Docosahexaenoic Acid Inhibits Osteoclastogenesis via FFAR4-Mediated Regulation of Inflammatory Cytokines
Abstract
1. Introduction
2. Molecular Mechanisms of DHA–FFAR4 Interaction
2.1. DHA–FFAR4 Activation of Gαq-PLCβ/IP3-Ca2+-ERK1/2 Pathway
2.2. DHA–FFAR4-Induced β-Arrestin-2/TAB1 Axis Mediates NF-κB Pathway
3. DHA Regulation of Immune Cytokines via FFAR4 Through Multiple Pathways
3.1. FFAR4-Mediated Suppression of Proinflammatory Cytokines
3.2. FFAR4-Mediated Anti-Inflammatory Polarization
3.3. FFAR4-Mediated Regulation of RANKL/OPG Ratio
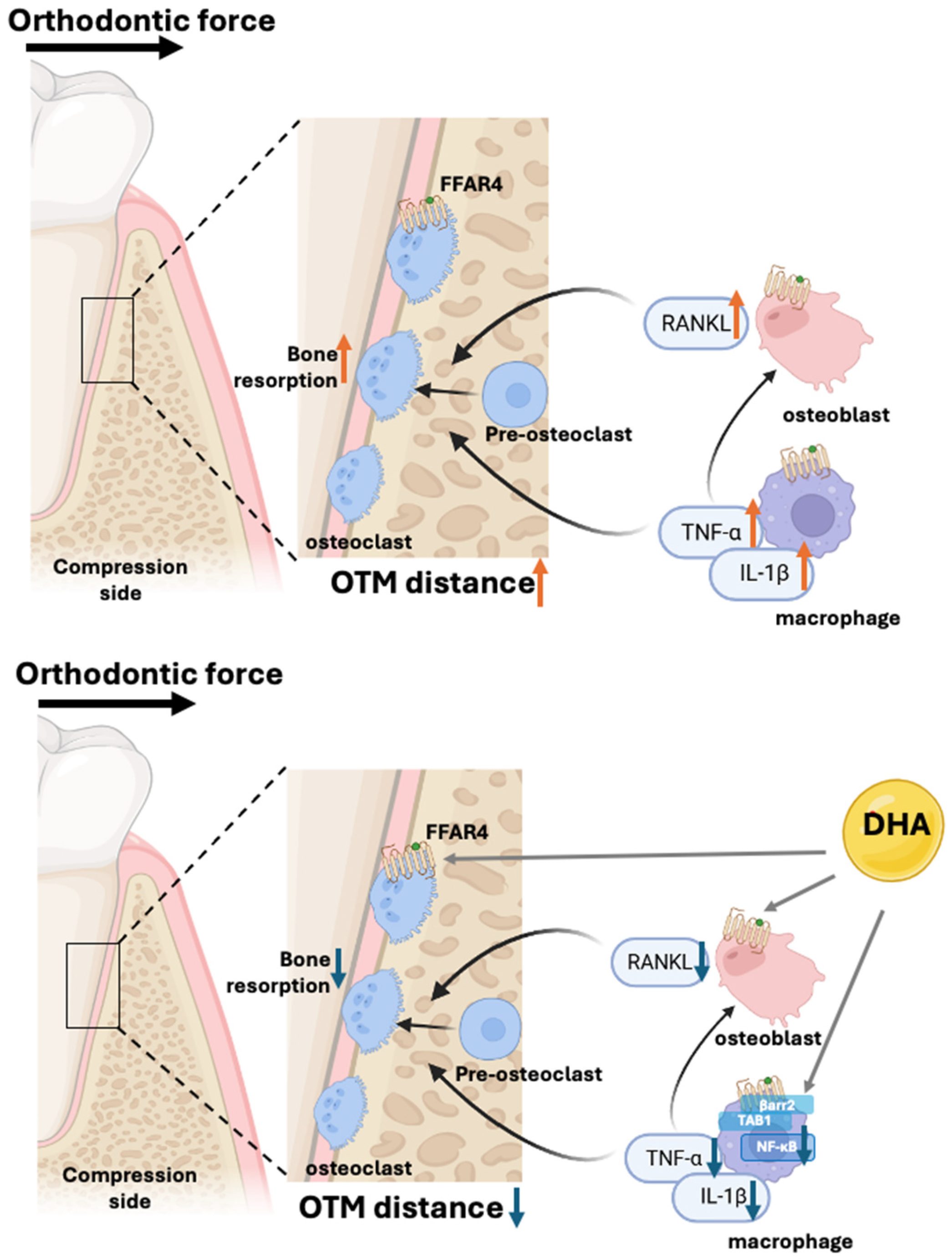
4. DHA-FFAR4 Signaling in Orthodontic Tooth Movement Model
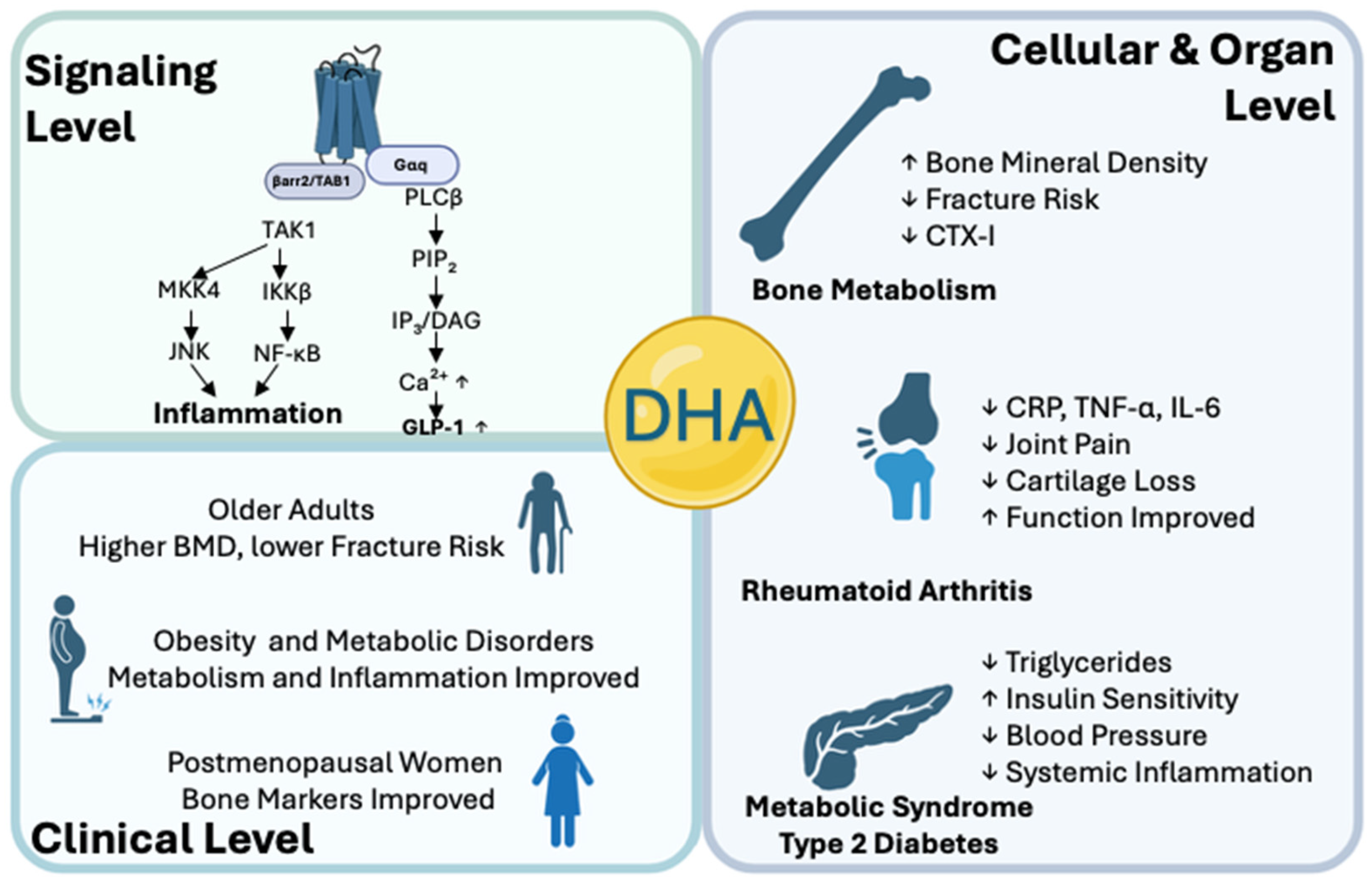
5. Preclinical and Clinical Evidence for DHA–FFAR4 in Bone Preservation
5.1. Roles of DHA–FFAR4 Signaling on Cellular Level
5.2. Roles of DHA–FFAR4 on Animal Models of Bone Diseases
5.3. Human Clinical Studies on the DHA–FFAR4 Pathway
6. Summary and Future Perspectives
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FFAR4 | Free Fatty Acid Receptor 4 |
| GPR120 | G Protein-Coupled Receptor 120 |
| DHA | Docosahexaenoic Acid |
| EPA | Eicosapentaenoic Acid |
| RANKL | Receptor Activator of Nuclear Factor-κB Ligand |
| OPG | Osteoprotegerin |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| MAPK | Mitogen-Activated Protein Kinase |
| NFATc1 | Nuclear Factor of Activated T-Cells, Cytoplasmic 1 |
| IL-10 | Interleukin-10 |
| TNF-α | Tumor Necrosis Factor-α |
| LPS | Lipopolysaccharide |
| OVX | Ovariectomized |
| TRAP | Tartrate-Resistant Acid Phosphatase |
| PLCβ | Phospholipase C Beta |
| TAK1 | Transforming Growth Factor β-Activated Kinase 1 |
| TAB1 | TAK1-Binding Protein 1 |
| ERK1/2 | Extracellular Signal-Regulated Kinases 1/2 |
| OTM | Orthodontic Tooth Movement |
| JNK | c-Jun N-terminal Kinase |
| MMP-9 | Matrix Metalloproteinase-9 |
| TRAF2 | TNF Receptor-Associated Factor 2 |
| DAG | Diacylglycerol |
| PKC | Protein Kinase C |
References
- Ono, T.; Nakashima, T. Recent Advances in Osteoclast Biology. Histochem. Cell Biol. 2018, 149, 325–341. [Google Scholar] [CrossRef]
- Lozano, C.; Duroux-Richard, I.; Firat, H.; Schordan, E.; Apparailly, F. MicroRNAs: Key Regulators to Understand Osteoclast Differentiation? Front. Immunol. 2019, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, M.; Song, S.; Zhao, T.; Zhou, B.; Wang, H.; Tian, W.; Zhao, W.; Zhao, J. Unraveling the Mechanisms That Regulate Osteoclast Differentiation: A Review of Current Advances. Genesis 2025, 63, e70012. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef] [PubMed]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Noguchi, T.; Mizoguchi, I. The Osteocyte and Its Osteoclastogenic Potential. Front. Endocrinol. 2023, 14, 1121727. [Google Scholar] [CrossRef] [PubMed]
- Negishi-Koga, T.; Takayanagi, H. Ca2+-NFATc1 Signaling Is an Essential Axis of Osteoclast Differentiation. Immunol. Rev. 2009, 231, 241–256. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhang, C.; Ni, S.; Ma, M.; Zhang, X.; Sang, W.; Lv, T.; Qian, Z.; Yi, C.; Yu, B. NFATc1-Mediated Expression of SLC7A11 Drives Sensitivity to TXNRD1 Inhibitors in Osteoclast Precursors. Redox Biol. 2023, 63, 102711. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef]
- Sun, J.; Sun, W.J.; Li, Z.Y.; Li, L.; Wang, Y.; Zhao, Y.; Wang, C.; Yu, L.R.; Li, L.Z.; Zhang, Y.L. Daidzein Increases OPG/RANKL Ratio and Suppresses IL-6 in MG-63 Osteoblast Cells. Int. Immunopharmacol. 2016, 40, 32–40. [Google Scholar] [CrossRef]
- Nishida, D.; Arai, A.; Zhao, L.; Yang, M.; Nakamichi, Y.; Horibe, K.; Hosoya, A.; Kobayashi, Y.; Udagawa, N.; Mizoguchi, T. RANKL/OPG Ratio Regulates Odontoclastogenesis in Damaged Dental Pulp. Sci. Rep. 2021, 11, 4575. [Google Scholar] [CrossRef]
- Feng, W.; Guo, J.; Li, M. RANKL-Independent Modulation of Osteoclastogenesis. J. Oral Biosci. 2019, 61, 16–21. [Google Scholar] [CrossRef]
- Wittrant, Y.; Théoleyre, S.; Chipoy, C.; Padrines, M.; Blanchard, F.; Heymann, D.; Rédini, F. RANKL/RANK/OPG: New Therapeutic Targets in Bone Tumours and Associated Osteolysis. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2004, 1704, 49–57. [Google Scholar] [CrossRef]
