Amino Acid Analysis and Cytotoxicity Study of Iraqi Ocimum basilicum Plant
Abstract
1. Introduction
2. Results
2.1. Amino Acid Detection
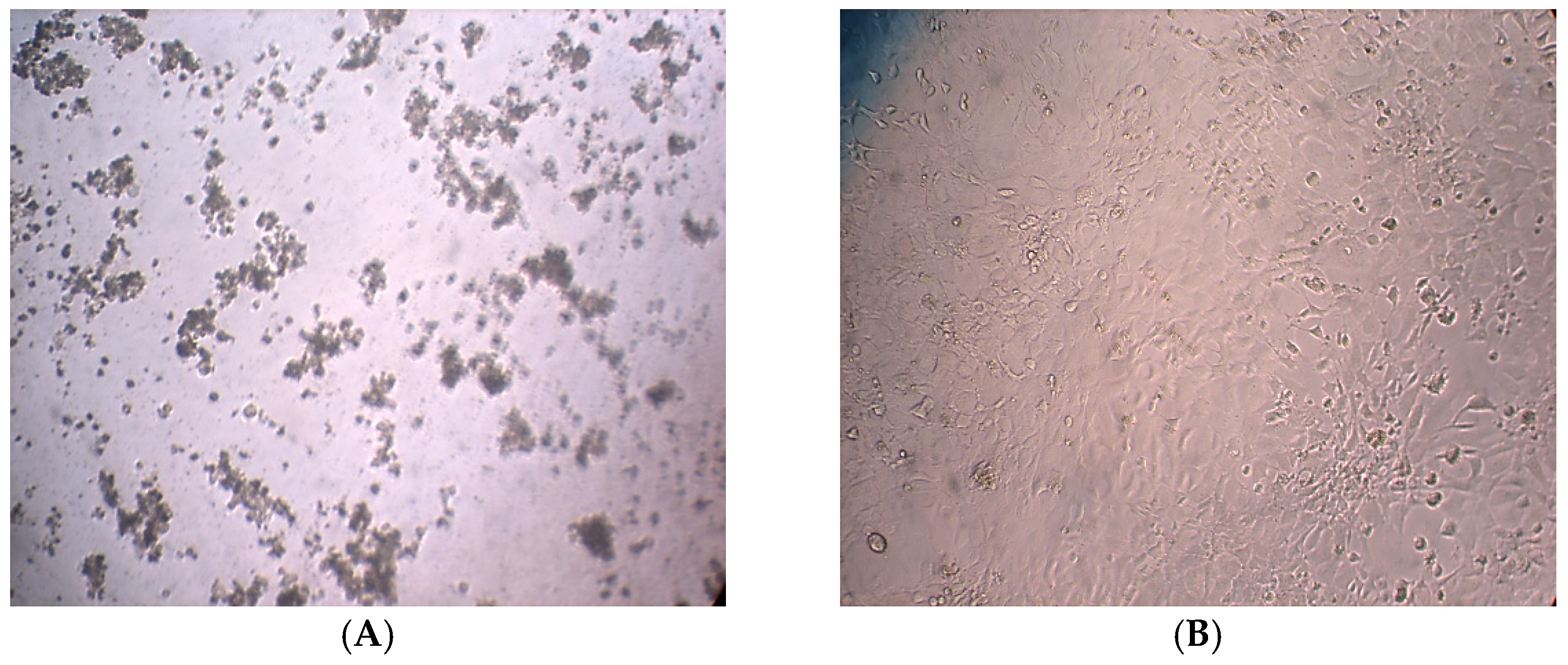
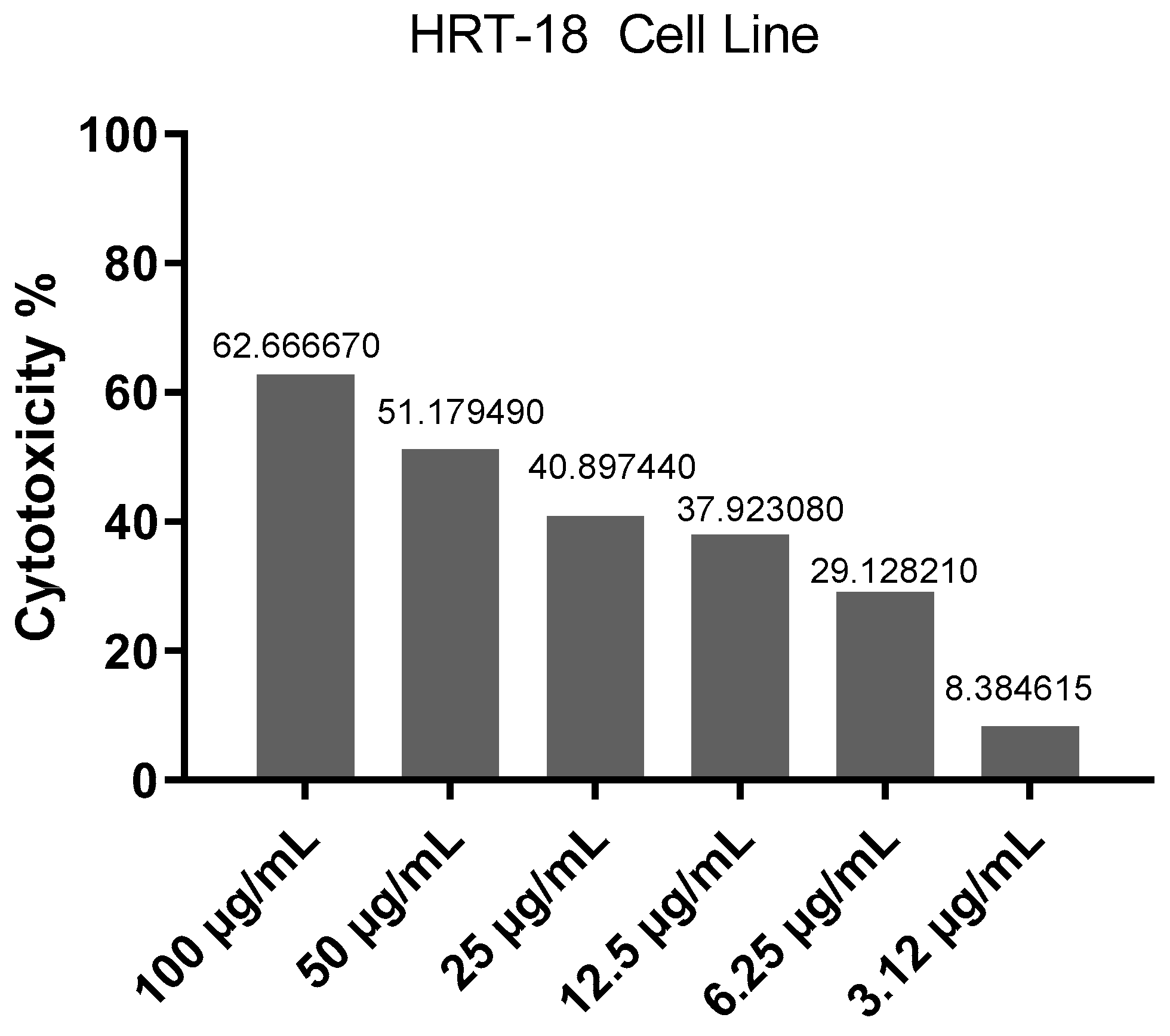
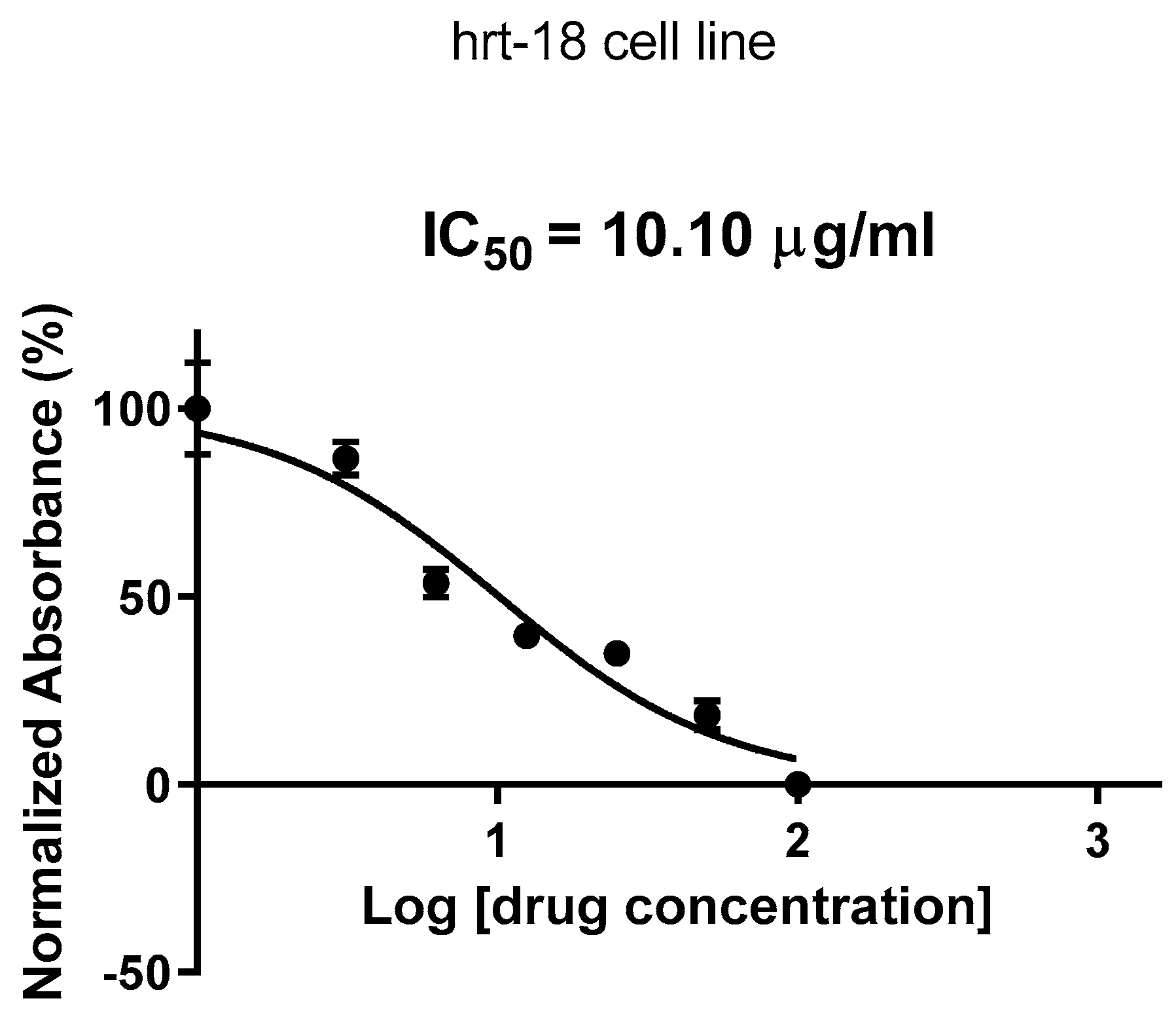
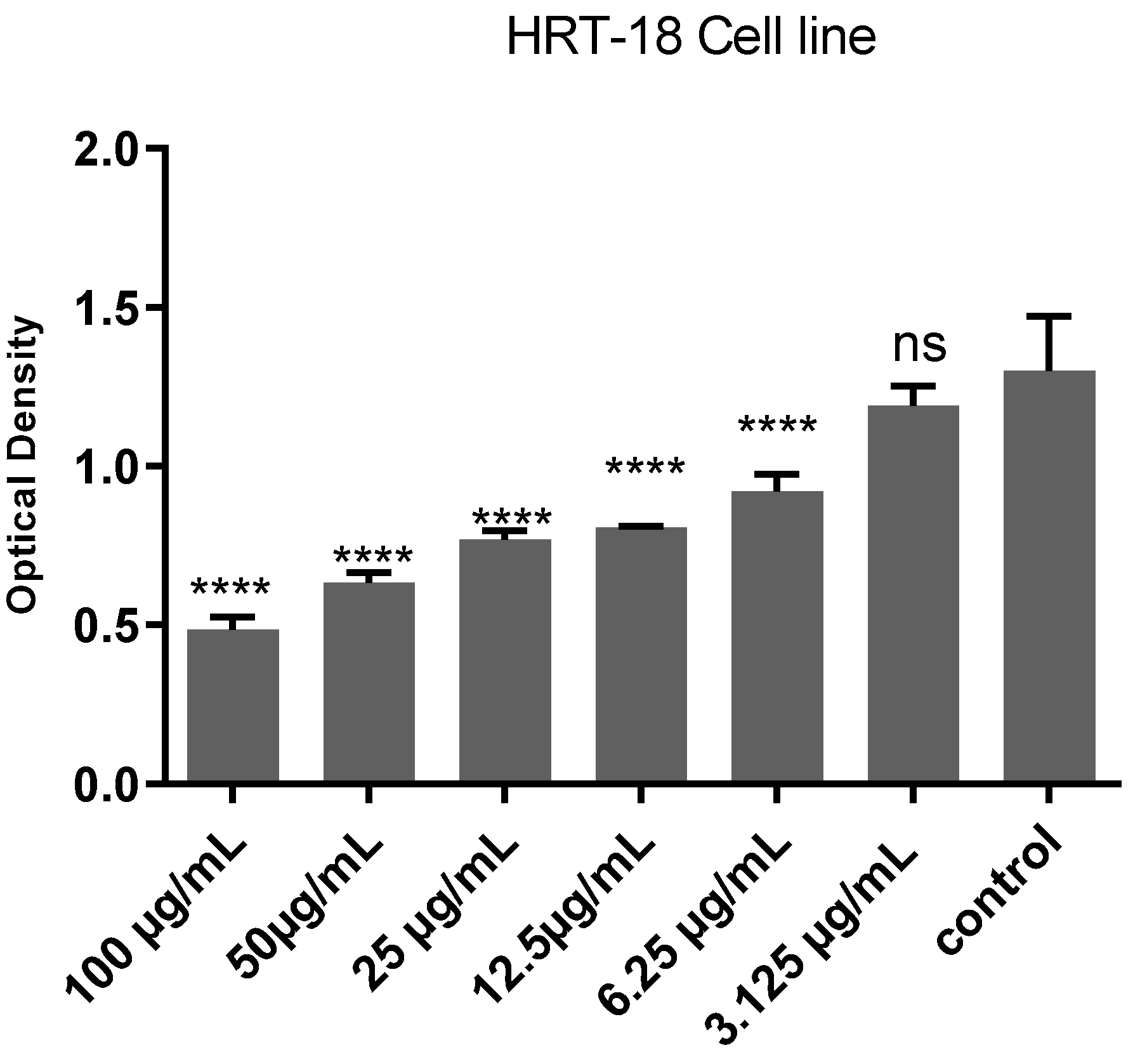
2.2. Cytotoxic Effects Evaluation
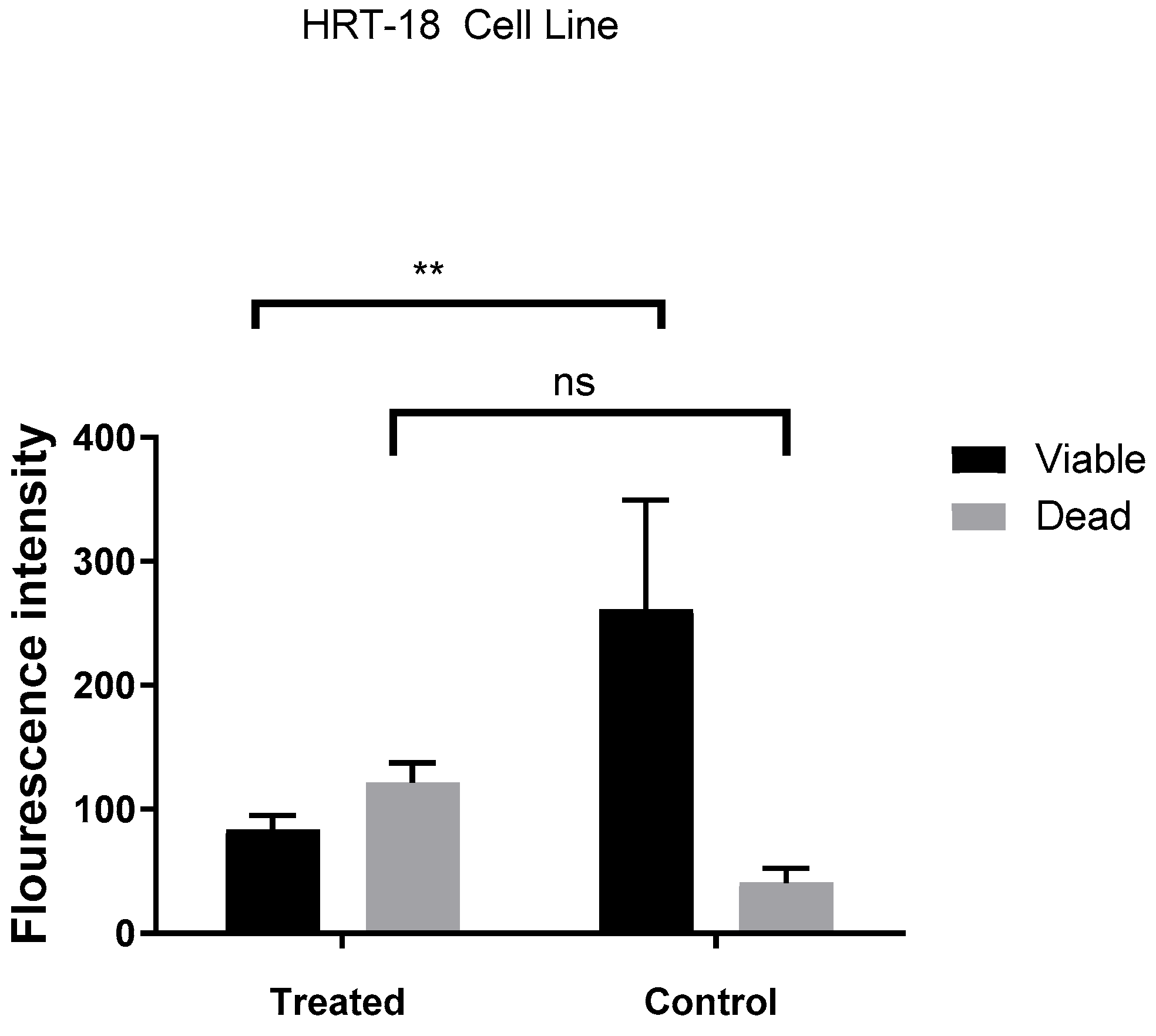
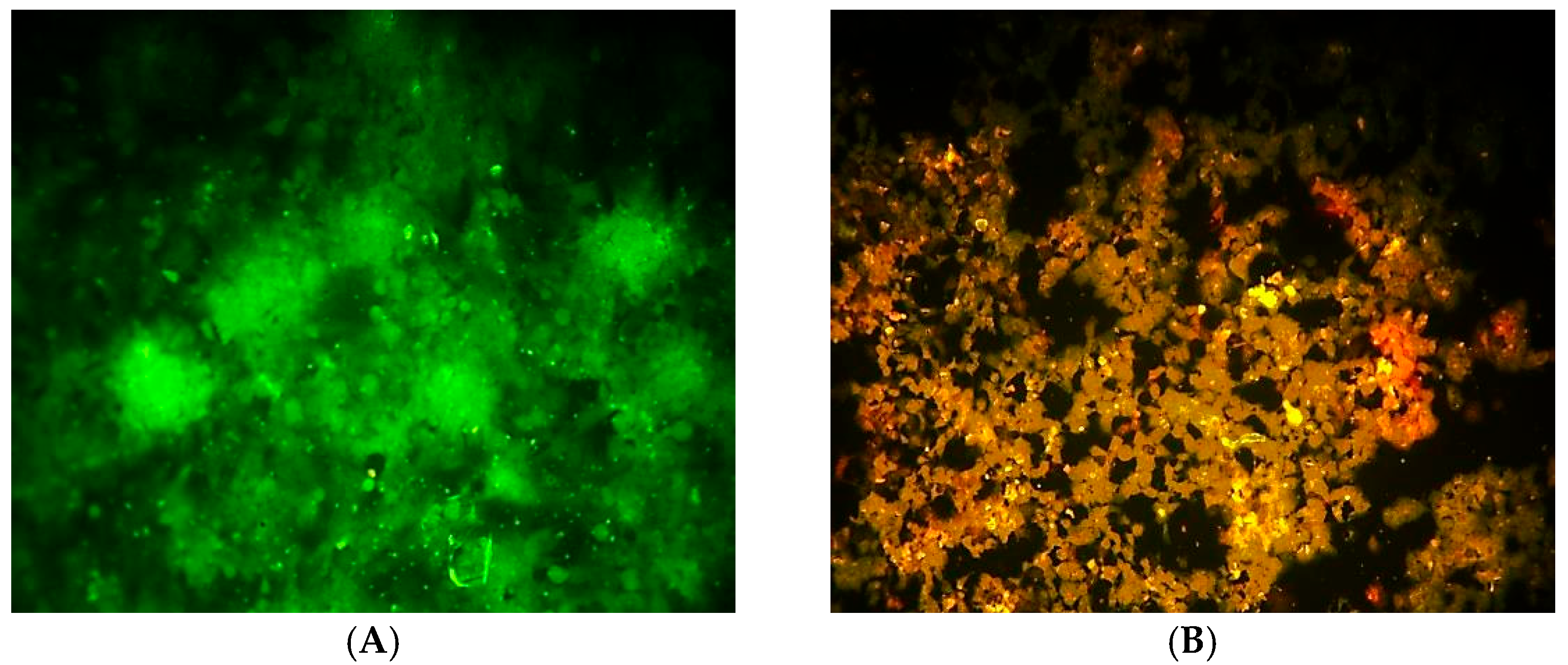
2.3. DNA Damage Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Work
4.2.1. Extraction Method (Cold Method)
