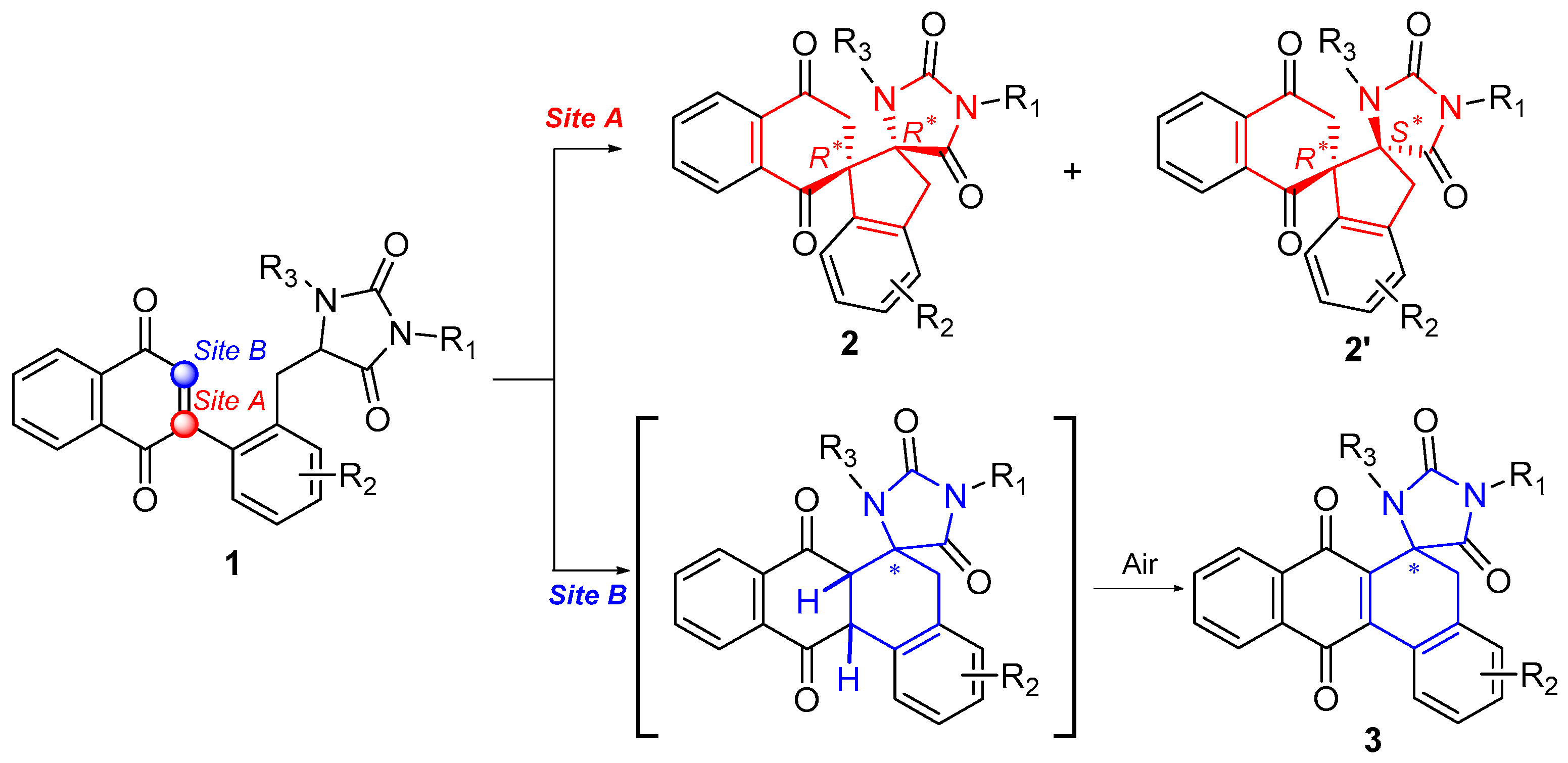
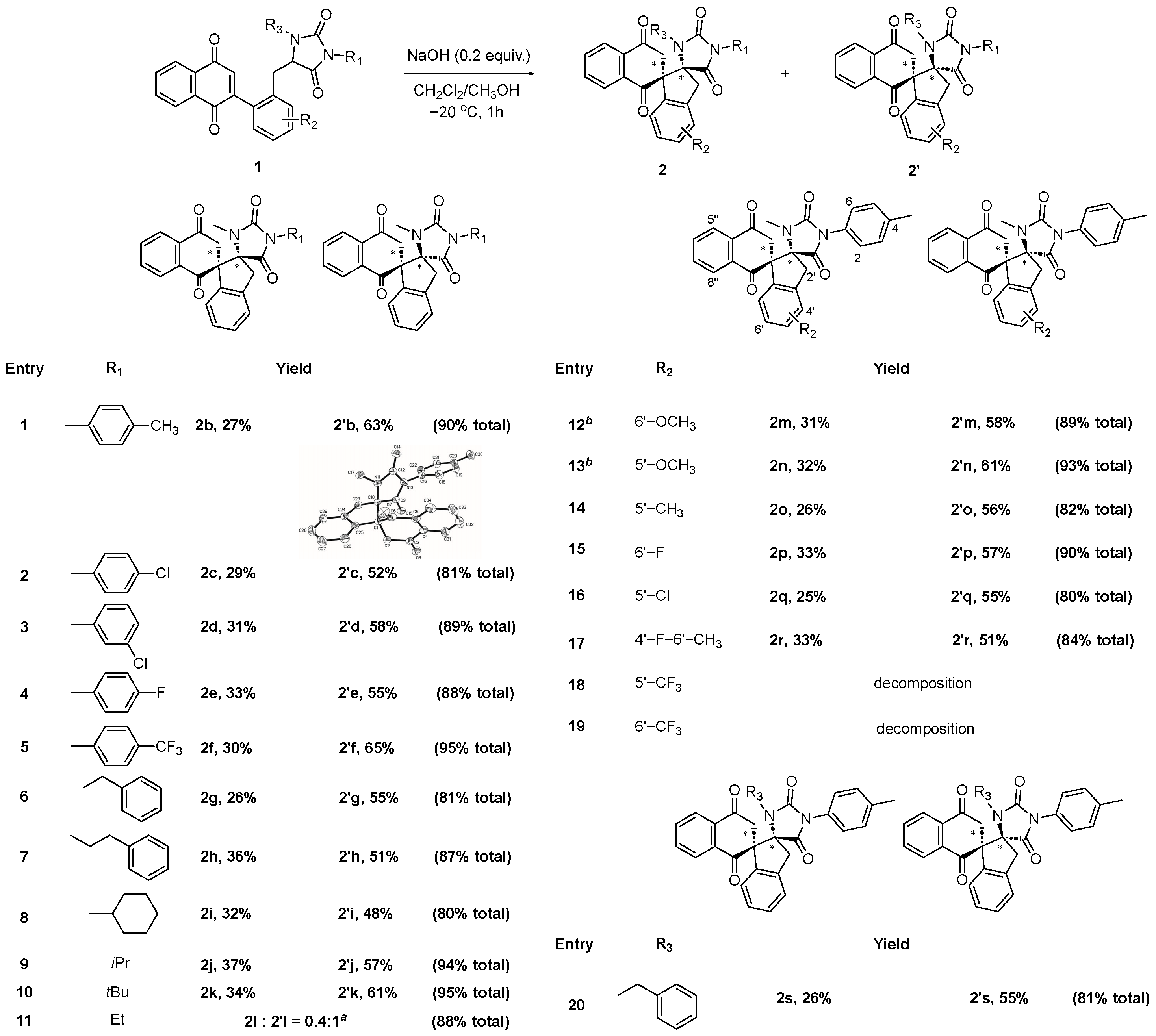
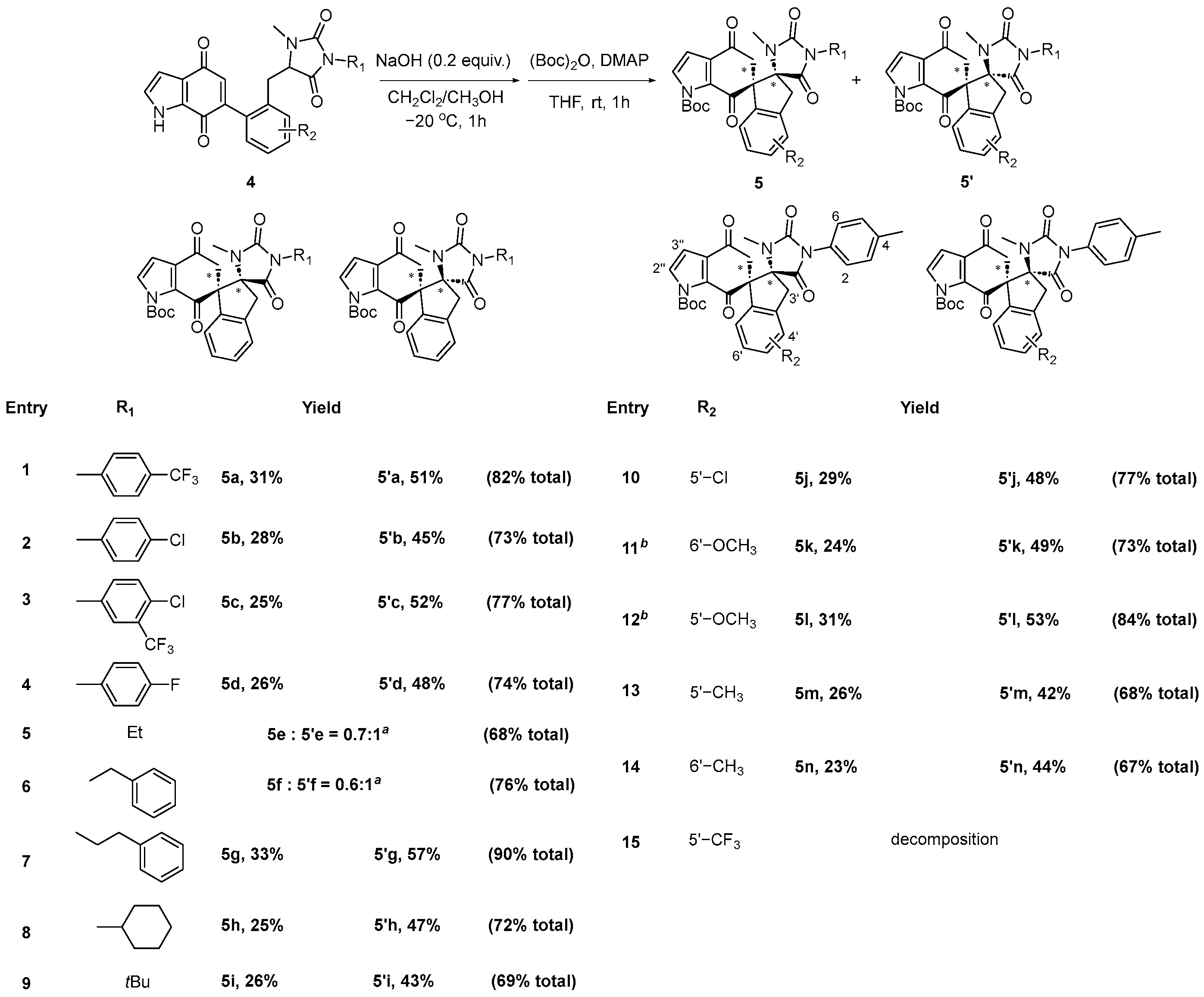
3.2. General Procedure for the Synthesis of Dispirocyclic Compounds
The general procedure for the preparation of (±)-(1′R*,4R*)-3-methyl-1-(m-tolyl)-1″H,3′H-dispiro [imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2a and (±)-(1′S*, 4R*)-3-methyl-1-(m-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′a is as follows:
To a solution of 5-(2-(1,4-dioxo-1,4-dihydronaphthalen-2-yl)benzyl)-1-methyl-3-(m-tolyl)imidazolidine-2,4-dione 1a (450 mg, 1.0 mmol) in CH2Cl2 (5 mL) and CH3OH (1 mL), NaOH (8 mg, 0.2 mmol) was added at −20 °C under argon. Then, the reaction was stirred at this temperature for 1 h. The mixture was quenched with 1N HCl solution (10 mL), extracted with CH2Cl2 (20 mL × 3), washed with brine (20 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography (silica gel, PET/EtOAc = 5:1) to give compound 2a (white solid, 158 mg, 35%) and 2′a (white solid, 261 mg, 58%).
