oxidative Stress, and Apoptosis in the Brain. Front. Pharmacol. 2023, 14, 1251917. [Google Scholar] [CrossRef]
- Alhowail, A.H.; Pinky, P.D.; Eggert, M.; Bloemer, J.; Woodie, L.N.; Buabeid, M.A.; Bhattacharya, S.; Jasper, S.L.; Bhattacharya, D.; Dhanasekaran, M.; et al. Doxorubicin Induces Dysregulation of AMPA Receptor and Impairs Hippocampal Synaptic Plasticity Leading to Learning and Memory Deficits. Heliyon 2021, 7, e07456. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Leal, Â.; Afonso, A.I.; Machado, F.; Shvachiy, L.; Rocha, I.; Outeiro, T.F.; Geraldes, V. Dose-Dependent Cognitive Decline, Anxiety, and Locomotor Impairments Induced by Doxorubicin: Evidence from an Animal Model. Biology 2024, 13, 939. [Google Scholar] [CrossRef]
- Abbasnezhad, A.; Salami, F.; Mohebbati, R. A Review: Systematic Research Approach on Toxicity Model of Liver and Kidney in Laboratory Animals. Anim. Models Exp. Med. 2022, 5, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Timm, K.N.; Ball, V.; Miller, J.J.; Savic, D.; West, J.A.; Griffin, J.L.; Tyler, D.J. Metabolic Effects of Doxorubicin on the Rat Liver Assessed with Hyperpolarized MRI and Metabolomics. Front. Physiol. 2022, 12, 782745. [Google Scholar] [CrossRef] [PubMed]
- Sedeman, M.; Christowitz, C.; de Jager, L.; Engelbrecht, A.-M. Obese Mammary Tumour-Bearing Mice Are Highly Sensitive to Doxorubicin-Induced Hepatotoxicity. BMC Cancer 2022, 22, 1240. [Google Scholar] [CrossRef]
- Gu, C.; Liu, Z.; Li, Y.; Yi, M.; Wang, S.; Fan, X.; Sun, D.; Zhang, C.; Yan, X.; Wu, G. Endogenous FGF1 Deficiency Aggravates Doxorubicin-Induced Hepatotoxicity. Toxics 2023, 11, 925. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, Y.; Xiong, H.; Zhao, M.; Wang, Q.; Zhu, Y.; Li, Y.; Tang, R.; Li, J. NMNATs Expression Inhibition Mediated NAD+ Deficiency Plays a Critical Role in Doxorubicin-Induced Hepatotoxicity in Mice. Toxicol. Appl. Pharmacol. 2024, 482, 116799. [Google Scholar] [CrossRef]
- Lu, C.; Wei, J.; Gao, C.; Sun, M.; Dong, D.; Mu, Z. Molecular Signaling Pathways in Doxorubicin-Induced Nephrotoxicity and Potential Therapeutic Agents. Int. Immunopharmacol. 2025, 144, 113373. [Google Scholar] [CrossRef]
- Patel, J.; Montalvo, R.; Boeno, F.; Doerr, V.; Smuder, A. The Effect of Exercise on Doxorubicin-Induced Nephrotoxicity in Male and Female Rats. Physiology 2023, 38, 5732553. [Google Scholar] [CrossRef]
- Amarasiri, S.S.; Attanayake, A.P.; Mudduwa, L.K.B.; Jayatilaka, K.A.P.W. Doxorubicin-Induced Nephrotoxicity Model in Wistar Rats: Characterization of Biochemical Parameters, Histological and Immunohistochemical Assessment. Ceylon J. Sci. 2022, 51, 471–479. [Google Scholar] [CrossRef]
- Alafifi, S.; Wahdan, S.; Elsherbiny, D.; Azab, S.S. Doxorubicin-Induced Testicular Toxicity: Possible Underlying Mechanisms and Promising Pharmacological Treatments in Experimental Models. Arch. Pharm. Sci. Ain Shams Univ. 2022, 6, 196–207. [Google Scholar] [CrossRef]
- Ağan, K.; Kaya, S.T.; Ağan, A.F.; Ağyar-Yoldaş, P.; Yoldaş, T.; İkinci-Keleş, A.; Çaprazlı, T.; Arıca, E.; Kekeçoglu, M. Alleviating Doxorubicin-Induced Reproductive Toxicity: Protective and Androgenic Effects of Drone Larvae on Sperm Morphology and Hormonal Balance. Toxicol. Res. 2025, 41, 149–165. [Google Scholar] [CrossRef]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. Gynotoxic Effects of Chemotherapy and Potential Protective Mechanisms. Cancers 2024, 16, 2288. [Google Scholar] [CrossRef]
- Babalola, A.A.; Adelowo, A.R.; Da-silva, O.F.; Ikeji, C.N.; Owoeye, O.; Rocha, J.B.T.; Adedara, I.A.; Farombi, E.O. Attenuation of Doxorubicin-Induced Hypothalamic-Pituitary-Testicular Axis Dysfunction by Diphenyl Diselenide Involves Suppression of Hormonal Deficits, Oxido-Inflammatory Stress and Caspase 3 Activity in Rats. J. Trace Elem. Med. Biol. 2023, 79, 127254. [Google Scholar] [CrossRef]