4.2.2. Preliminary Phytochemical Examination of Crude Extracts
- Alkaloid test: approximately 0.5 to 0.6 g of each of plant extract and fractions were mixed in 8 mL of 1% HCl (Sigma-Aldrich, St. Louis, MO, USA), warmed, and filtered. Two ml of the filtrate was treated separately with both reagents (Mayer’s and Dragendorff‘s, Sigma-Aldrich, St. Louis, MO, USA), after which it was observed whether the alkaloids were present or absent in the turbidity or precipitate formation.
- Coumarins test: 0.5 g of each of plant extract and fractions were mixed in a test tube. The mouth of the tube was covered with filter paper treated with 1 N NaOH (Sigma-Aldrich, St. Louis, MO, USA) solution. The test tube was placed for a few minutes in boiling water, and then the filter paper was removed and examined under the UV light detector (Thermo Fisher Scientific, Waltham, MA, USA) for yellow fluorescence indicating the presence of coumarins.
- Terpenoids test (Salkowski test): 5 mL of each of plant extract and fractions were mixed in 2 mL of chloroform (Sigma-Aldrich, St. Louis, MO, USA) followed by the careful addition of 3 mL concentrated (H2SO4) (Sigma-Aldrich, St. Louis, MO, USA). A layer of reddish-brown coloration was formed at the interface, thus indicating a positive result for the presence of terpenoids.
- Flavonoids test: A total of 0.5 g of each of plant extract and fractions were shaken with petroleum ether (Sigma-Aldrich, St. Louis, MO, USA) to remove the fatty materials (lipid layer). The defatted residue was dissolved in 20 mL of 80% ethanol and filtered. The filtrate was used for the following tests:
- (a)
- Three mL of the filtrate was mixed with 4 mL of 1% aluminum chloride (Sigma-Aldrich, St. Louis, MO, USA) in methanol in a test tube, and the color was observed. The formation of a yellow color indicated the presence of flavonoids.
- (b)
- Three mL of the filtrate was mixed with 4 mL of 1% potassium hydroxide (Sigma-Aldrich, St. Louis, MO, USA) in a test tube, and the color was observed. A dark yellow color indicated the presence of flavonoids.
