Macromycete Edible Fungi as a Functional Poultry Feed Additive: Influence on Health, Welfare, Eggs, and Meat Quality—Review
Abstract
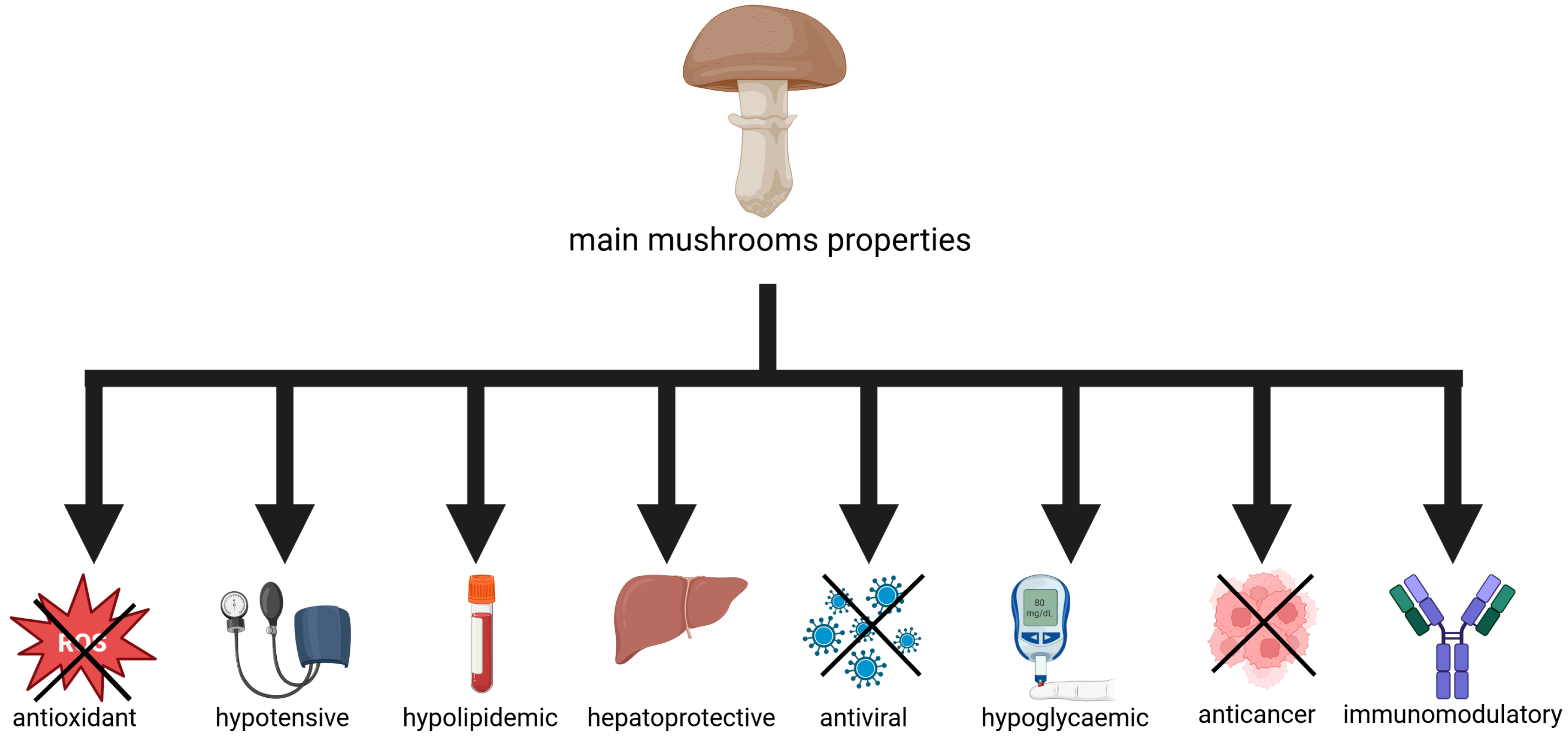
1. Introduction
2. Characteristics and Application of Selected Mushroom Species in the Nutrition of Laying Hens and Broiler Chickens
2.1. Lion’s Mane Mushroom (Hericium Erinaceus)
| (a) | ||
|---|---|---|
| Effect | Research Model | Type of Supplement and Feeding Time |
| Greater egg production | Hens | HEP from Hericium erinaceus (0.5 g/kg, 12 weeks) [30] |
| Greater weight gain | Broilers | Extract from Cordyceps sinensis (0.6 g/kg) with probiotics (6 g/kg, 42 days) [31] FPCM from Cordyceps militaris (1–4 g/kg feed, 42 days) [32] SMS from Flammulina velutipes (0.5–2.0 g/kg feed, 35 days) [33] Dried Ganoderma lucidum (2 g/kg feed, 21 days) [34] Dried Pleurotus ostreatus (0.3 g/kg feed, 35 days) [35] |
| Better feed utilization | Broilers | Extract from Cordyceps sinensis (0.6 g/kg) with probiotics (6 g/kg, 42 days) [31] SMS from Flammulina velutipes (0.5–2.0 g/kg feed, 35 days) [33] Agaricus bisporus stalks (100–150 g/kg feed, 56 days) [36] |
| Better muscle growth | Broilers | Extract from Cordyceps sinensis (0.6 g/kg) with probiotics (6 g/kg, 42 days) [31] FPCM from Cordyceps sinensis (1–4 g/kg feed, 42 days) [32] Dried Agaricus bisporus (20 g/kg feed, 60 days) [37] |
| Increased meat quality | Broilers | SMS from Flammulina velutipes (0.5–2.0 g/kg feed, 35 days) [33] Dried Pleurotus ostreatus (0.3 g/kg feed, 35 days) [35] |
| Increased egg quality | Hens | FVS from Flammulina velutipes (20–40 g/kg feed, 27 weeks) [38] Dried Agaricus bisporus (20 g/kg feed, 60 days) [37] Agaricus bisporus stalks (100–150 g/kg feed, 56 days) [36] |
| Increased strength of the eggshell | Hens | FVS from Flammulina velutipes (20–40 g/kg feed, 27 weeks) [38] |
| (b) | ||
| Effect | Research Model | Type of Supplement and Feeding Time |
| Beneficial effect on the immune system | Broilers Chickens Hens Broilers Broilers Chickens | Dried Ganoderma lucidum (2 g/kg feed, 32 days) [39] IOFP from Inonotus obliquus (8 g/kg feed, 35 days) [40] Extract from Lentinula edodes (0.02–0.03 g/kg feed, 21 days) [41] Dried Pleurotus ostreatus (0.3 g/kg feed, 35 days) [35] FSW from Flammulina velutipes (20 g/kg feed, 42 days) [42] Polysaccharides extract from Cordyceps militaris (CMP40, CMP50, 42 days) [43] |
| Beneficial effect on the intestinal and digestive function | Broilers Hens Broilers | Dried Ganoderma lucidum (2 g/kg feed, 32 days) [39] Extract from Lentinula edodes (0.02–0.03 g/kg feed, 21 days) [41] Dried Pleurotus ostreatus (0.3 g/kg feed, 35 days) [35] |
| Increases the number of beneficial bacteria | Broilers Hens Broilers Broilers Hens Broilers | Dried Ganoderma lucidum (2 g/kg feed, 21 days) [34] Extract from Lentinula edodes (0.02–0.03 g/kg feed, 21 days) [41] Dried Pleurotus ostreatus (0.3 g/kg feed, 35 days) [35] Dried Agaricus bisporus (10–20 g/kg feed, 42 days) [44] Dried Agaricus bisporus (10–20 g/kg feed, 60 days) [37] SMS from Flammulina velutipes (0.5–2.0 g/kg feed, 35 days) [33] |
| Increases the height of intestinal villi | Broilers | Dried Ganoderma lucidum (2 g/kg feed, 21 days) [34] |
| Better blood lipid profile | Broilers | HFC from Hericium erinaceus (6, 1.2, 1.8 g/kg, 42 days) [45] Dried Pleurotus ostreatus (0.3 g/kg feed, 35 days) [35] |
| Reduction in the cholesterol content in body | Broilers Hens Broilers | HFC from Hericium erinaceus (6, 1.2, 1.8 g/kg, 42 days) [45] FVS from Flammulina velutipes (20–40 g/kg feed, 27 weeks) [38] SMS from Flammulina velutipes (0.5–2.0 g/kg feed, 35 days) [33] |
| Reduced expression of the HMGR gene | Broilers | HFC from Hericium erinaceus (6, 1.2, 1.8 g/kg, 42 days) [45] |
| Maintenance of hormonal homeostasis and fat metabolism as well as liver and ovarian function | Hens | HEP from Hericium erinaceus (0.5 g/kg, 12 weeks) [30] |
| Less stress | Broilers | Dried Pleurotus ostreatus (0.3 g/kg feed, 35 days) [35] |
| Better antioxidant function | Broilers Hens Hens | Dried Agaricus bisporus (20 g/kg feed, 42 days) [46] Dried Agaricus bisporus (10–20 g/kg feed, 60 days) [37] ABSR from Agaricus bisporus (20–100 g/kg feed, 56 days) [36] |
