Investigating the Effect of Hydrogen Bonding on the Viscosity of an Aqueous Methanol Solution Using Raman Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
2.1. The Effect of Hydrogen Bonds on C-O, C-H, and O-H Bonds
2.2. The Effect of Hydrogen Bond Interactions on the Viscosity of the Solution
3. Experimental Methods
3.1. Sample and Preparation
3.2. Experiment Apparatus
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.-Y.; Le, J.-B.; Wang, Y.-H.; Chen, S.; Yang, Z.-L.; Li, J.-F.; Cheng, J.; Tian, Z.-Q. In situ probing electrified interfacial water structures at atomically flat surfaces. Nat. Mater. 2019, 18, 697–701. [Google Scholar] [CrossRef]
- Lang, X.; Shi, L.; Zhao, Z.; Min, W. Probing the structure of water in individual living cells. Nat. Commun. 2024, 15, 5271. [Google Scholar] [CrossRef]
- Westall, F.; André, B. The Importance of Water for Life. Space Sci. Rev. 2018, 214, 50. [Google Scholar] [CrossRef]
- Mishra, A.; Mohapatra, H.; Pradhan, M. Study of the perturbed hydrogen-bonded water structure of the hydration shell of N-Methyl-Acetamide by Raman multivariate curve resolution spectroscopy. J. Mol. Liquids. 2024, 410, 125574. [Google Scholar] [CrossRef]
- Zhou, G.Y. A Quantum Chemistry Study of Hydrogen Bonds (1). J. Adv. Phys. Chem. 2015, 4, 84–101. [Google Scholar] [CrossRef]
- Zhou, G.Y. A Quantum Chemistry Study of Hydrogen Bonds (2). J. Adv. Phys. Chem. 2016, 5, 58–74. [Google Scholar] [CrossRef]
