Garlic-Derived Allicin Attenuates Parkinson’s Disease via PKA/p-CREB/BDNF/DAT Pathway Activation and Apoptotic Inhibition
Abstract
1. Introduction
2. Results
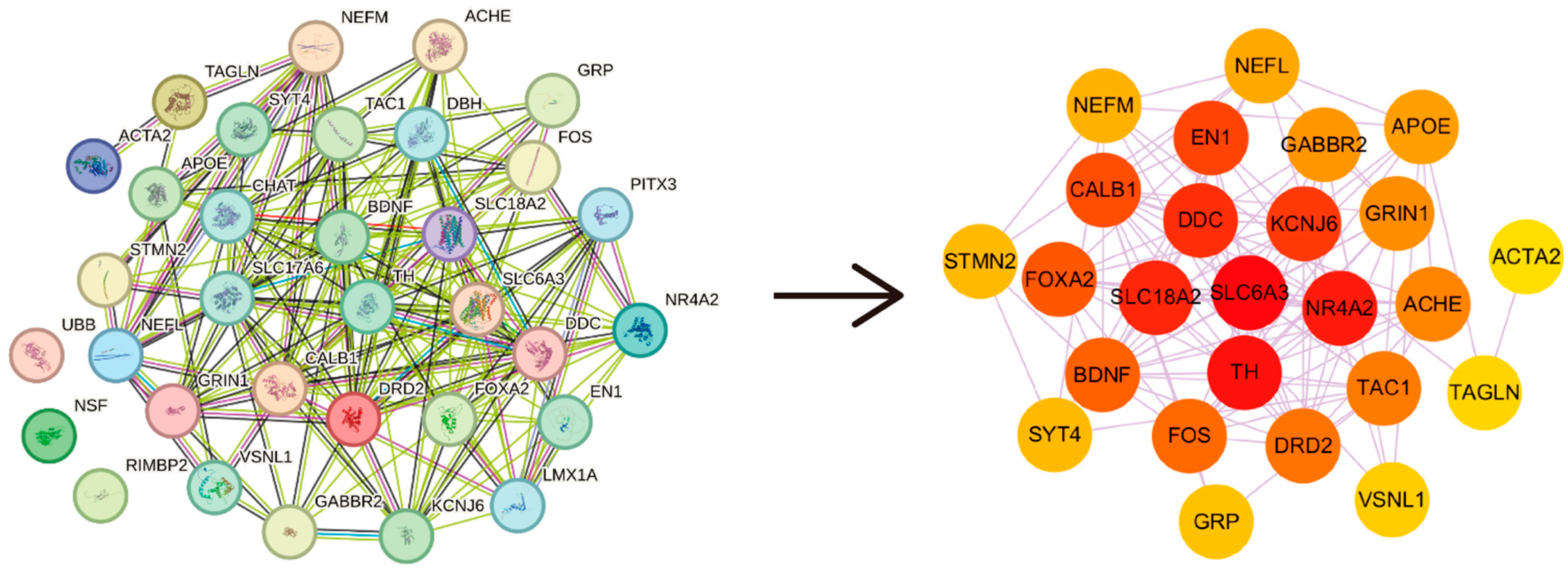
2.1. Identification of ALC Targets and PD Hub Genes
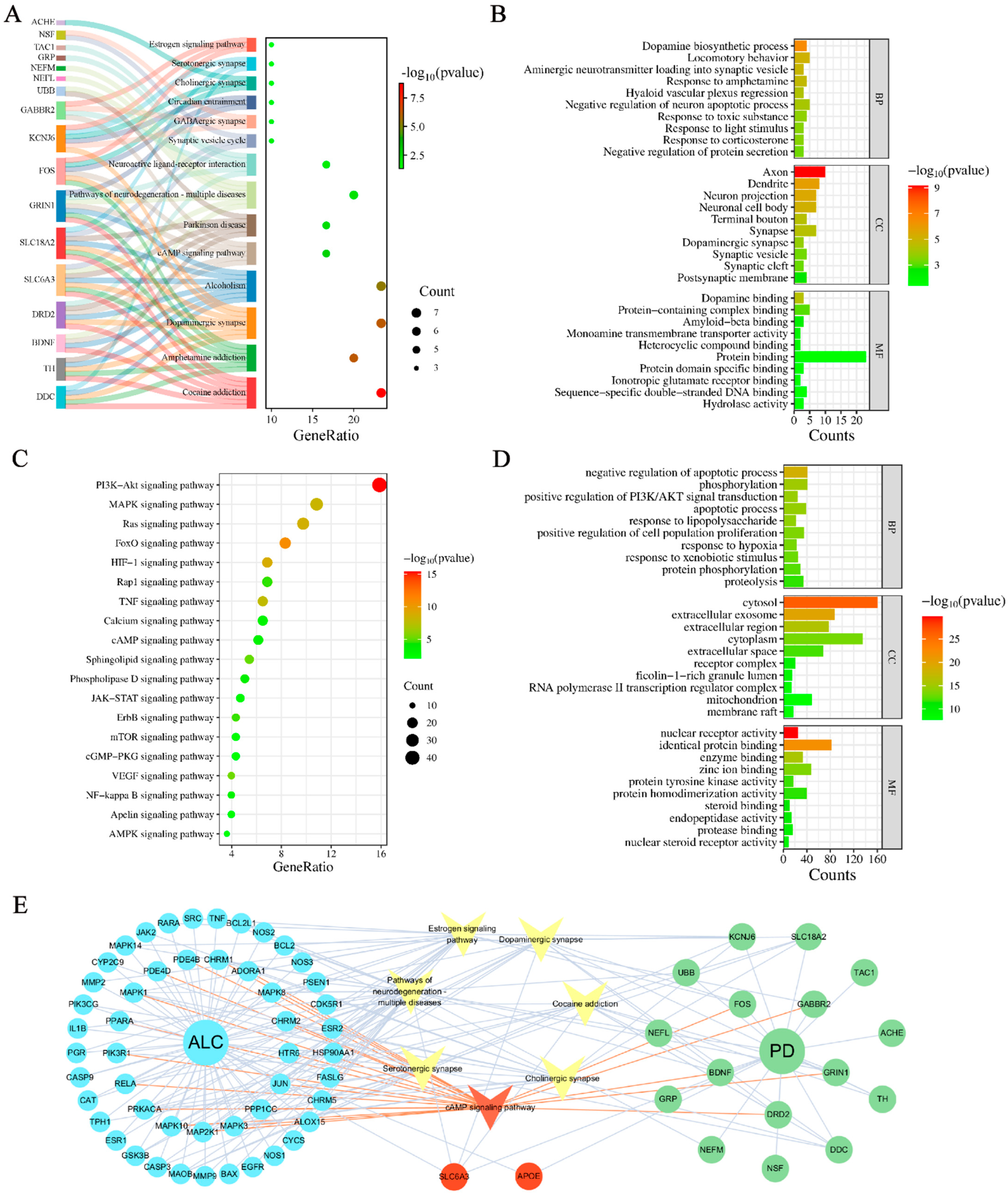
2.2. Mechanism Exploration of ALC Anti-PD
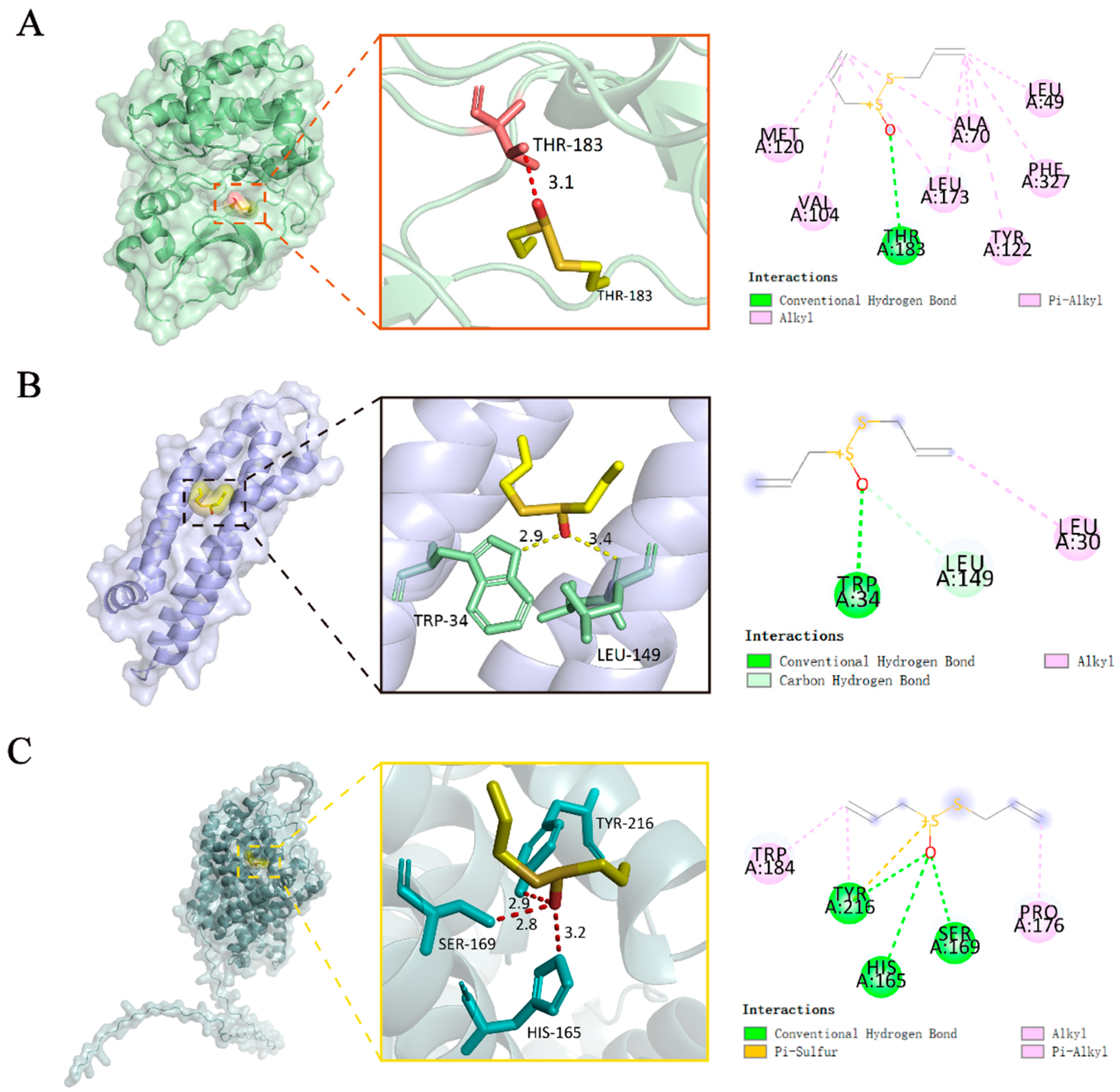
2.3. Molecular Docking
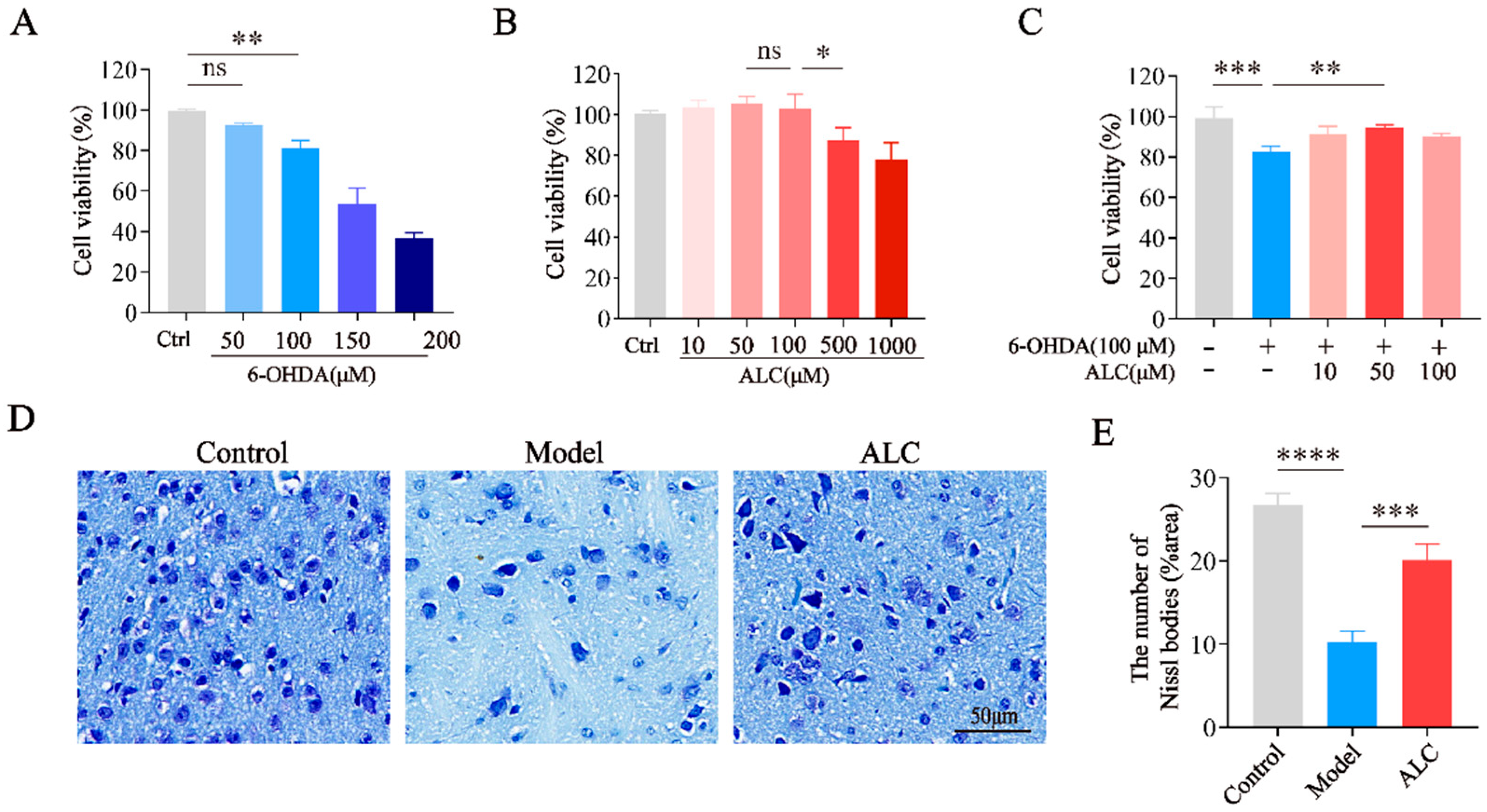
2.4. Neuroprotective Effect of ALC in 6-Hydroxydopamine (6-OHDA)-Induced Injury
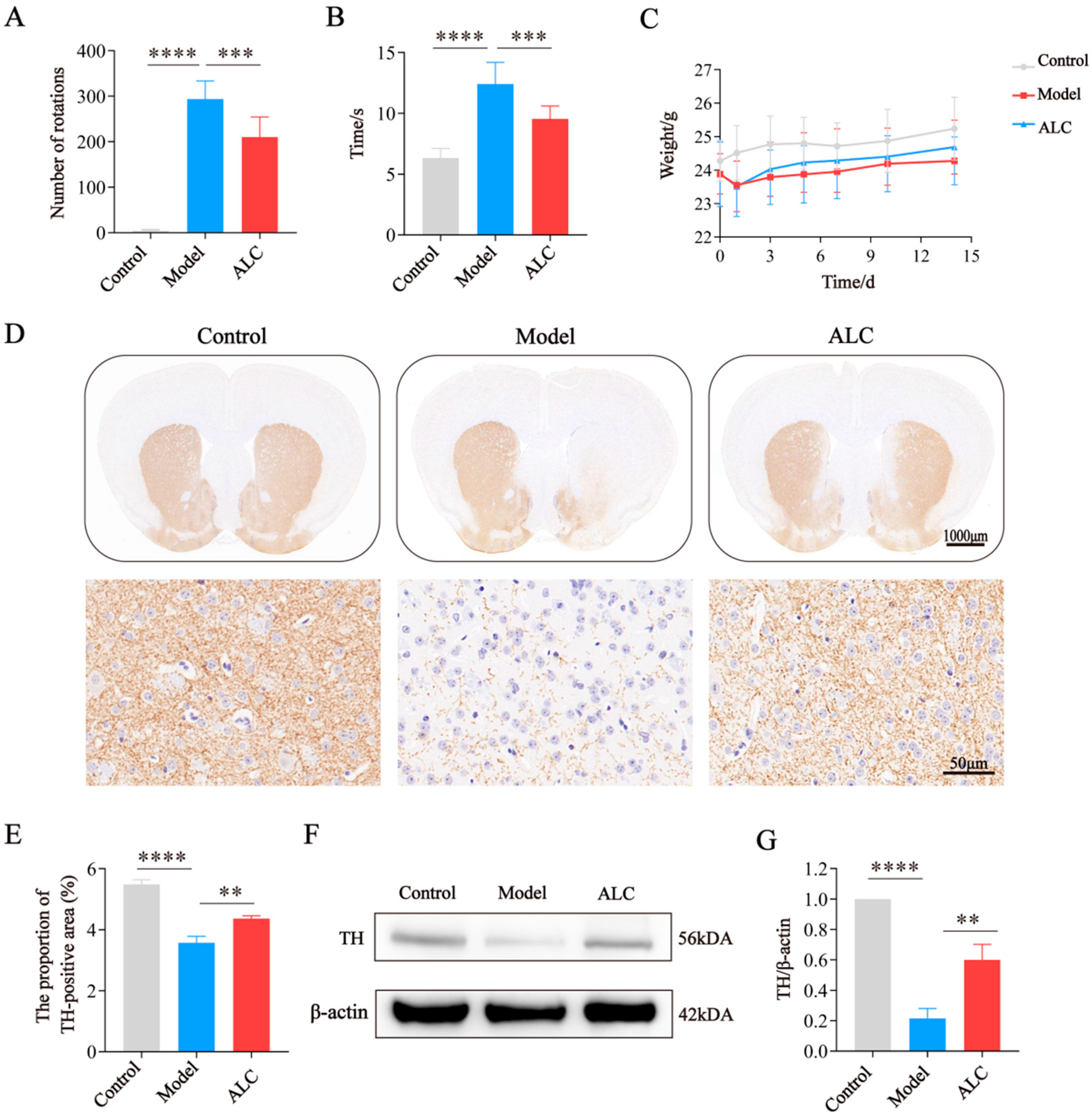
2.5. ALC Improves Motor Dysfunction and Dopaminergic Neuron Loss in PD Mice
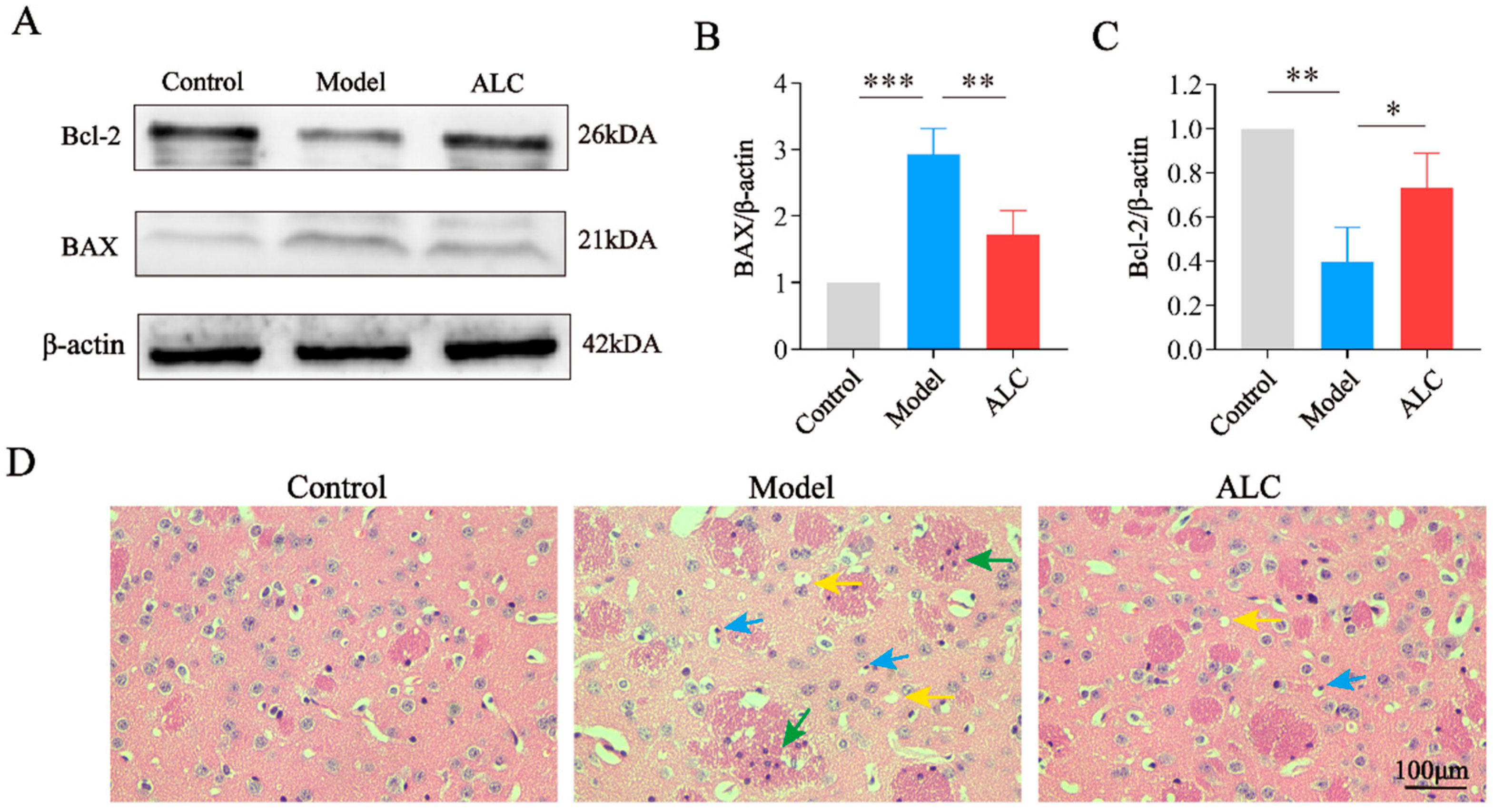
2.6. ALC Inhibits Neuronal Apoptosis and Improves Neuroinflammation
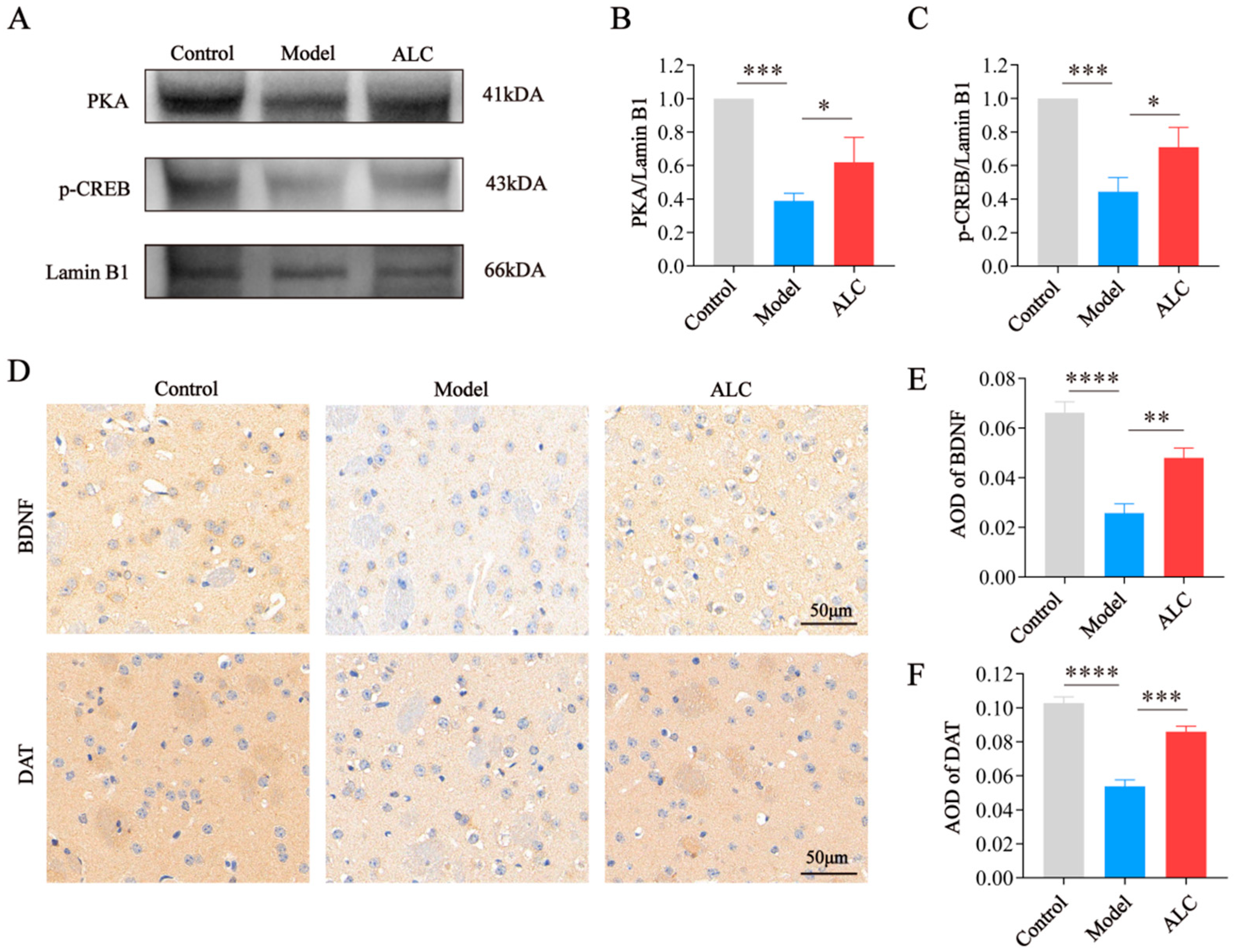
2.7. ALC Upregulates the PKA/p-CREB/BDNF Signaling Pathway and Restores DAT Expression
3. Discussion
4. Materials and Methods
4.1. Prediction of ALC Targets and Acquisition of PD-Related Genes
4.2. PPI Network Construction and Selection of PD Hub Genes
4.3. Pathway and Functional Enrichment
4.4. Visualization of Target-Pathway-Disease Interactions
4.5. Molecular Docking
4.6. Reagents and Preparation
4.7. Cell Culture and Viability Assay
4.8. Animals and Treatment
4.9. APO-Induced Rotation Experiment
4.10. Pole Climbing Test
4.11. Nissl Staining and H&E Staining
4.12. Western Blot
4.13. Immunohistochemistry
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.; Cui, Y.; Zhang, J.; Yan, R.; Su, D.; Zhao, D.; Wang, A.; Feng, T. Temporal Trends in the Prevalence of Parkinson’s Disease from 1980 to 2023: A Systematic Review and Meta-Analysis. Lancet Healthy Longev. 2024, 5, e464–e479. [Google Scholar] [CrossRef] [PubMed]
