Mechanical Properties of Biodegradable Fibers and Fibrous Mats: A Comprehensive Review
Abstract
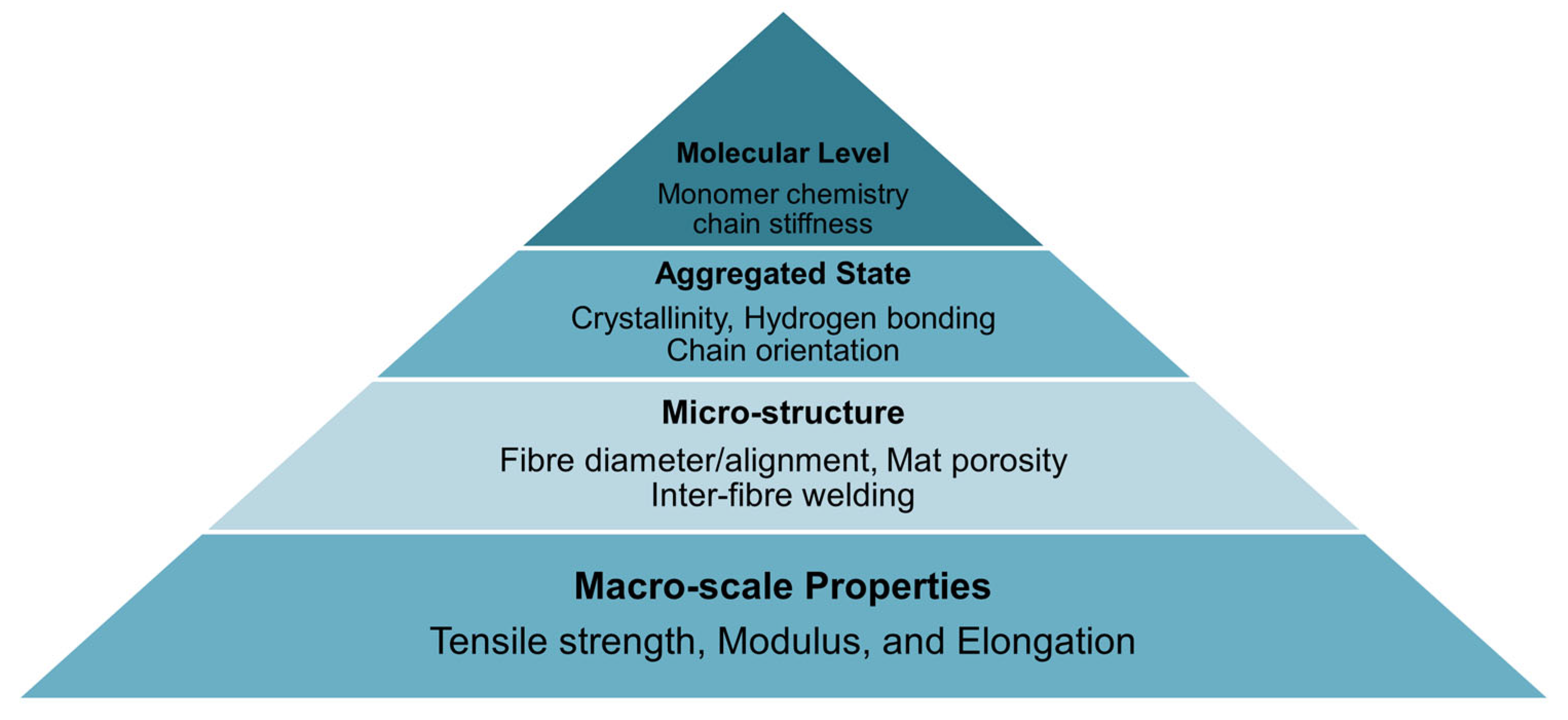
1. Introduction
2. Fiber Manufacturing Methods
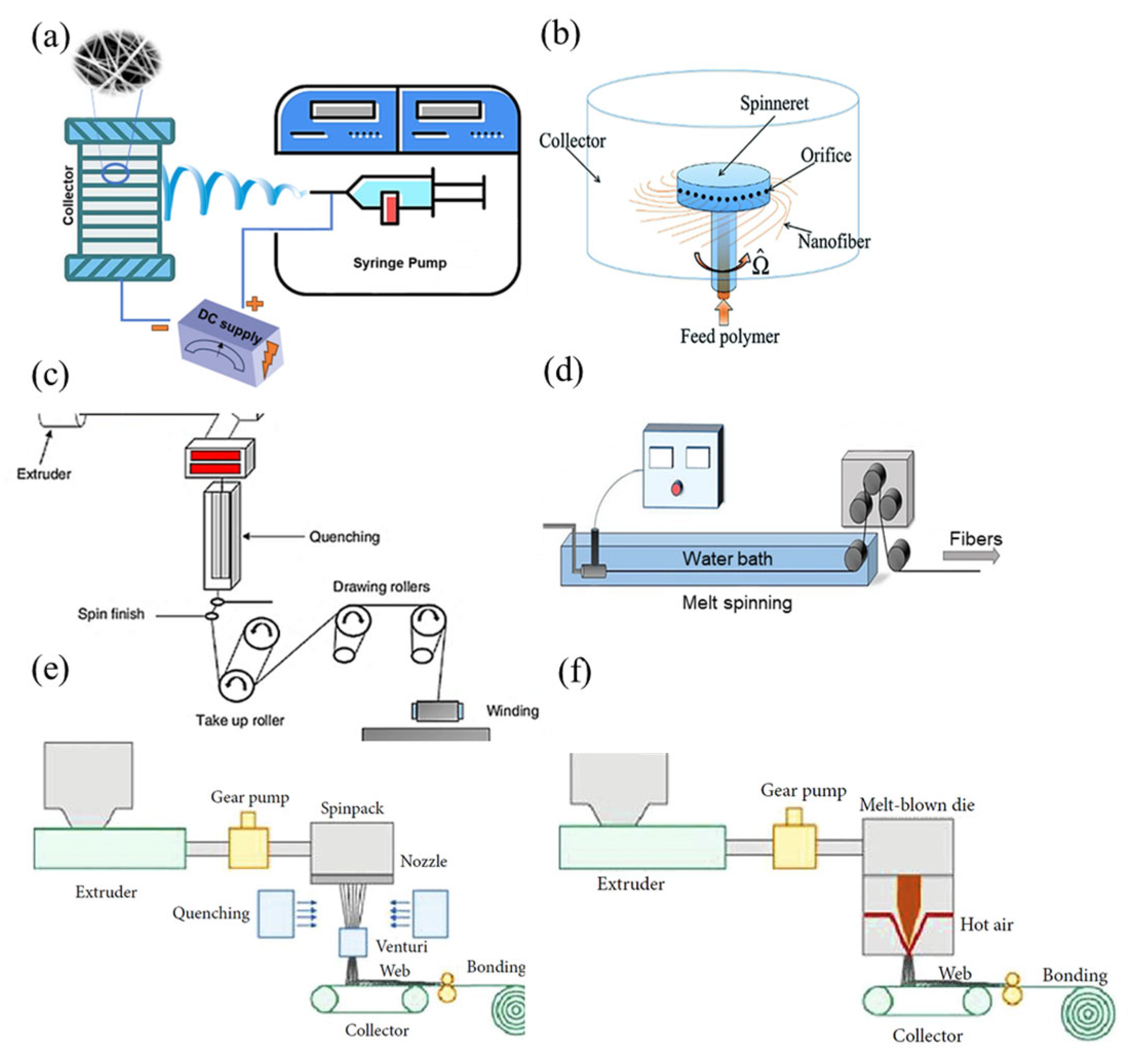
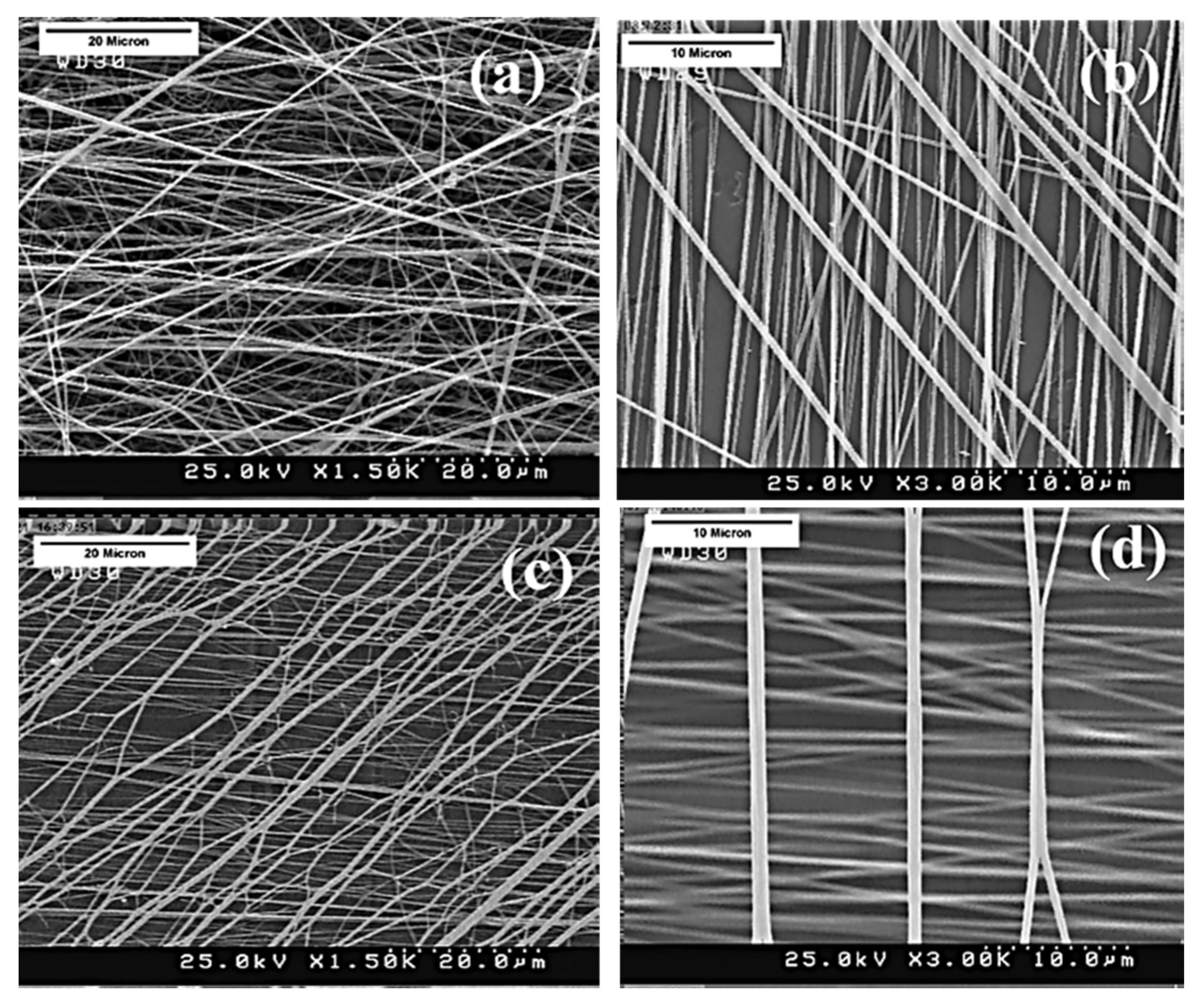
2.1. Electrospinning
2.2. Centrifugal Spinning
2.3. Melt Spinning
2.4. Wet Spinning
2.5. Melt Blowing
2.6. Spunbonding
3. Mechanical Property Testing
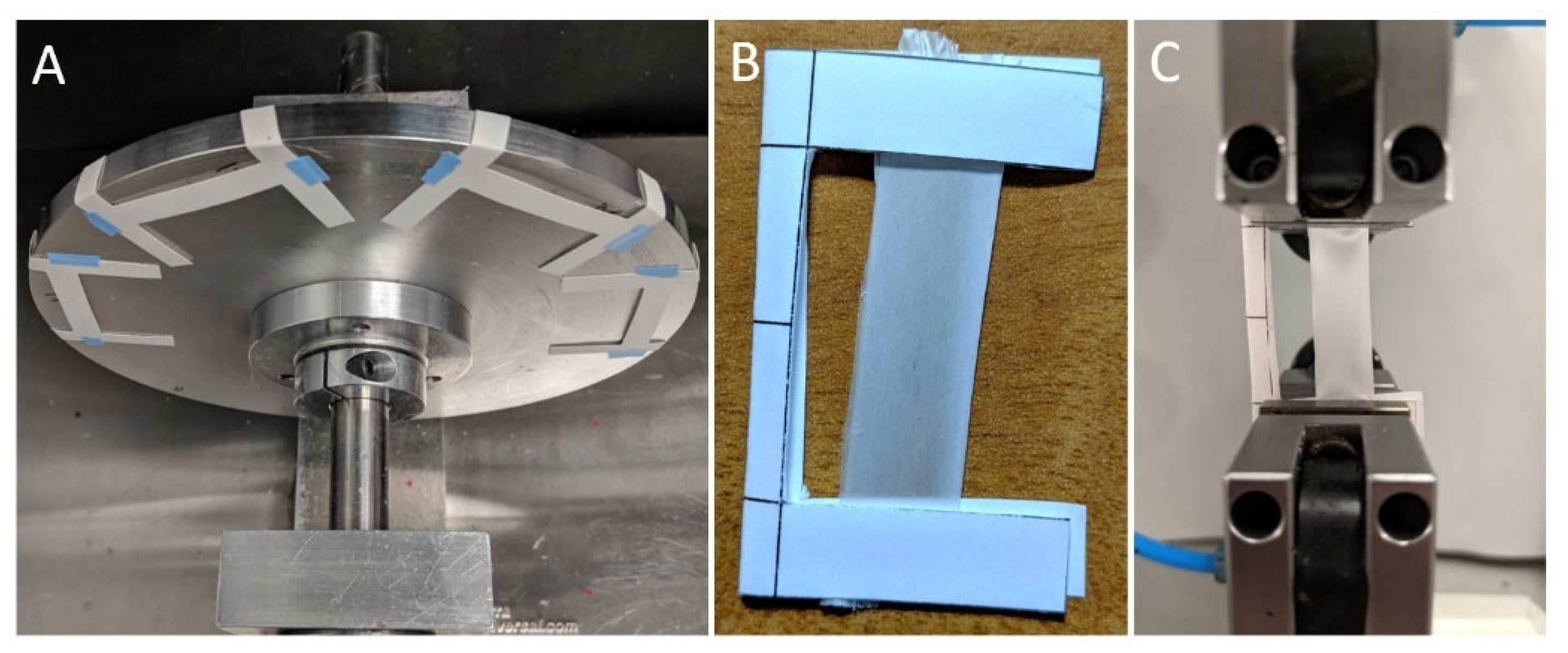
3.1. Tensile Testing Machines
3.2. Atomic Force Microscopy
3.3. Dynamic Mechanical Analysis
3.4. Other Mechanical Tests
4. Mechanical Properties of Biodegradable Polymer Fibers and Mats
4.1. Synthetic-Biodegradable-Polymer-Based Fibers
4.1.1. Polyvinyl Alcohol
4.1.2. Polyethylene Oxide
4.1.3. Polyglycolic Acid (PGA) and Poly(lactic-co-glycolic acid) (PLGA)
4.1.4. PLA, Poly(L-lactic acid) (PLLA), and Poly(D-lactic acid) (PDLA)
4.1.5. PCL
4.2. Natural-Biodegradable-Polymer-Based Fibers
4.2.1. Chitosan
4.2.2. Collagen
4.2.3. Cellulose
4.2.4. Starch
4.2.5. Silk Fibroin
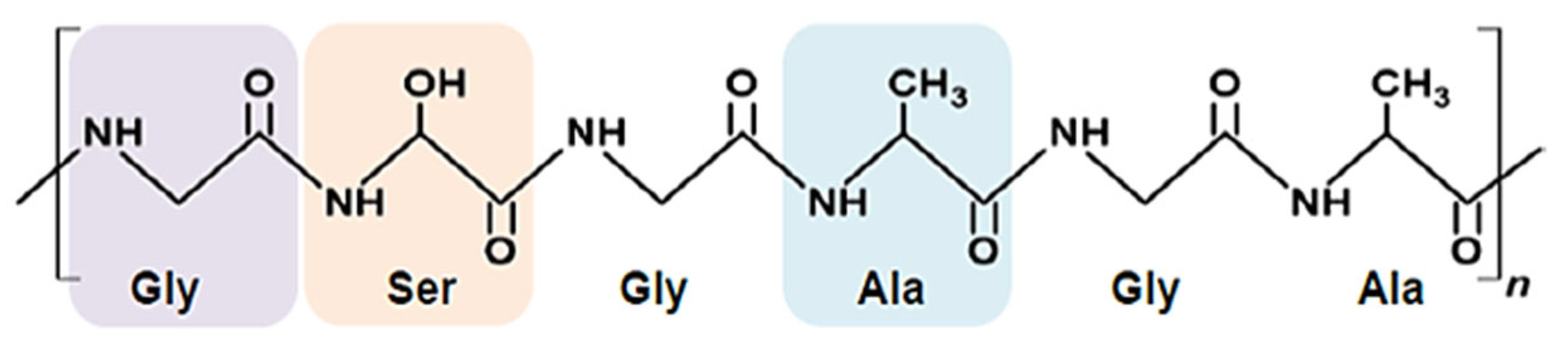
4.2.6. PHBV and PHB
5. Future Perspectives
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Meng, C.; Tang, D.; Liu, X.; Meng, J.; Wei, W.; Gong, R.H.; Li, J. Heterogeneous porous PLLA/PCL fibrous scaffold for bone tissue regeneration. Int. J. Biol. Macromol. 2023, 235, 123781. [Google Scholar] [CrossRef]
- Huang, Q.; Meng, C.; Liao, M.; Kou, T.; Zhou, F.; Lu, J.R.; Li, J.; Li, Y. Hierarchically porous, superhydrophobic plla/copper composite fibrous membranes for air filtration. ACS Appl. Polym. Mater. 2024, 6, 2381–2391. [Google Scholar] [CrossRef]
- Kaniuk, E.; Lechowska-Liszka, A.; Gajek, M.; Nikodem, A.; Ścisłowska-Czarnecka, A.; Rapacz-Kmita, A.; Stodolak-Zych, E. Correlation between porosity and physicochemical and biological properties of electrospinning PLA/PVA membranes for skin regeneration. Biomater. Adv. 2023, 152, 213506. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhao, Q.; Meng, C.; Meng, J.; Chen, Z.; Li, J. Hierarchical porous recycled PET nanofibers for high-efficiency aerosols and virus capturing. ACS Appl. Mater. Interfaces 2021, 13, 49380–49389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jian, Y.; Jiang, X.; Li, X.; Wu, X.; Zhong, J.; Jia, X.; Li, Q.; Wang, X.; Zhao, K.; et al. Stepwise degradable PGA-SF core-shell electrospinning scaffold with superior tenacity in wetting regime for promoting bone regeneration. Mater. Today Bio 2024, 26, 101023. [Google Scholar] [CrossRef]
- Cao, H.; Chai, S.; Tan, Z.; Wu, H.; Mao, X.; Wei, L.; Zhou, F.; Sun, R.; Liu, C. Recent advances in physical sensors based on electrospinning technology. ACS Mater. Lett. 2023, 5, 1627–1648. [Google Scholar] [CrossRef]
- Rahim Labbafzadeh, M.; Vakili, M.H. Application of magnetic electrospun polyvinyl alcohol/collagen nanofibres for drug delivery systems. Mol. Simul. 2022, 48, 1–7. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.-U. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef]
- Karthik, T.; Rathinamoorthy, R. Nonwovens: Process, Structure, Properties and Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Leal, A.A.; Deitzel, J.M.; Gillespie, J.W. Compressive Strength Analysis for High Performance Fibers with Different Modulus in Tension and Compression. J. Compos. Mater. 2009, 43, 661–674. [Google Scholar] [CrossRef]
- Cleeton, C.; Keirouz, A.; Chen, X.; Radacsi, N. Electrospun nanofibers for drug delivery and biosensing. ACS Biomater. Sci. Eng. 2019, 5, 4183–4205. [Google Scholar] [CrossRef]
- Rahim Labbafzadeh, M.; Vakili, M.H. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef]
- Ramazani, S.; Karimi, M. Aligned poly (ε-caprolactone)/graphene oxide and reduced graphene oxide nanocomposite nanofibers: Morphological, mechanical and structural properties. Mater. Sci. Eng. C 2015, 56, 325–334. [Google Scholar] [CrossRef]
- Meng, C.; Song, J.; Malekmohammadi, S.; Meng, J.; Wei, W.; Li, R.; Feng, J.; Gong, R.H.; Li, J. Hierarchical porous poly (L-lactic acid) fibrous vascular graft with controllable architectures and stable structure. Mater. Des. 2024, 240, 112829. [Google Scholar] [CrossRef]
- Pisani, S.; Dorati, R.; Conti, B.; Modena, T.; Bruni, G.; Genta, I. Design of copolymer PLA-PCL electrospun matrix for biomedical applications. React. Funct. Polym. 2018, 124, 77–89. [Google Scholar] [CrossRef]
- Nicknejad, E.T.; Ghoreishi, S.M.; Habibi, N. Electrospinning of cross-linked magnetic chitosan nanofibers for protein release. AAPS PharmSciTech 2015, 16, 1480–1486. [Google Scholar] [CrossRef]
- Merchiers, J.; Martínez Narváez, C.D.V.; Slykas, C.; Buntinx, M.; Deferme, W.; D’Haen, J.; Peeters, R.; Sharma, V.; Reddy, N.K. Centrifugally spun poly(ethylene oxide) fibers rival the properties of electrospun fibers. J. Polym. Sci. 2021, 59, 2754–2762. [Google Scholar] [CrossRef]
- Rahmat, M.; Karrabi, M.; Ghasemi, I.; Zandi, M.; Azizi, H. Silane crosslinking of electrospun poly (lactic acid)/nanocrystalline cellulose bionanocomposite. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 397–405. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Zarghami, N.; Melat Yar, H.; Akbarzadeh, A. Nanofiber: Synthesis and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Raksa, A.; Numpaisal, P.-o.; Ruksakulpiwat, Y. The effect of humidity during electrospinning on morphology and mechanical properties of SF/PVA nanofibers. Mater. Today Proc. 2021, 47, 3458–3461. [Google Scholar] [CrossRef]
- Nadaf, A.; Gupta, A.; Hasan, N.; Ahmad, S.; Kesharwani, P.; Ahmad, F.J. Recent update on electrospinning and electrospun nanofibers: Current trends and their applications. RSC Adv. 2022, 12, 23808–23828. [Google Scholar] [CrossRef] [PubMed]
