Casein-Based Biomaterials: Fabrication and Wound Healing Applications
Abstract
1. Casein: A Milk-Derived Protein
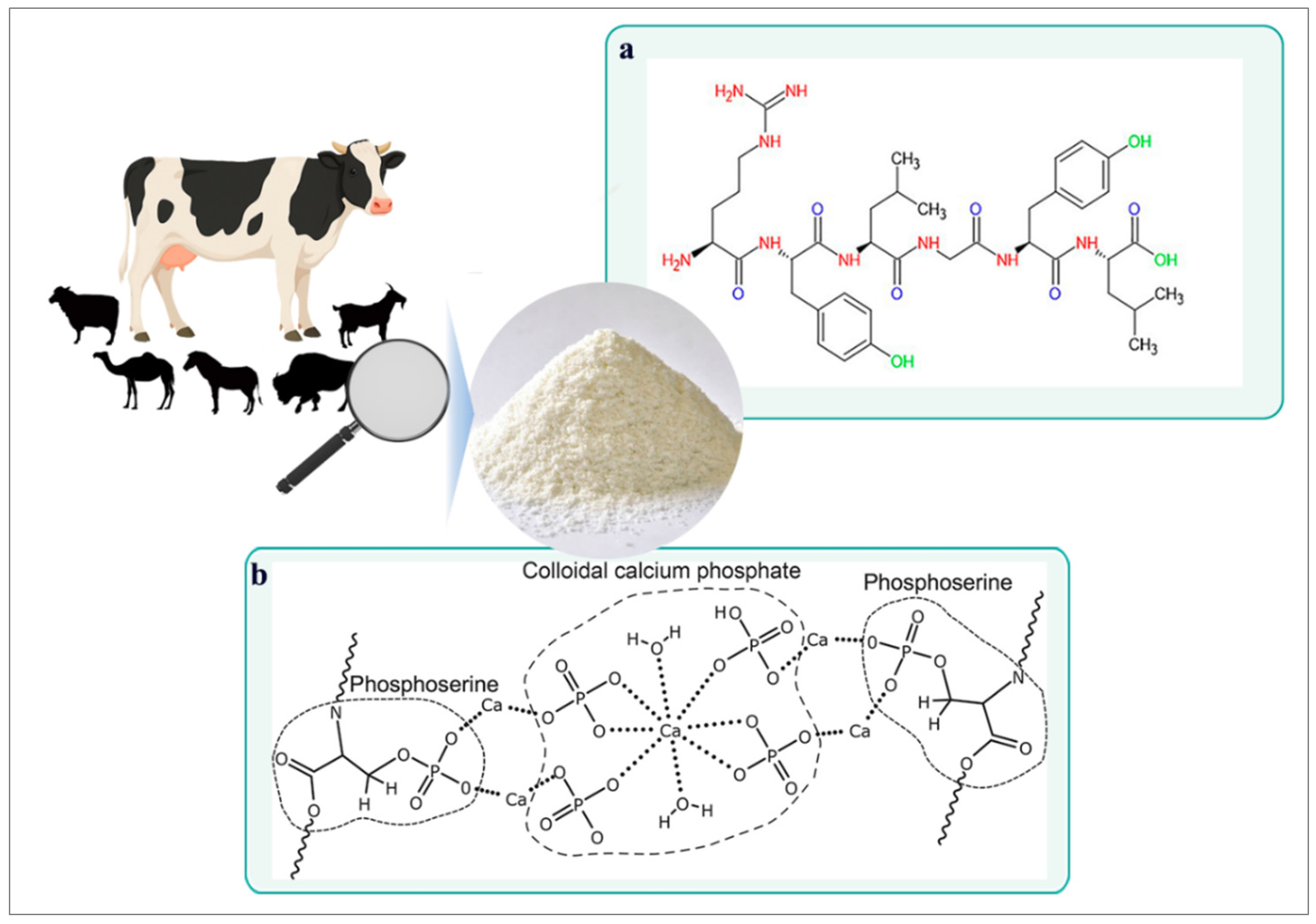
2. Structural and Functional Properties of Casein
2.1. Casein Micelle Structure
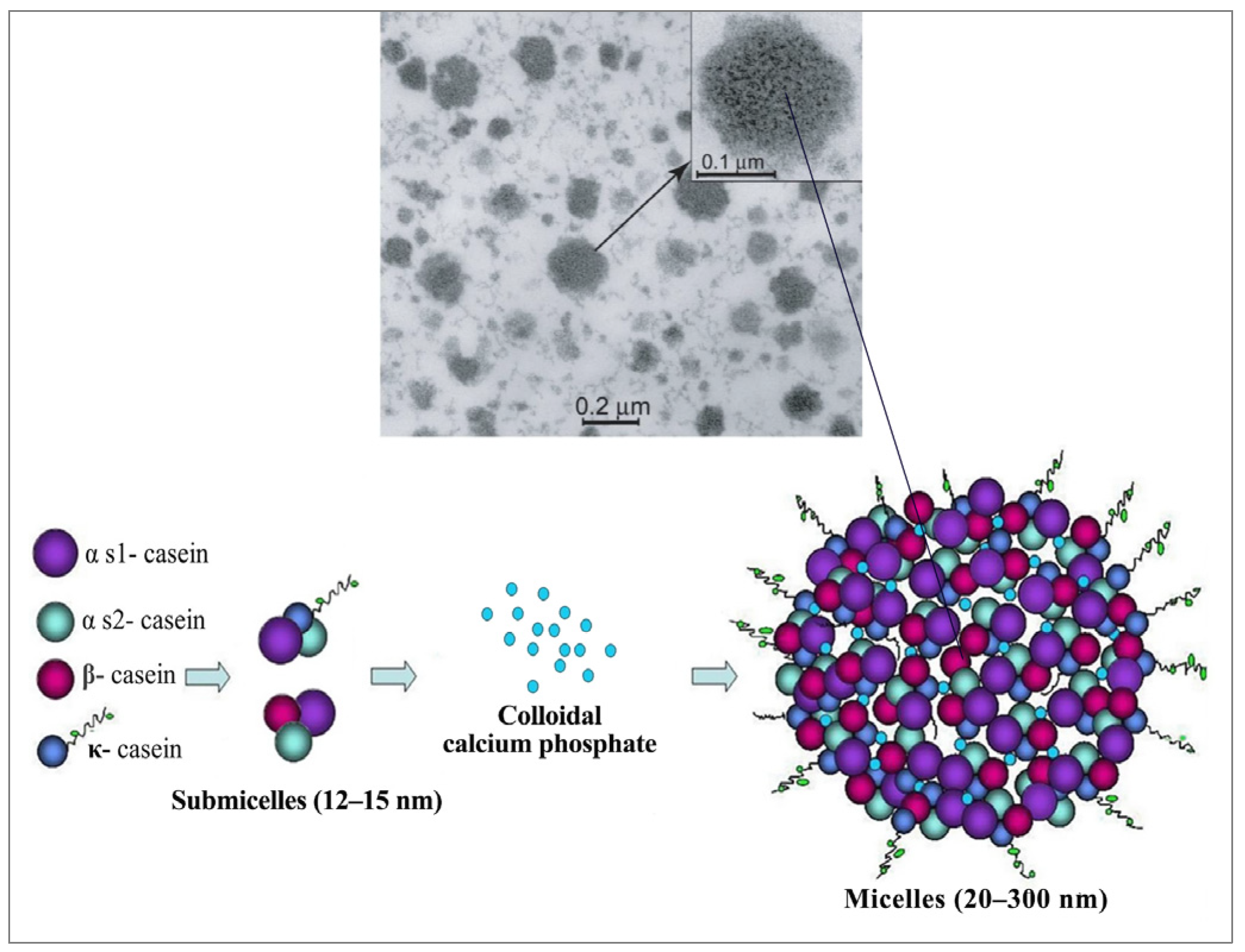
2.2. Casein-Based Biomaterials: Properties and Derivatives
3. Wound Healing and the Role of Casein-Based Biomaterials
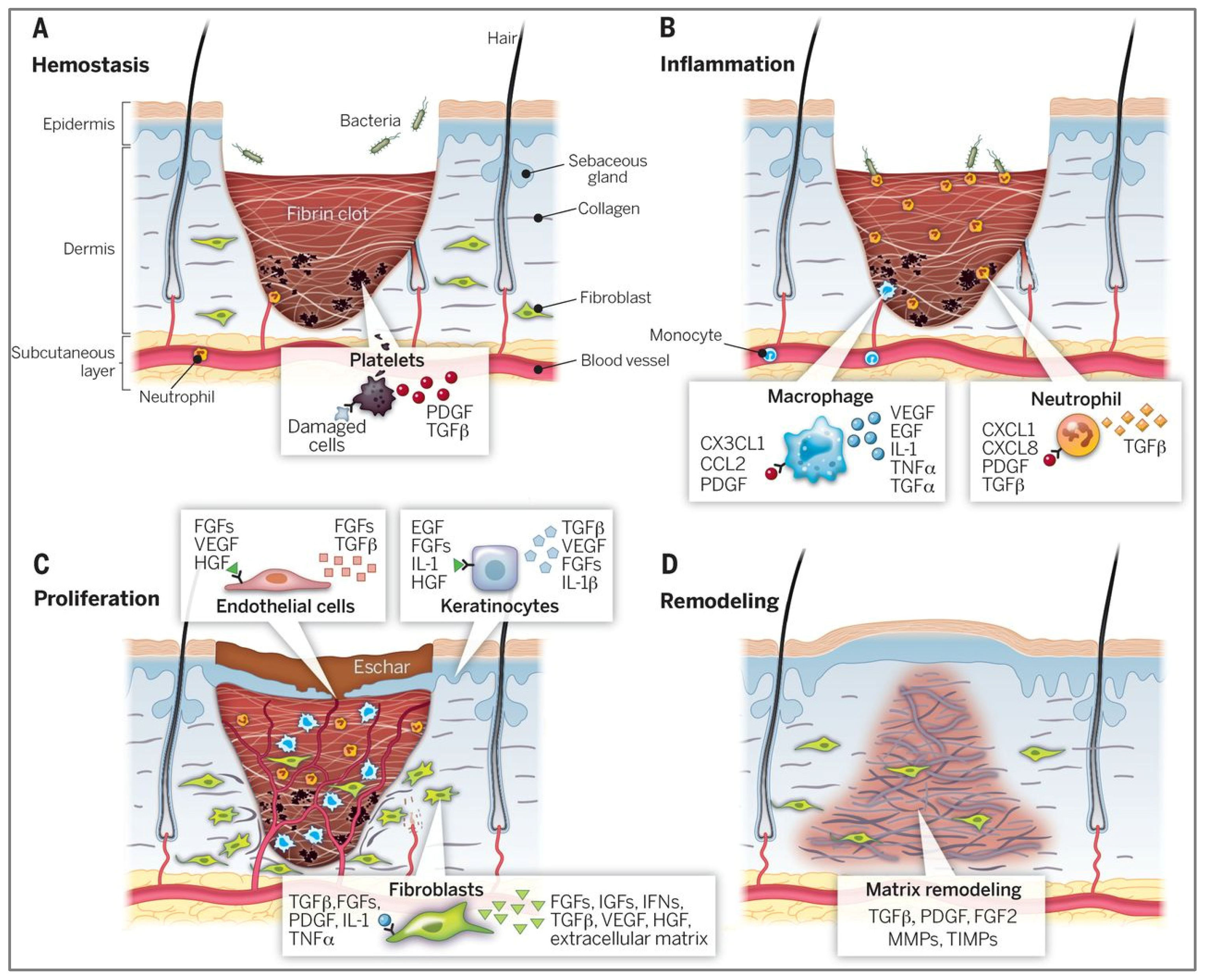
4. Fabrication of Casein-Based Wound Dressings
4.1. Casein-Based Hydrogels
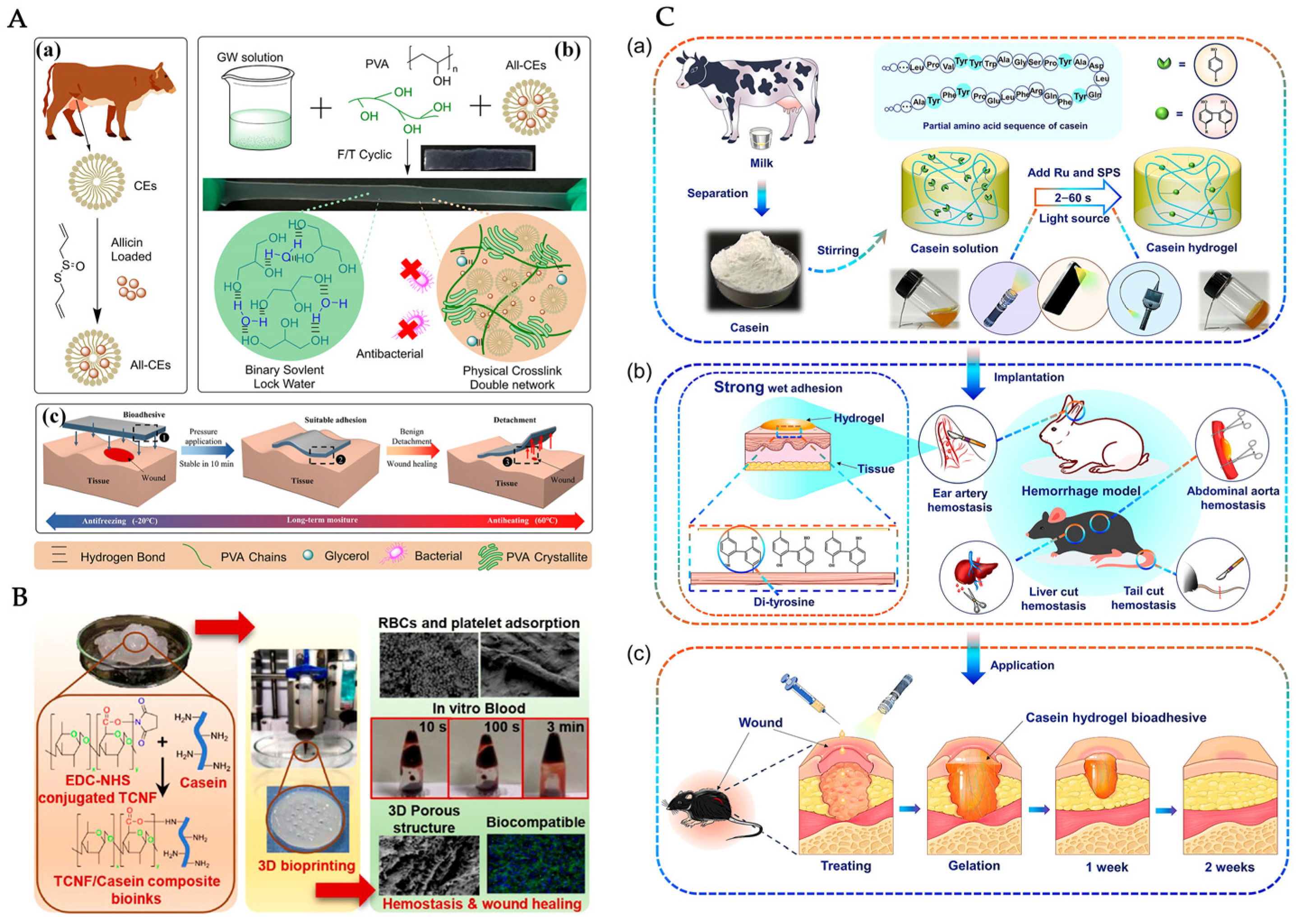
| Hydrogel Composition | Fabrication Method | Key Outcomes and Wound Healing Relevance | Ref. |
|---|---|---|---|
| Casein micelles, PVA, glycerol–water solvent | Organohydrogel formation via self-assembly | Mechanical strength, tissue adhesion for wound healing, moisture retention, and antibacterial properties | [87] |
| Cellulose nanofibrils, chitosan, casein | 3D bioprinting of composite bio-inks | Blood clotting acceleration and fibroblast proliferation; potential use in blood reduction for traumatic hemorrhage episodes | [88] |
| Casein, tris dichlororuthenium (II) hexahydrate (Ru), and sodium persulfate (SPS). | White-light-induced crosslinking using ruthenium-induced catalysis | Ultrafast gelation, bioadhesive, highly adaptable and suitable for first aid wound treatment | [72] |
| Sodium caseinate (Na Cas), gelatine, thyme oil | Essential oil encapsulation in Na Cas micelles via solvent evaporation method | Antibacterial activity through bacterial membrane disruption; in vivo wound healing potential, within ~15% faster epithelialization | [89] |
| Casein sodium salt, acid casein, Octiset® or polyhexanide | Synthesis via acrylamide polymerization and inducing casein micelle coagulation | Sustained drug release and antimicrobial activity against common wound pathogen | [74] |
| Polyacrylamide, casein micelles | High elasticity and durability; mechanical resilience and notch-insensitive gels for tissue engineering | [90] |
4.2. Films
4.3. Fibers/Mats
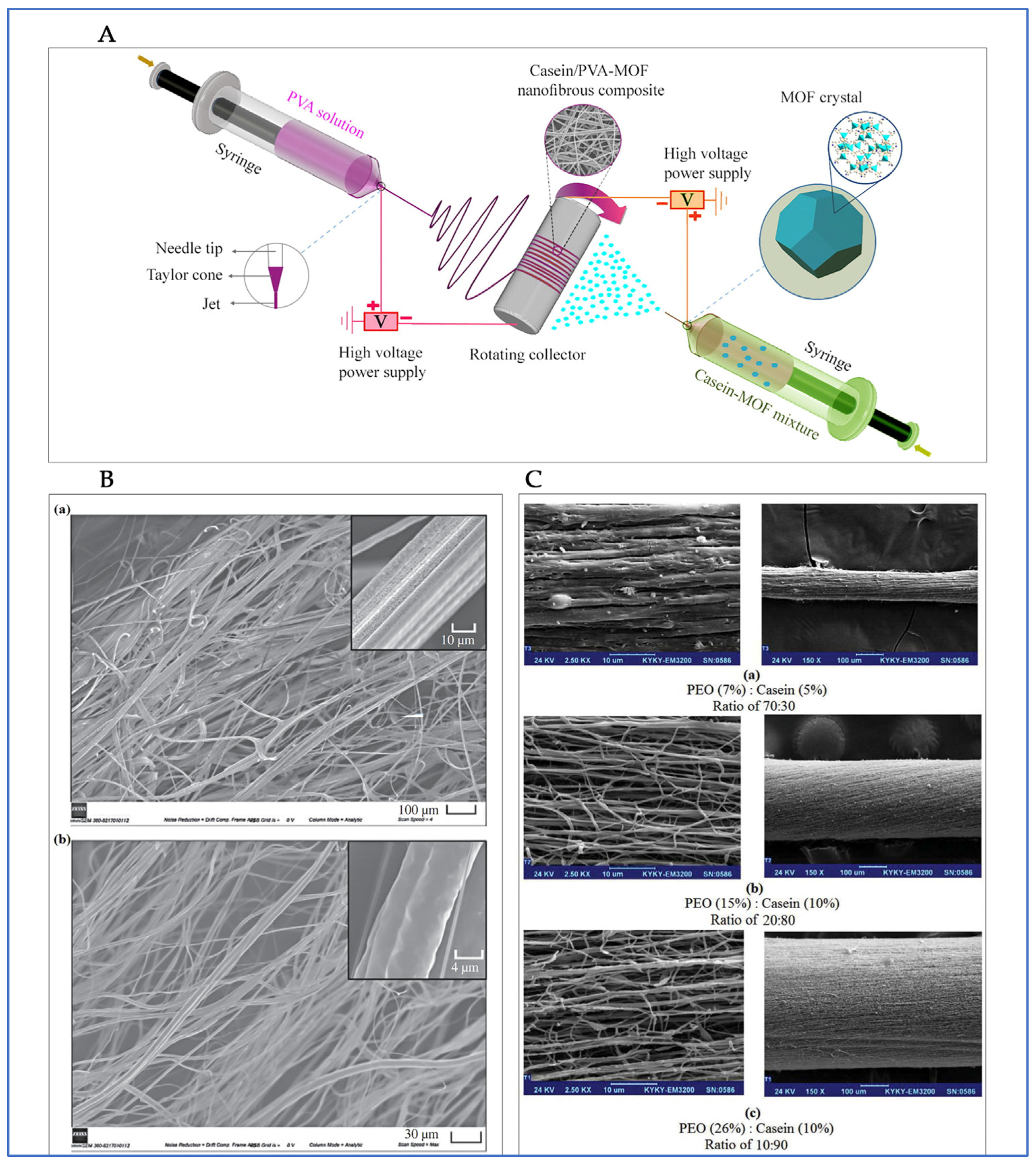
4.4. Other Casein-Based Delivery Systems
5. Functional Biological and Therapeutic Effects of Casein-Based Wound Dressings
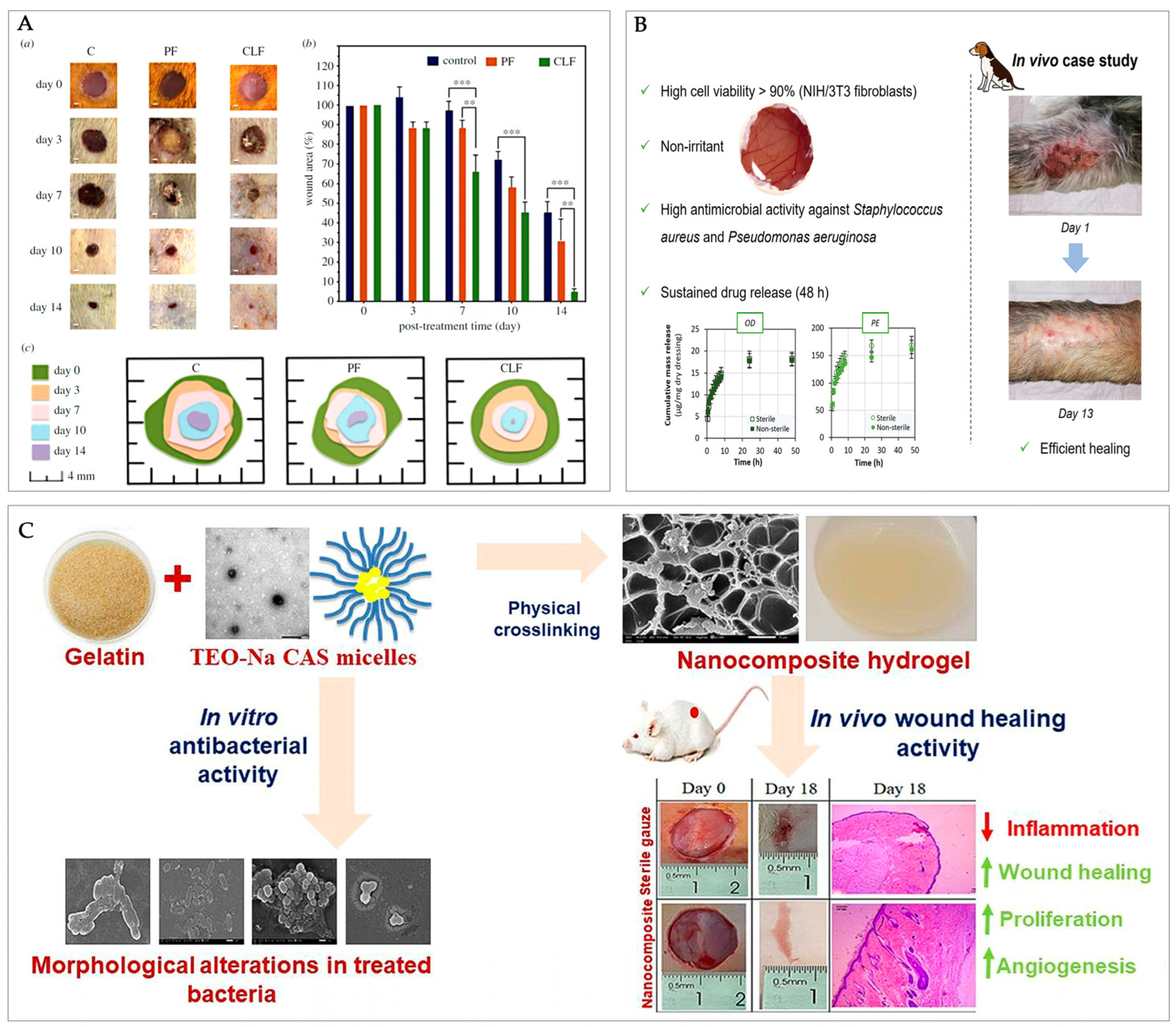
6. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Singh, L.R.; Bhat, M.Y.; Dar, T.A. Casein Proteins: Structural and Functional Aspects. In Milk Proteins-From Structure to Biological Properties and Health Aspects; Gigli, I., Ed.; IntechOpen: Rijeka, Croatia, 2016; p. 296. [Google Scholar]