2a:
m.p. = 182–184 °C; 1H NMR (500 MHz, CDCl3) δ 8.08–8.03 (m, 1H), 7.98 (dd, J = 7.6, 1.2 Hz, 1H), 7.73 (td, J = 7.6, 1.2 Hz, 1H), 7.66 (td, J = 7.6, 1.2 Hz, 1H), 7.41 (d, J = 6.4 Hz, 3H), 7.17 (q, J = 6.8, 5.6 Hz, 2H), 7.06 (d, J = 7.6 Hz, 1H), 6.79–6.73 (m, 1H), 6.67 (s, 1H), 3.85 (d, J = 16.8 Hz, 1H), 3.76 (d, J = 15.6 Hz, 1H), 3.18 (d, J = 16.8 Hz, 1H), 3.15 (d, J = 15.6 Hz, 1H), 2.73 (s, 3H), 2.27 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.7, 193.2, 170.6, 154.6, 140.2, 139.9, 138.8, 135.7, 135.3, 134.2, 134.0, 131.1, 129.7, 129.1, 128.8, 128.5 (2C), 126.1, 126.1, 124.5, 123.2, 122.6, 76.7, 68.6, 44.3, 39.9, 28.1, 21.4; HRMS (ESI) calcd for C28H23N2O4 [M + H]+ 451.1652, found 451.1655.
2′a:
m.p. = 181–183 °C; 1H NMR (500 MHz, CDCl3) δ 8.05 (dd, J = 7.5, 1.5 Hz, 1H), 8.00 (dd, J = 7.5, 1.5 Hz, 1H), 7.81 (td, J = 7.5, 1.5 Hz, 1H), 7.73 (td, J = 7.5, 1.5 Hz, 1H), 7.47–7.40 (m, 2H), 7.43–7.35 (m, 2H), 7.15 (t, J = 8.0 Hz, 1H), 7.06 (d, J = 8.0 Hz, 1H), 6.49 (d, J = 8.0 Hz, 1H), 6.43 (s, 1H), 3.91 (d, J = 16.5 Hz, 1H), 3.71 (d, J = 18.5 Hz, 1H), 3.23 (d, J = 16.5 Hz, 1H), 3.01 (d, J = 18.5 Hz, 1H), 2.54 (s, 3H), 2.27 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 195.0, 190.9, 171.3, 154.2, 140.7, 138.7, 137.8, 136.3, 135.3, 135.1, 134.7, 130.7, 129.4, 129.1, 128.6, 128.5, 127.3, 127.2, 126.2, 125.8, 124.6, 122.7, 73.4, 67.6, 46.3, 39.9, 27.2, 21.4; HRMS (ESI) calcd for C28H23N2O4 [M + H]+ 451.1652, found 451.1649.
(±)-(1′R*,4R*)-3-methyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2b and (±)-(1′S*,4R*)-3-methyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′b.
Compounds 2b and 2′b were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2b (white solid, 122 mg, 27%) and 2′b (white solid, 284 mg, 63%).
2b:
m.p. = 185–187 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (d, J = 7.6 Hz, 1H), 7.95 (d, J = 7.6 Hz, 1H), 7.71 (t, J = 7.6 Hz, 1H), 7.64 (t, J = 7.6 Hz, 1H), 7.46–7.37 (m, 3H), 7.15 (d, J = 7.2 Hz, 1H), 7.09 (d, J = 8.0 Hz, 2H), 6.83 (d, J = 8.0 Hz, 2H), 3.86 (d, J = 16.4 Hz, 1H), 3.75 (d, J = 15.6 Hz, 1H), 3.17 (d, J = 16.4 Hz, 1H), 3.14 (d, J = 15.6 Hz, 1H), 2.72 (s, 3H), 2.30 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 193.7, 193.2, 170.5, 154.7, 140.3, 139.9, 138.2, 135.6, 135.3, 134.2, 134.0, 129.6, 129.5 (2C), 128.6, 128.4, 128.4, 126.0, 125.3 (2C), 124.4, 123.2, 76.7, 68.5, 44.2, 39.8, 28.1, 21.2; HRMS (ESI) calcd for C28H23N2O4 [M + H]+ 451.1652, found 451.1651.
2′b:
m.p. = 186–188 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 7.6, 1.6 Hz, 1H), 7.98 (dd, J = 7.6, 1.6 Hz, 1H), 7.78 (td, J = 7.6, 1.6 Hz, 1H), 7.72 (td, J = 7.6, 1.6 Hz, 1H), 7.47–7.34 (m, 4H), 7.06 (d, J = 8.0 Hz, 2H), 6.57 (d, J = 8.0 Hz, 2H), 3.89 (d, J = 16.8 Hz, 1H), 3.70 (d, J = 18.8 Hz, 1H), 3.22 (d, J = 16.8 Hz, 1H), 3.01 (d, J = 18.8 Hz, 1H), 2.53 (s, 3H), 2.30 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 195.1, 190.9, 171.4, 154.3, 140.7, 138.2, 137.9, 136.3, 135.3, 135.1, 134.7, 129.4, 129.3 (2C), 128.5, 128.3, 127.2, 127.1, 125.8, 125.3 (2C), 124.5, 73.4, 67.5, 46.3, 39.9, 27.2, 21.2; HRMS (ESI) calcd for C28H23N2O4 [M + H]+ 451.1652, found 451.1655.
(±)-(1′R*,4R*)-1-(4-chlorophenyl)-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2c and (±)-(1′S*,4R*)-1-(4-chlorophenyl)-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′c.
Compounds 2c and 2′c were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2c (white solid, 137 mg, 29%) and 2′c (white solid, 245 mg, 52%).
2c:
m.p. = 186–188 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (d, J = 7.6 Hz, 1H), 7.91 (d, J = 7.6 Hz, 1H), 7.72 (t, J = 7.6 Hz, 1H), 7.63 (t, J = 7.6 Hz, 1H), 7.48–7.36 (m, 3H), 7.30–7.24 (m, 2H), 7.14 (d, J = 7.2 Hz, 1H), 6.95 (d, J = 8.4 Hz, 2H), 3.85 (d, J = 16.4 Hz, 1H), 3.77 (d, J = 15.6 Hz, 1H), 3.18 (d, J = 16.4 Hz, 1H), 3.13 (d, J = 15.6 Hz, 1H), 2.73 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.8, 193.1, 170.3, 154.1, 140.0, 139.7, 135.5, 135.4, 134.1, 134.1, 133.9, 129.8, 129.7, 129.1 (2C), 128.6, 128.4, 126.5 (2C), 125.9, 124.5, 123.2, 76.7, 68.7, 44.3, 39.6, 28.1; HRMS (ESI) calcd for C27H20ClN2O4 [M + H]+ 471.1106, found 471.1104.
2′c:
m.p. = 184–186 °C; 1H NMR (500 MHz, CDCl3) δ 8.03 (dd, J = 7.6, 1.2 Hz, 1H), 7.98 (dd, J = 7.6, 1.2 Hz, 1H), 7.78 (td, J = 7.6, 1.2 Hz, 1H), 7.71 (td, J = 7.6, 1.2 Hz, 1H), 7.50–7.33 (m, 4H), 7.26–7.20 (m, 2H), 6.87–6.61 (m, 2H), 3.90 (d, J = 16.8 Hz, 1H), 3.69 (d, J = 18.8 Hz, 1H), 3.23 (d, J = 16.8 Hz, 1H), 3.03 (d, J = 18.8 Hz, 1H), 2.55 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 194.9, 190.9, 171.1, 153.7, 140.5, 137.7, 136.3, 135.3, 135.1, 134.8, 133.9, 129.5, 128.9 (2C), 128.6, 127.2, 127.1, 126.5 (2C), 125.8, 124.6, 73.5, 67.7, 46.3, 39.8, 27.2; HRMS (ESI) calcd for C27H20ClN2O4 [M + H]+ 471.1106, found 471.1106.
(±)-(1′R*,4R*)-1-(3-chlorophenyl)-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2d and (±)-(1′S*,4R*)-1-(3-chlorophenyl)-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′d.
Compounds 2d and 2′d were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2d (white solid, 146 mg, 31%) and 2′d (white solid, 273 mg, 58%).
2d:
m.p. = 180–182 °C; 1H NMR (500 MHz, CDCl3) δ 8.06 (dd, J = 7.5, 1.5 Hz, 1H), 7.95 (dd, J = 7.5, 1.5 Hz, 1H), 7.75 (td, J = 7.5, 1.5 Hz, 1H), 7.68 (td, J = 7.5, 1.5 Hz, 1H), 7.47–7.39 (m, 3H), 7.24–7.21 (m, 2H), 7.17–7.12 (m, 1H), 6.96 (ddd, J = 6.0, 3.5, 2.0 Hz, 1H), 6.85–6.81 (m, 1H), 3.84 (d, J = 16.5 Hz, 1H), 3.77 (d, J = 15.5 Hz, 1H), 3.18 (d, J = 16.5 Hz, 1H), 3.13 (d, J = 15.5 Hz, 1H), 2.74 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.6, 193.1, 170.3, 153.9, 140.0, 139.7, 135.6, 135.5, 134.4, 134.3, 134.1, 132.3, 129.9, 129.7, 128.6, 128.5, 128.3, 126.1, 125.5, 124.5, 123.5, 123.3, 76.6, 68.9, 44.4, 39.7, 28.2; HRMS (ESI) calcd for C27H20ClN2O4 [M + H]+ 471.1106, found 471.1110.
2′d:
m.p. = 181–183 °C; 1H NMR (400 MHz, CDCl3) δ 8.08–8.02 (m, 1H), 8.01–7.96 (m, 1H), 7.82 (td, J = 7.6, 1.2 Hz, 1H), 7.74 (td, J = 7.6, 1.2 Hz, 1H), 7.47–7.37 (m, 4H), 7.25–7.17 (m, 2H), 6.77 (dt, J = 6.8, 2.0 Hz, 1H), 6.65–6.60 (m, 1H), 3.92 (d, J = 16.8 Hz, 1H), 3.69 (d, J = 18.8 Hz, 1H), 3.23 (d, J = 16.8 Hz, 1H), 3.02 (d, J = 18.8 Hz, 1H), 2.55 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 194.9, 190.8, 171.0, 153.5, 140.5, 137.7, 136.3, 135.3, 135.2, 134.9, 134.3, 131.9, 129.7, 129.5, 128.6, 128.4, 127.2 (2C), 125.8, 125.6, 124.6, 123.6, 73.5, 67.7, 46.3, 39.8, 27.2; HRMS (ESI) calcd for C27H20ClN2O4 [M + H]+ 471.1106, found 471.1102.
(±)-(1′R*,4R*)-1-(4-fluorophenyl)-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2e and (±)-(1′S*,4R*)-1-(4-fluorophenyl)-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′e.
Compounds 2e and 2′e were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2e (white solid, 150 mg, 33%) and 2′e (white solid, 250 mg, 55%).
2e:
m.p. = 186–188 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 7.6, 1.2 Hz, 1H), 7.92 (dd, J = 7.6, 1.2 Hz, 1H), 7.72 (td, J = 7.6, 1.2 Hz, 1H), 7.63 (td, J = 7.6, 1.2 Hz, 1H), 7.48–7.37 (m, 3H), 7.18–7.11 (m, 1H), 7.01–6.96 (m, 4H), 3.86 (d, J = 16.4 Hz, 1H), 3.77 (d, J = 15.6 Hz, 1H), 3.18 (d, J = 16.4 Hz, 1H), 3.14 (d, J = 15.6 Hz, 1H), 2.73 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.8, 193.1, 170.4, 162.9, 161.0, 154.3, 140.1, 139.8, 135.5, 135.4, 134.2, 134.1, 129.7, 128.6, 128.4, 127.3, 127.2, 127.2, 127.1, 125.9, 124.5, 123.2, 116.0, 115.8, 76.7, 68.6, 44.3, 39.7, 28.1; 19F{H} NMR (376 MHz, CDCl3) δ −112.8; HRMS (ESI) calcd for C27H20FN2O4 [M + H]+ 455.1402, found 455.1401.
2′e:
m.p. = 186–188 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 7.6, 1.2 Hz, 1H), 7.99 (dd, J = 7.6, 1.2 Hz, 1H), 7.79 (td, J = 7.6, 1.2 Hz, 1H), 7.72 (td, J = 7.6, 1.2 Hz, 1H), 7.47–7.35 (m, 4H), 6.99–6.91 (m, 2H), 6.77–6.70 (m, 2H), 3.90 (d, J = 16.8 Hz, 1H), 3.70 (d, J = 18.4 Hz, 1H), 3.23 (d, J = 16.8 Hz, 1H), 3.03 (d, J = 18.4 Hz, 1H), 2.55 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 194.9, 190.9, 171.3, 162.9, 160.9, 153.9, 140.5, 137.8, 136.3, 135.3, 135.1, 134.8, 129.5, 128.6, 127.3, 127.2, 127.2, 127.2, 126.9, 126.8, 125.8, 124.6, 115.9, 115.7, 73.5, 67.6, 46.3, 39.8, 27.2; 19F{H} NMR (376 MHz, CDCl3) δ −112.6; HRMS (ESI) calcd for C27H20FN2O4 [M + H]+ 455.1402, found 455.1400.
(±)-(1′R*,4R*)-3-methyl-1-(4-(trifluoromethyl)phenyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2f and (±)-(1′S*,4R*)-3-methyl-1-(4-(trifluoromethyl)phenyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′f.
Compounds 2f and 2′f were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2f (white solid, 152 mg, 30%) and 2′f (white solid, 328 mg, 65%).
