Progress and Prospects of Biomolecular Materials in Solar Photovoltaic Applications
Abstract
1. Introduction
2. Evolution of PV Technology
2.1. Solar Cells Generations and Advancements
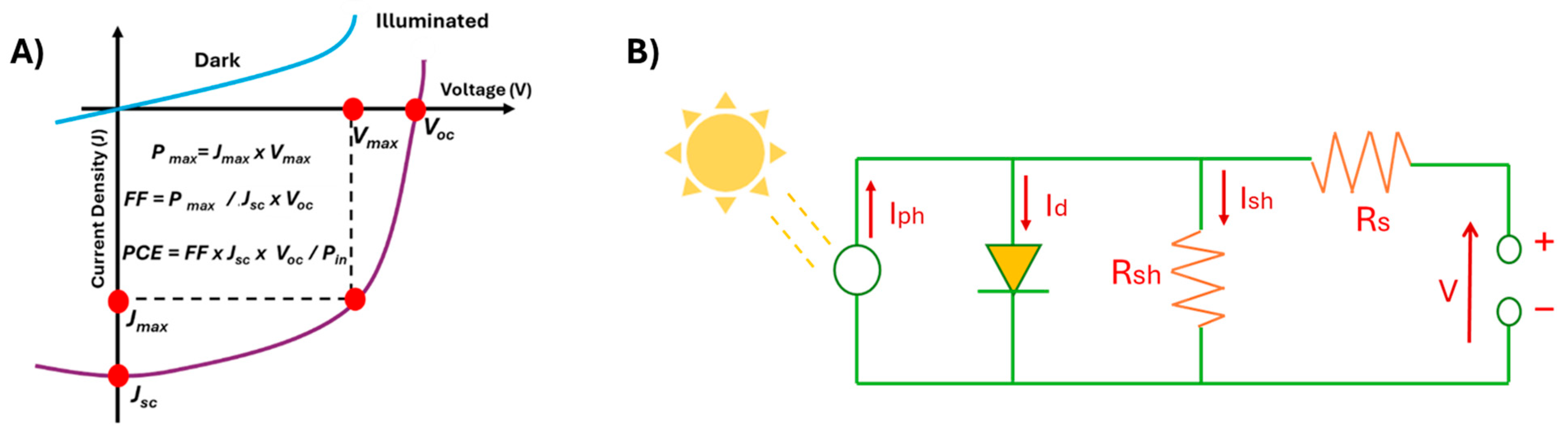
Solar Cells: General Aspects
2.2. Third Generation Solar Cells: Design Principles, Material Innovations
3. Introduction to Biomolecular Materials in Solar Applications
3.1. Roles of Biomolecules in PV
3.2. Pigments and Natural Small Molecules
3.2.1. Light Harvesting
3.2.2. Light Absorption and Charge Separation in Photovoltaics
3.2.3. Solar Cells Stabilization and Durability
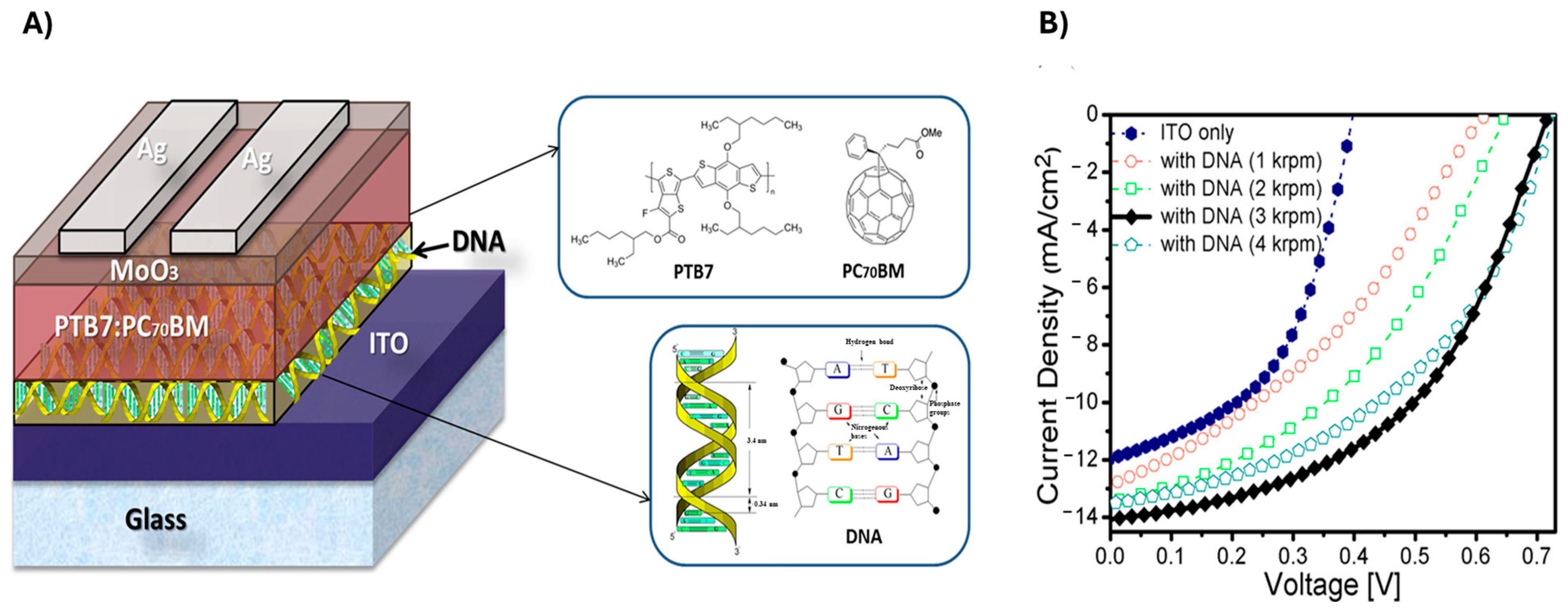
3.3. DNA-Based Nanostructures
DNA for Improved Charge Transfer
3.4. Proteins for Energy Conversion: From Natural Photosynthesis to Bio-Inspired Photoelectrochemical Systems
3.4.1. Proteins for Light Harvesting
3.4.2. Proteins for Improved Light Absorption in Photovoltaics
3.4.3. Amyloid from Neurodegenerative Pathways to Advanced Applications in PV
3.5. Polysaccharides
3.5.1. Cellulosic Materials as Supports for Solar Devices
3.5.2. Polysaccharides for Tuning Rheology
3.5.3. Polysaccharides for Charge Transfer
4. Summary and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassan, Q.; Viktor, P.; Al-Musawi, T.J.; Mahmood Ali, B.; Algburi, S.; Alzoubi, H.M.; Khudhair Al-Jiboory, A.; Zuhair Sameen, A.; Salman, H.M.; Jaszczur, M. The Renewable Energy Role in the Global Energy Transformations. Renew. Energy Focus 2024, 48, 100545. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Østergaard, P.A.; Duic, N.; Noorollahi, Y.; Kalogirou, S. Advances in Renewable Energy for Sustainable Development. Renew. Energy 2023, 219, 119377. [Google Scholar] [CrossRef]
- Scholes, G.D.; Fleming, G.R.; Olaya-Castro, A.; Van Grondelle, R. Lessons from Nature about Solar Light Harvesting. Nat. Chem. 2011, 3, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthi, B.N.C.V.; Hari Prasad, L.; Chavakula, R.L.; Vijetha Inti, V.V. Solar Energy Conversion Techniques and Practical Approaches to Design Solar PV Power Station. In Clean Energy Production Technologies Sustainable and Clean Energy Production Technologies; Springer Nature: Singapore, 2022; pp. 179–201. [Google Scholar] [CrossRef]
- Pourasl, H.H.; Barenji, R.V.; Khojastehnezhad, V.M. Solar Energy Status in the World: A Comprehensive Review. Energy Rep. 2023, 10, 3474–3493. [Google Scholar] [CrossRef]
- Becquerel, A.E. Recherches Sur Les Effets de La Radiation Chimique de La Lumiere Solaire Au Moyen Des Courants Electriques. CR Acad. Sci. 1839, 9, 145. [Google Scholar]
- Machín, A.; Márquez, F. Advancements in Photovoltaic Cell Materials: Silicon, Organic, and Perovskite Solar Cells. Materials 2024, 17, 1165. [Google Scholar] [CrossRef]
- Al-Ali, S.; Olabi, A.G.; Mahmoud, M. A Review of Solar Photovoltaic Technologies: Developments, Challenges, and Future Perspectives. Energy Convers. Manag. X 2025, 27, 101057. [Google Scholar] [CrossRef]
- Di Sabatino, M.; Hendawi, R.; Garcia, A.S. Silicon Solar Cells: Trends, Manufacturing Challenges, and AI Perspectives. Crystals 2024, 14, 167. [Google Scholar] [CrossRef]
- Martin, C.; Zhao, Q.; Patel, A.; Velasquez, E.; Chen, D.; Li, W. A Review of Advanced Roll-to-Roll Manufacturing: System Modeling and Control. J. Manuf. Sci. Eng. 2024, 147, 1087–1357. [Google Scholar] [CrossRef]
- Arrabito, G.; Errico, V.; De Ninno, A.; Cavaleri, F.; Ferrara, V.; Pignataro, B.; Caselli, F. Oil-in-Water FL Droplets by Interfacial Spontaneous Fragmentation and Their Electrical Characterization. Langmuir 2019, 35, 4936–4945. [Google Scholar] [CrossRef]
- Rajbongshi, B.M.; Verma, A. Emerging Nanotechnology for Third Generation Photovoltaic Cells. In Nanotechnology: Applications in Energy, Drug and Food; Springer: Cham, Switzerland, 2019; pp. 99–133. [Google Scholar] [CrossRef]
- Akinoglu, B.G.; Tuncel, B.; Badescu, V. Beyond 3rd Generation Solar Cells and the Full Spectrum Project. Recent Advances and New Emerging Solar Cells. Sustain. Energy Technol. Assess. 2021, 46, 101287. [Google Scholar] [CrossRef]
- Rehman, F.; Syed, I.H.; Khanam, S.; Ijaz, S.; Mehmood, H.; Zubair, M.; Massoud, Y.; Mehmood, M.Q. Fourth-Generation Solar Cells: A Review. Energy Adv. 2023, 2, 1239–1262. [Google Scholar] [CrossRef]
