Degradation of Emerging Plastic Pollutants from Aquatic Environments Using TiO2 and Their Composites in Visible Light Photocatalysis
Abstract
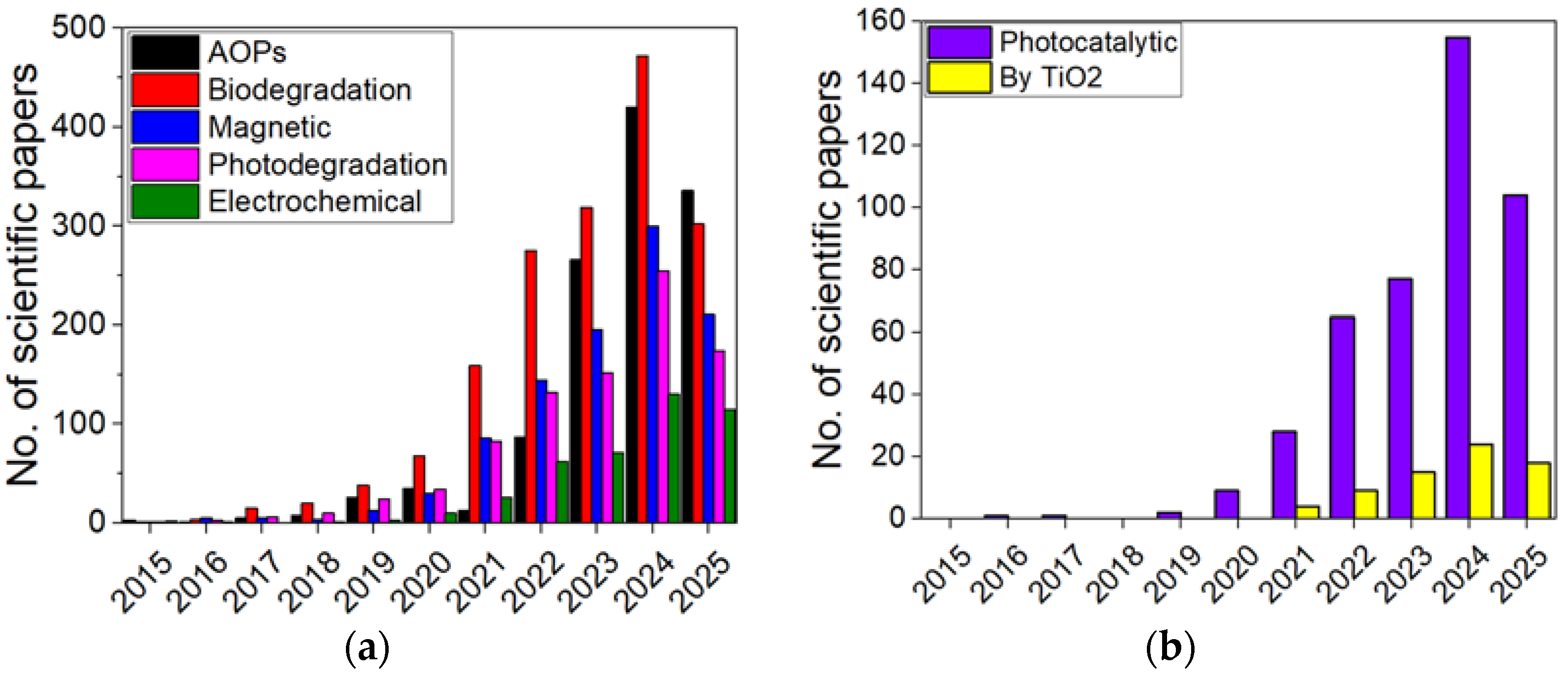
1. Introduction
2. Characteristics of TiO2 and Their Composites for the Photocatalytic Degradation of MPs and NPs
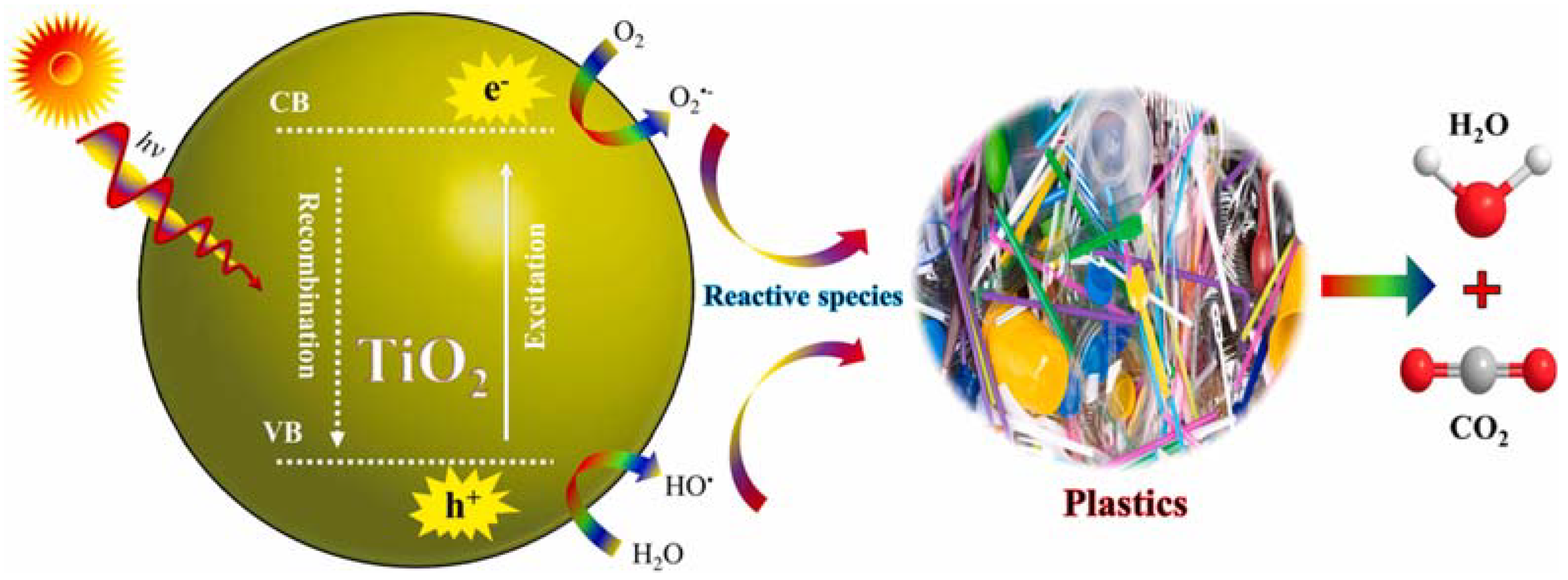
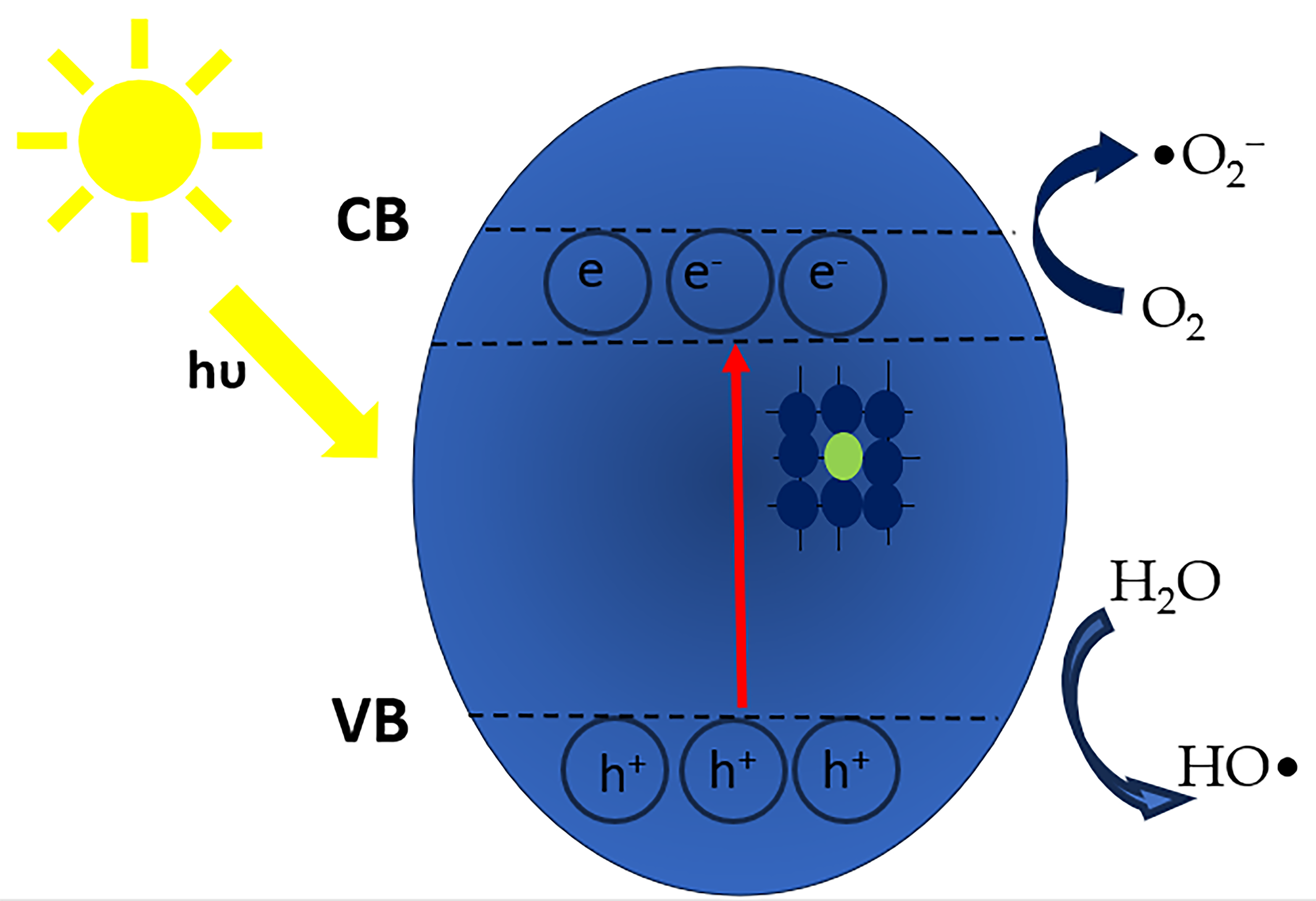
2.1. Principle of Photocatalysis
2.2. Strategies for the Photocatalytic Degradation in Visible Light
2.3. Synthesis of TiO2-Based Photocatalysts
| Semiconductor | Method | Main Characteristics | Plastic Pollutant/Solution for Degrading | Photocatalysis Conditions | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| TiO2 NPs | Sol–gel |
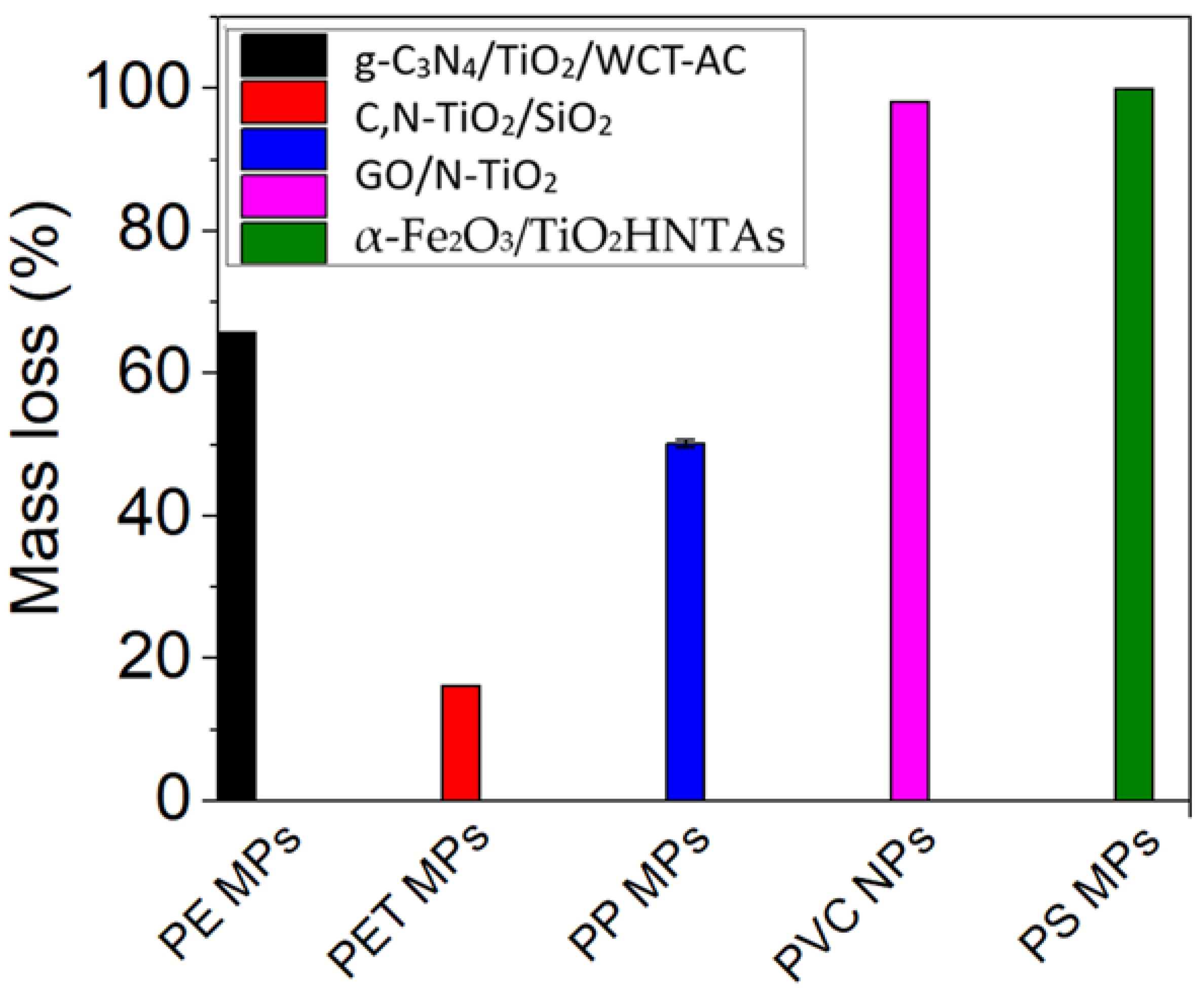
Bandgap of 2.93 eV. Size dimension ranged from 97.93 ± 145.79 nm (SEM). Anatase phase (XRD). | PP MPs/ A ratio of 1:1 between MPs to photocatalyst. This solution was added into buffer (pH∼3, 0.4% w/v dispersion of components) | Solar irradiation (pH 3 and 50 h); average light intensity of 5 kWh/m2 | Mass loss of 50.5 ± 0.5% | [91] |
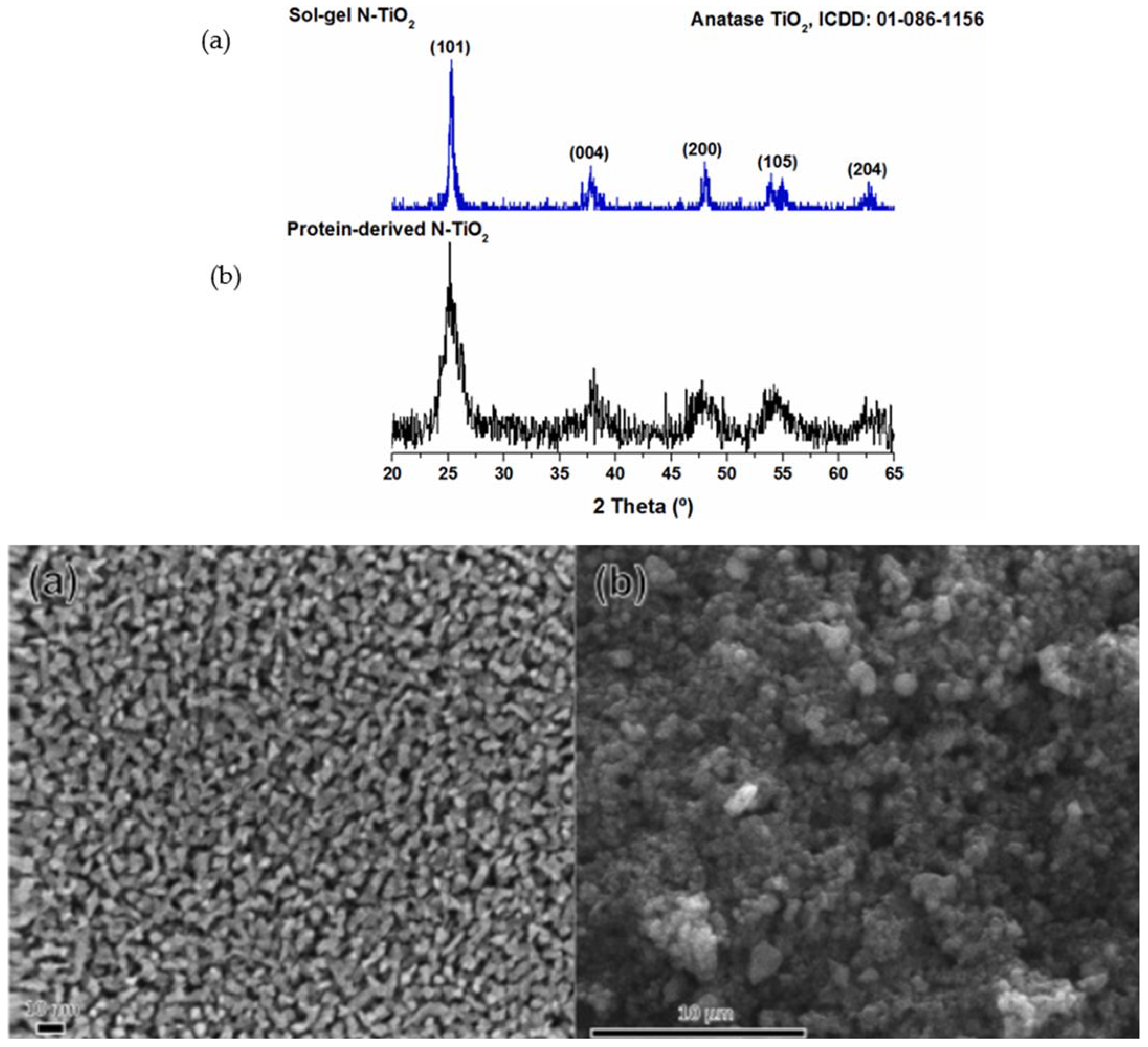
| N-TiO2 | I. Green synthesis; II. Sol–gel method | Protein-derived TiO2 powder was more amorphous than the sol–gel synthesized N-TiO2 (XRD). The diameters were in the range of 220–920 nm (FE-SEM). |
HDPE MPs/ 2 mg/mL of the extracted MPs in distilled water | Room temperature for 20 h, with samples placed at 120 mm distance of a 27 W fluorescent lamp after 8 h of irradiation with constant light emissions in the visible spectrum (400–800 nm) | Mass loss of 6.4% in aqueous environment | [89] |
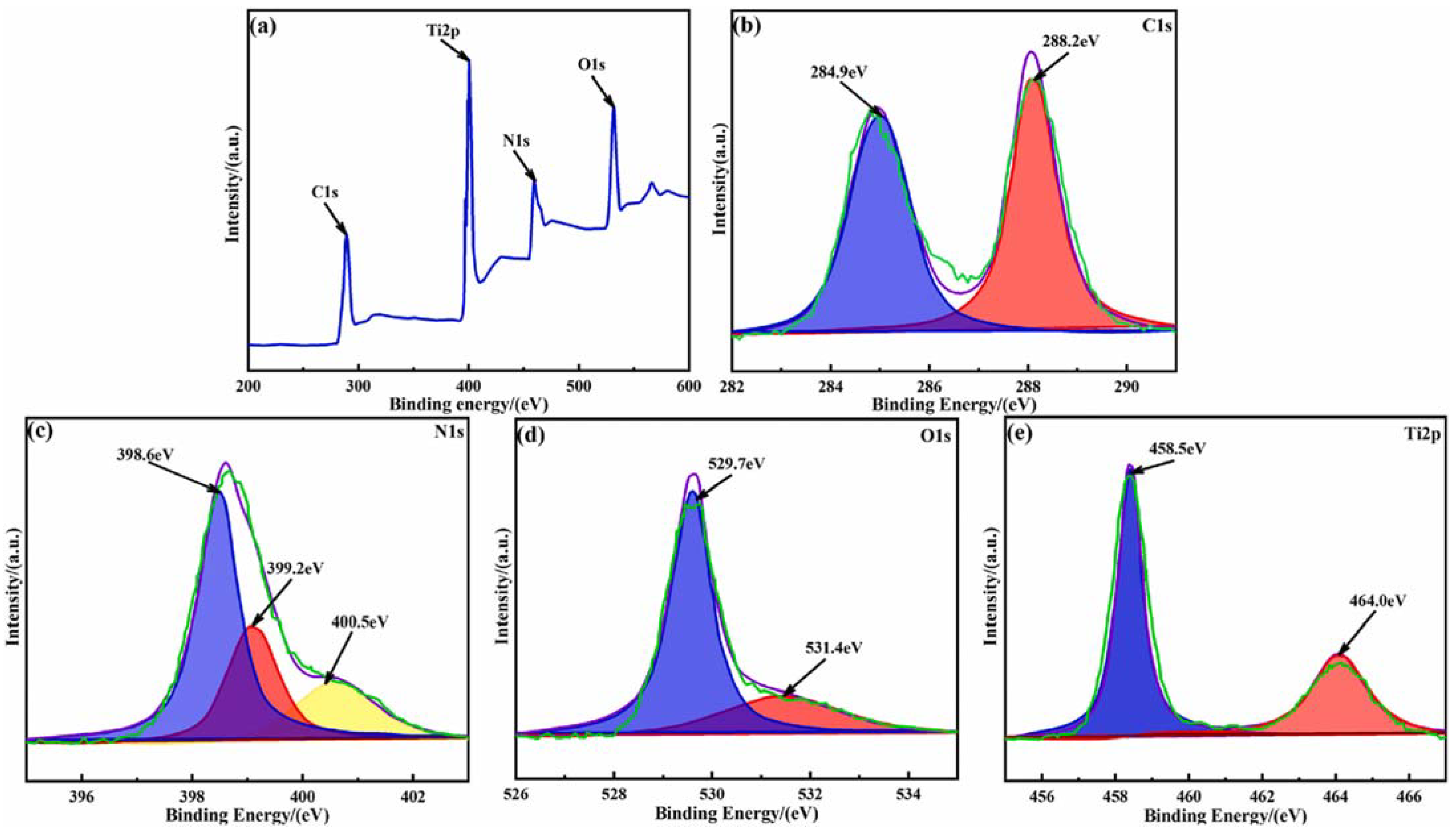
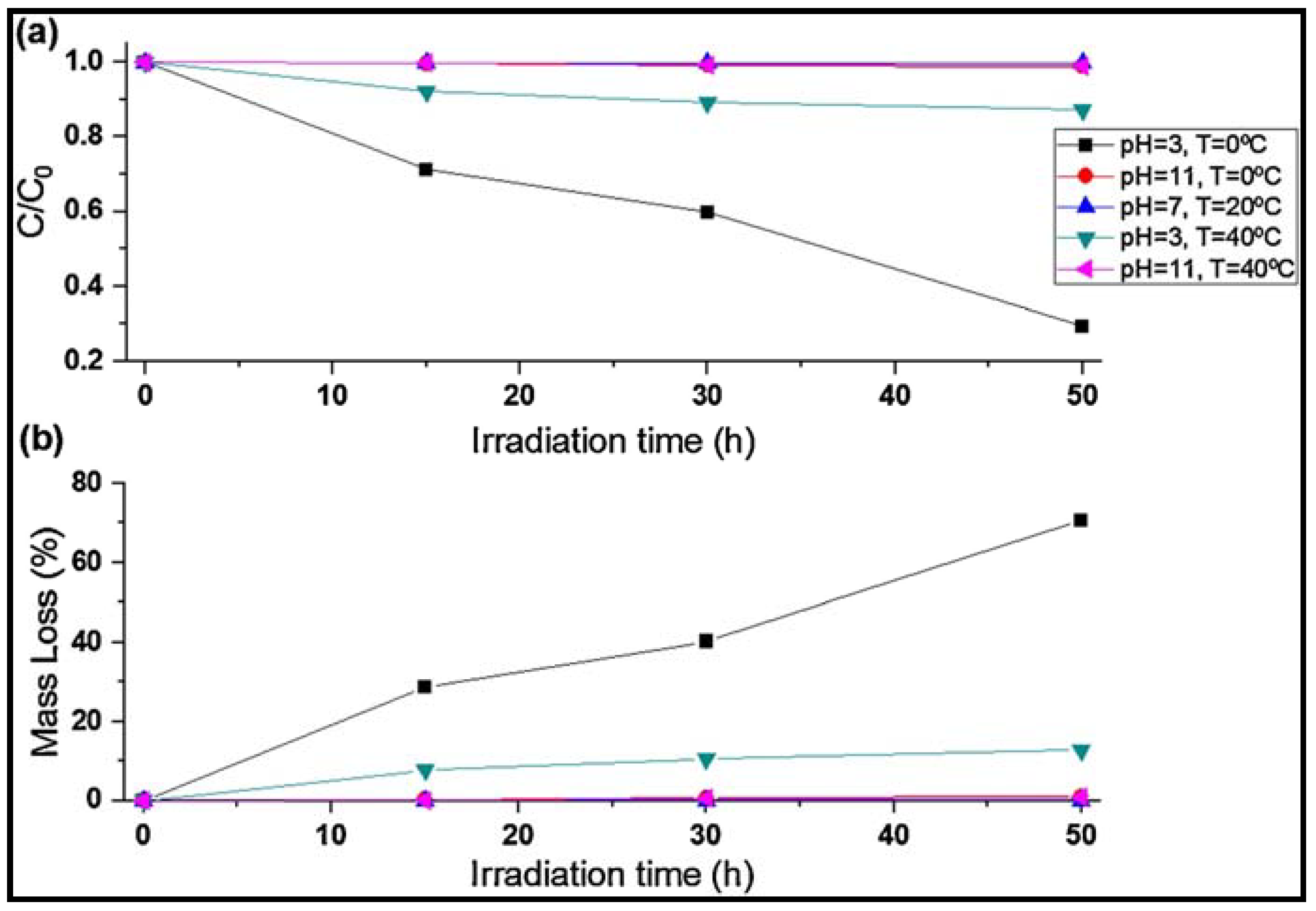
| C,N-TiO2 | Sol–gel; Solvothermal | Crystallite size of 4.92 nm. Eg of 2.9 eV. Surface area of 219.42 ± 1.82 m2/g (FEG-SEM micrographs). | Primary HDPE MPs | Irradiation at 428 nm, photocatalysis time of 50 h with continuous stirring at 300 rpm; 50 W LED lamp; absorbance at 428 nm; pH 3, 7 and 11, and temperature of 0, 20 and 40 ± 2 °C | Mass loss of 71.77 ± 1.88% at pH 3 and 0 °C | [38] |
| GO/N-TiO2 composites (at three different ratios 1:3, 1:1, and 3:1 w/w) | I. Sol–gel method for N-TiO2; II. Ultrasonication technique for composite preparation | Decrease in crystallite size from 13.69 nm (TiO2) to 6.13 nm, 4.35 nm, and 3.13 nm for composites (XRD). Eg in the range of 2.1–2.8 eV, 2.4–2.9 eV, and 2.6 eV for those three composites. Thermal stability increased with the N-TiO2 content (TGA). | PVC NPs/ 0.4 mg/mL concentration of catalyst in aqueous solution of PVC-NPs |
50 mL glass beaker;
natural room light conditions ( tungsten bulb and room light) at 446 nm; pH of 4, 7, and 10; irradiation durations of 30, 60, 90, 120, 150, and 180 min | 98.2% removal efficiency at pH 4 for 1:3 the ratio between components | [27] |
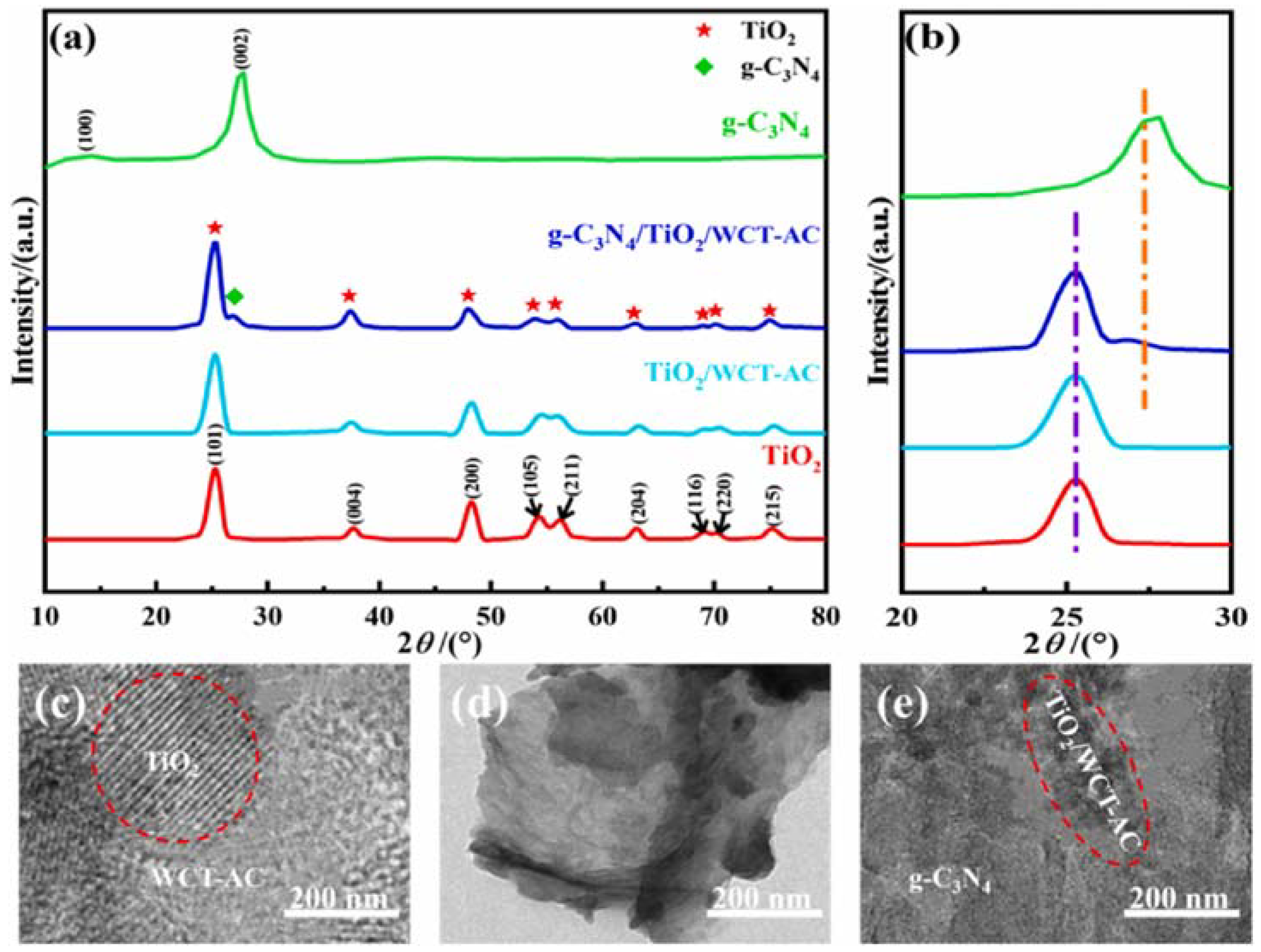
| g-C3N4/TiO2/waste cotton-based activated carbon (WCT-AC) composite | I. Sol–gel method for TiO2/WCT-AC; II. High-temperature thermal polymerization method from composite preparation |
Eg of the TiO2, g-C3N4, TiO2/WCT-AC and g-C3N4/TiO2/WCT-AC were 3.12, 2.65, 3.06 and 2.54 eV, respectively. | PE MPs/ 50 mg catalyst | VIS light irradiation provided by a 500 W xenon lamp light source (λ > 420 nm); photocatalysis time of 200 h, and system pH of 7.0; initial light intensity of 200 mW/cm2 and system temperature of 25 °C | Mass loss of 67.58% | [90] |
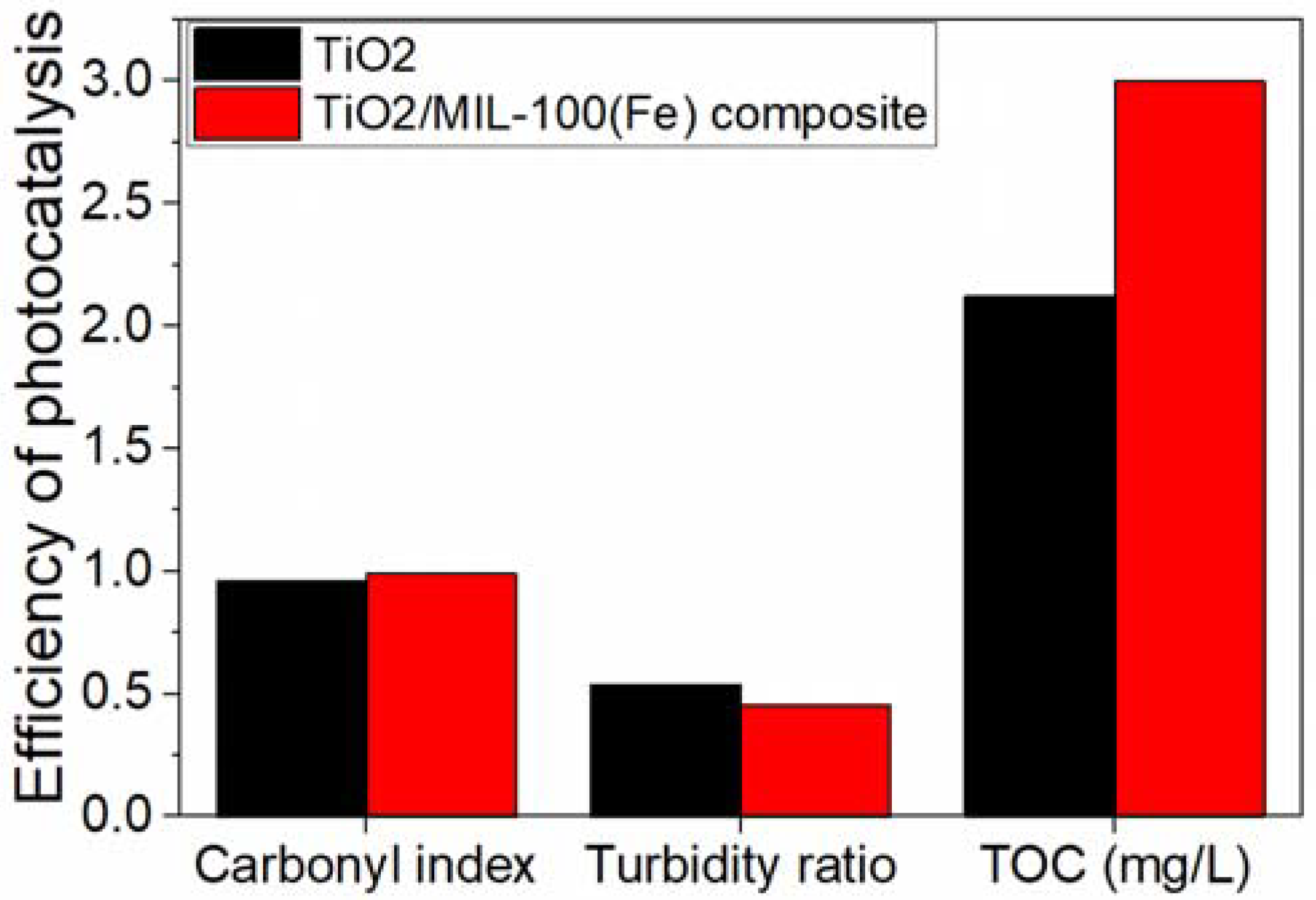
| TiO2/MIL-100(Fe) composites | Solvothermal/microwave methods and post-annealing technique. The mixtures were heated for 12 h at 180 °C in a 100 mL Teflon-lined stainless-steel autoclave; calcined in an air muffle furnace at 350 °C for 2 h | Spherical NPs (SEM). The particle sizes increased from 29 ± 6 nm (TiO2) to 54 ± 15 nm (SEM). TiO2 crystallite size slightly decreased from 4.0 to 3.0 nm (XRD). Eg of 2.21–2.65 eV (Tauc plots) compared with 3.03 for TiO2. BET of 179.0 m2/g compared with 128.2 m2/g for TiO2. |
PET NPs/ 0.1 mg/mL PET NPs in water suspension (pre-sonicated for 30 min, pH 3) and 0.125 g/L photocatalyst | A 200 mL batch reactor under simulated sunlight (Xe lamp 300–800 nm) at an intensity of 30 W/m2 (5 h reaction time) |
Increased CI (0.99); Reduction in the turbidity ratio (0.454); Increased TOC released (3.00 mg/L); Cavities in the NPs structure (SEM) | [92] |