2f:
m.p. = 176–178 °C; 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 7.6 Hz, 1H), 7.89 (d, J = 7.6 Hz, 1H), 7.73 (t, J = 7.6 Hz, 1H), 7.63 (t, J = 7.6 Hz, 1H), 7.57 (d, J = 8.4 Hz, 2H), 7.47–7.39 (m, 3H), 7.16 (t, J = 7.6 Hz, 3H), 3.87 (d, J = 16.4 Hz, 1H), 3.78 (d, J = 15.6 Hz, 1H), 3.20 (d, J = 16.4 Hz, 1H), 3.14 (d, J = 15.6 Hz, 1H), 2.75 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.7, 193.0, 170.3, 153.8, 139.9, 139.6, 135.5, 135.5, 134.4, 134.2 (2C), 129.9 (q, J = 35.0 Hz, 1C), 129.8, 128.6, 128.5, 126.0 (q, J = 4.0 Hz, 2C), 126.0, 125.3 (2C), 124.5, 123.8 (q, J = 272.5 Hz, 1C),123.2, 76.7, 68.8, 44.4, 39.6, 28.2; 19F{H} NMR (376 MHz, CDCl3) δ −62.6; HRMS (ESI) calcd for C28H20F3N2O4 [M + H]+ 505.1370, found 505.1366.
2′f:
m.p. = 179–181 °C; 1H NMR (400 MHz, CDCl3) δ 8.05–7.96 (m, 2H), 7.78 (td, J = 7.6, 1.2 Hz, 1H), 7.69 (td, J = 7.6, 1.2 Hz, 1H), 7.54 (d, J = 8.0 Hz, 2H), 7.48–7.35 (m, 4H), 7.00 (d, J = 8.0 Hz, 2H), 3.92 (d, J = 16.8 Hz, 1H), 3.70 (d, J = 18.4 Hz, 1H), 3.25 (d, J = 16.8 Hz, 1H), 3.05 (d, J = 18.4 Hz, 1H), 2.56 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 194.8, 190.9, 171.1, 153.4, 140.4, 137.6, 136.2, 135.2, 135.2, 134.8, 134.1, 129.9 (q, J = 32.5 Hz, 1C), 129.5, 128.7, 127.2, 127.1, 125.9 (q, J = 4.0 Hz, 2C), 125.8, 125.2 (2C), 124.6, 123.7 (q, J = 272.5 Hz, 1C) 73.5, 67.7, 46.3, 39.8, 27.3; 19F{H} NMR (376 MHz, CDCl3) δ −62.6; HRMS (ESI) calcd for C28H20F3N2O4 [M + H]+ 505.1370, found 505.1375.
(±)-(1′R*,4R*)-1-benzyl-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2g and (±)-(1′S*,4R*)-1-benzyl-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′g.
Compound 2g and 2′g was prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2g (white solid, 117 mg, 26%) and 2′g (white solid, 248 mg, 55%).
2g:
m.p. = 185–187 °C; 1H NMR (400 MHz, CDCl3) δ 8.02 (dd, J = 7.6, 1.2 Hz, 1H), 7.69 (td, J = 7.6, 1.2 Hz, 1H), 7.60 (dd, J = 7.6, 1.2 Hz, 1H), 7.54 (td, J = 7.6, 1.2 Hz, 1H), 7.44–7.32 (m, 3H), 7.29–7.21 (m, 3H), 7.16–7.08 (m, 3H), 4.26 (d, J = 14.4 Hz, 1H), 4.21 (d, J = 14.4 Hz, 1H), 3.74 (d, J = 16.4 Hz, 1H), 3.61 (d, J = 16.0 Hz, 1H), 3.03 (d, J = 16.4 Hz, 1H), 3.03 (d, J = 16.0 Hz, 1H), 2.63 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.2, 193.2, 171.3, 155.6, 140.4, 139.9, 135.3, 135.1, 134.9, 134.0, 133.9, 129.5, 129.1 (2C), 128.7 (2C), 128.4, 128.1, 127.9, 125.9, 124.4, 123.1, 77.4, 77.2, 76.9, 76.9, 67.8, 43.9, 42.8, 39.9, 28.0; HRMS (ESI) calcd for C28H23N2O4 [M + H]+ 451.1652, found 451.1649.
2′g:
m.p. = 186–188 °C; 1H NMR (400 MHz, CDCl3) δ 7.96 (dd, J = 7.6, 1.2 Hz, 1H), 7.84 (dd, J = 7.6, 1.2 Hz, 1H), 7.66 (td, J = 7.6, 1.2 Hz, 1H), 7.57 (td, J = 7.6, 1.2 Hz, 1H), 7.42–7.34 (m, 3H), 7.33–7.28 (m, 1H), 7.25–7.20 (m, 3H), 7.18–7.13 (m, 2H), 4.04 (d, J = 14.0 Hz, 1H), 3.93 (d, J = 14.0 Hz, 1H), 3.75 (d, J = 16.8 Hz, 1H), 3.64 (d, J = 18.4 Hz, 1H), 3.05 (d, J = 16.8 Hz, 1H), 2.95 (d, J = 18.4 Hz, 1H), 2.44 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 195.0, 191.1, 172.2, 155.0, 140.6, 137.9, 136.0, 135.3, 135.1, 134.8, 134.6, 129.3, 129.1 (2C), 128.7 (2C), 128.4, 128.0, 126.7, 126.6, 125.8, 124.5, 77.5, 77.2, 76.8, 73.7, 67.2, 46.1, 42.6, 39.5, 26.9; HRMS (ESI) calcd for C28H23N2O4 [M + H]+ 451.1652, found 451.1653.
(±)-(1′R*,4R*)-3-methyl-1-phenethyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2h and (±)-(1′S*,4R*)-3-methyl-1-phenethyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′h.
Compounds 2h and 2′h were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2h (white solid, 167 mg, 36%) and 2′h (white solid, 237 mg, 51%).
2h:
m.p. = 179–181 °C; 1H NMR (400 MHz, CDCl3) δ 8.21–8.03 (m, 1H), 8.00–7.93 (m, 1H), 7.79 (td, J = 7.6, 1.2 Hz, 1H), 7.70 (td, J = 7.6, 1.2 Hz, 1H), 7.43–7.33 (m, 3H), 7.26–7.15 (m, 3H), 7.14–7.03 (m, 3H), 3.72 (d, J = 16.8 Hz, 1H), 3.64 (d, J = 16.0 Hz, 1H), 3.41 (ddd, J = 13.2, 10.8, 5.6 Hz, 1H), 3.31 (ddd, J = 13.2, 10.8, 5.6 Hz, 1H), 3.01 (d, J = 16.0 Hz, 1H), 2.98 (d, J = 16.8 Hz, 1H), 2.68 (ddd, J = 13.2, 10.8, 5.6 Hz, 1H), 2.62 (s, 3H), 2.40 (ddd, J = 13.2, 10.8, 5.6 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 193.7, 193.1, 171.3, 155.4, 140.4, 140.0, 137.7, 135.6, 135.3, 134.4, 134.1, 129.6, 128.9 (2C), 128.6 (2C), 128.4 (2C), 126.7, 125.9, 124.5, 123.1, 76.8, 67.8, 44.0, 40.2, 39.7, 33.2, 27.8; HRMS (ESI) calcd for C29H25N2O4 [M + H]+ 465.1809, found 465.1806.
2′h:
m.p. = 181–183 °C; 1H NMR (400 MHz, CDCl3) δ 8.17–8.08 (m, 1H), 7.93–7.85 (m, 1H), 7.78–7.69 (m, 2H), 7.46–7.30 (m, 4H), 7.29–7.17 (m, 3H), 7.11–7.04 (m, 2H), 3.72 (d, J = 16.8 Hz, 1H), 3.64 (d, J = 18.4 Hz, 1H), 3.24–3.05 (m, 2H), 2.99 (d, J = 16.8 Hz, 1H), 2.96 (d, J = 18.4 Hz, 1H), 2.44 (s, 3H), 2.39–2.20 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 195.0, 191.0, 172.2, 154.9, 140.7, 137.9, 137.5, 136.3, 135.4, 135.0, 134.6, 129.3, 128.9 (2C), 128.6 (2C), 128.5, 127.1, 126.8 (2C), 125.7, 124.5, 73.5, 67.2, 46.2, 39.9, 39.4, 33.6, 26.8; HRMS (ESI) calcd for C29H25N2O4 [M + H]+ 465.1809, found 465.1807.
(±)-(1′R*,4R*)-1-cyclohexyl-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2i and (±)-(1′S*,4R*)-1-cyclohexyl-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′i.
Compounds 2i and 2′i were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2i (white solid, 142 mg, 32%) and 2i (white solid, 213 mg, 48%).
2i:
m.p. = 171–173 °C; 1H NMR (400 MHz, CDCl3) δ 8.09 (dd, J = 7.6, 1.2 Hz, 1H), 7.95 (dd, J = 7.6, 1.2 Hz, 1H), 7.78 (td, J = 7.6, 1.6 Hz, 1H), 7.70 (td, J = 7.6, 1.6 Hz, 1H), 7.43–7.33 (m, 3H), 7.11 (dd, J = 6.4, 2.8 Hz, 1H), 3.73 (d, J = 16.4 Hz, 1H), 3.67 (d, J = 15.6 Hz, 1H), 3.56 (tt, J = 12.4, 4.0 Hz, 1H), 3.07 (d, J = 15.6 Hz, 1H), 3.04 (d, J = 16.4 Hz, 1H), 2.59 (s, 3H), 1.85 (qd, J = 12.4, 3.6 Hz, 1H), 1.74–1.66 (m, 1H), 1.65–1.58 (m, 1H), 1.56–1.48 (m, 1H), 1.44–1.29 (m, 2H), 1.30–1.23 (m, 1H), 1.22–0.94 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 193.8, 193.3, 171.2, 155.5, 140.6, 140.1, 135.8, 135.2, 134.4, 133.8, 129.5, 128.4, 128.3, 126.1, 124.4, 123.0, 76.4, 67.5, 52.1, 43.9, 40.1, 28.6, 28.3, 27.9, 25.7, 25.6, 24.9; HRMS (ESI) calcd for C27H27N2O4 [M + H]+ 443.1965, found 443.1968.
2′i:
m.p. = 174–176 °C; 1H NMR (500 MHz, CDCl3) δ 8.13 (dd, J = 7.0, 2.0 Hz, 1H), 7.92 (dd, J = 7.0, 2.0 Hz, 1H), 7.82–7.65 (m, 2H), 7.48–7.31 (m, 4H), 3.77 (d, J = 16.5 Hz, 1H), 3.68 (d, J = 18.5 Hz, 1H), 3.53–3.45 (m, 1H), 3.06 (d, J = 16.5 Hz, 1H), 2.96 (d, J = 18.5 Hz, 1H), 2.44 (s, 3H), 1.86–1.41 (m, 5H), 1.15–1.01 (m, 3H), 0.86 (t, J = 13.3 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 195.1, 191.2, 172.2, 155.3, 141.2, 138.0, 136.3, 135.4, 135.0, 134.5, 129.2, 128.4, 127.3, 127.1, 125.6, 124.5, 72.9, 67.0, 51.8, 46.4, 40.0, 28.9, 28.5, 27.0, 25.7, 25.6, 24.8; HRMS (ESI) calcd for C27H27N2O4 [M + H]+ 443.1965, found 443.1961.
(±)-(1′R*,4R*)-1-isopropyl-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2j and (±)-(1′S*,4R*)-1-isopropyl-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′j.
Compounds 2j and 2′j were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2j (white solid, 149 mg, 37%) and 2′j (white solid, 230 mg, 57%).
2j:
m.p. = 178–180 °C; 1H NMR (400 MHz, CDCl3) δ 8.09 (dd, J = 7.6, 1.2 Hz, 1H), 7.96 (dd, J = 7.6, 1.6 Hz, 1H), 7.77 (td, J = 7.6, 1.6 Hz, 1H), 7.70 (td, J = 7.6, 1.2 Hz, 1H), 7.43–7.33 (m, 3H), 7.15–7.08 (m, 1H), 3.96 (p, J = 6.8 Hz, 1H), 3.73 (d, J = 16.4 Hz, 1H), 3.68 (d, J = 16.0 Hz, 1H), 3.05 (d, J = 16.0 Hz, 1H), 3.01 (d, J = 16.4 Hz, 1H), 2.59 (s, 3H), 1.16 (d, J = 6.8 Hz, 3H), 0.94 (d, J = 6.8 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 193.8, 193.3, 171.1, 155.4, 140.5, 140.1, 135.8, 135.2, 134.4, 133.9, 129.5, 128.4, 128.3, 126.0, 124.4, 123.0, 76.4, 67.6, 44.4, 43.9, 40.0, 27.8, 19.0, 18.8; HRMS (ESI) calcd for C24H23N2O4 [M + H]+ 403.1652, found 403.1648.