- Šišková, A.O.; Peer, P.; Andicsová, A.E.; Jordanov, I.; Rychter, P. Circulatory management of polymer waste: Recycling into fine fibers and their applications. Materials 2021, 14, 4694. [Google Scholar] [CrossRef]
- Maqsood, M.; Langensiepen, F.; Seide, G. Investigation of melt spinnability of plasticized polylactic acid biocomposites-containing intumescent flame retardant. J. Therm. Anal. Calorim. 2020, 139, 305–318. [Google Scholar] [CrossRef]
- Mirbaha, H.; Nourpanah, P.; Scardi, P.; D’incau, M.; Greco, G.; Valentini, L.; Bittolo Bon, S.; Arbab, S.; Pugno, N. The impact of shear and elongational forces on structural formation of polyacrylonitrile/carbon nanotubes composite fibers during wet spinning process. Materials 2019, 12, 2797. [Google Scholar] [CrossRef]
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.; Mao, L.; Wang, S.; Xue, K.; Yang, L.; et al. Face masks in the new COVID-19 normal: Materials, testing, and perspectives. Research 2020, 2020, 7286735. [Google Scholar] [CrossRef]
- Esmaeili, J.; Ghoraishizadeh, S.; Farzan, M.; Barati, A.; Salehi, E.; Ai, J. Fabrication and evaluation of a soy protein isolate/collagen/sodium alginate multifunctional bilayered wound dressing: Release of cinnamaldehyde, artemisia absinthium, and oxygen. ACS Appl. Bio Mater. 2024, 7, 5470–5482. [Google Scholar] [CrossRef]
- Castro, C.; Juárez, D.A.; Arizmendi-Morquecho, A.; Gonzalez-Perez, G.; Estrada-Villegas, G.M. The effects of relative humidity and salt concentration on the diameter of hydrophilic polymeric nanofibers obtained by electrospinning: Synergistic effect study by Central Composite Design (CCD). React. Funct. Polym. 2024, 203, 106013. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K. Electrospun nanofiber membranes for air filtration: A review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef]
- Zulkifli, M.Z.A.; Nordin, D.; Shaari, N.; Kamarudin, S.K. Overview of Electrospinning for Tissue Engineering Applications. Polymers 2023, 15, 2418. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Murillo, L.L.; Yang, K.; Wang, T.; Li, J.; Li, Y.; Chen, Y.; Chen, Z. Revisable and high-strength wheel-spun alginate/graphene oxide based fibrous rods towards a flexible and biodegradable rib internal fixation system. Int. J. Biol. Macromol. 2022, 219, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Liu, Y.; Bian, D.; He, F.; Li, Y.; Fan, W. Spinning solution viscosity reducing and wet spinning of carbon black-based elastic conductive fibers for sports monitoring and healthcare electrical heating. J. Mater. Res. Technol. 2025, 36, 1–12. [Google Scholar] [CrossRef]
- Mejía Suaza, M.L.; Hurtado Henao, Y.; Moncada Acevedo, M.E. Wet electrospinning and its applications: A review. TecnoLógicas 2022, 25, e2223. [Google Scholar] [CrossRef]
- Sikdar, P.; S Bhat, G.; Hinchliffe, D.; Islam, S.; Condon, B. Microstructure and physical properties of composite nonwovens produced by incorporating cotton fibers in elastic spunbond and meltblown webs for medical textiles. J. Ind. Text. 2022, 51 (Suppl. 4), 6028S–6050S. [Google Scholar] [CrossRef]
- Bunsell, A.R. Handbook of Tensile Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- kumar Gurajala, N.; Sanjeev, L.; Teja, B.; Kumar, T.S.; Manikanta, J.E. An overview of mechanical properties of biodegradable polymers and natural fibre materials. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Baker, S.R.; Banerjee, S.; Bonin, K.; Guthold, M. Determining the mechanical properties of electrospun poly-epsilon-caprolactone (PCL) nanofibers using AFM and a novel fiber anchoring technique. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T.U.; Gorga, R.E.; Krause, W.E. Mechanical Properties of Electrospun Fibers—A Critical Review. Adv. Eng. Mater. 2021, 23, 2100153. [Google Scholar] [CrossRef]
- Stachewicz, U.; Bailey, R.J.; Wang, W.; Barber, A.H. Size dependent mechanical properties of electrospun polymer fibers from a composite structure. Polymer 2012, 53, 5132–5137. [Google Scholar] [CrossRef]
- Costa, C.; Fonseca, A.; Serra, A.; Coelho, J. Dynamic mechanical thermal analysis of polymer composites reinforced with natural fibers. Polym. Rev. 2016, 56, 362–383. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M.; Alothman, O.Y.; Paridah, M. A review on dynamic mechanical properties of natural fibre reinforced polymer composites. Constr. Build. Mater. 2016, 106, 149–159. [Google Scholar] [CrossRef]
- Li, X.; Bhushan, B.; Takashima, K.; Baek, C.-W.; Kim, Y.-K. Mechanical characterization of micro/nanoscale structures for MEMS/NEMS applications using nanoindentation techniques. Ultramicroscopy 2003, 97, 481–494. [Google Scholar] [CrossRef]
- Santiago-Castillo, K.; Torres-Huerta, A.M.; Del Angel-Lopez, D.; Dominguez-Crespo, M.A.; Dorantes-Rosales, H.; Palma-Ramirez, D.; Willcock, H. In Situ Growth of Silver Nanoparticles on Chitosan Matrix for the Synthesis of Hybrid Electrospun Fibers: Analysis of Microstructural and Mechanical Properties. Polymers 2022, 14, 674. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Y.; Sun, C.; Zou, L.; He, J. The preparation and characterization of plasticized PVA fibres by a novel Glycerol/Pseudo Ionic Liquids system with melt spinning method. Eur. Polym. J. 2020, 133, 109768. [Google Scholar] [CrossRef]
- Bazzi, M.; Shabani, I.; Mohandesi, J.A. Enhanced mechanical properties and electrical conductivity of Chitosan/Polyvinyl Alcohol electrospun nanofibers by incorporation of graphene nanoplatelets. J. Mech. Behav. Biomed. Mater. 2022, 125, 104975. [Google Scholar] [CrossRef]
- Du, L.; Xu, H.; Li, T.; Zhang, Y.; Zou, F. Fabrication of silver nanoparticle/polyvinyl alcohol/polycaprolactone hybrid nanofibers nonwovens by two-nozzle electrospinning for wound dressing. Fibers Polym. 2017, 17, 1995–2005. [Google Scholar] [CrossRef]