| Higher levels of antibodies against Newcastle disease and infectious bursal disease | Broilers Chickens | FSW from Flammulina velutipes (20 g/kg feed, 42 days) [42] Polysaccharides extract from Cordyceps militaris (CMP40, CMP50, 42 days) [43] |
| Reduction in ovarian follicle atresia | Hens | FVS from Flammulina velutipes (20–40 g/kg feed, 27 weeks) [47] |
2.2. Genus Cordyceps
2.3. Winter Mushroom (Flammulina velutipes)
2.4. Chaga (Inonotus obliquus)
2.5. Ganoderma lucidum
2.6. Agaricus bisporus: Button Mushroom
2.7. Oyster Mushroom (Pleurotus ostreatus)
2.8. Shitake (Lentinula edodes)
3. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.S.; Thakur, M.; Peng, M.S.; Jiang, Y.; Frantz, L.A.F.; Li, M.; Zhang, J.J.; Wang, S.; Peters, J.; Otecko, N.O.; et al. 863 genomes reveal the origin and domestication of chicken. Cell Res. 2020, 30, 693–701. [Google Scholar] [CrossRef]
- Eda, M. Origin of the domestic chicken from modern biological and zooarchaeological approaches. Anim. Front. 2021, 11, 52–61. [Google Scholar] [CrossRef]
- Hata, A.; Nunome, M.; Suwanasopee, T.; Duengkae, P.; Chaiwatana, S.; Chamchumroon, W.; Suzuki, T.; Koonawootrittriron, S.; Matsuda, Y.; Srikulnath, K. Origin and evolutionary history of domestic chickens inferred from a large population study of Thai red junglefowl and indigenous chickens. Sci. Rep. 2021, 11, 2035. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Lebrasseur, O.; Irving-Pease, E.K.; Paxinos, P.D.; Best, J.; Smallman, R.; Callou, C.; Gardeisen, A.; Trixl, S.; Frantz, L.; et al. The biocultural origins and dispersal of domestic chickens. Proc. Natl. Acad. Sci. USA 2022, 119, e2121978119. [Google Scholar] [CrossRef] [PubMed]
- Renden, J.A.; McDaniel, G.R.; McGuire, J.A. Egg characteristics and production efficiency of dwarf (dw) White Leghorn hens divergently selected for body weight. Poult. Sci. 1984, 63, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Zuidhof, M.J.; Schneider, B.L.; Carney, V.L.; Korver, D.R.; Robinson, F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014, 93, 2970–2982. [Google Scholar] [CrossRef]
- Tiemann, I.; Hillemacher, S.; Wittmann, M. Are dual-purpose chickens twice as good? measuring performance and animal welfare throughout the fattening period. Animals 2020, 10, 1980. [Google Scholar] [CrossRef]
- Baxter, M.; Richmond, A.; Lavery, U.; O’Connell, N.E. A comparison of fast growing broiler chickens with a slower-growing breed type reared on Higher Welfare commercial farms. PLoS ONE 2021, 16, e0259333. [Google Scholar] [CrossRef]
- Hammershøj, M.; Kristiansen, G.H.; Steenfeldt, S. Dual-purpose poultry in organic egg production and effects on egg quality parameters. Foods 2021, 10, 897. [Google Scholar] [CrossRef]
- Erinle, T.J.; Adewole, D.I. Fruit pomaces—Their nutrient and bioactive components, effects on growth and health of poultry species, and possible optimization techniques. Anim. Nutr. 2022, 9, 357–377. [Google Scholar] [CrossRef]
- Jachimowicz, K.; Winiarska-Mieczan, A.; Tomaszewska, E. The impact of herbal additives for poultry feed on the fatty acid profile of meat. Animals 2022, 12, 1054. [Google Scholar] [CrossRef]
- Abd El-Aziz, A.; Abo Ghanima, M.; Mota-Rojas, D.; Sherasiya, A.; Ciani, F.; El-Sabrout, K. Bee Products for poultry and rabbits: Current challenges and perspectives. Animals 2023, 13, 3517. [Google Scholar] [CrossRef]
- Suberu, S.A.; Isikhuemhen, O.S.; Ogundare, T.E.; Ekunseitan, D.A.; Fasina, Y.O. Benefits of mushroom-based supplements on growth performance, immunocompetence, and meat quality in poultry. Animals 2024, 14, 1517. [Google Scholar] [CrossRef] [PubMed]
- Begna, R.; Masho, W. Valuation of livestock population and national feed security to enhance livestock productivity in Ethiopia. Vet. Med. Sci. 2024, 10, e1415. [Google Scholar] [CrossRef] [PubMed]
- More, S.J. European perspectives on efforts to reduce antimicrobial usage in food animal production. Ir. Vet. J. 2020, 73, 2. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.; Semedo-Lemsaddek, T.; Cunha, E.; Tavares, L.; Oliveira, M. Antimicrobial drug resistance in poultry production: Current status and innovative strategies for bacterial control. Microorganisms 2023, 11, 953. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal mushrooms: Their bioactive components, nutritional value and application in functional food production-a review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Chen, Y.; Liu, T.; Zhang, S.; Fan, H.; Liu, H.; Li, Y. Healthy function and high valued utilization of edible fungi. Food Sci. Hum. Wellness 2021, 10, 408–420. [Google Scholar] [CrossRef]