2′j:
m.p. = 178–180 °C; 1H NMR (400 MHz, CDCl3) δ 8.17–8.08 (m, 1H), 7.97–7.89 (m, 1H), 7.82–7.68 (m, 2H), 7.45–7.30 (m, 4H), 3.98–3.84 (m, 1H), 3.77 (d, J = 16.4 Hz, 1H), 3.68 (d, J = 18.4 Hz, 1H), 3.07 (d, J = 16.4 Hz, 1H), 2.97 (d, J = 18.4 Hz, 1H), 2.44 (s, 3H), 0.90 (d, J = 5.2 Hz, 3H), 0.88 (d, J = 5.2 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 195.1, 191.1, 172.1, 155.1, 141.2, 137.9, 136.2, 135.4, 135.0, 134.7, 129.2, 128.4, 127.3, 127.1, 125.6, 124.5, 72.9, 67.0, 46.4, 44.0, 40.0, 26.9, 19.3, 19.0; HRMS (ESI) calcd for C24H23N2O4 [M + H]+ 403.1652, found 403.1653.
(±)-(1′R*,4R*)-1-(tert-butyl)-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2k and (±)-(1′S*,4R*)-1-(tert-butyl)-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′k.
Compounds 2k and 2′k were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2k (white solid, 142 mg, 34%) and 2′k (white solid, 254 mg, 61%).
2k:
m.p. = 177–179 °C; 1H NMR (500 MHz, CDCl3) δ 8.09 (dd, J = 7.5, 1.5 Hz, 1H), 8.02 (dd, J = 7.5, 1.5 Hz, 1H), 7.78 (td, J = 7.5, 1.5 Hz, 1H), 7.73 (td, J = 7.5, 1.5 Hz, 1H), 7.40–7.35 (m, 3H), 7.11 (dd, J = 7.0, 2.0 Hz, 1H), 3.72 (d, J = 16.5 Hz, 1H), 3.67 (d, J = 16.0 Hz, 1H), 3.03 (d, J = 16.0 Hz, 1H), 2.99 (d, J = 16.5 Hz, 1H), 2.58 (s, 3H), 1.19 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 193.7, 193.5, 171.8, 156.2, 140.5, 140.3, 135.9, 135.1, 134.5, 133.9, 129.5, 128.5, 128.3, 126.1, 124.3, 123.1, 77.5, 77.2, 76.8, 76.4, 67.9, 58.4, 44.0, 40.1, 27.9 (3C), 27.6; HRMS (ESI) calcd for C25H25N2O4 [M + H]+ 417.1809, found 417.1807.
2′k:
m.p. = 176–177 °C; 1H NMR (500 MHz, CDCl3) δ 8.20 (dd, J = 7.5, 1.5 Hz, 1H), 8.00 (dd, J = 7.5, 1.5 Hz, 1H), 7.85 (td, J = 7.5, 1.5 Hz, 1H), 7.80 (td, J = 7.5, 1.5 Hz, 1H), 7.47–7.34 (m, 4H), 3.79 (d, J = 16.5 Hz, 1H), 3.69 (d, J = 18.5 Hz, 1H), 3.09 (d, J = 16.5 Hz, 1H), 3.01 (d, J = 18.5 Hz, 1H), 2.45 (s, 3H), 1.18 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 195.2, 191.3, 172.8, 155.9, 141.2, 138.2, 136.4, 135.5, 135.0, 134.6, 129.2, 128.3, 127.4, 127.1, 125.6, 124.4, 77.5, 77.2, 76.8, 72.8, 67.2, 58.2, 46.5, 40.1, 28.3 (3C), 26.8; HRMS (ESI) calcd for C25H25N2O4 [M + H]+ 417.1809, found 417.1807.
1-ethyl-3-methyl-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2l and 2′l.
Compounds 2l and 2′l were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2l and 2′l (white solid, 342 mg, 88%). m.p. = 181–183 °C; 1H NMR (400 MHz, CDCl3) δ 8.15–8.10 (m, 1H), 8.09–8.06 (m, 0.4H), 7.96–7.93 (m, 0.4H), 7.91–7.87 (m, 1H), 7.80–7.67 (m, 3H), 7.45–7.30 (m, 5H), 7.16–7.05 (m, 0.4H), 3.83–3.64 (m, 1.8H), 3.66 (d, J = 18.8 Hz, 1H), 3.26–3.11 (m, 0.8H), 3.08–2.91 (m, 4.8H), 2.61 (s, 1.2H), 2.44 (s, 3H), 0.81 (t, J = 7.2 Hz, 1.2H), 0.60 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 195.0, 193.6, 193.2, 191.0, 172.2, 171.3, 155.5, 155.1, 140.8, 140.4, 140.0, 137.9, 136.3, 135.7, 135.3, 135.2, 134.9, 134.6, 134.3, 134.0, 129.5, 129.2, 128.4, 128.4, 128.3, 127.1, 126.9, 125.9, 125.7, 124.5, 124.4, 123.1, 76.8, 73.5, 67.8, 67.2, 46.2, 44.0, 39.7, 39.5, 34.2, 33.9, 27.8, 26.8, 12.8, 12.4; HRMS (ESI) calcd for C23H21N2O4 [M + H]+ 389.1496, found 389.1496.
(±)-(1′R*,4R*)-6’-methoxy-3-methyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2m and (±)-(1′S*,4R*)-6’-methoxy-3-methyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′m.
Compounds 2m and 2′m were prepared according to the general procedure; the reaction was completed within 20 min. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2m (white solid, 149 mg, 31%) and 2′m (white solid, 279 mg, 58%).
2m:
m.p. = 185–187 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (d, J = 7.6 Hz, 1H), 7.95 (d, J = 7.6 Hz, 1H), 7.70 (td, J = 7.6, 1.6 Hz, 1H), 7.67–7.59 (m, 1H), 7.30 (d, J = 8.4 Hz, 1H), 7.09 (d, J = 8.0 Hz, 2H), 6.96 (dd, J = 8.4, 2.4 Hz, 1H), 6.66 (d, J = 2.4 Hz, 1H), 3.82 (s, 3H), 3.77 (d, J = 16.4 Hz, 1H), 3.70 (d, J = 15.6 Hz, 1H), 3.14 (d, J = 15.6 Hz, 1H), 3.10 (d, J = 16.4 Hz, 1H), 2.74 (s, 3H), 2.29 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.7, 193.1, 170.4, 160.1, 154.7, 141.6, 138.2, 135.6, 135.3, 134.2, 134.0, 131.7, 129.6 (2C), 128.6, 128.4, 126.0, 125.3 (2C), 125.1, 115.2, 108.9, 77.1, 68.5, 55.6, 44.2, 39.1, 28.1, 21.2; HRMS (ESI) calcd for C29H25N2O5 [M + H]+ 481.1758, found 481.1754.
2′m:
m.p. = 184–186 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 7.6, 1.2 Hz, 1H), 7.99 (dd, J = 7.6, 1.2 Hz, 1H), 7.79 (td, J = 7.6, 1.2 Hz, 1H), 7.72 (td, J = 7.6, 1.2 Hz, 1H), 7.26 (d, J = 8.4 Hz, 1H), 7.09–7.02 (m, 2H), 6.99–6.90 (m, 2H), 6.60–6.53 (m, 2H), 3.86 (s, 3H), 3.82 (d, J = 16.4 Hz, 1H), 3.71 (d, J = 18.8 Hz, 1H), 3.15 (d, J = 16.4 Hz, 1H), 3.02 (d, J = 18.8 Hz, 1H), 2.57 (s, 3H), 2.30 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 195.1, 190.9, 171.3, 160.0, 154.3, 142.1, 138.2, 136.3, 135.3, 135.1, 134.7, 129.5, 129.4 (2C), 128.2, 127.2, 127.1, 125.4 (2C), 125.2, 115.9, 110.6, 73.8, 67.6, 55.7, 46.2, 39.2, 27.2, 21.2; HRMS (ESI) calcd for C29H25N2O5 [M + H]+ 481.1758, found 481.1755.
(±)-(1′R*,4R*)-5’-methoxy-3-methyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2n and (±)-(1′S*,4R*)-5’-methoxy-3-methyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′n.
Compounds 2n and 2′n were prepared according to the general procedure; the reaction was completed within 20 min. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2n (white solid, 154 mg, 32%) and 2′n (white solid, 293 mg, 61%).
2n:
m.p. = 188–190 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (ddd, J = 7.6, 1.6, 0.8 Hz, 1H), 7.95 (ddd, J = 7.6, 1.6, 0.8 Hz, 1H), 7.70 (td, J = 7.6, 1.6 Hz, 1H), 7.63 (td, J = 7.6, 1.6 Hz, 1H), 7.12–7.06 (m, 2H), 7.04 (d, J = 9.2 Hz, 1H), 6.95–6.89 (m, 2H), 6.85–6.79 (m, 2H), 3.86 (s, 3H), 3.82 (d, J = 16.6 Hz, 1H), 3.69 (d, J = 15.6 Hz, 1H), 3.12 (d, J = 15.6 Hz, 1H), 3.11 (d, J = 16.6 Hz, 1H), 2.75 (s, 3H), 2.29 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.8, 193.6, 170.4, 160.9, 154.6, 141.3, 138.1, 135.6, 135.2, 134.2, 133.9, 132.2, 129.5 (2C), 128.5, 128.3, 125.9, 125.3 (2C), 124.0, 114.3, 109.7, 76.8, 67.9, 55.6, 44.4, 39.7, 28.0, 21.2; HRMS (ESI) calcd for C29H25N2O5 [M + H]+ 481.1758, found 481.1761.
2′n:
m.p. = 186–188 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 7.6, 1.2 Hz, 1H), 7.98 (dd, J = 7.6, 1.2 Hz, 1H), 7.78 (td, J = 7.6, 1.2 Hz, 1H), 7.72 (td, J = 7.6, 1.2 Hz, 1H), 7.31 (d, J = 8.4 Hz, 1H), 7.09–7.03 (m, 2H), 6.98 (dd, J = 8.4, 2.4 Hz, 1H), 6.88 (d, J = 2.4 Hz, 1H), 6.62–6.46 (m, 2H), 3.87 (s, 3H), 3.86 (d, J = 16.8 Hz, 1H), 3.69 (d, J = 18.8 Hz, 1H), 3.16 (d, J = 16.8 Hz, 1H), 2.98 (d, J = 18.8 Hz, 1H), 2.57 (s, 3H), 2.30 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 195.4, 191.1, 171.3, 160.7, 154.3, 139.4, 138.2, 136.4, 135.3, 135.0, 134.6, 132.7, 129.4 (2C), 128.2, 127.1, 127.1, 126.5, 125.3 (2C), 114.6, 109.6, 73.6, 66.9, 55.7, 46.5, 39.9, 27.2, 21.2; HRMS (ESI) calcd for C29H25N2O5 [M + H]+ 481.1758, found 481.1757.
(±)-(1′R*,4R*)-3,5’-dimethyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2o and (±)-(1′S*,4R*)-3,5’-dimethyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′o.
Compounds 2o and 2′o were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2o (white solid, 121 mg, 26%) and 2′o (white solid, 261 mg, 56%).
2o:
m.p. = 183–185 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 7.6, 1.2 Hz, 1H), 7.95 (dd, J = 7.6, 1.2 Hz, 1H), 7.70 (td, J = 7.6, 1.2 Hz, 1H), 7.63 (td, J = 7.6, 1.2 Hz, 1H), 7.24–7.17 (m, 2H), 7.10–7.06 (m, 2H), 7.02 (d, J = 7.6 Hz, 1H), 6.86–6.79 (m, 2H), 3.81 (d, J = 16.8 Hz, 1H), 3.72 (d, J = 15.6 Hz, 1H), 3.12 (d, J = 15.6 Hz, 1H), 3.12 (d, J = 16.8 Hz, 1H), 2.73 (s, 3H), 2.42 (s, 3H), 2.29 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.9, 193.4, 170.5, 154.7, 139.9, 139.7, 138.2, 137.4, 135.7, 135.3, 134.2, 133.9, 129.6 (2C), 129.3, 128.6, 128.4, 126.0, 125.3 (2C), 125.1, 122.9, 76.8, 68.3, 44.3, 39.7, 28.1, 21.6, 21.2; HRMS (ESI) calcd for C29H25N2O4 [M + H]+ 465.1809, found 465.1811.
2′o:
m.p. = 184–186 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (d, J = 7.6 Hz, 1H), 7.98 (d, J = 7.6 Hz, 1H), 7.78 (t, J = 7.6 Hz, 1H), 7.72 (t, J = 7.6 Hz, 1H), 7.33–7.22 (m, 2H), 7.18 (s, 1H), 7.06 (d, J = 8.0 Hz, 2H), 6.58 (d, J = 8.0 Hz, 2H), 3.85 (d, J = 16.8 Hz, 1H), 3.69 (d, J = 18.8 Hz, 1H), 3.17 (d, J = 16.8 Hz, 1H), 2.99 (d, J = 18.8 Hz, 1H), 2.55 (s, 3H), 2.42 (s, 3H), 2.30 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 195.3, 191.1, 171.4, 154.3, 139.4, 138.2, 138.0, 137.8, 136.4, 135.3, 135.0, 134.6, 129.4, 129.4 (2C), 128.2, 127.1, 127.1, 125.4, 125.3 (2C), 125.1, 73.5, 67.3, 46.4, 39.8, 27.2, 21.6, 21.2; HRMS (ESI) calcd for C29H25N2O4 [M + H]+ 465.1809, found 465.1805.