- Dhasmana, S.; Das, S.; Shrivastava, S. Potential nutraceuticals from the casein fraction of goat’s milk. J. Food Biochem. 2022, 46, e13982. [Google Scholar] [CrossRef] [PubMed]
- Rahmatalla, S.A.; Arends, D.; Brockmann, G.A. Review: Genetic and protein variants of milk caseins in goats. Front. Genet. 2022, 13, 995349. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, Structure, and Digestive Dynamics of Milk from Different Species—A Review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.K.; Verma, M. Post translational modifications of milk proteins in geographically diverse goat breeds. Sci. Rep. 2021, 11, 5619. [Google Scholar] [CrossRef]
- Goulding, D.A.; Fox, P.F.; O’Mahony, J.A. Chapter 2-Milk proteins: An overview. In Milk Proteins, 3rd ed.; Boland, M., Singh, H., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 21–98. [Google Scholar]
- Ji, Z.; Yu, Z.; Du, Q.; Fan, R.; Wang, J.; Han, R.; Yang, Y. Comparative proteomic study of casein micelles in human and animal milks for infant nutrition improvement. LWT 2025, 220, 117560. [Google Scholar] [CrossRef]
- Hindmarsh, J.P.; Watkinson, P. Experimental evidence for previously unclassified calcium phosphate structures in the casein micelle. J. Dairy Sci. 2017, 100, 6938–6948. [Google Scholar] [CrossRef]
- Hewa, B.; Pagel, C.N.; Raynes, J.K.; Ranadheera, C.S.; Logan, A. The effect of casein genetic variants, glycosylation and phosphorylation on bovine milk protein structure, technological properties, nutrition and product manufacture. Int. Dairy J. 2022, 133, 105440. [Google Scholar] [CrossRef]
- Broyard, C.; Gaucheron, F. Modifications of structures and functions of caseins: A scientific and technological challenge. Dairy Sci. Technol. 2015, 95, 831–862. [Google Scholar] [CrossRef]
- Ahmadi, E.; Markoska, T.; Huppertz, T.; Vasiljevic, T. Structural Properties of Casein Micelles with Adjusted Micellar Calcium Phosphate Content. Foods 2024, 13, 322. [Google Scholar] [CrossRef]
- Sadiq, U.; Gill, H.; Chandrapala, J. Casein Micelles as an Emerging Delivery System for Bioactive Food Components. Foods 2021, 10, 1965. [Google Scholar] [CrossRef]
- Markoska, T.; Daniloski, D.; Vasiljevic, T.; Huppertz, T. Structural Changes of beta-Casein Induced by Temperature and pH Analysed by Nuclear Magnetic Resonance, Fourier-Transform Infrared Spectroscopy, and Chemometrics. Molecules 2021, 26, 7650. [Google Scholar] [CrossRef] [PubMed]
