Photodegradation of Polyethylene Terephthalate and Bis(2-hydroxyethyl) Terephthalate Using Excimer Lamps and Hydrogen Peroxide: A Strategy for PET–Derived Waste Treatment
Abstract
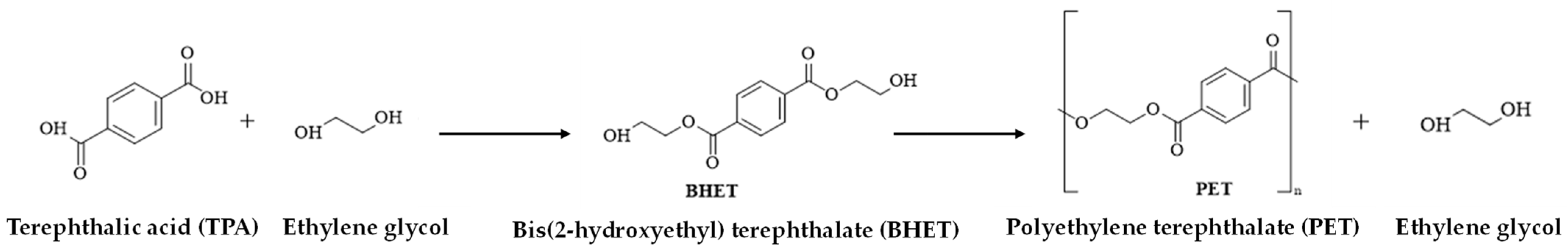
1. Introduction
2. Results