(±)-(1′R*,4R*)-6’-fluoro-3-methyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2p and (±)-(1′S*,4R*)-6’-fluoro-3-methyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′p.
Compounds 2p and 2′p were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2p (white solid, 155 mg, 33%) and 2′p (white solid, 267 mg, 57%).
2p:
m.p. = 181–183 °C; 1H NMR (400 MHz, CDCl3) δ 8.06–8.01 (m, 1H), 8.00–7.88 (m, 1H), 7.72 (td, J = 7.6, 1.6 Hz, 1H), 7.65 (td, J = 7.6, 1.6 Hz, 1H), 7.37 (dd, J = 8.4, 5.2 Hz, 1H), 7.17–7.06 (m, 3H), 6.86 (dd, J = 8.4, 2.4 Hz, 1H), 6.84–6.79 (m, 2H), 3.79 (d, J = 16.4 Hz, 1H), 3.67 (d, J = 15.6 Hz, 1H), 3.15 (d, J = 15.6 Hz, 1H), 3.14 (d, J = 16.4 Hz, 1H), 2.75 (s, 3H), 2.30 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.2, 192.8, 170.1, 163.11 (d, J = 246.8 Hz), 154.6, 142.31 (d, J = 7.7 Hz), 138.3, 135.4 (2C), 135.3 (d, J = 2.5 Hz), 134.2, 134.1, 129.6 (2C), 128.5, 128.4, 126.1, 125.70 (d, J = 8.7 Hz), 125.3 (2C), 116.81 (d, J = 22.6 Hz), 110.78 (d, J = 23.4 Hz), 77.0, 68.4, 44.1, 39.1, 28.1, 21.3; 19F{H} NMR (376 MHz, CDCl3) δ −113.2; HRMS (ESI) calcd for C28H22FN2O4 [M + H]+ 469.1558, found 469.1561.
2′p:
m.p. = 180–182 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 7.6, 1.6 Hz, 1H), 7.98 (dd, J = 7.6, 1.6 Hz, 1H), 7.80 (td, J = 7.6, 1.6 Hz, 1H), 7.74 (td, J = 7.6, 1.6 Hz, 1H), 7.37–7.29 (m, 1H), 7.16–7.08 (m, 2H), 7.06 (d, J = 8.0 Hz, 2H), 6.57 (d, J = 8.0 Hz, 2H), 3.84 (d, J = 16.4 Hz, 1H), 3.71 (d, J = 18.4 Hz, 1H), 3.19 (d, J = 16.4 Hz, 1H), 2.99 (d, J = 18.4 Hz, 1H), 2.56 (s, 3H), 2.30 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 194.6, 190.5, 170.9, 162.9 (d, J = 246.7 Hz), 154.2, 142.7 (d, J = 8.2 Hz), 138.3, 136.0, 135.3, 135.2, 134.9, 133.3 (d, J = 2.5 Hz), 129.4 (2C), 128.1, 127.2, 127.2, 125.8 (d, J = 8.8 Hz), 125.3 (2C), 116.7 (d, J = 22.7 Hz), 113.1 (d, J = 23.5 Hz), 73.8, 67.4, 45.9, 39.1, 27.2, 21.2; 19F{H} NMR (376 MHz, CDCl3) δ −112.8; HRMS (ESI) calcd for C28H22FN2O4 [M + H]+ 469.1558, found 469.1558.
(±)-(1′R*,4R*)-5’-chloro-3-methyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2q and (±)-(1′S*,4R*)-5’-chloro-3-methyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′q.
Compounds 2q and 2′q were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2q (white solid, 121 mg, 25%) and 2′q (white solid, 267 mg, 55%).
2q:
m.p. = 185–187 °C; 1H NMR (400 MHz, CDCl3) δ 8.03 (dd, J = 7.6, 1.2 Hz, 1H), 7.95 (dd, J = 7.6, 1.2 Hz, 1H), 7.71 (td, J = 7.6, 1.2 Hz, 1H), 7.65 (td, J = 7.6, 1.2 Hz, 1H), 7.45–7.35 (m, 2H), 7.12–7.05 (m, 3H), 6.85–6.75 (m, 2H), 3.82 (d, J = 16.8 Hz, 1H), 3.69 (d, J = 15.6 Hz, 1H), 3.15 (d, J = 16.8 Hz, 1H), 3.13 (d, J = 15.6 Hz, 1H), 2.75 (s, 3H), 2.30 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.3, 192.9, 170.1, 154.6, 141.7, 138.8, 138.4, 135.5, 135.4 (2C), 134.2, 134.1, 129.6 (2C), 128.8, 128.4 (2C), 126.1, 125.3 (2C), 124.8, 124.4, 76.7, 68.0, 44.1, 39.4, 28.2, 21.3; HRMS (ESI) (ESI) calcd for C28H22ClN2O4 [M + H]+ 485.1263, found 485.1261.
2′q:
m.p. = 184–186 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (d, J = 7.6 Hz, 1H), 7.98 (d, J = 7.6 Hz, 1H), 7.79 (t, J = 7.6 Hz, 1H), 7.73 (t, J = 7.6 Hz, 1H), 7.47–7.31 (m, 3H), 7.06 (d, J = 8.4 Hz, 2H), 6.58 (d, J = 8.4 Hz, 2H), 3.86 (d, J = 16.8 Hz, 1H), 3.69 (d, J = 18.4 Hz, 1H), 3.20 (d, J = 16.8 Hz, 1H), 2.98 (d, J = 18.4 Hz, 1H), 2.55 (s, 3H), 2.30 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 194.7, 190.6, 170.9, 154.2, 139.8, 139.2, 138.4, 136.1, 135.3 (2C), 135.2, 134.9, 129.4 (2C), 128.9, 128.1, 127.3, 127.2, 127.0, 125.3 (2C), 124.8, 73.4, 67.0, 46.1, 39.5, 27.2, 21.2; HRMS (ESI) calcd for C28H22ClN2O4 [M + H]+ 485.1263, found 485.1265.
(±)-(1′R*,4R*)-4’-fluoro-3,6’-dimethyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2r and (±)-(1′S*,4R*)-4’-fluoro-3,6’-dimethyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′r.
Compounds 2r and 2′r were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2r (white solid, 159 mg, 33%) and 2′r (white solid, 246 mg, 51%).
2r:
m.p. = 180–182 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 7.6, 1.2 Hz, 1H), 7.95 (dd, J = 7.6, 1.2 Hz, 1H), 7.71 (td, J = 7.6, 1.2 Hz, 1H), 7.64 (td, J = 7.6, 1.2 Hz, 1H), 7.13–7.05 (m, 2H), 6.95 (d, J = 9.6 Hz, 1H), 6.85–6.78 (m, 2H), 6.75 (s, 1H), 3.72 (d, J = 16.8 Hz, 1H), 3.71 (d, J = 15.6 Hz, 1H), 3.25 (d, J = 16.8 Hz, 1H), 3.13 (d, J = 15.6 Hz, 1H), 2.76 (s, 3H), 2.40 (s, 3H), 2.30 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.3, 192.8, 170.1, 158.4 (d, J = 248.8 Hz), 154.6, 143.3 (d, J = 5.8 Hz), 141.3 (d, J = 6.8 Hz), 138.3, 135.4, 135.4, 134.2 (2C), 129.6 (2C), 128.5, 128.4, 126.0, 125.3 (2C), 123.3 (d, J = 18.8 Hz), 119.6 (d, J = 2.6 Hz), 116.9 (d, J = 19.7 Hz), 76.8, 68.6, 44.2, 35.6, 28.0, 21.7, 21.2; 19F{H} NMR (376 MHz, CDCl3) δ −118.0; HRMS (ESI) calcd for C29H24FN2O4 [M + H]+ 483.1715, found 483.1714.
2′r:
m.p. = 184–186 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 8.0, 1.2 Hz, 1H), 8.02–7.96 (m, 1H), 7.84–7.77 (m, 1H), 7.77–7.70 (m, 1H), 7.07 (d, J = 8.0 Hz, 2H), 7.00 (s, 1H), 6.93 (d, J = 9.6 Hz, 1H), 6.62–6.55 (m, 2H), 3.76 (d, J = 16.8 Hz, 1H), 3.70 (d, J = 18.4 Hz, 1H), 3.30 (d, J = 16.8 Hz, 1H), 3.02 (d, J = 18.4 Hz, 1H), 2.58 (s, 3H), 2.44 (s, 3H), 2.30 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 194.7, 190.7, 171.0, 158.5 (d, J = 248.0 Hz), 154.2, 143.7 (d, J = 5.6 Hz), 141.5 (d, J = 6.4 Hz), 138.4, 136.1, 135.3, 135.1, 134.9, 129.4 (2C), 128.2, 127.2 (2C), 125.4 (2C), 121.9, 121.9 (d, J = 2.8 Hz), 121.4 (d, J = 18.8 Hz), 116.5 (d, J = 19.5 Hz), 73.6, 67.7, 46.1, 35.7, 27.1, 21.7, 21.2; 19F{H} NMR (376 MHz, CDCl3) δ −117.6; HRMS (ESI) (ESI) calcd for C29H24FN2O4 [M + H]+ 483.1715, found 483.1717.
(±)-(1′R*,4R*)-3-benzyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2s and (±)-(1′S*,4R*)-3-benzyl-1-(p-tolyl)-1″H,3′H-dispiro[imidazolidine-4,2′-indene-1′,2″-naphthalene]-1″,2,4″,5(3″H)-tetraone 2′s.
Compounds 2s and 2′s were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 2s (white solid, 137 mg, 26%) and 2′s (white solid, 290 mg, 55%).
2s:
m.p. = 182–184 °C; 1H NMR (400 MHz, CDCl3) δ 8.02 (d, J = 7.6 Hz, 1H), 7.92 (d, J = 7.6 Hz, 1H), 7.69 (t, J = 7.6 Hz, 1H), 7.61 (t, J = 7.6 Hz, 1H), 7.51–7.42 (m, 2H), 7.27–7.18 (m, 4H), 7.15–7.08 (m, 3H), 6.94–6.85 (m, 4H), 4.92 (d, J = 15.2 Hz, 1H), 3.70 (d, J = 16.8 Hz, 1H), 3.69 (d, J = 15.2 Hz, 1H), 3.68 (d, J = 16.0 Hz, 1H), 3.08 (d, J = 16.0 Hz, 1H), 2.86 (d, J = 16.8 Hz, 1H), 2.31 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 193.9, 193.2, 170.3, 155.3, 140.4, 140.3, 138.2, 137.1, 135.5, 135.3, 134.1, 134.0, 129.5 (2C), 129.5, 128.6 (2C), 128.5 (2C), 128.5 (2C), 128.4, 128.2, 126.0, 125.2, 125.2 (2C), 123.0, 78.0, 68.8, 45.7, 43.6, 39.9, 21.3; HRMS (ESI) calcd for C34H27N2O4 [M + H]+ 527.1965, found 527.1967.
2′s:
m.p. = 180–182 °C; 1H NMR (400 MHz, CDCl3) δ 8.06–7.99 (m, 2H), 7.81 (td, J = 7.6, 1.2 Hz, 1H), 7.72 (td, J = 7.6, 1.2 Hz, 1H), 7.47 (dd, J = 4.4, 1.2 Hz, 2H), 7.38 (dt, J = 8.4, 4.0 Hz, 1H), 7.22–7.13 (m, 3H), 7.07 (d, J = 7.6 Hz, 2H), 6.97 (d, J = 7.6 Hz, 1H), 6.80 (d, J = 7.2 Hz, 2H), 6.64 (d, J = 8.4 Hz, 2H), 4.88 (d, J = 16.0 Hz, 1H), 3.66 (d, J = 18.8 Hz, 2H), 3.52 (d, J = 16.0 Hz, 1H), 2.98 (d, J = 18.8 Hz, 1H), 2.76 (d, J = 16.8 Hz, 1H), 2.30 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 195.4, 190.8, 171.2, 154.7, 141.0, 138.2, 137.9, 137.5, 136.4, 135.3, 135.2, 134.9, 129.3 (2C), 129.0, 128.5, 128.5 (2C), 128.3, 128.1 (2C), 127.9, 127.3 (2C), 125.7, 125.4, 125.2 (2C), 74.4, 68.0, 46.6, 44.7, 40.4, 21.3; HRMS (ESI) calcd for C34H27N2O4 [M + H]+ 527.1965, found 527.1961.