- Suman; Sharma, P.; Goyal, P. Evolution of PV Technology from Conventional to Nano-Materials. Mater. Today Proc. 2020, 28, 1593–1597. [Google Scholar] [CrossRef]
- Singh, B.P.; Goyal, S.K.; Kumar, P. Solar Pv Cell Materials and Technologies: Analyzing the Recent Developments. Mater. Today Proc. 2021, 43, 2843–2849. [Google Scholar] [CrossRef]
- Chiappara, C.; Arrabito, G.; Ferrara, V.; Scopelliti, M.; Sancataldo, G.; Vetri, V.; Chillura Martino, D.F.; Pignataro, B. Improved Photocatalytic Activity of Polysiloxane TiO2Composites by Thermally Induced Nanoparticle Bulk Clustering and Dye Adsorption. Langmuir 2021, 37, 10354–10365. [Google Scholar] [CrossRef]
- Sartorio, C.; Scaramuzza, S.; Cataldo, S.; Vetri, V.; Scopelliti, M.; Leone, M.; Amendola, V.; Pignataro, B. Donor-Acceptor Interfaces by Engineered Nanoparticles Assemblies for Enhanced Efficiency in Plastic Planar Heterojunction Solar Cells. J. Phys. Chem. C 2016, 120, 26588–26599. [Google Scholar] [CrossRef]
- Bernardi, M.; Palummo, M.; Grossman, J.C. Extraordinary Sunlight Absorption and One Nanometer Thick Photovoltaics Using Two-Dimensional Monolayer Materials. Nano Lett. 2013, 13, 3664–3670. [Google Scholar] [CrossRef]
- Tsai, M.L.; Su, S.H.; Chang, J.K.; Tsai, D.S.; Chen, C.H.; Wu, C.I.; Li, L.J.; Chen, L.J.; He, J.H. Monolayer MoS2 Heterojunction Solar Cells. ACS Nano 2014, 8, 8317–8322. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, T.; Wang, Y.; Hahn, Y.B. Graphene and Its Derivatives for Solar Cells Application. Nano Energy 2018, 47, 51–65. [Google Scholar] [CrossRef]
- Solak, E.K.; Irmak, E. Advances in Organic Photovoltaic Cells: A Comprehensive Review of Materials, Technologies, and Performance. RSC Adv. 2023, 13, 12244–12269. [Google Scholar] [CrossRef]
- Wagenpfahl, A.; Rauh, D.; Binder, M.; Deibel, C.; Dyakonov, V. S-Shaped Current-Voltage Characteristics of Organic Solar Devices. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 82, 115306. [Google Scholar] [CrossRef]
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W. Solar Cell Efficiency Tables (Version 35). Prog. Photovolt. Res. Appl. 2010, 18, 144–150. [Google Scholar] [CrossRef]
- Bhatt, M.P.; Magurudeniya, H.D.; Rainbolt, E.A.; Huang, P.; Dissanayake, D.S.; Biewer, M.C.; Stefan, M.C. Poly(3-Hexylthiophene) Nanostructured Materials for Organic Electronics Applications. J. Nanosci. Nanotechnol. 2014, 14, 1033–1050. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.J.; He, Y.J.; Li, Y. 6.5% Efficiency of Polymer Solar Cells Based on Poly(3-Hexylthiophene) and Indene-C60 Bisadduct by Device Optimization. Adv. Mater. 2010, 22, 4355–4358. [Google Scholar] [CrossRef] [PubMed]
- Mativetsky, J.M.; Mativetsky, J.M. Supramolecular Approaches to Nanoscale Morphological Control in Organic Solar Cells. Int. J. Mol. Sci. 2015, 16, 13381–13406. [Google Scholar] [CrossRef]
- Fabiano, S.; Chen, Z.; Vahedi, S.; Facchetti, A.; Pignataro, B.; Loi, M.A. Role of Photoactive Layer Morphology in High Fill Factor All-Polymer Bulk Heterojunction Solar Cells. J. Mater. Chem. 2011, 21, 5891–5896. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- Grätzel, M. Recent Advances in Sensitized Mesoscopic Solar Cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef]
- Giannouli, M. Current Status of Emerging PV Technologies: A Comparative Study of Dye-Sensitized, Organic, and Perovskite Solar Cells. Int. J. Photoenergy 2021, 2021, 6692858. [Google Scholar] [CrossRef]
- Sharif, R.; Khalid, A.; Ahmad, S.W.; Rehman, A.; Qutab, H.G.; Akhtar, H.H.; Mahmood, K.; Afzal, S.; Saleem, F. A Comprehensive Review of the Current Progresses and Material Advances in Perovskite Solar Cells. Nanoscale Adv. 2023, 5, 3803–3833. [Google Scholar] [CrossRef]
- Luo, S.; Daoud, W.A. Recent Progress in Organic-Inorganic Halide Perovskite Solar Cells: Mechanisms and Material Design. J. Mater. Chem. A Mater. 2015, 3, 8992–9010. [Google Scholar] [CrossRef]
- Mombeshora, E.T.; Muchuweni, E.; Doolin, A.J.; Davies, M.L.; Martincigh, B.S.; Nyamori, V.O. The Prospects of Biologically Derived Materials in Perovskite Solar Cells. Appl. Mater. Today 2024, 40, 10246–102406. [Google Scholar] [CrossRef]
- Andrews, D. The Circular Economy, Design Thinking and Education for Sustainability. Local. Econ. 2015, 30, 305–315. [Google Scholar] [CrossRef]
- Arruda, E.H.; Melatto, R.A.P.B.; Levy, W.; Conti, D.; de Melo Conti, D. Circular Economy: A Brief Literature Review (2015–2020). Sustain. Oper. Comput. 2021, 2, 79–86. [Google Scholar] [CrossRef]
