New Method for the Determination of Lamotrigine in Human Saliva Using SPE-LC-DAD
Abstract
1. Introduction
2. Results
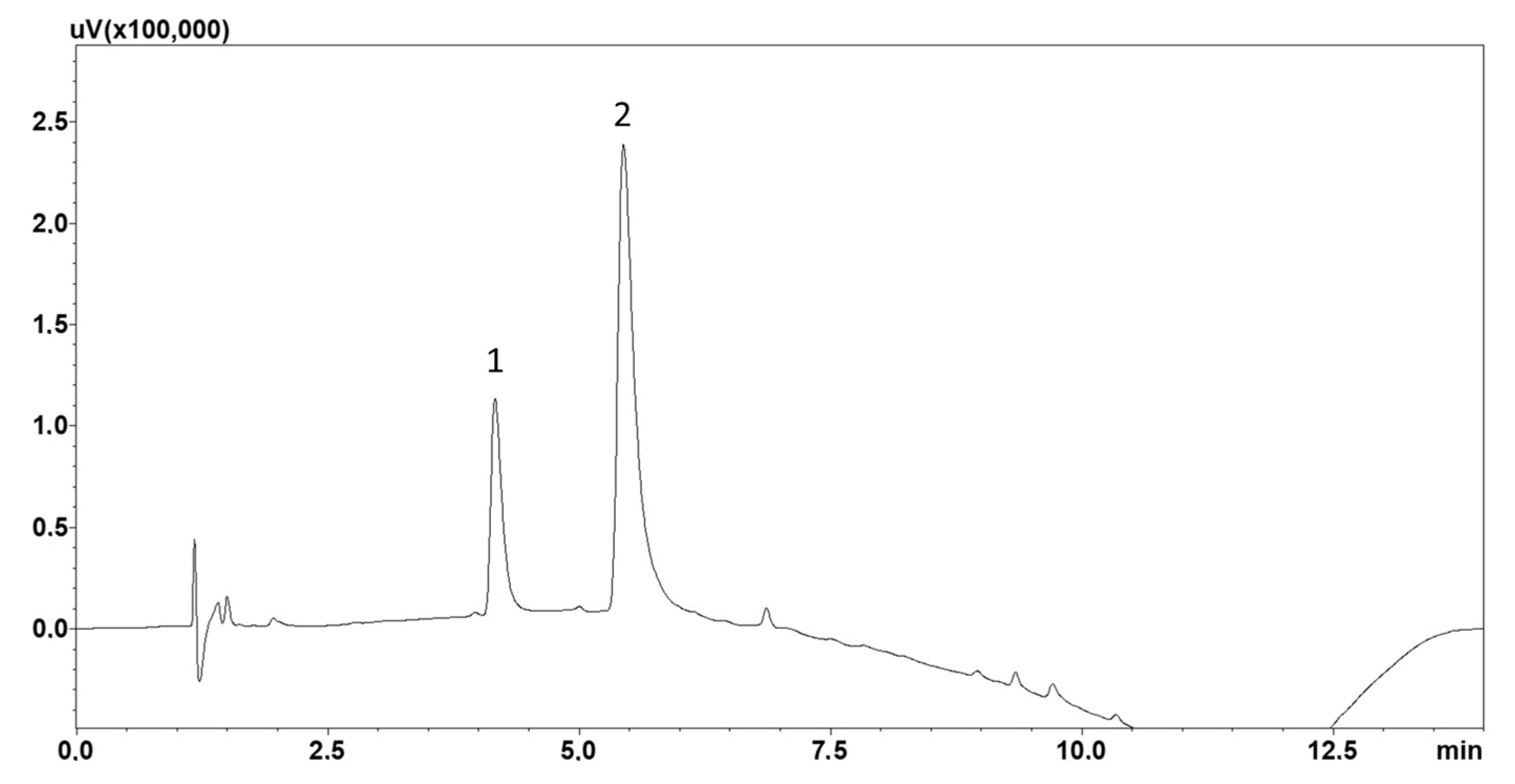
2.1. Chromatographic Analysis
2.2. Solid-Phase Extraction
2.3. Method Validation
2.3.1. Extraction and Absolute Recovery
2.3.2. Stability
2.4. Clinical Application
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solvents
4.2. Chromatographic Analysis
4.3. Standard and Quality Control (QC) Sample Preparation
4.4. Saliva Sample Collection
4.5. Extraction Procedure
4.6. Validation
4.6.1. Linearity
4.6.2. Intra- and Inter-Day Precision
4.6.3. Lower Limit of Quantification (LLOQ)
4.6.4. Selectivity
4.6.5. Extraction and Absolute Recovery
4.6.6. Stability
4.7. Clinical Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LC | liquid chromatography |
| DAD | diode array detector |
| CV | coefficient of variation |
| BD | bipolar disorder |
| LLE | liquid–liquid extraction |
| SPE | solid-phase extraction |
| QC | quality control |
| EMA | European Medicines Agency |
| LLOQ | lower limit of quantification |
References
- Drayton, S.J.; Fields, C.S. Bipolar Disorder. In Pharmacotherapy: A Pathophysiologic Approach, 11th ed.; DiPiro, J.T., Yee, G.C., Posey, L., Haines, S.T., Nolin, T.D., Ellingrod, V., Eds.; McGraw-Hill Education: Columbus, OH, USA, 2020; Available online: https://accesspharmacy-1mhmedical-1com-1aqoxlrdl019b.han.gumed.edu.pl/content.aspx?bookid=2577§ionid=219317751 (accessed on 6 June 2025).
- Miola, A.; Frye, M.A.; Tondo, L.; Baldessarini, R.J. Current status and treatment of rapid cycling bipolar disorder. J. Clin. Psychopharmacol. 2024, 44, 86–88. [Google Scholar] [CrossRef]
- Ratheesh, A.; Speed, M.; Salagre, E.; Berk, M.; Rohde, C.; Østergaard, S.D. Prior psychiatric morbidity and differential psychopharmacological treatment patterns: Exploring the heterogeneity of bipolar disorder in a nationwide study of 9594 patients. Bipolar Disord. 2024, 26, 570–583. [Google Scholar] [CrossRef]
- Dibué-Adjei, M.; Kamp, M.A.; Alpdogan, S.; Tevoufouet, E.E.; Neiss, W.F.; Hescheler, J.; Schneider, T. Cav2.3 (R-Type) calcium channels are critical for mediating anticonvulsive and neuroprotective properties of lamotrigine in vivo. Cell Physiol. Biochem. 2017, 44, 935–947. [Google Scholar] [CrossRef]