The general procedure for the preparation of (±)-tert-butyl (1′R*,4R*)-3-methyl-2,4″,5,7″-tetraoxo-1-(4-(trifluoromethyl)phenyl)-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylaten 5a and (±)-tert-butyl (1′S*,4R*)-3-methyl-2,4″,5,7″0-tetraoxo-1-(4-(trifluoromethyl)phenyl)-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5’a is as follows:
To a solution of 6-(2-((3-methyl-2,5-dioxo-1-(4-(trifluoromethyl)phenyl)imidazolidin-4-yl)methyl)phenyl)-1H-indole-4,7-dione 4a (493 mg, 1.0 mmol) in CH2Cl2 (5 mL) and CH3OH (1 mL), NaOH (8 mg, 0.2 mmol) was added at −20 °C under argon. Then, the reaction was stirred at this temperature for 1 h. The mixture was quenched with 1N HCl solution and extracted with CH2Cl2 (3 × 20 mL), washed with saturated NaCl solution, dried over anhydrous Na2SO4, and concentrated under reduced pressure.
The residue was dissolved in THF (10 mL), and DMAP (49 mg, 0.4 mmol) was added. The mixture was stirred, followed by the dropwise addition of (Boc)2O (0.46 mL, 2 mmol). The reaction was allowed to proceed at room temperature for 3 h. The mixture was quenched with hydrochloric acid, extracted with CH2Cl2 (3 × 20 mL), washed with saturated NaCl solution, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography (petroleum ether/EtOAc = 5:1) to afford compound 5a (white solid, 184 mg, 31%) and compound 5’a (white solid, 303 mg, 51%).
5a:
m.p. = 163–165 °C; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 3.2 Hz, 1H), 7.42–7.35 (m, 3H), 7.24–7.14 (m, 5H), 6.52 (d, J = 3.2 Hz, 1H), 3.79 (d, J = 16.4 Hz, 1H), 3.57 (d, J = 16.4 Hz, 1H), 3.21 (d, J = 16.4 Hz, 1H), 3.00 (d, J = 16.4 Hz, 1H), 2.71 (s, 3H), 1.50 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 190.1, 183.0, 170.6, 154.3, 148.4, 147.1, 139.7, 139.4, 134.3, 132.5, 130.0, 129.9, 129.5, 128.3, 127.2 (2C), 124.5, 123.7, 121.4 (2C), 120.4 (q, J = 248.8 Hz, 1C), 106.7, 86.7, 76.5, 70.4, 46.0, 39.0, 28.2, 27.5 (3C); 19F{H} NMR (376 MHz, CDCl3) δ −57.8; HRMS (ESI) calcd for C31H27F3N3O6 [M + H]+ 594.1846, found 594.1848.
5’a:
m.p. = 161–163 °C; 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 3.2 Hz, 1H), 7.44–7.35 (m, 4H), 7.22–7.17 (m, 2H), 7.11–7.06 (m, 2H), 6.62 (d, J = 3.2 Hz, 1H), 3.90 (d, J = 16.8 Hz, 1H), 3.56 (d, J = 18.4 Hz, 1H), 3.21 (d, J = 16.8 Hz, 1H), 2.90 (d, J = 18.4 Hz, 1H), 2.60 (s, 3H), 1.61 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 188.2, 183.6, 171.3, 153.4, 148.3, 146.7, 140.1, 137.5, 135.1, 133.7, 130.3, 129.7, 129.3, 128.4, 126.9 (2C), 126.1, 124.5, 121.4 (2C), 120.4 (q, J = 256.8 Hz, 1C), 107.8, 87.4, 73.6, 68.6, 48.5, 39.8, 27.8 (3C), 27.6; 19F{H} NMR (376 MHz, CDCl3) δ −57.8; HRMS (ESI) calcd for C31H27F3N3O6 [M + H]+ 594.1846, found 594.1847.
(±)-tert-butyl (1′R*,4R*)-1-(4-chlorophenyl)-3-methyl-2,4″,5,7″-tetraoxo-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5b and (±)-tert-butyl (1′S*,4R*)-1-(4-chlorophenyl)-3-methyl-2,4″,5,7″-tetraoxo-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5’b.
Compounds 5b and 5’b were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 5b (white solid, 157 mg, 28%) and 5’b (white solid, 252 mg, 45%).
5b:
m.p. = 163–165 °C; 1H NMR (400 MHz, CDCl3) δ 7.42 (d, J = 3.2 Hz, 1H), 7.40–7.36 (m, 3H), 7.37–7.29 (m, 2H), 7.17–7.14 (m, 1H), 7.13–7.06 (m, 2H), 6.51 (d, J = 3.2 Hz, 1H), 3.79 (d, J = 16.4 Hz, 1H), 3.57 (d, J = 16.4 Hz, 1H), 3.19 (d, J = 16.4 Hz, 1H), 2.99 (d, J = 16.4 Hz, 1H), 2.69 (s, 3H), 1.51 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 188.5, 181.5, 169.2, 153.3, 146.1, 138.9, 138.5, 133.6, 133.2, 131.7, 129.3, 129.2, 128.8, 128.4 (2C), 127.6, 126.3 (2C), 123.8, 123.1, 106.3, 86.6, 76.7, 70.6, 46.5, 39.7, 29.0, 28.3 (3C); HRMS (ESI) calcd for C30H27ClN3O6 [M + H]+ 560.1583, found 560.1581.
5’b:
m.p. = 165–167 °C; 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 3.2 Hz, 1H), 7.44–7.34 (m, 4H), 7.34–7.29 (m, 2H), 7.02–6.96 (m, 2H), 6.61 (d, J = 3.2 Hz, 1H), 3.89 (d, J = 16.4 Hz, 1H), 3.55 (d, J = 18.4 Hz, 1H), 3.20 (d, J = 16.4 Hz, 1H), 2.89 (d, J = 18.4 Hz, 1H), 2.59 (s, 3H), 1.61 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 188.2, 183.8, 171.3, 153.5, 146.7, 140.2, 137.7, 135.2, 133.8, 130.4, 129.9, 129.6, 129.3 (2C), 129.1, 128.4, 126.6 (2C), 126.1, 124.6, 107.9, 87.5, 73.6, 68.7, 48.5, 39.9, 27.9 (3C), 27.6; HRMS (ESI) calcd for C30H27ClN3O6 [M + H]+ 560.1583, found 560.1582.
(±)-tert-butyl (1′R*,4R*)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-methyl-2,4″,5,7″-tetraoxo-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5c and (±)-tert-butyl (1′S*,4R*)-1-(4-chloro-3-(trifluoromethyl)phenyl)-3-methyl-2,4″,5,7″-tetraoxo-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5’c.
Compounds 5c and 5’c were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 5c (white solid, 157 mg, 25%) and 5’c (white solid, 327 mg, 52%).
5c:
m.p. = 168–180 °C; 1H NMR (400 MHz, CDCl3) δ 7.51 (d, J = 8.8 Hz, 1H), 7.44 (d, J = 3.2 Hz, 1H), 7.42 (d, J = 2.4 Hz, 1H), 7.40–7.35 (m, 4H), 7.17–7.11 (m, 1H), 6.50 (d, J = 3.2 Hz, 1H), 3.78 (d, J = 16.4 Hz, 1H), 3.57 (d, J = 16.4 Hz, 1H), 3.20 (d, J = 16.4 Hz, 1H), 2.98 (d, J = 16.4 Hz, 1H), 2.71 (s, 3H), 1.50 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 190.1, 183.0, 170.6, 153.9, 147.0, 139.6, 139.3, 134.4, 132.5, 132.1, 131.8, 130.3, 130.3, 129.8, 129.7, 129.1 (q, J = 32.0 Hz), 128.4, 124.9 (q, J = 5.3 Hz), 124.6, 123.8, 122.4 (q, J = 273.4 Hz), 106.8, 86.9, 76.6, 70.8, 46.3, 39.0, 28.3, 27.5 (3C); 19F{H} NMR (376 MHz, CDCl3) δ −62.7; HRMS (ESI) calcd for C31H26ClF3N3O6 [M + H]+ 628.1457, found 628.1459.
5’c:
m.p. = 166–168 °C; 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 8.4 Hz, 1H), 7.46 (d, J = 3.2 Hz, 1H), 7.43–7.34 (m, 4H), 7.32–7.26 (m, 2H), 6.54 (d, J = 3.2 Hz, 1H), 3.90 (d, J = 16.8 Hz, 1H), 3.51 (d, J = 18.4 Hz, 1H), 3.21 (d, J = 16.8 Hz, 1H), 2.89 (d, J = 18.4 Hz, 1H), 2.59 (s, 3H), 1.61 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 188.0, 183.6, 171.1, 152.9, 146.5, 139.9, 137.5, 135.1, 133.8, 132.1, 131.7, 130.3, 130.1, 129.6, 129.4, 129.0 (q, J = 31.1 Hz), 128.5, 126.2, 124.6, 124.6 (q, J = 5.3 Hz), 122.35 (q, J = 273.8 Hz), 107.9, 87.6, 73.8, 68.8, 48.5, 39.7, 27.8 (3C), 27.6; 19F{H} NMR (376 MHz, CDCl3) δ −62.7; HRMS (ESI) calcd for C31H26ClF3N3O6 [M + H]+ 628.1457, found 628.1452.
(±)-tert-butyl (1′R*,4R*)-1-(4-fluorophenyl)-3-methyl-2,4″,5,7″-tetraoxo-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5d and (±)-tert-butyl (1′S*,4R*)-1-(4-fluorophenyl)-3-methyl-2,4″,5,7″-tetraoxo-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5’d.
Compounds 5d and 5’d were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 5d (white solid, 141 mg, 26%) and 5’d (white solid, 261 mg, 48%).
5d:
m.p. = 166–168 °C; 1H NMR (400 MHz, CDCl3) δ 7.42 (d, J = 3.2 Hz, 1H), 7.41–7.35 (m, 3H), 7.19–6.99 (m, 5H), 6.52 (d, J = 3.2 Hz, 1H), 3.79 (d, J = 16.4 Hz, 1H), 3.57 (d, J = 16.4 Hz, 1H), 3.19 (d, J = 16.4 Hz, 1H), 3.00 (d, J = 16.4 Hz, 1H), 2.69 (s, 3H), 1.50 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 190.3, 183.1, 170.8, 162.1 (d, J = 248.1 Hz), 154.7, 147.2, 139.8, 139.5, 134.5, 132.5, 130.1, 129.5, 128.3, 127.8 (d, J = 8.8 Hz, 2C), 127.4 (d, J = 3.1 Hz), 124.5, 123.8, 116.0 (d, J = 22.8 Hz, 2C), 106.7, 86.7, 76.7, 70.4, 46.0, 39.1, 28.2, 27.5 (3C); 19F{H} NMR (376 MHz, CDCl3) δ −112.7; HRMS (ESI) calcd for C30H27FN3O6 [M + H]+ 544.1878, found 544.1872.
5’d:
m.p. = 165–167 °C; 1H NMR (400 MHz, CDCl3) δ 7.51 (d, J = 3.2 Hz, 1H), 7.46–7.33 (m, 4H), 7.08–6.94 (m, 4H), 6.62 (d, J = 3.2 Hz, 1H), 3.89 (d, J = 17.2 Hz, 1H), 3.56 (d, J = 18.4 Hz, 1H), 3.20 (d, J = 17.2 Hz, 1H), 2.89 (d, J = 18.4 Hz, 1H), 2.59 (s, 3H), 1.61 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 188.2, 183.8, 171.5, 161.8 (d, J = 248.2 Hz), 153.7, 146.8, 140.2, 137.7, 135.3, 133.8, 130.3, 129.3, 128.4, 127.4 (d, J = 8.6 Hz, 2C), 127.3 (d, J = 3.1 Hz), 126.1, 124.6, 115.95 (d, J = 22.7 Hz, 2C), 107.9, 87.5, 73.6, 68.7, 48.5, 39.8, 27.8 (3C), 27.6; 19F{H} NMR (376 MHz, CDCl3) δ −112.8; HRMS (ESI) calcd for C30H27FN3O6 [M + H]+ 544.1878, found 544.1877.
(±)-tert-butyl 1-ethyl-3-methyl-2,4″,5,7″-tetraoxo-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5e and 5’e.
Compounds 5e and 5’e were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 5e and 5’e (white solid, 325 mg, 68%). m.p. = 161–163 °C; 1H NMR (500 MHz, CDCl3) δ 7.53 (d, J = 3.5 Hz, 1H), 7.49 (d, J = 3.5 Hz, 0.7H), 7.41–7.32 (m, 6.1H), 7.12 (d, J = 6.5 Hz, 0.7H), 6.65 (d, J = 3.5 Hz, 1H), 6.54 (d, J = 3.5 Hz, 0.7H), 3.79 (d, J = 17.0 Hz, 1H), 3.71 (d, J = 16.5 Hz, 0.7H), 3.52 (d, J = 18.5 Hz, 1H), 3.51 (d, J = 16.5 Hz, 0.7H), 3.43–3.21 (m, 3.4H), 3.05 (d, J = 17.0 Hz, 1H), 3.00 (d, J = 16.5 Hz, 0.7H), 2.91 (d, J = 16.5 Hz, 0.7H), 2.83 (d, J = 18.5 Hz, 1H), 2.55 (s, 2.1H), 2.47 (s, 3H), 1.61 (s, 9H), 1.58 (s, 6.3H), 0.97 (t, J = 7.0 Hz, 2.1H), 0.71 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 190.1, 188.4, 183.8, 183.4, 172.2, 171.5, 155.6, 154.9, 147.3, 146.9, 140.6, 139.9, 137.8, 136.7, 135.5, 134.7, 133.7, 132.3, 130.3, 129.4, 129.1, 128.3, 128.2, 127.7, 125.9, 124.5, 124.3, 123.5, 107.8, 106.7, 87.2, 86.4, 76.9, 73.7, 69.5, 68.2, 48.5, 45.3, 39.4, 39.3, 34.1, 33.8, 27.8 (3C), 27.7, 27.6 (3C), 27.3, 13.2, 12.8; HRMS (ESI) calcd for C26H28N3O6 [M + H]+ 478.1973, found 478.1976.