- Leite Silva, A.B.R.; Gonçalves de Oliveira, R.W.; Diógenes, G.P.; de Castro Aguiar, M.F.; Sallem, C.C.; Lima, M.P.P.; de Albuquerque Filho, L.B.; Peixoto de Medeiros, S.D.; Penido de Mendonça, L.L.; de Santiago Filho, P.C.; et al. Premotor, Nonmotor and Motor Symptoms of Parkinson’s Disease: A New Clinical State of the Art. Ageing Res. Rev. 2023, 84, 101834. [Google Scholar] [CrossRef]
- Hill, D.R.; Huters, A.D.; Towne, T.B.; Reddy, R.E.; Fogle, J.L.; Voight, E.A.; Kym, P.R. Parkinson’s Disease: Advances in Treatment and the Syntheses of Various Classes of Pharmaceutical Drug Substances. Chem. Rev. 2023, 123, 13693–13712. [Google Scholar] [CrossRef]
- Dionísio, P.A.; Amaral, J.D.; Rodrigues, C.M.P. Oxidative Stress and Regulated Cell Death in Parkinson’s Disease. Ageing Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef]
- Erekat, N.S. Apoptosis and Its Therapeutic Implications in Neurodegenerative Diseases. Clin. Anat. 2022, 35, 65–78. [Google Scholar] [CrossRef]
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular Mechanisms of Cell Death in Neurological Diseases. Cell Death Differ. 2021, 28, 2029–2044. [Google Scholar] [CrossRef]
- Tatton, W.G.; Chalmers-Redman, R.; Brown, D.; Tatton, N. Apoptosis in Parkinson’s Disease: Signals for Neuronal Degradation. Ann. Neurol. 2003, 53 (Suppl. 3), S61–S70; discussion S70–S72. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Michel, P.P.; Troadec, J.D.; Mouatt-Prigent, A.; Faucheux, B.A.; Ruberg, M.; Agid, Y.; Hirsch, E.C. Is Bax a Mitochondrial Mediator in Apoptotic Death of Dopaminergic Neurons in Parkinson’s Disease? J. Neurochem. 2001, 76, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Tatton, N.A. Increased Caspase 3 and Bax Immunoreactivity Accompany Nuclear GAPDH Translocation and Neuronal Apoptosis in Parkinson’s Disease. Exp. Neurol. 2000, 166, 29–43. [Google Scholar] [CrossRef]
- Blandini, F.; Mangiagalli, A.; Cosentino, M.; Marino, F.; Samuele, A.; Rasini, E.; Fancellu, R.; Martignoni, E.; Riboldazzi, G.; Calandrella, D.; et al. Peripheral Markers of Apoptosis in Parkinson’s Disease: The Effect of Dopaminergic Drugs. Ann. N. Y Acad. Sci. 2003, 1010, 675–678. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Park, J.-S.; Leem, Y.-H.; Park, J.-E.; Kim, D.-Y.; Choi, Y.-H.; Park, E.-M.; Kang, J.L.; Kim, H.-S. The Phosphodiesterase 10 Inhibitor Papaverine Exerts Anti-Inflammatory and Neuroprotective Effects via the PKA Signaling Pathway in Neuroinflammation and Parkinson’s Disease Mouse Models. J. Neuroinflammation 2019, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-L.; Li, L.; Yang, G.-M.; Zhang, Z.-B.; Zhao, Y.-N.; Zeng, X.-F.; Zhang, D.-X.; Yu, Y.; Shi, Z.-J.; Yan, Q.-W.; et al. Neuroprotective Effect of Gastrodin in Methamphetamine-Induced Apoptosis through Regulating cAMP/PKA/CREB Pathway in Cortical Neuron. Hum. Exp. Toxicol. 2020, 39, 1118–1129. [Google Scholar] [CrossRef]
