Nano-Phytomedicine: Harnessing Plant-Derived Phytochemicals in Nanocarriers for Targeted Human Health Applications
Abstract
1. Introduction
1.1. Background of Phytochemicals in Traditional and Modern Medicine
1.2. Limitations of Free Phytochemicals
1.3. Emergence of Nanotechnology in Phytomedicine
1.4. Objective and Scope of the Review
- To categorize major nanocarrier systems including lipid-based, polymeric, and inorganic nanomaterials used in phytochemical encapsulation and delivery, with an emphasis on their physicochemical properties and compatibility with bioactives.
- To elucidate molecular mechanisms of nano-phytochemical interactions with human cells, including uptake, intracellular trafficking, release kinetics, and downstream signaling modulation.
- To discuss therapeutic applications across key areas such as oncology, neurodegeneration, cardiovascular disease, metabolic disorders, and infectious diseases, highlighting both preclinical and clinical outcomes.
- To evaluate the pharmacokinetic and toxicological profiles of nanoformulated phytochemicals, considering parameters such as bioavailability, biodistribution, toxicity, and immunogenicity.
- To assess the current translational landscape, including clinical trials, regulatory barriers, commercialized products, and future scalability for global healthcare implementation.
- To explore future directions such as AI-guided formulation design, personalized nano-phytotherapy, smart delivery systems, and sustainable manufacturing.
2. Overview of Plant-Derived Phytochemicals Relevant to Human Health
2.1. Major Classes: Polyphenols, Alkaloids, Terpenoids, Flavonoids, and Glycosides
2.2. Therapeutic Properties: Antioxidant, Anticancer, Anti-Inflammatory, Antimicrobial
- Antioxidants: Polyphenols neutralize free radicals and reactive oxygen species (ROS) in vitro, though their direct antioxidant activity in vivo is less certain due to poor bioavailability [32]. Still, their metabolites may indirectly upregulate endogenous antioxidant pathways via Nrf2 signaling.
- Anticancer activity: Numerous phytochemicals modulate apoptosis, cell proliferation, angiogenesis, and metastasis. For example, catechin-3-gallate has been shown to enhance therapeutic efficacy through ROS induction and mitochondrial pathway activation in tumor cells [33]. Additionally, carrier-free self-assembled phytochemical nanoparticles (e.g., curcumin conjugates) demonstrate synergistic targeting and cytotoxicity in cancer models [34].
- Antimicrobial activity: Polyphenol-rich plant extracts and alkaloids (like berberine) show broad-spectrum antimicrobial effects. For instance, berberine disrupts bacterial cell membrane integrity, while flavonoids inhibit viral enzymes and microbial adhesion [37].
2.3. Bioavailability Challenges and Need for Delivery Enhancement
- Rapid metabolism and clearance: After absorption, phytochemicals are quickly subjected to phase I/II metabolism (e.g., glucuronidation, sulfation), yielding low plasma concentrations and short half-lives that necessitate frequent dosing [40].
- Low membrane permeability: Their molecular size, polarity, or lipophilicity often limits cellular uptake and penetration through biological barriers like the blood–brain barrier [41].
- Variability in bioavailability: Individual differences in gut microbiota, genetic polymorphisms in metabolic enzymes, and interactions with food can result in erratic absorption and therapeutic response [42].
3. Nanocarrier Systems for Phytochemical Delivery
3.1. Lipid-Based Carriers: Liposomes, Solid Lipid Nanoparticles (SLNs), and Nanostructured Lipid Carriers (NLCs)
3.2. Polymeric Nanoparticles: Natural and Synthetic Polymers
3.3. Inorganic Nanoparticles: Metal, Metal Oxide, and Silica Nanoparticles
3.4. Hybrid and Stimuli-Responsive Nanocarriers
3.5. Surface Functionalization and Targeting Strategies
4. Molecular Mechanisms of Action of Nano-Phytomedicine
4.1. Cellular Uptake and Intracellular Trafficking of Nanoformulations
4.2. Modulation of Redox Balance and Antioxidant Pathways
4.3. Regulation of Apoptosis, Cell Proliferation, and Inflammation
4.4. Interaction with Cellular Signaling
4.5. Epigenetic and Genomic Regulation by Nano-Phytochemicals
5. Therapeutic Applications in Human Health
5.1. Cancer Therapy: Tumor Targeting and Chemoprevention
- Improved tumor accumulation and retention: Nanocarriers exploit the Enhanced Permeability and Retention (EPR) effect, resulting in higher tumor uptake compared to free compounds. Lipid- and polymer-based nanoformulations of curcumin, resveratrol, and EGCG have shown significantly increased tumor accumulation and therapeutic efficacy in breast, prostate, pancreatic, and colon cancers compared to their native forms [130,131].
- Targeted delivery and cellular uptake: Surface functionalization strategies—such as folate receptors, peptides, or antibodies—enhance selective cellular uptake. Folic acid-decorated lipid carriers loaded with resveratrol exhibited over twice the uptake in ovarian cancer cells versus controls [132]. Similarly, HER2-targeted polymeric nanoparticles delivering phytochemical paclitaxel precursors demonstrated potent cytotoxicity in breast cancer models [133].
- Synergistic mechanisms in tumor suppression: Phyto-nanocarriers modulate multiple anticancer pathways including apoptotic induction, cell cycle arrest, anti-angiogenesis, and ferroptosis. For example, carriers inducing ferroptosis—an iron-dependent form of cell death—have been shown to synergize with photodynamic therapies, leveraging ROS accumulation and GPX4 inhibition [134].
- Clinical translation moving forward: While numerous phyto-nanodrugs are undergoing preclinical evaluation, a subset has entered clinical testing or is nearing approval [135]. The AuroLase™ gold nanoshells (for head and neck, lung, and prostate tumors) exemplify how phytochemical-functionalized inorganic nanocarriers can be translated into the clinic [136].
5.2. Cardiovascular Health: Anti-Atherogenic and Antihypertensive Effects
- Anti-atherogenic potential: Nanocarrier-delivered polyphenols (like resveratrol and catechins) and terpenoids mitigate oxidative stress and inflammation in endothelial cells. Enhanced bioavailability leads to reduced LDL oxidation, lower vascular smooth muscle proliferation, and decreased macrophage infiltration in atherosclerotic lesions [137,138]. Some systems leverage magnetic nanoparticles to localize phytochemicals to inflamed plaques under external guidance [139].
- Blood pressure modulation: Nanoformulations such as naringenin-loaded nanocarriers have demonstrated improved vasodilatory effects, enhanced endothelial nitric oxide synthesis, and reduced vascular resistance in hypertensive animal models. Compared to unencapsulated phytochemicals, these nanoformulations required lower doses for similar antihypertensive outcomes [140].
- Enhanced therapeutic indices: By increasing solubility and stability, nanocarriers enable sustained-release profiles, maintaining therapeutic concentrations over extended periods—crucial for managing chronic cardiovascular conditions [141].
- Safety and translational outlook: Lipid-based nanoemulsions and polymeric systems exhibit good biocompatibility and low toxicity in vivo. However, comprehensive long-term safety studies are needed. Clinical translational efforts are underway, though in vivo data and human trials are still limited [142].
5.3. Neurodegenerative Disorders: Blood–Brain Barrier Penetration and Neuroprotection
- Improved BBB permeability: Traditional phytochemicals like curcumin and resveratrol face poor CNS bioavailability due to the semi-permeable BBB. Encapsulation in liposomes or polymeric nanoparticles coated with surfactants (e.g., polysorbate-80) or targeted ligands (e.g., transferrin) has enhanced brain uptake by several-fold in rodent models [143].
- Neuroprotective effects: Once inside the CNS, these nano-phytomedicines exert antioxidant activity, suppress neuroinflammation, and support mitochondrial function. For example, curcumin-loaded nanoparticles attenuated microglial activation, decreased levels of neurotoxic cytokines (TNF-α, IL-6), and improved cognitive performance in Alzheimer’s disease (AD) models [144].
- Synergy with anti-aggregation mechanisms: Nano-encapsulated resveratrol and epigallocatechin gallate (EGCG) interfere with amyloid-β aggregation and tau phosphorylation, key pathological events in neurodegeneration. Targeted delivery increased accumulation near amyloid plaques and enhanced reduction in fibril load [145].
- Translational potential and safety: While preclinical data are promising, translating these technologies into clinical use requires rigorous assessment of long-term toxicity, dose optimization, scalable production, and regulatory compliance for CNS-targeted nanotherapies [146].
5.4. Metabolic Disorders: Anti-Diabetic and Anti-Obesity Actions
- Glycemic control and insulin sensitization: Nanosystems encapsulating berberine, quercetin, and cinnamon polyphenols have shown improved intestinal absorption and bioactivity, leading to reduced fasting glucose, enhanced insulin sensitivity, and decreased HbA1c in diabetic animal models [147]. Silver and chitosan nanoparticles aid in increasing pancreatic β-cell survival and functionality through their controlled release characteristics [148].
- Anti-obesity effects: Nanoformulations that improve the bioavailability of curcumin or catechins have been shown to inhibit adipogenesis, bolster thermogenesis, and reduce fat accumulation in high-fat-diet rodent models [149]. Lipid polymer hybrids loaded with anti-obesity phytochemicals have achieved sustained release in adipose tissues, improving satiety hormone profiles and suppressing inflammatory cytokines [150].
- Lipid profile modulation: Nano-delivered silymarin and resveratrol have demonstrated significant reductions in LDL and triglycerides, with concurrent increases in HDL cholesterol [151]. Liposomal formulations appear to amplify the cholesterol-lowering mechanisms of phytochemicals.
- Safety and therapeutic outcomes: Nanocarrier-based approaches typically show good tolerability in preclinical studies; however, long-term analyses are necessary to verify metabolic safety and assess off-target effects in chronic administration settings [152].
5.5. Infectious Diseases: Antibacterial, Antiviral, and Antifungal Applications
- Antibacterial strategies: Phytochemical-loaded nanoparticles combine the intrinsic antimicrobial properties of metal (e.g., Ag, ZnO) or polymeric materials with the bioactivity of plant compounds. Silver nanoparticle–curcumin conjugates, for instance, exhibit synergistic bactericidal effects against MRSA and E. coli, disrupting biofilm formation and increasing bacterial membrane disruption [157].
- Antiviral delivery systems: Nano-encapsulation of quercetin, baicalin, or cynanoside enhances antiviral activity against influenza, herpes simplex, and coronaviruses. Phytochemical–gold nanoparticle conjugates block viral entry and replication via actions like ACE2 receptor inhibition or protease binding [153].
- Antifungal efficacy: Nanocapsules containing eugenol, thymol, and other essential oils maintain effective drug concentrations in target tissues, adding precision to fungal membrane disruption and inhibiting virulence factors [154].
- Addressing antibiotic resistance: These systems employ multifunctional mechanisms—ROS production, membrane interaction, efflux pump inhibition—delivering therapeutic synergy at lower, safer doses while reducing the potential for resistance development [93].
- Stability and administration: Encapsulation ensures controlled release, improved bioavailability, and enhanced topical or inhalable delivery, offering versatility in treating cutaneous, pulmonary, and systemic infections [155].
