Classical Paal-Knorr Cyclization for Synthesis of Pyrrole-Based Aryl Hydrazones and In Vitro/In Vivo Evaluation on Pharmacological Models of Parkinson’s Disease
Abstract
1. Introduction
2. Results
2.1. Synthesis of the Target Molecules
2.1.1. Paal-Knorr Synthesis of the Initial Hydrazide
2.1.2. General Synthesis of the Target Hydrazones, Containing Phenyl/Substituted Phenyl Carbonyl Fragment
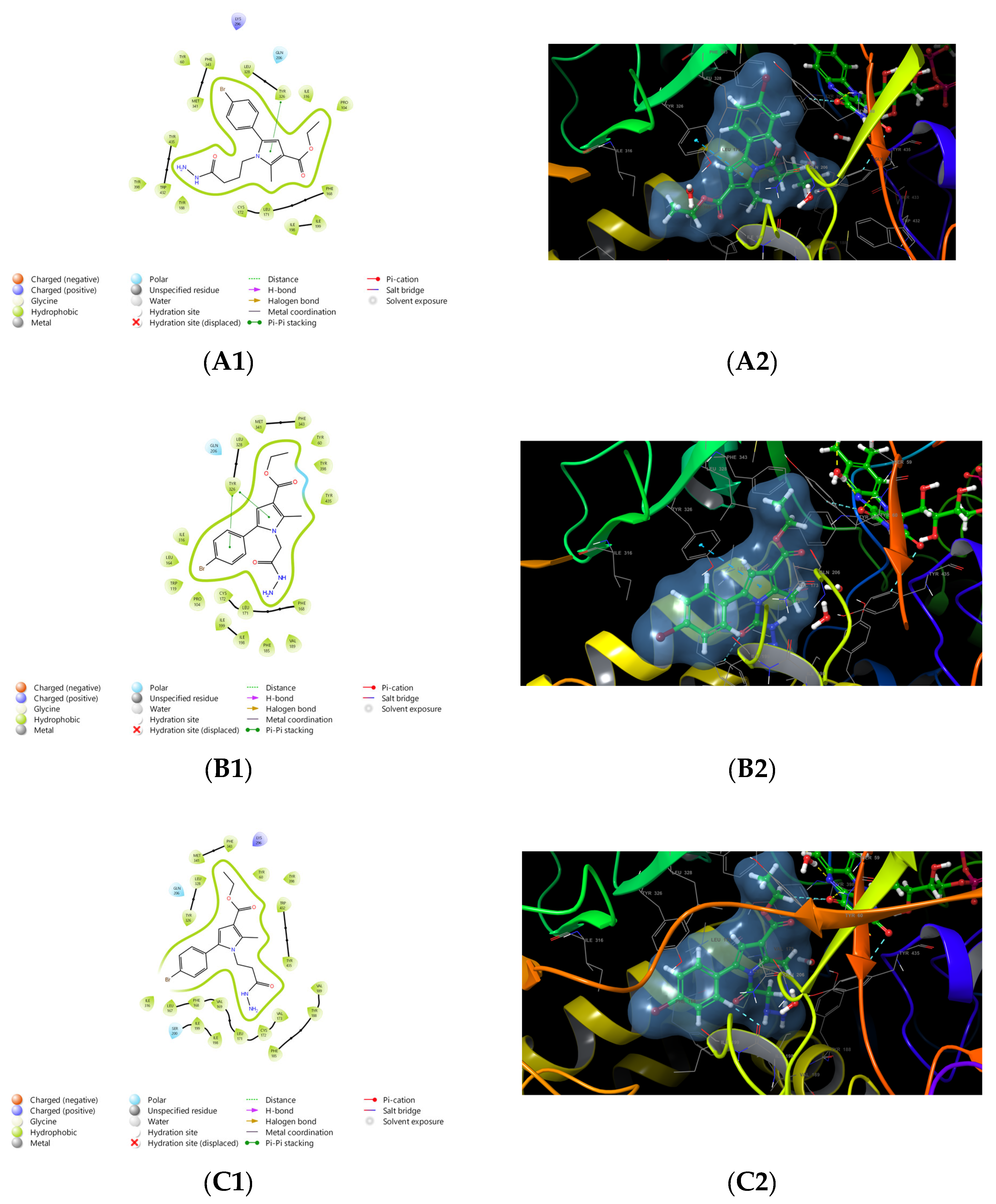
2.1.3. Molecular Docking Studies Results
2.2. Pharmacological Evaluations Results
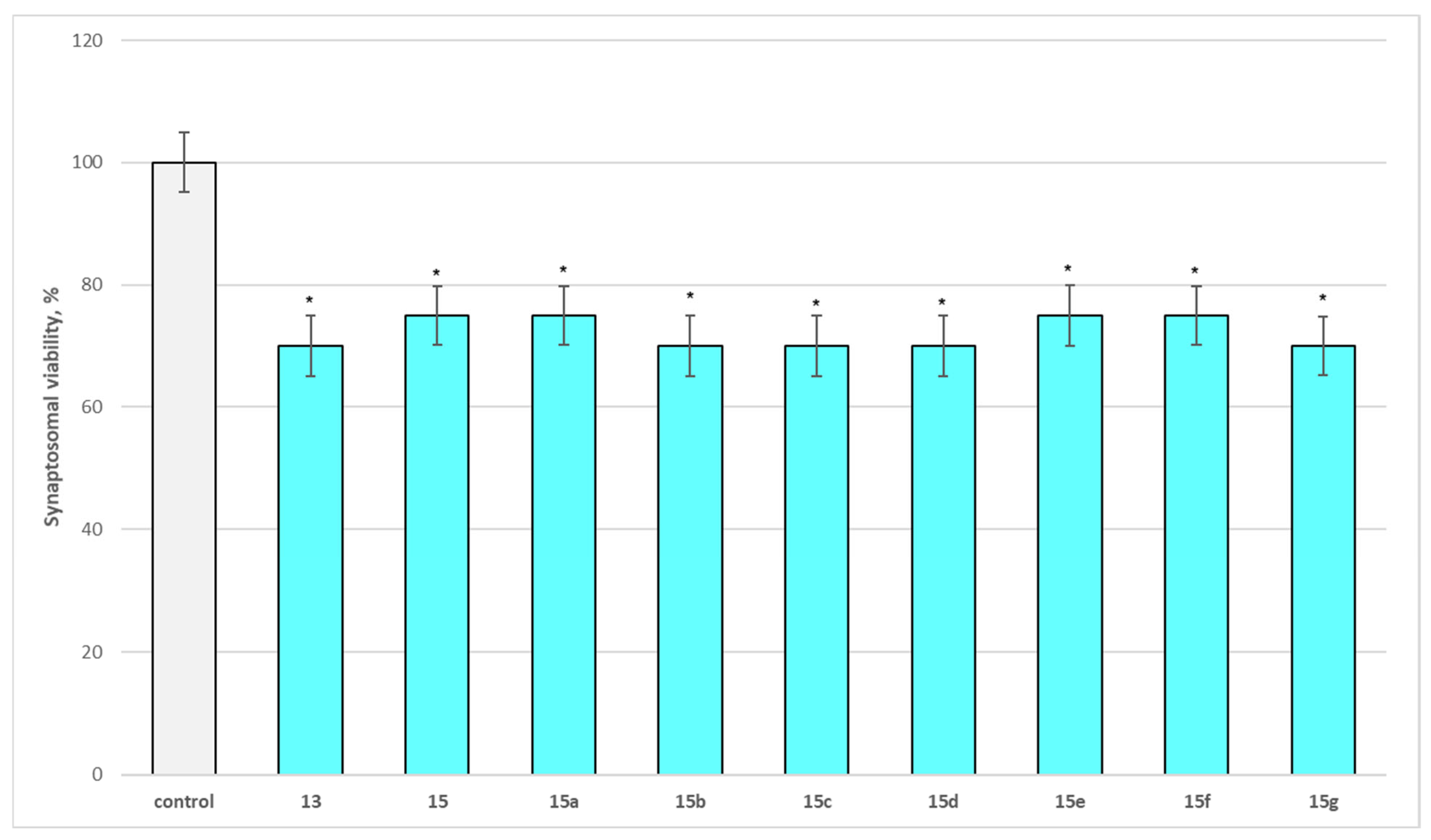
2.2.1. Neurotoxicity Assessment Results