- Verma, T.; Aggarwal, A.; Dey, P.; Chauhan, A.K.; Rashid, S.; Chen, K.-T.; Sharma, R. Medicinal and Therapeutic Properties of Garlic, Garlic Essential Oil, and Garlic-Based Snack Food: An Updated Review. Front. Nutr. 2023, 10, 1120377. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; García-Recio, E.; Ruiz, C.; De Luna-Bertos, E.; Illescas-Montes, R.; Costela-Ruiz, V.J. Biological Properties and Therapeutic Applications of Garlic and Its Components. Food Funct. 2022, 13, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Seol, J.; Kim, J.; Moon, S.M.; Jung, D.; Kang, C.; Chung, K.W.; Jung, Y.-S.; Chung, Y.-H.; Jung, Y.; Chung, H.Y.; et al. Preventive Effect of a Garlic Compound on Astrocyte-Mediated Neuroinflammation in Parkinson’s Disease. Neuropharmacology 2025, 275, 110494. [Google Scholar] [CrossRef]
- Yousefsani, B.S.; Ghobadi, A.; Shirani, K. Uncovering the Neuroprotective Powers of Allium Sativum: Exploring Its Potential to Alleviate Malathion- Associated Parkinson’s-like Behavioral Symptoms in a Rat Model. J. Med. Plants 2024, 23, 68–82. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Kazmi, I.; Ullah, I.; Muhammad, K.; Anwar, F. Allicin, an Antioxidant and Neuroprotective Agent, Ameliorates Cognitive Impairment. Antioxidants 2021, 11, 87. [Google Scholar] [CrossRef]
- Tedeschi, P.; Nigro, M.; Travagli, A.; Catani, M.; Cavazzini, A.; Merighi, S.; Gessi, S. Therapeutic Potential of Allicin and Aged Garlic Extract in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 6950. [Google Scholar] [CrossRef]
- Rakshit, D.; Nayak, S.; Kundu, S.; Angelopoulou, E.; Pyrgelis, E.-S.; Piperi, C.; Mishra, A. The Pharmacological Activity of Garlic (Allium Sativum) in Parkinson’s Disease: From Molecular Mechanisms to the Therapeutic Potential. ACS Chem. Neurosci. 2023, 14, 1033–1044. [Google Scholar] [CrossRef]