6. Pharmacokinetics and Toxicological Considerations
6.1. Bioavailability and Controlled Release Kinetics
6.2. In Vivo Biodistribution and Organ Targeting
6.3. Biocompatibility and Immunogenicity Assessment
6.4. Regulatory Guidelines for Herbal Nanomedicine Safety
7. Clinical Advancements and Translational Challenges
7.1. Preclinical Studies and Clinical Trials of Nano-Phytomedicines
7.2. Commercial Products and Patent Landscape
7.3. Scale-Up and Manufacturing Barriers
7.4. Cost, Accessibility, and Acceptance in Healthcare Systems
8. Future Prospects and Emerging Trends
8.1. Smart and Responsive Nano-Phytomedicine Systems
8.2. Artificial Intelligence and Computational Modeling in Formulation Design
8.3. Personalized Nano-Phytotherapy and Precision Medicine Approaches
8.4. Sustainable and Green Nanotechnology Integration
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paul, J.K.; Azmal, M.; Haque, A.S.N.B.; Talukder, O.F.; Meem, M.; Ghosh, A. Phytochemical-Mediated Modulation of Signaling Pathways: A Promising Avenue for Drug Discovery. Adv. Redox Res. 2024, 13, 100113. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and Challenges of Plant-Anticancer Compounds in Cancer Treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Herdiana, Y. Nanoparticles of Natural Product-Derived Medicines: Beyond the Pandemic. Heliyon 2025, 11, e42739. [Google Scholar] [CrossRef] [PubMed]
- Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rău, G.; Pîrvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocîlteu, M.V. Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics 2025, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Devaraji, M.; Thanikachalam, P.V. Phytoconstituents as Emerging Therapeutics for Breast Cancer: Mechanistic Insights and Clinical Implications. Cancer Pathog. Ther. 2025, in press. [CrossRef]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of Turmeric and Its Principal Bioactive Curcumin on Human Health: Pharmaceutical, Medicinal, and Food Applications: A Comprehensive Review. Front. Nutr. 2023, 9, 1040259. [Google Scholar] [CrossRef]
- de Luna, F.C.F.; Ferreira, W.A.S.; Casseb, S.M.M.; de Oliveira, E.H.C. Anticancer Potential of Flavonoids: An Overview with an Emphasis on Tangeretin. Pharmaceuticals 2023, 16, 1229. [Google Scholar] [CrossRef]
- Goktas, Z.; Zu, Y.; Abbasi, M.; Galyean, S.; Wu, D.; Fan, Z.; Wang, S. Recent Advances in Nanoencapsulation of Phytochemicals to Combat Obesity and Its Comorbidities. J. Agric. Food Chem. 2020, 68, 8119–8131. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhao, Y.-G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.; Roy, P. Soluble Nanoparticles Versatile Tools for Plant Tissue Imaging. J. Bionanoscience 2013, 7, 256–259. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Joo, S.W.; Park, S.-S.; Mandal, T.K. Recent Advances in the Characterized Identification of Mono-to-Multi-Layer Graphene and Its Biomedical Applications: A Review. Electronics 2022, 11, 3345. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K. Dually Emissive P,N-Co-Doped Carbon Dots for Fluorescent and Photoacoustic Tissue Imaging in Living Mice. Microchim. Acta 2017, 184, 1117–1125. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K. Synthesis of a Highly Fluorescence Nitrogen-Doped Carbon Quantum Dots Bioimaging Probe and Its In Vivo Clearance and Printing Applications. RSC Adv. 2016, 6, 18134–18140. [Google Scholar] [CrossRef]
- Mandal, T.K.; Parvin, N. Rapid Detection of Bacteria by Carbon Quantum Dots. J. Biomed. Nanotechnol. 2011, 7, 846–848. [Google Scholar] [CrossRef]
- Mandal, T.K.; Parvin, N.; Joo, S.W. PH-Responsive Biocompatible Fluorescent Core-Shell Nanogel for Intracellular Imaging and Control Drug Release. Part. Part. Syst. Charact. 2021, 38, 2100110. [Google Scholar] [CrossRef]
- Islam, S.; Ahmed, M.M.S.; Islam, M.A.; Hossain, N.; Chowdhury, M.A. Advances in Nanoparticles in Targeted Drug Delivery–A Review. Results Surf. Interfaces 2025, 19, 100529. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K.; Joo, S.-W. The Impact of COVID-19 on RNA Therapeutics: A Surge in Lipid Nanoparticles and Alternative Delivery Systems. Pharmaceutics 2024, 16, 1366. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Enhancing Vaccine Efficacy and Stability: A Review of the Utilization of Nanoparticles in MRNA Vaccines. Biomolecules 2024, 14, 1036. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Lee, R.J.; Xiang, G. Nano Encapsulated Curcumin: And Its Potential for Biomedical Applications. Int. J. Nanomed. 2020, 15, 3099–3120. [Google Scholar] [CrossRef]
- Bajracharya, R.; Song, J.G.; Patil, B.R.; Lee, S.H.; Noh, H.-M.; Kim, D.-H.; Kim, G.-L.; Seo, S.-H.; Park, J.-W.; Jeong, S.H.; et al. Functional Ligands for Improving Anticancer Drug Therapy: Current Status and Applications to Drug Delivery Systems. Drug Deliv. 2022, 29, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Ahmad, Z.; Ajaj, R.; Zhang, H.; Ibrahim, M.; Muhammad, N.; Al-Awthan, Y.S.; Bahattab, O.S.; Ullah, I. Green Synthesis an Eco-Friendly Route for the Synthesis of Iron Oxide Nanoparticles Using Aqueous Extract of Thevetia Peruviana and Their Biological Activities. Sci. Rep. 2025, 15, 18316. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Biodegradable and Stimuli-Responsive Nanomaterials for Targeted Drug Delivery in Autoimmune Diseases. J. Funct. Biomater. 2025, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.K.; Singh, V.V.; Solanki, M.K.; Kumar, A.; Ruokolainen, J.; Kesari, K.K. Smart Nanomaterials in Cancer Theranostics: Challenges and Opportunities. ACS Omega 2023, 8, 14290–14320. [Google Scholar] [CrossRef] [PubMed]
- Teli, D.; Satasia, R.; Patel, V.; Nair, R.; Khatri, R.; Gala, D.; Balar, P.C.; Patel, K.; Sharma, A.; Vadodariya, P.; et al. Nature Meets Technology: Harnessing Nanotechnology to Unleash the Power of Phytochemicals. Clin. Tradit. Med. Pharmacol. 2024, 5, 200139. [Google Scholar] [CrossRef]
- Ciupei, D.; Colişar, A.; Leopold, L.; Stănilă, A.; Diaconeasa, Z.M. Polyphenols: From Classification to Therapeutic Potential and Bioavailability. Foods 2024, 13, 4131. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and Their Role in Oxidative Stress, Inflammation, and Human Diseases. Chem. Biol. Interact. 2025, 413, 111489. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.M.; Alshawsh, M.A. Therapeutic Potential of Certain Terpenoids as Anticancer Agents: A Scoping Review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef]
- Kubatka, P.; Mazurakova, A.; Samec, M.; Koklesova, L.; Zhai, K.; AL-Ishaq, R.; Kajo, K.; Biringer, K.; Vybohova, D.; Brockmueller, A.; et al. Flavonoids against Non-Physiologic Inflammation Attributed to Cancer Initiation, Development, and Progression—3PM Pathways. EPMA J. 2021, 12, 559–587. [Google Scholar] [CrossRef]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A Review of Cardiac Glycosides: Structure, Toxicokinetics, Clinical Signs, Diagnosis and Antineoplastic Potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef]
- Bhuyan, U.; Handique, J.G. Plant Polyphenols as Potent Antioxidants: Highlighting the Mechanism of Antioxidant Activity and Synthesis/Development of Some Polyphenol Conjugates. In Bioactive Natural Products; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–266. [Google Scholar]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Sui, B.; Wang, M.; Hu, X.; Shi, S.; Xu, P. Carrier-Free Nanoassembly of Curcumin–Erlotinib Conjugate for Cancer Targeted Therapy. Adv. Healthc. Mater. 2020, 9, e2001128. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, Z.; Huang, D.; Sun, C.; Xie, J.; Chen, T.; Zhao, X.; Huang, Y.; Li, D.; Wu, B.; et al. Myricetin Inhibits TNF-α-Induced Inflammation in A549 Cells via the SIRT1/NF-ΚB Pathway. Pulm. Pharmacol. Ther. 2020, 65, 102000. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-C.; Huang, W.-C.; Pang, J.-H.S.; Wu, Y.-H.; Cheng, C.-Y. Quercetin Inhibits the Production of IL-1β-Induced Inflammatory Cytokines and Chemokines in ARPE-19 Cells via the MAPK and NF-ΚB Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 2957. [Google Scholar] [CrossRef]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Singh, M.; Devi, S.; Rana, V.S.; Mishra, B.B.; Kumar, J.; Ahluwalia, V. Delivery of Phytochemicals by Liposome Cargos: Recent Progress, Challenges and Opportunities. J. Microencapsul. 2019, 36, 215–235. [Google Scholar] [CrossRef]
- Stănciuc, N.; Râpeanu, G. Kinetics of Phytochemicals Degradation During Thermal Processing of Fruits Beverages. In Non-Alcoholic Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 407–440. [Google Scholar]
- Nikou, T.; Sakavitsi, M.E.; Kalampokis, E.; Halabalaki, M. Metabolism and Bioavailability of Olive Bioactive Constituents Based on In Vitro, In Vivo and Human Studies. Nutrients 2022, 14, 3773. [Google Scholar] [CrossRef]
- Rahat, I.; Yadav, P.; Singhal, A.; Fareed, M.; Purushothaman, J.R.; Aslam, M.; Balaji, R.; Patil-Shinde, S.; Rizwanullah, M. Polymer Lipid Hybrid Nanoparticles for Phytochemical Delivery: Challenges, Progress, and Future Prospects. Beilstein J. Nanotechnol. 2024, 15, 1473–1497. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.; Huang, W.; Zhou, H.; Zhang, W. Drug-Microbiota Interactions: An Emerging Priority for Precision Medicine. Signal Transduct. Target. Ther. 2023, 8, 386. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- Vosoughi, P.; Naghib, S.M.; Jafari, T.; Kangarshahi, B.M. Chitosan-Encapsulated Lipid-Based Nanovesicles for Therapeutic Applications and Tissue Engineering: A Review. Carbohydr. Polym. Technol. Appl. 2025, 10, 100805. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yuhong, J.; Xin, P.; Han, J.L.; Du, Y.; Yu, X.; Zhu, R.; Zhang, M.; Chen, W.; et al. Advances in Nanotechnology for Enhancing the Solubility and Bioavailability of Poorly Soluble Drugs. Drug Des. Dev. Ther. 2024, 18, 1469–1495. [Google Scholar] [CrossRef]
- Ahmad, K.; Zhang, Y.; Chen, P.; Yang, X.; Hou, H. Chitosan Interaction with Stomach Mucin Layer to Enhances Gastric Retention and Mucoadhesive Properties. Carbohydr. Polym. 2024, 333, 121926. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Cirri, M.; Mennini, N. Multiple Roles of Chitosan in Mucosal Drug Delivery: An Updated Review. Mar. Drugs 2022, 20, 335. [Google Scholar] [CrossRef] [PubMed]
- Bavnhøj, C.G.; Knopp, M.M.; Löbmann, K. Effect of Drug Loading in Mesoporous Silica on Amorphous Stability and Performance. Pharmaceutics 2024, 16, 163. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Lechanteur, A.; Cossais, F.; Bellefroid, C.; Arnold, P.; Lucius, R.; Held-Feindt, J.; Piel, G.; Hattermann, K. Liposomal Encapsulated Curcumin Effectively Attenuates Neuroinflammatory and Reactive Astrogliosis Reactions in Glia Cells and Organotypic Brain Slices. Int. J. Nanomed. 2020, 15, 3649–3667. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Akanda, M.; Mithu, M.S.H.; Douroumis, D. Solid Lipid Nanoparticles: An Effective Lipid-Based Technology for Cancer Treatment. J. Drug Deliv. Sci. Technol. 2023, 86, 104709. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Subroto, E.; Andoyo, R.; Indiarto, R. Solid Lipid Nanoparticles: Review of the Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds. Antioxidants 2023, 12, 633. [Google Scholar] [CrossRef]
- Mall, J.; Naseem, N.; Haider, M.F.; Rahman, M.A.; Khan, S.; Siddiqui, S.N. Nanostructured Lipid Carriers as a Drug Delivery System: A Comprehensive Review with Therapeutic Applications. Intell. Pharm. 2024, in press. [CrossRef]
- Panwar, P.; Kumar, S.; Chand, P.; Chauhan, A.S.; Jakhmola, V. Nanostructured Lipid Carriers (NLCs): A Comprehensive Review of Drug Delivery Advancements. J. Appl. Pharm. Res. 2025, 13, 20–38. [Google Scholar] [CrossRef]
- Uti, D.E.; Alum, E.U.; Atangwho, I.J.; Ugwu, O.P.-C.; Egbung, G.E.; Aja, P.M. Lipid-Based Nano-Carriers for the Delivery of Anti-Obesity Natural Compounds: Advances in Targeted Delivery and Precision Therapeutics. J. Nanobiotechnology 2025, 23, 336. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Solanki, J.; Kher, M.M.; Azagury, A. A Review: Surface Engineering of Lipid-Based Drug Delivery Systems. Small 2024, 20, e2401990. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Qazi, U.Y.; Mansha, A.; Bhatti, I.A.; Javaid, R.; Abbas, Q.; Nadeem, N.; Rehan, Z.A.; Noreen, S.; Zahid, M. Recent Developments for Antimicrobial Applications of Graphene-Based Polymeric Composites: A Review. J. Ind. Eng. Chem. 2021, 100, 40–58. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Jangde, R.K. Current Updated Review on Preparation of Polymeric Nanoparticles for Drug Delivery and Biomedical Applications. Next Nanotechnol. 2023, 2, 100013. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges. Polymers 2024, 16, 2536. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Montero, M.P. Enhancement of Oral Bioavailability of Natural Compounds and Probiotics by Mucoadhesive Tailored Biopolymer-Based Nanoparticles: A Review. Food Hydrocoll. 2021, 118, 106772. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Kunde, S.S.; Wairkar, S. Targeted Delivery of Albumin Nanoparticles for Breast Cancer: A Review. Colloids Surf. B Biointerfaces 2022, 213, 112422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Shen, W.; Li, M.; Wang, W.; Jin, X. Trend of Albumin Nanoparticles in Oncology: A Bibliometric Analysis of Research Progress and Prospects. Front. Pharmacol. 2024, 15, 1409163. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef]
- Pawlik, J.; Łukowicz, K.; Cholewa-Kowalska, K.; Osyczka, A.M. New Insights into the PLGA and PCL Blending: Physico-Mechanical Properties and Cell Response. Mater. Res. Express 2019, 6, 085344. [Google Scholar] [CrossRef]
- Misir, J.; Kassama, L. Nanoencapsulation of Lycopene by PLGA: Effect on Physicochemical Properties and Extended-Release Kinetics in a Simulated GIT System. LWT 2025, 225, 117747. [Google Scholar] [CrossRef]
- Arpagaus, C. PLA/PLGA Nanoparticles Prepared by Nano Spray Drying. J. Pharm. Investig. 2019, 49, 405–426. [Google Scholar] [CrossRef]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-Based Nanomedicines Manufacturing: Technologies Overview and Challenges in Industrial Scale-Up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef]
- Sivadasan, D.; Sultan, M.H.; Madkhali, O.; Almoshari, Y.; Thangavel, N. Polymeric Lipid Hybrid Nanoparticles (PLNs) as Emerging Drug Delivery Platform—A Comprehensive Review of Their Properties, Preparation Methods, and Therapeutic Applications. Pharmaceutics 2021, 13, 1291. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid Polymer Hybrid Nanoparticles: A Custom-Tailored Next-Generation Approach for Cancer Therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Alsehli, M. Polymeric Nanocarriers as Stimuli-Responsive Systems for Targeted Tumor (Cancer) Therapy: Recent Advances in Drug Delivery. Saudi Pharm. J. 2020, 28, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef] [PubMed]