(±)-tert-butyl 1-benzyl-3-methyl-2,4″,5,7″-tetraoxo-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5f and 5’f.
Compounds 5f and 5’f were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 5f and 5’f (white solid, 410 mg, 76%). m.p. = 166–178 °C; 1H NMR (400 MHz, CDCl3) δ 7.39–7.30 (m, 6.4H), 7.30–7.25 (m, 2.2H), 7.25–7.17 (m, 5.8H), 7.12–7.07 (m, 0.6H), 7.03 (d, J = 3.2 Hz, 1H), 6.23 (d, J = 3.2 Hz, 0.6H), 6.20 (d, J = 3.2 Hz, 1H), 4.42–4.39 (m, 3.2H), 3.80 (d, J = 16.8 Hz, 1H), 3.71 (d, J = 16.4 Hz, 0.6H), 3.48 (d, J = 18.4 Hz, 1H), 3.42 (d, J = 16.8 Hz, 0.6H), 3.04 (d, J = 16.8 Hz, 1H), 3.03 (d, J = 16.4 Hz, 0.6H), 2.91 (d, J = 16.8 Hz, 0.6H), 2.77 (d, J = 18.4 Hz, 1H), 2.58 (s, 1.8H), 2.47 (s, 3H), 1.61 (s, 9H), 1.58 (s, 5.2H); 13C NMR (125 MHz, CDCl3) 13C NMR (100 MHz, CDCl3) δ 189.7, 188.3, 183.5, 183.2, 172.0, 171.6, 155.8, 155.2, 147.3, 147.0, 140.8, 140.4, 139.7, 137.7, 135.7, 135.5, 135.4, 135.1, 130.5, 129.7, 129.3, 129.1 (2C), 129.0, 128.9 (2C), 128.7 (2C), 128.6 (2C), 128.4, 128.3, 128.3, 128.1, 127.9 (2C), 125.8, 124.5, 124.4, 123.6, 107.1, 106.7, 86.9, 86.4, 76.8, 73.9, 69.5, 68.0, 48.6, 45.3, 42.8, 42.6, 39.8, 39.6, 27.8, 27.8 (3C), 27.6 (3C), 27.5; HRMS (ESI) calcd for C31H30N3O6 [M + H]+ 540.2129, found 540.2129.
(±)-tert-butyl (1′R*,4R*)-3-methyl-2,4″,5,7″-tetraoxo-1-phenethyl-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5g and (±)-tert-butyl (1′S*,4R*)-3-methyl-2,4″,5,7″-tetraoxo-1-(p-tolyl)-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5’g.
Compounds 5g and 5’g were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 5g (white solid, 183 mg, 33%) and 5’g (white solid, 316 mg, 57%).
5g:
m.p. = 163–165 °C; 1H NMR (500 MHz, CDCl3) δ 7.50 (d, J = 3.0 Hz, 1H), 7.40–7.30 (m, 3H), 7.27–7.23 (m, 2H), 7.21–7.14 (m, 3H), 7.09 (d, J = 7.0 Hz, 1H), 6.55 (d, J = 3.0 Hz, 1H), 3.72 (d, J = 16.5 Hz, 1H), 3.57 (ddd, J = 13.5, 10.5, 6.0 Hz, 1H), 3.49 (ddd, J = 13.5, 10.5, 6.0 Hz, 1H), 3.41 (d, J = 16.5 Hz, 1H), 2.98 (d, J = 16.5 Hz, 1H), 2.85 (d, J = 16.5 Hz, 1H), 2.76 (td, J = 13.0, 11.0, 6.0 Hz, 1H), 2.62 (td, J = 13.0, 11.0, 6.0 Hz, 1H), 2.55 (s, 3H), 1.59 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 190.3, 183.1, 171.5, 155.6, 147.4, 140.5, 139.9, 137.9 (2C), 132.5, 130.1, 129.4, 129.0 (2C), 128.6 (2C), 128.2, 126.7, 124.5, 123.4, 106.8, 86.6, 77.6, 69.2, 45.2, 40.2, 39.4, 33.5, 27.8, 27.7 (3C); HRMS (ESI) calcd for C32H32N3O6 [M + H]+ 554.2286, found 554.2288.
5’g:
m.p. = 164–166 °C; 1H NMR (500 MHz, CDCl3) δ 7.50 (d, J = 3.2 Hz, 1H), 7.42–7.27 (m, 6H), 7.22 (t, J = 7.4 Hz, 1H), 7.14 (d, J = 7.4 Hz, 2H), 6.65 (d, J = 3.2 Hz, 1H), 3.75 (d, J = 16.8 Hz, 1H), 3.51 (d, J = 18.4 Hz, 1H), 3.47–3.38 (m, 2H), 2.99 (d, J = 16.8 Hz, 1H), 2.83 (d, J = 18.4 Hz, 1H), 2.50–2.42 (m, 4H), 2.30 (ddd, J = 12.4, 11.6, 5.6 Hz, 1H), 1.61 (s, 9H);13C NMR (125 MHz, CDCl3) δ 188.4, 183.9, 172.3, 154.8, 146.9, 140.5, 137.8, 137.6, 135.5, 133.8, 130.3, 129.2, 128.9 (2C), 128.7 (2C), 128.3, 126.9, 126.0, 124.6, 107.8, 87.3, 73.8, 68.3, 48.5, 40.1, 39.5, 34.1, 27.9 (3C), 27.4; HRMS (ESI) calcd for C32H32N3O6 [M + H]+ 554.2286, found 554.2282.
(±)-tert-butyl (1′R*,4R*)-1-cyclohexyl-3-methyl-2,4″,5,7″-tetraoxo-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5h and (±)-tert-butyl (1′S*,4R*)-1-cyclohexyl-3-methyl-2,4″,5,7″-tetraoxo-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5’h.
Compounds 5h and 5’h were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 5h (white solid, 133 mg, 25%) and 5’h (white solid, 250 mg, 47%).
5h:
m.p. = 167–169 °C; 1H NMR (400 MHz, CDCl3) δ 7.49 (d, J = 3.2 Hz, 1H), 7.41–7.30 (m, 3H), 7.14–7.07 (m, 1H), 6.54 (d, J = 3.2 Hz, 1H), 3.68 (d, J = 16.4 Hz, 1H), 3.66 (tt, J = 12.4, 4.0 Hz, 1H), 3.48 (d, J = 16.4 Hz, 1H), 3.00 (d, J = 16.4 Hz, 1H), 2.89 (d, J = 16.4 Hz, 1H), 2.54 (s, 3H), 1.96 (qd, J = 12.4, 3.6 Hz, 1H), 1.73 (dd, J = 20.4, 12.0 Hz, 3H), 1.59 (s, 9H), 1.64–1.53 (m, 2H), 1.51–1.35 (m, 2H), 1.27–1.06 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 190.2, 183.5, 171.5, 155.7, 147.4, 140.3, 140.0, 134.8, 132.4, 129.8, 129.3, 128.1, 124.4, 123.5, 106.8, 86.4, 76.4, 69.5, 51.9, 45.4, 39.6, 28.9, 28.8, 27.8, 27.7 (3C), 25.8, 25.8, 25.0; HRMS (ESI) calcd for C30H34N3O6 [M + H]+ 532.2442, found 532.2441.
5’h:
m.p. = 167–169 °C; 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 3.2 Hz, 1H), 7.41–7.29 (m, 4H), 6.65 (d, J = 3.2 Hz, 1H), 3.77 (d, J = 16.8 Hz, 1H), 3.67 (tt, J = 13.2, 4.0 Hz, 1H), 3.51 (d, J = 18.4 Hz, 1H), 3.03 (d, J = 16.8 Hz, 1H), 2.80 (d, J = 18.4 Hz, 1H), 2.45 (s, 3H), 1.95–1.82 (m, 1H), 1.81–1.62 (m, 3H), 1.60 (s, 9H), 1.24–1.01 (m, 6H); 13C NMR (125 MHz, CDCl3) δ 188.5, 184.0, 172.3, 154.9, 147.0, 140.8, 137.9, 135.6, 133.7, 130.2, 129.0, 128.2, 125.8, 124.5, 107.9, 87.2, 73.0, 68.1, 51.5, 48.6, 39.8, 29.2, 28.7, 27.8 (3C), 27.3, 25.8, 25.7, 24.9; HRMS (ESI) calcd for C30H34N3O6 [M + H]+ 532.2442, found 532.2445.
(±)-tert-butyl (1′R*,4R*)-1-(tert-butyl)-3-methyl-2,4″,5,7″-tetraoxo-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5i and (±)-tert-butyl (1′S*,4R*)-1-(tert-butyl)-3-methyl-2,4″,5,7″-tetraoxo-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5’i.
Compounds 5i and 5’i were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 5i (white solid, 132 mg, 26%) and 5’i (white solid, 217 mg, 43%).
5i:
m.p. = 166–168 °C; 1H NMR (400 MHz, CDCl3) δ 7.51 (d, J = 3.2 Hz, 1H), 7.41–7.29 (m, 3H), 7.14–7.06 (m, 1H), 6.59 (d, J = 3.2 Hz, 1H), 3.69 (d, J = 16.4 Hz, 1H), 3.50 (d, J = 16.4 Hz, 1H), 2.97 (d, J = 16.4 Hz, 1H), 2.88 (d, J = 16.4 Hz, 1H), 2.52 (s, 3H), 1.58 (s, 9H), 1.32 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 190.3, 184.0, 172.2, 156.3, 147.4, 140.1, 140.1, 135.0, 132.5, 129.7, 129.4, 128.1, 124.3, 123.7, 107.0, 86.4, 76.5, 69.9, 58.2, 45.6, 39.7, 28.1 (3C), 27.6 (3C), 27.5; HRMS (ESI) calcd for C28H32N3O6 [M + H]+ 506.2286, found 506.2288.
5’i:
m.p. = 164–166 °C; 1H NMR (400 MHz, CDCl3) δ 7.58 (d, J = 3.2 Hz, 1H), 7.39–7.29 (m, 4H), 6.69 (d, J = 3.2 Hz, 1H), 3.77 (d, J = 16.8 Hz, 1H), 3.47 (d, J = 18.4 Hz, 1H), 3.01 (d, J = 16.8 Hz, 1H), 2.80 (d, J = 18.4 Hz, 1H), 2.42 (s, 3H), 1.60 (s, 9H), 1.29 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 188.6, 184.1, 172.6, 155.5, 147.1, 140.8, 138.1, 135.7, 134.0, 130.4, 129.0, 128.2, 125.8, 124.5, 107.9, 87.2, 73.0, 68.4, 58.1, 48.7, 39.7, 28.4 (3C), 27.8 (3C), 27.1; HRMS (ESI) calcd for C28H32N3O6 [M + H]+ 506.2286, found 506.2287.
(±)-tert-butyl (1′R*,4R*)-5’-chloro-3-methyl-2,4″,5,7″-tetraoxo-1-(p-tolyl)-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5j and (±)-tert-butyl (1′S*,4R*)-5’-chloro-3-methyl-2,4″,5,7″-tetraoxo-1-(p-tolyl)-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5’j.
Compounds 5j and 5’j were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 5j (white solid, 166 mg, 29%) and 5’j (white solid, 276 mg, 48%).
5j:
m.p. = 163–165 °C; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 3.2 Hz, 1H), 7.36 (s, 1H), 7.34 (d, J = 8.4 Hz, 1H), 7.15 (d, J = 8.4 Hz, 2H), 7.09 (d, J = 8.4 Hz, 1H), 6.93 (d, J = 8.4 Hz, 2H), 6.54 (d, J = 3.2 Hz, 1H), 3.73 (d, J = 16.8 Hz, 1H), 3.47 (d, J = 16.4 Hz, 1H), 3.17 (d, J = 16.8 Hz, 1H), 2.98 (d, J = 16.4 Hz, 1H), 2.71 (s, 3H), 2.32 (s, 3H), 1.47 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 189.6, 182.9, 170.5, 154.9, 147.3, 141.5, 138.6, 138.4, 135.3, 134.2, 132.8, 130.2, 129.7 (2C), 128.8, 128.6, 125.8 (2C), 125.2, 124.8, 107.0, 86.7, 76.5, 70.0, 46.1, 38.8, 28.4, 27.5 (3C), 21.3; HRMS (ESI) calcd for C31H29ClN3O6 [M + H]+ 574.1740, found 574.1744.