- Kumkun, P.; Tuancharoensri, N.; Ross, G.; Mahasaranon, S.; Jongjitwimol, J.; Topham, P.D.; Ross, S. Green fabrication route of robust, biodegradable silk sericin and poly(vinyl alcohol) nanofibrous scaffolds. Polym. Int. 2019, 68, 1903–1913. [Google Scholar] [CrossRef]
- Alonso-Lopez, O.; Lopez-Ibanez, S.; Beiras, R. Assessment of Toxicity and Biodegradability of Poly(vinyl alcohol)-Based Materials in Marine Water. Polymers 2021, 13, 3742. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-Based Bio- and Nanocomposites: A Review. Int. J. Polym. Sci. 2011, 2011, 1–35. [Google Scholar] [CrossRef]
- Lee, J.; Deng, Y. Increased mechanical properties of aligned and isotropic electrospun PVA nanofiber webs by cellulose nanowhisker reinforcement. Macromol. Res. 2011, 20, 76–83. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Liao, N.; Cui, F.; Park, M.; Kim, H.Y. Fabrication and durable antibacterial properties of electrospun chitosan nanofibers with silver nanoparticles. Int. J. Biol. Macromol. 2015, 79, 638–643. [Google Scholar] [CrossRef]
- Cho, D.; Netravali, A.N.; Joo, Y.L. Mechanical properties and biodegradability of electrospun soy protein Isolate/PVA hybrid nanofibers. Polym. Degrad. Stab. 2012, 97, 747–754. [Google Scholar] [CrossRef]
- Ngadiman, N.H.; Yusof, N.M.; Idris, A.; Misran, E.; Kurniawan, D. Development of highly porous biodegradable gamma-Fe(2)O(3)/polyvinyl alcohol nanofiber mats using electrospinning process for biomedical application. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 520–534. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, N.; Li, L.; Wang, Q. Structure evolution of melt-spun poly(vinyl alcohol) fibers during hot-drawing. J. Appl. Polym. Sci. 2011, 124, 421–428. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Hodge, J.; Quint, C. The improvement of cell infiltration in an electrospun scaffold with multiple synthetic biodegradable polymers using sacrificial PEO microparticles. J. Biomed. Mater. Res. A 2019, 107, 1954–1964. [Google Scholar] [CrossRef]
- Kupka, V.; Dvorakova, E.; Manakhov, A.; Michlicek, M.; Petrus, J.; Vojtova, L.; Zajickova, L. Well-Blended PCL/PEO Electrospun Nanofibers with Functional Properties Enhanced by Plasma Processing. Polymers 2020, 12, 1403. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Li, R.; Wang, Y.; Xiang, X.; Zhang, C.; Wang, D.; Zhou, Y.; Liu, X.; Xu, W. Fabrication and properties of carboxymethyl chitosan/polyethylene oxide composite nonwoven mats by centrifugal spinning. Carbohydr. Polym. 2021, 251, 117037. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Hou, T.; Zhou, J.; Yang, B. Centrifugally spun ultrafine starch/PEO fibres as release formulation for poorly water-soluble drugs. Micro Nano Lett. 2018, 13, 1688–1692. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Aghdam, R.M.; Najarian, S.; Shakhesi, S.; Khanlari, S.; Shaabani, K.; Sharifi, S. Investigating the effect of PGA on physical and mechanical properties of electrospun PCL/PGA blend nanofibers. J. Appl. Polym. Sci. 2011, 124, 123–131. [Google Scholar] [CrossRef]
- Hajiali, H.; Shahgasempour, S.; Naimi-Jamal, M.R.; Peirovi, H. Electrospun PGA/gelatin nanofibrous scaffolds and their potential application in vascular tissue engineering. Int. J. Nanomed. 2011, 6, 2133–2141. [Google Scholar] [CrossRef]
- Ramdhanie, L.I.; Aubuchon, S.R.; Boland, E.D.; Knapp, D.C.; Barnes, C.P.; Simpson, D.G.; Wnek, G.E.; Bowlin, G.L. Thermal and Mechanical Characterization of Electrospun Blends of Poly(lactic acid) and Poly(glycolic acid). Polym. J. 2006, 38, 1137–1145. [Google Scholar] [CrossRef]
- Li, W.J.; Cooper, J.A., Jr.; Mauck, R.L.; Tuan, R.S. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006, 2, 377–385. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.R.; Bramson, M.T.K.; Puhl, D.L.; Johnson, J.; Corr, D.T.; Gilbert, R.J. Solvent retention in electrospun fibers affects scaffold mechanical properties. Electrospinning 2018, 2, 15–28. [Google Scholar] [CrossRef]
- Bazgir, M.; Saeinasab, M.; Zhang, W.; Zhang, X.; Min Tsui, K.; Maasoumi Sarvestani, A.; Nawaz, S.; Coates, P.; Youseffi, M.; Elies, J.; et al. Investigation of Cell Adhesion and Cell Viability of the Endothelial and Fibroblast Cells on Electrospun PCL, PLGA and Coaxial Scaffolds for Production of Tissue Engineered Blood Vessel. J. Funct. Biomater. 2022, 13, 282. [Google Scholar] [CrossRef]
- Chou, S.F.; Woodrow, K.A. Relationships between mechanical properties and drug release from electrospun fibers of PCL and PLGA blends. J. Mech. Behav. Biomed. Mater. 2017, 65, 724–733. [Google Scholar] [CrossRef]
- Elder, S.; Roberson, J.G.; Warren, J.; Lawson, R.; Young, D.; Stokes, S.; Ross, M.K. Evaluation of Electrospun PCL-PLGA for Sustained Delivery of Kartogenin. Molecules 2022, 27, 3739. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.C.; Wianny, F.; Livi, S.; Hidalgo, I.A.; Dehay, C.; Duchet-Rumeau, J.; Gerard, J.F. Development of Bioresorbable Hydrophilic-Hydrophobic Electrospun Scaffolds for Neural Tissue Engineering. Biomacromolecules 2016, 17, 3172–3187. [Google Scholar] [CrossRef]
- Arbeiter, D.; Kohse, S.; Eickner, T.; Grabow, N.; Schmitz, K.-P. Influence of additives on physico-chemical properties of electrospun poly(L-lactide). Curr. Dir. Biomed. Eng. 2018, 4, 493–496. [Google Scholar] [CrossRef]
- Qiao, T.; Song, P.; Guo, H.; Song, X.; Zhang, B.; Chen, X. Reinforced electrospun PLLA fiber membrane via chemical crosslinking. Eur. Polym. J. 2016, 74, 101–108. [Google Scholar] [CrossRef]
- Goreninskii, S.I.; Stankevich, K.S.; Nemoykina, A.L.; Bolbasov, E.N.; Tverdokhlebov, S.I.; Filimonov, V.D. A first method for preparation of biodegradable fibrous scaffolds containing iodine on the fibre surfaces. Bull. Mater. Sci. 2018, 41, 100. [Google Scholar] [CrossRef]