- Nadaf, S.J.; Jadhav, N.R.; Naikwadi, H.S.; Savekar, P.L.; Sapkal, I.D.; Kambli, M.M.; Desai, I.A. Green Synthesis of Gold and Silver Nanoparticles: Updates on Research, Patents, and Future Prospects. OpenNano 2022, 8, 100076. [Google Scholar] [CrossRef]
- Serdar, G.; Gül Kılınç, G.; Mazlum Şen, T. Green One-Pot Synthesis of Silver and Gold Nanoparticles Using Catechin Extracts: Influence of Temperature and Antioxidant Activity Evaluation. Plasmonics 2025, 1–20. [Google Scholar] [CrossRef]
- Sadalage, P.S.; Patil, R.V.; Havaldar, D.V.; Gavade, S.S.; Santos, A.C.; Pawar, K.D. Optimally Biosynthesized, PEGylated Gold Nanoparticles Functionalized with Quercetin and Camptothecin Enhance Potential Anti-Inflammatory, Anti-Cancer and Anti-Angiogenic Activities. J. Nanobiotechnology 2021, 19, 84. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; De Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in Silver Nanoparticles: A Comprehensive Review on Their Potential as Antimicrobial Agents and Their Mechanisms of Action Elucidated by Proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef]
- Perera, W.P.T.D.; Dissanayake, R.K.; Ranatunga, U.I.; Hettiarachchi, N.M.; Perera, K.D.C.; Unagolla, J.M.; De Silva, R.T.; Pahalagedara, L.R. Curcumin Loaded Zinc Oxide Nanoparticles for Activity-Enhanced Antibacterial and Anticancer Applications. RSC Adv. 2020, 10, 30785–30795. [Google Scholar] [CrossRef]
- Turrina, C.; Schoenen, M.; Milani, D.; Klassen, A.; Rojas Gonzaléz, D.M.; Cvirn, G.; Mela, P.; Berensmeier, S.; Slabu, I.; Schwaminger, S.P. Application of Magnetic Iron Oxide Nanoparticles: Thrombotic Activity, Imaging and Cytocompatibility of Silica-Coated and Carboxymethyl Dextrane-Coated Particles. Colloids Surf. B Biointerfaces 2023, 228, 113428. [Google Scholar] [CrossRef]
- Aulifa, D.L.; Amarilis, B.; Ichsani, L.N.; Maharani, D.S.; Shabrina, A.M.; Hanifah, H.; Wulandari, R.P.; Rusdin, A.; Subra, L.; Budiman, A. A Comprehensive Review: Mesoporous Silica Nanoparticles Greatly Improve Pharmacological Effectiveness of Phytoconstituent in Plant Extracts. Pharmaceuticals 2024, 17, 1684. [Google Scholar] [CrossRef]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Nanomaterial-Based Encapsulation for Controlled Gastrointestinal Delivery of Viable Probiotic Bacteria. Nanoscale Adv. 2021, 3, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhou, Y.; Fan, T.; Lin, Y.; Zhang, H.; Mei, L. Inorganic Nano-Carriers Based Smart Drug Delivery Systems for Tumor Therapy. Smart Mater. Med. 2020, 1, 32–47. [Google Scholar] [CrossRef]
- Unnikrishnan, G.; Joy, A.; Megha, M.; Kolanthai, E.; Senthilkumar, M. Exploration of Inorganic Nanoparticles for Revolutionary Drug Delivery Applications: A Critical Review. Discov. Nano 2023, 18, 157. [Google Scholar] [CrossRef]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-Polymer Hybrid Nanoparticles: Synthesis Strategies and Biomedical Applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yu, Q.; Tan, L.; Wang, Q.; Zhu, W. Tumor Microenvironment-Responsive Polymer Delivery Platforms for Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2025, 64, e202503776. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Ma, Y.; Xu, X.; Xie, J.; Ju, S. Stimuli-Responsive Polymeric Nanoplatforms for Cancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 707319. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Pawar, A.; Mahadik, K.R.; Gajbhiye, V. PEGylated Nanocarriers: A Promising Tool for Targeted Delivery to the Brain. Colloids Surf. B Biointerfaces 2020, 187, 110770. [Google Scholar] [CrossRef]
- Sadeghzadeh, F.; Motavalizadehkakhky, A.; Mehrzad, J.; Zhiani, R.; Homayouni Tabrizi, M. Folic Acid Conjugated-Chitosan Modified Nanostructured Lipid Carriers as Promising Carriers for Delivery of Umbelliprenin to Cancer Cells: In Vivo and In Vitro. Eur. Polym. J. 2023, 186, 111849. [Google Scholar] [CrossRef]
- Kim, S.M.; Patel, M.; Patel, R. PLGA Core-Shell Nano/Microparticle Delivery System for Biomedical Application. Polymers 2021, 13, 3471. [Google Scholar] [CrossRef]
- Idoudi, S.; Ismail, R.; Rachid, O.; Elhissi, A.; Alkilany, A.M. The Golden Liposomes: Preparation and Biomedical Applications of Gold-Liposome Nanocomposites. J. Nanotheranostics 2023, 4, 201–227. [Google Scholar] [CrossRef]
- Koga, K.; Tagami, T.; Ozeki, T. Gold Nanoparticle-Coated Thermosensitive Liposomes for the Triggered Release of Doxorubicin, and Photothermal Therapy Using a near-Infrared Laser. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127038. [Google Scholar] [CrossRef]
- Luiz, M.T.; Dutra, J.A.P.; Viegas, J.S.R.; de Araújo, J.T.C.; Tavares Junior, A.G.; Chorilli, M. Hybrid Magnetic Lipid-Based Nanoparticles for Cancer Therapy. Pharmaceutics 2023, 15, 751. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Viscardi, S.; Szewczyk, W.; Topola, E. Natural Alkaloids in Cancer Therapy: Berberine, Sanguinarine and Chelerythrine against Colorectal and Gastric Cancer. Int. J. Mol. Sci. 2024, 25, 8375. [Google Scholar] [CrossRef]
- Isaković, S.; Senćanski, M.; Perović, V.; Stevanović, K.; Prodić, I. Bioinformatic Selection of Mannose-Specific Lectins from Allium Genus as SARS-CoV-2 Inhibitors Analysing Protein–Protein Interaction. Life 2025, 15, 162. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Martín del Valle, E. Nature-Inspired Nanoparticles as Paclitaxel Targeted Carrier for the Treatment of HER2-Positive Breast Cancer. Cancers 2021, 13, 2526. [Google Scholar] [CrossRef]
- Chen, B.-Q.; Zhao, Y.; Zhang, Y.; Pan, Y.-J.; Xia, H.-Y.; Kankala, R.K.; Wang, S.-B.; Liu, G.; Chen, A.-Z. Immune-Regulating Camouflaged Nanoplatforms: A Promising Strategy to Improve Cancer Nano-Immunotherapy. Bioact. Mater. 2023, 21, 1–19. [Google Scholar] [CrossRef]
- Vadevoo, S.M.P.; Gurung, S.; Lee, H.-S.; Gunassekaran, G.R.; Lee, S.-M.; Yoon, J.-W.; Lee, Y.-K.; Lee, B. Peptides as Multifunctional Players in Cancer Therapy. Exp. Mol. Med. 2023, 55, 1099–1109. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-Dependent Internalization of Particles via the Pathways of Clathrin- and Caveolae-Mediated Endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Y.; Chong, K.; Cui, M.; Cao, Z.; Tang, C.; Tian, Z.; Hu, Y.; Zhao, Y.; Jiang, S. Recent Advances in Lipid Nanoparticles and Their Safety Concerns for MRNA Delivery. Vaccines 2024, 12, 1148. [Google Scholar] [CrossRef]
- Shirsat, S.D.; Londhe, P.V.; Gaikwad, A.P.; Rizwan, M.; Laha, S.S.; Khot, V.M.; Achal, V.; Tabish, T.A.; Thorat, N.D. Endosomal Escape in Magnetic Nanostructures: Recent Advances and Future Perspectives. Mater. Today Adv. 2024, 22, 100484. [Google Scholar] [CrossRef]
- Ni, J.; Tian, F.; Dahmani, F.Z.; Yang, H.; Yue, D.; He, S.; Zhou, J.; Yao, J. Curcumin-Carboxymethyl Chitosan (CNC) Conjugate and CNC/LHR Mixed Polymeric Micelles as New Approaches to Improve the Oral Absorption of P-Gp Substrate Drugs. Drug Deliv. 2016, 23, 3424–3435. [Google Scholar] [CrossRef]
- Majrashi, T.A.; Alshehri, S.A.; Alsayari, A.; Bin Muhsinah, A.; Alrouji, M.; Alshahrani, A.M.; Shamsi, A.; Atiya, A. Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics. Nutrients 2023, 15, 1704. [Google Scholar] [CrossRef]
- Karnwal, A.; Jassim, A.Y.; Mohammed, A.A.; Sharma, V.; Al-Tawaha, A.R.M.S.; Sivanesan, I. Nanotechnology for Healthcare: Plant-Derived Nanoparticles in Disease Treatment and Regenerative Medicine. Pharmaceuticals 2024, 17, 1711. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan Meenakshi, D.; Narde, G.K.; Ahuja, A.; Al Balushi, K.; Francis, A.P.; Khan, S.A. Therapeutic Applications of Nanoformulated Resveratrol and Quercetin Phytochemicals in Colorectal Cancer—An Updated Review. Pharmaceutics 2024, 16, 761. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Chang, J.-H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-Drug Loaded Nanoparticles of Epigallocatechin-3-Gallate (EGCG)/Ascorbic Acid Enhance Therapeutic Efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s Disease Mice Model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Sharma, R.; Sharma, S.; Mittal, A.; Deodhar, N. Drug–Polymer Conjugate Tailoring by Disulfide Linkage for Controlled and Targeted Drug Delivery. In Polymer-Drug Conjugates; Elsevier: Amsterdam, The Netherlands, 2023; pp. 171–192. [Google Scholar]
- Solanki, R.; Jodha, B.; Prabina, K.E.; Aggarwal, N.; Patel, S. Recent Advances in Phytochemical Based Nano-Drug Delivery Systems to Combat Breast Cancer: A Review. J. Drug Deliv. Sci. Technol. 2022, 77, 103832. [Google Scholar] [CrossRef]
- Chowdhury, S.; Kumari, G.; Jit, T. Nano-Formulation of Phytochemicals to Combat Neurodegenerative Diseases: Current and Thorough Strategies. Pharmacol. Res.—Nat. Prod. 2025, 7, 100249. [Google Scholar] [CrossRef]
- Cherian, E.; Rosamma; Anju, K.A.; Naushad, B. Nanotechnology in Energy Supplements: Sustained Endurance. In Nanofuel: The Future of Sports Nutrition; Springer: Singapore, 2025; pp. 201–208. [Google Scholar]
- Sun, S.; Lv, W.; Li, S.; Zhang, Q.; He, W.; Min, Z.; Teng, C.; Chen, Y.; Liu, L.; Yin, J.; et al. Smart Liposomal Nanocarrier Enhanced the Treatment of Ischemic Stroke through Neutrophil Extracellular Traps and Cyclic Guanosine Monophosphate-Adenosine Monophosphate Synthase-Stimulator of Interferon Genes (CGAS-STING) Pathway Inhibition of Ischemic Pen. ACS Nano 2023, 17, 17845–17857. [Google Scholar] [CrossRef]
- Trofin, A.-M.; Scripcariu, D.V.; Filipiuc, S.-I.; Neagu, A.-N.; Filipiuc, L.-E.; Tamba, B.-I.; Palaghia, M.M.; Uritu, C.M. From Nature to Nanomedicine: Enhancing the Antitumor Efficacy of Rhein, Curcumin, and Resveratrol. Medicina 2025, 61, 981. [Google Scholar] [CrossRef]
- Cavalcante de Freitas, P.G.; Rodrigues Arruda, B.; Araújo Mendes, M.G.; Barroso de Freitas, J.V.; da Silva, M.E.; Sampaio, T.L.; Petrilli, R.; Eloy, J.O. Resveratrol-Loaded Polymeric Nanoparticles: The Effects of D-α-Tocopheryl Polyethylene Glycol 1000 Succinate (TPGS) on Physicochemical and Biological Properties against Breast Cancer In Vitro and In Vivo. Cancers 2023, 15, 2802. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current Advance of Nanotechnology in Diagnosis and Treatment for Malignant Tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Rizwanullah, M.; Altamimi, A.S.A.; Alossaimi, M.A.; Kamal, M.; Ahmad, J. Harnessing Natural Polysaccharides-Based Nanoparticles for Oral Delivery of Phytochemicals: Knocking down the Barriers. J. Drug Deliv. Sci. Technol. 2023, 82, 104368. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, D.; Xue, G.; Yu, S.; Yuan, C.; Huang, M.; Jiang, L. Improved Therapeutic Efficacy of Quercetin-Loaded Polymeric Nanoparticles on Triple-Negative Breast Cancer by Inhibiting UPA. RSC Adv. 2020, 10, 34517–34526. [Google Scholar] [CrossRef]
- Cunha, C.; Daniel-da-Silva, A.L.; Oliveira, H. Drug Delivery Systems and Flavonoids: Current Knowledge in Melanoma Treatment and Future Perspectives. Micromachines 2022, 13, 1838. [Google Scholar] [CrossRef]
- Tu, J.; Xu, Y.; Xu, J.; Ling, Y.; Cai, Y. Chitosan Nanoparticles Reduce LPS-Induced Inflammatory Reaction via Inhibition of NF-ΚB Pathway in Caco-2 Cells. Int. J. Biol. Macromol. 2016, 86, 848–856. [Google Scholar] [CrossRef]
- Singh Patel, P.; Srivastava, R.; Panchawat, S. Role of Apoptotic-Targeted Phytoconstitutent-Loaded Antipsoriatic Nanobiocomposites. Recent Pat. Nanotechnol. 2024, 18, 220–236. [Google Scholar] [CrossRef]
- Hedayati, N.; Safari, M.H.; Milasi, Y.E.; Kahkesh, S.; Farahani, N.; Khoshnazar, S.M.; Dorostgou, Z.; Alaei, E.; Alimohammadi, M.; Rahimzadeh, P.; et al. Modulation of the PI3K/Akt Signaling Pathway by Resveratrol in Cancer: Molecular Mechanisms and Therapeutic Opportunity. Discov. Oncol. 2025, 16, 669. [Google Scholar] [CrossRef]
- Nurkolis, F.; Taslim, N.A.; Syahputra, R.A.; d’Arqom, A.; Tjandrawinata, R.R.; Purba, A.K.R.; Mustika, A. Food Phytochemicals as Epigenetic Modulators in Diabetes: A Systematic Review. J. Agric. Food Res. 2025, 21, 101873. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Momeni, M.R. Epigallocatechin-3-Gallate and Its Nanoformulation in Cervical Cancer Therapy: The Role of Genes, MicroRNA and DNA Methylation Patterns. Cancer Cell Int. 2023, 23, 335. [Google Scholar] [CrossRef] [PubMed]
- Minafra, L.; Porcino, N.; Bravatà, V.; Gaglio, D.; Bonanomi, M.; Amore, E.; Cammarata, F.P.; Russo, G.; Militello, C.; Savoca, G.; et al. Radiosensitizing Effect of Curcumin-Loaded Lipid Nanoparticles in Breast Cancer Cells. Sci. Rep. 2019, 9, 11134. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Z.; Qiu, Y.; Liu, Y.; Ding, M.; Zhang, Y. Anti-MiRNA21 and Resveratrol-Loaded Polysaccharide-Based Mesoporous Silica Nanoparticle for Synergistic Activity in Gastric Carcinoma. J. Drug Target. 2019, 27, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Di Pompo, G.; Cortini, M.; Palomba, R.; Di Francesco, V.; Bellotti, E.; Decuzzi, P.; Baldini, N.; Avnet, S. Curcumin-Loaded Nanoparticles Impair the Pro-Tumor Activity of Acid-Stressed MSC in an In Vitro Model of Osteosarcoma. Int. J. Mol. Sci. 2021, 22, 5760. [Google Scholar] [CrossRef]
- Roy, S.; Deka, D.; Kondaveeti, S.B.; Ayyadurai, P.; Siripragada, S.; Philip, N.; Pathak, S.; Duttaroy, A.K.; Banerjee, A. An Overview of Potential of Natural Compounds to Regulate Epigenetic Modifications in Colorectal Cancer: A Recent Update. Epigenetics 2025, 20, 2491316. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Efficacy of Polymer-Based Nanocarriers for Co-Delivery of Curcumin and Selected Anticancer Drugs. Nanomaterials 2020, 10, 1556. [Google Scholar] [CrossRef]
- Caon, T.; Mazzarino, L.; Simões, C.M.O.; Senna, E.L.; Silva, M.A.S. Lipid- and Polymer-Based Nanostructures for Cutaneous Delivery of Curcumin. AAPS PharmSciTech 2017, 18, 920–925. [Google Scholar] [CrossRef]
- Wongrakpanich, A.; Bui Thi Thu, H.; Sakchaisri, K.; Taresco, V.; Crucitti, V.C.; Bunsupa, S.; Suksiriworapong, J. Co-Delivery of Curcumin and Resveratrol by Folic Acid-Conjugated Poly(Glycerol Adipate) Nanoparticles for Enhanced Synergistic Anticancer Effect against Osteosarcoma. J. Drug Deliv. Sci. Technol. 2024, 95, 105610. [Google Scholar] [CrossRef]
- Sitia, L.; Sevieri, M.; Signati, L.; Bonizzi, A.; Chesi, A.; Mainini, F.; Corsi, F.; Mazzucchelli, S. HER-2-Targeted Nanoparticles for Breast Cancer Diagnosis and Treatment. Cancers 2022, 14, 2424. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, X.; Zhang, J.; Zhu, M.; Tian, M.; Zhao, P. Advances in Understanding Ferroptosis Mechanisms and Their Impact on Immune Cell Regulation and Tumour Immunotherapy. Discov. Oncol. 2025, 16, 153. [Google Scholar] [CrossRef]