- Imtiaj, A.; Jayasinghe, C.; Lee, G.W.; Shim, M.J.; Rho, H.S.; Lee, H.S.; Hur, H.; Lee, M.W.; Lee, U.Y.; Lee, T.S. Vegetative growth of four strains of Hericium erinaceus collected from different habitats. Mycobiology 2008, 36, 88–92. [Google Scholar] [CrossRef]
- Qiu, Y.; Lin, G.; Liu, W.; Zhang, F.; Linhardt, R.J.; Wang, X.; Zhang, A. Bioactive substances in Hericium erinaceus and their biological properties: A review. Food Sci. Hum. Wellness 2024, 13, 1825–1844. [Google Scholar] [CrossRef]
- Wang, M.; Kanako, N.; Zhang, Y.; Xiao, X.; Gao, Q.; Tetsuya, K. A unique polysaccharide purified from Hericium erinaceus mycelium prevents oxidative stress induced by H2O2 in human gastric mucosa epithelium cell. PLoS ONE 2017, 12, e0181546. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Ando, M.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y.; Ukai, N.; Furukawa, S. Hericenones C, D and E, stimulators of nerve growth factor (NGF)-synthesis, from the mushroom Hericium erinaceum. Tetrahedron Lett. 1991, 32, 4561. [Google Scholar] [CrossRef]
- Lee, K.F.; Chen, J.H.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; Lu, C.C.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; et al. Protective effects of Hericium erinaceus mycelium and its isolated erinacine A against ischemia-injury-induced neuronal cell death via the inhibition of iNOS/p38 MAPK and nitrotyrosine. Int. J. Mol. Sci. 2014, 15, 15073–15089. [Google Scholar] [CrossRef]
- Li, I.C.; Lee, L.Y.; Tzeng, T.T.; Chen, W.P.; Chen, Y.P.; Shiao, Y.J.; Chen, C.C. Neurohealth properties of Hericium erinaceus mycelia enriched with erinacines. Behav. Neurol. 2018, 2018, 5802634. [Google Scholar] [CrossRef]
- Szućko-Kociuba, I.; Trzeciak-Ryczek, A.; Kupnicka, P.; Chlubek, D. Neurotrophic and neuroprotective effects of Hericium erinaceus. Int. J. Mol. Sci. 2023, 24, 15960. [Google Scholar] [CrossRef]
- Zhang, C.C.; Cao, C.Y.; Kubo, M.; Harada, K.; Yan, X.T.; Fukuyama, Y.; Gao, J.M. Chemical constituents from Hericium erinaceus promote neuronal survival and potentiate neurite outgrowth via the TrkA/Erk1/2 pathway. Int. J. Mol. Sci. 2017, 18, 1659. [Google Scholar] [CrossRef]
- Dai, H.; Lv, Z.; Huang, Z.; Ye, N.; Li, S.; Jiang, J.; Cheng, Y.; Shi, F. Dietary hawthorn-leaves flavonoids improves ovarian function and liver lipid metabolism in aged breeder hens. Poult. Sci. 2021, 100, 101499. [Google Scholar] [CrossRef]
- Wu, L.; Lv, Y.; Ge, C.; Luo, X.; Hu, Z.; Huang, W.; Zhan, S.; Shen, X.; Yu, D.; Liu, B. Polysaccharide from Hericium erinaceus improved laying performance of aged hens by promoting yolk precursor synthesis and follicle development via liver-blood-ovary axis. Poult. Sci. 2024, 103, 103810. [Google Scholar] [CrossRef]
- Khalid Shihab, S.; Hkmat Nafea, H. Effect of adding Cordyceps sinensis extract and probiotic to the diet on productive performance of broiler. Arch. Razi Inst. 2023, 78, 659–666. [Google Scholar] [CrossRef]
- Han, J.C.; Qu, H.X.; Wang, J.G.; Yan, Y.F.; Zhang, J.L.; Yang, L.; Zhang, M.; Cheng, Y.H. Effects of fermentation products of Cordyceps militaris on growth performance and bone mineralization of broiler chicks. J. Appl. Anim. Res. 2014, 43, 236–241. [Google Scholar] [CrossRef]
- Srinual, O.; Kanmanee, C.; Srinual, P.; Chaiyaso, T.; Yachai, M.; Tapingkae, T.; Tapingkae, W. Innovation and utilization of functional feed additives from maize by-products in broiler chickens. Animals 2024, 14, 3198. [Google Scholar] [CrossRef]
- Pinzón-Osorio, C.A.; Álvarez-Mira, D.M.; Betancourt-López, L.L. Effect of the Inclusion of Ganoderma spp. on gut morphometry and growth performance of broiler chickens. Rev. Bras. Zootec. 2023, 52, e20210215. [Google Scholar] [CrossRef]