- Walstra, P. Casein sub-micelles: Do they exist? Int. Dairy J. 1999, 9, 189–192. [Google Scholar] [CrossRef]
- Rebouillat, S.; Ortega-Requena, S. Potential Applications of Milk Fractions and Valorization of Dairy By-Products: A Review of the State-of-the-Art Available Data, Outlining the Innovation Potential from a Bigger Data Standpoint. J. Biomater. Nanobiotechnology 2015, 06, 176–203. [Google Scholar] [CrossRef]
- Runthala, A.; Mbye, M.; Ayyash, M.; Xu, Y.; Kamal-Eldin, A. Caseins: Versatility of Their Micellar Organization in Relation to the Functional and Nutritional Properties of Milk. Molecules 2023, 28, 2023. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.S. Chapter 6-Casein micelle structure and stability. In Milk Proteins, 3rd ed.; Boland, M., Singh, H., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 213–250. [Google Scholar]
- Gangnard, S.; Zuev, Y.; Gaudin, J.-C.; Fedotov, V.; Choiset, Y.; Axelos, M.A.V.; Chobert, J.-M.; Haertlé, T. Modifications of the charges at the N-terminus of bovine β-casein: Consequences on its structure and its micellisation. Food Hydrocoll. 2007, 21, 180–190. [Google Scholar] [CrossRef]
- Cippollini, R.; France, T.C.; Huppertz, T.; O’Mahony, J.A. Artificial and reformed casein micelles as encapsulation vehicles: Approaches and applications. Crit. Rev. Food Sci. Nutr. 2025. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z. Improved encapsulation capacity of casein micelles with modified structure. J. Food Eng. 2022, 333, 111138. [Google Scholar] [CrossRef]
- Portnaya, I.; Avni, S.; Kesselman, E.; Boyarski, Y.; Sukenik, S.; Harries, D.; Dan, N.; Cogan, U.; Danino, D. Competing processes of micellization and fibrillization in native and reduced casein proteins. Phys. Chem. Chem. Phys. 2016, 18, 22516–22525. [Google Scholar] [CrossRef]
- Carver, J.A.; Holt, C. Functional and dysfunctional folding, association and aggregation of caseins. Adv. Protein Chem. Struct. Biol. 2019, 118, 163–216. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Jiang, C.; Xie, A.; Yue, X.; Li, M. A review of casein phosphopeptides: From enrichment identification to biological properties. Food Biosci. 2024, 59, 104217. [Google Scholar] [CrossRef]
- Markoska, T. Influence of pH and solids content on heat-induced changes in structural arrangements of proteins in milk. Mljekarstvo 2021, 71, 95–105. [Google Scholar] [CrossRef]