- Nagamune, T. Biomolecular Engineering for Nanobio/Bionanotechnology. Nano Converg. 2017, 4, 9. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; et al. Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Arrabito, G.; Aleeva, Y.; Ferrara, V.; Prestopino, G.; Chiappara, C.; Pignataro, B. On the Interaction between 1d Materials and Living Cells. J. Funct. Biomater. 2020, 11, 40. [Google Scholar] [CrossRef]
- Ferrara, V.; Marchetti, M.; Alfieri, D.; Targetti, L.; Scopelliti, M.; Pignataro, B.; Pavone, F.; Vetri, V.; Sancataldo, G. Blue Light Activated Photodegradation of Biomacromolecules by N-Doped Titanium Dioxide in a Chitosan Hydrogel Matrix. J. Photochem. Photobiol. A Chem. 2023, 437, 114451. [Google Scholar] [CrossRef]
- Wang, R.; Xue, J.; Wang, K.-L.; Wang, Z.-K.; Luo, Y.; Fenning, D.; Xu, G.; Nuryyeva, S.; Huang, T.; Zhao, Y.; et al. Constructive Molecular Configurations for Surface-Defect Passivation of Perovskite Photovoltaics. Science 2019, 366, 1509–1513. [Google Scholar] [CrossRef]
- Kaymaz, S.V.; Nobar, H.M.; Sarıgül, H.; Soylukan, C.; Akyüz, L.; Yüce, M. Nanomaterial Surface Modification Toolkit: Principles, Components, Recipes, and Applications. Adv. Colloid. Interface Sci. 2023, 322, 103035. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, K.; Yang, D.; Jiang, Y.; Yennawar, N.; Wang, K.; Sanghadasa, M.; Wu, C.; Priya, S. Enhanced Performance and Stability in DNA-Perovskite Heterostructure-Based Solar Cells. ACS Energy Lett. 2019, 4, 2646–2655. [Google Scholar] [CrossRef]
- Kazim, S.; Haris, M.P.U.; Ahmad, S. Peptide-Perovskite Based Bio-Inspired Materials for Optoelectronics Applications. Adv. Sci. 2025, 12, 2408919. [Google Scholar] [CrossRef]
- Limo, M.J.; Sola-Rabada, A.; Boix, E.; Thota, V.; Westcott, Z.C.; Puddu, V.; Perry, C.C. Interactions between Metal Oxides and Biomolecules: From Fundamental Understanding to Applications. Chem. Rev. 2018, 118, 11118–11193. [Google Scholar] [CrossRef]
- Song, P.; Wang, H. High-Performance Polymeric Materials through Hydrogen-Bond Cross-Linking. Adv. Mater. 2020, 32, 1901244. [Google Scholar] [CrossRef]
- Andrew, J.J.; Dhakal, H.N. Sustainable Biobased Composites for Advanced Applications: Recent Trends and Future Opportunities—A Critical Review. Compos. Part. C Open Access 2022, 7, 100220. [Google Scholar] [CrossRef]
- Miettunen, K.; Hadadian, M.; García, J.V.; Lawrynowicz, A.; Akulenko, E.; Rojas, O.J.; Hummel, M.; Vapaavuori, J. Bio-Based Materials for Solar Cells. Wiley Interdiscip. Rev. Energy Environ. 2024, 13, e508. [Google Scholar] [CrossRef]
- Altassan, A. Sustainable Integration of Solar Energy, Behavior Change, and Recycling Practices in Educational Institutions: A Holistic Framework for Environmental Conservation and Quality Education. Sustainability 2023, 15, 15157. [Google Scholar] [CrossRef]
- Peng, M.; Dong, B.; Cai, X.; Wang, W.; Jiang, X.; Wang, Y.; Yang, Y.; Zou, D. Organic Dye-Sensitized Photovoltaic Fibers. Sol. Energy 2017, 150, 161–165. [Google Scholar] [CrossRef]
- Zhang, D.; Lanier, S.M.; Downing, J.A.; Avent, J.L.; Lum, J.; McHale, J.L. Betalain Pigments for Dye-Sensitized Solar Cells. J. Photochem. Photobiol. A Chem. 2008, 195, 72–80. [Google Scholar] [CrossRef]
- Hadmojo, W.T.; Lee, U.H.; Yim, D.; Kim, H.W.; Jang, W.D.; Yoon, S.C.; Jung, I.H.; Jang, S.Y. High-Performance Near-Infrared Absorbing n-Type Porphyrin Acceptor for Organic Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 41344–41349. [Google Scholar] [CrossRef]
- Dagar, J.; Scavia, G.; Scarselli, M.; Destri, S.; De Crescenzi, M.; Brown, T.M. Coating ZnO Nanoparticle Films with DNA Nanolayers for Enhancing the Electron Extracting Properties and Performance of Polymer Solar Cells. Nanoscale 2017, 9, 19031–19038. [Google Scholar] [CrossRef]
- Dagar, J.; Scarselli, M.; De Crescenzi, M.; Brown, T.M. Solar Cells Incorporating Water/Alcohol- Soluble Electron-Extracting DNA Nanolayers. ACS Energy Lett. 2016, 1, 510–515. [Google Scholar] [CrossRef]
- Yusoff, A.R.b.M.; Kim, J.; Jang, J.; Nazeeruddin, M.K. New Horizons for Perovskite Solar Cells Employing DNA-CTMA as the Hole-Transporting Material. ChemSusChem 2016, 9, 1736–1742. [Google Scholar] [CrossRef]