- Shiotari, A.; Sugimoto, Y. Characterization of two-and one-dimensional water networks on Ni (111) via atomic force microscopy. Phys. Rev. Mater. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Li, S.; Zhou, R.-Y.; Zheng, S.; Zhang, Y.-J.; Dong, J.-C.; Yang, Z.-L.; Pan, F.; Tian, Z.-Q.; Li, J.-F. In situ electrochemical Raman spectroscopy and ab initio molecular dynamics study of interfacial water on a single-crystal surface. Nat. Protoc. 2023, 18, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Nilson, A.; Pettersson, L.G.M. The structural origin of anomalous properties of liquid water. Nat. Commun. 2015, 6, 8998. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Liu, F.; Zhang, T.; Xu, W.; Zhang, Y.; Wu, N.; Ouyang, S. Study of microwave non-thermal effects on hydrogen bonding in water by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 1386–1425. [Google Scholar] [CrossRef] [PubMed]
- Iachen, L.; Yun, K.; Yongtao, M.; Xin, T.; Wei-Hsuan, H.; Xiao, Z.; Aowen, L.; Mingquan, X.; Wu, Z.; Ching-Shun, K.; et al. Electroreduction of CO2 to formate on copper based electrocatalyst at high pressures with high energy conversion efficiency. J. Am. Chem. Soc. 2020, 142, 7276–7282. [Google Scholar]
- Xue, X.; He, Z.-Z.; Liu, J. Detection of water-ice phase transition based on Raman spectrum. J. Raman Spectrosc. 2019, 44, 1045–1048. [Google Scholar] [CrossRef]