- Chouchana, M.; Delage, C.; Godin, O.; Fontan, J.E.; Bellivier, F.; Gard, S.; Aubin, V.; Belzeaux, R.; Dubertret, C.; Haffen, E.; et al. FondaMental Advanced Center of Expertise for Bipolar Disorders (FACE-BD). Factors associated with lamotrigine concentration/dose ratio in individuals with bipolar disorders. Eur. Neuropsychopharmacol. 2023, 73, 75–81. [Google Scholar] [CrossRef]
- Ding, Y.; Tan, X.; Zhang, S.; Guo, Y. Pharmacokinetic changes and therapeutic drug monitoring of lamotrigine during pregnancy. Brain Behav. 2019, 9, e01315. [Google Scholar] [CrossRef]
- Kuczynska, J.; Karas-Ruszczyk, K.; Zakrzewska, A.; Dermanowski, M.; Sienkiewicz-Jarosz, H.; Kurkowska-Jastrzebska, I.; Bienkowski, P.; Konopko, M.; Dominiak, M.; Mierzejewski, P. Comparison of plasma, saliva, and hair lamotrigine concentrations. Clin. Biochem. 2019, 74, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Grim, S.A.; Miles, M.V.; Tang, P.H.; Fakhoury, T.A.; Strawsburg, R.H.; deGrauw, T.J.; Baumann, R.J. Correlation of lamotrigine concentrations between serum and saliva. Pharmacotherapy 2003, 23, 1550–1557. [Google Scholar] [CrossRef]
- Shah, H.J.; Subbaiah, G.; Patel, D.M.; Suhagia, B.N.; Patel, C.N. Rapid quantification of lamotrigine in human plasma by two LC systems connected with tandem MS. J. Chromatogr. Sci. 2010, 48, 375–381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Itabashi, S.; Bito, R.; Nishina, M.; Fukumoto, M.; Soda, M.; Doi, M.; Usui, S.; Kitaichi, K. Determination of lamotrigine in human plasma using liquid chromatography-tandem mass spectrometry. Neuropsychopharmacol. Rep. 2019, 39, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Pucci, V.; Bugamelli, F.; Baccini, C.; Raggi, M.A. Analysis of lamotrigine and its metabolites in human plasma and urine by micellar electrokinetic capillary chromatography. Electrophoresis 2005, 26, 935–942. [Google Scholar] [CrossRef]
- Asadi, M.; Dadfarnia, S.; Haji Shabani, A.M.; Abbasi, B. Simultaneous extraction and quantification of lamotrigine, phenobarbital, and phenytoin in human plasma and urine samples using solidified floating organic drop microextraction and high-performance liquid chromatography. J. Sep. Sci. 2015, 38, 2510–2516. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, T.; Edelbroek, P.M. Robust isocratic high performance liquid chromatographic method for simultaneous determination of seven antiepileptic drugs including lamotrigine, oxcarbazepine and zonisamide in serum after solid-phase extraction. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 857, 40–46. [Google Scholar] [CrossRef]