4.3. Amino Acid Determination
4.4. Cytotoxicity Assay
4.4.1. Cell Lines Used
4.4.2. MTT Cytotoxicity Assay
Cell Culture Conditions
MTT Procedure
4.5. DNA Damage
Apoptosis Estimation (Propidium Iodide/Acridine Orange Assay)
4.6. Statistical Analysis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Javanmardi, J.; Khalighi, A.; Kashi, A.; Bais, H.P.; Vivanco, J.M. Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. J. Agric. Food Chem. 2002, 50, 5878–5883. [Google Scholar] [CrossRef]
- Muralidharan, A.; Dhananjayan, R. Cardiac stimulant activity of Ocimum basilicum Linn. extracts. Indian J. Pharmacol. 2004, 36, 163–166. [Google Scholar]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Chang, X.; Alderson, P.G.; Wright, C.J. Variation in the essential oils in different leaves of Basil (Ocimum basilicum L.) at day time. Open Hortic. J. 2009, 2, 13–16. [Google Scholar] [CrossRef]
- Jayaweera, D.M.A. Medicinal Plants, (Indigenous and Exotic) Used in Ceylon; Part III; The National Science Foundation of Sri Lanka: Colombo, Sri Lanka, 1981; pp. 101–103. [Google Scholar]
- Bhatti, H.A.; Tehseen, Y.; Maryam, K.; Uroos, M.; Siddiqui, B.S.; Hameed, A.; Iqbal, J. Identification of new potent inhibitor of aldose reductase from Ocimum basilicum. Bioorg. Chem. 2017, 75, 62–70. [Google Scholar] [CrossRef]
- El-Dakar, A.Y.; Shalaby, S.M.; Nemetallah, B.R.; Saleh, N.E.; Sakr, E.M.; Toutou, M.M. Possibility of using basil (Ocimum basilicum) supplementation in Gilthead sea bream (Sparus aurata) diet. Egypt. J. Aquat. Res. 2015, 41, 203–210. [Google Scholar] [CrossRef]
- Dong, L.; He, J.; Luo, L.; Wang, K. Targeting the Interplay of Autophagy and ROS for Cancer Therapy: An Updated Overview on Phytochemicals. Pharmaceuticals 2023, 16, 92. [Google Scholar] [CrossRef]
- Devika, T.; Shashi, V. Pharmacognostical and phytochemical investigation of Tulsi plants available in Western Bareilly region. Glob. J. Pharmacol. 2016, 9, 60–63. [Google Scholar]
- Ahmed, A.S.; Fanokh, A.K.M.; Mahdi, M.A. Phytochemical Identification and Anti-Oxidant Study of Essential Oil Constituents of Ocimum basilicum L. Growing in Iraq. Pharmacogn. J. 2019, 11, 724–729. [Google Scholar] [CrossRef]
- Calderón Bravo, H.; Vera Céspedes, N.; Zura-Bravo, L.; Muñoz, L.A. Basil Seeds as a Novel Food, Source of Nutrients and Functional Ingredients with Beneficial Properties: A Review. Foods 2021, 10, 1467. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2015, 25, 41–59. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hauptmann, S.; Budianto, D.; Denkert, C.; Köbel, M.; Borsi, L.; Siri, A. Adhesion and migration of HRT-18 colorectal carcinoma cells on extracellular matrix components typical for the desmoplastic stroma of colorectal adenocarcinomas. Oncology 2003, 65, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Origin and Roles of Alanine and Glutamine in Gluconeogenesis in the Liver, Kidneys, and Small Intestine under Physiological and Pathological Conditions. Int. J. Mol. Sci. 2024, 25, 7037. [Google Scholar] [CrossRef] [PubMed]