| HKUST-1(Cu/Fe)-derived CuO/TiO2 (TCFH) composites containing 5, 10, and 15 wt % of MOF | Solvothermal method; Temperature of 180 °C for 18 h with a heating ramp of 5 °C min−1; calcined at 350 °C for 2 h | Crystallite sizes for: TCFH 95:5–53.0 nm. TCFH 90:10–54.2. TCFH 85:15–58.5 (XRD). Specific surface area for: TCFH 95:5–168.54 m2/g. TCFH 90:10–158.08 m2/g. TCFH 85:15–161.95 m2/g, compared with 152.09 m2/g for TiO2 (BET). | Nylon 6 MPs/ 0.2 mg/mL of nylon 6 MPs suspension |
Stirring at 250 rpm and irradiated with a UV–vis Hg lamp (350–700 nm, 32.3 W/m2) positioned at 9 cm; ambient temperature for 5 h | TOC of 11.012 mg/L for 15% wt (Cu/Fe) HKUST-1, at pH 7 | [30] |
| Mesoporous N–TiO2 coating | Evaporation-induced self-assembly (EISA) | Anatase shape (XRD). Eg of 3.1 eV. A thickness of the microstructure of 146 ± 3 nm. The coating had a grid-like structure composed of NPs of 12 ± 3 nm and pores with a diameter of approximately 10 nm. BET surface area of 74.7 ± 0.2 m2/g. |
HDPE and LDPE MPs/ 0.4 wt/v% of MPs dispersion in a CH3COONa/CH3COOH buffer (pH 3) |
Glass container; a 215 mm distance of dispersion with catalyst from the visible LED; irradiation with a lamp of 50 W (400–800 nm) for 50 h, with continuous stirring at 300 rpm, at room temperature |
Mass losses of 0.22 ± 0.02% and 4.65 ± 0.35% for two HDPE MPs with different sizes, and 0.97 ± 0.32% and 1.38 ± 0.13% for two LDPE MPs with different dimensions; CI of 0.80 and 0.45 for HDPE MPs and 1.25 for LDPE MPs | [94] |
| Mesoporous C,N-TiO2/SiO2 |
Solvothermal method; I. Preparation of C,N-TiO2 semiconductor, designed TS-ME, by mineralization; II. Preparation of C,N-TiO2/SiO2 semiconductor, designed TS-MG, by thermal treatment in an autoclave | TS-ME: Eg of 2.41 eV and BET of 313 m2/g. TS-MG: Eg of 2.93 eV and BET of 332 m2/g. | Secondary PET MPs/ 1:1 (wt %) of PET MPs to photocatalyst in buffer solution with a pH 6 or 8 | Batch-type glass container, 50 W LED visible light lamp, 500 W/m2 light irradiance; 120 h of irradiation at room temperature under 350 rpm | Mass loss values ranging from 9.35 to 16.22% | [93] |
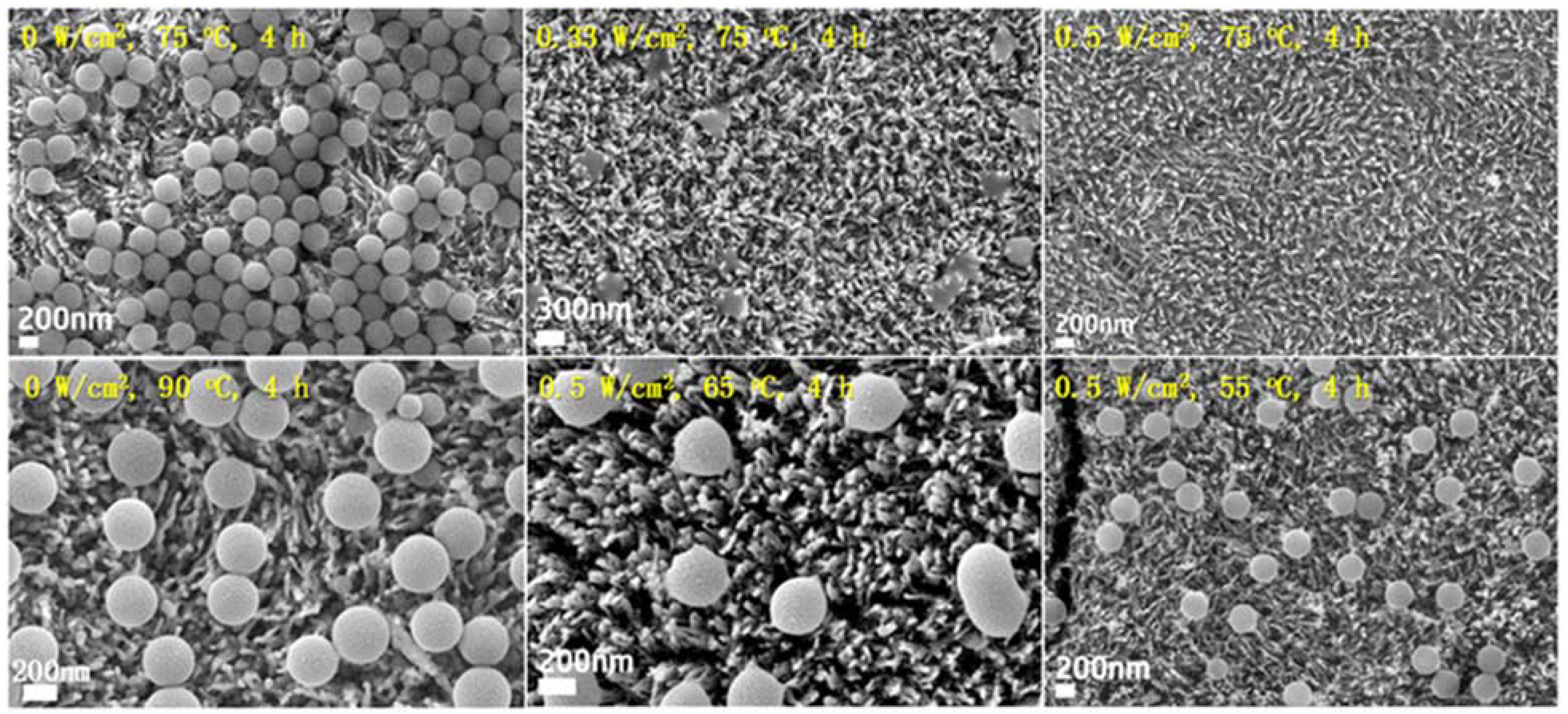
| α-Fe2O3/TiO2HNTAs | Two-step anodic oxidation process; Hydrothermal method | TiO2 anatase phase. The average pore size for TiO2HNTAs of 120 nm. The average length of the bottom nanotube array of 4.3–4.4 μm. The average thickness for α-Fe2O3/TiO2HNT ranging from 260 nm to 330 nm. | PS MPs spheres/0.11% v/v of MPs in ultrapure water | Halogen lamp with a light intensity of 0.5 W/cm2 from 2 h, 3 h to 4 h at an induced temperature of 75 °C | 100% degradation after 4 h of irradiation | [95] |
2.4. Characteristics of TiO2-Based Photocatalysts
3. Performance of TiO2-Based Photocatalysts for MP/NP Degradation Under Visible Light Irradiation
3.1. Plastic Pollutant Types
3.2. Methods for MP/NP Degradation Evaluation
3.3. Doping TiO2 Photocatalysts
Mechanism of MP/NP Degradation Using Doped TiO2
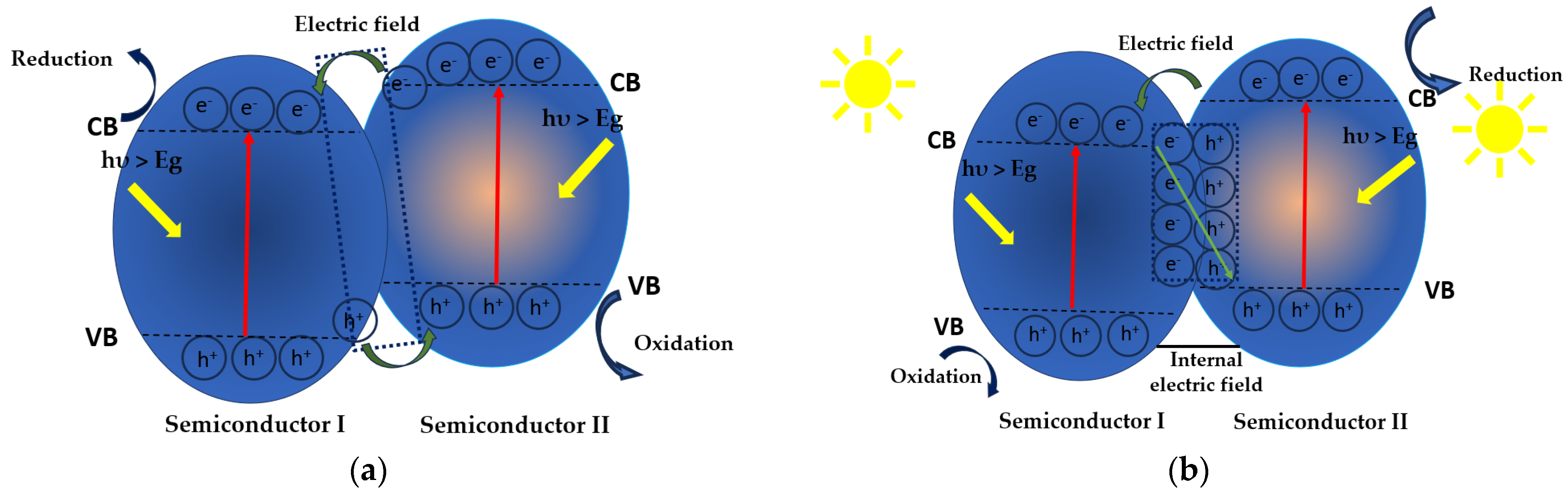
3.4. Heterojunction with TiO2
Mechanism of MP/NP Degradation Using TiO2 Heretostructures
3.5. Inhibition of Reactive Species
4. Challenges
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Villarrubia-Gómez, P.; Cornell, S.E.; Fabres, J. Marine plastic pollution as a planetary boundary threat—The drifting piece in the sustainability puzzle. Mar. Policy 2018, 96, 213–220. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Diehl, A.; Lewandowski, A.; Gopalakrishnan, K.; Baker, T. Removal efficiency of micro- and nanoplastics (180 nm–125 μm) during drinking water treatment. Sci. Total Environ. 2020, 720, 137383. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.K.; Samaei, S.H.A.; Chen, J.F.; Doucet, A.; Ng, K.T.W. What have we known so far about microplastics in drinking water treatment? A timely review. Front. Environ. Sci. Eng. 2022, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Kim, S.; Kim, Y.S.; Jeong, S.; Lee, J. Tracing microplastics from raw water to drinking water treatment plants in Busan, South Korea. Sci. Total Environ. 2022, 825, 154015. [Google Scholar] [CrossRef]
- Pojar, I.; Tiron Dutu, L.; Pop, C.I.; Dutu, F. Hydrodynamic observations on microplastic abundances and morphologies in the Danube delta, Romania. Agrolife Sci. J. 2021, 10, 142–149. [Google Scholar] [CrossRef]
- Zhou, X.X.; He, S.; Gao, Y.; Chi, H.Y.; Wang, D.J.; Li, Z.C.; Yan, B. Quantitative Analysis of Polystyrene and Poly(methyl methacrylate) Nanoplastics in Tissues of Aquatic Animals. Environ. Sci. Technol. 2021, 55, 3032–3040. [Google Scholar] [CrossRef]