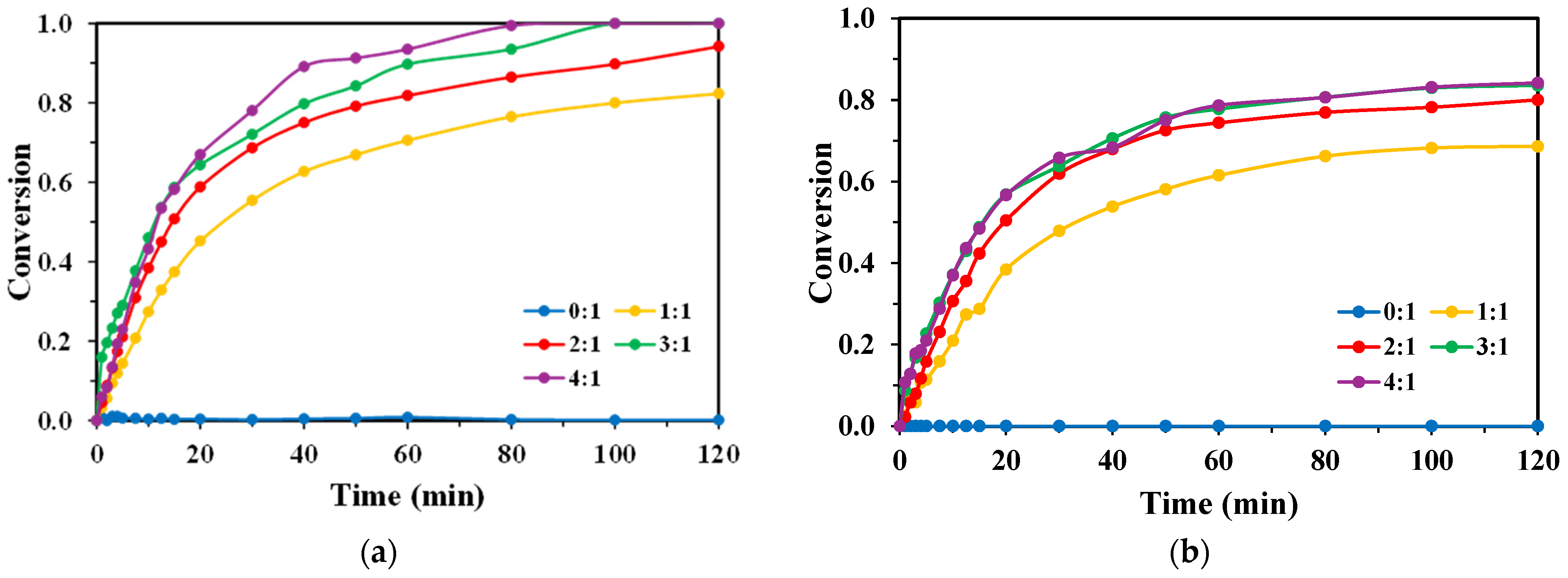
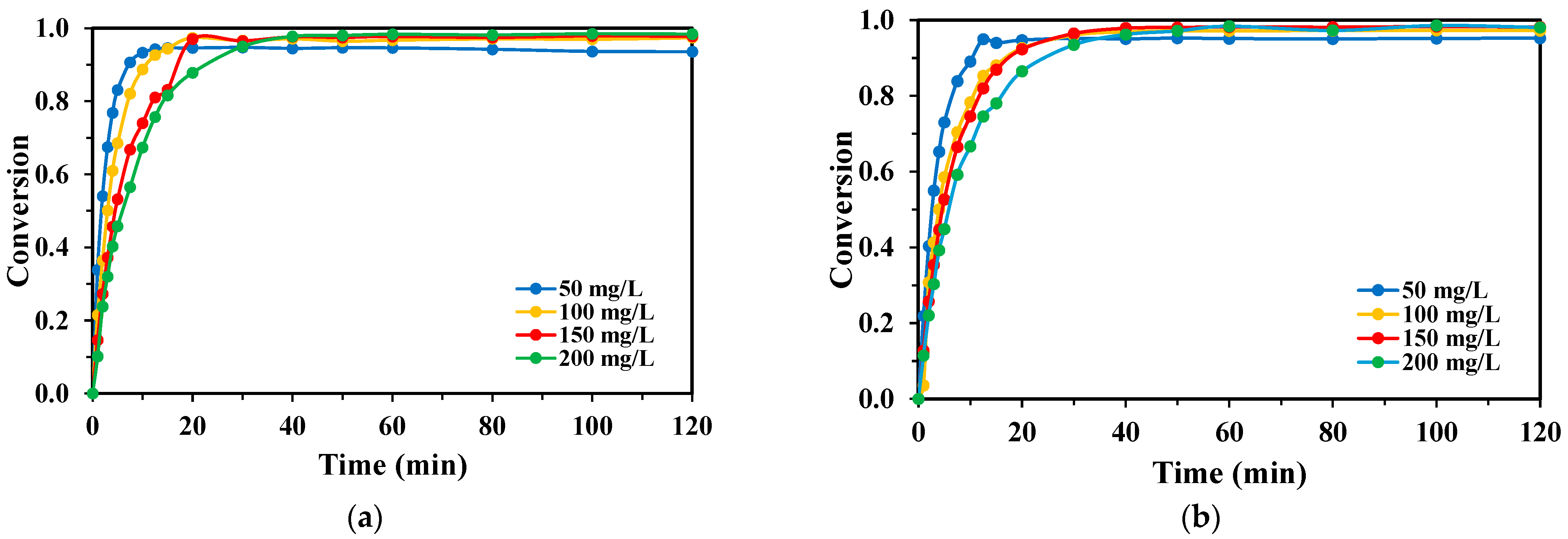
2.1. Effect of H2O2/Monomer Mass Ratio
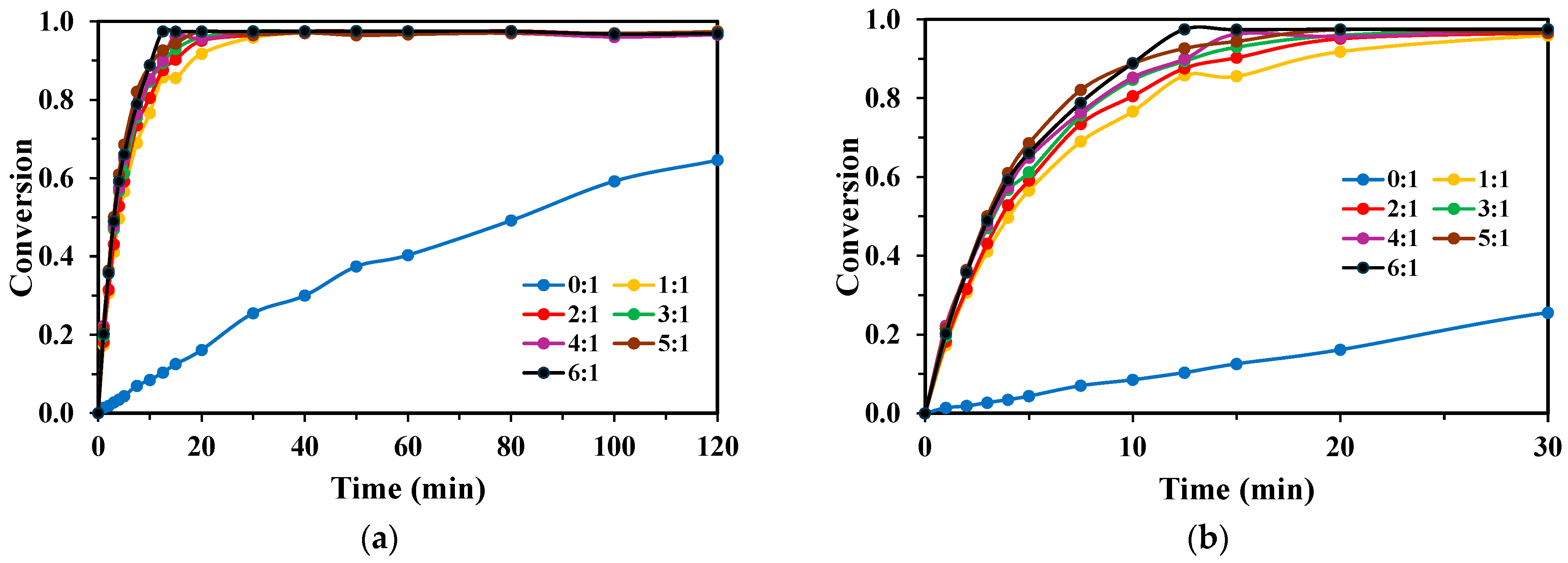
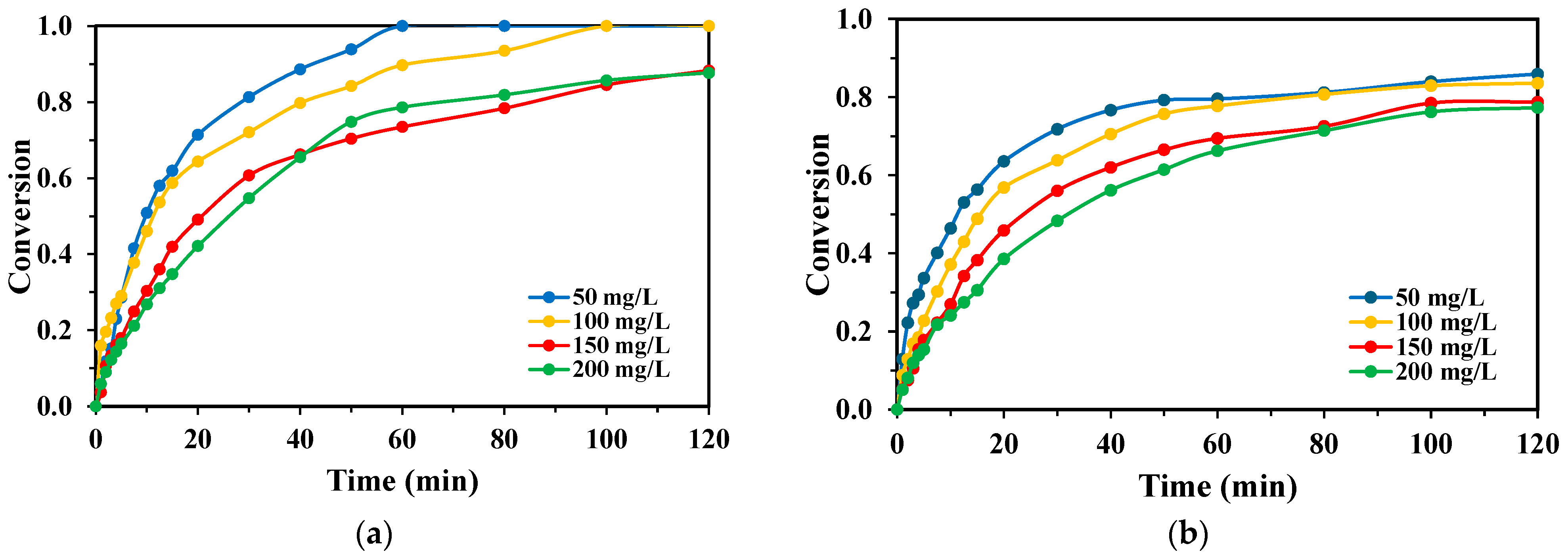
2.2. Effect of Initial Monomer Concentration