- O’Mahony, J.A.; Fox, P.F. Milk Proteins: Introduction and Historical Aspects. In Advanced Dairy Chemistry; Springer: Berlin/Heidelberg, Germany, 2013; pp. 43–85. [Google Scholar]
- Wu, J.; Chen, S.; Van der Meeren, P. Heat Stability Assessment of Milk: A Review of Traditional and Innovative Methods. Foods 2024, 13, 2236. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.; Moraru, C.I. Heat stability of micellar casein concentrates as affected by temperature and pH. J. Dairy Sci. 2012, 95, 6339–6350. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; Gebhardt, R. Influence of Post-Treatment Temperature on the Stability and Swelling Behavior of Casein Microparticles. Macromol. Mater. Eng. 2023, 308, 2200661. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Z. Acid and rennet-induced coagulation behavior of casein micelles with modified structure. Food Chem. 2019, 291, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Sinaga, H.; Bansal, N.; Bhandari, B. Effects of milk pH alteration on casein micelle size and gelation properties of milk. Int. J. Food Prop. 2016, 20, 179–197. [Google Scholar] [CrossRef]
- Sarode, A.R.; Sawale, P.D.; Khedkar, C.D.; Kalyankar, S.D.; Pawshe, R.D. Casein and Caseinate: Methods of Manufacture. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 676–682. [Google Scholar]
- Chen, X.; Fan, R.; Wang, Y.; Munir, M.; Li, C.; Wang, C.; Hou, Z.; Zhang, G.; Liu, L.; He, J. Bovine milk β-casein: Structure, properties, isolation, and targeted application of isolated products. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13311. [Google Scholar] [CrossRef]
- Fuchs, A.; Stroinski, D.; Gruman, A.; Lewis, G. Casein Functionalization Using High-Pressure Homogenization and Emulsifying Salts. Polymers 2025, 17, 931. [Google Scholar] [CrossRef]
- Ye, R.; Harte, F. High pressure homogenization to improve the stability of casein–hydroxypropyl cellulose aqueous systems. Food Hydrocoll. 2014, 35, 670–677. [Google Scholar] [CrossRef]
- Wang, C.; Ma, Y.; Liu, B.; Kang, Z.; Geng, S.; Wang, J.; Wei, L.; Ma, H. Effects of dynamic ultra-high pressure homogenization on the structure and functional properties of casein. Int. J. Agric. Biol. Eng. 2019, 12, 229–234. [Google Scholar] [CrossRef]
- Ma, B.; Al-Wraikat, M.; Shu, Q.; Yang, X.; Liu, Y. An Overview of Interactions between Goat Milk Casein and Other Food Components: Polysaccharides, Polyphenols, and Metal Ions. Foods 2024, 13, 2903. [Google Scholar] [CrossRef]