- Shruti, V.C.; Pérez-Guevara, F.; Elizalde-Martinez, I.; Kutralam-Muniasamy, G. Toward a unified framework for investigating micro(nano)plastics in packaged beverages intended for human consumption. Environ. Pollut. 2021, 268, 115811. [Google Scholar] [CrossRef]
- Cecchi, T.; Poletto, D.; Berbecaru, A.; Carstea, E.; Rapa, M. Assessing Microplastics and Nanoparticles in the Surface Seawater of Venice Lagoon-Part I: Methodology of Research. Materials 2024, 17, 1759. [Google Scholar] [CrossRef]
- Râpa, M.; Matei, E.; Cârstea, E.; Popa, C.; Matic, M.; Kosic, D. Unraveling the Plastic Pollution in the Aquatic Environment of the Croatian Krk Island. Water 2025, 17, 785. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, B.; Sadhu, S.D. Microplastic profusion in food and drinking water: Are microplastics becoming a macroproblem? Environ. Sci. Process. Impacts 2022, 24, 992–1009. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.R.; Chen, Z.; Chen, Y.L.; Yang, F.W.; Yao, W.R.; Xie, Y.F. Microplastics and Nanoplastics: Emerging Contaminants in Food. J. Agric. Food Chem. 2021, 69, 10450–10468. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.; Nyakuma, B.B.; Wong, K.Y.; Lee, C.T.; Lee, T.H.; Lee, C.H. Microplastics and nanoplastics in global food webs: A bibliometric analysis (2009-2019). Mar. Pollut. Bull. 2020, 158, 111432. [Google Scholar] [CrossRef] [PubMed]
- Myszograj, M. Microplastic in Food and Drinking Water- Environmental Monitoring Data. Civ. Environ. Eng. Rep. 2020, 30, 201–209. [Google Scholar] [CrossRef]
- Okoffo, E.D.; O’Brien, S.; Ribeiro, F.; Burrows, S.D.; Toapanta, T.; Rauert, C.; O’Brien, J.W.; Tscharke, B.J.; Wang, X.Y.; Thomas, K.V. Plastic particles in soil: State of the knowledge on sources, occurrence and distribution, analytical methods and ecological impacts. Environ. Sci. -Process. Impacts 2021, 23, 240–274. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Chen, L.; Chao, J.Y.; Teng, J.; Wang, Q. Microplastics in soils: A review of possible sources, analytical methods and ecological impacts. J. Chem. Technol. Biotechnol. 2020, 95, 2052–2068. [Google Scholar] [CrossRef]
- Jia, W.Q.; Karapetrova, A.; Zhang, M.J.; Xu, L.B.; Li, K.; Huang, M.K.; Wang, J.; Huang, Y. Automated identification and quantification of invisible microplastics in agricultural soils. Sci. Total Environ. 2022, 844, 156853. [Google Scholar] [CrossRef]
- Rhodes, C.J. Solving the plastic problem: From cradle to grave, to reincarnation. Sci. Prog. 2019, 102, 218–248. [Google Scholar] [CrossRef]
- Ding, J.F.; Sun, C.J.; He, C.F.; Zheng, L.; Dai, D.J.; Li, F.M. Atmospheric microplastics in the Northwestern Pacific Ocean: Distribution, source, and deposition. Sci. Total Environ. 2022, 829, 154337. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, Fate, Behavior and Ecotoxicological State of Phthalates in Different Environmental Matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef]
- Paluselli, A.; Fauvelle, V.; Galgani, F.; Sempéré, R. Phthalate Release from Plastic Fragments and Degradation in Seawater. Environ. Sci. Technol. 2019, 53, 166–175. [Google Scholar] [CrossRef]
- Björnsdotter, M.K.; Jonker, W.; Legradi, J.; Kool, J.; Ballesteros-Gómez, A. Bisphenol A alternatives in thermal paper from the Netherlands, Spain, Sweden and Norway. Screening and potential toxicity. Sci. Total Environ. 2017, 601, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.R.; Kauffman, A.E.; Li, L.; McFee, W.; Cai, B.; Weinstein, J.; Lead, J.R.; Chatterjee, S.; Scott, G.I.; Xiao, S. Health impacts of environmental contamination of micro- and nanoplastics: A review. Environ. Health Prev. Med. 2020, 25, 29. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, R.M.; Kanwar, R.S.; Jovanovi, B. Past, present, and possible future policies on plastic use in the United States, particularly microplastics and nanoplastics: A review. Integr. Environ. Assess. Manag. 2023, 19, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Almeida, M.; Miguel, I. A micro(nano)plastic boomerang tale: A never ending story? Trac-Trends Anal. Chem. 2019, 112, 196–200. [Google Scholar] [CrossRef]
- Kaur, K.; Kaur, H. Visible light induced photocatalytic degradation of polyvinyl chloride from water using graphene oxide/nitrogen-doped TiO2 nanocomposite. J. Environ. Chem. Eng. 2025, 13, 115664. [Google Scholar] [CrossRef]