- Cengiz Mat, O.; Alisan Suna, P.; Baran, M.; Ceyhan, A.; Yay, A. Studies on the Ameliorative Potential of Dietary Supplemented Different Dose of Selenium on Doxorubicin-Induced Ovarian Damage in Rat. J. Biochem. Mol. Toxicol. 2024, 38, e23522. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A Review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Ghahremanloo, A.; Erfani, B.; Asgharzadeh, F.; Mansoori, S.; Gheybi, F.; Hashemy, S.I. Reducing Toxicity and Enhancing Efficacy of Doxorubicin by Liposomal Doxorubicin and Aprepitant in Breast Cancer. Sci. Rep. 2025, 15, 9798. [Google Scholar] [CrossRef] [PubMed]
- Arzt, M.; Gao, B.; Mozneb, M.; Pohlman, S.; Cejas, R.B.; Liu, Q.; Huang, F.; Yu, C.; Zhang, Y.; Fan, X.; et al. Protein-Encapsulated Doxorubicin Reduces Cardiotoxicity in hiPSC-Cardiomyocytes and Cardiac Spheroids While Maintaining Anticancer Efficacy. Stem Cell Rep. 2023, 18, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Drinković, N.; Beus, M.; Barbir, R.; Debeljak, Ž.; Lovaković, B.T.; Kalčec, N.; Ćurlin, M.; Bekavac, A.; Gorup, D.; Mamić, I.; et al. Novel PLGA-Based Nanoformulation Decreases Doxorubicin-Induced Cardiotoxicity. Nanoscale 2024, 16, 9412–9425. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, N.; Mokhtar, F.; Zaazaa, A.; Mahmoud, M. Nano-Encapsulation of Doxorubicin Using Pectin: Safety an Activity on Chemotherapy-Induced Cardiotoxicity in Carcinoma Mice. Lett. Appl. NanoBioScience 2023, 12, 107. [Google Scholar] [CrossRef]
- de Oliveira Silva, J.; Fernandes, R.S.; de Alcântara Lemos, J.; Cassali, G.D.; de Paula Sabino, A.; Townsend, D.M.; Oliveira, M.C.; de Barros, A.L.B. Evaluation of Acute Toxicity and in Vitro Antitumor Activity of a Novel Doxorubicin-Loaded Folate-Coated pH-Sensitive Liposome. Biomed. Pharmacother. 2023, 165, 115280. [Google Scholar] [CrossRef]
- Shetake, N.G.; Ali, M.; Kumar, A.; Bellare, J.; Pandey, B.N. Theranostic Magnetic Nanoparticles Enhance DNA Damage and Mitigate Doxorubicin-Induced Cardio-Toxicity for Effective Multi-Modal Tumor Therapy. Biomater. Adv. 2022, 142, 213147. [Google Scholar] [CrossRef]
- Yang, S.; Shim, M.K.; Kim, W.J.; Choi, J.; Nam, G.-H.; Kim, J.; Kim, J.; Moon, Y.; Kim, H.Y.; Park, J.; et al. Cancer-Activated Doxorubicin Prodrug Nanoparticles Induce Preferential Immune Response with Minimal Doxorubicin-Related Toxicity. Biomaterials 2021, 272, 120791. [Google Scholar] [CrossRef]
- Naik, H.; Sonju, J.J.; Singh, S.; Chatzistamou, I.; Shrestha, L.; Gauthier, T.; Jois, S. Lipidated Peptidomimetic Ligand-Functionalized HER2 Targeted Liposome as Nano-Carrier Designed for Doxorubicin Delivery in Cancer Therapy. Pharmaceuticals 2021, 14, 221. [Google Scholar] [CrossRef]
- Mal, A.; Prabhuraj, R.S.; Malhotra, R.; Valvi, S.K.; Ingle, A.; Srivastava, R.; De, A.; Bandyopadhyaya, R. pH-Responsive Sustained Delivery of Doxorubicin Using Aminated and PEGylated Mesoporous Silica Nanoparticles Leads to Enhanced Antitumor Efficacy in Pre-Clinical Orthotopic Breast Cancer Model. J. Drug Deliv. Sci. Technol. 2022, 77, 103800. [Google Scholar] [CrossRef]
- Gomes, E.R.; Souza, F.R.; Cassali, G.D.; Sabino, A.d.P.; Barros, A.L.B.d.; Oliveira, M.C. Investigation of the Antitumor Activity and Toxicity of Tumor-Derived Exosomes Fused with Long-Circulating and pH-Sensitive Liposomes Containing Doxorubicin. Pharmaceuticals 2022, 14, 2256. [Google Scholar] [CrossRef] [PubMed]