- Li, W.; Pu, Y.; Ge, B.; Wang, Y.; Yu, D.; Qin, S. Dye-Sensitized Solar Cells Based on Natural and Artificial Phycobiliproteins to Capture Low Light Underwater. Int. J. Hydrogen Energy 2019, 44, 1182–1191. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Shi, Z.; Pan, M.; Yu, D. Protein-Encapsulated Chlorophyll a Molecules for Biological Solar Cells. Mater. Des. 2020, 195, 108983. [Google Scholar] [CrossRef]
- Das, S.; Wu, C.; Song, Z.; Hou, Y.; Koch, R.; Somasundaran, P.; Priya, S.; Barbiellini, B.; Venkatesan, R. Bacteriorhodopsin Enhances Efficiency of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 30728–30734. [Google Scholar] [CrossRef] [PubMed]
- Barrau, S.; Zhang, F.; Herland, A.; Mammo, W.; Andersson, M.R.; Inganäs, O. Integration of Amyloid Nanowires in Organic Solar Cells. Appl. Phys. Lett. 2008, 93, 023307. [Google Scholar] [CrossRef]
- Bolisetty, S.; Adamcik, J.; Heier, J.; Mezzenga, R. Amyloid Directed Synthesis of Titanium Dioxide Nanowires and Their Applications in Hybrid Photovoltaic Devices. Adv. Funct. Mater. 2012, 22, 3424–3428. [Google Scholar] [CrossRef]
- Gao, C.; Yuan, S.; Cui, K.; Qiu, Z.; Ge, S.; Cao, B.; Yu, J. Flexible and Biocompatibility Power Source for Electronics: A Cellulose Paper Based Hole-Transport-Materials-Free Perovskite Solar Cell. Solar RRL 2018, 2, 175. [Google Scholar] [CrossRef]
- Giuri, A.; Rolston, N.; Colella, S.; Listorti, A.; Esposito Corcione, C.; Elmaraghi, H.; Lauciello, S.; Dauskardt, R.H.; Rizzo, A. Robust, High-Performing Maize-Perovskite-Based Solar Cells with Improved Stability. ACS Appl. Energy Mater. 2021, 4, 11194–11203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Wang, L.; Su, Z.; Xu, Y.; Fan, L.; Yao, G.; Qian, X.; Lin, J.Y. Enhanced Fill Factor and Stability of Perovskite Solar Cells with a Multifunctional Additive of Starch-Iodine Complex. J. Power Sources 2024, 602, 234383. [Google Scholar] [CrossRef]
- Brunetti, F.; Operamolla, A.; Castro-Hermosa, S.; Lucarelli, G.; Manca, V.; Farinola, G.M.; Brown, T.M. Printed Solar Cells and Energy Storage Devices on Paper Substrates. Adv. Funct. Mater. 2019, 29, 1806798. [Google Scholar] [CrossRef]
- Green, M.A. Crystalline and Thin-Film Silicon Solar Cells: State of the Art and Future Potential. Solar Energy 2003, 74, 181–192. [Google Scholar] [CrossRef]
- Ibn-Mohammed, T.; Koh, S.C.L.; Reaney, I.M.; Acquaye, A.; Schileo, G.; Mustapha, K.B.; Greenough, R. Perovskite Solar Cells: An Integrated Hybrid Lifecycle Assessment and Review in Comparison with Other Photovoltaic Technologies. Renew. Sustain. Energy Rev. 2017, 80, 1321–1344. [Google Scholar] [CrossRef]
- Laquai, F.; Andrienko, D.; Mauer, R.; Blom, P.W.M. Charge Carrier Transport and Photogeneration in P3HT:PCBM Photovoltaic Blends. Macromol. Rapid Commun. 2015, 36, 1001–1025. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.; Zhang, J. Investigation of UV Aging Influences on the Crystallization of Ethylene-Vinyl Acetate Copolymer via Successive Self-Nucleation and Annealing Treatment. J. Polym. Res. 2010, 17, 827–836. [Google Scholar] [CrossRef]
- Long, B.; Zhou, X.; Cao, H.; Chen, R.; He, N.; Chi, L.; Fan, P.; Chen, X. Excellent Stability of Perovskite Solar Cells Encapsulated With Paraffin/Ethylene-Vinyl Acetate/Paraffin Composite Layer. Front. Mater. 2022, 9, 892657. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The Role of Photosynthesis Related Pigments in Light Harvesting, Photoprotection and Enhancement of Photosynthetic Yield in Planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Sanjay, P.; Isaivani, I.; Deepa, K.; Madhavan, J.; Senthil, S. The Preparation of Dye Sensitized Solar Cells Using Natural Dyes Extracted from Phytolacca Icosandra and Phyllanthus Reticulatus with ZnO as Photoanode. Mater. Lett. 2019, 244, 142–146. [Google Scholar] [CrossRef]
- Peng, X.; Lu, H.; Zhuang, J.; Liu, X.; Ma, Z.; Wang, H.; Guo, Z.; Wang, Q.; Zhang, H.; Zhao, S. Enhanced Performance of Perovskite Solar Cells Using DNA-Doped Mesoporous-TiO2 as Electron Transporting Layer. Sol. Energy 2020, 206, 855–863. [Google Scholar] [CrossRef]
- He, K.; Zheng, C.; Mohammadi, S. High Efficient Perovskite Solar Cells Enhancement via Photosystem I Proteins. Opt. Mater. 2023, 137, 113566. [Google Scholar] [CrossRef]