- Chen, X.; Ma, H.; Ji, X.; Han, R.; Pang, K.; Yang, Z.; Liu, Z.; Peng, S. Engineering green MOF-based superhydrophobic sponge for efficiently synchronous removal of microplastics and pesticides from high-salinity water. Water Res. 2023, 243, 120314. [Google Scholar] [CrossRef]
- Poerio, T.; Lavorato, C.; Severino, A.; Russo, B.; Molinari, R.; Argurio, P.; Figoli, A. Combined membrane separation and photocatalysis process for the recovery and decomposition of micro/nanoplastics from polyester fabrics. J. Environ. Chem. Eng. 2024, 12, 113310. [Google Scholar] [CrossRef]
- Rodríguez-Olivares, A.; Guzmán-Mar, J.; Quero-Jiménez, P.; Montemayor, S.; Maya-Treviño, L.; Hinojosa-Reyes, L. Analytical approaches to track nylon 6 microplastic fiber degradation using HKUST-1(Cu/Fe)-derived CuO/TiO2 photocatalyst. J. Water Process Eng. 2025, 71, 107192. [Google Scholar] [CrossRef]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Johnson, L.M.; Grieger, K.D.; Ambroso, J.L.; Fennell, T.R. Biological interactions between nanomaterials and placental development and function following oral exposure. Reprod. Toxicol. 2019, 90, 150–165. [Google Scholar] [CrossRef]
- Gambino, I.; Bagordo, F.; Grassi, T.; Panico, A.; De Donno, A. Occurrence of Microplastics in Tap and Bottled Water: Current Knowledge. Int. J. Environ. Res. Public Health 2022, 19, 5283. [Google Scholar] [CrossRef]
- Aghaei, Z.; Sled, J.G.; Kingdom, J.C.; Baschat, A.A.; Helm, P.A.; Jobst, K.J.; Cahill, L.S. Maternal Exposure to Polystyrene Micro- and Nanoplastics Causes Fetal Growth Restriction in Mice. Environ. Sci. Technol. Lett. 2022, 9, 426–430. [Google Scholar] [CrossRef]
- Banerjee, A.; Billey, L.O.; McGarvey, A.M.; Shelver, W.L. Effects of polystyrene micro/nanoplastics on liver cells based on particle size, surface functionalization, concentration and exposure period. Sci. Total Environ. 2022, 836, 155621. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.F.; Lin, S.Y.; Cao, G.D.; Wu, J.B.; Jin, H.B.; Wang, C.; Wong, M.H.; Yang, Z.; Cai, Z.W. Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J. Hazard. Mater. 2022, 437, 129361. [Google Scholar] [CrossRef] [PubMed]
- Hasan Anik, A.; Hossain, S.; Alam, M.; Binte Sultan, M.; Hasnine, M.D.T.; Rahman, M.M. Microplastics pollution: A comprehensive review on the sources, fates, effects, and potential remediation. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100530. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.; Villarreal-Chiu, J.; Hernández-López, J.; De la Rosa, J.; Barbieri, V.; Siligardi, C.; Cedillo-González, E. Microplastic pollution reduction by a carbon and nitrogen-doped TiO2: Effect of pH and temperature in the photocatalytic degradation process. J. Hazard. Mater. 2020, 395, 122632. [Google Scholar] [CrossRef]
- Anyame Bawa, S.; Chan, A.; Wrobel-Tobiszewska, A.; Hardie, M.; Towns, C. A review of methods for mitigating microplastic contamination in biosolids from wastewater treatment plants before agricultural soil application. Sci. Total Environ. 2024, 957, 177360. [Google Scholar] [CrossRef]
- Hansda, A.; Chand, S.; Pradhan, B.; Chand, S.; Shukla, A.; Rout, P. Toxicological Impacts and Microbial-Mediated Degradation Processes of Microplastics. J. Hazard. Toxic Radioact. Waste 2025, 29, 2. [Google Scholar] [CrossRef]
- Ngaba, M.; Rennenberg, H.; Hu, B. Insights Into the Efficiency and Health Impacts of Emerging Microplastic Bioremediation Approaches. Glob. Change Biol. 2025, 31, e70226. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, R.; Wang, Y.; Zhang, C.; Zheng, J.; Ning, P.; Shan, D.; Wang, B. Light-driven degradation of microplastics: Mechanisms, technologies, and future directions. J. Hazard. Mater. Adv. 2025, 17, 100628. [Google Scholar] [CrossRef]
- Râpa, M.; Cârstea, E.; Saulean, A.; Popa, C.; Matei, E.; Predescu, A.; Predescu, C.; Dontu, S.; Dinca, A. An Overview of the Current Trends in Marine Plastic Litter Management for a Sustainable Development. Recycling 2024, 9, 30. [Google Scholar] [CrossRef]