- Abo-ser, M.M.; Toson, E.-S.A.; El-Bindary, A.A.; Schlatter, G.; Shoueir, K.R. Smart Chitosan Nanogel for Targeted Doxorubicin Delivery, Ensuring Precise Release, and Minimizing Side Effects in Ehrlich Ascites Carcinoma-Bearing Mice. Int. J. Biol. Macromol. 2024, 267, 131390. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Tian, D.; Liu, J.; Wang, C.; Wan, Y. Doxorubicin-Bound Hydroxyethyl Starch Conjugate Nanoparticles with pH/Redox Responsive Linkage for Enhancing Antitumor Therapy. Int. J. Nanomed. 2021, 16, 4527–4544. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, J.; Pan, H.; Sang, Y.; Wang, D.; Wang, Z.; Ai, J.; Lin, B.; Chen, L. pH-Redox Responsive Polymer-Doxorubicin Prodrug Micelles Studied by Molecular Dynamics, Dissipative Particle Dynamics Simulations and Experiments. J. Drug Deliv. Sci. Technol. 2022, 69, 103136. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, S.; Zhu, P.; Liu, W.; Du, J. Fabrication of pH/Redox Dual-Responsive Mixed Polyprodrug Micelles for Improving Cancer Chemotherapy. Front. Pharmacol. 2022, 12, 802785. [Google Scholar] [CrossRef]
- Abdulkareem Aljumaily, S.A.; Demir, M.; Elbe, H.; Yigitturk, G.; Bicer, Y.; Altinoz, E. Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Effects of Crocin against Doxorubicin-Induced Myocardial Toxicity in Rats. Environ. Sci. Pollut. Res. 2021, 28, 65802–65813. [Google Scholar] [CrossRef]
- Sirwi, A.; Shaik, R.A.; Alamoudi, A.J.; Eid, B.G.; Elfaky, M.A.; Ibrahim, S.R.M.; Mohamed, G.A.; Abdallah, H.M.; Abdel-Naim, A.B. Mokko Lactone Alleviates Doxorubicin-Induced Cardiotoxicity in Rats via Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities. Nutrients 2022, 14, 733. [Google Scholar] [CrossRef] [PubMed]
- Cheah, I.K.; Tang, R.M.Y.; Wang, X.; Sachaphibulkij, K.; Chong, S.Y.; Lim, L.H.K.; Wang, J.-W.; Halliwell, B. Protection against Doxorubicin-Induced Cardiotoxicity by Ergothioneine. Antioxidants 2023, 12, 320. [Google Scholar] [CrossRef]
- Dulf, P.L.; Coadă, C.A.; Florea, A.; Moldovan, R.; Baldea, I.; Dulf, D.V.; Blendea, D.; Filip, A.G. Mitigating Doxorubicin-Induced Cardiotoxicity through Quercetin Intervention: An Experimental Study in Rats. Antioxidants 2024, 13, 1068. [Google Scholar] [CrossRef] [PubMed]
- Naderi, Y.; Khosraviani, S.; Nasiri, S.; Hajiaghaei, F.; Aali, E.; Jamialahmadi, T.; Banach, M.; Sahebkar, A. Cardioprotective Effects of Minocycline against Doxorubicin-Induced Cardiotoxicity. Biomed. Pharmacother. 2023, 158, 114055. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, F.K.; Alshehri, Z.S.; Alshehri, F.F.; Alhajlah, S.; Khalifa, H.A.; Dahran, N.; Ghonimi, W.A.M. The Role of Hesperidin as a Cardioprotective Strategy against Doxorubicin-Induced Cardiotoxicity: The Antioxidant, Anti-Inflammatory, Antiapoptotic, and Cytoprotective Potentials. Open Vet. J. 2023, 13, 1718–1728. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; Ahmed, K.A. Curcumin Ameliorates Doxorubicin-Induced Cardiotoxicity and Hepatotoxicity Via Suppressing Oxidative Stress and Modulating iNOS, NF-κB, and TNF-α in Rats. Cardiovasc. Toxicol. 2022, 22, 152–166. [Google Scholar] [CrossRef]
- Tungalag, T.; Kang, H.-S.; Yang, D.K. Sinapic Acid Ameliorates Doxorubicin-Induced Cardiotoxicity in H9c2 Cardiomyoblasts by Inhibiting Oxidative Stress Through Activation of the Nrf2 Signaling Pathway. Antioxidants 2025, 14, 337. [Google Scholar] [CrossRef]
- Negm, A.; Mersal, E.A.; Dawood, A.F.; Abd El-Azim, A.O.; Hasan, O.; Alaqidi, R.; Alotaibi, A.; Alshahrani, M.; Alheraiz, A.; Shawky, T.M. Multifaceted Cardioprotective Potential of Reduced Glutathione Against Doxorubicin-Induced Cardiotoxicity via Modulating Inflammation–Oxidative Stress Axis. Int. J. Mol. Sci. 2025, 26, 3201. [Google Scholar] [CrossRef]
- Lin, Z.-H.; Xiang, H.-Q.; Yu, Y.-W.; Xue, Y.-J.; Wu, C.; Lin, C.; Ji, K.-T. Dihydroartemisinin Alleviates Doxorubicin-Induced Cardiotoxicity and Ferroptosis by Activating Nrf2 and Regulating Autophagy. FASEB J. 2024, 38, e23677. [Google Scholar] [CrossRef]
- Tadokoro, T.; Ikeda, M.; Abe, K.; Ide, T.; Miyamoto, H.D.; Furusawa, S.; Ishimaru, K.; Watanabe, M.; Ishikita, A.; Matsushima, S.; et al. Ethoxyquin Is a Competent Radical-Trapping Antioxidant for Preventing Ferroptosis in Doxorubicin Cardiotoxicity. J. Cardiovasc. Pharmacol. 2022, 80, 690. [Google Scholar] [CrossRef]