- Pelcová, M.; Ďurčová, V.; Šmak, P.; Strýček, O.; Štolcová, M.; Peš, O.; Glatz, Z.; Šištík, P.; Juřica, J. Non-invasive therapeutic drug monitoring: LC-MS validation for lamotrigine quantification in dried blood spot and oral fluid/saliva. J. Pharm. Biomed. Anal. 2025, 262, 116877. [Google Scholar] [CrossRef]
- Malone, S.A.; Eadie, M.J.; Addison, R.S.; Wright, A.W.; Dickinson, R.G. Monitoring salivary lamotrigine concentrations. J. Clin. Neurosci. 2006, 13, 902–907. [Google Scholar] [CrossRef]
- Ohman, I.; Vitols, S.; Tomson, T. Lamotrigine in pregnancy: Pharmacokinetics during delivery, in the neonate, and during lactation. Epilepsia 2000, 41, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.; Rodrigues, M.; Pousinho, S.; Falcão, A.; Alves, G. Determination of lamotrigine in human plasma and saliva using microextraction by packed sorbent and high performance liquid chromatography–diode array detection: An innovative bioanalytical tool for therapeutic drug monitoring. Microchem. J. 2017, 130, 221–228. [Google Scholar] [CrossRef]
- Tsiropoulos, I.; Kristensen, O.; Klitgaard, N.A. Saliva and serum concentration of lamotrigine in patients with epilepsy. Ther. Drug Monit. 2000, 22, 517–521. [Google Scholar] [CrossRef]
- Kruizinga, M.D.; Stuurman, F.E.; Driessen, G.J.A.; Cohen, A.F.; Bergmann, K.R.; Van Esdonk, M.J. Theoretical performance of nonlinear mixed-effect models incorporating saliva as an alternative sampling matrix for therapeutic drug monitoring in pediatrics: A simulation study. Ther. Drug Monit. 2021, 43, 546–554. [Google Scholar] [CrossRef]
- Sabença, R.; Bicker, J.; Silva, R.; Carona, A.; Silva, A.; Santana, I.; Sales, F.; Falcão, A.; Fortuna, A. Development and application of an HPLC-DAD technique for human plasma concentration monitoring of perampanel and lamotrigine in drug-resistant epileptic patients. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1162, 122491. [Google Scholar] [CrossRef]
- Namdev, K.K.; Dwivedi, J.; Chilkoti, D.C.; Sharma, S. A simple, rapid and stability indicating validated method for quantification of lamotrigine in human plasma and dry plasma spot using LC-ESI-MS/MS: Application in clinical study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1072, 362–369. [Google Scholar] [CrossRef]