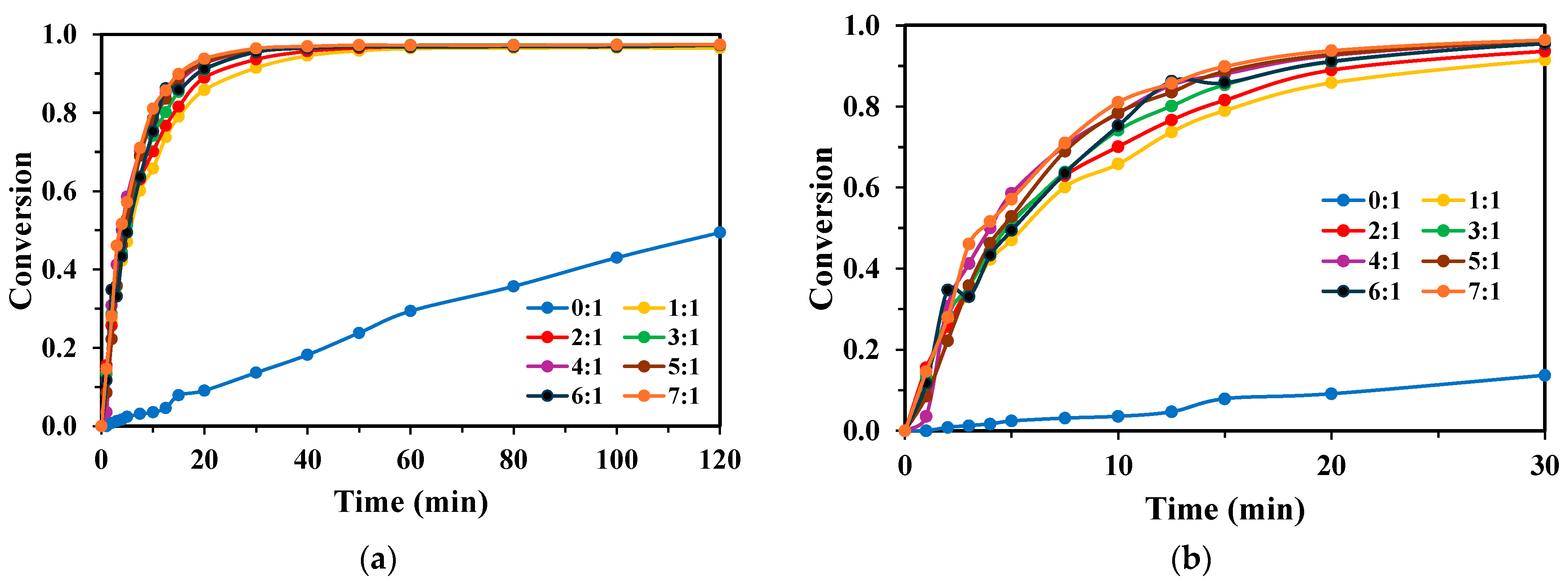
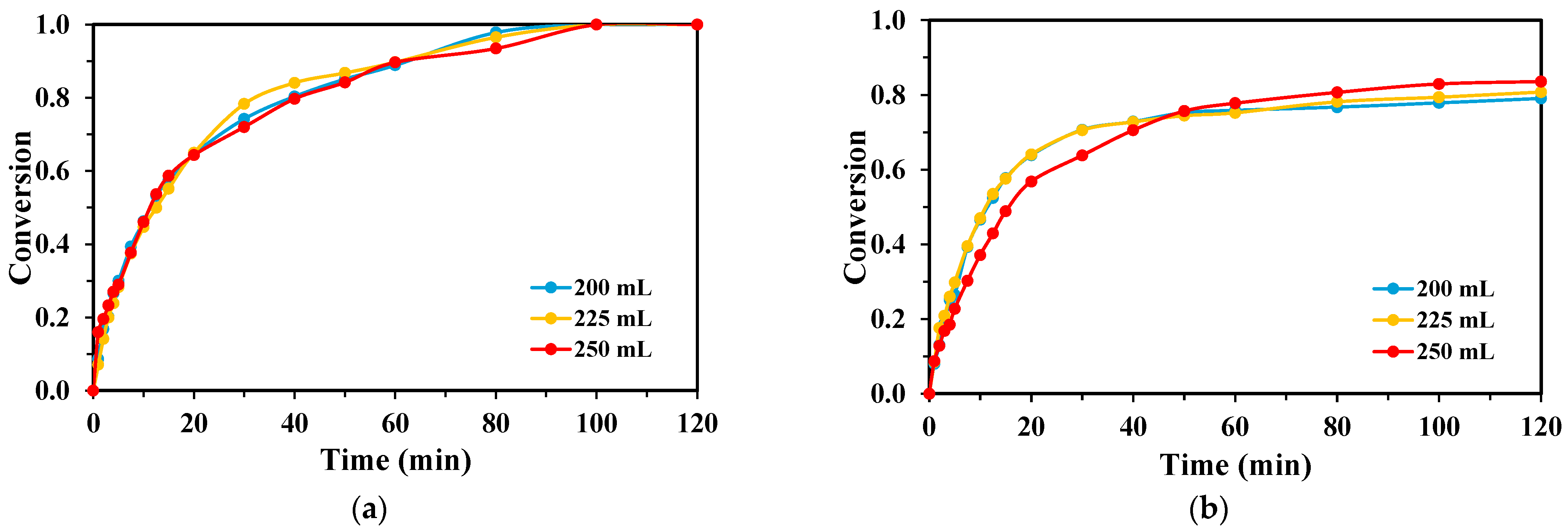
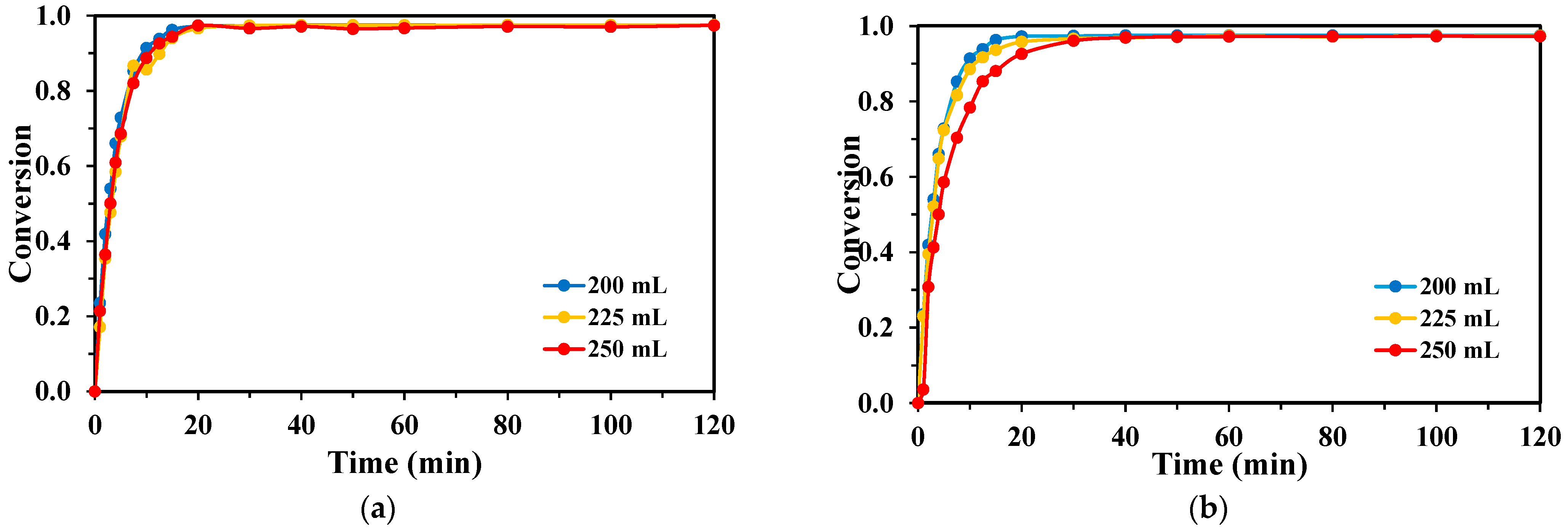
2.3. Effect of Reaction Volume
2.4. Chemical Oxygen Demand
3. Discussion
3.1. Experimental Series
3.1.1. Superior Efficacy of the KrCl Excimer Lamp