- Bormon, C.C.; Akib, G.; Rifat, A.; Hossain, M.; Uddin, N.; Hossain, F.M.A.; Azzam, M.M.; Farouk, M.H.; Das, R.; Mahfuz, S.U. Effects of oyster mushroom (Pleurotus ostreatus) stem residue supplementation on growth performance, meat quality and health status of broilers. Poult. Sci. 2024, 103, 104054. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhao, G.; Wang, L.; Liu, S.; Tang, J. Effects of the Agaricus bisporus stem residue on performance, nutrients digestibility and antioxidant activity of laying hens and its effects on egg storage. Anim. Biosci. 2021, 34, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Han, K.L.; Yan, Y.; Li, Y.; Li, S.T.; Jia, R.L.; He, C.N.; Wang, Z.B.; Zhang, T.W. Effect of Agaricus bisporus stalk replacement of soybean on productive performance, egg quality and intestinal microbiota of laying hens. Vet. Med. Sci. 2025, 11, e70185. [Google Scholar] [CrossRef]
- Sun, C.; Wu, H.; Nguepi Tsopmejio, I.S.; Jin, Z.; Song, H. Effect of dietary Flammulina velutipes (Curt.: Fr.) stem waste on ovarian follicles development in laying hens. Ital. J. Anim. Sci. 2023, 22, 200–213. [Google Scholar] [CrossRef]
- Avain, A.; Azad, M.A.K.; García, Y.; García, Y.; Martínez, Y. Effects of Ganoderma lucidum powder on the growth performance, immune organ weights, cecal microbiology, serum immunoglobulins, and tibia minerals of broiler chickens. Vet. Sci. 2024, 11, 675. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Li, H.; Yu, S.; Bai, J.; Ding, Z.; Wu, J. Immunopotentiating Effect of Inonotus obliquus fermentation products administered at vaccination in chickens. Mol. Cell Probes 2018, 41, 43–51. [Google Scholar] [CrossRef]
- Guo, F.C.; Williams, B.A.; Kwakkel, R.P.; Li, H.S.; Li, X.P.; Luo, J.Y.; Li, W.K.; Verstegen, M.W.A. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on the cecal microbial ecosystem in broiler chickens. Poult. Sci. 2004, 83, 175–182. [Google Scholar] [CrossRef]
- Mahfuz, S.; He, T.; Liu, S.; Wu, D.; Long, S.; Piao, X. Dietary inclusion of mushroom (Flammulina velutipes) stem waste on growth performance, antibody response, immune status, and serum cholesterol in broiler chickens. Animals 2019, 9, 692. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Meng, X.; Yang, R.; Qin, T.; Li, Y.; Zhang, L.; Fei, C.; Zen, W.; Zhang, K.; Wang, X.; et al. Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken. Int. J. Biol. Macromol. 2013, 59, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Tontis, D.; Tsalie, E.; Chronis, E.F.; Doukas, D.; Kyriazakis, I. Influence of dietary mushroom Agaricus bisporus on intestinal morphology and microflora composition in broiler chickens. Res. Vet. Sci. 2010, 89, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.M.; Zhao, J.C.; Guo, Y.; Zhang, H.X.; Song, H. Effects of supplementing feed with fermentation concentrate of Hericium caput-medusae (Bull.: Fr.) Pers. on cholesterol deposition in broiler chickens. Livest. Sci. 2020, 235, 104009. [Google Scholar] [CrossRef]
- Giannenas, I.; Pappas, I.S.; Mavridis, S.; Kontopidis, G.; Skoufos, J.; Kyriazakis, I. Performance and antioxidant status of broiler chickens supplemented with dried mushrooms (Agaricus bisporus) in their diet. Poult. Sci. 2010, 89, 303–311. [Google Scholar] [CrossRef]
- Géry, A.; Dubreule, C.; André, V.; Rioult, J.P.; Bouchart, V.; Heutte, N.; Eldin de Pécoulas, P.; Krivomaz, T.; Garon, D. Chaga (Inonotus obliquus), a Future Potential Medicinal Fungus in Oncology? A Chemical Study and a Comparison of the Cytotoxicity Against Human Lung Adenocarcinoma Cells (A549) and Human Bronchial Epithelial Cells (BEAS-2B). Integr. Cancer Ther. 2018, 17, 832–843. [Google Scholar] [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Mfoundou, J.D.L.; Guo, Y.J.; Liu, M.M.; Ran, X.R.; Fu, D.H.; Yan, Z.Q.; Li, M.N.; Wang, X.R. The morphological and histological study of chicken left ovary during growth and development among Hy-line brown layers of different ages. Poult. Sci. 2021, 100, 101191. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Xiang, W. Mechanisms of ovarian aging in women: A review. J. Ovarian Res. 2023, 16, 67. [Google Scholar] [CrossRef]