- Yang, J.; Dettori, R.; Nunes, J.P.F.; List, N.H.; Biasin, E.; Centurion, M.; Chen, Z.; Cordones, A.A.; Deponte, D.P.; Heinz, T.F.; et al. Direct observation of ultrafast hydrogen bond strengthening in liquid water. Nature 2001, 596, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Errington, J.R.; Debenedetti, P.G. Relationship between structural order and the anomalies of liquid water. Nature 2001, 409, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhao, H.; Ouyang, S. Understanding water structure from Raman spectra of isotopic substitution H2O/D2O up to 573 K. Phys. Chem. Chem. Phys. 2017, 19, 21540–21547. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Huang, B.; Wu, N.; Ouyang, S. Raman Spectroscopy for the Competition of Hydrogen Bonds in Ternary (H2O-THF-DMSO) Aqueous Solutions. Molecules 2019, 24, 3666. [Google Scholar] [CrossRef]
- Han, L.; Sun, Y.; Wang, S.; Su, T.; Cai, W.; Shao, X. Understanding the water structures by near-infrared and Raman spectroscopy. J. Raman Spectrosc. 2022, 53, 1686–1693. [Google Scholar] [CrossRef]
- Balos, V.; Kaliannan, N.K.; Elgabarty, H.; Wolf, M.; Kühne, T.D.; Sajadi, M. Time-resolved terahertz–Raman spectroscopy reveals that cations and anions distinctly modify intermolecular interactions of water. Nat. Chem. 2022, 14, 1031–1037. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zheng, S.; Yang, W.-M.; Zhou, R.-Y.; He, Q.-F.; Radjenovic, P.; Dong, J.-C.; Li, S.; Zheng, J.; Yang, Z.-L.; et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 2021, 600, 81. [Google Scholar] [CrossRef]