- Ann, M.; Oliver, C.M.; Hemar, Y. Casein, Caseinates, and Milk Protein Concentrates. In Dairy Ingredients for Food Processing; Wiley-Blackwell: Oxford, UK, 2011; pp. 161–178. [Google Scholar]
- Madan, J.R.; Ansari, I.N.; Dua, K.; Awasthi, R. Formulation and In Vitro Evaluation of Casein Nanoparticles as Carrier for Celecoxib. Adv. Pharm. Bull. 2020, 10, 408–417. [Google Scholar] [CrossRef]
- Liu, S.; Yu, H.; Huang, K. Structural characteristics and biocompatibility of a casein-based nanocomposite for potential biomedical applications. J. Mater. Sci. 2017, 53, 3959–3971. [Google Scholar] [CrossRef]
- Dumitriu, R.P.; Stoleru, E.; Rosnes, J.T.; Sharmin, N.; Doroftei, F.; Brebu, M. Rheological properties influence on the electrospinning of caseinate for loading with antioxidant rosemary extract. Food Hydrocoll. 2024, 151, 109883. [Google Scholar] [CrossRef]
- Pojanavaraphan, T.; Magaraphan, R.; Chiou, B.S.; Schiraldi, D.A. Development of biodegradable foamlike materials based on casein and sodium montmorillonite clay. Biomacromolecules 2010, 11, 2640–2646. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Di, L.; Ren, Q.-S.; Wang, J.-Y. Applications and Degradation of Proteins Used as Tissue Engineering Materials. Materials 2009, 2, 613–635. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Bulk Drug Substances Nominated for Use in Compounding Under Section 503A of the Federal Food, Drug, and Cosmetic Act; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2024.
- Yaşayan, G.; Alarçin, E.; Bal-Öztürk, A.; Avci-Adali, M. Chapter 11-Natural polymers for wound dressing applications. In Studies in Natural Products Chemistry; Rahman, A.-U., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 74, pp. 367–441. [Google Scholar]
- Abdallah, F.; Mijouin, L.; Pichon, C. Skin Immune Landscape: Inside and Outside the Organism. Mediat. Inflamm. 2017, 2017, 5095293. [Google Scholar] [CrossRef] [PubMed]
- Nagle, S.M.; Stevens, K.A.; Wilbraham, S.C. Wound Assessment. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482198/ (accessed on 8 July 2025).
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of Wound Healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, AU, USA, 2011; p. 23. [Google Scholar]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470443/ (accessed on 8 July 2025).