- Koklesova, L.; Jakubikova, J.; Cholujova, D.; Samec, M.; Mazurakova, A.; Šudomová, M.; Pec, M.; Hassan, S.T.S.; Biringer, K.; Büsselberg, D.; et al. Phytochemical-Based Nanodrugs Going beyond the State-of-the-Art in Cancer Management—Targeting Cancer Stem Cells in the Framework of Predictive, Preventive, Personalized Medicine. Front. Pharmacol. 2023, 14, 1121950. [Google Scholar] [CrossRef]
- Gaur, S.; Stein, E.B.; Schneider, D.K.; Masotti, M.; Davenport, M.S.; George, A.K.; Ellis, J.H. Gold Nanoshells for Prostate Cancer Treatment: Evidence for Deposition in Abdominal Organs. Abdom. Radiol. 2024, 49, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Zhou, L.; Li, L.; Zhou, P.; Shen, Z. Nano-Based Drug Delivery of Polyphenolic Compounds for Cancer Treatment: Progress, Opportunities, and Challenges. Pharmaceuticals 2023, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Tie, S.; Tan, M. Current Advances in Multifunctional Nanocarriers Based on Marine Polysaccharides for Colon Delivery of Food Polyphenols. J. Agric. Food Chem. 2022, 70, 903–915. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.; Gómez-Pastora, J.; et al. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small 2024, 20, e2304848. [Google Scholar] [CrossRef] [PubMed]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef]
- Javid-Naderi, M.J.; Abbasi, Z.; Fathi-karkan, S.; Shahgolzari, M.; Maleki-baladi, R.; Shayegh, F.; Ebrahimzadeh, A.; Banimohamad-Shotorbani, B.; Rahdar, A.; Babaei, M.; et al. Advancements in Nanocarrier-Mediated Sunitinib Delivery: Addressing Obstacles and Revealing Its Therapeutic Promise in Oncological Treatment. J. Drug Deliv. Sci. Technol. 2024, 100, 106107. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Sedaghat, M.; Hoseini, A.; Mohammadi, N.; Bodaghi, M. Combinational System of Lipid-Based Nanocarriers and Biodegradable Polymers for Wound Healing: An Updated Review. J. Funct. Biomater. 2023, 14, 115. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Surface-Modified Lipid Nanocarriers for Crossing the Blood-Brain Barrier (BBB): A Current Overview of Active Targeting in Brain Diseases. Colloids Surf. B Biointerfaces 2023, 221, 112999. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-Loaded Nanoparticles: A Novel Therapeutic Strategy in Treatment of Central Nervous System Disorders. Int. J. Nanomed. 2019, 14, 4449–4460. [Google Scholar] [CrossRef]
- Payne, A.; Nahashon, S.; Taka, E.; Adinew, G.M.; Soliman, K.F.A. Epigallocatechin-3-Gallate (EGCG): New Therapeutic Perspectives for Neuroprotection, Aging, and Neuroinflammation for the Modern Age. Biomolecules 2022, 12, 371. [Google Scholar] [CrossRef]
- Patel, V.; Chavda, V.; Shah, J. Nanotherapeutics in Neuropathologies: Obstacles, Challenges and Recent Advancements in CNS Targeted Drug Delivery Systems. Curr. Neuropharmacol. 2021, 19, 693–710. [Google Scholar] [CrossRef]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Behzadifar, S.; Barras, A.; Plaisance, V.; Pawlowski, V.; Szunerits, S.; Abderrahmani, A.; Boukherroub, R. Polymer-Based Nanostructures for Pancreatic Beta-Cell Imaging and Non-Invasive Treatment of Diabetes. Pharmaceutics 2023, 15, 1215. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, R.; Kondapavuluri, B.K.; Thangavelu, L.; Thiruvengadam, M.; Hatami, M.; Kim, J.H. Nanoparticle-Based Strategies with Bioactive Compounds for Targeting Oxidative Stress in Therapeutic Interventions: A Comprehensive Review. Ind. Crops Prod. 2025, 227, 120804. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Udeanu, D.; Coc, L.; Pop, A.; Cioates Negut, C.; Tanase, C.; Stan, R.; Meghea, A. Improved Anti-Obesity Effect of Herbal Active and Endogenous Lipids Co-Loaded Lipid Nanocarriers: Preparation, In Vitro and In Vivo Evaluation. Mater. Sci. Eng. C 2019, 99, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Padmanaban, S.; Baek, J.-W.; Chamarthy, S.S.; Chandrasekaran, S.; Samrot, A.V.; Gosu, V.; Park, I.-K.; Radhakrishnan, K.; Kim, D.-K. Nanoparticle-Based Therapeutic Strategies for Chronic Liver Diseases: Advances and Insights. Liver Res. 2025, 9, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing Nanotechnology to Improve Targeted Cancer Treatment: Overcoming Hurdles in Its Clinical Implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Sharma, G.; Song, L.F.; Merz, K.M. Effect of an Inhibitor on the ACE2-Receptor-Binding Domain of SARS-CoV-2. J. Chem. Inf. Model. 2022, 62, 6574–6585. [Google Scholar] [CrossRef]
- Hajibonabi, A.; Yekani, M.; Sharifi, S.; Nahad, J.S.; Dizaj, S.M.; Memar, M.Y. Antimicrobial Activity of Nanoformulations of Carvacrol and Thymol: New Trend and Applications. OpenNano 2023, 13, 100170. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Consoli, G.M.L.; Cafiso, V.; Stefani, S.; Geraci, C. Essential Oils Encapsulated in Polymer-Based Nanocapsules as Potential Candidates for Application in Food Preservation. Food Chem. 2018, 269, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.S. Developing Nanocarrier-Based Formulations of Antidiabetic Drugs Derived from Medicinal Plants: A Systemic Review. Pharmacol. Res. Nat. Prod. 2024, 2, 100004. [Google Scholar] [CrossRef]
- Dube, E.; Okuthe, G.E. Silver Nanoparticle-Based Antimicrobial Coatings: Sustainable Strategies for Microbial Contamination Control. Microbiol. Res. 2025, 16, 110. [Google Scholar] [CrossRef]
- Hallan, S.S.; Ferrara, F.; Cortesi, R.; Sguizzato, M. Potential of the Nano-Encapsulation of Antioxidant Molecules in Wound Healing Applications: An Innovative Strategy to Enhance the Bio-Profile. Molecules 2025, 30, 641. [Google Scholar] [CrossRef]
- Pattnaik, S.; Mohanty, S.; Sahoo, S.K.; Mohanty, C. A Mechanistic Perspective on the Role of Phytoconstituents-Based Pharmacotherapeutics and Their Topical Formulations in Chronic Wound Management. J. Drug Deliv. Sci. Technol. 2023, 84, 104546. [Google Scholar] [CrossRef]
- Spleis, H.; Sandmeier, M.; Claus, V.; Bernkop-Schnürch, A. Surface Design of Nanocarriers: Key to More Efficient Oral Drug Delivery Systems. Adv. Colloid Interface Sci. 2023, 313, 102848. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-Based Biodegradable Microspheres in Drug Delivery: Recent Advances in Research and Application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Pareek, A.; Kapoor, D.U.; Yadav, S.K.; Rashid, S.; Fareed, M.; Akhter, M.S.; Muteeb, G.; Gupta, M.M.; Prajapati, B.G. Advancing Lipid Nanoparticles: A Pioneering Technology in Cosmetic and Dermatological Treatments. Colloid Interface Sci. Commun. 2025, 64, 100814. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.; Zoulikha, M.; Boafo, G.F.; Magar, K.T.; Ju, Y.; He, W. Multifunctional Nanoparticle-Mediated Combining Therapy for Human Diseases. Signal Transduct. Target. Ther. 2024, 9, 1. [Google Scholar] [CrossRef]
- Kim, M.; Shin, M.; Zhao, Y.; Ghosh, M.; Son, Y. Transformative Impact of Nanocarrier-Mediated Drug Delivery: Overcoming Biological Barriers and Expanding Therapeutic Horizons. Small Sci. 2024, 4, 2400280. [Google Scholar] [CrossRef]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a Highway to the Brain: A Review on Nose-to-Brain Drug Delivery Using Nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Cheng, X.; Han, X.; Si, J.; Dong, C.; Ji, Z.; Zhao, S.; Wu, X.; Li, H.; Jin, X. Cationic Curcumin Nanocrystals Liposomes for Improved Oral Bioavailability: Formulation Development, Optimization, In Vitro and In Vivo Evaluation. Pharmaceutics 2024, 16, 1155. [Google Scholar] [CrossRef] [PubMed]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.-M. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Whittaker, A.K.; Han, F.Y.; Smith, M.T. Journey to the Market: The Evolution of Biodegradable Drug Delivery Systems. Appl. Sci. 2022, 12, 935. [Google Scholar] [CrossRef]
- Zandieh, M.A.; Farahani, M.H.; Daryab, M.; Motahari, A.; Gholami, S.; Salmani, F.; Karimi, F.; Samaei, S.S.; Rezaee, A.; Rahmanian, P.; et al. Stimuli-Responsive (Nano)Architectures for Phytochemical Delivery in Cancer Therapy. Biomed. Pharmacother. 2023, 166, 115283. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-Responsive Nanocarriers for Drug Delivery, Tumor Imaging, Therapy and Theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, C.; Wang, J.; Zhao, L.; Li, G. Research Progress in Nano-Drug Delivery Systems Based on the Characteristics of the Liver Cancer Microenvironment. Biomed. Pharmacother. 2024, 170, 116059. [Google Scholar] [CrossRef]
- Shi, Y.; Li, X.; Li, Z.; Sun, J.; Gao, T.; Wei, G.; Guo, Q. Nano-Formulations in Disease Therapy: Designs, Advances, Challenges, and Future Directions. J. Nanobiotechnology 2025, 23, 396. [Google Scholar] [CrossRef]
- Vagena, I.-A.; Malapani, C.; Gatou, M.-A.; Lagopati, N.; Pavlatou, E.A. Enhancement of EPR Effect for Passive Tumor Targeting: Current Status and Future Perspectives. Appl. Sci. 2025, 15, 3189. [Google Scholar] [CrossRef]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or Not to PEGylate: Immunological Properties of Nanomedicine’s Most Popular Component, Polyethylene Glycol and Its Alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of Polyethylene Glycol on the Surface of Nanoparticles for Targeted Drug Delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef]
- Shakori Poshteh, S.; Alipour, S.; Varamini, P. Harnessing Curcumin and Nanotechnology for Enhanced Treatment of Breast Cancer Bone Metastasis. Discov. Nano 2024, 19, 177. [Google Scholar] [CrossRef] [PubMed]
- Madamsetty, V.S.; Pal, K.; Keshavan, S.; Caulfield, T.R.; Dutta, S.K.; Wang, E.; Fadeel, B.; Mukhopadhyay, D. Development of Multi-Drug Loaded PEGylated Nanodiamonds to Inhibit Tumor Growth and Metastasis in Genetically Engineered Mouse Models of Pancreatic Cancer. Nanoscale 2019, 11, 22006–22018. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Ritter, C.A.; von Woedtke, T.; Bekeschus, S.; Wende, K. Package Delivered: Folate Receptor-Mediated Transporters in Cancer Therapy and Diagnosis. Chem. Sci. 2024, 15, 1966–2006. [Google Scholar] [CrossRef] [PubMed]
- Perumal, V.; Ravula, A.R.; Agas, A.; Kannan, M.; Liu, X.; I, S.S.; Vijayaraghavalu, S.; Haorah, J.; Zhang, Y.; Chandra, N. Transferrin-Grafted Albumin Nanoparticles for the Targeted Delivery of Apocynin and Neuroprotection in an In Vitro Model of the BBB. Micro 2023, 3, 84–106. [Google Scholar] [CrossRef]
- Ow, V.; Lin, Q.; Wong, J.H.M.; Sim, B.; Tan, Y.L.; Leow, Y.; Goh, R.; Loh, X.J. Understanding the Interplay between PH and Charges for Theranostic Nanomaterials. Nanoscale 2025, 17, 6960–6980. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Rarokar, N.; Yadav, S.; Saoji, S.; Bramhe, P.; Agade, R.; Gurav, S.; Khedekar, P.; Subramaniyan, V.; Wong, L.S.; Kumarasamy, V. Magnetic Nanosystem a Tool for Targeted Delivery and Diagnostic Application: Current Challenges and Recent Advancement. Int. J. Pharm. X 2024, 7, 100231. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Z.; Huang, Y.; Zhang, X.; Huang, J.; Cui, Y.; Yue, X.; Ma, C.; Fu, F.; Wang, W.; et al. Pulmonary Delivery Nanomedicines towards Circumventing Physiological Barriers: Strategies and Characterization Approaches. Adv. Drug Deliv. Rev. 2022, 185, 114309. [Google Scholar] [CrossRef]
- Chow, J.C.L. Nanomaterial-Based Molecular Imaging in Cancer: Advances in Simulation and AI Integration. Biomolecules 2025, 15, 444. [Google Scholar] [CrossRef]
- Kumar, M.; Kulkarni, P.; Liu, S.; Chemuturi, N.; Shah, D.K. Nanoparticle Biodistribution Coefficients: A Quantitative Approach for Understanding the Tissue Distribution of Nanoparticles. Adv. Drug Deliv. Rev. 2023, 194, 114708. [Google Scholar] [CrossRef] [PubMed]
- Sarcan, E.T. Nanoparticles for Dual Imaging: Pet and Fluorescence Imaging. Ank. Univ. Eczac. Fak. Derg. 2024, 48, 658–671. [Google Scholar] [CrossRef]
- Skotland, T.; Iversen, T.G.; Llorente, A.; Sandvig, K. Biodistribution, Pharmacokinetics and Excretion Studies of Intravenously Injected Nanoparticles and Extracellular Vesicles: Possibilities and Challenges. Adv. Drug Deliv. Rev. 2022, 186, 114326. [Google Scholar] [CrossRef] [PubMed]
- Kurul, F.; Turkmen, H.; Cetin, A.E.; Topkaya, S.N. Nanomedicine: How Nanomaterials Are Transforming Drug Delivery, Bio-Imaging, and Diagnosis. Next Nanotechnol. 2025, 7, 100129. [Google Scholar] [CrossRef]
- Sairam, A.B.; Sanmugam, A.; Pushparaj, A.; Mahesh Kumar, G.; Sundarapandian, N.; Balaji, S.; Nallal, M.; Park, K.H. Toxicity of Polymeric Nanodrugs as Drug Carriers. ACS Chem. Health Saf. 2023, 30, 236–250. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-Based Nanoparticles of Targeted Drug Delivery System in Breast Cancer Treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef]
- Sukubo, N.G.; Bigini, P.; Morelli, A. Nanocarriers and Macrophage Interaction: From a Potential Hurdle to an Alternative Therapeutic Strategy. Beilstein J. Nanotechnol. 2025, 16, 97–118. [Google Scholar] [CrossRef]
- Gutiérrez-Cruz, S.G.; Muñoz-Diosdado, A.; Gutiérrez-Calleja, R.A.; Rodríguez-Cortés, O.; Ortiz-Reyez, A.E.; Flores-Mejía, R. Influence of Physicochemical Factors on the Interaction of Metallic Nanoparticles with Immune System Cells. Front. Nanotechnol. 2025, 6, 1496230. [Google Scholar] [CrossRef]
- Makharadze, D.; del Valle, L.J.; Katsarava, R.; Puiggalí, J. The Art of PEGylation: From Simple Polymer to Sophisticated Drug Delivery System. Int. J. Mol. Sci. 2025, 26, 3102. [Google Scholar] [CrossRef]
- Miao, G.; He, Y.; Lai, K.; Zhao, Y.; He, P.; Tan, G.; Wang, X. Accelerated Blood Clearance of PEGylated Nanoparticles Induced by PEG-Based Pharmaceutical Excipients. J. Control. Release 2023, 363, 12–26. [Google Scholar] [CrossRef]
- Yang, L.; Lin, X.; Zhou, J.; Hou, S.; Fang, Y.; Bi, X.; Yang, L.; Li, L.; Fan, Y. Cell Membrane-Biomimetic Coating via Click-Mediated Liposome Fusion for Mitigating the Foreign-Body Reaction. Biomaterials 2021, 271, 120768. [Google Scholar] [CrossRef]
- de la Harpe, K.; Kondiah, P.; Choonara, Y.; Marimuthu, T.; du Toit, L.; Pillay, V. The Hemocompatibility of Nanoparticles: A Review of Cell–Nanoparticle Interactions and Hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef]
- Mehrizi, T.Z.; Kafiabad, S.A.; Eshghi, P. Effects and Treatment Applications of Polymeric Nanoparticles on Improving Platelets’ Storage Time: A Review of the Literature from 2010 to 2020. Blood Res. 2021, 56, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Pizzoli, G.; Gargaro, M.; Drava, G.; Voliani, V. Inorganic Nanomaterials Meet the Immune System: An Intricate Balance. Adv. Healthc. Mater. 2025, 14, e2404795. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Saifi, M.A.; Singh, S.B.; Godugu, C. In Vivo Studies: Toxicity and Biodistribution of Nanocarriers in Organisms. In Nanotoxicity; Elsevier: Amsterdam, The Netherlands, 2020; pp. 41–70. [Google Scholar]
- Available online: https://www.iso.org/standard/68936.html (accessed on 29 June 2025).