- Yang, B.; Cao, X.; Lang, H.; Wang, S.; Sun, C. Study on hydrogen bonding network in aqueous methanol solution by Raman spectroscopy. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2020, 225, 117488. [Google Scholar] [CrossRef]
- Ebukuro, T.; Takami, A.; Oshima, Y.; Koda, S. Raman spectroscopic studies on hydrogen bonding in methanol and methanol/water mixtures under high temperature and pressure. J. Supercrit. Fluids 1999, 15, 73–78. [Google Scholar] [CrossRef]
- Liu, H.; Chen, G.; Gu, J.; Zhu, C.; Song, X.; Zhang, X. Raman spectroscopic analysis and fast identification of several saturated monohydroxy alcohols. Spectrosc. Lett. 2017, 50, 347–351. [Google Scholar] [CrossRef]
- Ouyang, S.; Zhang, M.; Hu, Q.; Wei, H. Hydrogen Bonding Effect on the Surface Tension and Viscosity of DMSO Aqueous Solutions Studied by Raman Spectroscopy. Spectrosc. Spectr. Anal. 2018, 38, 2778–2781. [Google Scholar]
- Hsin, W.L.; Sheng, Y.-J.; Lin, S.-Y.; Tsao, H.-K. Surface tension increment due to solute addition. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2004, 69, 031605. [Google Scholar] [CrossRef] [PubMed]
- David, R.; Neumann, A.W. A Theory for the Surface Tensions and Contact Angles of Hydrogen Bonding Liquids. Langmuir 2014, 30, 11634–11639. [Google Scholar] [CrossRef]
- Dixit, S.; Poon, W.C.K.; Crain, J. Hydration of methanol in aqueous solutions: A Raman spectroscopic study. J. Phys. Condens. Matter 2000, 12, 323–328. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. Raman Intensities and Detection Limits of Geochemically Relevant Gas Mixtures for a Laser Raman Microprobe. Anal. Chem. 1987, 59, 2165–2170. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q. The Quantitative Analysis of Raman Spectroscopy to Suifate Ion in Aqueous Solution. Spectrosc. Spectr. Anal. 2016, 36, 430–435. [Google Scholar]
- Ouyang, S.; Zhou, M.; Cao, B.; Lu, G. Raman Spectral Analysis of the Anti-freezing Mechanism of Dimethyl Sulfoxide. Chem. J. Chin. Univ. 2008, 29, 2055–2058. [Google Scholar]
- Zou, Q.; Du, X.-Y.; Zhang, C.; Li, X.-C.; Li, Y. Vibrational Assignment Analysis of Raman Spectra of Fatty Alcohols. Spectrosc. Spectr. Anal. 2013, 33, 106–110. [Google Scholar]
- Weng, S. Fourier Transform Infrared Spectral Analysis; Chemical Industry Press: Beijing, China, 2016; pp. 6–8. [Google Scholar]
- Mir, J.M.; Itoo, F.A. Experimental-DFT interface of hydrogen bonding description of 1:10 methanol-water solution. J. Mol. Liq. 2017, 247, 1–5. [Google Scholar] [CrossRef]
- Bakó, I.; Megyes, T.; Bálint, S.; Grósz, T.; Chihaia, V. Water-methanol mixtures: Topology of hydrogen bonded network. Phys. Chem. Chem. Phys. 2008, 10, 5004–5011. [Google Scholar] [CrossRef]
- Dixit, S.; Crain, J.; Poon, W.; Finney, J.L.; Soper, A.K. Molecular segregation observed in a concentrated alcohol-water solution. Nature 2002, 6838, 829–832. [Google Scholar] [CrossRef]
- Matisz, G.; Kelterer, A.M.; Fabian, W.M.F.; Kunsági-Máté, S. Structural properties of methanol-water binary mixtures within the quantum cluster equilibrium model. Phys. Chem. Chem. Phys. 2015, 17, 8467–8479. [Google Scholar] [CrossRef]