- Croce, R.; Van Amerongen, H. Natural Strategies for Photosynthetic Light Harvesting. Nat. Chem. Biol. 2014, 10, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Li, M.; Duan, S.; Fu, M.; Dong, X.; Liu, B.; Feng, D.; Wang, J.; Wang, H. Bin Optimization of Light-Harvesting Pigment Improves Photosynthetic Efficiency. Plant Physiol. 2016, 172, 1720–1731. [Google Scholar] [CrossRef]
- Yu, J.M.; Jang, J.W. Organic Semiconductor-Based Photoelectrochemical Cells for Efficient Solar-to-Chemical Conversion. Catalysts 2023, 13, 814. [Google Scholar] [CrossRef]
- Li, W.; Su, H.N.; Pu, Y.; Chen, J.; Liu, L.N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular Structure, Production, Applications, and Prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef]
- Cardona, M.M.; Gonzalez, A.O.; García, A. Fundamentals in Organic Dyes for Perovskite Solar Cells. Curr. Opin. Colloid. Interface Sci. 2024, 74, 101869. [Google Scholar] [CrossRef]
- Zdyb, A.; Krawczyk, S. Natural Flavonoids as Potential Photosensitizers for Dye-Sensitized Solar Cells. Ecol. Chem. Eng. S 2019, 26, 29–36. [Google Scholar] [CrossRef]
- Okello, A.; Owuor, B.O.; Namukobe, J.; Okello, D.; Mwabora, J. Influence of the PH of Anthocyanins on the Efficiency of Dye Sensitized Solar Cells. Heliyon 2022, 8, e09921. [Google Scholar] [CrossRef]
- Cavinato, L.M.; Fresta, E.; Ferrara, S.; Costa, R.D. Merging Biology and Photovoltaics: How Nature Helps Sun-Catching. Adv. Energy Mater. 2021, 11, 2100520. [Google Scholar] [CrossRef]
- Hazebroucq, S.; Labat, F.; Lincot, D.; Adamo, C. Theoretical Insights on the Electronic Properties of Eosin Y, an Organic Dye for Photovoltaic Applications. J. Phys. Chem. A 2008, 112, 7264–7270. [Google Scholar] [CrossRef]
- Liu, X.; Yeow, E.K.L.; Velate, S.; Steer, R.P. Photophysics and Spectroscopy of the Higher Electronic States of Zinc Metalloporphyrins: A Theoretical and Experimental Study. Phys. Chem. Chem. Phys. 2006, 8, 1298–1309. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Ge, H.; Li, A.; Wang, X.F. Recent Research Progress and Perspectives on Porphyrin and Phthalocyanine Analogues for Perovskite Solar Cell Applications. Energy Fuels 2024, 38, 13685–13703. [Google Scholar] [CrossRef]
- Giovannetti, R. The Use of Spectrophotometry UV-Vis for the Study of Porphyrins. Macro Nano Spectrosc. 2012, 6, 87–108. [Google Scholar]
- Roohi, H.; Mohtamadifar, N. The Role of the Donor Group and Electron-Accepting Substitutions Inserted in π-Linkers in Tuning the Optoelectronic Properties of D-π-A Dye-Sensitized Solar Cells: A DFT/TDDFT Study. RSC Adv. 2022, 12, 11557–11573. [Google Scholar] [CrossRef] [PubMed]
- Gkini, K.; Balis, N.; Papadakis, M.; Verykios, A.; Skoulikidou, M.C.; Drivas, C.; Kennou, S.; Golomb, M.; Walsh, A.; Coutsolelos, A.G.; et al. Manganese Porphyrin Interface Engineering in Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 7353–7363. [Google Scholar] [CrossRef]
- Wu, H.; Hou, Y.; Yoon, J.; Knoepfel, A.M.; Zheng, L.; Yang, D.; Wang, K.; Qian, J.; Priya, S.; Wang, K. Down-Selection of Biomolecules to Assemble “Reverse Micelle” with Perovskites. Nat. Commun. 2024, 15, 772. [Google Scholar] [CrossRef]
- Hu, J.; Xu, X.; Chen, Y.; Wu, S.; Wang, Z.; Wang, Y.; Jiang, X.; Cai, B.; Shi, T.; Brabec, C.J.; et al. Overcoming Photovoltage Deficitvianatural Amino Acid Passivation for Efficient Perovskite Solar Cells and Modules. J. Mater. Chem. A Mater. 2021, 9, 5857–5865. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhou, B.; Fu, Y.; Li, D.; Guo, C.; Wang, H.; Chen, C.; Cai, J.; Zhang, X.; et al. Self-Assembled Biomolecule Interlayer for Enhanced Efficiency and Stability of Inverted Organic Solar Cells. ACS Mater. Lett. 2023, 5, 321–329. [Google Scholar] [CrossRef]
- Guan, C.; Zhu, X.; Feng, C. Dna Nanodevice-Based Drug Delivery Systems. Biomolecules 2021, 11, 1855. [Google Scholar] [CrossRef]
- Neidle, S. Beyond the Double Helix: DNA Structural Diversity and the PDB. J. Biol. Chem. 2021, 296, 100553. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Yoo, K.-H.; Kim, J.; Kim, J.-J.; Kim, S.K. Electrical Transport Properties of DNA Molecules. J. Korean Phys. Soc. 2001, 39, 56–58. [Google Scholar]
- Wilkins, M.H.F.; Stokes, A.R.; Wilson, H. Molecular Structure of Deoxypentose Nucleic Acids. Nature 1953, 171, 738. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.E.; Channell, G.; Phillips-Jones, M.K. The Discovery of Hydrogen Bonds in DNA and a Re-Evaluation of the 1948 Creeth Two-Chain Model for Its Structure. Biochem. Soc. Trans. 2018, 46, 1171–1182. [Google Scholar] [CrossRef]