3.1.2. Influence of H2O2/Monomer Ratio and Oxidant Scavenging
3.1.3. Effect of Initial Monomer Concentration and Inner Filter Effect
3.2. Chemical Oxygen Demand Analysis
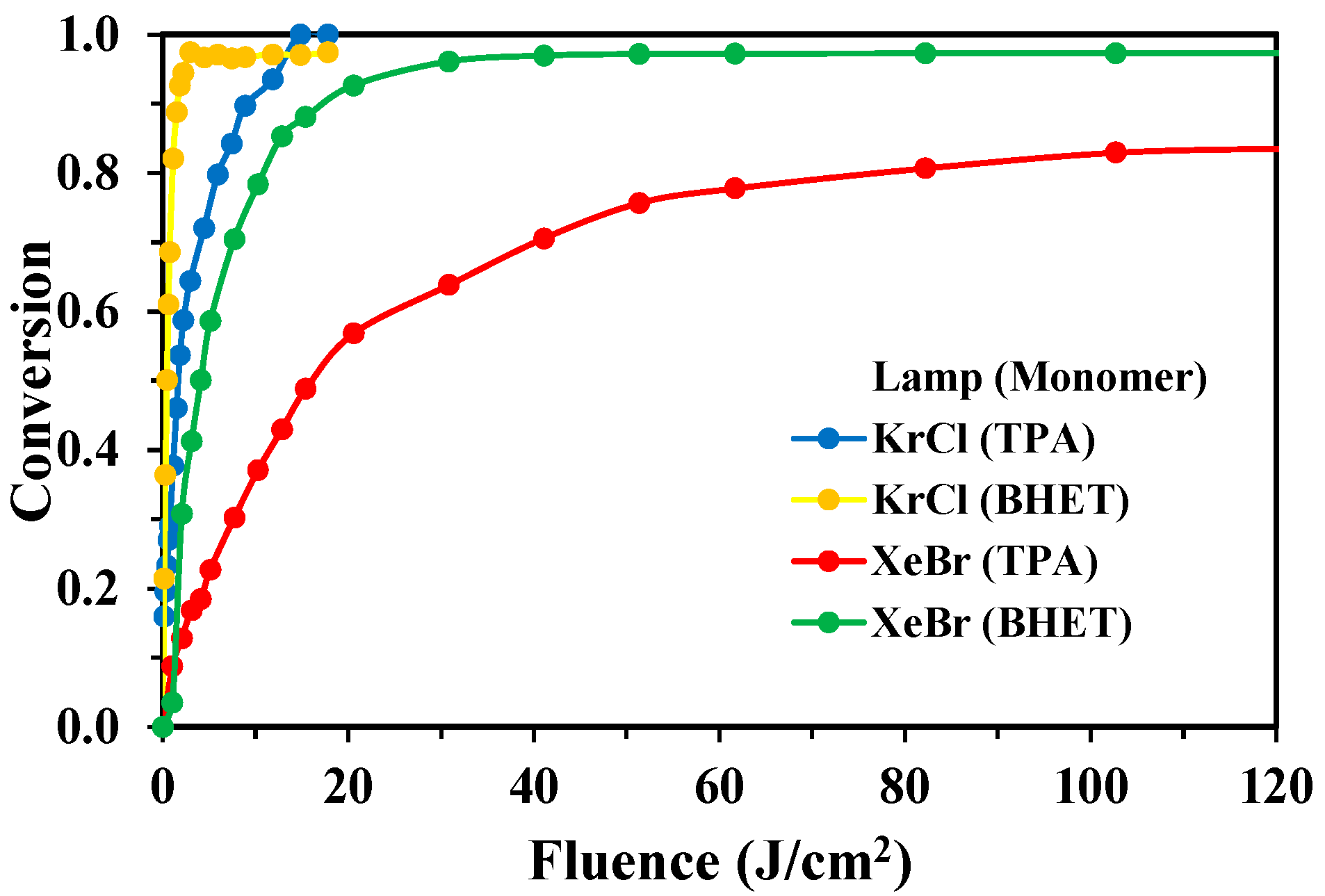
3.3. Fluence-Based Analysis of Degradation Efficiency
4. Materials and Methods
4.1. Materials
4.2. Experimental Setup
- A Krypton Chloride (KrCl) excimer lamp with a peak monochromatic emission at 222 nm and an average radiation intensity of 2.47 mW cm−2 at the solution surface.