- Devi, M.K.; Karmegam, N.; Manikandan, S.; Subbaiya, R.; Song, H.; Kwon, E.E.; Sarkar, B.; Bolan, N.; Kim, W.; Rinklebe, J.; et al. Removal of nanoplastics in water treatment processes: A review. Sci. Total Environ. 2022, 845, 157168. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.; Song, K.; Zhou, Y.W. Performance evaluation of MBR in treating microplastics polyvinylchloride contaminated polluted surface water. Mar. Pollut. Bull. 2020, 150, 110724. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.M.A.; Sheng, J.; Zimba, P.V.; Zhu, L.; Fadare, O.O.; Haley, C.; Wang, M.C.; Phillips, T.D.; Conkle, J.; Xu, W. Testing an Iron Oxide Nanoparticle-Based Method for Magnetic Separation of Nanoplastics and Microplastics from Water. Nanomaterials 2022, 12, 2348. [Google Scholar] [CrossRef]
- Flores-Díaz, A.; Arango, J.; Calvo, D.; Rangel-Mendez, R.; Ontiveros-Valencia, A. Physicochemical and microbial treatments for plastics, microplastics, and nanoplastics. Environ. Dev. 2025, 54, 101181. [Google Scholar] [CrossRef]
- Sutrisna, P.; Riadi, L.; Buana, P.; Khoiruddin, K.; Boopathy, R.; Wenten, I.; Siagian, U. Membrane and membrane-integrated processes for nanoplastics removal and remediation. Polym. Degrad. Stab. 2024, 220, 110635. [Google Scholar] [CrossRef]
- López-Maldonado, E.; Khan, N.; Singh, S.; Ramamurthy, P.; Kabak, B.; Baudrit, J.; Alkahtan, M.; alvarez-Torrellas, S.; Varshney, R.; Serra-Pérez, E.; et al. Magnetic polymeric composites: Potential for separating and degrading micro/nano plastics. Desalin. Water Treat. 2024, 317, 100198. [Google Scholar] [CrossRef]
- Rizvi, N.; Sarwar, A.; Waheed, S.; Iqbal, Z.; Imran, M.; Javaid, A.; Kim, T.; Khan, M. Nano-based remediation strategies for micro and nanoplastic pollution. J. Contam. Hydrol. 2024, 265, 104380. [Google Scholar] [CrossRef]
- Pondala, S.; Mohan Botsa, S. Physical, thermal, chemical and biological approaches for plastics degradation–A review. Clean. Chem. Eng. 2025, 11, 100162. [Google Scholar] [CrossRef]
- Leppänen, I.; Lappalainen, T.; Lohtander, T.; Jonkergouw, C.; Arola, S.; Tammelin, T. Capturing colloidal nano- and microplastics with plant-based nanocellulose networks. Nat. Commun. 2022, 13, 1814. [Google Scholar] [CrossRef]
- Arenas, L.R.; Gentile, S.R.; Zimmermann, S.; Stoll, S. Nanoplastics adsorption and removal efficiency by granular activated carbon used in drinking water treatment process. Sci. Total Environ. 2021, 791, 148175. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.L.; Liu, Z.Z.; Jiang, W. Transport behavior of nanoplastics in activated carbon column. Environ. Sci. Pollut. Res. 2023, 30, 26256–26269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.Y.; Jarvis, P.; Zhou, W.; Zhang, J.P.; Chen, J.P.; Tan, Q.W.; Tian, Y. Occurrence, removal and potential threats associated with microplastics in drinking water sources. J. Environ. Chem. Eng. 2020, 8, 104527. [Google Scholar] [CrossRef]
- Hofman-Caris, C.H.M.; Bauerlein, P.S.; Siegers, W.G.; Mintenig, S.M.; Messina, R.; Dekker, S.C.; Bertelkamp, C.; Cornelissen, E.R.; van Wezel, A.P. Removal of nanoparticles (both inorganic nanoparticles and nanoplastics) in drinking water treatment–coagulation/flocculation/sedimentation, and sand/granular activated carbon filtration. Environ. Sci. Water Res. Technol. 2022, 8, 1675–1686. [Google Scholar] [CrossRef]
- Elfgen, R.; Gehrke, S.; Holloczki, O. Ionic Liquids as Extractants for Nanoplastics. Chemsuschem 2020, 13, 5449–5459. [Google Scholar] [CrossRef]
- Arenas, L.R.; Gentile, S.R.; Zimmermann, S.; Stoll, S. Coagulation of TiO2, CeO2 nanoparticles, and polystyrene nanoplastics in bottled mineral and surface waters. Effect of water properties, coagulant type, and dosage. Water Environ. Res. 2020, 92, 1184–1194. [Google Scholar] [CrossRef]
- Ding, H.J.; Zhang, J.; He, H.; Zhu, Y.; Dionysiou, D.D.; Liu, Z.; Zhao, C. Do membrane filtration systems in drinking water treatment plants release nano/microplastics? Sci. Total Environ. 2021, 755, 142658. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, B.; Sun, F.; Zhang, Z.; Kong, Y.; Liu, X.; Cui, Y.; Ma, Y.; Wu, Y.; Fan, J.; et al. Interactions between the co-contamination system of oxcarbazepine-polypropylene microplastics and Chlorella sp. FACHB-9: Toxic effects and biodegradation. J. Environ. Manag. 2025, 376, 124434. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the degradation of (micro)plastics: Degradation methods, influencing factors, environmental impacts. Sci. Total Environ. 2022, 806, 151312. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Ding, J.; Song, Z.R.; Yang, B.C.; Zhang, C.M.; Guan, B.H. Degradation of nano-sized polystyrene plastics by ozonation or chlorination in drinking water disinfection processes. Chem. Eng. J. 2022, 427, 131690. [Google Scholar] [CrossRef]