- Cerqueira, N.M.; Oliveira, E.F.; Gesto, D.S.; Santos-Martins, D.; Moreira, C.; Moorthy, H.N.; Ramos, M.J.; Fernandes, P.A. Cholesterol biosynthesis: A mechanistic overview. Biochemistry 2016, 55, 5483–5506. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the european atherosclerosis society consensus panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Go, T.H.; Kwak, K.I.; Jang, J.Y.; Yu, M.; Kim, H.S.; Kim, J.Y.; Koh, S.B.; Kang, D.R. Inference of a causal relation between low-density lipoprotein cholesterol and hypertension using Mendelian randomization analysis. Clin. Hypertens. 2021, 27, 7. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, A.D.; Mehta, A.; Dhindsa, D.S.; Virani, S.S.; Orringer, C.E.; Blumenthal, R.S.; Stone, N.J.; Sperling, L.S. How low is safe? The frontier of very low (<30 mg/dL) LDL cholesterol. Eur. Heart J. 2021, 42, 2154–2169. [Google Scholar] [CrossRef]
- Lin, C.W.; Huang, T.W.; Peng, Y.J.; Lin, Y.Y.; Mersmann, H.J.; Ding, S.T. A Novel chicken model of fatty liver disease induced by high cholesterol and low choline diets. Poult. Sci. 2021, 100, 100869. [Google Scholar] [CrossRef]
- Riber, A.B.; Wurtz, K.E. Impact of growth rate on the welfare of broilers. Animals 2024, 14, 3330. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.S.; Leyva-Gómez, G.; Prado-Audelo, M.L.D.; Cortes, H.; Singh, Y.D.; Panda, M.K.; Mishra, A.P.; Nigam, M.; Saklani, S.; et al. Cordyceps spp.: A Review on Its immune-stimulatory and other biological potentials. Front. Pharmacol. 2021, 11, 602364. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, N.; An, S.S.A. Unique bioactives from zombie fungus (cordyceps) as promising multitargeted neuroprotective Agents. Nutrients 2024, 16, 102. [Google Scholar] [CrossRef]
- Lindblom, G.B.; Sjörgren, E.; Kaijser, B. Natural campylobacter colonization in chickens raised under different environmental conditions. Epidemiol. Infect. 1986, 96, 385–391. [Google Scholar] [CrossRef]
- Santos, M.N.; Rothschild, D.; Widowski, T.M.; Barbut, S.; Kiarie, E.G.; Mandell, I.; Guerin, M.T.; Edwards, A.M.; Torrey, S. In pursuit of a better broiler: Carcass traits and muscle myopathies in conventional and slower-growing strains of broiler chickens. Poult. Sci. 2021, 100, 101309. [Google Scholar] [CrossRef]
- Biesek, J.; Banaszak, M.; Kądziołka, K.; Wlaźlak, S.; Adamski, M. Growth of broiler chickens, and physical features of the digestive system, and leg bones after aluminosilicates used. Sci. Rep. 2022, 12, 20425. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Arif, M.; Sajjad, N.; Al-Ghadi, M.Q.; Alagawany, M.; Abd El-Hack, M.E.; Alhimadi, A.R.; Elnesr, S.S.; Almutari, B.O.; Amran, E.O.S.; et al. Dietary effect of probiotics and prebiotics on broiler performance, carcass, and immunity. Poult. Sci. 2020, 99, 6946–6953. [Google Scholar] [CrossRef] [PubMed]
- Chaves Hernández, A.J. Poultry and Avian Diseases. In Encyclopedia of Agriculture and Food Systems; Academic Press: Oxford, UK, 2014; pp. 504–520. [Google Scholar] [CrossRef]
- Zhang, D.; Ding, Z.; Xu, X. Pathologic mechanisms of the Newcastle disease virus. Viruses 2023, 15, 864. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, K.M.; Afonso, C.L.; Yu, Q.; Miller, P.J. Newcastle disease vaccines—A solved problem or a continuous challenge? Vet. Microbiol. 2017, 206, 126–136. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory effects of edible and medicinal mushrooms and their bioactive immunoregulatory products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef]
- Yeh, M.Y.; Ko, W.C.; Lin, L.Y. Hypolipidemic and antioxidant activity of enoki mushrooms (Flammulina velutipes). Biomed. Res. Int. 2014, 2014, 352385. [Google Scholar] [CrossRef]
- Tang, C.; Hoo, P.C.; Tan, L.T.; Pusparajah, P.; Khan, T.M.; Lee, L.H.; Goh, B.H.; Chan, K.G. Golden needle mushroom: A culinary medicine with evidence-based biological activities and health promoting properties. Front. Pharmacol. 2016, 7, 474. [Google Scholar] [CrossRef]
- Patangia, D.V.; Ryan, C.A.; Dempsey, E.; Ross, R.P.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. MicrobiologyOpen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Liu, X.T.; Lin, X.; Mi, Y.L.; Zeng, W.D.; Zhang, C.Q. Age-related changes of yolk precursor formation in the liver of laying hens. J. Zhejiang Univ. Sci. B 2018, 19, 390–399. [Google Scholar] [CrossRef]
- Arulnathan, V.; Turner, I.; Bamber, N.; Ferdous, J.; Grassauer, F.; Doyon, M.; Pelletier, N. A systematic review of potential productivity, egg quality, and animal welfare implications of extended lay cycles in commercial laying hens in Canada. Poult. Sci. 2024, 103, 103475. [Google Scholar] [CrossRef]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. β-1,3/1,6-glucans and immunity: State of the art and future directions. Mol. Nutr. Food Res. 2021, 65, e1901071. [Google Scholar] [CrossRef]