- De Luca, I.; Pedram, P.; Moeini, A.; Cerruti, P.; Peluso, G.; Di Salle, A.; Germann, N. Nanotechnology Development for Formulating Essential Oils in Wound Dressing Materials to Promote the Wound-Healing Process: A Review. Appl. Sci. 2021, 11, 1713. [Google Scholar] [CrossRef]
- Jin, S.; Newton, M.A.A.; Cheng, H.; Zhang, Q.; Gao, W.; Zheng, Y.; Lu, Z.; Dai, Z.; Zhu, J. Progress of Hydrogel Dressings with Wound Monitoring and Treatment Functions. Gels 2023, 9, 694. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Norahan, M.H.; Pedroza-Gonzalez, S.C.; Sanchez-Salazar, M.G.; Alvarez, M.M.; Trujillo de Santiago, G. Structural and biological engineering of 3D hydrogels for wound healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Zhao, Z.; Li, J.; Huang, Y.; Chen, W. Multifunctional dressings for wound exudate management. Progress. Mater. Sci. 2024, 146, 101328. [Google Scholar] [CrossRef]
- Bishop, S.M.; Walker, M.; Rogers, A.A.; Chen, W.Y. Importance of moisture balance at the wound-dressing interface. J. Wound Care 2003, 12, 125–128. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.M.; Gan, Y.C.; Qiu, X.Z.; Gao, Z.C.; Wang, H.; Chen, S.X.; Xiong, Y.; Liu, G.H.; Lin, S.E.; et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: New biosynthesis methods, recent advances, and emerging applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef]
- Pei, J.; Zhu, Q.; Zhu, Y.; Guo, C.; Valencak, T.G.; Tang, S.Y.; Ren, T.; Ren, D. Milk protein-based hydrogels: Development and biomedical applications. Biomater. Transl. 2025, 6, 127–150. [Google Scholar] [CrossRef]
- Folliero, V.; Lama, S.; Franci, G.; Giugliano, R.; D’Auria, G.; Ferranti, P.; Pourjula, M.; Galdiero, M.; Stiuso, P. Casein-derived peptides from the dairy product kashk exhibit wound healing properties and antibacterial activity against Staphylococcus aureus: Structural and functional characterization. Food Res. Int. 2022, 153, 110949. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Larki-Harchegani, A.; Shabib, S.; Jalali, A.; Rezaei, A.; Housmand, G. Wound healing property of milk in full thickness wound model of rabbit. Int. J. Surg. 2018, 54, 133–140. [Google Scholar] [CrossRef]
- Gundes, S.E.; Orenay Boyacioglu, S.; Abbak, M.; Bulca, S.; Boyacioglu, O. Whey proteins from camel’s milk have higher in vitro wound-healing effect than whey proteins from cow’s milk. J. Sci. Food Agric. 2025, 105, 2552–2558. [Google Scholar] [CrossRef]
- Xin, P.; Han, S.; Huang, J.; You, X.; Wu, J. Natural Soybean Milk-Derived Bioactive Coatings for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 34480–34487. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.E.; Han, G.; Lim, N.R.; Kim, E.H.; Jang, Y.; Cho, H.; Jang, H.; Kim, K.H.; Kim, S.H.; et al. Harnessing the Natural Healing Power of Colostrum: Bovine Milk-Derived Extracellular Vesicles from Colostrum Facilitating the Transition from Inflammation to Tissue Regeneration for Accelerating Cutaneous Wound Healing. Adv. Healthc. Mater. 2022, 11, e2102027. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Ma, X.; Liu, B.; Yang, Y.; Yang, Y.; Ren, T.; Li, Y. Antioxidant-Engineered Milk-Derived Extracellular Vesicles for Accelerating Wound Healing via Regulation of the PI3K-AKT Signaling Pathway. Adv. Healthc. Mater. 2023, 12, e2301865. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.D.; You, G.; Lee, M.; Mao, W.; So, C.; Byeon, C.; Hong, S.; Mok, H.; Yoo, H.S. Milk exosome-infused fibrous matrix for treatment of acute wound. J. Control Release 2024, 376, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Xia, Y.; Ma, Y.; Xu, X.; Luo, S.; Yi, J.; Wu, B.; Chen, R.; Wang, H.; Yu, H.; et al. Milk-derived exosomes as functional nanocarriers in wound healing: Mechanisms, applications, and future directions. Mater. Today Bio 2025, 32, 101715. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-H.; Hong, W.-J.; Li, C.-N.; Huang, Y.-H.; Tsai, J.-H.; Huang, C.-Y.; Wu, J.-C.; Kuo, C.-Y.; Kuo, W.-C. Colostrum-Derived Exosomal Lactoferrin Promotes Skin Fibroblast Regeneration by Suppressing Inflammatory Responses. Curr. Issues Mol. Biol. 2025, 47, 549. [Google Scholar] [CrossRef]
- Samuel, M.; Chisanga, D.; Liem, M.; Keerthikumar, S.; Anand, S.; Ang, C.S.; Adda, C.G.; Versteegen, E.; Jois, M.; Mathivanan, S. Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci. Rep. 2017, 7, 5933. [Google Scholar] [CrossRef]
- Kazimierska, K.; Kalinowska-Lis, U. Milk Proteins-Their Biological Activities and Use in Cosmetics and Dermatology. Molecules 2021, 26, 3253. [Google Scholar] [CrossRef]
- Han, G.; Kim, H.; Kim, D.E.; Ahn, Y.; Kim, J.; Jang, Y.J.; Kim, K.; Yang, Y.; Kim, S.H. The Potential of Bovine Colostrum-Derived Exosomes to Repair Aged and Damaged Skin Cells. Pharmaceutics 2022, 14, 307. [Google Scholar] [CrossRef]
- Rashidi, M.; Bijari, S.; Khazaei, A.H.; Shojaei-Ghahrizjani, F.; Rezakhani, L. The role of milk-derived exosomes in the treatment of diseases. Front. Genet. 2022, 13, 1009338. [Google Scholar] [CrossRef]
- Kocic, H.; Langerholc, T.; Kostic, M.; Stojanovic, S.; Najman, S.; Krstic, M.; Nesic, I.; Godic, A.; Wollina, U. The Regenerative Potential of Donkey and Human Milk on the Redox-Sensitive and Proliferative Signaling Pathways of Skin Fibroblasts. Oxid. Med. Cell Longev. 2020, 2020, 5618127. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, X.; Zhang, Y.; Ye, D.; Yu, K.; Cao, W.; Zhang, L.; Zheng, H.; Sun, Z.; Guo, C.; et al. White-light crosslinkable milk protein bioadhesive with ultrafast gelation for first-aid wound treatment. Biomater. Res. 2023, 27, 6. [Google Scholar] [CrossRef]
- Sali, S.S.; Gould, M.L.; Qasim, M.; Ali, M.A. Biodegradable methacrylated casein for cardiac tissue engineering applications. J. Mater. Chem. B 2021, 9, 1557–1567. [Google Scholar] [CrossRef]
- Garcia, L.V.; Silva, D.; Costa, M.M.; Armes, H.; Salema-Oom, M.; Saramago, B.; Serro, A.P. Antiseptic-Loaded Casein Hydrogels for Wound Dressings. Pharmaceutics 2023, 15, 334. [Google Scholar] [CrossRef] [PubMed]
- Muse, M.E.; Shumway, K.R.; Crane, J.S. Physiology, Epithelialization. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532977/ (accessed on 8 July 2025).