- Ramos, T.I.; Villacis-Aguirre, C.A.; López-Aguilar, K.V.; Santiago Padilla, L.; Altamirano, C.; Toledo, J.R.; Santiago Vispo, N. The Hitchhiker’s Guide to Human Therapeutic Nanoparticle Development. Pharmaceutics 2022, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Ji, X.; Luo, J. Rational Nanocarrier Design towards Clinical Translation of Cancer Nanotherapy. Biomed. Mater. 2021, 16, 032005. [Google Scholar] [CrossRef]
- Ahmad, J.; Ameeduzzafar; Ahmad, M.Z.; Akhter, H. Surface-Engineered Cancer Nanomedicine: Rational Design and Recent Progress. Curr. Pharm. Des. 2020, 26, 1181–1190. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.D.; Monferrer, D.; Penon, O.; Rivera-Gil, P. Regulatory Pathways and Guidelines for Nanotechnology-Enabled Health Products: A Comparative Review of EU and US Frameworks. Front. Med. 2025, 12, 1544393. [Google Scholar] [CrossRef]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- DA, B. Exploring Diverse Techniques for Phytochemical Extraction from Plant Sources a Comprehensive Review. Int. J. Pharmacogn. Chin. Med. 2024, 8, 1–11. [Google Scholar] [CrossRef]
- Havelikar, U.; Ghorpade, K.B.; Kumar, A.; Patel, A.; Singh, M.; Banjare, N.; Gupta, P.N. Comprehensive Insights into Mechanism of Nanotoxicity, Assessment Methods and Regulatory Challenges of Nanomedicines. Discov. Nano 2024, 19, 165. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The Synergistic and Antagonistic Antioxidant Interactions of Dietary Phytochemical Combinations. Crit. Rev. Food Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef]
- Powell, L.G.; Gillies, S.; Fernandes, T.F.; Murphy, F.; Giubilato, E.; Cazzagon, V.; Hristozov, D.; Pizzol, L.; Blosi, M.; Costa, A.L.; et al. Developing Integrated Approaches for Testing and Assessment (IATAs) in Order to Support Nanomaterial Safety. Nanotoxicology 2022, 16, 484–499. [Google Scholar] [CrossRef]
- Hu, L.; Luo, Y.; Yang, J.; Cheng, C. Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability. Molecules 2025, 30, 1184. [Google Scholar] [CrossRef] [PubMed]
- Petcov, T.E.; Silberschmidt, V.V.; Pandele, M.A.; Chiticaru, E.A.; Ioniță, M.; Manole, M. Nanostructures: An Efficient Drug Delivery Platform for Therapy of Multiple Myeloma. Eur. J. Med. Chem. Rep. 2025, 14, 100263. [Google Scholar] [CrossRef]
- Ma, X.; Tian, Y.; Yang, R.; Wang, H.; Allahou, L.W.; Chang, J.; Williams, G.; Knowles, J.C.; Poma, A. Nanotechnology in Healthcare, and Its Safety and Environmental Risks. J. Nanobiotechnology 2024, 22, 715. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lee, S.-L.; Taylor, C.; Li, J.; Chan, Y.-M.; Agarwal, R.; Temple, R.; Throckmorton, D.; Tyner, K. Scientific and Regulatory Approach to Botanical Drug Development: A U.S. FDA Perspective. J. Nat. Prod. 2020, 83, 552–562. [Google Scholar] [CrossRef]
- Takechi-Haraya, Y.; Ohgita, T.; Demizu, Y.; Saito, H.; Izutsu, K.; Sakai-Kato, K. Current Status and Challenges of Analytical Methods for Evaluation of Size and Surface Modification of Nanoparticle-Based Drug Formulations. AAPS PharmSciTech 2022, 23, 150. [Google Scholar] [CrossRef]
- Caputo, F.; Favre, G.; Borchard, G.; Calzolai, L.; Fisicaro, P.; Frejafon, E.; Günday-Türeli, N.; Koltsov, D.; Minelli, C.; Nelson, B.C.; et al. Toward an International Standardisation Roadmap for Nanomedicine. Drug Deliv. Transl. Res. 2024, 14, 2578–2588. [Google Scholar] [CrossRef]
- Ali, F.; Neha, K.; Parveen, S. Current Regulatory Landscape of Nanomaterials and Nanomedicines: A Global Perspective. J. Drug Deliv. Sci. Technol. 2023, 80, 104118. [Google Scholar] [CrossRef]
- Thomas, J.; Kumar, V.; Sharma, N.; John, N.; Umesh, M.; Kumar Dasarahally Huligowda, L.; Kaur, K.; Utreja, D. Recent Approaches in Nanotoxicity Assessment for Drug Delivery Applications: Challenges and Prospects. Med. Drug Discov. 2025, 25, 100204. [Google Scholar] [CrossRef]
- Abdel-Mageed, H.M.; AbuelEzz, N.Z.; Ali, A.A.; Abdelaziz, A.E.; Nada, D.; Abdelraouf, S.M.; Fouad, S.A.; Bishr, A.; Radwan, R.A. Newly Designed Curcumin-Loaded Hybrid Nanoparticles: A Multifunctional Strategy for Combating Oxidative Stress, Inflammation, and Infections to Accelerate Wound Healing and Tissue Regeneration. BMC Biotechnol. 2025, 25, 49. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Buck, A.C.; D’ Souza, S.; Dube, A.; Bardien, S. Nanophytomedicines as Therapeutic Agents for Parkinson’s Disease. ACS Omega 2023, 8, 42045–42061. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, S.; Zhuang, H.; Niu, M.; Pan, F.; Wang, N.; Sha, S.; Wang, Q.; Wang, J. LC–MS/MS Quantification of 20(S)-Protopanaxadiol in Complex Biological Matrices for Bioanalytical Method Validation and Pharmacokinetic Analysis. Sci. Rep. 2025, 15, 16640. [Google Scholar] [CrossRef]
- Dannhorn, A.; Kazanc, E.; Hamm, G.; Swales, J.G.; Strittmatter, N.; Maglennon, G.; Goodwin, R.J.A.; Takats, Z. Correlating Mass Spectrometry Imaging and Liquid Chromatography-Tandem Mass Spectrometry for Tissue-Based Pharmacokinetic Studies. Metabolites 2022, 12, 261. [Google Scholar] [CrossRef]
- Bimonte, S.; Barbieri, A.; Leongito, M.; Piccirillo, M.; Giudice, A.; Pivonello, C.; De Angelis, C.; Granata, V.; Palaia, R.; Izzo, F. Curcumin AntiCancer Studies in Pancreatic Cancer. Nutrients 2016, 8, 433. [Google Scholar] [CrossRef]
- Conte, R.; Valentino, A.; Sepe, F.; Gianfreda, F.; Condò, R.; Cerroni, L.; Calarco, A.; Peluso, G. Resveratrol-Loaded Solid Lipid Nanoparticles Reinforced Hyaluronic Hydrogel: Multitarget Strategy for the Treatment of Diabetes-Related Periodontitis. Biomedicines 2025, 13, 1059. [Google Scholar] [CrossRef]
- Zhang, X.; Chan, H.W.; Shao, Z.; Wang, Q.; Chow, S.; Chow, S.F. Navigating Translational Research in Nanomedicine: A Strategic Guide to Formulation and Manufacturing. Int. J. Pharm. 2025, 671, 125202. [Google Scholar] [CrossRef]
- Singh, G.; Thakur, N.; Kumar, R. Nanoparticles in Drinking Water: Assessing Health Risks and Regulatory Challenges. Sci. Total Environ. 2024, 949, 174940. [Google Scholar] [CrossRef]
- Saji, C.V.; Mohanakumar, K.P.; Shenoi, R.A. Phytomedicine Nanoformulations for Parkinson’s Disease. Phytomedicine Plus 2025, 5, 100704. [Google Scholar] [CrossRef]
- Chavda, V.P.; Nalla, L.V.; Balar, P.; Bezbaruah, R.; Apostolopoulos, V.; Singla, R.K.; Khadela, A.; Vora, L.; Uversky, V.N. Advanced Phytochemical-Based Nanocarrier Systems for the Treatment of Breast Cancer. Cancers 2023, 15, 1023. [Google Scholar] [CrossRef]
- Anas, M.; Falak, A.; Khan, A.; Khattak, W.A.; Nisa, S.G.; Aslam, Q.; Khan, K.A.; Saleem, M.H.; Fahad, S. Therapeutic Potential and Agricultural Benefits of Curcumin: A Comprehensive Review of Health and Sustainability Applications. J. Umm Al-Qura Univ. Appl. Sci. 2024, 1–16. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Multifunctional and Stimuli-Responsive Nanocarriers for Targeted Therapeutic Delivery. Expert Opin. Drug Deliv. 2021, 18, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Dureja, H. Recent Patents on Polymeric Nanoparticles for Cancer Therapy. Recent Pat. Nanotechnol. 2018, 12, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Mehan, S.; Arora, N.; Bhalla, S.; Khan, A.; Rehman, M.U.; Alghamdi, B.S.; Al Zughaibi, T.; Ashraf, G.M. Involvement of Phytochemical-Encapsulated Nanoparticles’ Interaction with Cellular Signalling in the Amelioration of Benign and Malignant Brain Tumours. Molecules 2022, 27, 3561. [Google Scholar] [CrossRef] [PubMed]
- Benderski, K.; Lammers, T.; Sofias, A.M. Analysis of Multi-Drug Cancer Nanomedicine. Nat. Nanotechnol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Awlqadr, F.H.; Majeed, K.R.; Altemimi, A.B.; Hassan, A.M.; Qadir, S.A.; Saeed, M.N.; Faraj, A.M.; Salih, T.H.; Abd Al-Manhel, A.J.; Najm, M.A.A.; et al. Nanotechnology-Based Herbal Medicine: Preparation, Synthesis, and Applications in Food and Medicine. J. Agric. Food Res. 2025, 19, 101661. [Google Scholar] [CrossRef]
- Datta, D.; Priyanka Bandi, S.; Colaco, V.; Dhas, N.; Siva Reddy, D.; Vora, L.K. Fostering the Unleashing Potential of Nanocarriers-Mediated Delivery of Ocular Therapeutics. Int. J. Pharm. 2024, 658, 124192. [Google Scholar] [CrossRef]
- Pérez-Pérez, V.; Jiménez-Martínez, C.; González-Escobar, J.L.; Corzo-Ríos, L.J. Exploring the Impact of Encapsulation on the Stability and Bioactivity of Peptides Extracted from Botanical Sources: Trends and Opportunities. Front. Chem. 2024, 12, 1423500. [Google Scholar] [CrossRef]
- Nunziata, G.; Borroni, A.; Rossi, F. Advanced Microfluidic Strategies for Core-Shell Nanoparticles: The Next-Generation of Polymeric and Lipid-Based Drug Nanocarriers. Chem. Eng. J. Adv. 2025, 22, 100759. [Google Scholar] [CrossRef]
- Bezelya, A.; Küçüktürkmen, B.; Bozkır, A. Microfluidic Devices for Precision Nanoparticle Production. Micro 2023, 3, 822–866. [Google Scholar] [CrossRef]
- Das, A.; Prajapati, P. Navigating Pharmaceuticals: Microfluidic Devices in Analytical and Formulation Sciences. Discov. Chem. 2025, 2, 49. [Google Scholar] [CrossRef]
- Nogueira, S.S.; Samaridou, E.; Simon, J.; Frank, S.; Beck-Broichsitter, M.; Mehta, A. Analytical Techniques for the Characterization of Nanoparticles for MRNA Delivery. Eur. J. Pharm. Biopharm. 2024, 198, 114235. [Google Scholar] [CrossRef] [PubMed]
- Gerzon, G.; Sheng, Y.; Kirkitadze, M. Process Analytical Technologies—Advances in Bioprocess Integration and Future Perspectives. J. Pharm. Biomed. Anal. 2022, 207, 114379. [Google Scholar] [CrossRef] [PubMed]