- Fordjour, E.; Manful, C.F.; Javed, R.; Galagedara, L.W.; Cuss, C.W.; Cheema, M.; Thomas, R. Chaga mushroom: A super-fungus with countless facets and untapped potential. Front. Pharmacol. 2023, 14, 1273786. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Skóra, B.; Pomianek, T.; Gmiński, J. Inonotus obliquus-from folk medicine to clinical use. J. Tradit. Complement. Med. 2020, 11, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.J.; Cho, S.Y.; Kim, K.M.; Cha, D.S.; Park, H.J. A Comparative study of analytical methods for alkali-soluble β-glucan in medicinal mushroom, Chaga (Inonotus obliquus). LWT-Food Sci. Technol. 2008, 41, 545–549. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, Y.; Xue, Z.; Li, N.; Liu, J.; Chen, H. Recent developments in Inonotus obliquus (Chaga mushroom) polysaccharides: Isolation, structural characteristics, biological activities and application. Polymers 2021, 13, 1441. [Google Scholar] [CrossRef]
- Ern, P.T.Y.; Quan, T.Y.; Yee, F.S.; Yin, A.C.Y. Therapeutic properties of Inonotus obliquus (Chaga mushroom): A review. Mycology 2023, 15, 144–161. [Google Scholar] [CrossRef]
- Nguyen, P.C.; Nguyen, M.T.T.; Truong, B.T.; Kim, D.-R.; Shin, S.; Kim, J.-E.; Park, K.-B.; Park, J.-H.; Tran, P.L.; Ban, S.-Y.; et al. Isolation, physicochemical characterization, and biological properties of inotodiol, the potent pharmaceutical oxysterol from Chaga mushroom. Antioxidants 2023, 12, 447. [Google Scholar] [CrossRef]
- Oke, M.A.; Afolabi, F.J.; Oyeleke, O.O.; Kilani, T.A.; Adeosun, A.R.; Olanbiwoninu, A.A.; Adebayo, E.A. Ganoderma lucidum: Unutilized natural medicine and promising future solution to emerging diseases in Africa. Front. Pharmacol. 2022, 13, 952027. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, S.; Peng, B.; Tan, D.; Wu, M.; Wei, J.; Wang, Y.; Luo, H. Ganoderma lucidum: A comprehensive review of phytochemistry, efficacy, safety and clinical study. Food Sci. Hum. Wellness 2024, 13, 568–596. [Google Scholar] [CrossRef]
- Simonić, J.; Stajic, M.; Vukojevic, J. Ganoderma lucidum–from tradition to modern medicine. Zb. Matice Srp. Prir. Nauk. 2017, 133, 151–161. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Chen, H.; Chen, X.; Lan, J.; Liu, C. Complete mitochondrial genome of the medicinal mushroom Ganoderma lucidum. PLoS ONE 2013, 8, e72038. [Google Scholar] [CrossRef] [PubMed]
- Sheng, F.; Wang, S.; Luo, X.; Xiao, J.; Hu, L.; Li, P. Simultaneous determination of ten nucleosides and bases in Ganoderma by micellar electrokinetic chromatography. Food Sci. Hum. Wellness 2022, 11, 263–268. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, L.; Ding, K. Structure elucidation and anti-tumor activity in vivo of a polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Int. J. Biol. Macromol. 2019, 141, 693–699. [Google Scholar] [CrossRef]
- Dai, C.; He, L.; Ma, B.; Chen, T. Facile nanolization strategy for therapeutic Ganoderma lucidum spore oil to achieve enhanced protection against radiation-induced heart disease. Small 2019, 15, e1902642. [Google Scholar] [CrossRef]
- Wen, L.; Sheng, Z.; Wang, J.; Jiang, Y.; Yang, B. Structure of water-soluble polysaccharides in spore of Ganoderma lucidum and their anti-inflammatory activity. Food Chem. 2022, 373 Pt A, 131374. [Google Scholar] [CrossRef]
- Ma, H.T.; Hsieh, J.F.; Chen, S.T. Anti-diabetic effects of Ganoderma lucidum. Phytochemistry 2015, 114, 109–113. [Google Scholar] [CrossRef]
- Yin, Z.; Liang, Z.; Li, C.; Wang, J.; Ma, C.; Kang, W. Immunomodulatory effects of polysaccharides from edible fungus: A review. Food Sci. Hum. Wellness 2021, 10, 393–400. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Xu, J.P.; Callac, P.; Chen, M.Y.; Wu, Q.; Wach, M.; Mata, G.; Zhao, R.L. insight into the evolutionary and domesticated history of the most widely cultivated mushroom Agaricus bisporus via mitogenome sequences of 361 global strains. BMC Genom. 2023, 24, 182. [Google Scholar] [CrossRef]