- Liu, H.; Mao, P.; Wang, J.; Wang, T.; Xie, C.-H. Allicin Protects PC12 Cells Against 6-OHDA-Induced Oxidative Stress and Mitochondrial Dysfunction via Regulating Mitochondrial Dynamics. Cell Physiol. Biochem. 2015, 36, 966–979. [Google Scholar] [CrossRef]
- Dovonou, A.; Bolduc, C.; Soto Linan, V.; Gora, C.; Peralta Iii, M.R.; Lévesque, M. Animal Models of Parkinson’s Disease: Bridging the Gap between Disease Hallmarks and Research Questions. Transl. Neurodegener. 2023, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, M.M.; Basgall, E.J.; Zamrini, E.; Hill, W.D. Apoptotic-like Changes in Lewy-Body-Associated Disorders and Normal Aging in Substantia Nigral Neurons. Am. J. Pathol. 1997, 150, 119–131. [Google Scholar]
- Zhou, Y.; Li, W.; Han, H.; Gao, D.; He, X.; Li, L.; Song, J.; Fei, Z. Allicin Protects Rat Cortical Neurons against Mechanical Trauma Injury by Regulating Nitric Oxide Synthase Pathways. Brain Res. Bull. 2014, 100, 14–21. [Google Scholar] [CrossRef]
- Atef, Y.; Kinoshita, K.; Ichihara, Y.; Ushida, K.; Hirata, Y.; Kurauchi, Y.; Seki, T.; Katsuki, H. Therapeutic Effect of Allicin in a Mouse Model of Intracerebral Hemorrhage. J. Pharmacol. Sci. 2023, 153, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Cheng, B.; Fang, B.; Meng, Z.; Zheng, Y.; Tian, X.; Guan, S. Protective Effects of Allicin on 1,3-DCP-Induced Lipid Metabolism Disorder in HepG2 Cells. Biomed. Pharmacother. 2017, 96, 1411–1417. [Google Scholar] [CrossRef]