- Hashizume, M.; Hayakawa, N.; Mihara, M. IL-6 Trans-Signalling Directly Induces RANKL on Fibroblast-like Synovial Cells and Is Involved in RANKL Induction by TNF- and IL-17. Rheumatology 2008, 47, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zheng, T.; Zhao, B. Cytokine-Mediated Immunomodulation of Osteoclastogenesis. Bone 2022, 164, 116540. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jin, H.M.; Kim, K.; Song, I.; Youn, B.U.; Matsuo, K.; Kim, N. The Mechanism of Osteoclast Differentiation Induced by IL-1. J. Immunol. 2009, 183, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Li, L.; Sun, Y.; Wang, W.; Wang, X.; Ye, Y.; Chen, X.; Xu, Y. Interleukin-17 Regulates the Expressions of RANKL and OPG in Human Periodontal Ligament Cells via TRAF 6/ TBK 1-JNK / NF-κB Pathways. Immunology 2015, 144, 472–485. [Google Scholar] [CrossRef]
- Li, J.-Y.; Yu, M.; Tyagi, A.M.; Vaccaro, C.; Hsu, E.; Adams, J.; Bellido, T.; Weitzmann, M.N.; Pacifici, R. IL-17 Receptor Signaling in Osteoblasts/Osteocytes Mediates PTH-Induced Bone Loss and Enhances Osteocytic RANKL Production. J. Bone Miner. Res. 2019, 34, 349–360. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.-S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Xie, L.; Crane, J.; Zhen, G.; Mishina, Y.; Deng, R.; Gao, B.; Chen, H.; Liu, S.; et al. Inhibition of Overactive TGF-β Attenuates Progression of Heterotopic Ossification in Mice. Nat. Commun. 2018, 9, 551. [Google Scholar] [CrossRef]
- Lee, B.; Oh, Y.; Jo, S.; Kim, T.-H.; Ji, J.D. A Dual Role of TGF-β in Human Osteoclast Differentiation Mediated by Smad1 versus Smad3 Signaling. Immunol. Lett. 2019, 206, 33–40. [Google Scholar] [CrossRef]
- Gao, J.; Xie, C.; Yang, J.; Tian, C.; Zhang, M.; Lu, Z.; Meng, X.; Cai, J.; Guo, X.; Gao, T. The Effects of N-3 PUFA Supplementation on Bone Metabolism Markers and Body Bone Mineral Density in Adults: A Systematic Review and Meta-Analysis of RCTs. Nutrients 2023, 15, 2806. [Google Scholar] [CrossRef]
- Shawl, M.; Geetha, T.; Burnett, D.; Babu, J. Omega-3 Supplementation and Its Effects on Osteoarthritis. Nutrients 2024, 16, 1650. [Google Scholar] [CrossRef]
- Freitas, R.; Campos, M.M. Protective Effects of Omega-3 Fatty Acids in Cancer-Related Complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Sharma, A.; Maheshwari, R.; Pachori, G.; Kumari, P.; Mandal, C.C. Docosahexaenoic Acid (DHA) Inhibits Bone Morphogenetic Protein-2 (BMP-2) Elevated Osteoblast Potential of Metastatic Breast Cancer (MDA-MB-231) Cells in Mammary Microcalcification. Nutr. Cancer 2020, 72, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Pros and Cons of Long-Chain Omega-3 Polyunsaturated Fatty Acids in Cardiovascular Health. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Abou-Saleh, H.; Ouhtit, A.; Halade, G.V.; Rahman, M.M. Bone Benefits of Fish Oil Supplementation Depend on Its EPA and DHA Content. Nutrients 2019, 11, 2701. [Google Scholar] [CrossRef]
- Yang, W.; Liu, H.; Xu, L.; Yu, T.; Zhao, X.; Yao, S.; Zhao, Q.; Barnes, S.; Cohn, S.M.; Dann, S.M.; et al. GPR120 Inhibits Colitis Through Regulation of CD4+ T Cell Interleukin 10 Production. Gastroenterology 2022, 162, 150–165. [Google Scholar] [CrossRef]
- Rahman, M.M.; Veigas, J.M.; Williams, P.J.; Fernandes, G. DHA Is a More Potent Inhibitor of Breast Cancer Metastasis to Bone and Related Osteolysis than EPA. Breast Cancer Res. Treat. 2013, 141, 341–352. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, Y.; Wang, Q.; Fu, M.; Xue, C.; Wang, J. Comparative Study of DHA with Different Molecular Forms for Ameliorating Osteoporosis by Promoting Chondrocyte-to-Osteoblast Transdifferentiation in the Growth Plate of Ovariectomized Mice. J. Agric. Food Chem. 2021, 69, 10562–10571. [Google Scholar] [CrossRef]
- Dicks, L.M.T. How Important Are Fatty Acids in Human Health and Can They Be Used in Treating Diseases? Gut Microbes 2024, 16, 2420765. [Google Scholar] [CrossRef]