- Feng, T.; Wu, Y.; Zhang, Z.; Song, S.; Zhuang, H.; Xu, Z.; Yao, L.; Sun, M. Purification, identification, and sensory evaluation of kokumi peptides from Agaricus bisporus mushroom. Foods 2019, 8, 43. [Google Scholar] [CrossRef]
- Muszyńska, B.; Kała, K.; Rojowski, J.; Grzywacz, A.; Opoka, W. Composition and biological properties of Agaricus bisporus fruiting bodies—A review. Pol. J. Food Nutr. Sci. 2017, 67, 173–181. [Google Scholar] [CrossRef]
- Rutckeviski, R.; Corso, C.R.; Fonseca, A.S.; Rodrigues, M.L.; Román-Ochoa, Y.; Cipriani, T.R.; Cavalli, L.R.; Cadena, S.M.S.C.; Smiderle, F.R. Anti-cancer potential of linear β-(1→6)-d-glucan from Agaricus bisporus on estrogen receptor-positive (ER+) breast cancer cells. Molecules 2024, 29, 4781. [Google Scholar] [CrossRef] [PubMed]
- Jankov, M.; Léguillier, V.; Gašić, U.; Anba-Mondoloni, J.; Ristivojević, M.K.; Radoičić, A.; Dimkić, I.; Ristivojević, P.; Vidic, J. Antibacterial activities of Agaricus bisporus extracts and their synergistic effects with the antistaphylococcal drug AFN-1252. Foods 2024, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Muszynska, B.; Grzywacz, A.; Kala, K.; Gdula-Argasinska, J. Anti-inflammatory potential of in vitro cultures of the white button mushroom, Agaricus bisporus (Agaricomycetes), in Caco-2 cells. Int. J. Med. Mushrooms 2018, 20, 129–139. [Google Scholar] [CrossRef]
- Liu, J.; Jia, L.; Kan, J.; Jin, C.H. In Vitro and In Vivo Antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [CrossRef]
- Lopez-Tejedor, D.; Claveria-Gimeno, R.; Velazquez-Campoy, A.; Abian, O.; Palomo, J.M. In Vitro antiviral activity of tyrosinase from mushroom Agaricus bisporus against hepatitis c virus. Pharmaceuticals 2021, 14, 759. [Google Scholar] [CrossRef]
- Huang, J.; Ou, Y.; Yew, T.W.; Liu, J.; Leng, B.; Lin, Z.; Su, Y.; Zhuang, Y.; Lin, J.; Li, X.; et al. Hepatoprotective effects of polysaccharide isolated from Agaricus bisporus industrial wastewater against CCl4-induced hepatic injury in mice. Int. J. Biol. Macromol. 2016, 82, 678–686. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications; Petre, M., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Ferdousi, J.; Riyadh, Z.A.; Hossain, M.I.; Saha, S.R.; Zakaria, M. Mushroom production benefits, status, challenges and opportunities in Bangladesh: A review. Annu. Res. Rev. Biol. 2020, 34, 1–13. [Google Scholar] [CrossRef]
- Roy, D.N.; Azad, A.K.; Sultana, F.; Anisuzzaman, A.S.M.; Khondkar, P. Nutritional profile and mineral composition of two edible mushroom varieties consumed and cultivated in Bangladesh. J. Phytopharmacol. 2015, 4, 217–220. [Google Scholar] [CrossRef]
- Ejigu, N.; Sitotaw, B.; Girmay, S.; Assaye, H. Evaluation of oyster mushroom (Pleurotus ostreatus) production using water hyacinth (Eichhornia crassipes) biomass supplemented with agricultural wastes. Int. J. Food Sci. 2022, 2022, 9289043. [Google Scholar] [CrossRef]
- Lesa, K.; Khandaker, M.; Faruque, M.R.; Sharma, R.; Mitra, S.; Emran, T. Nutritional Value, Medicinal Importance, and Health-Promoting Effects of Dietary Mushroom (Pleurotus ostreatus). J. Food Qual. 2022, 2022, 2454180. [Google Scholar] [CrossRef]
- Adams, S.; Che, D.; Hailong, J.; Zhao, B.; Rui, H.; Danquah, K.; Qin, G. Effects of pulverized oyster mushroom (Pleurotus ostreatus) on diarrhea incidence, growth performance, immunity, and microbial composition in piglets. J. Sci. Food Agric. 2019, 99, 3616–3627. [Google Scholar] [CrossRef]
- Sierra-Patev, S.; Min, B.; Naranjo-Ortiz, M.; Looney, B.; Konkel, Z.; Slot, J.C.; Sakamoto, Y.; Steenwyk, J.L.; Rokas, A.; Carro, J.; et al. A global phylogenomic analysis of the shiitake genus Lentinula. Proc. Natl. Acad. Sci. USA 2023, 120, e2214076120. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Arif, M.; Xu, M.; Zhang, J.; Ding, Y.; Lyu, F. Therapeutic values and nutraceutical properties of shiitake mushroom (Lentinula edodes): A review. Trends Food Sci. Technol. 2023, 134, 123–135. [Google Scholar] [CrossRef]
- Singh, M.; Kamal, S.; Sharma, V.P. Status and trends in world mushroom production-III world production of different mushroom species in 21st century. Mushroom Res. 2020, 29, 75–111. [Google Scholar] [CrossRef]
- Song, X.; Shang, X.; Zhang, M.; Yu, H.; Zhang, D.; Tan, Q.; Song, C. Cultivation methods and biology of Lentinula edodes. Appl. Microbiol. Biotechnol. 2025, 109, 63. [Google Scholar] [CrossRef]