- El-Kurdi, N.; El-Shatoury, S.; Elbaghdady, K.; Hammad, S.; Ghazy, M. Biodegradation of polystyrene nanoplastics by Achromobacter xylosoxidans M9 offers a mealworm gut-derived solution for plastic pollution. Arch. Microbiol. 2024, 206, 238. [Google Scholar] [CrossRef]
- Pham, T.; Do, H.; Thi, L.; Singh, P.; Raizada, P.; Wu, J.; Nguyen, V. Global challenges in microplastics: From fundamental understanding to advanced degradations toward sustainable strategies. Chemosphere 2021, 267, 129275. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.; Souza, K.; Motteran, F.; de Araújo, L.; Singh, R.; Bhadouria, R.; de Oliveira, M. Exploring biodegradative efficiency: A systematic review on the main microplastic-degrading bacteria. Front. Microbiol. 2024, 15, 1360844. [Google Scholar] [CrossRef]
- Xu, R.; Cui, L.; Kang, S. Countering microplastics pollution with photocatalysis: Challenge and prospects. Prog. Nat. Sci. Mater. Int. 2023, 33, 251–266. [Google Scholar] [CrossRef]
- Anirudhan, T.; Shainy, F.; Mohan, A. Fabrication of zinc oxide nanorod incorporated carboxylic graphene/polyaniline composite and its photocatalytic activity for the effective degradation of diuron from aqueous solutions. Sol. Energy 2018, 171, 534–546. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, F.; Shen, Y.; Duan, X.; Zhao, S.; Chen, X.; Liang, J. Removal of Emerging Organic Pollutants by Zeolite Mineral (Clinoptilolite) Composite Photocatalysts in Drinking Water and Watershed Water. Catalysts 2024, 14, 216. [Google Scholar] [CrossRef]
- Feng, H.; Liang, L.; Wu, W.; Huang, Z.; Liu, Y. Architecting epitaxial-lattice-mismatch-free (LMF) zinc oxide/bismuth oxyiodide nano-heterostructures for efficient photocatalysis. J. Mater. Chem. C 2020, 8, 11263–11273. [Google Scholar] [CrossRef]
- He, Y.; Rehman, A.; Xu, M.; Not, C.; Ng, A.; Djurisic, A. Photocatalytic degradation of different types of microplastics by TiOx/ZnO tetrapod photocatalysts. Heliyon 2023, 9, e22562. [Google Scholar] [CrossRef]
- Nguyen, T.; Edalati, K. Impact of high-pressure columbite phase of titanium dioxide (TiO2) on catalytic photoconversion of plastic waste and simultaneous hydrogen (H2) production. J. Alloys Compd. 2024, 1008, 176722. [Google Scholar] [CrossRef]
- Edirisooriya, E.; Senanayake, P.; Ahasan, T.; Xu, P.; Wang, H. Comprehensive Insights into Photoreforming of Waste Plastics for Hydrogen Production. Catalysts 2025, 15, 453. [Google Scholar] [CrossRef]
- Edirisooriya, E.; Senanayake, P.; Wang, H.; Talipov, M.; Xu, P.; Wang, H. Photo-reforming and degradation of waste plastics under UV and visible light for H2 production using nanocomposite photocatalysts. J. Environ. Chem. Eng. 2023, 11, 109580. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Edalati, K. Brookite TiO2 as an active photocatalyst for photoconversion of plastic wastes to acetic acid and simultaneous hydrogen production: Comparison with anatase and rutile. Chemosphere 2024, 355, 141785. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Jaimes, L.; Cedillo-González, E.; Luévano-Hipólito, E.; Acuña-Bedoya, J.; Hernández-López, J. Degradation of primary nanoplastics by photocatalysis using different anodized TiO2 structures. J. Hazard. Mater. 2021, 413, 125452. [Google Scholar] [CrossRef]
- Garcia-Munoz, P.; Alle, P.; Bertoloni, C.; Torres, A.; de la Orden, M.; Urreaga, J.; Dziurla, M.; Fresno, F.; Robert, D.; Keller, N. Photocatalytic degradation of polystyrene nanoplastics in water. A methodological study. J. Environ. Chem. Eng. 2022, 10, 108195. [Google Scholar] [CrossRef]
- Nabi, I.; Bacha, A.; Ahmad, F.; Zhang, L. Application of titanium dioxide for the photocatalytic degradation of macro- and micro-plastics: A review. J. Environ. Chem. Eng. 2021, 9, 105964. [Google Scholar] [CrossRef]
- Feizpoor, S.; Habibi-Yangjeh, A.; Luque, R. Preparation of TiO2/Fe-MOF n.n heterojunction photocatalysts for visible-light degradation of tetracycline hydrochloride. Chemosphere 2023, 336, 139101. [Google Scholar] [CrossRef]
- Díez, A.; Pazos, M.; Sanromán, M.; Naranjo, H.; Mayer, J.; Kolen’ko, Y. Photocatalytic solid-phase degradation of polyethylene with fluoride-doped titania under low consumption ultraviolet radiation. J. Environ. Manag. 2023, 329, 117044. [Google Scholar] [CrossRef]