- Demir, M.; Altinoz, E.; Koca, O.; Elbe, H.; Onal, M.O.; Bicer, Y.; Karayakali, M. Antioxidant and Anti-Inflammatory Potential of Crocin on the Doxorubicin Mediated Hepatotoxicity in Wistar Rats. Tissue Cell 2023, 84, 102182. [Google Scholar] [CrossRef]
- alherz, F.A.; Negm, W.A.; El-Masry, T.A.; Elmorshedy, K.E.; El-Kadem, A.H. The Potential Beneficial Role of Ginkgetin in Doxorubicin-Induced Hepatotoxicity: Elucidating the Underlying Claim. Biomed. Pharmacother. 2023, 165, 115010. [Google Scholar] [CrossRef]
- Özturk, N.; Ceylan, H.; Demir, Y. The Hepatoprotective Potential of Tannic Acid against Doxorubicin-Induced Hepatotoxicity: Insights into Its Antioxidative, Anti-Inflammatory, and Antiapoptotic Mechanisms. J. Biochem. Mol. Toxicol. 2024, 38, e23798. [Google Scholar] [CrossRef]
- Kizir, D.; Yeşilkent, E.N.; Öztürk, N.; Karağaç, M.S.; Isıyel, M.; Tosun, H.; Karadaş, H.; Ceylan, H.; Karaman, M.; Demir, Y. The Protective Effects of Esculetin against Doxorubicin-Induced Hepatotoxicity in Rats: Insights into the Modulation of Caspase, FOXOs, and Heat Shock Protein Pathways. J. Biochem. Mol. Toxicol. 2024, 38, e23861. [Google Scholar] [CrossRef]
- Köroğlu, Z.; Kizir, D.; Karaman, M.; Demir, Y.; Türkeş, C.; Beydemir, Ş. Protective Effects of Esculetin against Doxorubicin-Induced Toxicity Correlated with Oxidative Stress in Rat Liver: In Vivo and in Silico Studies. J. Biochem. Mol. Toxicol. 2024, 38, e23702. [Google Scholar] [CrossRef]
- Abdrabou, R.D.; Salama, R.M.; El-Naga, R.N.; Azab, S.S. Luteolin Mitigates Doxorubicin-Induced Hepatotoxicity and Neurotoxicity: Modulating the Liver–Brain Axis via IRE1α/GRP78/ATF6 Endoplasmic Reticulum Stress Pathways and miRNA-199a-5p Expression. Future J. Pharm. Sci. 2025, 11, 44. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Elkomy, M.H.; Fahim, H.I.; Ashour, M.B.; Naguib, I.A.; Alghamdi, B.S.; Mahmoud, H.U.R.; Ahmed, N.A. Rutin and Quercetin Counter Doxorubicin-Induced Liver Toxicity in Wistar Rats via Their Modulatory Effects on Inflammation, Oxidative Stress, Apoptosis, and Nrf2. Oxid. Med. Cell. Longev. 2022, 2022, 2710607. [Google Scholar] [CrossRef] [PubMed]
- Makipour, A.; Hosseinifar, S.; Khazaeel, K.; Tabandeh, M.R.; Jamshidian, J. Protective Effect of Chlorella vulgaris on Testicular Damage, Sperm Parameters, Androgen Production, Apoptosis and Oxidative Stress Index in Male Rats Following Doxorubicin Administration. Reprod. Toxicol. 2024, 128, 108653. [Google Scholar] [CrossRef] [PubMed]
- Güleş, Ö.; Doğan, G.; Ercins, U.H.; Eren, Ü. Effects of Quercetin against Doxorubicin-Induced Testicular Toxicity in Male Rats. Biol. Bull. 2022, 49, 203–213. [Google Scholar] [CrossRef]
- Samare-Najaf, M.; Zal, F.; Jamali, N.; Vakili, S.; Khodabandeh, Z. Do Quercetin and Vitamin E Properties Preclude Doxorubicin-Induced Stress and Inflammation in Reproductive Tissues? Curr. Cancer Ther. Rev. 2022, 18, 292–302. [Google Scholar] [CrossRef]
- dos Santos Silva, R.L.; Lins, T.L.B.G.; do Monte, A.P.O.; de Andrade, K.O.; de Sousa Barberino, R.; da Silva Pereira Campinho, D.; Junior, R.C.P.; de Matos, M.H.T. Protective Effect of Gallic Acid on Doxorubicin-Induced Ovarian Toxicity in Mouse. Reprod. Toxicol. 2023, 115, 147–156. [Google Scholar] [CrossRef]
- Alsyaad, K.M. Ameliorative Impacts of Propolis against Testicular Toxicity Promoted by Doxorubicin. Vet. World 2024, 17, 421–426. [Google Scholar] [CrossRef]
- Herrero, Y.; Velázquez, C.; Pascuali, N.; May, M.; Abramovich, D.; Scotti, L.; Parborell, F. Resveratrol Alleviates Doxorubicin-Induced Damage in Mice Ovary. Chem. Biol. Interact. 2023, 376, 110431. [Google Scholar] [CrossRef]