- Ogbuagu, O.O.; Mbata, A.O.; Balogun, O.D.; Oladapo, O.; Ojo, O.O.; Muonde, M. Quality Assurance in Pharmaceutical Manufacturing: Bridging the Gap between Regulations, Supply Chain, and Innovations. Int. J. Multidiscip. Res. Growth Eval. 2023, 4, 823–831. [Google Scholar] [CrossRef]
- Carroccio, S.C.; Scarfato, P.; Bruno, E.; Aprea, P.; Dintcheva, N.T.; Filippone, G. Impact of Nanoparticles on the Environmental Sustainability of Polymer Nanocomposites Based on Bioplastics or Recycled Plastics—A Review of Life-Cycle Assessment Studies. J. Clean. Prod. 2022, 335, 130322. [Google Scholar] [CrossRef]
- Elayaperumal, S.; Sivamani, Y.; Agarwal, P.; Srivastava, N. Plant-Based Nanotherapeutics: A New Frontier in Disease Management and Prevention. Nano TransMed 2025, 4, 100086. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of Nanotechnology in Medical Field: A Brief Review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Khanna, N.N.; Maindarkar, M.A.; Viswanathan, V.; Fernandes, J.F.E.; Paul, S.; Bhagawati, M.; Ahluwalia, P.; Ruzsa, Z.; Sharma, A.; Kolluri, R.; et al. Economics of Artificial Intelligence in Healthcare: Diagnosis vs. Treatment. Healthcare 2022, 10, 2493. [Google Scholar] [CrossRef]
- Dutta, N.; Dhar, D. Understanding Medical Technology Innovation in Low- and Middle-Income Countries: Factors, Impact, and a Model Proposal. She Ji J. Des. Econ. Innov. 2024, 10, 192–222. [Google Scholar] [CrossRef]
- Cheng, T.-M.; Chu, H.-Y.; Huang, H.-M.; Li, Z.-L.; Chen, C.-Y.; Shih, Y.-J.; Whang-Peng, J.; Cheng, R.H.; Mo, J.-K.; Lin, H.-Y.; et al. Toxicologic Concerns with Current Medical Nanoparticles. Int. J. Mol. Sci. 2022, 23, 7597. [Google Scholar] [CrossRef]
- Damasco, J.A.; Ravi, S.; Perez, J.D.; Hagaman, D.E.; Melancon, M.P. Understanding Nanoparticle Toxicity to Direct a Safe-by-Design Approach in Cancer Nanomedicine. Nanomaterials 2020, 10, 2186. [Google Scholar] [CrossRef]
- Shajar, F.; Saleem, S.; Mushtaq, N.U.; Shah, W.H.; Rasool, A.; Padder, S.A.; Tahir, I.; Rehman, R.U. Regulatory and Ethical Issues Raised by the Utilization of Nanomaterials. In Interaction of Nanomaterials with Living Cells; Springer Nature: Singapore, 2023; pp. 899–924. [Google Scholar]
- Gupta, A.P.; Pathak, A.; Pandey, P. Challenges and Future of Nanotechnology in Global Herbal Medicine Practices. In Herbal Medicine Phytochemistry; Springer: Cham, Switzerland, 2024; pp. 1627–1653. [Google Scholar]
- Tian, M.; Xin, X.; Wu, R.; Guan, W.; Zhou, W. Advances in Intelligent-Responsive Nanocarriers for Cancer Therapy. Pharmacol. Res. 2022, 178, 106184. [Google Scholar] [CrossRef]
- Sabit, H.; Pawlik, T.M.; Radwan, F.; Abdel-Hakeem, M.; Abdel-Ghany, S.; Wadan, A.-H.S.; Elzawahri, M.; El-Hashash, A.; Arneth, B. Precision Nanomedicine: Navigating the Tumor Microenvironment for Enhanced Cancer Immunotherapy and Targeted Drug Delivery. Mol. Cancer 2025, 24, 160. [Google Scholar] [CrossRef]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. PH-Responsive High Stability Polymeric Nanoparticles for Targeted Delivery of Anticancer Therapeutics. Commun. Biol. 2020, 3, 95. [Google Scholar] [CrossRef]
- Meng, X.; Shen, Y.; Zhao, H.; Lu, X.; Wang, Z.; Zhao, Y. Redox-Manipulating Nanocarriers for Anticancer Drug Delivery: A Systematic Review. J. Nanobiotechnology 2024, 22, 587. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.; Torbett-Dougherty, M.; Islam, A.; Soleimani, S.; Bruce-Tagoe, T.A.; Johnson, J.A. Magnetic Nanoparticles and Drug Delivery Systems for Anti-Cancer Applications: A Review. Nanomaterials 2025, 15, 285. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, Q.; Li, J.; Peng, S.; Wang, X.; Cai, R. Near-Infrared Photoactivated Nanomedicines for Photothermal Synergistic Cancer Therapy. Nano Today 2021, 37, 101073. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Qiu, S. Nanomaterials in Stimulus-Responsive Drug Delivery Systems Facilitate Precise Therapy for Hematologic Diseases. J. Mater. Chem. B 2025, 13, 7953–7972. [Google Scholar] [CrossRef]
- Mousavi-Kiasary, S.M.S.; Senabreh, A.; Zandi, A.; Pena, R.; Cruz, F.; Adibi, A.; Hooshmand, N. Synergistic Cancer Therapies Enhanced by Nanoparticles: Advancing Nanomedicine Through Multimodal Strategies. Pharmaceutics 2025, 17, 682. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, Z.; Ma, S.; He, Y.; He, Y.; Ma, L.; Lei, N.; Deng, W.; Wang, F. Microenvironment-Responsive Nanosystems for Ischemic Stroke Therapy. Theranostics 2024, 14, 5571–5595. [Google Scholar] [CrossRef]
- Han, H.S.; Koo, S.Y.; Choi, K.Y. Emerging Nanoformulation Strategies for Phytocompounds and Applications from Drug Delivery to Phototherapy to Imaging. Bioact. Mater. 2022, 14, 182–205. [Google Scholar] [CrossRef] [PubMed]
- Kopac, T. Leveraging Artificial Intelligence and Machine Learning for Characterizing Protein Corona, Nanobiological Interactions, and Advancing Drug Discovery. Bioengineering 2025, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.U.; Sharma, J.B.; Gandhi, S.M.; Prajapati, B.G.; Thanawuth, K.; Limmatvapirat, S.; Sriamornsak, P. AI-Driven Design and Optimization of Nanoparticle-Based Drug Delivery Systems. Sci. Eng. Health Stud. 2024, 18, 24010003. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Mihandoost, S.; Rezaiee, M. Machine Learning Assisted Exploration of the Influential Parameters on the PLGA Nanoparticles. Sci. Rep. 2024, 14, 1114. [Google Scholar] [CrossRef]
- Zeynalov, N.; Taghiyev, D.; Taghiyev, S.; Mikayilov, E. Role of Computational Modeling in the Design and Development of Nanotechnology-Based Drug Delivery Systems. Chem. Biochem. Eng. Q. 2024, 38, 97–110. [Google Scholar] [CrossRef]
- Chou, W.-C.; Chen, Q.; Yuan, L.; Cheng, Y.-H.; He, C.; Monteiro-Riviere, N.A.; Riviere, J.E.; Lin, Z. An Artificial Intelligence-Assisted Physiologically-Based Pharmacokinetic Model to Predict Nanoparticle Delivery to Tumors in Mice. J. Control. Release 2023, 361, 53–63. [Google Scholar] [CrossRef]
- Samathoti, P.; Kumarachari, R.K.; Bukke, S.P.N.; Rajasekhar, E.S.K.; Jaiswal, A.A.; Eftekhari, Z. The Role of Nanomedicine and Artificial Intelligence in Cancer Health Care: Individual Applications and Emerging Integrations—A Narrative Review. Discov. Oncol. 2025, 16, 697. [Google Scholar] [CrossRef]
- Fu, C.; Chen, Q. The Future of Pharmaceuticals: Artificial Intelligence in Drug Discovery and Development. J. Pharm. Anal. 2025, 101248, in press. [Google Scholar] [CrossRef]
- Zeng, J.; Jia, X. Systems Theory-Driven Framework for AI Integration into the Holistic Material Basis Research of Traditional Chinese Medicine. Engineering 2024, 40, 28–50. [Google Scholar] [CrossRef]
- Mienye, I.D.; Obaido, G.; Jere, N.; Mienye, E.; Aruleba, K.; Emmanuel, I.D.; Ogbuokiri, B. A Survey of Explainable Artificial Intelligence in Healthcare: Concepts, Applications, and Challenges. Inform. Med. Unlocked 2024, 51, 101587. [Google Scholar] [CrossRef]
- Serrano, D.R.; Luciano, F.C.; Anaya, B.J.; Ongoren, B.; Kara, A.; Molina, G.; Ramirez, B.I.; Sánchez-Guirales, S.A.; Simon, J.A.; Tomietto, G.; et al. Artificial Intelligence (AI) Applications in Drug Discovery and Drug Delivery: Revolutionizing Personalized Medicine. Pharmaceutics 2024, 16, 1328. [Google Scholar] [CrossRef]
- Shrestha, B.; Tang, L.; Hood, R.L. Nanotechnology for Personalized Medicine. In Nanomedicine; Springer: Singapore, 2022; pp. 1–48. [Google Scholar]
- Kim, J.H.; Dareowolabi, B.O.; Thiruvengadam, R.; Moon, E.-Y. Application of Nanotechnology and Phytochemicals in Anticancer Therapy. Pharmaceutics 2024, 16, 1169. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Hamid, S.; Tuan, T.T.; Park, J.-E.; Chung, Y.S. Advance Computational Tools for Multiomics Data Learning. Biotechnol. Adv. 2024, 77, 108447. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, S.; Liu, F.-Y.; Liu, M. Advancing Oral Cancer Care: Nanomaterial-Driven Diagnostic and Therapeutic Innovations. Cell Biol. Toxicol. 2025, 41, 90. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Mohammadnejad, J.; Salamat, S.; Beiram Zadeh, Z.; Tanhaei, M.; Ramakrishna, S. Theranostic Polymeric Nanoparticles as a New Approach in Cancer Therapy and Diagnosis: A Review. Mater. Today Chem. 2023, 29, 101400. [Google Scholar] [CrossRef]
- Rahmani Dabbagh, S.; Rezapour Sarabi, M.; Birtek, M.T.; Mustafaoglu, N.; Zhang, Y.S.; Tasoglu, S. 3D Bioprinted Organ-on-chips. Aggregate 2023, 4, e197. [Google Scholar] [CrossRef]
- Wu, X.; Shi, W.; Liu, X.; Gu, Z. Recent Advances in 3D-Printing-Based Organ-on-a-Chip. EngMedicine 2024, 1, 100003. [Google Scholar] [CrossRef]
- Wainwright, C.L.; Teixeira, M.M.; Adelson, D.L.; Braga, F.C.; Buenz, E.J.; Campana, P.R.V.; David, B.; Glaser, K.B.; Harata-Lee, Y.; Howes, M.-J.R.; et al. Future Directions for the Discovery of Natural Product-Derived Immunomodulating Drugs: An IUPHAR Positional Review. Pharmacol. Res. 2022, 177, 106076. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Oladzadabbasabadi, N.; Dheyab, M.A.; Lalabadi, M.A.; Sheibani, S.; Ghasemlou, M.; Esmaeili, Y.; Barrow, C.J.; Naebe, M.; Timms, W. Emerging Trends in Smart and Sustainable Nano-Biosensing: The Role of Green Nanomaterials. Ind. Crops Prod. 2025, 223, 120108. [Google Scholar] [CrossRef]