- Song, J.; Zhang, B.; Lu, Z.; Xin, Z.; Liu, T.; Wei, W.; Zia, Q.; Pan, K.; Gong, R.H.; Bian, L.; et al. Hierarchical porous poly (l-lactic acid) nanofibrous membrane for ultrafine particulate aerosol filtration. ACS Appl. Mater. Interfaces 2019, 11, 46261–46268. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, B.; Gong, H.; Li, J. Fabrication of hierarchical porous poly (l-lactide)(PLLA) fibrous membrane by electrospinning. Polymer 2021, 226, 123797. [Google Scholar] [CrossRef]
- Didekhani, R.; Sohrabi, M.R.; Seyedjafari, E.; Soleimani, M.; Hanaee-Ahvaz, H. Electrospun composite PLLA/Oyster shell scaffold enhances proliferation and osteogenic differentiation of stem cells. Biologicals 2018, 54, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Du, G.; Guo, Q.; Li, R.; Li, C.; He, H. Fabrication and Characterization of Polylactic Acid Electrospun Wound Dressing Modified with Polyethylene Glycol, Rosmarinic Acid and Graphite Oxide. Nanomaterials 2023, 13, 2000. [Google Scholar] [CrossRef]
- Meng, C.; Liu, X.; Li, J. Hierarchical porous PLLA/ACP fibrous membrane towards bone tissue scaffold. J. Mech. Behav. Biomed. Mater. 2024, 152, 106455. [Google Scholar] [CrossRef]
- Kai, D.; Ren, W.; Tian, L.; Chee, P.L.; Liu, Y.; Ramakrishna, S.; Loh, X.J. Engineering Poly(lactide)–Lignin Nanofibers with Antioxidant Activity for Biomedical Application. ACS Sustain. Chem. Eng. 2016, 4, 5268–5276. [Google Scholar] [CrossRef]
- Shamsah, A.H.; Cartmell, S.H.; Richardson, S.M.; Bosworth, L.A. Material Characterization of PCL:PLLA Electrospun Fibers Following Six Months Degradation In Vitro. Polymers 2020, 12, 700. [Google Scholar] [CrossRef]
- Feng, J. Preparation and properties of poly(lactic acid) fiber melt blown non-woven disordered mats. Mater. Lett. 2017, 189, 180–183. [Google Scholar] [CrossRef]
- Zhou, H.; Tang, Y.; Wang, Z.; Zhang, P.; Zhu, Q. Cotton-like micro- and nanoscale poly(lactic acid) nonwoven fibers fabricated by centrifugal melt-spinning for tissue engineering. RSC Adv. 2018, 8, 5166–5179. [Google Scholar] [CrossRef]
- Boschetto, F.; Doan, H.N.; Vo, P.P.; Zanocco, M.; Zhu, W.; Sakai, W.; Kinashi, K.; Marin, E.; Pezzotti, G. Effect of BaTiO3 on the aging process of PLA fibers obtained by centrifugal spinning. Mater. Today Chem. 2021, 20, 100461. [Google Scholar] [CrossRef]
- Chen, X.-X.; Yu, J.-C.; Chen, K.; Ji, P.; Chen, X.-L.; Pan, Z.-J. Facile and Large-scale Fabrication of Biodegradable Thermochromic Fibers Based on Poly(lactic acid). Chin. J. Polym. Sci. 2022, 40, 1242–1251. [Google Scholar] [CrossRef]
- Hassan, E.A.M.; Elarabi, S.E.; Wei, Y.; Yu, M. Biodegradable poly (lactic acid)/poly (butylene succinate) fibers with high elongation for health care products. Text. Res. J. 2017, 88, 1735–1744. [Google Scholar] [CrossRef]
- Wu, J.-h.; Hu, T.-g.; Wang, H.; Zong, M.-h.; Wu, H.; Wen, P. Electrospinning of PLA nanofibers: Recent advances and its potential application for food packaging. J. Agric. Food Chem. 2022, 70, 8207–8221. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Jia, X.; Qi, Y.; Miao, D.; Yan, X. Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics. e-Polymers 2023, 23, 20230030. [Google Scholar] [CrossRef]
- Neffe, A.T.; Zhang, Q.; Hommes-Schattmann, P.J.; Wang, W.; Xu, X.; Ahmad, B.S.; Williams, G.R.; Lendlein, A. Functionalizable coaxial PLLA/PDLA nanofibers with stereocomplexes at the internal interface. J. Mater. Res. 2021, 36, 2995–3009. [Google Scholar] [CrossRef]
- Kheradvar Kolour, A.; Ghoraishizadeh, S.; Zaman, M.S.; Alemzade, A.; Banavand, M.; Esmaeili, J.; Shahrousvand, M. Janus films wound dressing comprising electrospun gelatin/PCL nanofibers and gelatin/honey/curcumin thawed layer. ACS Appl. Bio Mater. 2024, 7, 8642–8655. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Alharbi, N.; Daraei, A.; Lee, H.; Guthold, M. The effect of molecular weight and fiber diameter on the mechanical properties of single, electrospun PCL nanofibers. Mater. Today Commun. 2023, 35, 105773. [Google Scholar] [CrossRef]
- Mobarakeh, A.I.; Ramsheh, A.S.; Khanshan, A.; Aghaei, S.; Mirbagheri, M.S.; Esmaeili, J. Fabrication and evaluation of a bi-layered electrospun PCL/PVA patch for wound healing: Release of vitamins and silver nanoparticle. Heliyon 2024, 10, e33178. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar] [CrossRef]
- Yalcin Enis, I.; Gok Sadikoglu, T.; Horakova, J.; Lukas, D. The post-morphological analysis of electrospun vascular grafts following mechanical testing. J. Polym. Eng. 2018, 38, 525–535. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, D.; Zhao, L.; Lin, J. Composite fibrous membrane comprising PLA and PCL fibers for biomedical application. Compos. Commun. 2022, 34, 101268. [Google Scholar] [CrossRef]
- Sharma, D.; Satapathy, B.K. Tuning structural-response of PLA/PCL based electrospun nanofibrous mats: Role of dielectric-constant and electrical-conductivity of the solvent system. J. Biomater. Sci. Polym. Ed. 2022, 33, 1759–1793. [Google Scholar] [CrossRef]
- Maleki, H.; Gharehaghaji, A.A.; Moroni, L.; Dijkstra, P.J. Influence of the solvent type on the morphology and mechanical properties of electrospun PLLA yarns. Biofabrication 2013, 5, 035014. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Satapathy, B.K. Understanding release kinetics and collapse proof suture retention response of curcumin loaded electrospun mats based on aliphatic polyesters and their blends. J. Mech. Behav. Biomed. Mater. 2021, 120, 104556. [Google Scholar] [CrossRef]
- Volokhova, A.A.; Kudryavtseva, V.L.; Spiridonova, T.I.; Kolesnik, I.; Goreninskii, S.I.; Sazonov, R.V.; Remnev, G.E.; Tverdokhlebov, S.I. Controlled drug release from electrospun PCL non-woven scaffolds via multi-layering and e-beam treatment. Mater. Today Commun. 2021, 26, 102134. [Google Scholar] [CrossRef]