- Vetvicka, V.; Teplyakova, T.V.; Shintyapina, A.B.; Korolenko, T.A. Effects of medicinal fungi-derived β-glucan on tumor progression. J. Fungi 2021, 7, 250. [Google Scholar] [CrossRef]
- Wasser, S.P. Shiitake (Lentinus edodes). In Encyclopedia of Dietary Supplements; CRC Press: Boca Raton, FL, USA, 2004; pp. 653–664. [Google Scholar]
- Finimundy, T.C.; Dillon, A.J.P.; Henriques, J.A.P.; Ely, M.R. A review on general nutritional compounds and pharmacological properties of the Lentinula edodes mushroom. Food Nutr. Sci. 2014, 5, 1095–1105. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, D.; Zhang, L.; Li, Q.; Song, C.; Shang, X.; Bao, D.; Tan, Q.; Chen, H.; Lv, B. Corncob as a substrate for the cultivation of Lentinula edodes. Preprints 2021, 13, 929–939. [Google Scholar] [CrossRef]
- Hwang, J.A.; Hossain, M.E.; Yun, D.H.; Moon, S.T.; Kim, G.M.; Yang, C.J. Effect of shiitake (Lentinula edodes (Berk.) Pegler) mushroom on laying performance, egg quality, fatty acid composition and cholesterol concentration of eggs in layer chickens. J. Med. Plants Res. 2012, 6, 146–153. [Google Scholar] [CrossRef][Green Version]
- Mahfuz, S.; Piao, X. Use of medicinal mushrooms in layer ration. Animals 2019, 9, 1014. [Google Scholar] [CrossRef]
- Ma, X.; Yan, S.; Wang, M. Spent Mushroom Substrate: A Review on Present and Future of Green Applications. J. Environ. Manag. 2025, 373, 123970. [Google Scholar] [CrossRef]
- Cunha Zied, D.; Sánchez, J.E.; Noble, R.; Pardo-Giménez, A. Use of Spent Mushroom Substrate in New Mushroom Crops to Promote the Transition towards a Circular Economy. Agronomy 2020, 10, 1239. [Google Scholar] [CrossRef]
- Sadigov, R. Rapid Growth of the World Population and Its Socioeconomic Results. Sci. World J. 2022, 2022, 8110229. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Kourampi, I.; Umar, T.P.; Almansoor, Z.R.; Anand, A.; Ur Rehman, M.E.; Jain, S.; Reinis, A. Global Population Surpasses Eight Billion: Are We Ready for the Next Billion? AIMS Public Health 2023, 10, 849–866. [Google Scholar] [CrossRef] [PubMed]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water Scarcity in Agriculture: An Overview of Causes, Impacts and Approaches for Reducing the Risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef]
- Martín, C.; Zervakis, G.I.; Xiong, S.; Koutrotsios, G.; Strætkvern, K.O. Spent Substrate from Mushroom Cultivation: Exploitation Potential toward Various Applications and Value-Added Products. Bioengineered 2023, 14, 2252138. [Google Scholar] [CrossRef]
- Whitton, C.; Bogueva, D.; Marinova, D.; Phillips, C.J.C. Are We Approaching Peak Meat Consumption? Analysis of Meat Consumption from 2000 to 2019 in 35 Countries and Its Relationship to Gross Domestic Product. Animals 2021, 11, 3466. [Google Scholar] [CrossRef]
- Broom, D.M. Land and Water Usage in Beef Production Systems. Animals 2019, 9, 286. [Google Scholar] [CrossRef]
- von Holst, C.; Robouch, P.; Bellorini, S.; González de la Huebra, M.J.; Ezerskis, Z. A Review of the Work of the EU Reference Laboratory Supporting the Authorisation Process of Feed Additives in the EU. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 66–77. [Google Scholar] [CrossRef][Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda, D.; Jaszcza, K.; Bernaś, E. Macromycete Edible Fungi as a Functional Poultry Feed Additive: Influence on Health, Welfare, Eggs, and Meat Quality—Review. Molecules 2025, 30, 3241. https://doi.org/10.3390/molecules30153241
Duda D, Jaszcza K, Bernaś E. Macromycete Edible Fungi as a Functional Poultry Feed Additive: Influence on Health, Welfare, Eggs, and Meat Quality—Review. Molecules. 2025; 30(15):3241. https://doi.org/10.3390/molecules30153241
Chicago/Turabian StyleDuda, Damian, Klaudia Jaszcza, and Emilia Bernaś. 2025. "Macromycete Edible Fungi as a Functional Poultry Feed Additive: Influence on Health, Welfare, Eggs, and Meat Quality—Review" Molecules 30, no. 15: 3241. https://doi.org/10.3390/molecules30153241
APA StyleDuda, D., Jaszcza, K., & Bernaś, E. (2025). Macromycete Edible Fungi as a Functional Poultry Feed Additive: Influence on Health, Welfare, Eggs, and Meat Quality—Review. Molecules, 30(15), 3241. https://doi.org/10.3390/molecules30153241