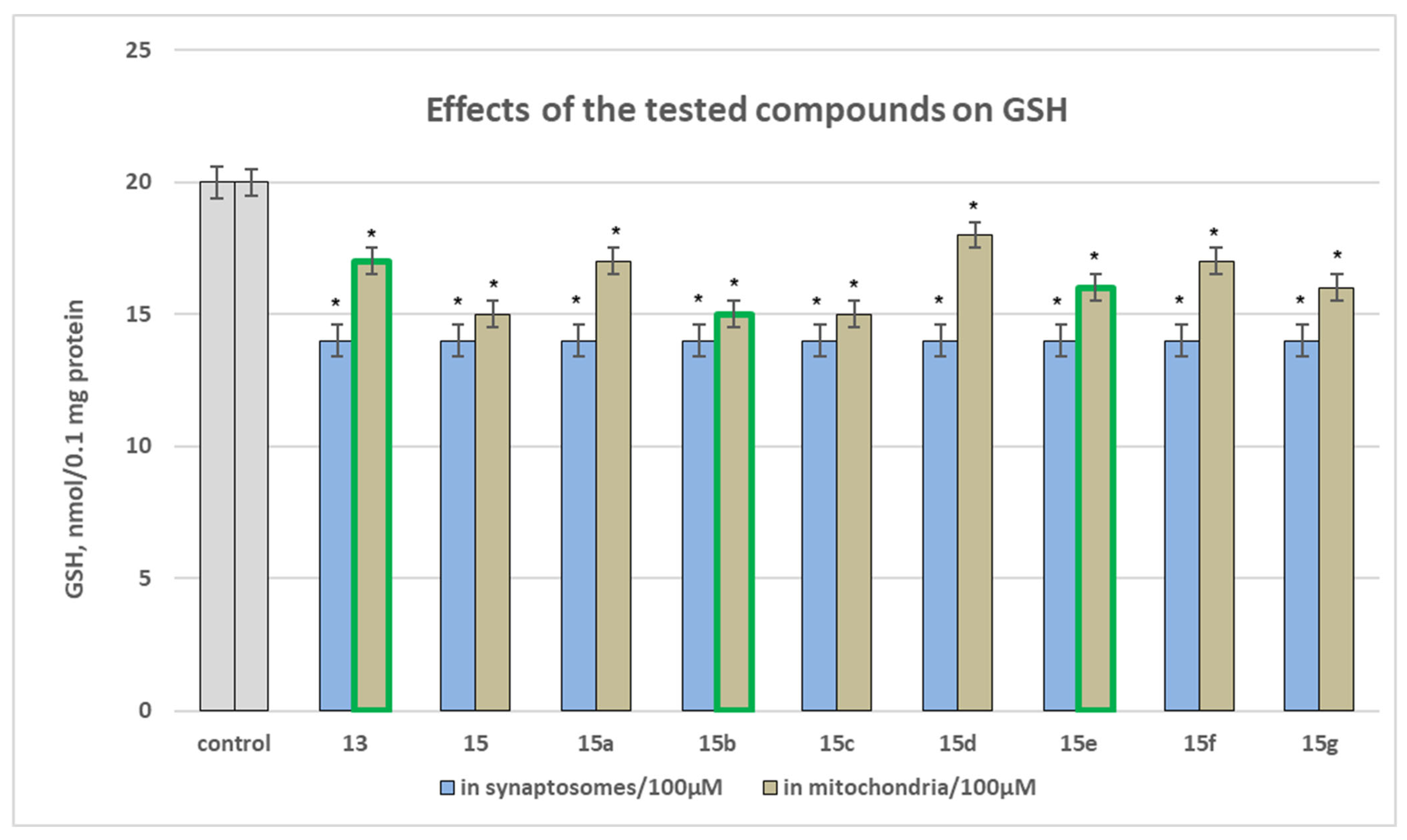
Effect of Substances Administered Alone on Biomarkers Characterizing the Functional-Metabolic Profile of Brain Synaptosomes (Measured as Synaptosomal Vitality and GSH Level), Mitochondria (Measured as GSH Level and MDA Production), and Microsomes (Measured as MDA Production)