- Banaszak, M.; Dobrzyńska, M.; Kawka, A.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Role of Omega-3 Fatty Acids Eicosapentaenoic (EPA) and Docosahexaenoic (DHA) as Modulatory and Anti-Inflammatory Agents in Noncommunicable Diet-Related Diseases—Reports from the Last 10 Years. Clin. Nutr. ESPEN 2024, 63, 240–258. [Google Scholar] [CrossRef]
- So, J.; Wu, D.; Lichtenstein, A.H.; Tai, A.K.; Matthan, N.R.; Maddipati, K.R.; Lamon-Fava, S. EPA and DHA Differentially Modulate Monocyte Inflammatory Response in Subjects with Chronic Inflammation in Part via Plasma Specialized Pro-Resolving Lipid Mediators: A Randomized, Double-Blind, Crossover Study. Atherosclerosis 2021, 316, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Talamonti, E.; Jacobsson, A.; Chiurchiù, V. Impairment of Endogenous Synthesis of Omega-3 DHA Exacerbates T-Cell Inflammatory Responses. Int. J. Mol. Sci. 2023, 24, 3717. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Kitaura, H.; Ogawa, S.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; Pramusita, A.; Kinjo, R.; Kanou, K.; et al. Docosahexaenoic Acid Inhibits TNF-α-Induced Osteoclast Formation and Orthodontic Tooth Movement through GPR120. Front. Immunol. 2023, 13, 929690. [Google Scholar] [CrossRef] [PubMed]
- Di Petrillo, A.; Kumar, A.; Onali, S.; Favale, A.; Fantini, M.C. GPR120/FFAR4: A Potential New Therapeutic Target for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2023, 29, 1981–1989. [Google Scholar] [CrossRef]
- Burns, R.N.; Singh, M.; Senatorov, I.S.; Moniri, N.H. Mechanisms of Homologous and Heterologous Phosphorylation of FFA Receptor 4 (GPR120): GRK6 and PKC Mediate Phosphorylation of Thr347, Ser350, and Ser357 in the C-Terminal Tail. Biochem. Pharmacol. 2014, 87, 650–659. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Hayashi, M.; Nagamatsu, K.; Ono, T.; Kamakura, M.; Iwata, T.; Nakashima, T. The Key Royal Jelly Component 10-Hydroxy-2-Decenoic Acid Protects against Bone Loss by Inhibiting NF-κB Signaling Downstream of FFAR4. J. Biol. Chem. 2020, 295, 12224–12232. [Google Scholar] [CrossRef]
- Milligan, G.; Alvarez-Curto, E.; Hudson, B.D.; Prihandoko, R.; Tobin, A.B. FFA4/GPR120: Pharmacology and Therapeutic Opportunities. Trends Pharmacol. Sci. 2017, 38, 809–821. [Google Scholar] [CrossRef]
- Davenport, A.P.; Alexander, S.P.H.; Sharman, J.L.; Pawson, A.J.; Benson, H.E.; Monaghan, A.E.; Liew, W.C.; Mpamhanga, C.P.; Bonner, T.I.; Neubig, R.R.; et al. International Union of Basic and Clinical Pharmacology. LXXXVIII. G Protein-Coupled Receptor List: Recommendations for New Pairings with Cognate Ligands. Pharmacol. Rev. 2013, 65, 967–986. [Google Scholar] [CrossRef]
- Kim, H.; Yoon, H.; Kim, B.K.; Kang, W.Y.; Seong, S.J.; Lim, M.; Kim, S.; Yoon, Y. G Protein-Coupled Receptor 120 Signaling Negatively Regulates Osteoclast Differentiation, Survival, and Function. J. Cell. Physiol. 2016, 231, 844–851. [Google Scholar] [CrossRef]
- Chang, C.-K.; Chen, P.-K.; Chen, C.-C.; Chang, S.-H.; Chen, C.-H.; Chen, D.-Y. Increased Levels of Omega-3 Fatty Acids and DHA Are Linked to Pain Reduction in Rheumatoid Arthritis Patients Treated with Janus Kinase Inhibitors. Nutrients 2021, 13, 3050. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, J.; Tan, X.; Shen, Y.; Xu, L.; Li, T.; Ma, W.; Wu, J. GPR120 Agonist Ameliorated Insulin Resistance and Improved Ovarian Function. Zygote 2022, 30, 380–385. [Google Scholar] [CrossRef]
- Su, Y.; Han, Y.; Choi, H.S.; Lee, G.-Y.; Cho, H.W.; Choi, H.; Choi, J.H.; Jang, Y.-S.; Seo, J.-W. Lipid Mediators Obtained from Docosahexaenoic Acid by Soybean Lipoxygenase Attenuate RANKL-Induced Osteoclast Differentiation and Rheumatoid Arthritis. Biomed. Pharmacother. 2024, 171, 116153. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Song, S.; Niu, Y.; Meng, M.; Wang, C. Eicosapentaenoic Acid (EPA) Induced Macrophages Activation through GPR120-Mediated Raf-ERK1/2-IKKβ-NF-κB P65 Signaling Pathways. Nutrients 2017, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shao, J.; Brandenburger, I.; Qian, W.; Hahnefeld, L.; Bonnavion, R.; Cho, H.; Wang, S.; Hidalgo, J.; Wettschureck, N.; et al. FFAR4-Mediated IL-6 Release from Islet Macrophages Promotes Insulin Secretion and Is Compromised in Type-2 Diabetes. Nat. Commun. 2025, 16, 3422. [Google Scholar] [CrossRef] [PubMed]