- Minchin, S.; Lodge, J. Understanding Biochemistry: Structure and Function of Nucleic Acids. Essays Biochem. 2019, 63, 433–456. [Google Scholar] [CrossRef]
- Kumar, C.V.; Duff, M.R. DNA-Based Supramolecular Artificial Light Harvesting Complexes. J. Am. Chem. Soc. 2009, 131, 16024–16026. [Google Scholar] [CrossRef]
- Hemmig, E.A.; Creatore, C.; Wünsch, B.; Hecker, L.; Mair, P.; Parker, M.A.; Emmott, S.; Tinnefeld, P.; Keyser, U.F.; Chin, A.W. Programming Light-Harvesting Efficiency Using DNA Origami. Nano Lett. 2016, 16, 2369–2374. [Google Scholar] [CrossRef]
- Gomez, E.F.; Steckl, A.J. Engineering DNA and Nucleobases for Present and Future Device Applications. In Green Materials for Electronics; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 191–233. [Google Scholar] [CrossRef]
- Volkov, I.L.; Reveguk, Z.V.; Serdobintsev, P.Y.; Ramazanov, R.R.; Kononov, A.I. DNA as UV Light-Harvesting Antenna. Nucleic Acids Res. 2018, 46, 3543–3551. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.W.; Schönenberger, C. Electrical Conduction through DNA Molecules. Nature 1999, 398, 407–410. [Google Scholar] [CrossRef]
- Ratner, M. Electronic Motion in DNA. Nature 1999, 397, 480–481. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Wilkinson, E.A.; Karuppannan, S.K.; Bailey, L.; Vilan, A.; Zhang, Z.; Qi, D.C.; Tadich, A.; Tuite, E.M.; Pike, A.R.; et al. Role of Order in the Mechanism of Charge Transport across Single-Stranded and Double-Stranded DNA Monolayers in Tunnel Junctions. J. Am. Chem. Soc. 2021, 143, 20309–20319. [Google Scholar] [CrossRef]
- Genereux, J.C.; Barton, J.K. Mechanisms for DNA Charge Transport. Chem. Rev. 2010, 110, 1642–1662. [Google Scholar] [CrossRef]
- Su, W.; Schuster, M.; Bagshaw, C.R.; Rant, U.; Burley, G.A. Site-Specific Assembly of DNA-Based Photonic Wires by Using Programmable Polyamides. Angew. Chem.—Int. Ed. 2011, 50, 2712–2715. [Google Scholar] [CrossRef] [PubMed]
- Olejko, L.; Bald, I. FRET Efficiency and Antenna Effect in Multi-Color DNA Origami-Based Light Harvesting Systems. RSC Adv. 2017, 7, 23924–23934. [Google Scholar] [CrossRef]
- Nizioł, J.; Makyła-Juzak, K.; Marzec, M.M.; Ekiert, R.; Marzec, M.; Gondek, E. Thermal Stability of the Solid DNA as a Novel Optical Material. Opt. Mater. 2017, 66, 344–350. [Google Scholar] [CrossRef]
- Müller, S.; Manger, F.; Graf von Reventlow, L.; Colsmann, A.; Wagenknecht, H.A. Molecular Chromophore-DNA Architectures With Fullerenes: Optical Properties and Solar Cells. Front. Chem. 2021, 9, 645006. [Google Scholar] [CrossRef] [PubMed]
- Lavan, D.A.; Cha, J.N. Approaches for Biological and Biomimetic Energy ConversionProc. Natl. Acad. Sci. USA 2006, 103, 5251–5255. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, J.R.; Waigh, T.A. Electronics of Peptide- and Protein-Based Biomaterials. Adv. Colloid. Interface Sci. 2021, 287, 102319. [Google Scholar] [CrossRef]
- Mali, M.G.; Yoon, H.; Joshi, B.N.; Park, H.; Al-Deyab, S.S.; Lim, D.C.; Ahn, S.J.; Nervi, C.; Yoon, S.S. Enhanced Photoelectrochemical Solar Water Splitting Using a Platinum-Decorated CIGS/CdS/ZnO Photocathode. ACS Appl. Mater. Interfaces 2015, 7, 21619–21625. [Google Scholar] [CrossRef] [PubMed]
- Ihssen, J.; Braun, A.; Faccio, G.; Gajda-Schrantz, K.; Thöny-Meyer, L. Light Harvesting Proteins for Solar Fuel Generation in Bioengineered Photoelectrochemical Cells. Curr. Protein Pept. Sci. 2014, 15, 374–384. [Google Scholar] [CrossRef]
- Lima-Melo, Y.; Alencar, V.T.C.B.; Lobo, A.K.M.; Sousa, R.H.V.; Tikkanen, M.; Aro, E.M.; Silveira, J.A.G.; Gollan, P.J. Photoinhibition of Photosystem i Provides Oxidative Protection during Imbalanced Photosynthetic Electron Transport in Arabidopsis Thaliana. Front. Plant Sci. 2019, 10, 916. [Google Scholar] [CrossRef] [PubMed]
- Schuergers, N.; Werlang, C.; Ajo-Franklin, C.M.; Boghossian, A.A. A Synthetic Biology Approach to Engineering Living Photovoltaics. Energy Environ. Sci. 2017, 10, 1102–1115. [Google Scholar] [CrossRef]
- Busch, A.; Petersen, J.; Webber-Birungi, M.T.; Powikrowska, M.; Lassen, L.M.M.; Naumann-Busch, B.; Nielsen, A.Z.; Ye, J.; Boekema, E.J.; Jensen, O.N.; et al. Composition and Structure of Photosystem I in the Moss Physcomitrella Patens. J. Exp. Bot. 2013, 64, 2689–2699. [Google Scholar] [CrossRef]