- Palacios-Magaña, C.V.; Romero-Tejeda, E.M.; Fajardo-Robledo, N.S.; González-Ortiz, L.J.; González-Mendez, J.G.; Pacheco-Moisés, F.P. Lamotrigine extraction and quantification by UPLC-DAD in plasma from patients with bipolar disorder. Int. J. Anal. Chem. 2022, 2022, 3288646. [Google Scholar] [CrossRef] [PubMed]
- Choong, E.; Vassallo, P.; Aícua-Rapún, I.; Stampfli, C.; André, P.; Rossetti, A.O.; Buclin, T.; Novy, J.; Decosterd, L.A. Clinical value of saliva therapeutic drug monitoring of newer antiseizure medications. Seizure 2025, 125, 106–112. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en (accessed on 11 July 2025).


| Calibration Curve y = ax + b (n = 4) | |
|---|---|
| Range (ng/mL) | 10–2000 |
| Determination coefficient (R2) | 0.9996 ± 0.0002 |
| Slope a ± Δa | 0.0003 ± 0.0000 |
| Intercept b ± Δb | −0.0042 ± 0.0024 |
| LLOQ (ng/mL) | 10.0 |
| QC (ng/mL) | Intra-Day (CV%) | Inter-Day (CV%) | Extraction Recovery (%) | Absolute Recovery (%) | Stability (Difference %) | |
|---|---|---|---|---|---|---|
| 8 °C | −21 °C | |||||
| 10 | 12.97 | 14.39 | 101.82 | 99.48 | −3.46 | −0.82 |
| 100 | 7.18 | 10.18 | 100.45 | 100.03 | −3.70 | −0.64 |
| 800 | 2.50 | 6.62 | 95.42 | 93.53 | −5.96 | −1.08 |
| 1500 | 5.20 | 6.92 | 93.63 | 90.95 | −4.63 | −0.98 |
| Patient | Age | Lamotrigine (ng/mL) |
|---|---|---|
| 1 | 48 | 320.7 |
| 2 | 52 | 461.8 |
| 3 | 56 | 228.6 |
| 4 | 46 | 1058.0 |
| 5 | 39 | 539.2 |
| 6 | 53 | 445.1 |
| 7 | 60 | 628.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziurkowska, E.; Michalak, A.; Plenis, A.; Dziurkowski, M. New Method for the Determination of Lamotrigine in Human Saliva Using SPE-LC-DAD. Molecules 2025, 30, 3237. https://doi.org/10.3390/molecules30153237
Dziurkowska E, Michalak A, Plenis A, Dziurkowski M. New Method for the Determination of Lamotrigine in Human Saliva Using SPE-LC-DAD. Molecules. 2025; 30(15):3237. https://doi.org/10.3390/molecules30153237
Chicago/Turabian StyleDziurkowska, Ewelina, Aleksandra Michalak, Alina Plenis, and Maciej Dziurkowski. 2025. "New Method for the Determination of Lamotrigine in Human Saliva Using SPE-LC-DAD" Molecules 30, no. 15: 3237. https://doi.org/10.3390/molecules30153237
APA StyleDziurkowska, E., Michalak, A., Plenis, A., & Dziurkowski, M. (2025). New Method for the Determination of Lamotrigine in Human Saliva Using SPE-LC-DAD. Molecules, 30(15), 3237. https://doi.org/10.3390/molecules30153237