- Reda, A.T.; Park, Y.T. Sustainable Synthesis of Functional Nanomaterials: Renewable Resources, Energy-Efficient Methods, Environmental Impact and Circular Economy Approaches. Chem. Eng. J. 2025, 516, 163894. [Google Scholar] [CrossRef]
- Kazemi, S.; Hosseingholian, A.; Gohari, S.D.; Feirahi, F.; Moammeri, F.; Mesbahian, G.; Moghaddam, Z.S.; Ren, Q. Recent Advances in Green Synthesized Nanoparticles: From Production to Application. Mater. Today Sustain. 2023, 24, 100500. [Google Scholar] [CrossRef]
- Singh, P.; Garg, A.; Pandit, S.; Mokkapati, V.R.S.S.; Mijakovic, I. Antimicrobial Effects of Biogenic Nanoparticles. Nanomaterials 2018, 8, 1009. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Ahmed, S.; Abbasi, A.; Khan, M.T.; Subhan, M.; Bukhari, N.A.; Hatamleh, A.A.; Abdelsalam, N.R. Plant Mediated Fabrication of Silver Nanoparticles, Process Optimization, and Impact on Tomato Plant. Sci. Rep. 2023, 13, 18048. [Google Scholar] [CrossRef]
- Kučuk, N.; Primožič, M.; Knez, Ž.; Leitgeb, M. Sustainable Biodegradable Biopolymer-Based Nanoparticles for Healthcare Applications. Int. J. Mol. Sci. 2023, 24, 3188. [Google Scholar] [CrossRef] [PubMed]
- Nizam, N.U.M.; Hanafiah, M.M.; Woon, K.S. A Content Review of Life Cycle Assessment of Nanomaterials: Current Practices, Challenges, and Future Prospects. Nanomaterials 2021, 11, 3324. [Google Scholar] [CrossRef] [PubMed]
- Alli, Y.A.; Bamisaye, A.; Bamidele, M.O.; Etafo, N.O.; Chkirida, S.; Lawal, A.; Hammed, V.O.; Akinfenwa, A.S.; Hanson, E.; Nwakile, C.; et al. Transforming Waste to Wealth: Harnessing Carbon Dioxide for Sustainable Solutions. Results Surf. Interfaces 2024, 17, 100321. [Google Scholar] [CrossRef]
- Jeffcoat, P.; Di Lernia, C.; Hardy, C.; New, E.J.; Chrzanowski, W. (Re)Imagining Purpose: A Framework for Sustainable Nanotechnology Innovation. NanoImpact 2024, 35, 100511. [Google Scholar] [CrossRef]
- Pokrajac, L.; Abbas, A.; Chrzanowski, W.; Dias, G.M.; Eggleton, B.J.; Maguire, S.; Maine, E.; Malloy, T.; Nathwani, J.; Nazar, L.; et al. Nanotechnology for a Sustainable Future: Addressing Global Challenges with the International Network4Sustainable Nanotechnology. ACS Nano 2021, 15, 18608–18623. [Google Scholar] [CrossRef]
- Kumar, A.; Tyagi, P.K.; Tyagi, S.; Ghorbanpour, M. Integrating Green Nanotechnology with Sustainable Development Goals: A Pathway to Sustainable Innovation. Discov. Sustain. 2024, 5, 364. [Google Scholar] [CrossRef]
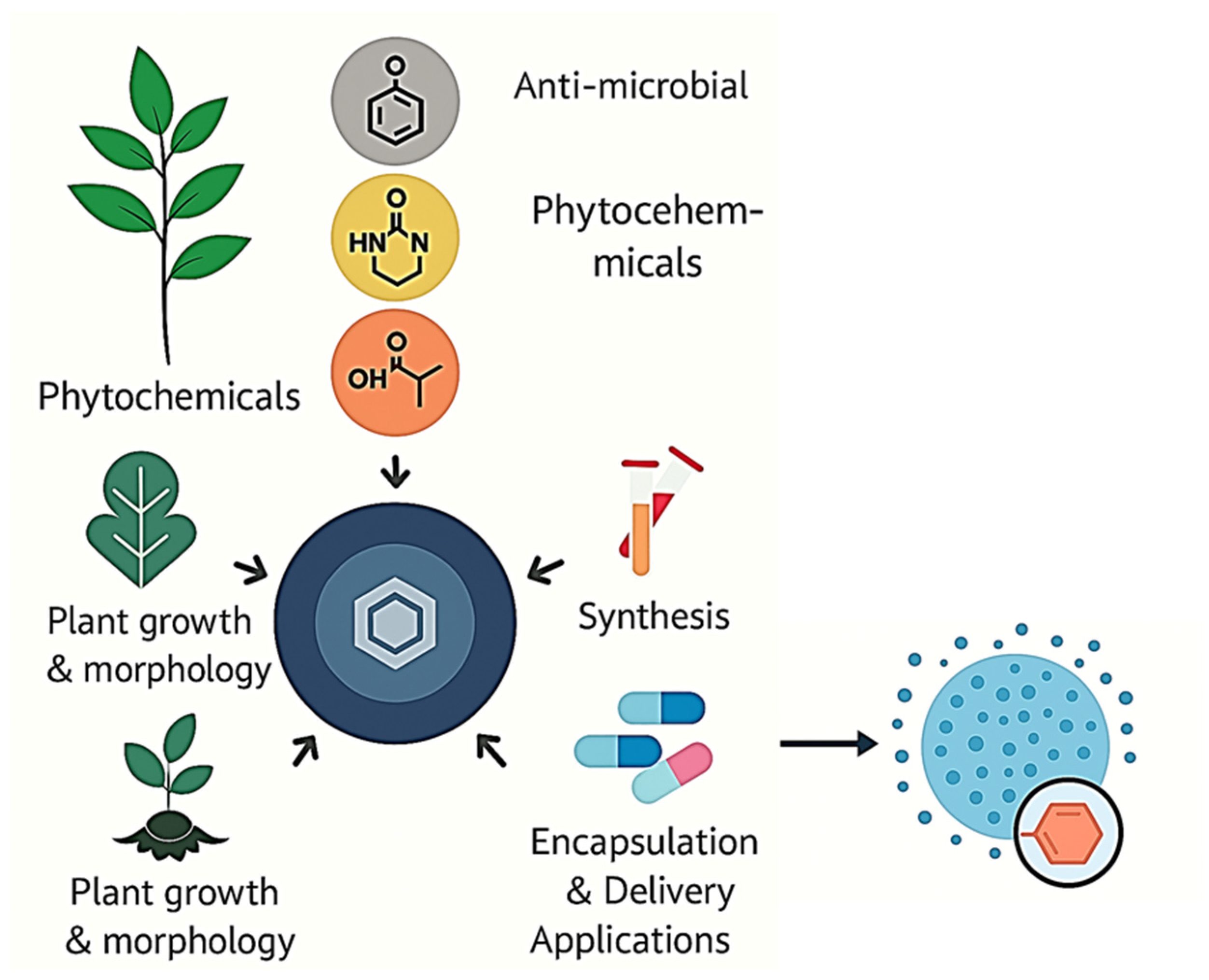
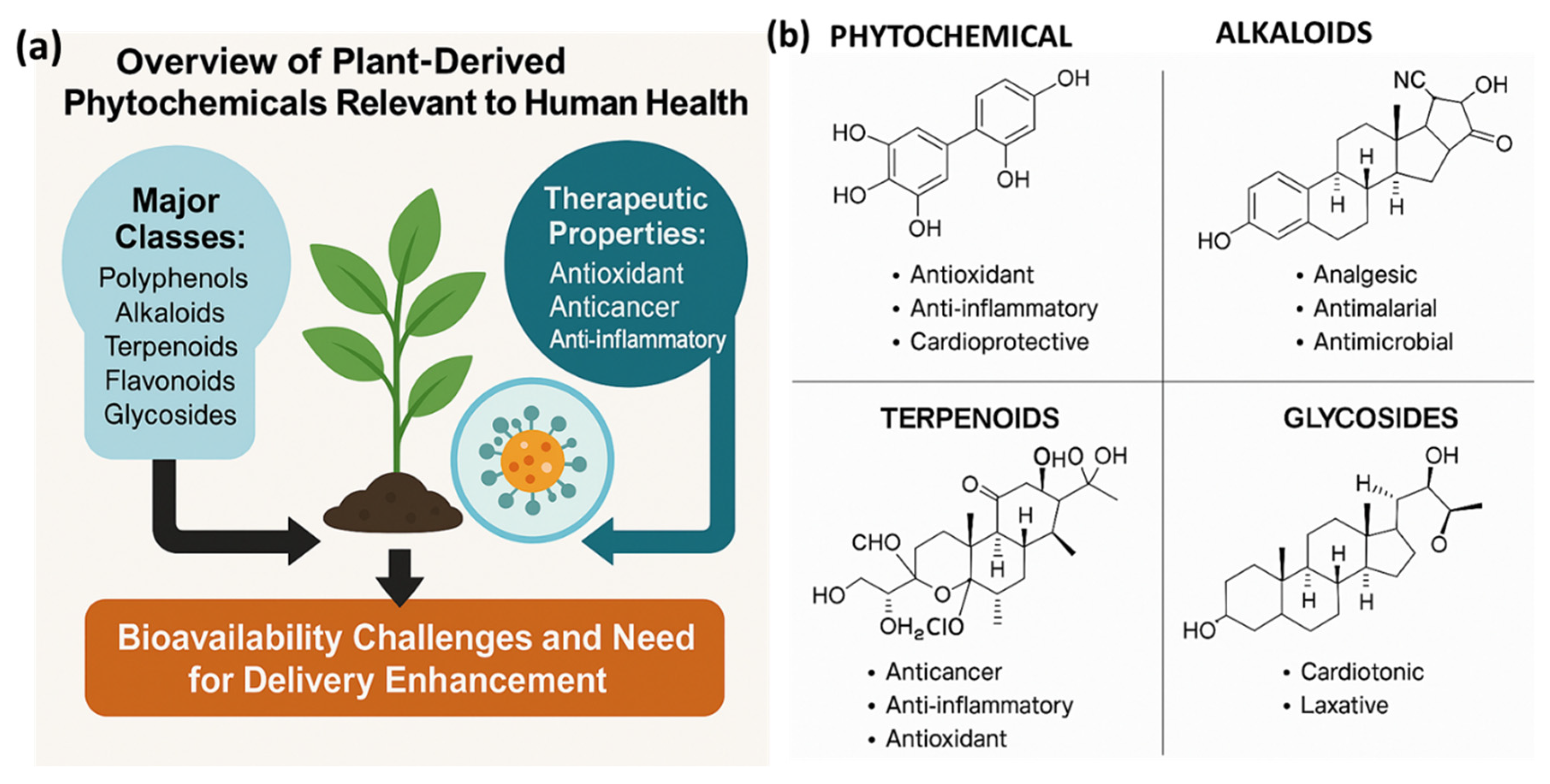


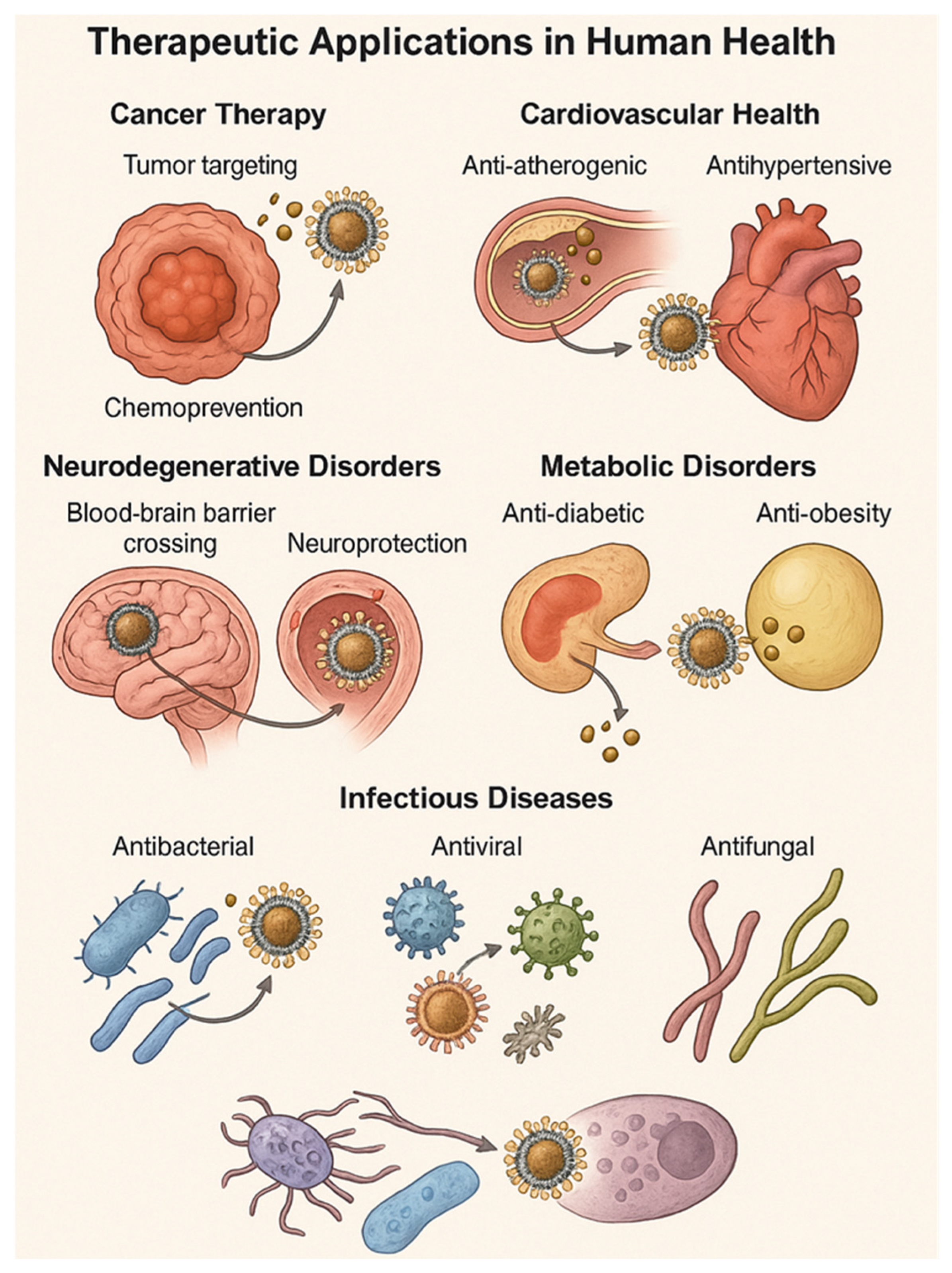
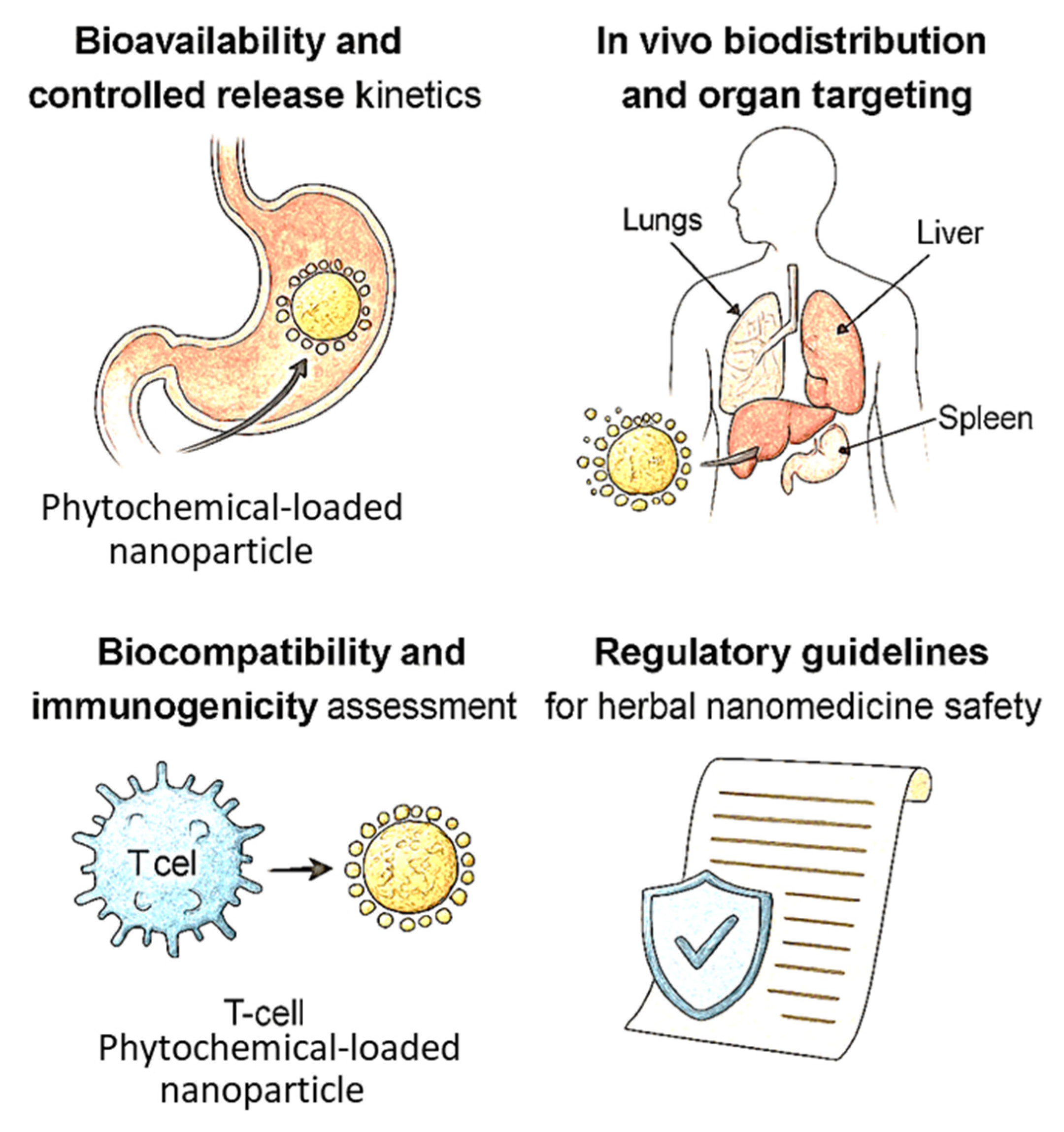
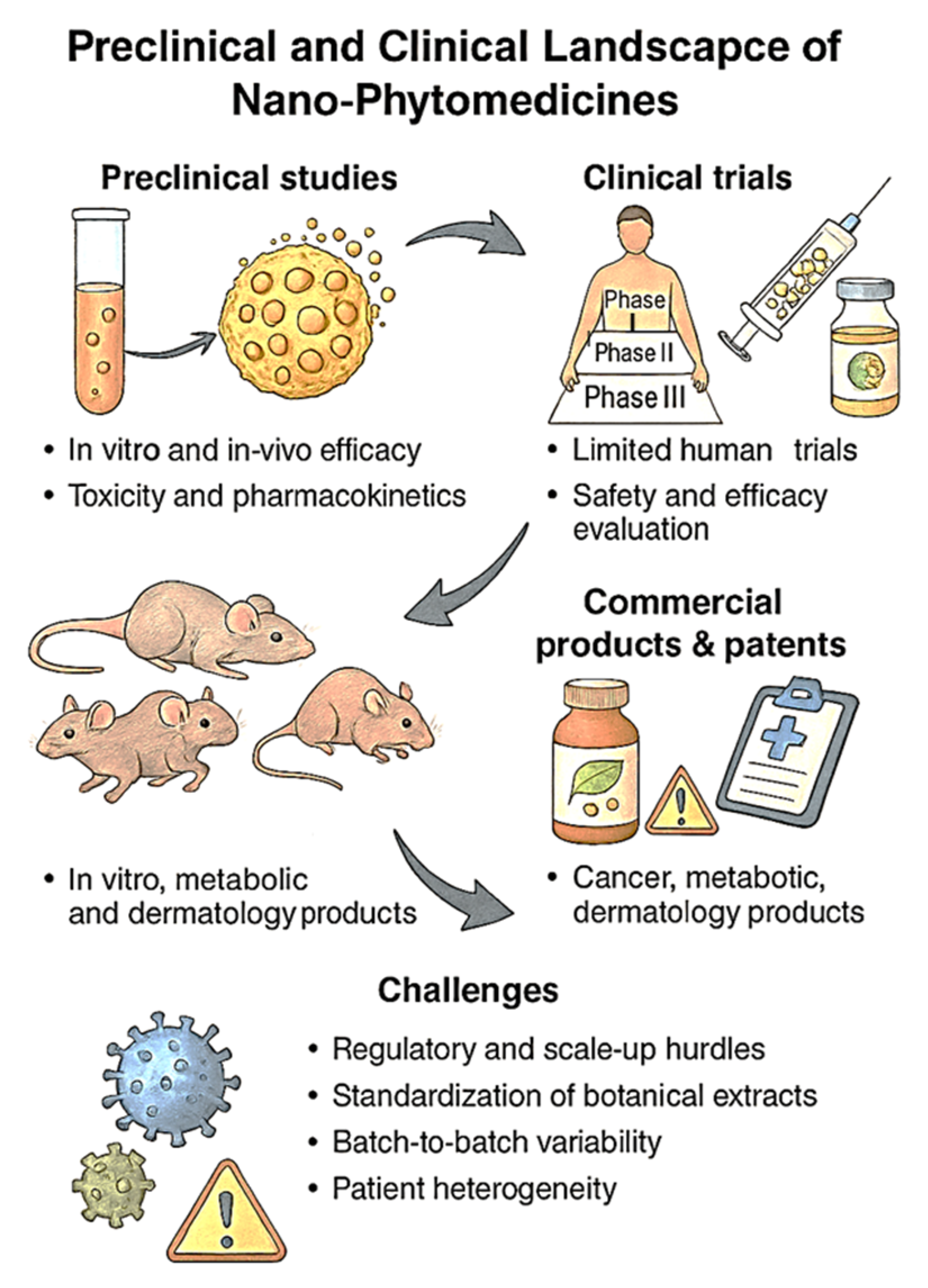
| Phytochemical Class | Example Compounds | Primary Therapeutic Properties | Delivery Challenges | References |
|---|---|---|---|---|
| Polyphenols | Curcumin, Resveratrol, EGCG | Antioxidant, anti-inflammatory, anticancer | Poor aqueous solubility, rapid metabolism, low absorption | [30,32] |
| Alkaloids | Berberine, Morphine, Vincristine | Antibacterial, antidiabetic, anticancer | Narrow therapeutic index, high toxicity at higher doses | [28,37] |
| Terpenoids | Limonene, Paclitaxel, Carotenoids | Anticancer, antimicrobial, cardioprotective | Oxidative instability, poor bioavailability | [29] |
| Flavonoids | Quercetin, Kaempferol, Naringenin | Antioxidant, neuroprotective, anti-inflammatory | Low membrane permeability, rapid phase II metabolism | [30,37] |
| Glycosides | Digoxin, Sennosides, Stevioside | Cardiotonic, laxative, metabolic regulation | Limited stability in GI tract, slow release kinetics | [31] |
| Nanocarrier Type | Key Features | Advantages in Phytochemical Delivery | Limitations/Challenges | Representative Applications | References |
|---|---|---|---|---|---|
| Liposomes | Phospholipid bilayer vesicles with hydrophilic core and lipophilic shell | Biocompatibility, can carry both hydrophilic and lipophilic phytochemicals, enhanced absorption | Physical instability, oxidation of lipids, rapid clearance without PEGylation | Curcumin, resveratrol, EGCG for cancer and inflammation | [49] |
| Solid Lipid Nanoparticles (SLNs) | Nanocarriers made of solid lipids stabilized by surfactants | High loading of lipophilic phytochemicals, protection from degradation, scalable production | Limited drug loading, potential for polymorphic transitions affecting stability | Silymarin, quercetin for liver protection and metabolic disorders | [52,53,54] |
| Nanostructured Lipid Carriers (NLCs) | Mix of solid and liquid lipids to form a less ordered matrix | Improved drug loading, prolonged release, better stability than SLNs | Complex formulation optimization needed, possibility of lipid phase separation | Berberine, naringenin for metabolic and cardiovascular diseases | [55,56] |
| Natural Polymeric NPs | Polysaccharide (e.g., chitosan, alginate) or protein-based carriers | Biodegradable, mucoadhesive, enhances intestinal uptake and stability | Batch variability, lower mechanical strength than synthetic carriers | Chitosan-curcumin NPs for oral anti-inflammatory therapy | [63,64] |
| Synthetic Polymeric NPs | PLGA, PCL, PEG-based systems designed via emulsion or nanoprecipitation | Customizable release profile, high encapsulation efficiency, FDA-accepted polymers | Requires organic solvents, potential accumulation of degradation products | PLGA-resveratrol or quercetin NPs for cancer and neurodegenerative diseases | [67,68] |
| Inorganic NPs—Metal | Gold (AuNPs), Silver (AgNPs), synthesized via green or chemical methods | Excellent stability, imaging compatibility, tunable size/shape, antimicrobial synergy with phytochemicals | Potential cytotoxicity, accumulation risk, surface functionalization required for targeting | AgNP–berberine conjugates for antimicrobial activity | [76,77] |
| Inorganic NPs—Metal Oxide | ZnO, Fe3O4, TiO2, often used with plant extract functionalization | Intrinsic bioactivity (e.g., ROS generation), magnetic guidance, enhanced dissolution | Long-term toxicity concerns, environmental persistence | ZnO–curcumin for wound healing, Fe3O4–EGCG for brain targeting | [81] |
| Inorganic NPs—Silica (MSNs) | Mesoporous silica with tunable pore size and surface area | High surface loading, pH-sensitive release, good biocompatibility | Non-biodegradable, risk of particle accumulation and chronic exposure | MSNs with polyphenols for anticancer or gut delivery | [83] |
| Hybrid Nanocarriers | Lipid-polymer combinations, metal-lipid or polymer-metal hybrids | Combines benefits of each system, controlled release, structural stability, multi-functional capability | Complex synthesis, scalability challenges | PLGA–lipid hybrid loaded with curcumin for dual-release in tumors | [87] |
| Stimuli-Responsive Systems | pH, redox, enzyme, temperature, or light-triggered release platforms | On-demand release at disease sites, reduced systemic toxicity | Stimuli-specific tuning required, clinical validation is limited | pH-sensitive quercetin NPs for tumor microenvironments | [88,89] |
| Surface-Functionalized NPs | Nanoparticles modified with ligands (folate, peptides, antibodies, aptamers) for active targeting | Receptor-specific delivery, improved uptake in diseased cells/tissues | Ligand synthesis cost, potential immune response, ligand detachment in vivo | Folic acid-decorated NLCs for ovarian cancer therapy | [90,91] |
| Mechanism | Key Features | Nano-Phytomedicine Role | Advantages of Nanoformulation | Representative Phytochemicals | References |
|---|---|---|---|---|---|
| Cellular Uptake and Intracellular Trafficking | Involves endocytosis (clathrin, caveolin, macropinocytosis), endosomal escape, and organelle targeting | Enables cytosolic/nuclear delivery; bypasses degradation; improves uptake in target cells | Enhanced retention, subcellular targeting, P-gp bypass, prolonged release | Curcumin, resveratrol, quercetin | [101,102] |
| Redox Balance and Antioxidant Pathways | Modulation of oxidative stress via Nrf2 pathway, ROS scavenging, and GSH regulation | Improves intracellular antioxidant levels, activates protective genes (e.g., HO-1, NQO1) | Site-specific ROS scavenging, redox-responsive release, mitochondrial targeting | EGCG, curcumin, sulforaphane | [106,107,108,109,110] |
| Apoptosis, Proliferation and Inflammation | Targets intrinsic/extrinsic apoptotic pathways, NF-κB, and cytokine networks | Triggers caspase cascade, downregulates Bcl-2, inhibits COX-2 and TNF-α production | Selective cancer cell apoptosis, reduced systemic toxicity, enhanced anti-inflammatory effects | Berberine, apigenin, quercetin | [111] |
| Signaling Pathway Regulation | Interference with PI3K/Akt, MAPK, and NF-κB cascades | Inhibits survival/proliferation signals; enhances apoptosis and immune responses | Sustained pathway inhibition, synergistic effects with ligands or co-drugs | Kaempferol, luteolin, curcumin | [118], |
| Epigenetic and Genomic Regulation | Involves DNA methylation, histone acetylation, miRNA modulation | Modulates DNMTs, HDACs, and epigenetic markers for long-term gene expression control | Nuclear delivery, miRNA-targeted therapy, chromatin remodeling | Resveratrol, sulforaphane, EGCG | [124] |
| Therapeutic Area | Mechanism of Action/Benefit | Nanocarrier Strategies | Representative Phytochemicals | References |
|---|---|---|---|---|