- Sengupta, U.; Kayed, R. Amyloid β, Tau, and α-Synuclein Aggregates in the Pathogenesis, Prognosis, and Therapeutics for Neurodegenerative Diseases. Prog. Neurobiol. 2022, 214, 102270. [Google Scholar] [CrossRef] [PubMed]
- Schenk, D.; Basi, G.S.; Pangalos, M.N. Treatment Strategies Targeting Amyloid β-Protein. Cold Spring Harb. Perspect. Med. 2012, 2, a006387. [Google Scholar] [CrossRef]
- Nelson, R.; Sawaya, M.R.; Balbirnie, M.; Madsen, A.Ø.; Riekel, C.; Grothe, R.; Eisenberg, D. Structure of the Cross-β Spine of Amyloid-like Fibrils. Nature 2005, 435, 773–778. [Google Scholar] [CrossRef]
- Sawaya, M.R.; Sambashivan, S.; Nelson, R.; Ivanova, M.I.; Sievers, S.A.; Apostol, M.I.; Thompson, M.J.; Balbirnie, M.; Wiltzius, J.J.W.; McFarlane, H.T.; et al. Atomic Structures of Amyloid Cross-β Spines Reveal Varied Steric Zippers. Nature 2007, 447, 453–457. [Google Scholar] [CrossRef]
- Anselmo, S.; Sancataldo, G.; Vetri, V. Deciphering Amyloid Fibril Molecular Maturation through FLIM-Phasor Analysis of Thioflavin, T. Biophys. Rep. 2024, 4, 100145. [Google Scholar] [CrossRef] [PubMed]
- Vetri, V.; Foderà, V. The Route to Protein Aggregate Superstructures: Particulates and Amyloid-like Spherulites. FEBS Lett. 2015, 589, 2448–2463. [Google Scholar] [CrossRef]
- Das, S.; Jacob, R.S.; Patel, K.; Singh, N.; Maji, S.K. Amyloid Fibrils: Versatile Biomaterials for Cell Adhesion and Tissue Engineering Applications. Biomacromolecules 2018, 19, 1826–1839. [Google Scholar] [CrossRef]
- Hauser, C.A.E.; Maurer-Stroh, S.; Martins, I.C. Amyloid-Based Nanosensors and Nanodevices. Chem. Soc. Rev. 2014, 43, 5326–5345. [Google Scholar] [CrossRef]
- Jacob, R.S.; Ghosh, D.; Singh, P.K.; Basu, S.K.; Jha, N.N.; Das, S.; Sukul, P.K.; Patil, S.; Sathaye, S.; Kumar, A.; et al. Self Healing Hydrogels Composed of Amyloid Nano Fibrils for Cell Culture and Stem Cell Differentiation. Biomaterials 2015, 54, 97–105. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Mezzenga, R. Amyloid Fibrils as Building Blocks for Natural and Artificial Functional Materials. Adv. Mater. 2016, 28, 6546–6561. [Google Scholar] [CrossRef]
- Anselmo, S.; Fricano, A.; Sancataldo, G.; Vetri, V. Sustainable Formation of Gold Nanoparticle-Decorated Amyloid Fibrils for the Development of Functional Hybrid Materials. Langmuir 2025, 41, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Acar, H.; Garifullin, R.; Aygun, L.E.; Okyay, A.K.; Guler, M.O. Amyloid-like Peptide Nanofiber Templated Titania Nanostructures as Dye Sensitized Solar Cell Anodic Materials. J. Mater. Chem. A Mater. 2013, 1, 10979–10984. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Urtecho, A.; Soto, P.; Pachas, B. Recent Progress in Polysaccharide-Based Materials for Energy Applications: A Review. ACS Appl. Mater. Interfaces 2024, 17, 13179–13196. [Google Scholar] [CrossRef]
- Ferrara, V.; Vetri, V.; Pignataro, B.; Chillura Martino, D.F.; Sancataldo, G. Phasor-FLIM Analysis of Cellulose Paper Ageing Mechanism with Carbotrace 680 Dye. Int. J. Biol. Macromol. 2024, 260, 129452. [Google Scholar] [CrossRef]
- Ani, P.C.; Nzereogu, P.U.; Agbogu, A.C.; Ezema, F.I.; Nwanya, A.C. Cellulose from Waste Materials for Electrochemical Energy Storage Applications: A Review. Appl. Surf. Sci. Adv. 2022, 11, 100298. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Arias, A.C.; Steingart, D.A. Recent Progress on Printed Flexible Batteries: Mechanical Challenges, Printing Technologies, and Future Prospects. Energy Technol. 2014, 3, 305–328. [Google Scholar] [CrossRef]
- Vicente, A.; Águas, H.; Mateus, T.; Araújo, A.; Lyubchyk, A.; Siitonen, S.; Fortunato, E.; Martins, R. Solar Cells for Self-Sustainable Intelligent Packaging. J. Mater. Chem. A Mater. 2015, 3, 13226–13236. [Google Scholar] [CrossRef]
- Liu, W.; Liu, K.; Du, H.; Zheng, T.; Zhang, N.; Xu, T.; Pang, B.; Zhang, X.; Si, C.; Zhang, K. Cellulose Nanopaper: Fabrication, Functionalization, and Applications. Nanomicro Lett. 2022, 14, 104. [Google Scholar] [CrossRef]
- Operamolla, A. Recent Advances on Renewable and Biodegradable Cellulose Nanopaper Substrates for Transparent Light-Harvesting Devices: Interaction with Humid Environment. Int. J. Photoenergy 2019, 2019, 3057929. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, M.; Jungstedt, E.; Xu, B.; Sun, L.; Berglund, L. Optically Transparent Wood Substrate for Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 6061–6067. [Google Scholar] [CrossRef]