- Li, H.; Zhu, C.; Xue, J.; Ke, Q.; Xia, Y. Enhancing the Mechanical Properties of Electrospun Nanofiber Mats through Controllable Welding at the Cross Points. Macromol. Rapid Commun. 2017, 38, 1600723. [Google Scholar] [CrossRef]
- Rajzer, I.; Menaszek, E.; Castano, O. Electrospun polymer scaffolds modified with drugs for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 493–499. [Google Scholar] [CrossRef]
- Khunova, V.; Pavlinak, D.; Safarik, I.; Skratek, M.; Ondreas, F. Multifunctional Electrospun Nanofibers Based on Biopolymer Blends and Magnetic Tubular Halloysite for Medical Applications. Polymers 2021, 13, 3870. [Google Scholar] [CrossRef]
- Chlanda, A.; Kijeńska, E.; Święszkowski, W. Microscopic Methods for Characterization of Selected Surface Properties of Biodegradable, Nanofibrous Tissue Engineering Scaffolds. Mater. Sci. Forum 2017, 890, 213–216. [Google Scholar] [CrossRef]
- Loordhuswamy, A.M.; Krishnaswamy, V.R.; Korrapati, P.S.; Thinakaran, S.; Rengaswami, G.D. Fabrication of highly aligned fibrous scaffolds for tissue regeneration by centrifugal spinning technology. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Milanesi, G.; Bruni, G.; Ferrari, F. Coated electrospun alginate-containing fibers as novel delivery systems for regenerative purposes. Int. J. Nanomed. 2018, 13, 6531–6550. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Yang, J.M.; Chang, Y.H.; Su, W.W. Modification of different molecular weights of chitosan by p-Coumaric acid: Preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Albanna, M.Z.; Bou-Akl, T.H.; Blowytsky, O.; Walters, H.L., 3rd; Matthew, H.W. Chitosan fibers with improved biological and mechanical properties for tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2013, 20, 217–226. [Google Scholar] [CrossRef]
- Li, Z.; Mei, S.; Dong, Y.; She, F.; Kong, L. High Efficiency Fabrication of Chitosan Composite Nanofibers with Uniform Morphology via Centrifugal Spinning. Polymers 2019, 11, 1550. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, Y.; Lu, A.; Fu, Q.; Hu, J.; Zhang, L. Cellulose/Chitosan Composite Multifilament Fibers with Two-Switch Shape Memory Performance. ACS Sustain. Chem. Eng. 2019, 7, 6981–6990. [Google Scholar] [CrossRef]
- Barnes, C.P.; Sell, S.A.; Knapp, D.C.; Walpoth, B.H.; Brand, D.D.; Bowlin, G.L. Preliminary investigation of electros pun collagen and polydioxanone for vascular tissue engineering applications. Int. J. Electrospun Nanofibers Appl. 2020, 6, 81. [Google Scholar]
- Qin, Z.; Jia, X.; Liu, Q.; Kong, B.; Wang, H. Enhancing physical properties of chitosan/pullulan electrospinning nanofibers via green crosslinking strategies. Carbohydr. Polym. 2020, 247, 116734. [Google Scholar] [CrossRef]
- Santiago-Castillo, K.; Del Angel-López, D.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Palma-Ramírez, D.; Willcock, H.; Brachetti-Sibaja, S.B. Effect on the processability, structure and mechanical properties of highly dispersed in situ ZnO:CS nanoparticles into PVA electrospun fibers. J. Mater. Res. Technol. 2021, 11, 929–945. [Google Scholar] [CrossRef]
- Abbas, W.A.; Sharafeldin, I.M.; Omar, M.M.; Allam, N.K. Novel mineralized electrospun chitosan/PVA/TiO(2) nanofibrous composites for potential biomedical applications: Computational and experimental insights. Nanoscale Adv. 2020, 2, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Roether, J.A.; Schubert, D.W.; Beier, J.P.; Boccaccini, A.R. Bi-layered porous constructs of PCL-coated 45S5 bioactive glass and electrospun collagen-PCL fibers. J. Porous Mater. 2015, 22, 1215–1226. [Google Scholar] [CrossRef]
- Tuan, R.S. Biology of developmental and regenerative skeletogenesis. Clin. Orthop. Relat. Res. 2004, 427, S105–S117. [Google Scholar] [CrossRef]
- Chandika, P.; Oh, G.W.; Heo, S.Y.; Kim, S.C.; Kim, T.H.; Kim, M.S.; Jung, W.K. Electrospun porous bilayer nano-fibrous fish collagen/PCL bio-composite scaffolds with covalently cross-linked chitooligosaccharides for full-thickness wound-healing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111871. [Google Scholar] [CrossRef]
- Panwar, P.; Du, X.; Sharma, V.; Lamour, G.; Castro, M.; Li, H.; Bromme, D. Effects of cysteine proteases on the structural and mechanical properties of collagen fibers. J. Biol. Chem. 2013, 288, 5940–5950. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Khalf, A.; Singarapu, K.; Madihally, S.V. Cellulose acetate core–shell structured electrospun fiber: Fabrication and characterization. Cellulose 2015, 22, 1389–1400. [Google Scholar] [CrossRef]
- Shi, Q.; Zhou, C.; Yue, Y.; Guo, W.; Wu, Y.; Wu, Q. Mechanical properties and in vitro degradation of electrospun bio-nanocomposite mats from PLA and cellulose nanocrystals. Carbohydr. Polym. 2012, 90, 301–308. [Google Scholar] [CrossRef]
- Sheng, L.; Jiang, R.; Zhu, Y.; Ji, Y. Electrospun Cellulose Nanocrystals/Polycaprolactone Nanocomposite Fiber Mats. J. Macromol. Sci. Part B 2014, 53, 820–828. [Google Scholar] [CrossRef]
- Hossain, K.M.; Hasan, M.S.; Boyd, D.; Rudd, C.D.; Ahmed, I.; Thielemans, W. Effect of cellulose nanowhiskers on surface morphology, mechanical properties, and cell adhesion of melt-drawn polylactic Acid fibers. Biomacromolecules 2014, 15, 1498–1506. [Google Scholar] [CrossRef]
- Aydogdu, M.O.; Altun, E.; Ahmed, J.; Gunduz, O.; Edirisinghe, M. Fiber Forming Capability of Binary and Ternary Compositions in the Polymer System: Bacterial Cellulose-Polycaprolactone-Polylactic Acid. Polymers 2019, 11, 1148. [Google Scholar] [CrossRef]
- Hemamalini, T.; Giri Dev, V.R. Comprehensive review on electrospinning of starch polymer for biomedical applications. Int. J. Biol. Macromol. 2018, 106, 712–718. [Google Scholar] [CrossRef]