- Teyani, R.L.; Moniri, N.H. Biased Agonism at Free-Fatty Acid Receptor-4 (FFA4/GPR120). Pharmacol. Ther. 2025, 266, 108784. [Google Scholar] [CrossRef]
- Kamato, D.; Thach, L.; Bernard, R.; Chan, V.; Zheng, W.; Kaur, H.; Brimble, M.; Osman, N.; Little, P.J. Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Gα/q, 11. Front. Cardiovasc. Med. 2015, 2, 14. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, D.; Ho, K.W.; Lin, S.; Suen, W.C.-W.; Zhang, H.; Zha, Z.; Li, G.; Leung, P.S. GPR120 Is an Important Inflammatory Regulator in the Development of Osteoarthritis. Arthritis Res. Ther. 2018, 20, 163. [Google Scholar] [CrossRef]
- Nakamoto, K.; Tokuyama, S. Docosahexaenoic Acid Attenuates the Progression of Nonalcoholic Steatohepatitis by Suppressing the Adipocyte Inflammation via the G Protein-Coupled Receptor 120/Free Fatty Acid Receptor 4 Pathway. Pharmacology 2022, 107, 330–338. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Zong, Y.; Yang, X. DHA Protects Hepatocytes from Oxidative Injury through GPR120/ERK-Mediated Mitophagy. Int. J. Mol. Sci. 2021, 22, 5675. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-Inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Jordy, A.B.; Kiens, B. Regulation of Exercise-induced Lipid Metabolism in Skeletal Muscle. Exp. Physiol. 2014, 99, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.J.; Edelman, A.M.; Turcotte, L.P. CaMKK Is an Upstream Signal of AMP-Activated Protein Kinase in Regulation of Substrate Metabolism in Contracting Skeletal Muscle. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, R1724–R1732. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, S.; Shen, W.; Liu, J.; Liu, Y.; Li, Y.; Zhang, F.; Li, T.; Zhang, X.; Tian, W.; et al. α-Linolenic Acid Mitigates Microglia-Mediated Neuroinflammation of Schizophrenia in Mice by Suppressing the NF-κB/NLRP3 Pathway via Binding GPR120-β-Arrestin 2. Int. Immunopharmacol. 2024, 142, 113047. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bhattacharya, A.; Fernandes, G. Docosahexaenoic Acid Is More Potent Inhibitor of Osteoclast Differentiation in RAW 264.7 Cells than Eicosapentaenoic Acid. J. Cell. Physiol. 2008, 214, 201–209. [Google Scholar] [CrossRef]
- Sithole, C.; Pieterse, C.; Howard, K.; Kasonga, A. GPR120 Inhibits RANKL-Induced Osteoclast Formation and Resorption by Attenuating Reactive Oxygen Species Production in RAW264.7 Murine Macrophages. Int. J. Mol. Sci. 2021, 22, 10544. [Google Scholar] [CrossRef]
- Gao, B.; Han, Y.-H.; Wang, L.; Lin, Y.-J.; Sun, Z.; Lu, W.-G.; Hu, Y.-Q.; Li, J.-Q.; Lin, X.-S.; Liu, B.-H.; et al. Eicosapentaenoic Acid Attenuates Dexamethasome-Induced Apoptosis by Inducing Adaptive Autophagy via GPR120 in Murine Bone Marrow-Derived Mesenchymal Stem Cells. Cell Death Dis. 2016, 7, e2235. [Google Scholar] [CrossRef]
- Zhang, B.; Cui, J.; Zhang, X.; Pan, Z.; Du, L.; Ye, R.; Wen, L.; Zhai, W.; Huang, L.; Li, D.; et al. Autophagy: Regulating the Seesaw of Bone–Fat Balance. Front. Cell Dev. Biol. 2025, 13, 1465092. [Google Scholar] [CrossRef]
- Ahn, S.H.; Park, S.-Y.; Baek, J.-E.; Lee, S.-Y.; Baek, W.-Y.; Lee, S.-Y.; Lee, Y.-S.; Yoo, H.J.; Kim, H.; Lee, S.H.; et al. Free Fatty Acid Receptor 4 (GPR120) Stimulates Bone Formation and Suppresses Bone Resorption in the Presence of Elevated n-3 Fatty Acid Levels. Endocrinology 2016, 157, 2621–2635. [Google Scholar] [CrossRef]
- Hansen, M.S.; Madsen, K.; Price, M.; Søe, K.; Omata, Y.; Zaiss, M.M.; Gorvin, C.M.; Frost, M.; Rauch, A. Transcriptional Reprogramming during Human Osteoclast Differentiation Identifies Regulators of Osteoclast Activity. Bone Res. 2024, 12, 5. [Google Scholar] [CrossRef]
- Ahn, S.; Kaipparettu, B.A. G-Protein Coupled Receptors in Metabolic Reprogramming and Cancer. Pharmacol. Ther. 2025, 270, 108849. [Google Scholar] [CrossRef]
- Al Mahri, S.; Malik, S.S.; Al Ibrahim, M.; Haji, E.; Dairi, G.; Mohammad, S. Free Fatty Acid Receptors (FFARs) in Adipose: Physiological Role and Therapeutic Outlook. Cells 2022, 11, 750. [Google Scholar] [CrossRef]