- Junker, J.P.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nakamura, S.; Kitts, D.D. Antioxidant Properties of Casein Phosphopeptides (CPP) and Maillard-Type Conjugated Products. Antioxidants 2020, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, Q.; Wang, X.; Bai, Z.; Xu, X.; Ma, J. Casein-based hydrogels: Advances and prospects. Food Chem. 2024, 447, 138956. [Google Scholar] [CrossRef] [PubMed]
- Zahra, D.; Shokat, Z.; Ahmad, A.; Javaid, A.; Khurshid, M.; Ashfaq, U.A.; Nashwan, A.J. Exploring the recent developments of alginate silk fibroin material for hydrogel wound dressing: A review. Int. J. Biol. Macromol. 2023, 248, 125989. [Google Scholar] [CrossRef]
- Tagrida, M.; Gulzar, S.; Nilsuwan, K.; Prodpran, T.; Zhang, B.; Benjakul, S. Polylactic Acid Film Coated with Electrospun Gelatin/Chitosan Nanofibers Containing Betel Leaf Ethanolic Extract: Properties, Bioactivities, and Use for Shelf-Life Extension of Tilapia Slices. Molecules 2022, 27, 5877. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fan, Z.; Duan, L.; Gao, G. A tough, stretchable, and extensively sticky hydrogel driven by milk protein. Polym. Chem. 2018, 9, 2617–2624. [Google Scholar] [CrossRef]
- Mezzenga, R.; Fischer, P. The self-assembly, aggregation and phase transitions of food protein systems in one, two and three dimensions. Rep. Prog. Phys. 2013, 76, 046601. [Google Scholar] [CrossRef]
- Guntero, V.A.; Acuña, M.C.; Aon, Y.S.; Gutierrez, L.G.; Ferretti, C.A. Formulation of Casein Hydrogels. In Proceedings of the 28th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-28), Online, 15–30 November 2024; Volume 16. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Q.; Ma, J.; Deng, Y.; An, W.; Yan, K.; Zong, Y.; Zhang, F. Progress in Protein-Based Hydrogels for Flexible Sensors: Insights from Casein. ACS Sens. 2024, 9, 5642–5664. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Chen, T.; Wang, Y.; Xu, Q.; Feng, B.; Weng, J.; Peng, W.; Wang, J. By Endowing Polyglutamic Acid/Polylysine Composite Hydrogel with Super Intrinsic Characteristics to Enhance its Wound Repair Potential. Macromol. Biosci. 2021, 21, e2000367. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.L.; Celli, A.; Mauro, T.; Chang, W. Calcium-Sensing Receptor Regulates Epidermal Intracellular Ca2+ Signaling and Re-Epithelialization after Wounding. J. Investig. Dermatol. 2019, 139, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Wang, Y.; An, M.; Fan, Y. Casein micelles embedded composite organohydrogel as potential wound dressing. Int. J. Biol. Macromol. 2022, 211, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Biranje, S.S.; Sun, J.; Cheng, L.; Cheng, Y.; Shi, Y.; Yu, S.; Jiao, H.; Zhang, M.; Lu, X.; Han, W.; et al. Development of Cellulose Nanofibril/Casein-Based 3D Composite Hemostasis Scaffold for Potential Wound-Healing Application. ACS Appl. Mater. Interfaces 2022, 14, 3792–3808. [Google Scholar] [CrossRef]
- Alsakhawy, S.A.; Baghdadi, H.H.; El-Shenawy, M.A.; Sabra, S.A.; El-Hosseiny, L.S. Encapsulation of thymus vulgaris essential oil in caseinate/gelatin nanocomposite hydrogel: In vitro antibacterial activity and in vivo wound healing potential. Int. J. Pharm. 2022, 628, 122280. [Google Scholar] [CrossRef]
- Ma, J.; Lee, J.; Han, S.S.; Oh, K.H.; Nam, K.T.; Sun, J.Y. Highly Stretchable and Notch-Insensitive Hydrogel Based on Polyacrylamide and Milk Protein. ACS Appl. Mater. Interfaces 2016, 8, 29220–29226. [Google Scholar] [CrossRef]
- Ding, G.-J.; Zhu, Y.-J.; Cheng, G.-F.; Ruan, Y.-J.; Qi, C.; Lu, B.-Q.; Chen, F.; Wu, J. Porous Microspheres of Casein/Amorphous Calcium Phosphate Nanocomposite: Room Temperature Synthesis and Application in Drug Delivery. Curr. Nanosci. 2015, 12, 70–78. [Google Scholar] [CrossRef]
- Picchio, M.L.; Cuggino, J.C.; Nagel, G.; Wedepohl, S.; Minari, R.J.; Alvarez Igarzabal, C.I.; Gugliotta, L.M.; Calderón, M. Crosslinked casein-based micelles as a dually responsive drug delivery system. Polym. Chem. 2018, 9, 3499–3510. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Bajpai, M.; Shah, F.F. Alginate dialdehyde (AD)-crosslinked casein films: Synthesis, characterization and water absorption behavior. Des. Monomers Polym. 2016, 19, 406–419. [Google Scholar] [CrossRef]
- Hadj Kaddour, N.E.; Iles, N.; Biran Ay, S.; Blekhalfa, H.; Belalem, A.K.; Lekoui, F.; Bachari, K. Silver-casein composite films: Towards a new antibacterial coating. Polym. Bull. 2025. [Google Scholar] [CrossRef]
- Kolahreez, D.; Ghasemi-Mobarakeh, L.; Quartinello, F.; Liebner, F.W.; Guebitz, G.M.; Ribitsch, D. Multifunctional Casein-Based Wound Dressing Capable of Monitoring and Moderating the Proteolytic Activity of Chronic Wounds. Biomacromolecules 2024, 25, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Minaei, F.; Ravandi, S.A.H.; Hejazi, S.M.; Alihosseini, F. The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning. e-Polymers 2019, 19, 154–167. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Li, B.; Yang, C.; Shen, J.; Wang, N.; Gu, R.; Wang, D.; Chen, D.; Hu, H.; et al. Robust Biological Fibers Based on Widely Available Proteins: Facile Fabrication and Suturing Application. Small 2020, 16, e1907598. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Guler, E.; Sinemcan Ozcan, G.; Emin Cam, M.; Homer-Vanniasinkam, S.; Edirisinghe, M. Casein fibres for wound healing. J. R. Soc. Interface 2023, 20, 20230166. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, B.; Wang, Y.; Deng, B.; Wang, D.; Tang, B.Z. As Fiber Meets with AIE: Opening a Wonderland for Smart Flexible Materials. Adv. Mater. 2023, 35, e2210085. [Google Scholar] [CrossRef] [PubMed]
- Bairagi, S.; Banerjee, S.; Kalyaniyan, P.; Dhanalakshmi, G.; Ali, S.W. New Insights toward Casein/Polyvinyl Alcohol Electrospun Nanofibrous Webs as a Piezoelectric-Cum-Triboelectric Energy Harvester. ACS Appl. Electron. Mater. 2021, 3, 4348–4361. [Google Scholar] [CrossRef]
- Movahedi, M.; Salehi, A.O.M.; Hajipour, F.P.; Etemad, S. Casein release and characterization of electrospun nanofibres for cartilage tissue engineering. Bull. Mater. Sci. 2022, 45, 76. [Google Scholar] [CrossRef]
- Ghosh, A.; Ali, M.A.; Dias, G.J. Effect of Cross-Linking on Microstructure and Physical Performance of Casein Protein. Biomacromolecules 2009, 10, 1681–1688. [Google Scholar] [CrossRef]
- Russo, M.; Langella, A.; Sansone, L.; Ricciardi, M.R. Casein matrix composites reinforced with recycled cellulose and cellulose acetate fibers: Formulation and mechanical performance for sustainable applications. Cellulose 2025, 32, 3761–3776. [Google Scholar] [CrossRef]
- Sharma, D.; Ziegler, G.R.; Harte, F.M. Fabrication and physicomechanical performance of κ-carrageenan/casein nanofibers. Food Hydrocoll. 2025, 160, 110855. [Google Scholar] [CrossRef]
- Hassabo, A.; Khaleed, N.; Shaker, S.; Abd El-Salam, N.; Mohamed, N.; Gouda, N.; Abd El-Aziz, E. Significance of Casein fibre in textile technology. J. Text. Color. Polym. Sci. 2023, 21, 63–73. [Google Scholar] [CrossRef]