- A Xenon Bromide (XeBr) excimer lamp with a peak monochromatic emission at 283 nm and an average radiation intensity of 17.12 mW cm−2 at the solution surface.
4.3. Experimental Design
4.3.1. Series 1: Effect of H2O2/Monomer Mass Ratio
4.3.2. Series 2: Effect of Initial Monomer Concentration
4.3.3. Series 3: Effect of Reaction Volume
4.4. Analytical Methods
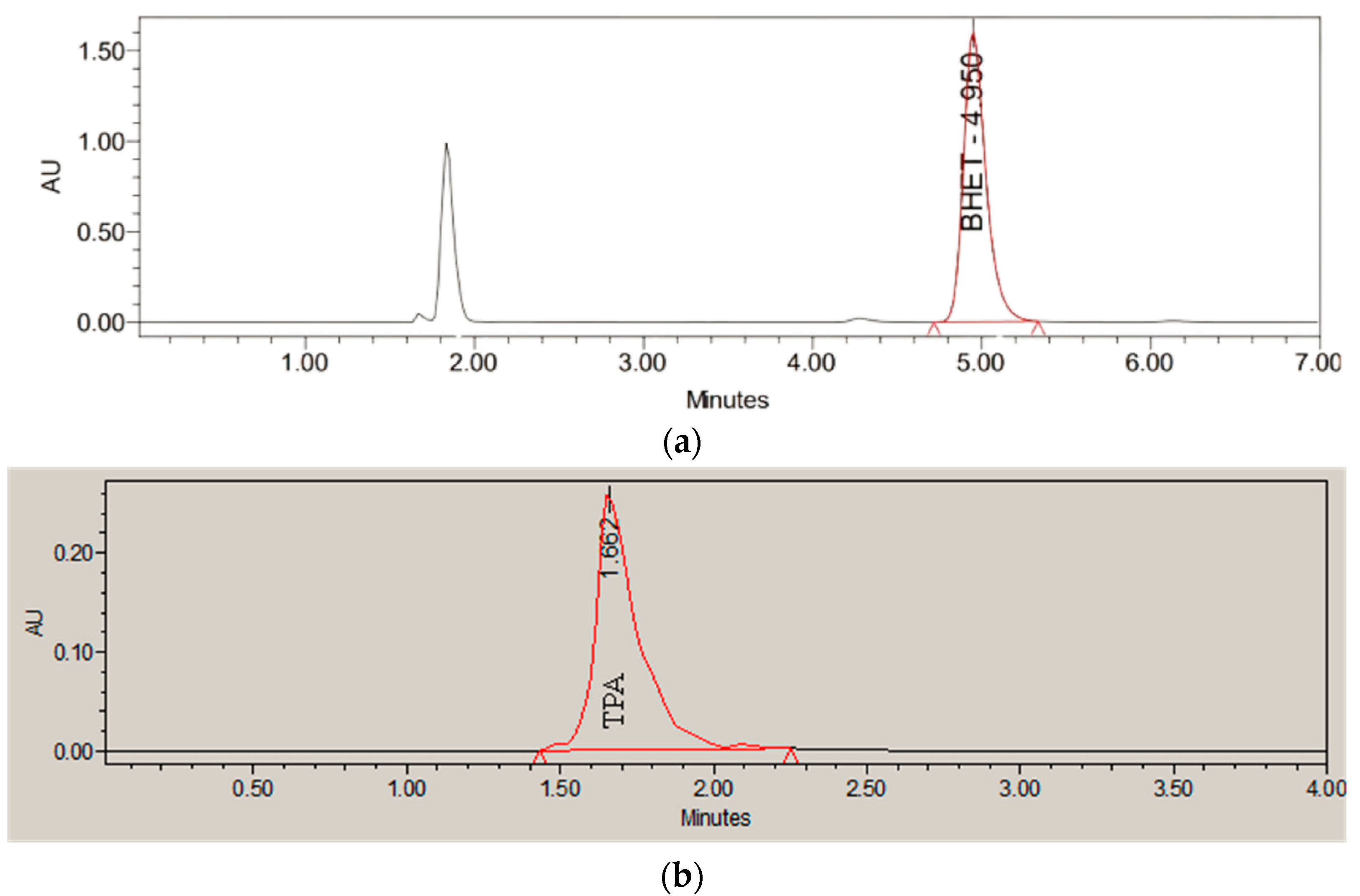
4.4.1. Monomer Quantification
4.4.2. Chemical Oxygen Demand Quantification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CSIC. Combatir La Contaminación Por Plásticos. 2023. Available online: https://www.idaea.csic.es/wp-content/uploads/2023/11/Ciencia-para-las-politicas-publicas-CSIC_-Combatir-la-contaminacion-por-plasticos.-2023.pdf (accessed on 4 May 2025).