- Giuri, A.; Masi, S.; Listorti, A.; Gigli, G.; Colella, S.; Esposito Corcione, C.; Rizzo, A. Polymeric Rheology Modifier Allows Single-Step Coating of Perovskite Ink for Highly Efficient and Stable Solar Cells. Nano Energy 2018, 54, 400–408. [Google Scholar] [CrossRef]
- Bisconti, F.; Giuri, A.; Vanni, N.; Carallo, S.; Spera, S.; Marrazzo, R.; Po’, R.; Biagini, P.; Paci, B.; Generosi, A.; et al. Inclusion of Polysaccharides in Perovskite Thin Films: From in-Solution Interaction to Film Formation and Stability. Nanoscale Adv. 2025, 7, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Tavormina, F.; Quadrivi, E.; Biagini, P.; Po, R.; Marrazzo, R.; Loi, M.A.; Barba, L.; Masciocchi, N.; Guagliardi, A. Crystal Orientation, Strain, and Microstrain of Perovskite Films in a Complex Compositional Parameter Space. Chem. Mater. 2024, 36, 8880–8893. [Google Scholar] [CrossRef]
- Muscarella, L.A.; Hutter, E.M.; Sanchez, S.; Dieleman, C.D.; Savenije, T.J.; Hagfeldt, A.; Saliba, M.; Ehrler, B. Crystal Orientation and Grain Size: Do They Determine Optoelectronic Properties of MAPbI3 Perovskite? J. Phys. Chem. Lett. 2019, 10, 6010–6018. [Google Scholar] [CrossRef] [PubMed]
- Vanni, N.; Giuri, A.; Calora, M.; Podda, E.; Caricato, A.P.; Sparnacci, K.; Suhonen, R.; Ylikunnari, M.; Covarelli, A.; Gregori, L.; et al. Camphorsulfonic-Salified Chitosan Allowing MACl-Free Stabilization of Pure FAPbI3 α-Phase via Gravure Printing in Ambient Air. Solar RRL 2024, 8, 2400612. [Google Scholar] [CrossRef]
- Li, J.; Qiao, X.; He, B.; Zhang, Y.; Pal, S.; Sun, L.; Bilal, M.; Su, Z.; Gao, X.; Briscoe, J.; et al. Biomass-Derived Functional Additive for Highly Efficient and Stable Lead Halide Perovskite Solar Cells with Built-in Lead Immobilisation. Energy Environ. Sci. 2025, 18, 5632–5642. [Google Scholar] [CrossRef]
- He, J.; Xu, X.; Dai, Y.; Xue, D.; Zhang, P.; Niu, Q. Polysaccharide-Modified SnO2 for Highly Efficient Perovskite Solar Cells. Solar RRL 2024, 8, 2400080. [Google Scholar] [CrossRef]






| Material Type | Device Type | Function | PCE (%) | Cost | Stability | Sustainability | Refs. |
|---|---|---|---|---|---|---|---|
| Biomolecules | |||||||
| Pigments | DSSC, OSC, PSC | Sensitizers, Light absorber, Improves charge transport | ~2–18 | Low | Moderate (photo-degradable) | High | [51,52,53] |
| DNA | OSC, PSC | Template for layer organization, Charge transport | ~5–15 | Low–Moderate | High (sensitive to UV) | High (biocompatible, renewable) | [54,55,56] |
| Proteins | DSSC PSC OSC | Structural scaffold | ~0.34–14 | Low–Moderate | Low–Moderate | High | [57,58,59,60,61] |
| Polysaccharides | PSC OSC | Encapsulant | ~3–22 | High | High | Very High (biodegradable, abundant) | [62,63,64,65] |
| Tradizional Materials | |||||||
| Silicon | Si-PV | Light absorber | 20–27 | High | Very High (25+ years) | Low (energy-intensive fabrication) | [66] |
| Perovskite (e.g., MAPbI3) | PSC | Light absorber | 22–25 | Moderate | Moderate | Low | [67] |
| P3HT/PCBM | OSCs | Charge transport | ~3–5 | Moderate | Moderate (photochemical degradation possible) | Moderate | [68] |
| Ethylene vinyl acetate (EVA) | Si-PV PSCs | Encapsulant | 20–25 | Moderate | Moderate | Moderate | [69,70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fricano, A.; Tavormina, F.; Pignataro, B.; Vetri, V.; Ferrara, V. Progress and Prospects of Biomolecular Materials in Solar Photovoltaic Applications. Molecules 2025, 30, 3236. https://doi.org/10.3390/molecules30153236
Fricano A, Tavormina F, Pignataro B, Vetri V, Ferrara V. Progress and Prospects of Biomolecular Materials in Solar Photovoltaic Applications. Molecules. 2025; 30(15):3236. https://doi.org/10.3390/molecules30153236
Chicago/Turabian StyleFricano, Anna, Filippo Tavormina, Bruno Pignataro, Valeria Vetri, and Vittorio Ferrara. 2025. "Progress and Prospects of Biomolecular Materials in Solar Photovoltaic Applications" Molecules 30, no. 15: 3236. https://doi.org/10.3390/molecules30153236
APA StyleFricano, A., Tavormina, F., Pignataro, B., Vetri, V., & Ferrara, V. (2025). Progress and Prospects of Biomolecular Materials in Solar Photovoltaic Applications. Molecules, 30(15), 3236. https://doi.org/10.3390/molecules30153236