- Mustafa, S.; Ijaz, M.U.; ul Ain, Q.; Afsar, T.; Almajwal, A.; Shafique, H.; Razak, S. Isorhamnetin: A Flavonoid, Attenuated Doxorubicin-Induced Testicular Injury via Regulation of Steroidogenic Enzymes and Apoptotic Signaling Gene Expression in Male Rats. Toxicol. Res. 2022, 11, 475–485. [Google Scholar] [CrossRef]
- Malayeri, A.; Birgani, S.M.; Basir, Z.; Kalantar, H. Protective Effects of Diosmin on Doxorubicin-Induced Testicular Toxicity in Rat. Naunyn. Schmiedebergs Arch. Pharmacol. 2024, 397, 7881–7890. [Google Scholar] [CrossRef] [PubMed]
- Gad, N.S.; Shabana, S.M.; Amer, M.E.; Othman, A.I.; El-Missiry, M.A. Naringin Mitigated Doxorubicin-Induced Kidney Injury by the Reduction of Oxidative Stress and Inflammation with a Synergistic Anticancer Effect. BMC Pharmacol. Toxicol. 2025, 26, 121. [Google Scholar] [CrossRef]
- Mohamed, H.K.; Mobasher, M.A.; Ebiya, R.A.; Hassen, M.T.; Hagag, H.M.; El-Sayed, R.; Abdel-Ghany, S.; Said, M.M.; Awad, N.S. Anti-Inflammatory, Anti-Apoptotic, and Antioxidant Roles of Honey, Royal Jelly, and Propolis in Suppressing Nephrotoxicity Induced by Doxorubicin in Male Albino Rats. Antioxidants 2022, 11, 1029. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.H.; Atta, S.A.; Khattab, M.S.; El-Aziz, T.H.A.; Mouneir, S.M.; Ibrahim, M.A.; Nasr, S.M.; Emam, S.R. Ceratonia Siliqua Pods (Carob) Methanol Extract Alleviates Doxorubicin-Induced Nephrotoxicity via Antioxidant, Anti-Inflammatory and Anti-Apoptotic Pathways in Rats. Environ. Sci. Pollut. Res. 2023, 30, 83421–83438. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, R.A.; Sadek, A.S.; Khattab, R.T.; Saad, D.Z.; Shawky, N.O.; Abdelfattah, H.A. Could Hesperetin Ameliorate Doxorubicin-Induced Nephrotoxicity in Rats via Its Antioxidant, Antiapoptotic, and Anti-Inflammatory Properties? Tissue Cell 2025, 96, 102951. [Google Scholar] [CrossRef]
- AlAsmari, A.F.; Ali, N.; Alharbi, M.; Alqahtani, F.; Alasmari, F.; Almoqbel, D.; AlSwayyed, M.; Alshammari, A.; Alanazi, M.M.; Alhoshani, A.; et al. Geraniol Ameliorates Doxorubicin-Mediated Kidney Injury through Alteration of Antioxidant Status, Inflammation, and Apoptosis: Potential Roles of NF-κB and Nrf2/Ho-1. Nutrients 2022, 14, 1620. [Google Scholar] [CrossRef]
- Yalcın, T.; Kaya, S.; Kuloğlu, T. Resveratrol May Dose-Dependently Modulate Nephrin and OTULIN Levels in a Doxorubicin-Induced Nephrotoxicity Model. Toxicol. Mech. Methods 2024, 34, 98–108. [Google Scholar] [CrossRef]
- Karadogan, M.T.; Cicek, B.; Cinisli, K.T.; Mendil, A.S.; Ozkaraca, M.; Yilmaz, F.; Suleyman, H. Myricetin Protects against Doxorubicin-Induced Acute Kidney Injury in Rats by Mitigating Oxidative Damage and Apoptotic Response. Front. Pharmacol. 2025, 16, 1601628. [Google Scholar] [CrossRef]
- Ijaz, M.U.; Alvi, K.; Khan, H.A.; Imran, M.; Afsar, T.; Almajwal, A.; Amor, H.; Razak, S. Gossypetin Mitigates Doxorubicin-Induced Nephrotoxicity: A Histopathological and Biochemical Evaluation. J. King Saud Univ.-Sci. 2023, 35, 102830. [Google Scholar] [CrossRef]
- Okudan, N.; Belviranlı, M.; Sezer, T. Potential Protective Effect of Coenzyme Q10 on Doxorubicin-Induced Neurotoxicity and Behavioral Disturbances in Rats. Neurochem. Res. 2022, 47, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Lal, R.; Dharavath, R.N.; Chopra, K. Alpha-Lipoic Acid Ameliorates Doxorubicin-Induced Cognitive Impairments by Modulating Neuroinflammation and Oxidative Stress via NRF-2/HO-1 Signaling Pathway in the Rat Hippocampus. Neurochem. Res. 2023, 48, 2476–2489. [Google Scholar] [CrossRef] [PubMed]
- Aldubayan, M.A.; Alsharidah, A.S.; Alenezi, S.K.; Alhowail, A.H. Galantamine Mitigates Neurotoxicity Caused by Doxorubicin via Reduced Neuroinflammation, Oxidative Stress, and Apoptosis in Rat Model. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 805–813. [Google Scholar] [CrossRef]
- Alsikhan, R.S.; Aldubayan, M.A.; Almami, I.S.; Alhowail, A.H. Protective Effect of Galantamine against Doxorubicin-Induced Neurotoxicity. Brain Sci. 2023, 13, 971. [Google Scholar] [CrossRef]