- Edres, H.A.; Taha, N.M.; Lebda, M.A.; Elfeky, M.S. The Potential Neuroprotective Effect of Allicin and Melatonin in Acrylamide-Induced Brain Damage in Rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 58768–58780. [Google Scholar] [CrossRef] [PubMed]
- Lal, R.; Chopra, K. Experimental Models of Parkinson’s Disease: Challenges and Opportunities. Eur. J. Pharmacol. 2024, 980, 176819. [Google Scholar] [CrossRef]
- Mackie, P.; Lebowitz, J.; Saadatpour, L.; Nickoloff, E.; Gaskill, P.; Khoshbouei, H. The Dopamine Transporter: An Unrecognized Nexus for Dysfunctional Peripheral Immunity and Signaling in Parkinson’s Disease. Brain Behav. Immun. 2018, 70, 21–35. [Google Scholar] [CrossRef]
- Kim, H.I.; Lee, S.; Lim, J.; Chung, S.; Koo, T.-S.; Ji, Y.-G.; Suh, Y.-G.; Son, W.S.; Kim, S.-H.; Choi, H.J. ERRγ Ligand HPB2 Upregulates BDNF-TrkB and Enhances Dopaminergic Neuronal Phenotype. Pharmacol. Res. 2021, 165, 105423. [Google Scholar] [CrossRef]
- Badanjak, K.; Fixemer, S.; Smajić, S.; Skupin, A.; Grünewald, A. The Contribution of Microglia to Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4676. [Google Scholar] [CrossRef]
- Long-Smith, C.M.; Sullivan, A.M.; Nolan, Y.M. The Influence of Microglia on the Pathogenesis of Parkinson’s Disease. Prog. Neurobiol. 2009, 89, 277–287. [Google Scholar] [CrossRef]
- Goyal, A.; Agrawal, A.; Verma, A.; Dubey, N. The PI3K-AKT Pathway: A Plausible Therapeutic Target in Parkinson’s Disease. Exp. Mol. Pathol. 2023, 129, 104846. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Yan, Y. Allicin Attenuates UVB-Induced Photodamage of Keratinocytes by Inhibiting NLRP3 Inflammasomes and Activating the PI3K/Akt Pathway. Arch. Dermatol. Res. 2024, 317, 124. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 Update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, F.; Yang, K.; Fang, S.; Bu, D.; Li, H.; Sun, L.; Hu, H.; Gao, K.; Wang, W.; et al. SymMap: An Integrative Database of Traditional Chinese Medicine Enhanced by Symptom Mapping. Nucleic Acids Res. 2019, 47, D1110–D1117. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 Update: A Web Server for Potential Drug Target Identification with a Comprehensive Target Pharmacophore Database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef]
- Davis, A.P.; Wiegers, T.C.; Sciaky, D.; Barkalow, F.; Strong, M.; Wyatt, B.; Wiegers, J.; McMorran, R.; Abrar, S.; Mattingly, C.J. Comparative Toxicogenomics Database’s 20th Anniversary: Update 2025. Nucleic Acids Res. 2024, 53, D1328–D1334. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Santos, A.; von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting Protein-Chemical Interaction Networks with Tissue and Affinity Data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets--Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING V10: Protein-Protein Interaction Networks, Integrated over the Tree of Life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A Free Online Platform for Data Visualization and Graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An Interactive Venn Diagram Viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.-X.; Cao, Y. CB-Dock2: Improved Protein-Ligand Blind Docking by Integrating Cavity Detection, Docking and Homologous Template Fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Hu, X.-M.; Long, Y.-F.; Huang, H.-R.; He, Y.; Xu, Z.-R.; Qi, Z.-Q. Treatment of Parkinson’s Disease Model with Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Loaded with BDNF. Life Sci. 2024, 356, 123014. [Google Scholar] [CrossRef]
- Gao, W.; Wang, W.; Liu, G.; Zhang, J.; Yang, J.; Deng, Z. Allicin Attenuated Chronic Social Defeat Stress Induced Depressive-like Behaviors through Suppression of NLRP3 Inflammasome. Metab. Brain Dis. 2019, 34, 319–329. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, W.; Wang, Y.; Liu, Y.; Liu, X.; Qi, Z. Garlic-Derived Allicin Attenuates Parkinson’s Disease via PKA/p-CREB/BDNF/DAT Pathway Activation and Apoptotic Inhibition. Molecules 2025, 30, 3265. https://doi.org/10.3390/molecules30153265
Zeng W, Wang Y, Liu Y, Liu X, Qi Z. Garlic-Derived Allicin Attenuates Parkinson’s Disease via PKA/p-CREB/BDNF/DAT Pathway Activation and Apoptotic Inhibition. Molecules. 2025; 30(15):3265. https://doi.org/10.3390/molecules30153265
Chicago/Turabian StyleZeng, Wanchen, Yingkai Wang, Yang Liu, Xiaomin Liu, and Zhongquan Qi. 2025. "Garlic-Derived Allicin Attenuates Parkinson’s Disease via PKA/p-CREB/BDNF/DAT Pathway Activation and Apoptotic Inhibition" Molecules 30, no. 15: 3265. https://doi.org/10.3390/molecules30153265
APA StyleZeng, W., Wang, Y., Liu, Y., Liu, X., & Qi, Z. (2025). Garlic-Derived Allicin Attenuates Parkinson’s Disease via PKA/p-CREB/BDNF/DAT Pathway Activation and Apoptotic Inhibition. Molecules, 30(15), 3265. https://doi.org/10.3390/molecules30153265