- Singh, V.; Park, S.; Choi, J.; Kim, C. Photocatalytic Degradation of Polyethylene, Polypropylene, and Sulfathiazole Using a UV/TiO2/Oxidant System. Water Air Soil Pollut. 2025, 236, 166. [Google Scholar] [CrossRef]
- Wang, X.; Teo, S.; Shamsuddin, M.; Wid, N. Influence of Ag-concentration on TiO2-supported catalysts for photocatalytic degradation of microplastics PA66 in marine. Reg. Stud. Mar. Sci. 2025, 81, 103922. [Google Scholar] [CrossRef]
- García-Montelongo, X.; Martínez-de la Cruz, A.; Vázquez-Rodríguez, S.; Torres-Martínez, L. Photo-oxidative degradation of TiO2/polypropylene films. Mater. Res. Bull. 2014, 51, 56–62. [Google Scholar] [CrossRef]
- Silva, D.; Marques, A. TiO2-based photocatalytic degradation of microplastics in water: Current status, challenges and future perspectives. J. Water Process Eng. 2025, 72, 107465. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Luo, C.; Li, Y.; Zhang, L.; Li, C.; Zhang, X.; Liao, J.; Zhou, W. Cs3Bi2Br9/BiOCl S-scheme heterojunction photocatalysts with solid built-in electric field for efficient polystyrene microplastics degradation. Appl. Catal. B-Environ. Energy 2025, 371, 125288. [Google Scholar] [CrossRef]
- Cartraud, A.; Le Corre, M.; Turquet, J.; Tourmetz, J. Plastic ingestion in seabirds of the western Indian Ocean. Mar. Pollut. Bull. 2019, 140, 308–314. [Google Scholar] [CrossRef]
- Fa, W.; Zan, L.; Gong, C.; Zhong, J.; Deng, K. Solid-phase photocatalytic degradation of polystyrene with TiO2 modified by iron(II) phthalocyanine. Appl. Catal. B-Environ. 2008, 79, 216–223. [Google Scholar] [CrossRef]
- Shang, J.; Chai, M.; Zhu, Y. Photocatalytic degradation of polystyrene plastic under fluorescent light. Environ. Sci. Technol. 2003, 37, 4494–4499. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, C.; Li, X.; Yang, Q.; Wang, D.; Duan, A.; Ding, J.; Rong, S.; Chen, Z.; Luo, J.; et al. Enhanced photodegradation of tetracycline hydrochloride by hexameric AgBr/Zn-Al MMO S-scheme heterojunction photocatalysts: Low metal leaching, degradation mechanism and intermediates. Chem. Eng. J. 2022, 446, 137371. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.; Villarreal-Chiu, J.; Barbieri, V.; Siligardi, C.; Cedillo-González, E. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, L.; Nekliudov, A. Construction of loading g-C3N4/TiO2 on waste cotton-based activated carbon S-scheme heterojunction for enhanced photocatalytic degradation of microplastics: Performance, DFT calculation and mechanism study. Opt. Mater. 2024, 154, 115786. [Google Scholar] [CrossRef]
- Jeyaraj, J.; Baskaralingam, V.; Stalin, T.; Muthuvel, I. Mechanistic vision on polypropylene microplastics degradation by solar radiation using TiO2 nanoparticle as photocatalyst. Environ. Res. 2023, 233, 16366. [Google Scholar] [CrossRef]
- Rojas-Guerrero, C.; Villanueva-Rodríguez, M.; Guzmán-Mar, J.; Hernández-Ramírez, A.; Cedillo-González, E.; Rodríguez, F.; Hinojosa-Reyes, L. Solar photocatalytic degradation of polyethylene terephthalate nanoplastics: Evaluation of the applicability of the TiO2/MIL-100(Fe) composite material. J. Environ. Chem. Eng. 2023, 11, 110415. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.; Siligardi, C.; Carreón-López, H.; Valdéz-Cerda, J.; Pozzi, P.; Kaushik, G.; Villarreal-Chiu, J.; Cedillo-González, E. Low environmental impact remediation of microplastics: Visible-light photocatalytic degradation of PET microplastics using bio-inspired C, N-TiO2/SiO2 photocatalysts. Mar. Pollut. Bull. 2023, 193, 115206. [Google Scholar] [CrossRef] [PubMed]
- Llorente-García, B.; Hernández-López, J.; Zaldívar-Cadena, A.; Siligardi, C.; Cedillo-González, E. First Insights into Photocatalytic Degradation of HDPE and LDPE Microplastics by a Mesoporous N-TiO2Coating: Effect of Size and Shape of Microplastics. Coatings 2020, 10, 658. [Google Scholar] [CrossRef]
- Xue, Z.; Yu, X.; Ke, X.; Zhao, J. A novel route for microplastic mineralization: Visible-light-driven heterogeneous photocatalysis and photothermal Fenton-like reaction. Environ. Sci. Nano 2024, 11, 113–122. [Google Scholar] [CrossRef]
- Lin, P.; Lu, X.; Deka, B.; Shang, J.; Wu, H.; Sun, J.; Yi, C.; Farid, M.; An, A.; Guo, J. Research progress in the preparation of electrospinning MOF nanofiber membranes and applications in the field of photocatalysis. Sep. Purif. Technol. 2025, 356, 129948. [Google Scholar] [CrossRef]