- Laaksonen, A.; Kusalik, P.G.; Svishchev, I.M. Three-dimensional structure in water- methanol mixtures. J. Phys. Chem. A 1997, 101, 5910–5918. [Google Scholar] [CrossRef]
- Tomza, P.; Wrzeszcz, W.; Mazurek, S.; Szostak, R.; Czarnecki, M.A. Microheterogeneity in binary mixtures of water with CH3OH and CD3OH: ATR-IR spectroscopic, chemometric and DFT studies. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2018, 197, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Tinker, D.; Lesher, C.E.; Baxter, G.M.; Uchida, T.; Wang, Y. High-pressure viscometry of polymerized silicate melts and limitations of the Eyring equation. Am. Mineral. 2004, 89, 1701–1708. [Google Scholar] [CrossRef]
- Vogel, H.; Weiss, A. Transport Properties of Liquids, III. Viscosity of Athermal Liquid Mixtures. Ztschrift Fr. Elektrochem. Berichte Der Bunsenges. Fr. Phys. Chem. 2010, 86, 193–198. [Google Scholar] [CrossRef]
- He, Y.; Xu, X.; Yang, L.; Ding, B. Viscosity modeling for ionic liquid solutions by Eyring-Wilson equation. Chem. Ind. Chem. Eng. Q. 2012, 18, 441–447. [Google Scholar] [CrossRef]
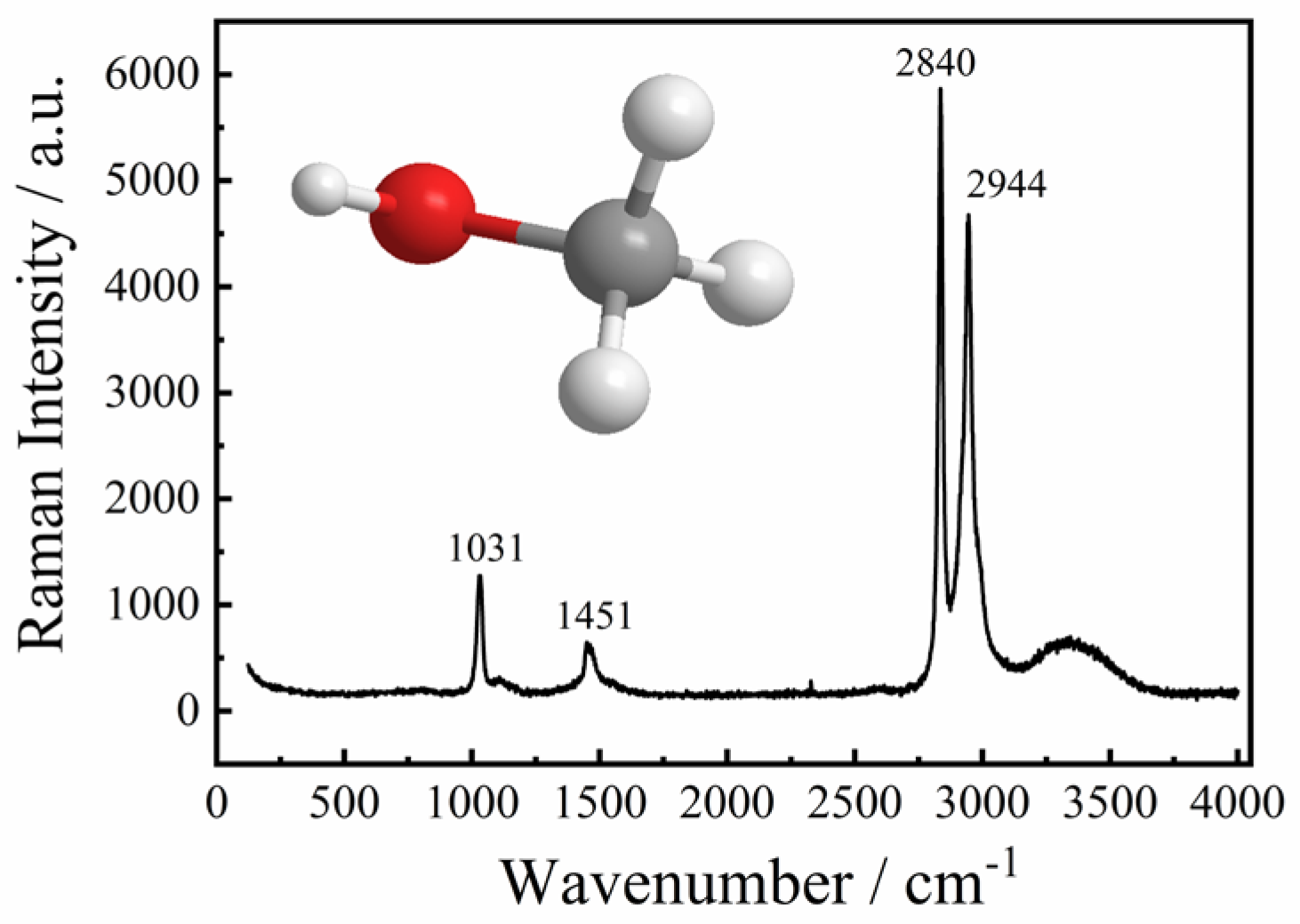
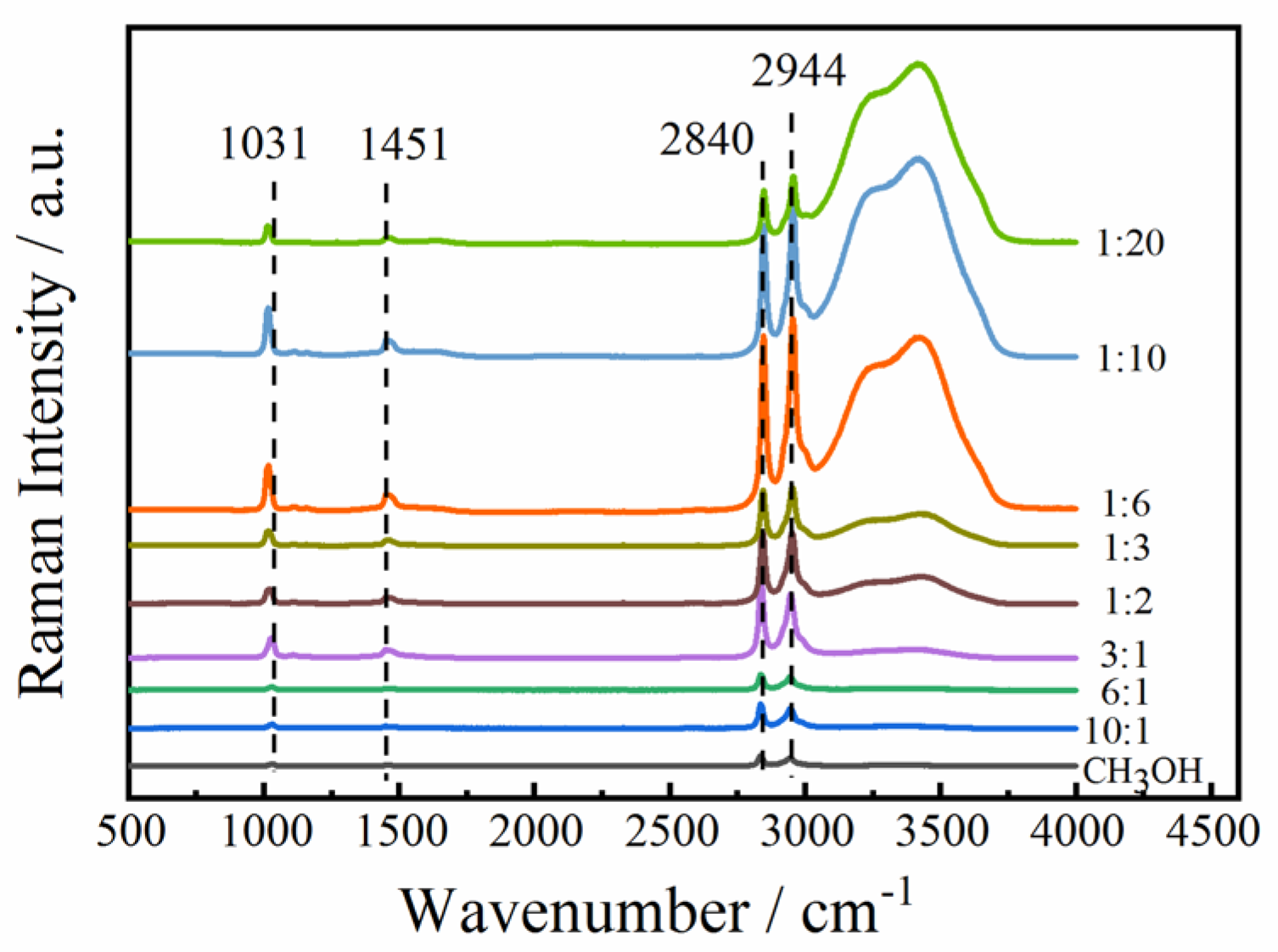
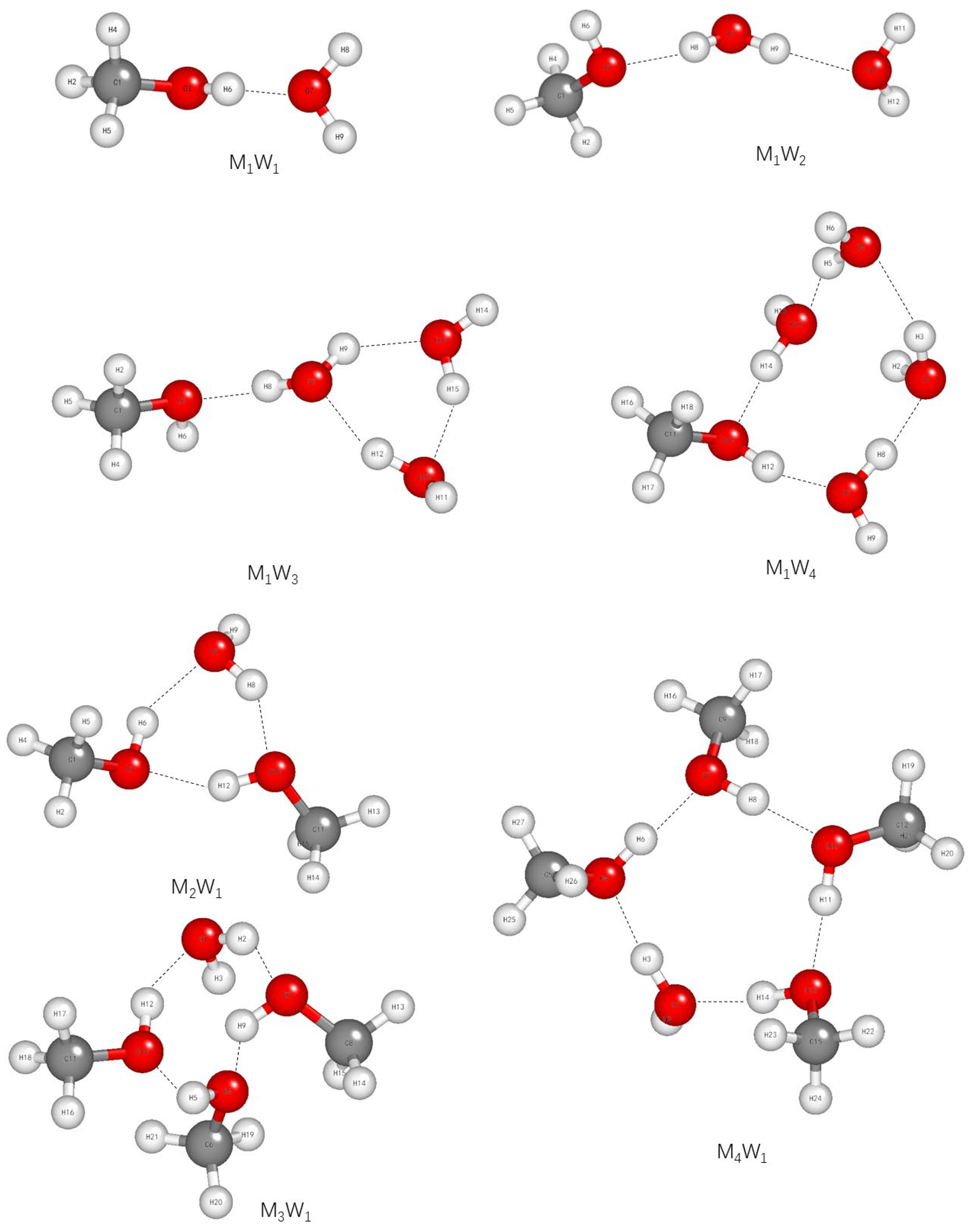

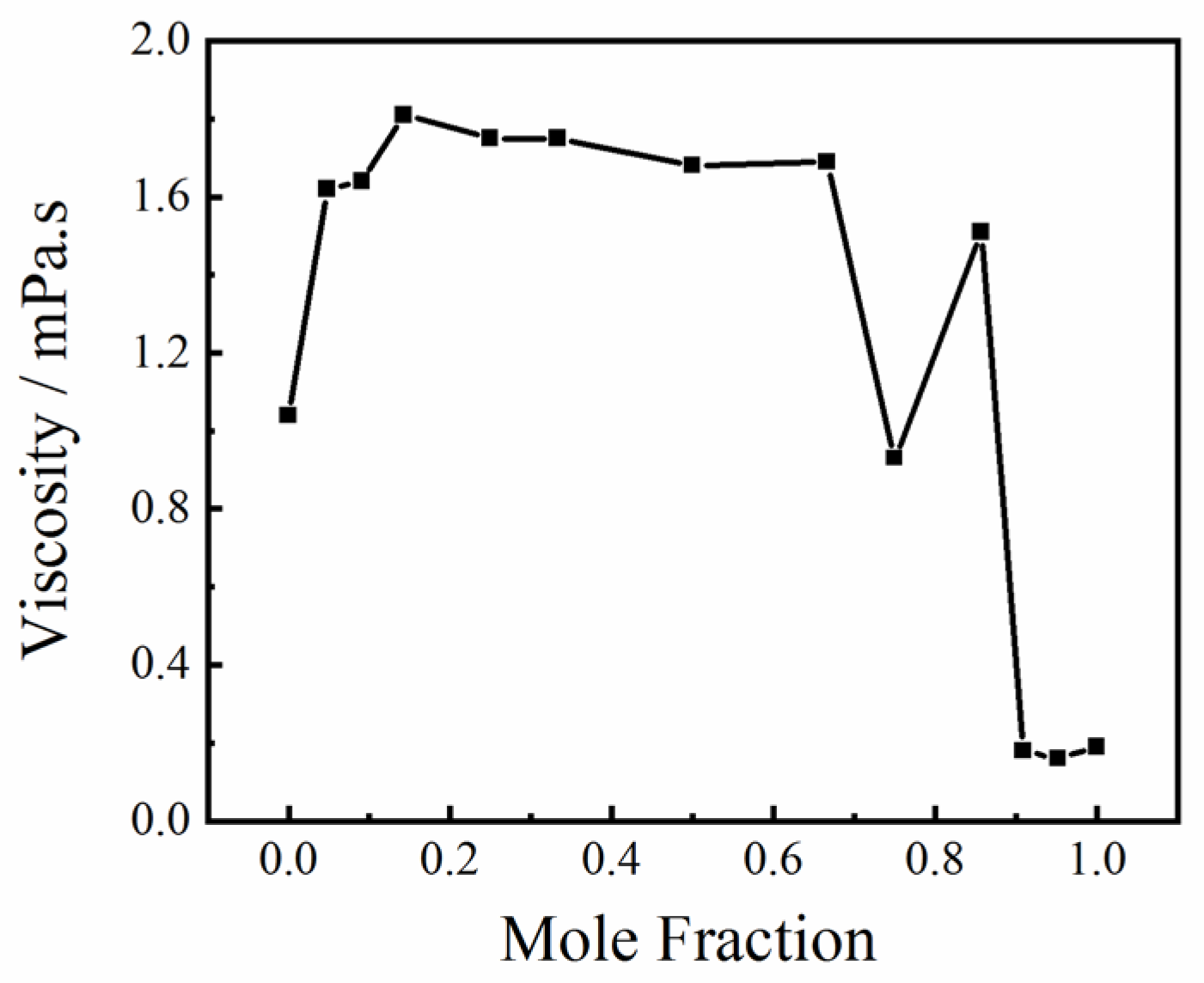
| Serial Number | Wavenumber, m−1 | Assignments | Ref. |
|---|---|---|---|
| 1 | 1031 | C-O stretching vibration | [20,24,25] |