- Liao, D.; Shangguan, D.; Wu, Y.; Chen, Y.; Liu, N.; Tang, J.; Yao, D.; Shi, Y. Curcumin Protects against Doxorubicin Induced Oxidative Stress by Regulating the Keap1-Nrf2-ARE and Autophagy Signaling Pathways. Psychopharmacology 2023, 240, 1179–1190. [Google Scholar] [CrossRef]
- El-Shetry, E.S.; Ibrahim, I.A.; Kamel, A.M.; Abdelwahab, O.A. Quercetin Mitigates Doxorubicin-Induced Neurodegenerative Changes in the Cerebral Cortex and Hippocampus of Rats; Insights to DNA Damage, Inflammation, Synaptic Plasticity. Tissue Cell 2024, 87, 102313. [Google Scholar] [CrossRef] [PubMed]
- Ramalingayya, G.V.; John, J.; Gourishetti, K.; Nayak, P.G.; Rao, C.M.; Kishore, A.; Alnasser, S.M.; Hussain, S.M.; Krishnadas, N. Amelioration of Doxorubicin-Induced Cognitive Impairment by Quercetin in a Rat Model of Breast Cancer. Rev. Bras. Farmacogn. 2023, 33, 153–163. [Google Scholar] [CrossRef]
- Wei, T.; Wang, L.; Tang, J.; Ashaolu, T.J.; Olatunji, O.J. Protective Effect of Juglanin against Doxorubicin-Induced Cognitive Impairment in Rats: Effect on Oxidative, Inflammatory and Apoptotic Machineries. Metab. Brain Dis. 2022, 37, 1185–1195. [Google Scholar] [CrossRef]
- Ebrahim, N.A.; Elnagar, M.R.; El-Gamal, R.; Habotta, O.A.; Albadawi, E.A.; Albadrani, M.; Bahashwan, A.S.; Hassan, H.M. Melatonin Mitigates Doxorubicin Induced Chemo Brain in a Rat Model in a NRF2/P53–SIRT1 Dependent Pathway. Heliyon 2024, 10, e38081. [Google Scholar] [CrossRef] [PubMed]
- Gelen, V.; Başeğmez, M.; Dursun, İ.; Çinar, I.; Kara, A. Propolis Extract Reduces Doxorubucin-Induced Brain Damage by Regulating Inflammation, ER Stress, Oxidative Stress, and Apoptosis. Food Sci. Nutr. 2025, 13, e70194. [Google Scholar] [CrossRef] [PubMed]
- Da-silva, O.F.; Adelowo, A.R.; Babalola, A.A.; Ikeji, C.N.; Owoeye, O.; Rocha, J.B.T.; Adedara, I.A.; Farombi, E.O. Diphenyl Diselenide Through Reduction of Inflammation, Oxidative Injury and Caspase-3 Activation Abates Doxorubicin-Induced Neurotoxicity in Rats. Neurochem. Res. 2024, 49, 1076–1092. [Google Scholar] [CrossRef]
- Soliman, A.G.; Mahmoud, B.; Eldin, Z.E.; El-Shahawy, A.A.G.; Abdel-Gabbar, M. Optimized Synthesis Characterization and Protective Activity of Quercetin and Quercetin–Chitosan Nanoformula against Cardiotoxicity That Was Induced in Male Wister Rats via Anticancer Agent: Doxorubicin. Cancer Nanotechnol. 2023, 14, 10. [Google Scholar] [CrossRef]
- Shao, Z.; Li, R.; Shao, D.; Tang, H.; Han, Y. Albumin-Based Zn (II)-Quercetin Enzyme Mimic Scavenging ROS for Protection against Cardiotoxicity Induced by Doxorubicin. Pharmaceuticals 2022, 15, 1524. [Google Scholar] [CrossRef]
- Li, J.; Zeng, Y.; Liu, F.; Liao, X.; Zhong, C.; Dong, S.; Cai, Y.; Yang, P. Erythrocyte Membrane-Camouflaged Xanthohumol Nanoparticles Mitigate Doxorubicin-Induced Cardiotoxicity by Inhibiting Ferroptosis. ACS Biomater. Sci. Eng. 2025, 11, 2727–2738. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, E.A.; Ahmed, S.M.; Masoud, M.A.; Mohamed, F.A.; Mohammed, H.S. Cardioprotective Potential of Moringa Oleifera Leaf Extract Loaded Niosomes Nanoparticles—Against Doxorubicin Toxicity in Rats. Curr. Pharm. Biotechnol. 2025, 26, 289–301. [Google Scholar] [CrossRef]
- Md, S.; Rahman Mahrous, H.A.; Alhakamy, N.A.; Shaik, R.A.; Eid, B.G. Protective Effect of Statistically Designed and Optimized Icariin Nanoemulsion on Doxorubicin-Induced Cardiotoxicity: Inhibition of Oxidative Stress, Inflammation, and Apoptosis. J. Drug Deliv. Sci. Technol. 2023, 81, 104297. [Google Scholar] [CrossRef]
- Metwally, A.A.; Ganguly, S.; Biomi, N.; Yao, M.; Elbayoumi, T. Cationic Vitamin E-TPGS Mixed Micelles of Berberine to Neutralize Doxorubicin-Induced Cardiotoxicity via Amelioration of Mitochondrial Dysfunction and Impeding Apoptosis. Molecules 2024, 29, 1155. [Google Scholar] [CrossRef]