- Price, M.L.; Wyatt, R.A.; Correia, J.; Areej, Z.; Hinds, M.; Crastin, A.; Hardy, R.S.; Frost, M.; Gorvin, C.M. Identification of Anti-Resorptive GPCRs by High-Content Imaging in Human Osteoclasts. J. Mol. Endocrinol. 2025, 74, e240143. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of Energy Metabolism by Long-Chain Fatty Acids. Progress Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Kishikawa, A.; Kitaura, H.; Kimura, K.; Ogawa, S.; Qi, J.; Shen, W.-R.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; et al. Docosahexaenoic Acid Inhibits Inflammation-Induced Osteoclast Formation and Bone Resorption in Vivo Through GPR120 by Inhibiting TNF-α Production in Macrophages and Directly Inhibiting Osteoclast Formation. Front. Endocrinol. 2019, 10, 157. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Hu, M.; Huang, J.; Yu, S.; Zeng, H.; Mao, L. EPA and DHA Differentially Improve Insulin Resistance by Reducing Adipose Tissue Inflammation—Targeting GPR120/PPARγ Pathway. J. Nutr. Biochem. 2024, 130, 109648. [Google Scholar] [CrossRef]
- McMurray, D.N.; Jolly, C.A.; Chapkin, R.S. Effects of Dietary N-3 Fatty Acids on T Cell Activation and T Cell Receptor-Mediated Signaling in a Murine Model. J. Infect. Dis. 2000, 182, S103–S107. [Google Scholar] [CrossRef]
- Hong, L.; Zahradka, P.; Taylor, C.G. Differential Modulation by Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) of Mesenteric Fat and Macrophages and T Cells in Adipose Tissue of Obese Fa/Fa Zucker Rats. Nutrients 2024, 16, 1311. [Google Scholar] [CrossRef] [PubMed]
- Salaga, M.; Bartoszek, A.; Binienda, A.; Krajewska, J.B.; Fabisiak, A.; Mosińska, P.; Dziedziczak, K.; Niewinna, K.; Talar, M.; Tarasiuk, A.; et al. Activation of Free Fatty Acid Receptor 4 Affects Intestinal Inflammation and Improves Colon Permeability in Mice. Nutrients 2021, 13, 2716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Wu, Y.; Guo, B.; Li, J.; Huang, X.; Zhang, F.; Li, M.; Yang, P.; Zheng, X. Activation of Free Fatty Acid Receptors, FFAR1 and FFAR4, Ameliorates Ulcerative Colitis by Promote Fatty Acid Metabolism and Mediate Macrophage Polarization. Int. Immunopharmacol. 2024, 130, 111778. [Google Scholar] [CrossRef] [PubMed]
- Kasonga, A.E.; Kruger, M.C.; Coetzee, M. Free Fatty Acid Receptor 4-β-Arrestin 2 Pathway Mediates the Effects of Different Classes of Unsaturated Fatty Acids in Osteoclasts and Osteoblasts. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 281–289. [Google Scholar] [CrossRef]
- Cho, H.J.; Ahn, S.H.; Lee, Y.-S.; Lee, S.H.; Im, D.-S.; Kim, I.; Koh, J.-M.; Kim, S.; Kim, B.-J. Free Fatty Acid Receptor 4 Mediates the Beneficial Effects of N-3 Fatty Acids on Body Composition in Mice. Calcif. Tissue Int. 2017, 101, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Ohori, F.; Kitaura, H.; Marahleh, A.; Ma, J.; Miura, M.; Ren, J.; Narita, K.; Fan, Z.; Lin, A.; Mizoguchi, I. Osteocyte Necroptosis Drives Osteoclastogenesis and Alveolar Bone Resorption during Orthodontic Tooth Movement. Sci. Rep. 2025, 15, 19413. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Nara, Y.; Pramusita, A.; Kinjo, R.; Ma, J.; Kanou, K.; Mizoguchi, I. Role of the Interaction of Tumor Necrosis Factor-α and Tumor Necrosis Factor Receptors 1 and 2 in Bone-Related Cells. Int. J. Mol. Sci. 2022, 23, 1481. [Google Scholar] [CrossRef] [PubMed]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Noguchi, T.; Nara, Y.; Pramusita, A.; Kinjo, R.; Ma, J.; Kanou, K.; Mizoguchi, I. Effect of TNF-a on Osteocyte RANKL Expression during Orthodontic Tooth Movement. J. Dent. Sci. 2021, 16, 1191–1197. [Google Scholar] [CrossRef]
- Noguchi, T.; Kitaura, H.; Ogawa, S.; Qi, J.; Shen, W.-R.; Ohori, F.; Marahleh, A.; Nara, Y.; Pramusita, A.; Mizoguchi, I. TNF-α Stimulates the Expression of RANK during Orthodontic Tooth Movement. Arch. Oral Biol. 2020, 117, 104796. [Google Scholar] [CrossRef]
- Kanou, K.; Kitaura, H.; Noguchi, T.; Ohori, F.; Marahleh, A.; Kinjo, R.; Ma, J.; Ren, J.; Ogasawara, K.; Mizoguchi, I. Effect of Age on Orthodontic Tooth Movement in Mice. J. Dent. Sci. 2024, 19, 828–836. [Google Scholar] [CrossRef]