- Dingledine, R.; McBain, C.J. Glutamate and Aspartate Are the Major Excitatory Transmitters in the Brain. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Siegel, G.J., Agranoff, B.W., Albers, R.W., Fisher, S.K., Uhler, M.D., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Chiang, F.-F.; Chao, T.-H.; Huang, S.-C.; Cheng, C.-H.; Tseng, Y.-Y.; Huang, Y.-C. Cysteine Regulates Oxidative Stress and Glutathione-Related Antioxidative Capacity before and after Colorectal Tumor Resection. Int. J. Mol. Sci. 2022, 23, 9581. [Google Scholar] [CrossRef]
- Kurhaluk, N.; Tkaczenko, H. L-Arginine and Nitric Oxide in Vascular Regulation—Experimental Findings in the Context of Blood Donation. Nutrients 2025, 17, 665. [Google Scholar] [CrossRef]
- Bakhtiar, Z.; Mirjalili, M.H.; Hassandokht, M. Nutritional composition of sweet basil (Ocimum basilicum L.) agro-ecotypic populations: Prospecting and selecting for use in food products. Food Humanit. 2024, 3, 100445. [Google Scholar] [CrossRef]
- Enegide, C.; Charles, C.O. Ocimum species: Ethnomedicinal uses, phytochemistry and pharmacological importance. Int. J. Curr. Res. Physiol. Pharmacol. (IJCRPP) 2021, 5, 1–12. [Google Scholar] [CrossRef]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Physician 2016, 8, 1832–1842. [Google Scholar] [CrossRef]
- Sivak, K.V.; Stosman, K.I.; Lesiovskaya, E.E. Bioactive compounds of medicinal plants with anti-herpes effect (part 1). Rastitelnye Resursy 2024, 60, 3–20. [Google Scholar] [CrossRef]
- Pattanayak, P.; Behera, P.; Das, D.; Panda, S.K. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn. Rev. 2010, 4, 95–105. [Google Scholar] [CrossRef]
- D’archivio, M.; Santangelo, C.; Scazzocchio, B.; Varì, R.; Filesi, C.; Masella, R.; Giovannini, C. Modulatory effects of polyphenols on apoptosis induction: Relevance for cancer prevention. Int. J. Mol. Sci. 2008, 9, 213–228. [Google Scholar] [CrossRef]
- Fitsiou, E.; Pappa, A. Anticancer Activity of Essential Oils and Other Extracts from Aromatic Plants Grown in Greece. Antioxidants 2019, 8, 290. [Google Scholar] [CrossRef]
- Perna, S.; Alawadhi, H.; Riva, A.; Allegrini, P.; Petrangolini, G.; Gasparri, C.; Alalwan, T.A.; Rondanelli, M. In Vitro and In Vivo Anticancer Activity of Basil (Ocimum spp.): Current Insights and Future Prospects. Cancers 2022, 14, 2375. [Google Scholar] [CrossRef]
- Miller, M.A.; Zachary, J.F. Mechanisms and Morphology of Cellular Injury, Adaptation, and Death. Pathol. Basis Vet. Dis. 2017, 2–43.e19. [Google Scholar] [CrossRef]
- Parekh, J.; Chanda, S. In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk. J. Biol. 2007, 31, 53–58. [Google Scholar]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Dahl-Lassen, R.; van Hecke, J.; Jørgensen, H.; Bukh, C.; Andersen, B.; Schjoerring, J.K. High-throughput analysis of amino acids in plant materials by single quadrupole mass spectrometry. Plant Methods 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Scriver, C.R.; Beaudet, A.L.; Valle, D.; Sly, W.S.; Childs, B.; Kinzler, K.W.; Vogelstein, B. (Eds.) The Metabolic and Molecular Bases of Inherited Disease, 8th ed.; McGraw-Hill, Inc.: New York, NY, USA, 2001; pp. 1665–2105. [Google Scholar]