| Cancer Therapy | Tumor targeting via EPR effect, apoptosis induction, ferroptosis, and anti-angiogenesis | Liposomes, polymeric nanoparticles, gold nanoshells; HER2, folate-targeted systems | Curcumin, resveratrol, EGCG, paclitaxel precursors | [130,131,132,133,134,135,136] |
| Cardiovascular Health | Anti-atherogenic (↓ LDL oxidation, ↓ VSMC proliferation), antihypertensive (↑ NO release, ↓ vascular resistance), anti-inflammatory | Nanoemulsions, lipid-polymer hybrids, magnetic targeting nanoparticles | Resveratrol, catechins, naringenin | [137,138,139,140,141,142] |
| Neurodegenerative Disorders | BBB penetration, microglial modulation, anti-amyloid aggregation, antioxidant and anti-inflammatory neuroprotection | Liposomes, polymeric nanoparticles with surfactant/ligand coating (e.g., polysorbate-80, transferrin) | Curcumin, resveratrol, EGCG | [143,144,145,146] |
| Metabolic Disorders | Glycemic control, insulin sensitization, adipogenesis inhibition, lipid modulation, anti-inflammatory and thermogenic effects | Chitosan, silver NPs, lipid-polymer hybrids, nanoencapsulation for GI protection and sustained release | Berberine, quercetin, curcumin, silymarin, catechins | [147,148,149,150,151,152] |
| Infectious Diseases | Antibacterial (biofilm disruption, membrane damage), antiviral (entry blockade, protease inhibition), antifungal (membrane disruption), resistance mitigation (efflux inhibition) | Metal–phytochemical NPs (e.g., Ag, ZnO), polymeric nanocapsules, gold conjugates, inhalable/topical delivery formats | Curcumin, baicalin, quercetin, thymol, eugenol | [153,154,155,156] |
| Pharmacological/Toxicological Dimension | Scientific Insight and Mechanistic Role | Nano-Phytomedicine Strategy/Advantage | Emerging Barriers/Optimization Needs | References |
|---|---|---|---|---|
| Kinetic Modulation via Nano-Architecture | Nanoform morphology (e.g., core-shell, hollow, matrix) influences degradation and release rates through interfacial drug diffusion and structural disintegration | PLGA matrix vs. lipid vesicles: predictable vs. burst release kinetics; core–shell hybrids improve tunability | Nano–drug mismatch (e.g., rapid-release from hydrophobic matrices), cross-linking irregularity | [156,159,160] |
| Gastrointestinal Stability and Transit Behavior | Nano-encapsulation delays premature phytochemical degradation by gastric enzymes and bile salts, improving residence time and epithelial transport | Enteric-coated or mucin-adhering nanoparticles preserve payload integrity and enhance absorption | GI transit variability, impact of digestive enzymes on carrier integrity | [159,163,165] |
| Epithelial Transport and Endocytosis Pathways | Nanoform size/charge influences endocytic route: clathrin/caveolae-mediated or macropinocytosis; smaller particles often cross via paracellular or tight junctions | Surface-tuned nanoparticles exploit paracellular pathways (e.g., chitosan opens tight junctions), enhancing transepithelial flux | Excessive opening of junctions may increase toxicity; variability in transporter expression among individuals | [163,165,166] |
| Organ-Specific Accumulation Dynamics | Organotropism is governed by anatomical microenvironment, vascular permeability, and nanoparticle deformability or elasticity | Flexible or elongated nanoparticles exhibit greater tumor margination and accumulation; stealth PEG coating extends circulation | Shape-induced immune clearance, variability in microvascular permeability in disease states | [171,173,181] |
| Mononuclear Phagocyte System (MPS) Interactions | Recognition by Kupffer cells and splenic macrophages remains a major clearance mechanism, influenced by opsonization and protein corona formation | PEGylation or biomimetic cloaking (e.g., RBC membrane) reduces MPS uptake and enhances plasma half-life | Accelerated clearance after repeated dosing (anti-PEG antibodies); need for personalized stealth coatings | [174,176,186] |
| Cytokine and Complement Response Mechanisms | Nanoparticles can activate TLRs or complement system, leading to cytokine storms or hypersensitivity depending on their physicochemical profile | Using neutral/zwitterionic polymers lowers immune activation; shape and surface control mitigate pro-inflammatory signaling | Risk of chronic low-grade inflammation; inter-individual differences in immune recognition pathways | [191,193,198] |
| Metabolic Fate and Excretion Pathways | Surface functionalization and hydrodynamic diameter define renal (≤10 nm) vs. hepatobiliary clearance (≥50 nm); metabolism by macrophage enzymes modifies excretion patterns | Size-tuned nanoparticles designed for controlled hepatic metabolism or renal filtration; active efflux modulation is possible | Risk of hepatic overloading or kidney retention with chronic use; lack of data on long-term excretion metabolites | [169,184,199] |
| Standardization for Regulatory Approval | ISO 10993 and ICH M3(R2) guidelines emphasize safety, biodegradation, genotoxicity, and immunotoxicity profiling in nanomedicine development | Nanoformulations undergo systematic hemolysis, cytotoxicity, and cytokine profiling prior to preclinical testing | Absence of phytochemical-specific nanotoxicity thresholds; inter-lab variability in compliance methodologies | [28] |
| Focus Area | Scientific Highlights | Challenges and Strategic Needs | References |
|---|---|---|---|
| Preclinical and Clinical Trials | Demonstrated enhanced efficacy in murine models (e.g., liposomal curcumin, polymeric resveratrol); improved ADME profiles via nanoencapsulation | Limited human trials, complex regulatory pathways, need for predictive preclinical models and adaptive clinical designs | [218,219,220,221,222,223,224,225,226] |
| Commercial Products and Patent Landscape | Several liposomal/polymeric products on market as supplements; rising patents in targeted nanocarriers and controlled release technologies | Regulatory heterogeneity between supplements and drugs; IP protection of complex phytochemical systems remains difficult | [227,228,229,230,231,232,233] |
| Scale-Up and Manufacturing Barriers | Techniques like high-pressure homogenization and microfluidics can enhance reproducibility; PAT tools support QbD compliance | Batch-to-batch variation, costly GMP production, lack of industrial scalability for many prototype nanoformulations | [234,235,236,237,238,239,240,241,242,243] |
| Cost, Accessibility, and Healthcare Acceptance | Nanoformulations may reduce dosing and improve outcomes; potential for long-term cost-effectiveness with fewer side effects and improved patient adherence | High initial cost, unequal access in low-income regions, lack of awareness and acceptance among healthcare providers and patients | [244,245,246,247,248,249,250] |
| Emerging Trend | Key Features | Translational Advantages | References |
|---|---|---|---|
| Smart and Responsive Nano-Phytomedicine Systems | Stimuli-responsive release triggered by pH, ROS, enzymes, light, or magnetic fields; enables site-specific and on-demand phytochemical delivery. | Enhanced spatiotemporal control, reduced off-target toxicity, and improved efficacy in complex disease microenvironments. | [251,252,253,254,255,256,257,258,259,260] |
| Artificial Intelligence and Computational Modeling in Formulation Design | Machine learning and modeling tools used to predict formulation outcomes, optimize nanocarrier parameters, and reduce trial-and-error cycles. | Accelerated development timelines, reduced costs, improved reproducibility, and predictive safety/toxicity screening. | [261,262,263,264,265,266,267,268,269,270] |
| Personalized Nano-Phytotherapy and Precision Medicine Approaches | Stratified treatments using biomarker profiling, genomics, and pharmacogenomics; theranostics and companion diagnostics enhance personalization. | Customized therapies for heterogeneous patient populations, improved adherence, reduced side effects, and dynamic dose optimization. | [271,272,273,274,275,276,277,278] |
| Sustainable and Green Nanotechnology Integration | Green synthesis using biogenic routes; biodegradable carriers; aqueous-based processes and LCA-driven design minimize environmental impact. | Improved public acceptance, regulatory alignment, reduced ecological burden, and compliance with sustainability mandates. | [279,280,281,282,283,284,285,286,287,288,289] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, N.; Aslam, M.; Joo, S.W.; Mandal, T.K. Nano-Phytomedicine: Harnessing Plant-Derived Phytochemicals in Nanocarriers for Targeted Human Health Applications. Molecules 2025, 30, 3177. https://doi.org/10.3390/molecules30153177
Parvin N, Aslam M, Joo SW, Mandal TK. Nano-Phytomedicine: Harnessing Plant-Derived Phytochemicals in Nanocarriers for Targeted Human Health Applications. Molecules. 2025; 30(15):3177. https://doi.org/10.3390/molecules30153177
Chicago/Turabian StyleParvin, Nargish, Mohammad Aslam, Sang Woo Joo, and Tapas Kumar Mandal. 2025. "Nano-Phytomedicine: Harnessing Plant-Derived Phytochemicals in Nanocarriers for Targeted Human Health Applications" Molecules 30, no. 15: 3177. https://doi.org/10.3390/molecules30153177
APA StyleParvin, N., Aslam, M., Joo, S. W., & Mandal, T. K. (2025). Nano-Phytomedicine: Harnessing Plant-Derived Phytochemicals in Nanocarriers for Targeted Human Health Applications. Molecules, 30(15), 3177. https://doi.org/10.3390/molecules30153177