- Thill, S.; Gebhardt, R. Effect of Glycerol, Calcium and Transglutaminase Post-Treatment on the Properties of Regenerated Fibers from Rennet-Treated Casein Micelles. Colloids Interfaces 2022, 6, 17. [Google Scholar] [CrossRef]
- Thill, S.; Schmidt, T.; Wöll, D.; Gebhardt, R. A regenerated fiber from rennet-treated casein micelles. Colloid. Polym. Sci. 2021, 299, 909–914. [Google Scholar] [CrossRef]
- Flores-Nieves, M.M.; Castellanos-Espinoza, R.; Estevez, M.; Baldenegro-Pérez, L.A.; Trejo, J.F.G.; García, M.E.; Cano, B.M.; Soto-Zarazúa, G.M.; España-Sánchez, B.L. Electrospun Casein fibers obtained from revalued milk with mechanical and antibacterial properties. Arab. J. Chem. 2022, 15, 104201. [Google Scholar] [CrossRef]
- Taheri, P.; Jahanmardi, R.; Koosha, M.; Abdi, S. Physical, mechanical and wound healing properties of chitosan/gelatin blend films containing tannic acid and/or bacterial nanocellulose. Int. J. Biol. Macromol. 2020, 154, 421–432. [Google Scholar] [CrossRef]
- Lamei, E.; Hasanzadeh, M. Concurrent electrospinning of microporous metal-organic framework-laden casein/PVA nanofibrous composites for potential antibacterial wound dressing applications. Int. J. Polym. Mater. Polym. Biomater. 2023, 73, 637–645. [Google Scholar] [CrossRef]
- Biranje, S.; Madiwale, P.; Adivarekar, R.V. Porous electrospun Casein/PVA nanofibrous mat for its potential application as wound dressing material. J. Porous Mater. 2018, 26, 29–40. [Google Scholar] [CrossRef]
- Şendil, Ö.; Samatya Yilmaz, S.; Yazici Ozcelik, E.; Uzuner, H.; Aytac, A. Cross-linked electrospun polyvinyl alcohol/sodium caseinate nanofibers for antibacterial applications. J. Vinyl Addit. Technol. 2022, 29, 48–65. [Google Scholar] [CrossRef]
- Samatya Yilmaz, S.; Uzuner, H.; Aytac, A. Potential Wound Dressing with Antibacterial Effect from Cross-Linked Polyvinyl Alcohol/Sodium Caseinate/CuO NP Electrospun Mat. ChemistrySelect 2024, 9, e202400233. [Google Scholar] [CrossRef]
- Samatya Yilmaz, S.; Aytac, A. Antibacterial wound dressing with cross-linked electrospun surface from reduced graphene oxide doped polyvinyl alcohol/sodium caseinate blends. Biopolymers 2024, 115, e23579. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Thangam, R.; Fathima, N.N. Electrospinning of casein nanofibers with silver nanoparticles for potential biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, C.; Zhang, X.; Bian, W.; Liu, N.; Yin, S.; Yang, M.; Luo, M.; Tang, J.; Yang, X. A short peptide potentially promotes the healing of skin wound. Biosci. Rep. 2019, 39, BSR20181734. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, G.; Qi, X.; Guo, W.; Wang, C.; Liu, L.; Hou, Z.; Li, C.; He, J. Improved protective and controlled releasing effect of flaxseed oil microcapsules with glycosylated casein as wall materials. LWT 2024, 191, 115687. [Google Scholar] [CrossRef]
- Rajanna, D.; Pushpadass, H.A.; Emerald, F.M.E.; Padaki, N.V.; Nath, B.S. Nanoencapsulation of casein-derived peptides within electrospun nanofibres. J. Sci. Food Agric. 2022, 102, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Das, S.; Sharma, C.; Singh, A.; Das, S.; Das, M.K.; Tyagi, P.K.; Khurana, N. A novel nano-emulsion cream of casein isolated from raw milk for thermal and acid burn wounds. Braz. J. Pharm. Sci. 2025, 61, e24233. [Google Scholar] [CrossRef]
- Kumari, M.; Dwivedi, M.; Kumar, K.J.; Pattnaik, A.K. Bioinspired Chitosan-Based Patches Enriched With Lipid-Casein Nanocarriers: An Innovative Approach for Wound Management and Evaluation in a Rat Model. J. Pharm. Innov. 2025, 20, 41. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, X.; Zhao, Y.; Tian, Y.; Ma, Y.; Zhang, X.; Li, F.; Zhao, W.; Ma, J.; Xu, Q.; et al. A self-powered casein hydrogel E-dressing with synergistic photothermal therapy, electrical stimulation, and antibacterial effects for chronic wound management. Acta Biomater. 2025, 198, 63–84. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, H.; Qian, Y.; Shen, J.; Zhang, Z. Protein-Based Multifunctional Hydrogel Adhesive for Wound Healing. Macromol. Biosci. 2025, e00205. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, X.; Wang, Z.; Ren, D.; Ren, T. Milk-Derived Injectable Wound Dressing with Sequential Photoactivatable Antibacterial Property through In Situ Biomineralization. Small Sci. 2025, 2500026. [Google Scholar] [CrossRef]
- Bonnaillie, L.; Zhang, H.; Akkurt, S.; Yam, K.; Tomasula, P. Casein Films: The Effects of Formulation, Environmental Conditions and the Addition of Citric Pectin on the Structure and Mechanical Properties. Polymers 2014, 6, 2018–2036. [Google Scholar] [CrossRef]
- Monaci, L.; Pilolli, R.; Quintieri, L.; Caputo, L.; Luparelli, A.; De Angelis, E. Chapter 21-Casein: Allergenicity and molecular properties. In Casein; El-Bakry, M., Mehta, B.M., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 363–382. [Google Scholar]
- Liang, X.; Qu, Y.; Zhou, W.; Qin, R.; Bai, J.; Cao, T.; Pu, X.; Chu, Y.; Gu, M.; Wang, J.; et al. Changes in allergenicity characteristics of bovine casein by enzymatic hydrolysis treatment. J. Food Meas. Charact. 2024, 18, 7213–7223. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Liu, X.; Guan, W.; Lu, C. Aggregation-induced emission-active micelles: Synthesis, characterization, and applications. Chem. Soc. Rev. 2023, 52, 1456–1490. [Google Scholar] [CrossRef] [PubMed]
- Rainho, P.; Salema-Oom, M.; Pinto, C.A.; Saraiva, J.A.; Saramago, B.; Silva, D.C.; Serro, A.P. Polyvinyl alcohol/casein hydrogels with oxymatrine eluting ability for cancer-related wound management. Biomater. Sci. 2025, 13, 2755–2766. [Google Scholar] [CrossRef]
- El-Kattawy, A.M.; Algezawy, O.; Alfaifi, M.Y.; Noseer, E.A.; Hawsawi, Y.M.; Alzahrani, O.R.; Algarni, A.; Kahilo, K.A.; El-Magd, M.A. Therapeutic potential of camel milk exosomes against HepaRG cells with potent apoptotic, anti-inflammatory, and anti-angiogenesis effects for colostrum exosomes. Biomed. Pharmacother. 2021, 143, 112220. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez Mesa, N.E.; Vasilev, K.; Tang, Y. Casein-Based Biomaterials: Fabrication and Wound Healing Applications. Molecules 2025, 30, 3278. https://doi.org/10.3390/molecules30153278
Gomez Mesa NE, Vasilev K, Tang Y. Casein-Based Biomaterials: Fabrication and Wound Healing Applications. Molecules. 2025; 30(15):3278. https://doi.org/10.3390/molecules30153278
Chicago/Turabian StyleGomez Mesa, Nikolay Estiven, Krasimir Vasilev, and Youhong Tang. 2025. "Casein-Based Biomaterials: Fabrication and Wound Healing Applications" Molecules 30, no. 15: 3278. https://doi.org/10.3390/molecules30153278
APA StyleGomez Mesa, N. E., Vasilev, K., & Tang, Y. (2025). Casein-Based Biomaterials: Fabrication and Wound Healing Applications. Molecules, 30(15), 3278. https://doi.org/10.3390/molecules30153278