- Temesgen, S.; Rennert, M.; Tesfaye, T.; Nase, M. Review on Spinning of Biopolymer Fibers from Starch. Polymers 2021, 13, 1121. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Azam, F.; Ahmad, S.; Khaliq, Z.; Shahzad, A.; Qadir, M.B.; Hai, A.M. Development and characterization of biodegradable starch-based fibre by wet extrusion. Cellulose 2021, 28, 2039–2051. [Google Scholar] [CrossRef]
- Maqsood, M.; Seide, G. Improved Thermal Processing of Polylactic Acid/Oxidized Starch Composites and Flame-Retardant Behavior of Intumescent Non-Wovens. Coatings 2020, 10, 291. [Google Scholar] [CrossRef]
- Lancuski, A.; Vasilyev, G.; Putaux, J.L.; Zussman, E. Rheological Properties and Electrospinnability of High-Amylose Starch in Formic Acid. Biomacromolecules 2015, 16, 2529–2536. [Google Scholar] [CrossRef]
- Promnil, S.; Ruksakulpiwat, Y.; Ruksakulpiwat, C.; Numpaisal, P.-O. Effect of silk fibroin content on physical and mechanical properties of electrospun poly (lactic acid)/silk fibroin nanofibers for meniscus tissue engineering scaffold. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2022; p. 012016. [Google Scholar]
- Song, J.; Chen, Z.; Murillo, L.L.; Tang, D.; Meng, C.; Zhong, X.; Wang, T.; Li, J. Hierarchical porous silk fibroin/poly(L-lactic acid) fibrous membranes towards vascular scaffolds. Int. J. Biol. Macromol. 2021, 166, 1111–1120. [Google Scholar] [CrossRef]
- Lee, H.; Kim, G. Biocomposites electrospun with poly(epsilon-caprolactone) and silk fibroin powder for biomedical applications. J. Biomater. Sci. Polym. Ed. 2010, 21, 1687–1699. [Google Scholar] [CrossRef]
- Dodel, M.; Nejad, N.H.; Bahrami, S.; Soleimani, M.; Hanaee-Ahvaz, H. Modifying the mechanical properties of silk nanofiber scaffold by knitted orientation for regenerative medicine applications. Cell. Mol. Biol. 2016, 62, 16–25. [Google Scholar]
- Amiraliyan, N.; Nouri, M.; Haghighat Kish, M. Structural characterization and mechanical properties of electrospun silk fibroin nanofiber mats. Polym. Sci. Ser. A 2010, 52, 407–412. [Google Scholar] [CrossRef]
- Lu, L.X.; Wang, Y.Y.; Mao, X.; Xiao, Z.D.; Huang, N.P. The effects of PHBV electrospun fibers with different diameters and orientations on growth behavior of bone-marrow-derived mesenchymal stem cells. Biomed. Mater. 2012, 7, 015002. [Google Scholar] [CrossRef]
- Castro Mayorga, J.L.; Fabra Rovira, M.J.; Cabedo Mas, L.; Sánchez Moragas, G.; Lagarón Cabello, J.M. Antimicrobial nanocomposites and electrospun coatings based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and copper oxide nanoparticles for active packaging and coating applications. J. Appl. Polym. Sci. 2017, 135, 45673. [Google Scholar] [CrossRef]
- Kuppan, P.; Vasanthan, K.S.; Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Development of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) fibers for skin tissue engineering: Effects of topography, mechanical, and chemical stimuli. Biomacromolecules 2011, 12, 3156–3165. [Google Scholar] [CrossRef]
- Sombatmankhong, K.; Suwantong, O.; Waleetorncheepsawat, S.; Supaphol, P. Electrospun fiber mats of poly(3-hydroxybutyrate), poly(3-hydroxybutyrate-co-3-hydroxyvalerate), and their blends. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2923–2933. [Google Scholar] [CrossRef]
- Wang, X.; Yan, H.; Shen, Y.; Tang, H.; Yi, B.; Qin, C.; Zhang, Y. Shape Memory and Osteogenesis Capabilities of the Electrospun Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Modified Poly(l-Lactide) Fibrous Mats. Tissue Eng. Part A 2021, 27, 142–152. [Google Scholar] [CrossRef]
- Upson, S.J.; O’Haire, T.; Russell, S.J.; Dalgarno, K.; Ferreira, A.M. Centrifugally spun PHBV micro and nanofibres. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 190–195. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Fabra, M.J.; Cabedo, L.; Lagaron, J.M. On the Use of the Electrospinning Coating Technique to Produce Antimicrobial Polyhydroxyalkanoate Materials Containing In Situ-Stabilized Silver Nanoparticles. Nanomaterials 2017, 7, 4. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.; Fabra, M.; Lagaron, J. Stabilized nanosilver based antimicrobial poly (3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites of interest in active food packaging. Innov. Food Sci. Emerg. Technol. 2016, 33, 524–533. [Google Scholar] [CrossRef]
- Mohamadi, F.; Ebrahimi-Barough, S.; Reza Nourani, M.; Ali Derakhshan, M.; Goodarzi, V.; Sadegh Nazockdast, M.; Farokhi, M.; Tajerian, R.; Faridi Majidi, R.; Ai, J. Electrospun nerve guide scaffold of poly(epsilon-caprolactone)/collagen/nanobioglass: An in vitro study in peripheral nerve tissue engineering. J. Biomed. Mater. Res. A 2017, 105, 1960–1972. [Google Scholar] [CrossRef]
- Chakrapani, V.Y.; Gnanamani, A.; Giridev, V.R.; Madhusoothanan, M.; Sekaran, G. Electrospinning of type I collagen and PCL nanofibers using acetic acid. J. Appl. Polym. Sci. 2012, 125, 3221–3227. [Google Scholar] [CrossRef]
- Roy, T.; Maity, P.P.; Rameshbabu, A.P.; Das, B.; John, A.; Dutta, A.; Ghorai, S.K.; Chattopadhyay, S.; Dhara, S. Core-Shell Nanofibrous Scaffold Based on Polycaprolactone-Silk Fibroin Emulsion Electrospinning for Tissue Engineering Applications. Bioengineering 2018, 5, 68. [Google Scholar] [CrossRef]
- Rajasekaran, R.; Dutta, A.; Ray, P.G.; Seesala, V.S.; Ojha, A.K.; Dogra, N.; Roy, S.; Banerjee, M.; Dhara, S. High fibroin-loaded silk-PCL electrospun fiber with core-shell morphology promotes epithelialization with accelerated wound healing. J. Mater. Chem. B 2022, 10, 9622–9638. [Google Scholar] [CrossRef]
- Mamun, A.; Blachowicz, T.; Sabantina, L. Electrospun Nanofiber Mats for Filtering Applications—Technology, Structure and Materials. Polymers 2021, 13, 1368. [Google Scholar] [CrossRef]
- Roldán, E.; Reeves, N.D.; Cooper, G.; Andrews, K. Machine learning to predict morphology, topography and mechanical properties of sustainable gelatin-based electrospun scaffolds. Sci. Rep. 2024, 14, 21017. [Google Scholar] [CrossRef]