- Zhang, Q.; Wang, X.; Chen, Y.; Song, G.; Zhang, H.; Huang, K.; Luo, Y.; Cheng, N. Discovery and solution for microplastics: New risk carriers in food. Food Chem. 2025, 471, 142784. [Google Scholar] [CrossRef]
- Dhaka, V.; Singh, S.; Anil, A.G.; Sunil Kumar Naik, T.S.; Garg, S.; Samuel, J.; Kumar, M.; Ramamurthy, P.C.; Singh, J. Occurrence, Toxicity and Remediation of Polyethylene Terephthalate Plastics. A Review. Environ. Chem. Lett. 2022, 20, 1777–1800. [Google Scholar] [CrossRef]
- Ho, K.L.; Yeap, S.P.; Lee, K.M. critical review of microplastics and nanoplastics in wastewater: Insights into adsorbent-based remediation strategies. Environ. Pollut. 2025, 382, 126658. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Kovačić, M.; Tomić, A.; Tonković, S.; Pulitika, A.; Papac Zjačić, J.; Katančić, Z.; Genorio, B.; Kušić, H.; Lončarić Božić, A. Pristine and UV-Weathered PET Microplastics as Water Contaminants: Appraising the Potential of the Fenton Process for Effective Remediation. Processes 2024, 12, 844. [Google Scholar] [CrossRef]
- Homin, K.; Yeojoon, Y.; Tae-Mun, H. Changes in physical and chemical properties of microplastics by ozonation. Process Saf. Environ. Prot. 2024, 192, 1062–1072. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, J.; Wang, J.H. Chemical degradation and recycling of polyethylene terephthalate (PET): A review. RSC Sustain. 2025, 3, 2111–2133. [Google Scholar] [CrossRef]
- Bermúdez, L.A.; Pascual, J.M.; Martínez, M.D.M.M.; Poyatos Capilla, J.M. Effectiveness of Advanced Oxidation Processes in Wastewater Treatment: State of the Art. Water 2021, 13, 2094. [Google Scholar] [CrossRef]
- Najafinejad, M.S.; Chianese, S.; Fenti, A.; Iovino, P.; Musmarra, D. Application of Electrochemical Oxidation for Water and Wastewater Treatment: An Overview. Molecules 2023, 28, 4208. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Chen, J.; Jinzhou, L.; Yang, J. Persulfate-based advanced oxidation reforming of polyethylene terephthalate fiber into formate via singlet oxygen activation. Adv. Fiber Mater. 2025, 7, 664–677. [Google Scholar] [CrossRef]
- Bouzayani, B.; Elaoud, S.C.; Sanromán, M.Á. Current progress in advanced oxidation processes for the removal of contaminants of emerging concern using peracetic acid as an effective oxidant. Catalysts 2025, 15, 469. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Pishbin, E. Ozone-based advanced oxidation processes in water treatment: Recent advances, challenges, and perspective. Environ. Sci. Pollut. Res. 2025, 32, 3531–3570. [Google Scholar] [CrossRef]
- Kosar Hikmat, H.A.; Fryad, S.M.; Mozart, A.H.K. Pharmaceutical pollution in the aquatic environment: Advanced oxidation processes as efficient treatment approaches: A review. Mater. Adv. 2023, 6, 3433–3454. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Dionysiou, D.D. Advanced oxidation processes for removal of emerging contaminants in water. Water 2023, 15, 398. [Google Scholar] [CrossRef]
- Conradie, W.; Dorfling, C.; Chimphango, A.; Booth, A.M.; Sørensen, L.; Akdogan, G. Investigating the Physicochemical Property Changes of Plastic Packaging Exposed to UV Irradiation and Different Aqueous Environments. Microplastics 2022, 1, 456–476. [Google Scholar] [CrossRef]