- Fan, Z.; Kitaura, H.; Noguchi, T.; Ohori, F.; Marahleh, A.; Ma, J.; Ren, J.; Lin, A.; Narita, K.; Mizoguchi, I. Exacerbating Orthodontic Tooth Movement in Mice with Salt-Sensitive Hypertension. J. Dent. Sci. 2025, 20, 764–769. [Google Scholar] [CrossRef]
- Ndemuweda, T.; Kitaura, H.; Ohori, F.; Marahleh, A.; Ma, J.; Fan, Z.; Lin, A.; Narita, K.; Itou, A.; Mizoguchi, I. Evaluation of the Effects for Root Resorption in Orthodontic Tooth Movement with Micro-Osteoperforations in Mice. J. Dent. Sci. 2025, 20, 1415–1421. [Google Scholar] [CrossRef]
- Kitaura, H.; Ohori, F.; Marahleh, A.; Ma, J.; Lin, A.; Fan, Z.; Narita, K.; Murakami, K.; Kanetaka, H. The Role of Cytokines in Orthodontic Tooth Movement. Int. J. Mol. Sci. 2025, 26, 6688. [Google Scholar] [CrossRef]
- Miroult, C.; Lasserre, J.; Toma, S. Effects of Omega-3 as an Adjuvant in the Treatment of Periodontal Disease: A Systematic Review and Meta-analysis. Clin. Exp. Dent. Res. 2023, 9, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Van Ravensteijn, M.M.; Timmerman, M.F.; Brouwer, E.A.G.; Slot, D.E. The Effect of Omega-3 Fatty Acids on Active Periodontal Therapy: A Systematic Review and Meta-analysis. J. Clin. Periodontol. 2022, 49, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, S.; Fontana, S.; Salaroglio, I.C.; Anobile, D.P.; Digiovanni, S.; Akman, M.; Jafari, N.; Godel, M.; Costamagna, C.; Corbet, C.; et al. Increasing Membrane Polyunsaturated Fatty Acids Sensitizes Non-Small Cell Lung Cancer to Anti-PD-1/PD-L1 Immunotherapy. Cancer Lett. 2024, 604, 217221. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Shao, J.; Zhou, S.; Zhao, Z.; Li, F.; Xiang, R.; Zhao, A.Z.; Pan, J. Inhibition of Lung Cancer Growth and Metastasis by DHA and Its Metabolite, RvD1, through miR-138-5p/FOXC1 Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 479. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Li, X.-C.; Liang, X.-Y.; Zhao, Y.-Y.; Xie, R.; Zhang, L.-J.; Zhang, X.-C.; Chen, C. GPR120 Regulates Pancreatic Polypeptide Secretion from Male Mouse Islets via PLC-Mediated Calcium Mobilization. Endocrinology 2020, 161, bqaa157. [Google Scholar] [CrossRef]
- Ali, Z.; Al-Ghouti, M.A.; Abou-Saleh, H.; Rahman, M.M. Unraveling the Omega-3 Puzzle: Navigating Challenges and Innovations for Bone Health and Healthy Aging. Mar. Drugs 2024, 22, 446. [Google Scholar] [CrossRef]
- Sun, D.; Krishnan, A.; Zaman, K.; Lawrence, R.; Bhattacharya, A.; Fernandes, G. Dietary N-3 Fatty Acids Decrease Osteoclastogenesis and Loss of Bone Mass in Ovariectomized Mice. J. Bone Miner. Res. 2003, 18, 1206–1216. [Google Scholar] [CrossRef]
- Poulsen, R.C.; Firth, E.C.; Rogers, C.W.; Moughan, P.J.; Kruger, M.C. Specific Effects of γ-Linolenic, Eicosapentaenoic, and Docosahexaenoic Ethyl Esters on Bone Post-Ovariectomy in Rats. Calcif. Tissue Int. 2007, 81, 459–471. [Google Scholar] [CrossRef]
- Jeong, M.; Shin, J.-I.; Cho, J.; Jeon, Y.-J.; Kim, J.-H.; Youn, J.; Lee, K. DHA Induces Cell Death through the Production of ROS and the Upregulation of CHOP in Fibroblast-like Synovial Cells from Human Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2023, 24, 1734. [Google Scholar] [CrossRef]
- Olson, M.V.; Liu, Y.-C.; Dangi, B.; Paul Zimmer, J.; Salem Jr, N.; Nauroth, J.M. Docosahexaenoic Acid Reduces Inflammation and Joint Destruction in Mice with Collagen-Induced Arthritis. Inflamm. Res. 2013, 62, 1003–1013. [Google Scholar] [CrossRef]
- Dawczynski, C.; Dittrich, M.; Neumann, T.; Goetze, K.; Welzel, A.; Oelzner, P.; Völker, S.; Schaible, A.M.; Troisi, F.; Thomas, L.; et al. Docosahexaenoic Acid in the Treatment of Rheumatoid Arthritis: A Double-Blind, Placebo-Controlled, Randomized Cross-over Study with Microalgae vs. Sunflower Oil. Clin. Nutr. 2018, 37, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Kolahi, S.; Ghorbanihaghjo, A.; Alizadeh, S.; Rashtchizadeh, N.; Argani, H.; Khabazzi, A.-R.; Hajialilo, M.; Bahreini, E. Fish Oil Supplementation Decreases Serum Soluble Receptor Activator of Nuclear Factor-Kappa B Ligand/Osteoprotegerin Ratio in Female Patients with Rheumatoid Arthritis. Clin. Biochem. 2010, 43, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Q.; Liao, X.; Elbelt, U.; Weylandt, K.H. The Effects of Omega-3 Fatty Acids in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Prostaglandins Leukot. Essent. Fat. Acids 2022, 182, 102456. [Google Scholar] [CrossRef]