- Roldán, E.; Reeves, N.D.; Cooper, G.; Andrews, K. Machine learning to mechanically assess 2D and 3D biomimetic electrospun scaffolds for tissue engineering applications: Between the predictability and the interpretability. J. Mech. Behav. Biomed. Mater. 2024, 157, 106630. [Google Scholar] [CrossRef]
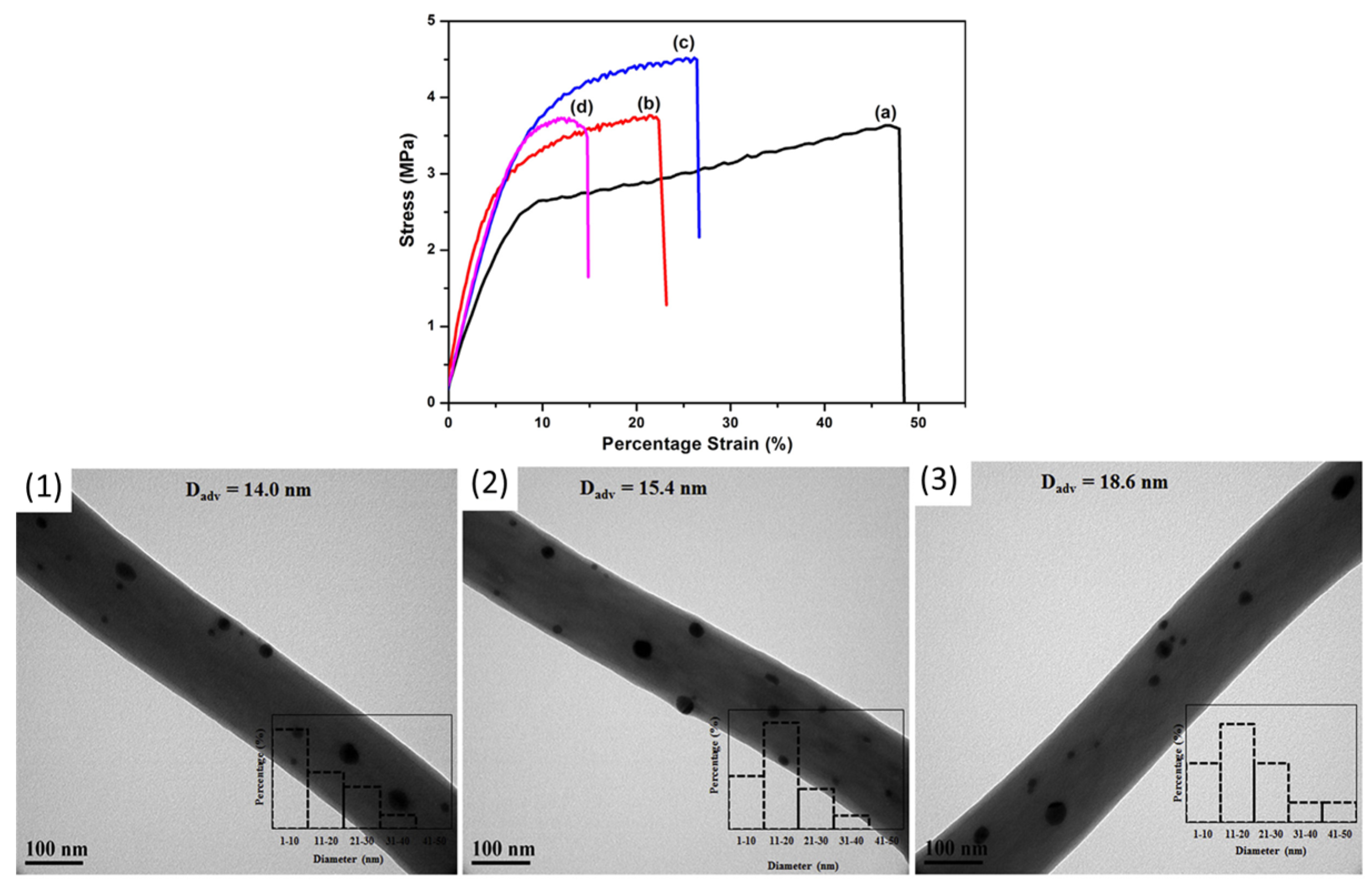



| Main Polymer | Fiber Components | Average Fiber Diameter (nm) | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) | Application and Processing Technique | Ref. |
|---|---|---|---|---|---|---|---|
| PVA | Isotropic PVA | 292 | 4.21 | - | 64.7 | Separation filters, wound dressing materials, tissue scaffold Electrospinning | [50] |
| PVA/cellulose nanowhisker | - | 7.84 | - | 132.8 | |||
| aligned PVA | 166 | 5.36 | - | 87.7 | |||
| PVA/cellulose nanowhisker | 149 | 10.5 | - | 190.5 | |||
| PVA/cellulose nanowhisker | - | 7.84 | - | 132.8 | |||
| PVA/CS | 155 | 3.65 | - | - | Antibacterial activity Electrospinning | [42] | |
| PVA/CS/Ag P (0.25%) | 148 | 3.78 | - | - | |||
| PVA/CS/Ag NPs (0.5%) | 144 | 4.52 | - | - | |||
| PVA/CS/Ag NPs (1%) | 139 | 3.74 | - | - | |||
| PVA | 328 | 7.31 | 186 | 29.2 | Wound healing Electrospinning | [45] | |
| PVA/AgNPs | 285 | 9.19 | 31 | 182.4 | |||
| PVA–silk sericin (non-heated) | 60–500 | 0.7 | 5 | - | Biomedical applications (skin) Electrospinning | [46] | |
| PVA–silk sericin (heated) | 3.7 | 35 | - | ||||
| PVA/γ-Fe2O3 porosity: 76.94% | 301 | 60.31 | - | 114.53 | Soft tissue engineering Scaffold Electrospinning | [53] | |
| Porosity: 80.68% | 298 | 57.48 | - | 111.62 | |||
| Porosity: 85.53% | 260 | 54.93 | - | 108.80 | |||
| Porosity: 90.85% | 235 | 51.45 | - | 105.97 | |||
| Porosity: 91.24% | 220 | 50.28 | - | 105.27 | |||
| PEO | PEO | 670–1620 | 1.8–4.23 | 445–517 | 4.88–15.3 | General applications Centrifugal spinning | [17] |
| CMCS-PEO | 1.91–3.22 µm | 0.8–2.2 | 11.2–22.8% | 10.5–26.6 | Wound dressing Centrifugal spinning | [58] | |
| Starch–PEO | 2 µm | 0.39–1.8 | - | - | Drug delivery Centrifugal spinning | [59] | |
| PCL/PEO (75/25) | 250–450 | 6.5 | - | 12–30 | Surface Functionality | [57] | |
| PGA And PLGA | PGA-PLA | 900–1300 | 2.2–11 | 70–330 | 3.4–140 | Biomedical applications Electrospinning | [63] |
| PGA | 300–1500 | 2.7 | 2.1 | 138 | Tissue engineering Electrospinning | [64] | |
| PLGA (50/50) | 2.8 | 2.2 | 144 | ||||
| PLGA (85/15) | 1.9 | 1.95 | 114 | ||||
| PLGA (85/15) | 1.9 | 1.95 | 114 | ||||
| PGA | 480 | - | 3.5 | 1013.4 | General application Electrospinning | [65] | |
| PGA (14 days) | - | - | 1.5 | 2989 | |||
| PGA | 666 | 3.44 | 152 | 74 | Tissue engineering (cell infiltration) Electrospinning | [56] | |
| PLGA | 2211 | 5.9 | 276 | 141 | |||
| PGA (sacrificial PEO) | 609 | 3.32 | 171 | 66 | |||
| PLGA (sacrificial PEO) | 2294 | 6.04 | 144 | 86 | |||
| PCL | 1165 | 7.05 | 453 | 28 | |||
| PCL (sacrificial PEO) | 1171 | 2.57 | 488 | 9 | |||
| PCL | 245 | 1.02 | 32.43 | 7.75 | Tissue engineering (blood vessel) Electrospinning | [66] | |
| PLGA | 163 | 3.1 | 3.1 | 13.51 | |||
| (PCL-PLGA) Coaxial | 256 | 2.89 | 2.89 | 9.18 | |||
| PLA And PLLA | PLLA | 850 | 2.7 | 261 | 1.15 | Medical applications Electrospinning | [73] |
| PLLA/TEAC | 270 | 27.2 | 79 | 2.8 | |||
| PLLA/formic acid | 520 | 9.5 | 150 | 1.5 | |||
| PLLA/TX-100 | 570 | 7.1 | 255 | 1.7 | |||
| PLA | - | 1.17 | 144 | - | Bone tissue engineering Electrospinning | [78] | |
| PLA-OS | 1.72 | 103 | - | ||||
| PLA | 910 | 0.8 | 125 | - | Wound healing Electrospinning | [79] | |
| PLA/Rosa/GO | 720 | 2.6 | 50 | - | |||
| PLA/PEG/RosA/GO | - | 2.3 | 15 | - | |||
| PLA | ~360 | 1.5 | - | - | Hard tissue engineering Electrospinning | [18] | |
| PLA/NCC | ~280 | 3.3 | - | - | |||
| PLLA | 712 | 3.49 | 49 | 56.8 | Cell adhesion and growth Electrospinning | [107] | |
| PLLA/PLA-Lig10 | 485 | 3.25 | 21 | 55.2 | |||
| PLLA/PLA-Lig20 | 525 | 2.49 | 19 | 56.1 | |||
| PLLA/PLA-Lig30 | 480 | 2.55 | 20 | 54.3 | |||
| PLLA/PLA-Lig40 | 417 | 2.58 | 16 | 66 | |||
| PLLA/PLA-Lig50 | 350 | 2.56 | 11 | 66.8 | |||
| PLA (CF/DMF (80:20 v/v)) | - | 5.8 | 29.8 | 305 | Biomedical applications Electrospinning | [98] | |
| PCL | - | 1.6 | 82.1 | 7.5 | |||
| PLA-PCL (70/30 w/w) | - | 2.5 | 155 | 67.5 | |||
| PLLA (treated at 120 C) | 796 | 5.6 | 56.9 | 165 | Tissue engineering Electrospinning | [74] | |
| PLLA (DCP crosslinkers) | 895 | 8.2 | 59.4 | 203 | |||
| PLLA (DCP-TAIC crosslinkers) | - | 8.78 | 47.3 | 208 | |||
| PLLA | 323 | 3.8 | 76.1 | 91.7 | |||
| PLLA | 323 | 3.8 | 76.1 | 91.7 | Tissue regeneration (human annulus fibrosus tissue) Electrospinning | [82] | |
| (PLLA) After 180 days in PBS | 330 | 5.2 | 38.9 | 136.4 | |||
| PCL | PCL | 219 | 2.9 | 190 | 16.3 | ||
| (PCL) | 214 | 3.5 | 81.4 | 21.6 | |||
| PLLA-PCL | 720 | 4.1 | 148.3 | 71.6 | |||
| PLLA-PCL After 180 days in PBS | 671 | 6.2 | 87.3 | 125.6 | |||
| PLA | 2500–7500 | 0.18 | - | - | Cell culture (melt-blown) | [83] | |
| PLA | 20,000 | 3.77 cN/dtex | 18 | 80.43 cN/dtex | Packaging Melt spinning | [86,87] | |
| PLA, 350 rpm | 9980 | 1.51 | 7.77 | 26.18 | Tissue engineering Centrifugal spanning | [84] | |
| PLA, 900 rpm | 8270 | 2.34 | 8.47 | 46.21 | |||
| PLA, 1500 rpm | 5990 | 3.31 | 7.47 | 66.77 | |||
| PLA (CF/DMF (80:20 v/v)) | - | 5.8 | 29.8 | 305 | Biomedical application Electrospinning | [98] | |
| PCL | - | 1.6 | 82.1 | 7.5 | |||
| PLA-PCL (70/30 w/w) | - | 2.5 | 155 | 67.5 | |||
| PCL | 1830 | 4.1 | 222 | 48 | Drug delivery Electrospinning | [101] | |
| PCL (E-beam treatment) | 1870 | 1.3 | 175 | 5 | |||
| PCL | 721 | 11.53 | - | 8.41 | General Electrospinning | [102] | |
| PCL (DCM vapor treatment) | 693 | 21.4 | - | 16.5 | |||
| PCL/Gel | - | 1.2 | 28 | - | Medical application Electrospinning | [104] | |