| 2 | 1451 | CH3 asymmetrical deformation vibration | [20,24,25] |
| 3 | 2840 | CH2 symmetrical stretching vibration | [20,24,25] |
| 4 | 2944 | CH3 asymmetrical deformation vibration | [20,24,25] |
| 5 | 3100–3800 | OH stretching vibration | [20,24,25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.-N.; Liu, F.; Li, Z.; Qiu, Z.; Li, X.; Huang, J.; Li, B.; Qiu, J.; Ouyang, S.-L. Investigating the Effect of Hydrogen Bonding on the Viscosity of an Aqueous Methanol Solution Using Raman Spectroscopy. Molecules 2025, 30, 3204. https://doi.org/10.3390/molecules30153204
Wu N-N, Liu F, Li Z, Qiu Z, Li X, Huang J, Li B, Qiu J, Ouyang S-L. Investigating the Effect of Hydrogen Bonding on the Viscosity of an Aqueous Methanol Solution Using Raman Spectroscopy. Molecules. 2025; 30(15):3204. https://doi.org/10.3390/molecules30153204
Chicago/Turabian StyleWu, Nan-Nan, Fang Liu, Zonghang Li, Ziyun Qiu, Xiaofan Li, Junhui Huang, Bohan Li, Junxi Qiu, and Shun-Li Ouyang. 2025. "Investigating the Effect of Hydrogen Bonding on the Viscosity of an Aqueous Methanol Solution Using Raman Spectroscopy" Molecules 30, no. 15: 3204. https://doi.org/10.3390/molecules30153204
APA StyleWu, N.-N., Liu, F., Li, Z., Qiu, Z., Li, X., Huang, J., Li, B., Qiu, J., & Ouyang, S.-L. (2025). Investigating the Effect of Hydrogen Bonding on the Viscosity of an Aqueous Methanol Solution Using Raman Spectroscopy. Molecules, 30(15), 3204. https://doi.org/10.3390/molecules30153204