- Salman, M.I.; Emran, M.A.; Al-Shammari, A.M. Spheroid-formation 3D engineering model assay for in vitro assessment and expansion of cancer cells. In Proceedings of the AIP Conference Proceedings, Yogyakarta, Indonesia, 10–11 November 2021; AIP Publishing: Melville, NY, USA, 2021; Volume 2372. No.1. [Google Scholar] [CrossRef]
- Al-Shammari, A.M.; Salman, M.I. Antimetastatic and antitumor activities of oncolytic NDV AMHA1 in a 3D culture model of breast cancer. Front. Mol. Biosci. 2024, 11, 1331369. [Google Scholar] [CrossRef]
- Salman, M.I.; Al-Shammari, A.M.; Emran, M.A. 3-Dimensional coculture of breast cancer cell lines with adipose tissue–Derived stem cells reveals the efficiency of oncolytic Newcastle disease virus infection via labeling technology. Front. Mol. Biosci. 2022, 9, 754100. [Google Scholar] [CrossRef] [PubMed]
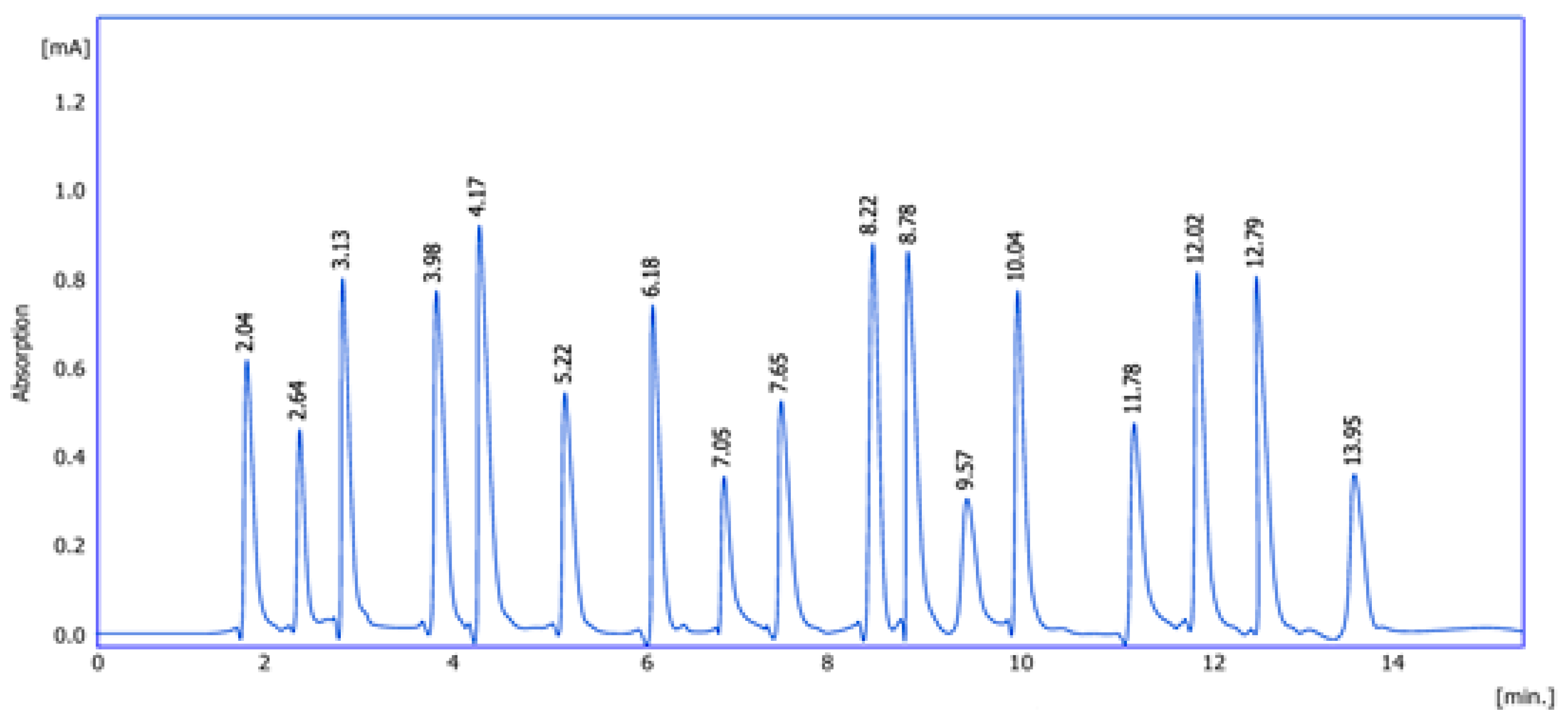
| No | Reten. Time [min] | Area [mAU·s] | Height [mAU] | Amount (g/100 g) | Calculation | Peak Type | Compound Name |
|---|---|---|---|---|---|---|---|
| 1 | 2.04 | 4562.9 | 604.7 | 17.45 | Calibration curve | Order | Aspartic acid |
| 2 | 2.64 | 4152.6 | 643.6 | 26.09 | Calibration curve | Order | Glycine |
| 3 | 3.13 | 6325.9 | 794.9 | 24.12 | Calibration curve | Order | Lysine |
| 4 | 3.98 | 5854.0 | 789.8 | 27.88 | Calibration curve | Order | Serine |
| 5 | 4.17 | 6214.5 | 835.7 | 23.65 | Calibration curve | Order | Threonine |
| 6 | 5.22 | 5062.6 | 594.4 | 23.54 | Calibration curve | Order | Isoleucine |
| 7 | 6.18 | 6541.8 | 781.1 | 29.08 | Calibration curve | Order | Alanine |
| 8 | 7.05 | 4562.6 | 386.5 | 35.65 | Calibration curve | Order | Valine |
| 9 | 7.65 | 4369.0 | 549.6 | 18.99 | Calibration curve | Order | Tyrosine |
| 10 | 8.22 | 7125.8 | 827.4 | 26.14 | Calibration curve | Order | Arginine |
| 11 | 8.78 | 10,325.6 | 812.7 | 24.15 | Calibration curve | Order | Cysteine |
| 12 | 9.57 | 6521.4 | 284.8 | 19.08 | Calibration curve | Order | Methionine |
| 13 | 10.04 | 10,568.9 | 741.9 | 21.65 | Calibration curve | Order | Proline |
| 14 | 11.78 | 8542.6 | 418.5 | 28.9 | Calibration curve | Order | Histidine |
| 15 | 12.02 | 6985.8 | 719.4 | 30.65 | Calibration curve | Order | Lucien |
| 16 | 12.79 | 11,256.6 | 712.1 | 27.44 | Calibration curve | Order | Glutamic acid |
| 17 | 13.95 | 3565.0 | 362.3 | 38.0 | Calibration curve | Order | Phenylalanine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, O.H. Amino Acid Analysis and Cytotoxicity Study of Iraqi Ocimum basilicum Plant. Molecules 2025, 30, 3232. https://doi.org/10.3390/molecules30153232
Ahmed OH. Amino Acid Analysis and Cytotoxicity Study of Iraqi Ocimum basilicum Plant. Molecules. 2025; 30(15):3232. https://doi.org/10.3390/molecules30153232
Chicago/Turabian StyleAhmed, Omar Hussein. 2025. "Amino Acid Analysis and Cytotoxicity Study of Iraqi Ocimum basilicum Plant" Molecules 30, no. 15: 3232. https://doi.org/10.3390/molecules30153232
APA StyleAhmed, O. H. (2025). Amino Acid Analysis and Cytotoxicity Study of Iraqi Ocimum basilicum Plant. Molecules, 30(15), 3232. https://doi.org/10.3390/molecules30153232