| PCL/Gel/HNTs | - | 2.3 | 67 | - | |||
| PCL | 824 | 3.14 | - | 40.76 | Wound dressing Centrifugal spinning | [106] | |
| PCL/Gel (70/30) | 534 | 2.91 | - | 84.87 | |||
| PCL/Gel (50/50) | 367 | 1.7 | - | 82.70 | |||
| PCL/Gel (30/70) | 265 | 1.22 | - | 72.76 | |||
| PCL fiber (AFM) | 440–1040 | 12 | 98 | 62 | Biomedical devices Electrospinning | [36] | |
| PCL fiber (AFM) | - | - | - | 4 | Tissue engineering Scaffolds Electrospinning | [105] | |
| PCL fiber/HAp (AFM) | - | - | - | 20 | |||
| PCL fiber (AFM) | 20–25 | 257–340 | 127–133 | 500–3000 | General application Electrospinning | [93] |
| Main Polymer | Fiber Components | Average Fiber Diameter (nm) | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) | Application and Processing Technique | Ref. |
|---|---|---|---|---|---|---|---|
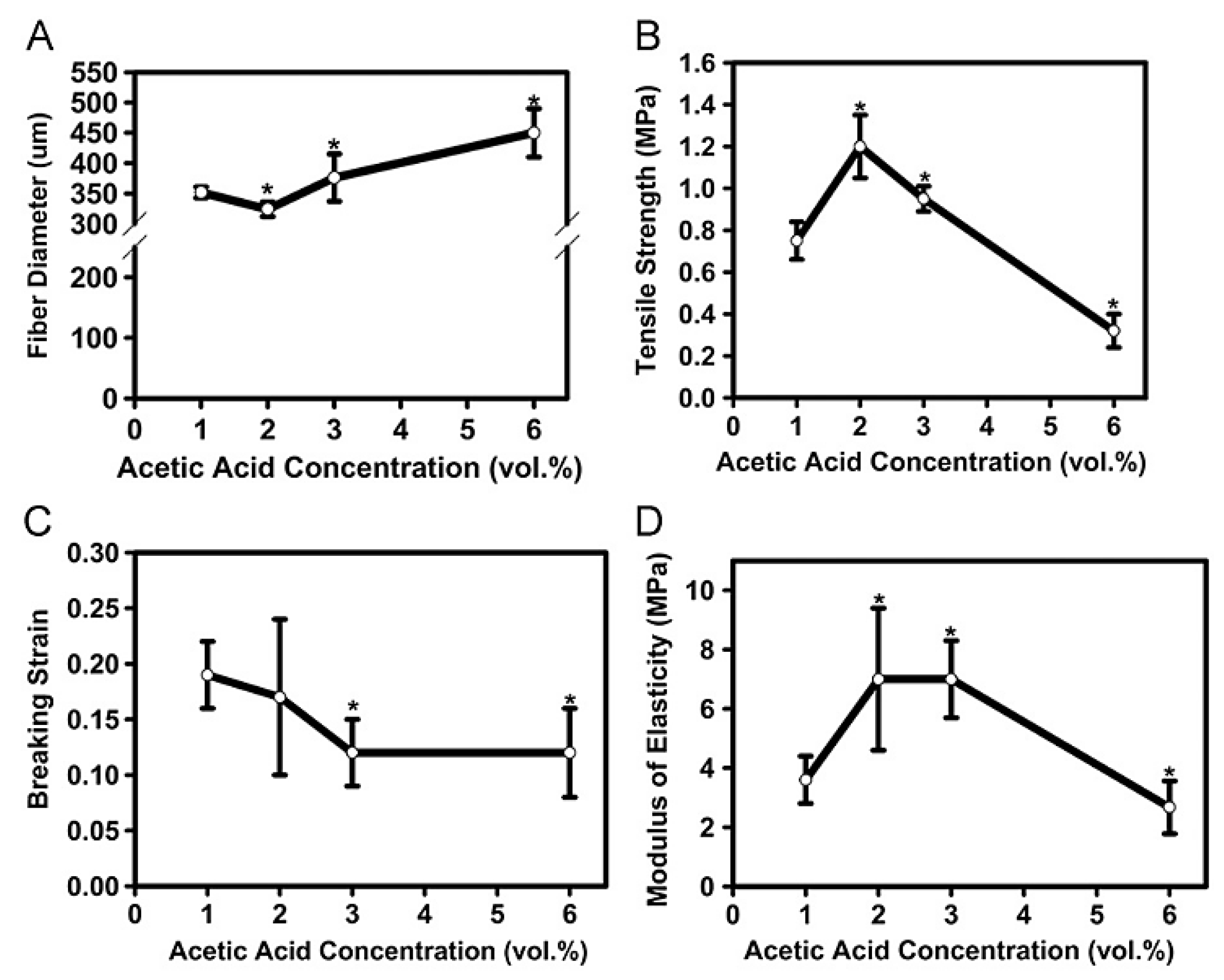
| Chitosan | CS (1% acetic acid) | 325 | 0.7 | 19 | 3.6 | Tissue engineering Electrospinning | [109] |
| CS (2% acetic acid) | 350 | 1.2 | 17 | 7 | |||
| CS (6% acetic acid) | 450 | 0.3 | 12 | 7 | |||
| Chitosan (10–30%)–PDO | 0.26–0.31 µm | 4.6–6.3 | - | 14–68 MPa | Vascular tissue engineering Electrospinning | [112] | |
| CS–pullulan | - | 11.36 | 4.53 | 344.3 | Active packaging Electrospinning | [113] | |
| CS–pullulan–crosslinker (cinnamaldehyde) | - | 22 | 2.83 | 1150.9 | |||
| CS-PVA | 155 | 3.65 | - | - | Biomedical application Electrospinning | [51] | |
| CS-PVA-AgNPs (0.25%) | 148 | 3.78 | - | - | |||
| CS-PVA-AgNPs (0.5%) | 144 | 4.52 | -- | ||||
| Collagen | Collagen (AFM) | 60–80 µm | 650 | - | 3.2 GPa | Tissue engineering Electrospinning | [119] |
| Collagen–cathepsin K (AFM) | 241 | 39 | 1.9 Gpa | ||||
| Collagen–PCL 9:1 | 318 | 4.2 | 4.74 | - | Wound-healing applications Electrospinning | [118] | |
| Collagen–PCL–crosslinker (5/5) | 388 | 7.65 | 8.93 | - | Nerve tissue engineering Electrospinning | [144] | |
| Collagen–PCL–crosslinker (9/1) | 480 | 12.61 | 11.76 | - | Tissue engineering Electrospinning | [145] | |
| Cellulose | PLA/nanocrystalline cellulose (NCC) (0–2%) | 280–320 | 1.5–3.3 | - | - | Tissue engineering Electrospinning | [18] |
| PLA/nanocrystalline cellulose (NCC) | 600–700 | 0.5–2.1 | - | - | Tissue engineering Electrospinning | [121] | |
| PCL–cellulose nanocrystals (CNCs) (0–20%) | 350–600 | 2.7–7.2 | 110–180% | - | General application Electrospinning | [123] | |
| PLA/nanocrystalline cellulose (NCC) 5% | 642 | 6.3 | 70 | 125.6 | Tissue engineering scaffold Electrospinning | [122] | |
| PCL–cellulose nanocrystals (CNCs) 65–95% (AFM) | 11.2 µm | 705 | - | 4.9 GPa | Tissue engineering Melt spinning | [124] | |
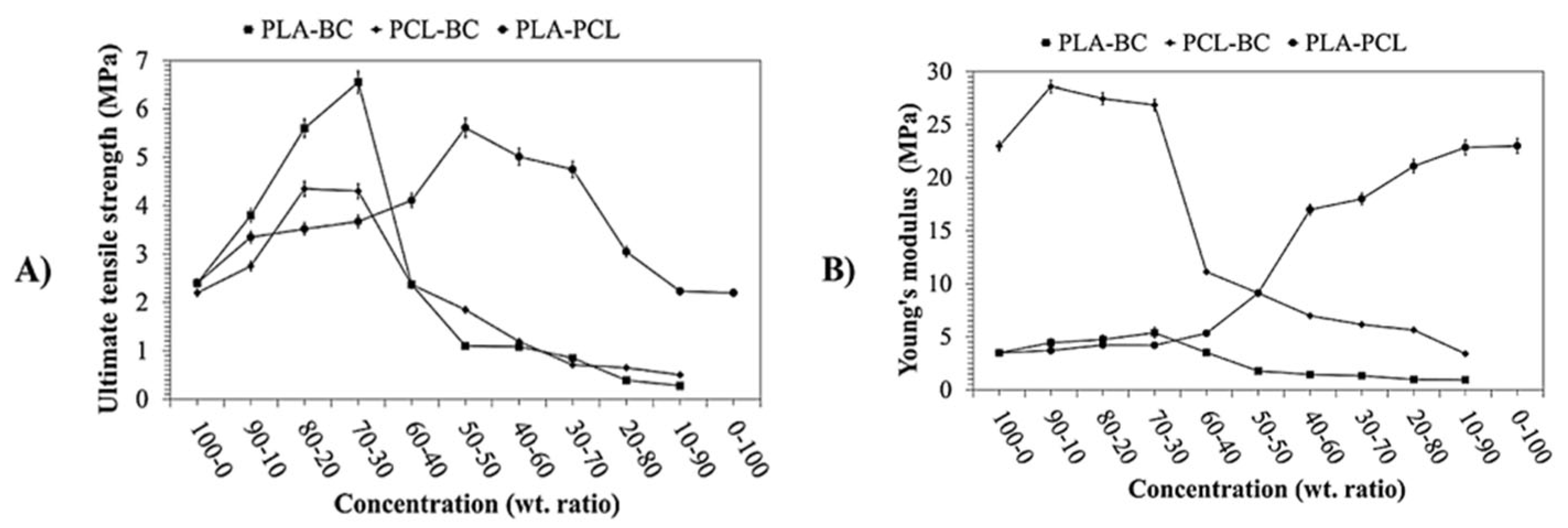
| (PLA-PCL)-BC 70/30 | 5–18.5 µm | 9.05 | - | 19.61 | Wound healing Centrifugal spinning | [125] | |
| Starch | Starch (in formic acid solution) | 80–300 | 9.38 | 26 | 264 | General application Electrospinning | [130] |
| Starch–PCL | 156–476 µm | 13 | - | 20 | Tissue engineering Electrospinning | [109] | |
| Starch–PLA (50) | - | 27 | - | 33 | |||
| Starch–PLA (70) | - | 40 | - | 162 | |||
| Starch–EVA | - | 25 | - | 85 | |||
| Starch–PVA | 247–301 µm | 0.88–11.54 | - | - |
Wound dressings Wet extrusion | [128] | |
| SF |
SF (10%) SF after treatment with methanol | 85–206 |
16.03 25.41 |
21.7 11.9 |
253 MPa 490 MPa | Air permeability Electrospinning | [135] |
| SF (20%) (central angle 60°) | 230 | 4.4 | 7.99 | 30 | Tissue engineering Electrospinning | [134] | |
| SF after treatment with methanol | 15.3 | 12.3 | 150 | ||||
| 2 wt% PCL/SF | - | 1.3 | 28.27 | 27.67 | Biomedical application Electrospinning | [133] | |
| PCL | - | 1.3 | 70.47 | 14.06 | |||
| PCL/SF (70/30) | 539 | 4.7 | 62.7 | 12.73 | Tissue engineering Electrospinning | [146] | |
| PCL/SF | 242–618 | 11.2 | 70 | Wound healing Electrospinning | [147] | ||
| PLA | 10,365 | 1.14 | 15.97 | 70.21 | Tissue engineering scaffold Electrospinning | [131] | |
| PLA75/SF25 | 665 | 0.47 | 26.22 | 20.51 | |||
| PLA50/SF50 | 693 | 1.05 | 17.51 | 16.49 | |||
| SF/PVA | 60–500 | 0.7 | 5 | - | Biomedical application Electrospinning | [46] | |
| SF/PVA (thermal treated) | - | 3.7 | 35 | - | |||
| PHBV | PHBV fiber | 724 | 1.4 | - | 1028 | Skin tissue engineering Electrospinning | [138] |
| PHBV 2D film | - | 1.57 | - | 5619 | |||
| PHBV | 105 µm | 1.6 | - | 127 | Antibacterial fibers Electrospinning | [139] | |
| PHB | 383–5200 | 1.8 | - | 147 | Bone tissue engineering Electrospinning | [136] | |
| PHBV-PHB (25/75) | 2.45 | 140 | |||||
| PHBV (chloroform solution) | 1.17 | 3.73 | |||||
| PHBV (TFE solution) | 1.88 | 25.54 | |||||
| PHBV-PLA (30/70) | 2890 | 73.5 | Bone regeneration Electrospinning | [140] | |||
| PLA | - | - | 41.5 | ||||
| PHBV | 0.5–3 μm | 3 | - | 100 | Biomedical application Centrifugal methods | [141] | |
| PHBV | - | 1.76 | - | 125.7 | Tissue engineering | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niknejad, E.; Jafari, R.; Valipour Motlagh, N. Mechanical Properties of Biodegradable Fibers and Fibrous Mats: A Comprehensive Review. Molecules 2025, 30, 3276. https://doi.org/10.3390/molecules30153276
Niknejad E, Jafari R, Valipour Motlagh N. Mechanical Properties of Biodegradable Fibers and Fibrous Mats: A Comprehensive Review. Molecules. 2025; 30(15):3276. https://doi.org/10.3390/molecules30153276
Chicago/Turabian StyleNiknejad, Ehsan, Reza Jafari, and Naser Valipour Motlagh. 2025. "Mechanical Properties of Biodegradable Fibers and Fibrous Mats: A Comprehensive Review" Molecules 30, no. 15: 3276. https://doi.org/10.3390/molecules30153276
APA StyleNiknejad, E., Jafari, R., & Valipour Motlagh, N. (2025). Mechanical Properties of Biodegradable Fibers and Fibrous Mats: A Comprehensive Review. Molecules, 30(15), 3276. https://doi.org/10.3390/molecules30153276