- Mei, Z.; Chen, G.-C.; Hu, J.; Lin, C.; Sun, Z.; Liu, C.; Geng, X.; Yuan, C.; Qi, Q.; Zheng, Y. Habitual Use of Fish Oil Supplements, Genetic Predisposition, and Risk of Fractures: A Large Population-Based Study. Am. J. Clin. Nutr. 2021, 114, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rizzolo, D.A.; Serra, A.; Colungo, C.; Sala-Vila, A.; Sisó-Almirall, A.; Gomis, R. Type 2 Diabetes Preventive Effects with a 12-Months Sardine-Enriched Diet in Elderly Population with Prediabetes: An Interventional, Randomized and Controlled Trial. Clin. Nutr. 2021, 40, 2587–2598. [Google Scholar] [CrossRef]
- Holub, B.J. Docosahexaenoic Acid (DHA) and Cardiovascular Disease Risk Factors. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 199–204. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Libby, P.; Budoff, M.J.; Bhatt, D.L.; Mason, R.P. Role of Omega-3 Fatty Acids in Cardiovascular Disease: The Debate Continues. Curr. Atheroscler. Rep. 2023, 25, 1–17. [Google Scholar] [CrossRef]
- Félix-Soriano, E.; Martínez-Gayo, A.; Cobo, M.J.; Pérez-Chávez, A.; Ibáñez-Santos, J.; Palacios Samper, N.; Goikoetxea Galarza, I.; Cuervo, M.; García-Unciti, M.; González-Muniesa, P.; et al. Effects of DHA-Rich n-3 Fatty Acid Supplementation and/or Resistance Training on Body Composition and Cardiometabolic Biomarkers in Overweight and Obese Post-Menopausal Women. Nutrients 2021, 13, 2465. [Google Scholar] [CrossRef]
- Lu, A.-X.; Lin, Y.; Li, J.; Liu, J.-X.; Yan, C.-H.; Zhang, L. Effects of Food-Borne Docosahexaenoic Acid Supplementation on Bone Lead Mobilisation, Mitochondrial Function and Serum Metabolomics in Pre-Pregnancy Lead-Exposed Lactating Rats. Environ. Pollut. 2023, 337, 122613. [Google Scholar] [CrossRef]
- Dong, H.; Hutchins-Wiese, H.; Kleppinger, A.; Annis, K.; Liva, E.; Lammi-Keefe, C.; Durham, H.; Feinn, R.; Kenny, A.M. Effects of Omega-3 Polyunsaturated Fatty Acid Supplementation on Bone Turnover in Older Women. Int. J. Vitam. Nutr. Res. 2014, 84, 124–132. [Google Scholar] [CrossRef]
- Farina, E.K.; Kiel, D.P.; Roubenoff, R.; Schaefer, E.J.; Cupples, L.A.; Tucker, K.L. Protective Effects of Fish Intake and Interactive Effects of Long-Chain Polyunsaturated Fatty Acid Intakes on Hip Bone Mineral Density in Older Adults: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2011, 93, 1142–1151. [Google Scholar] [CrossRef]
- Koren, N.; Simsa-Maziel, S.; Shahar, R.; Schwartz, B.; Monsonego-Ornan, E. Exposure to Omega-3 Fatty Acids at Early Age Accelerate Bone Growth and Improve Bone Quality. J. Nutr. Biochem. 2014, 25, 623–633. [Google Scholar] [CrossRef]
- Kelly, O.J.; Gilman, J.C.; Kim, Y.; Ilich, J.Z. Long-Chain Polyunsaturated Fatty Acids May Mutually Benefit Both Obesity and Osteoporosis. Nutr. Res. 2013, 33, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Feehan, O.; Magee, P.J.; Pourshahidi, L.K.; Armstrong, D.J.; Slevin, M.M.; Allsopp, P.J.; Conway, M.C.; Strain, J.J.; McSorley, E.M. Associations of Long Chain Polyunsaturated Fatty Acids with Bone Mineral Density and Bone Turnover in Postmenopausal Women. Eur. J. Nutr. 2023, 62, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Y.; Zhou, J.; Zang, Y. Effects of Omega-3 Supplementation on Lipid Metabolism, Inflammation, and Disease Activity in Rheumatoid Arthritis: A Meta-Analysis of Randomized Controlled Trials. Clin. Rheumatol. 2024, 43, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 Polyunsaturated Fatty Acids and Inflammatory Processes: Nutrition or Pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Vranou, P.; Gkoutzourelas, A.; Athanatou, D.; Zafiriou, E.; Grammatikopoulou, M.G.; Bogdanos, D.P. Let Food Be Thy Medicine: The Case of The Mediterranean Diet in Rheumatoid Arthritis. Mediterr. J. Rheumatol. 2020, 31, 325. [Google Scholar] [CrossRef]
- Parolini, C. The Role of Marine N-3 Polyunsaturated Fatty Acids in Inflammatory-Based Disease: The Case of Rheumatoid Arthritis. Mar. Drugs 2023, 22, 17. [Google Scholar] [CrossRef]