- Mohana, A.; Rahman, M.; Sarker, S.; Haque, N.; Gao, L.; Pramanik, B. Nano/microplastics: Fragmentation, interaction with co-existing pollutants and their removal from wastewater using membrane processes. Chemosphere 2022, 309, 136682. [Google Scholar] [CrossRef]
- He, J.; Cao, M.; Gao, Y.; Zhu, M.; Zhang, J.; Wei, X.; Dong, Y.; Sun, C.; Qu, M. Ni-MOF-doped superhydrophobic sponge with photothermal conversion properties for enhanced removal of oil pollutants and microplastics from aqueous environments. Sep. Purif. Technol. 2025, 362, 131734. [Google Scholar] [CrossRef]
- Kong, E.; Lai, C.; Juan, J.; Pang, Y.; Khe, C.; Badruddin, I.; Gapsari, F.; Anam, K. Recent advances in titanium dioxide bio-derived carbon photocatalysts for organic pollutant degradation in wastewater. Iscience 2025, 28, 112368. [Google Scholar] [CrossRef]
- Montefalcon, L.; Ramoy, M.; Montallana, A.; Vasquez, M.J. Fabrication of Silver-decorated Titanium Dioxide 6Nanofibers for Visible-light Photodegradation of Methylene Blue. e-J. Surf. Sci. Nanotechnol. 2025, 23, 1–7. [Google Scholar] [CrossRef]
- Ashina, C.; Pugazhenthiran, N.; Mangalaraja, R.; Sathishkumar, P. Review on enhancing solar photocatalysis for sustainable degradation of invisible environmental pollutants. Renew. Sustain. Energy Rev. 2025, 214, 115490. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, X.; Liu, Z.; Lu, J.; Sheng, X.; Feng, X. Interfacial Microenvironment Engineering Based on Ordered TiO2 Porous Films for Enhanced Visible Light Driven Photocatalysis. Chin. J. Chem. 2025, 43, 1961–1967. [Google Scholar] [CrossRef]
- Kumari, S.; Yadav, D.; Yadav, S.; Selvaraj, M.; Sharma, G.; Karnwal, A.; Yadav, S. From macro to micro: The key parameters influencing the degradation mechanism and the toxicity of microplastics in the environment. Polym. Degrad. Stab. 2025, 233, 111174. [Google Scholar] [CrossRef]
- Surana, M.; Pattanayak, D.; Yadav, V.; Singh, V.; Pal, D. An insight decipher on photocatalytic degradation of microplastics: Mechanism, limitations, and future outlook. Environ. Res. 2024, 247, 118268. [Google Scholar] [CrossRef]
- Janani, R.; Bhuvana, S.; Geethalakshmi, V.; Jeyachitra, R.; Sathishkumar, K.; Balu, R.; Ayyamperumal, R. Micro and nano plastics in food: A review on the strategies for identification, isolation, and mitigation through photocatalysis, and health risk assessment. Environ. Res. 2024, 241, 117666. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Ojha, A.; Kansal, S.; Gupta, N.; Swart, H.; Cho, J.; Kuznetsov, A.; Sun, S.; Prakash, J. Advances in powder nano-photocatalysts as pollutant removal and as emerging contaminants in water: Analysis of pros and cons on health and environment. Adv. Powder Mater. 2024, 3, 100233. [Google Scholar] [CrossRef]
- Bevacqua, E.; Occhiuzzi, M.; Grande, F.; Tucci, P. TiO2-NPs Toxicity and Safety: An Update of the Findings Published over the Last Six Years. Mini-Rev. Med. Chem. 2023, 23, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Feng, D.; Li, Y.; Liu, L.; Tang, J. Innovations in chemical degradation technologies for the removal of micro/nano-plastics in water: A comprehensive review. Ecotoxicol. Environ. Saf. 2024, 271, 115979. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stancu, A.G.; Râpă, M.; Popa, C.L.; Donțu, S.I.; Matei, E.; Covaliu-Mirelă, C.I. Degradation of Emerging Plastic Pollutants from Aquatic Environments Using TiO2 and Their Composites in Visible Light Photocatalysis. Molecules 2025, 30, 3186. https://doi.org/10.3390/molecules30153186
Stancu AG, Râpă M, Popa CL, Donțu SI, Matei E, Covaliu-Mirelă CI. Degradation of Emerging Plastic Pollutants from Aquatic Environments Using TiO2 and Their Composites in Visible Light Photocatalysis. Molecules. 2025; 30(15):3186. https://doi.org/10.3390/molecules30153186
Chicago/Turabian StyleStancu, Alexandra Gabriela, Maria Râpă, Cristina Liana Popa, Simona Ionela Donțu, Ecaterina Matei, and Cristina Ileana Covaliu-Mirelă. 2025. "Degradation of Emerging Plastic Pollutants from Aquatic Environments Using TiO2 and Their Composites in Visible Light Photocatalysis" Molecules 30, no. 15: 3186. https://doi.org/10.3390/molecules30153186
APA StyleStancu, A. G., Râpă, M., Popa, C. L., Donțu, S. I., Matei, E., & Covaliu-Mirelă, C. I. (2025). Degradation of Emerging Plastic Pollutants from Aquatic Environments Using TiO2 and Their Composites in Visible Light Photocatalysis. Molecules, 30(15), 3186. https://doi.org/10.3390/molecules30153186