- Rawal, S.; Gupta, P.; Bhatnagar, P.; Yadav, H.N.; Dinda, A.K. Solid Lipid Nanoformulation of Berberine Attenuates Doxorubicin Triggered in Vitro Inflammation in H9c2 Rat Cardiomyocytes. Comb. Chem. High Throughput Screen. 2022, 25, 1695–1706. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Helmy, M.W.; Mahmoud, H.E.; Embaby, A.M.; Haroun, M.; Sabra, S.A. Cinnamaldehyde /Naringin Co-Loaded into Lactoferrin/ Casienate-Coated Zein Nanoparticles as a Gastric Resistance Oral Carrier for Mitigating Doxorubicin-Induced Hepatotoxicity. J. Drug Deliv. Sci. Technol. 2024, 96, 105688. [Google Scholar] [CrossRef]
- Abdelhady, S.; Hassanein, K.; Taha, M. Thymoquinone Nanotherapy Abrogates Hepatotoxicity-Induced By Doxorubicin In Male Albino Rats. Assiut Vet. Med. J. 2023, 69, 99–109. [Google Scholar] [CrossRef]
- ALRashdi, B.M.; Hussein, M.M.; Mohammed, R.M.; Abdelhamed, N.W.; Asaad, M.E.; Alruwaili, M.; Alrashidi, S.M.; Habotta, O.A.; Moneim, A.E.A.; Ramadan, S.S. Turmeric Extract-Loaded Selenium Nanoparticles Counter Doxorubicin-Induced Hepatotoxicity in Mice via Repressing Oxidative Stress, Inflammatory Cytokines, and Cell Apoptosis. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chem.—Anti-Cancer Agents 2024, 24, 443–453. [Google Scholar] [CrossRef]
- Fu, Y.; Yuan, P.; Wang, M.; Zheng, Y.; Zhang, Y.; Zhao, L.; Ma, Q.; Wang, P.; Sun, X.; Zheng, X.; et al. Eugenol Nanoparticles Ameliorate Doxorubicin-Induced Spermatogenic Dysfunction by Inhibiting the PINK1/Parkin and BNIP3/NIX Signaling Pathways. Int. J. Nanomed. 2024, 19, 13287–13300. [Google Scholar] [CrossRef] [PubMed]
- Erdem Guzel, E.; Kaya Tektemur, N.; Tektemur, A.; Acay, H.; Yildirim, A. The Antioxidant and Anti-Apoptotic Potential of Pleurotus Eryngii Extract and Its Chitosan-Loaded Nanoparticles against Doxorubicin-Induced Testicular Toxicity in Male Rats. Andrologia 2021, 53, e14225. [Google Scholar] [CrossRef]
- Mohammed, R.; Al-Okaily, B. Alterations of VEGF Expression and Ovarian Follicles in Doxorubicin Treated Rats: Role of Curcumin Selenium Nanoparticles. Adv. Anim. Vet. Sci. 2024, 12, 2185–2194. [Google Scholar] [CrossRef]
- Alhusaini, A.M.; Fadda, L.M.; Alanazi, A.M.; Sarawi, W.S.; Alomar, H.A.; Ali, H.M.; Hasan, I.H.; Ali, R.A. Nano-Resveratrol: A Promising Candidate for the Treatment of Renal Toxicity Induced by Doxorubicin in Rats Through Modulation of Beclin-1 and mTOR. Front. Pharmacol. 2022, 13, 826908. [Google Scholar] [CrossRef]
- Alherz, F.A.; El-Masry, T.A.; Oriquat, G.A.; Elekhnawy, E.; Al-Shaalan, N.H.; Gaballa, M.M.S.; El Zahaby, E.I.; El-Nagar, M.M.F. Hesperidin Nanoformulation: A Potential Strategy for Reducing Doxorubicin-Induced Renal Damage via the Sirt-1/HIF1-α/VEGF/NF-κB Signaling Cascade. Pharmaceuticals 2024, 17, 1144. [Google Scholar] [CrossRef]
- Arora, S.; Kumar, V.; Kapil, L.; Agrawal, A.K.; Singh, A.; Singh, C. Piperine Loaded Metal Organic Frameworks Reverse Doxorubicin Induced Chemobrain in Adult Zebrafish. J. Control. Release 2023, 355, 259–272. [Google Scholar] [CrossRef]
- Dorostkar, H.; Haghiralsadat, B.F.; Hemati, M.; Safari, F.; Hassanpour, A.; Naghib, S.M.; Roozbahani, M.H.; Mozafari, M.R.; Moradi, A. Reduction of Doxorubicin-Induced Cardiotoxicity by Co-Administration of Smart Liposomal Doxorubicin and Free Quercetin: In Vitro and In Vivo Studies. Pharmaceuticals 2023, 15, 1920. [Google Scholar] [CrossRef] [PubMed]
- Siva, D.; Abinaya, S.; Rajesh, D.; Archunan, G.; Padmanabhan, P.; Gulyás, B.; Achiraman, S. Mollification of Doxorubicin (DOX)-Mediated Cardiotoxicity Using Conjugated Chitosan Nanoparticles with Supplementation of Propionic Acid. Nanomaterials 2022, 12, 502. [Google Scholar] [CrossRef] [PubMed]
- Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tzankova, V.; Yoncheva, K. Double-Loaded Doxorubicin/Resveratrol Polymeric Micelles Providing Low Toxicity on Cardiac Cells and Enhanced Cytotoxicity on Lymphoma Cells. Pharmaceuticals 2023, 15, 1287. [Google Scholar] [CrossRef]
- Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tzankova, V.; Yoncheva, K. Double Encapsulation of Resveratrol and Doxorubicin in Composite Nanogel—An Opportunity to Reduce Cardio- and Neurotoxicity of Doxorubicin. Gels 2024, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Pogorzelska, A.; Mazur, M.; Świtalska, M.; Wietrzyk, J.; Sigorski, D.; Fronczyk, K.; Wiktorska, K. Anticancer Effect and Safety of Doxorubicin and Nutraceutical Sulforaphane Liposomal Formulation in Triple-Negative Breast Cancer (TNBC) Animal Model. Biomed. Pharmacother. 2023, 161, 114490. [Google Scholar] [CrossRef]
- Zhang, T.; Li, N.; Wang, R.; Sun, Y.; He, X.; Lu, X.; Chu, L.; Sun, K. Enhanced Therapeutic Efficacy of Doxorubicin against Multidrug-Resistant Breast Cancer with Reduced Cardiotoxicity. Drug Deliv. 2023, 30, 2189118. [Google Scholar] [CrossRef]
- Wang, K.; Geng, S.; Wang, F.; Fang, B.; Qian, H.; Li, Y.; Zhou, Y.; Chen, Y.; Yu, Z. Natural Epigallocatechin-3-Gallocarboxylate Nanoformulation Loaded Doxorubicin to Construct a Novel and Low Cardiotoxicity Chemotherapeutic Drug for High-Efficiency Breast Cancer Therapy. J. Nanobiotechnol. 2024, 22, 793. [Google Scholar] [CrossRef]
- Gomaa, S.; Nassef, M.; Tabl, G.; Zaki, S.; Abdel-Ghany, A. Doxorubicin and Folic Acid-Loaded Zinc Oxide Nanoparticles-Based Combined Anti-Tumor and Anti-Inflammatory Approach for Enhanced Anti-Cancer Therapy. BMC Cancer 2024, 24, 34. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Dang, W.; Xing, B.; Yu, C.; Guo, P.; Pi, J.; Deng, X.; Qi, D.; Liu, Z. The Anti-Tumor and Renoprotection Study of E-[c(RGDfK)2]/Folic Acid Co-Modified Nanostructured Lipid Carrier Loaded with Doxorubicin Hydrochloride/Salvianolic Acid A. J. Nanobiotechnol. 2022, 20, 425. [Google Scholar] [CrossRef]
- Dragojevic, S.; Ryu, J.S.; Hall, M.E.; Raucher, D. Targeted Drug Delivery Biopolymers Effectively Inhibit Breast Tumor Growth and Prevent Doxorubicin-Induced Cardiotoxicity. Molecules 2022, 27, 3371. [Google Scholar] [CrossRef]
- Mozaffari, S.; Salehi, D.; Mahdipoor, P.; Beuttler, R.; Tiwari, R.; Aliabadi, H.M.; Parang, K. Design and Application of Hybrid Cyclic-Linear Peptide-Doxorubicin Conjugates as a Strategy to Overcome Doxorubicin Resistance and Toxicity. Eur. J. Med. Chem. 2021, 226, 113836. [Google Scholar] [CrossRef] [PubMed]
- Lages, E.B.; Fernandes, R.S.; Andrade, M.M.S.; Paiyabhroma, N.; de Oliveira, R.B.; Fernandes, C.; Cassali, G.D.; Sicard, P.; Richard, S.; Branco de Barros, A.L.; et al. pH-Sensitive Doxorubicin-Tocopherol Succinate Prodrug Encapsulated in Docosahexaenoic Acid-Based Nanostructured Lipid Carriers: An Effective Strategy to Improve Pharmacokinetics and Reduce Toxic Effects. Biomed. Pharmacother. 2021, 144, 112373. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radeva, L.; Yoncheva, K. Doxorubicin Toxicity and Recent Approaches to Alleviating Its Adverse Effects with Focus on Oxidative Stress. Molecules 2025, 30, 3311. https://doi.org/10.3390/molecules30153311
Radeva L, Yoncheva K. Doxorubicin Toxicity and Recent Approaches to Alleviating Its Adverse Effects with Focus on Oxidative Stress. Molecules. 2025; 30(15):3311. https://doi.org/10.3390/molecules30153311
Chicago/Turabian StyleRadeva, Lyubomira, and Krassimira Yoncheva. 2025. "Doxorubicin Toxicity and Recent Approaches to Alleviating Its Adverse Effects with Focus on Oxidative Stress" Molecules 30, no. 15: 3311. https://doi.org/10.3390/molecules30153311
APA StyleRadeva, L., & Yoncheva, K. (2025). Doxorubicin Toxicity and Recent Approaches to Alleviating Its Adverse Effects with Focus on Oxidative Stress. Molecules, 30(15), 3311. https://doi.org/10.3390/molecules30153311