Effect of Substances Administered Alone on Biomarkers Characterizing the Functional-Metabolic Profile of Brain Synaptosomes
Effect of Newly Synthesized Hydrazones, Administered Alone, on Biomarkers Characterizing the Functional-Metabolic Profile of Brain Mitochondria
Effect of Newly Synthesized Hydrazones, Administered Alone, on Biomarkers Characterizing the Functional Metabolic Profile of Brain Microsomes
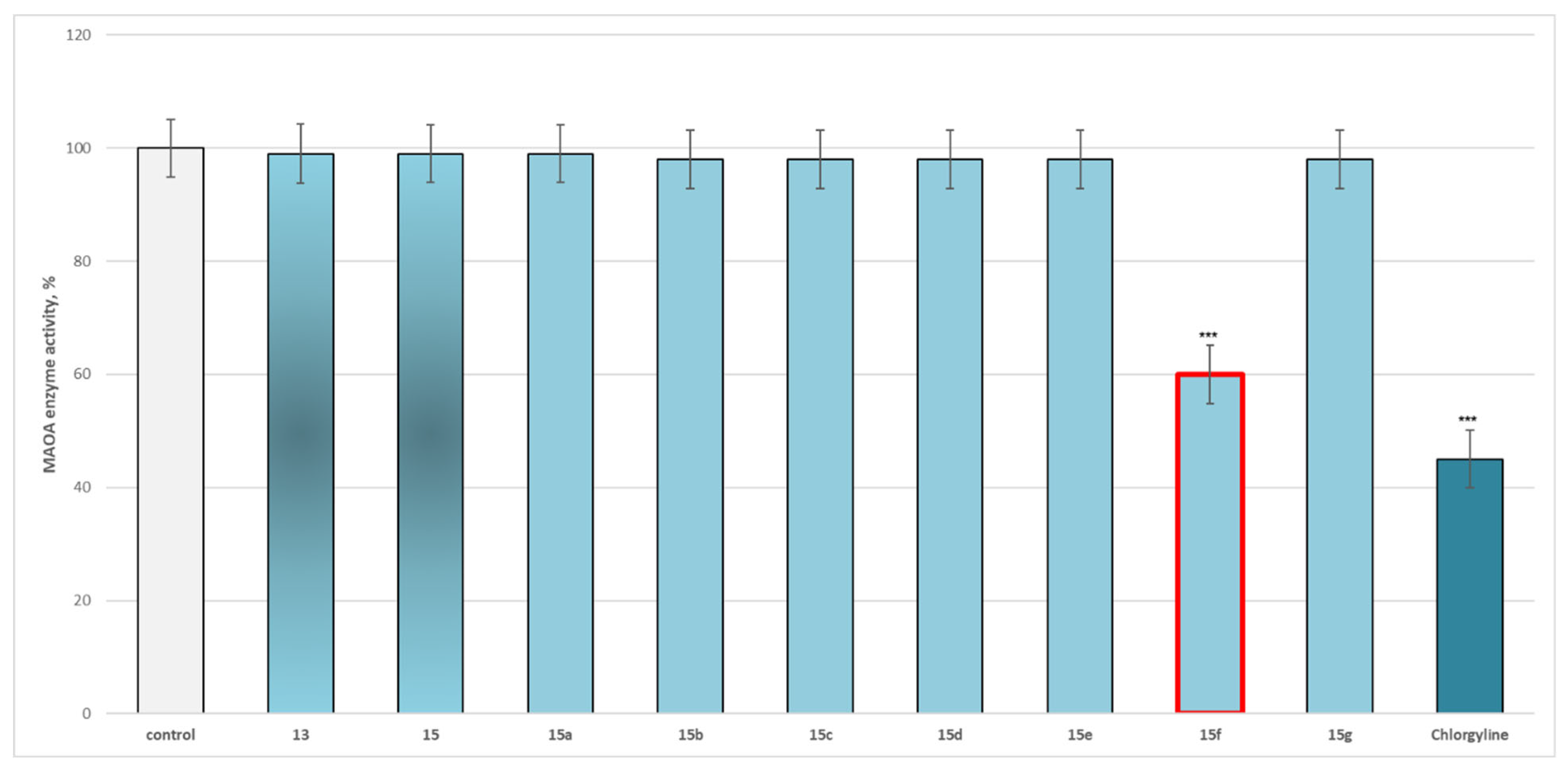
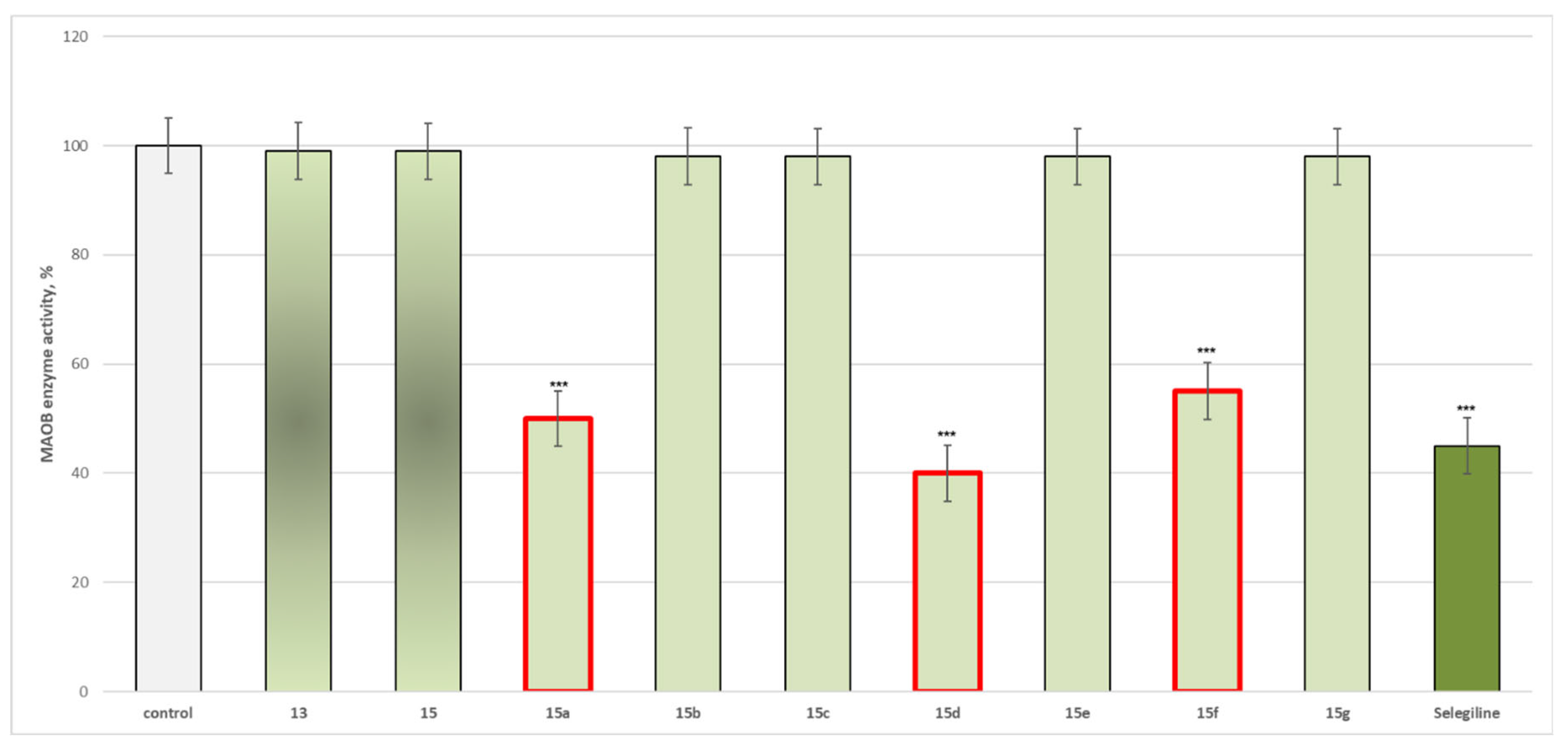
Effects of the Target New Compounds 13, 15, 15a–15g on Human Recombinant MAOA/MAOB Enzyme Results