- Marchand, N.E.; Choi, M.Y.; Oakes, E.G.; Cook, N.R.; Stevens, E.; Gomelskaya, N.; Kotler, G.; Manson, J.E.; Lasky-Su, J.; Mora, S.; et al. Over-the-Counter Fish Oil Supplementation and pro-Resolving and pro-Inflammatory Lipid Mediators in Rheumatoid Arthritis. Prostaglandins Leukot. Essent. Fat. Acids 2023, 190, 102542. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Wang, D.; Yan, N.; Li, C.; Wu, M.; Wang, F.; Mi, B.; Chen, F.; Jia, W.; et al. Omega-3 Polyunsaturated Fatty Acid Biomarkers and Risk of Type 2 Diabetes, Cardiovascular Disease, Cancer, and Mortality. Clin. Nutr. 2022, 41, 1798–1807. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, P.; Sun, Y.; Sun, J.; Dong, J.-N.; Wang, H.-G.; Zuo, L.-G.; Gong, J.-F.; Li, Y.; Gu, L.-L.; et al. DHA Protects against Experimental Colitis in IL-10-Deficient Mice Associated with the Modulation of Intestinal Epithelial Barrier Function. Br. J. Nutr. 2015, 114, 181–188. [Google Scholar] [CrossRef]
| Citation | Model | Treatment Dose | Duration | Effect on Bone Resorption |
|---|---|---|---|---|
| Cell Sithole et al., 2021 [56] | RAW264.7 macrophages | TUG-891 (GPR120 agonist) 20–100 µM | 5 days | Inhibits osteoclast activity—blocks RANKL-induced TRAP+ multinuclear cells |
| Kasonga et al., 2019 [71] | RAW264.7 MC3T3-E1 | DHA 40 µM | ≤5 days | Inhibits osteoclast activity—DHA-activated GPR120/β-arrestin-2 blocks RANKL-NF-κB/MAPK |
| Animal Tsuchiya et al., 2020 [37] | OVX mice (and OC cultures) | In vivo: 10H2DA (GPR120 agonist) 40 mg kg−1 In vitro: 500 µM | 4 weeks | Inhibits osteoclast activity—10H2DA blocks RANKL-NF-κB/NFATc1, reducing bone loss |
| Ahn et al., 2016 [59] | In vivo: fat-1 × Ffar4-/- mice (±OVX) with calvarial injection In vitro: RAW264.7 pre-osteoclasts | Endogenously elevated n-3 fatty acids In vivo: DHA 50–250 µM In vitro: DHA 40 µM | 4–8 wk experimental periods ≤5 d (culture) | Inhibits bone resorption |
| Sun et al., 2003 [87] | OVX mice (and BMM cultures) | In vivo: 5% fish oil diet In vitro: DHA/EPA 40 µM | 4 months | Inhibits osteoclast activity—prevents trabecular bone loss; suppresses NF-κB and osteoclast formation |
| Su et al., 2024 [43] | RAW264.7 macrophages and CAIA mice | In vitro: lipid mediator mix from DHA 1 µg mL−1 In vivo: 10 µg kg−1 day−1 oral | 5-day culture 10-day dosing | Inhibits osteoclast activity—DHA-derived lipid mediators suppress RANKL-induced TRAP and CTSK via NF-κB and reduce bone erosion |
| Human | ||||
| Xiao et al., 2022 [93] | Meta-analysis 46 RCTs, n = 4991 | Fish-oil ≤ 2 vs. >2 g d−1 | 4–12 months | Improves lipid profile |
| Mei et al., 2021 [94] | Prospective cohort, UK 40–70 y, n = 378,018 | With or without fish oil supplement | 12 years | Reduces fracture risk |
| Díaz-Rizzolo et al., 2021 [95] | RCT ≥ 65 y, n = 152 | Sardine 200 g week−1 (≈3 g EPA + DHA) | 12 months | Lowers risk of type 2 diabetes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Kitaura, H.; Ohori, F.; Marahleh, A.; Fan, Z.; Lin, A.; Narita, K.; Murakami, K.; Kanetaka, H. Docosahexaenoic Acid Inhibits Osteoclastogenesis via FFAR4-Mediated Regulation of Inflammatory Cytokines. Molecules 2025, 30, 3180. https://doi.org/10.3390/molecules30153180
Ma J, Kitaura H, Ohori F, Marahleh A, Fan Z, Lin A, Narita K, Murakami K, Kanetaka H. Docosahexaenoic Acid Inhibits Osteoclastogenesis via FFAR4-Mediated Regulation of Inflammatory Cytokines. Molecules. 2025; 30(15):3180. https://doi.org/10.3390/molecules30153180
Chicago/Turabian StyleMa, Jinghan, Hideki Kitaura, Fumitoshi Ohori, Aseel Marahleh, Ziqiu Fan, Angyi Lin, Kohei Narita, Kou Murakami, and Hiroyasu Kanetaka. 2025. "Docosahexaenoic Acid Inhibits Osteoclastogenesis via FFAR4-Mediated Regulation of Inflammatory Cytokines" Molecules 30, no. 15: 3180. https://doi.org/10.3390/molecules30153180
APA StyleMa, J., Kitaura, H., Ohori, F., Marahleh, A., Fan, Z., Lin, A., Narita, K., Murakami, K., & Kanetaka, H. (2025). Docosahexaenoic Acid Inhibits Osteoclastogenesis via FFAR4-Mediated Regulation of Inflammatory Cytokines. Molecules, 30(15), 3180. https://doi.org/10.3390/molecules30153180