- Boyd, I.W.; Liaw, I.I. Development and application of UV excimer lamps from 354 nm–126 nm. Proc. High-Power Laser Ablation VI 2006, 6261, 27–42. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Boyd, I.W. Lifetime investigation of excimer UV sources. Appl. Surf. Sci. 2020, 168, 296–299. [Google Scholar] [CrossRef]
- Murcia, M.D.; Gómez, M.; Gómez, E.; Gomez, J.L.; Hidalgo, A.M.; Murcia, S.; Campos, D. Comparison of two excilamps and two reactor configurations in the UV-H2O2 removal process of amaranth. J. Water Process Eng. 2020, 33, 101051. [Google Scholar] [CrossRef]
- Wu, S.; Wu, Y.; Lu, X.; Hu, X.; Brighwell, G. Challenges of Far UVC 222 nm radiation for food safety applications. Food Control 2025, 171, 111132. [Google Scholar] [CrossRef]
- Murcia, M.D.; Gómez, M.; Gómez, E.; Gómez, J.L.; Christofi, N. Photodegradation of congo red using XeBr, KrCl and Cl2 barrier discharge excilamps: A kinetics study. Desalination 2011, 281, 364–371. [Google Scholar] [CrossRef]
- Gómez, M.; Murcia, M.D.; Gómez, E.; Gómez, J.L.; Christofi, N. Degradation of phenolic pollutants using KrCl and XeBr excilamps in the presence of dye: A comparative study. Desalination 2011, 274, 156–163. [Google Scholar] [CrossRef]
- Gomez, M.; Murcia, M.D.; Christofi, N.; Gomez, E.; Gomez, J.L. Photodegradation of 4-chlorophenol using XeBr, KrCl and Cl2 barrier-discharge excilamps: A comparative study. Chem. Eng. J. 2010, 158, 120–128. [Google Scholar] [CrossRef]
- Talinli, I.; Anderson, G.K. Interference of hydrogen peroxide on the standard cod test. Water Res. 1992, 26, 107–110. [Google Scholar] [CrossRef]
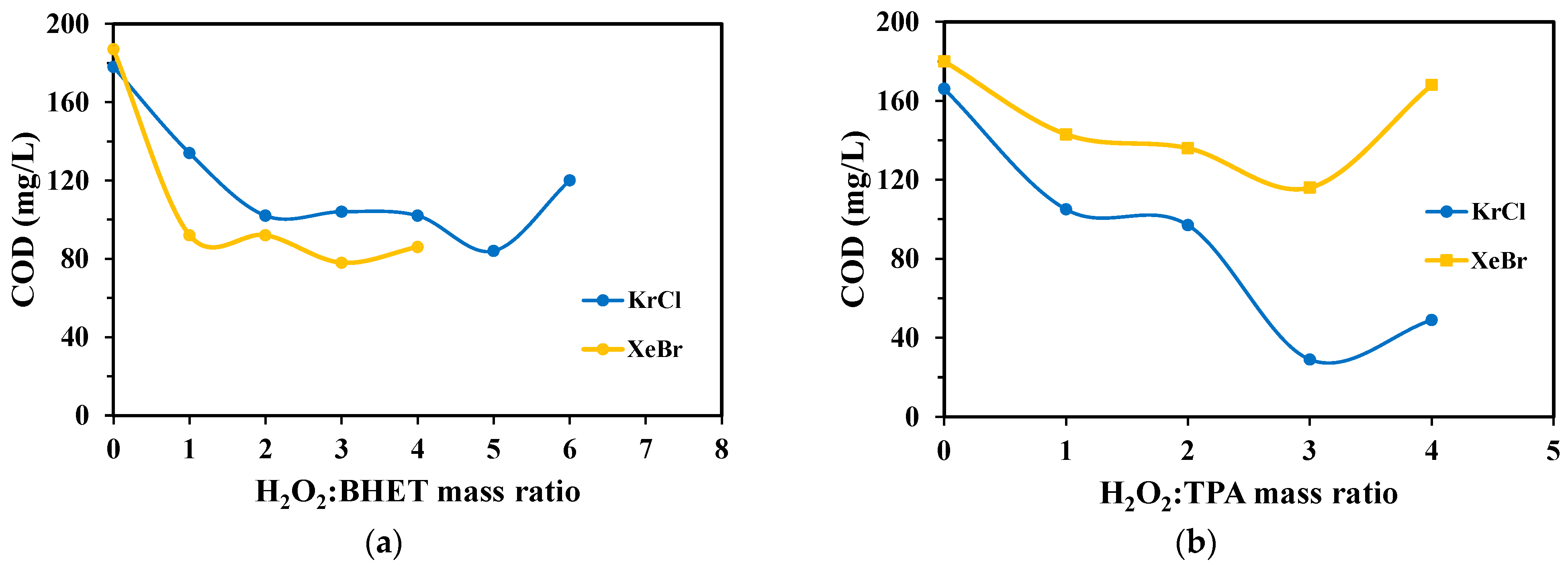
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-García, Á.; Gómez, M.; Murcia, M.D.; Gómez, E.; Hidalgo, A.M.; Dorado, L.A.; Bastida, J. Photodegradation of Polyethylene Terephthalate and Bis(2-hydroxyethyl) Terephthalate Using Excimer Lamps and Hydrogen Peroxide: A Strategy for PET–Derived Waste Treatment. Molecules 2025, 30, 3302. https://doi.org/10.3390/molecules30153302
Navarro-García Á, Gómez M, Murcia MD, Gómez E, Hidalgo AM, Dorado LA, Bastida J. Photodegradation of Polyethylene Terephthalate and Bis(2-hydroxyethyl) Terephthalate Using Excimer Lamps and Hydrogen Peroxide: A Strategy for PET–Derived Waste Treatment. Molecules. 2025; 30(15):3302. https://doi.org/10.3390/molecules30153302
Chicago/Turabian StyleNavarro-García, Ángel, María Gómez, María D. Murcia, Elisa Gómez, Asunción M. Hidalgo, Luis A. Dorado, and Josefa Bastida. 2025. "Photodegradation of Polyethylene Terephthalate and Bis(2-hydroxyethyl) Terephthalate Using Excimer Lamps and Hydrogen Peroxide: A Strategy for PET–Derived Waste Treatment" Molecules 30, no. 15: 3302. https://doi.org/10.3390/molecules30153302
APA StyleNavarro-García, Á., Gómez, M., Murcia, M. D., Gómez, E., Hidalgo, A. M., Dorado, L. A., & Bastida, J. (2025). Photodegradation of Polyethylene Terephthalate and Bis(2-hydroxyethyl) Terephthalate Using Excimer Lamps and Hydrogen Peroxide: A Strategy for PET–Derived Waste Treatment. Molecules, 30(15), 3302. https://doi.org/10.3390/molecules30153302