5’j:
m.p. = 162–164 °C; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 3.2 Hz, 1H), 7.43–7.29 (m, 3H), 7.17–7.11 (m, 2H), 6.86–6.80 (m, 2H), 6.62 (d, J = 3.2 Hz, 1H), 3.86 (d, J = 16.8 Hz, 1H), 3.54 (d, J = 18.4 Hz, 1H), 3.17 (d, J = 16.8 Hz, 1H), 2.83 (d, J = 18.4 Hz, 1H), 2.59 (s, 3H), 2.33 (s, 3H), 1.61 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 187.8, 183.5, 171.1, 153.9, 146.7, 139.8, 138.9, 138.3, 135.4, 135.1, 133.5, 130.5, 129.6 (2C), 128.7, 128.6, 127.3, 125.5 (2C), 124.8, 108.1, 87.5, 73.6, 68.1, 48.3, 39.5, 27.9 (3C), 27.6, 21.3; HRMS (ESI) calcd for C31H29ClN3O6 [M + H]+ 574.1740, found 574.1737.
(±)-tert-butyl (1′R*,4R*)-6’-methoxy-3-methyl-2,4″,5,7″-tetraoxo-1-(p-tolyl)-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5k and (±)-tert-butyl (1′S*,4R*)-6’-methoxy-3-methyl-2,4″,5,7″-tetraoxo-1-(p-tolyl)-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5’k.
Compounds 5k and 5’k were prepared according to the general procedure; the reaction was completed within 20 min. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 5k (white solid, 137 mg, 24%) and 5’k (white solid, 279 mg, 49%).
5k:
m.p. = 166–168 °C; 1H NMR (400 MHz, CDCl3) δ 7.51 (d, J = 3.2 Hz, 1H), 7.24 (d, J = 8.4 Hz, 1H), 7.13 (d, J = 8.4 Hz, 2H), 6.97–6.90 (m, 2H), 6.86–6.79 (m, 2H), 6.62 (d, J = 3.2 Hz, 1H), 3.85 (s, 3H), 3.81 (d, J = 16.4 Hz, 1H), 3.56 (d, J = 18.4 Hz, 1H), 3.12 (d, J = 16.4 Hz, 1H), 2.88 (d, J = 18.4 Hz, 1H), 2.61 (s, 3H), 2.32 (s, 3H), 1.61 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 190.1, 183.1, 170.7, 159.9, 155.0, 147.3, 141.4, 138.3, 134.5, 132.6, 131.5, 130.0, 129.7 (2C), 128.9, 125.9 (2C), 125.1, 115.2, 109.5, 106.8, 86.6, 76.9, 70.4, 55.6, 46.0, 38.5, 28.2, 27.6 (3C), 21.3; HRMS (ESI) calcd for C32H32N3O7 [M + H]+ 570.2235, found 570.2240.
5’k:
m.p. = 165–167 °C; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 3.2 Hz, 1H), 7.26 (d, J = 8.4 Hz, 1H), 7.16 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 8.4 Hz, 2H), 6.93 (dd, J = 8.4, 2.4 Hz, 1H), 6.68 (d, J = 2.4 Hz, 1H), 6.54 (d, J = 3.2 Hz, 1H), 3.81 (s, 3H), 3.71 (d, J = 16.4 Hz, 1H), 3.51 (d, J = 16.4 Hz, 1H), 3.11 (d, J = 16.4 Hz, 1H), 3.00 (d, J = 16.4 Hz, 1H), 2.70 (s, 3H), 2.33 (s, 3H), 1.50 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 188.3, 183.9, 171.5, 159.8, 154.1, 146.9, 141.8, 138.2, 135.4, 133.8, 130.3, 129.6 (2C), 129.5, 128.7, 125.6 (2C), 125.2, 115.8, 110.9, 108.0, 87.4, 74.0, 68.6, 55.7, 48.5, 39.2, 27.9 (3C), 27.6, 21.3; HRMS (ESI) calcd for C32H32N3O7 [M + H]+ 570.2235, found 570.2233.
(±)-tert-butyl (1′R*,4R*)-5’-methoxy-3-methyl-2,4″,5,7″-tetraoxo-1-(p-tolyl)-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5l and (±)-tert-butyl (1′S*,4R*)-5’-methoxy-3-methyl-2,4″,5,7″-tetraoxo-1-(p-tolyl)-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5’l.
Compounds 5l and 5’l were prepared according to the general procedure; the reaction was completed within 20 min. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 5l (white solid, 177 mg, 31%) and 5’l (white solid, 302 mg, 53%).
5l:
m.p. = 169–171 °C; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 3.2 Hz, 1H), 7.16 (d, J = 8.0 Hz, 2H), 7.05 (d, J = 9.2 Hz, 1H), 6.96 (d, J = 8.0 Hz, 2H), 6.90 (d, J = 6.8 Hz, 1H), 6.89 (s, 1H), 6.54 (d, J = 3.2 Hz, 1H), 3.85 (s, 3H), 3.74 (d, J = 16.4 Hz, 1H), 3.48 (d, J = 16.4 Hz, 1H), 3.14 (d, J = 16.4 Hz, 1H), 2.99 (d, J = 16.4 Hz, 1H), 2.72 (s, 3H), 2.33 (s, 3H), 1.49 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 190.3, 183.7, 170.8, 160.8, 155.0, 147.3, 141.1, 138.3, 134.6, 132.7, 132.0, 129.9, 129.7 (2C), 128.8, 125.9 (2C), 124.7, 114.1, 109.8, 106.8, 86.5, 76.8, 69.8, 55.6, 46.4, 39.2, 28.2, 27.5 (3C), 21.3; HRMS (ESI) calcd for C32H32N3O7 [M + H]+ 570.2235, found 570.2232.
5’l:
m.p. = 166–168 °C; 1H NMR (400 MHz, CDCl3) δ 7.52–7.49 (m, 1H), 7.33 (d, J = 8.8 Hz, 1H), 7.13 (d, J = 7.8 Hz, 2H), 6.95 (d, J = 8.8 Hz, 1H), 6.90–6.78 (m, 3H), 6.63–6.59 (m, 1H), 3.85 (s, 3H), 3.85 (d, J = 16.4 Hz, 1H), 3.54 (d, J = 18.4 Hz, 1H), 3.13 (d, J = 16.4 Hz, 1H), 2.84 (d, J = 18.4 Hz, 1H), 2.61 (s, 3H), 2.33 (s, 3H), 1.60 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 188.5, 184.3, 171.4, 160.5, 154.1, 146.9, 139.3, 138.2, 135.3, 133.9, 132.4, 130.3, 129.6 (2C), 128.7, 126.8, 125.5 (2C), 114.3, 109.6, 108.0, 87.2, 73.8, 67.9, 55.6, 48.8, 40.0, 27.9 (3C), 27.6, 21.3; HRMS (ESI) calcd for C32H32N3O7 [M + H]+ 570.2235, found 570.2237.
(±)-tert-butyl (1′R*,4R*)-3,5’-dimethyl-2,4″,5,7″-tetraoxo-1-(p-tolyl)-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5m and (±)-tert-butyl (1′S*,4R*)-3,5’-dimethyl-2,4″,5,7″-tetraoxo-1-(p-tolyl)-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5’m.
Compounds 5m and 5’m were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 5m (white solid, 144 mg, 26%) and 5’m (white solid, 232 mg, 42%).
5m:
m.p. = 161–163 °C; 1H NMR (500 MHz, CDCl3) δ 7.43 (d, J = 3.0 Hz, 1H), 7.18 (d, J = 8.0 Hz, 3H), 7.15 (s, 1H), 7.03 (d, J = 8.0 Hz, 1H), 6.96 (d, J = 8.0 Hz, 2H), 6.55 (d, J = 3.0 Hz, 1H), 3.74 (d, J = 16.5 Hz, 1H), 3.51 (d, J = 16.5 Hz, 1H), 3.14 (d, J = 16.5 Hz, 1H), 2.99 (d, J = 16.5 Hz, 1H), 2.70 (s, 3H), 2.41 (s, 3H), 2.33 (s, 3H), 1.48 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 190.3, 183.6, 170.9, 155.1, 147.4, 139.7, 139.5, 138.3, 137.1, 134.6, 132.6, 129.9, 129.7 (2C), 129.1, 128.9, 125.9 (2C), 125.1, 123.5, 106.8, 86.5, 76.7, 70.2, 46.2, 39.1, 28.3, 27.5 (3C), 21.6, 21.3; HRMS (ESI) calcd for C32H32N3O6 [M + H]+ 554.2286, found 554.2281.
5’m:
m.p. = 164–166 °C; 1H NMR (400 MHz, CDCl3) δ 7.51 (d, J = 3.2 Hz, 1H), 7.30 (d, J = 8.0 Hz, 1H), 7.22 (d, J = 8.0 Hz, 1H), 7.17 (s, 1H), 7.13 (d, J = 8.0 Hz, 2H), 6.83 (d, J = 8.0 Hz, 2H), 6.62 (d, J = 3.2 Hz, 1H), 3.84 (d, J = 16.8 Hz, 1H), 3.54 (d, J = 18.4 Hz, 1H), 3.14 (d, J = 16.8 Hz, 1H), 2.85 (d, J = 18.4 Hz, 1H), 2.59 (s, 3H), 2.41 (s, 3H), 2.33 (s, 3H), 1.60 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 188.4, 184.1, 171.6, 154.1, 146.9, 139.2, 138.1, 137.9, 137.5, 135.3, 133.8, 130.3, 129.6 (2C), 129.2, 128.7, 125.7, 125.5 (2C), 125.1, 108.0, 87.3, 73.7, 68.4, 48.6, 39.8, 27.9 (3C), 27.6, 21.5, 21.2; HRMS (ESI) calcd for C32H32N3O6 [M + H]+ 554.2286, found 554.2286.
(±)-tert-butyl (1′R*,4R*)-3,6’-dimethyl-2,4″,5,7″-tetraoxo-1-(p-tolyl)-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5n and (±)-tert-butyl (1′S*,4R*)-3,6’-dimethyl-2,4″,5,7″-tetraoxo-1-(p-tolyl)-4″,7″-dihydro-3′H-dispiro[imidazolidine-4,2′-indene-1′,6″-indole]-1″(5″H)-carboxylate 5’n.
Compounds 5n and 5’n were prepared according to the general procedure. Purification by column chromatography (silica gel, PET/EtOAc = 5:1, v/v) generated compounds 5n (white solid, 127 mg, 23%) and 5’n (white solid, 244 mg, 44%).
5n:
m.p. = 162–164 °C; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 3.2 Hz, 1H), 7.25–7.13 (m, 4H), 7.01–6.89 (m, 3H), 6.54 (d, J = 3.2 Hz, 1H), 3.74 (d, J = 16.4 Hz, 1H), 3.53 (d, J = 16.4 Hz, 1H), 3.13 (d, J = 16.4 Hz, 1H), 2.99 (d, J = 16.4 Hz, 1H), 2.68 (s, 3H), 2.39 (s, 3H), 2.33 (s, 3H), 1.50 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 190.3, 183.4, 170.8, 155.0, 147.3, 140.1, 138.3, 138.0, 136.7, 134.6, 132.6, 130.4, 130.0, 129.7 (2C), 128.9, 125.9 (2C), 124.4, 124.1, 106.8, 86.5, 77.4, 77.2, 76.9, 70.2, 46.0, 38.9, 28.2, 27.5 (4C), 21.7, 21.3; HRMS (ESI) calcd for C32H32N3O6 [M + H]+ 554.2286, found 554.2288.
5’n:
m.p. = 165–167 °C; 1H NMR (500 MHz, CDCl3) δ 7.51 (d, J = 3.2 Hz, 1H), 7.25–7.17 (m, 3H), 7.13 (d, J = 8.0 Hz, 2H), 6.83 (d, J = 8.4 Hz, 2H), 6.62 (d, J = 3.2 Hz, 1H), 3.83 (d, J = 16.8 Hz, 1H), 3.54 (d, J = 18.4 Hz, 1H), 3.14 (d, J = 16.8 Hz, 1H), 2.87 (d, J = 18.4 Hz, 1H), 2.59 (s, 3H), 2.42 (s, 3H), 2.32 (s, 3H), 1.61 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 188.3, 184.1, 171.6, 154.1, 146.9, 140.5, 138.1, 138.1, 135.4, 134.8, 133.8, 130.3, 130.1, 129.6 (2C), 128.7, 126.6, 125.5 (2C), 124.2, 107.9, 87.3, 73.8, 68.5, 48.6, 39.6, 27.9 (3C), 27.6, 21.7, 21.3; HRMS (ESI) calcd for C32H32N3O6 [M + H]+ 554.2286, found 554.2285.
48.6, 39.6, 27.9 (3C), 27.6, 21.7, 21.3; HRMS (ESI) calcd for C32H32N3O6 [M + H]+ 554.2286, found 554.2283.