2.2.2. Neuroprotection Effects Results
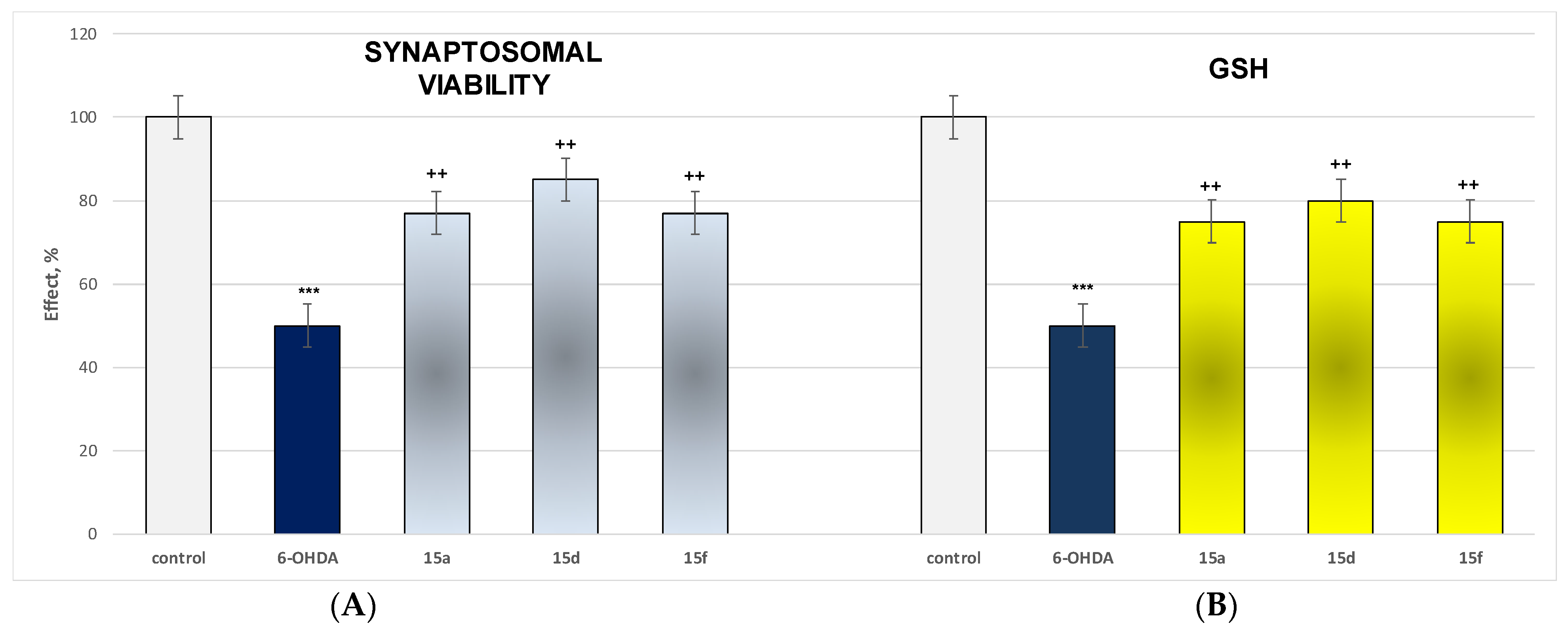
Effect of Most Active and Least Toxic Substances in a Model of 6-OHDA-Induced Neurotoxicity on Parameters Characterizing the Functional-Metabolic Profile of Brain Synaptosomes
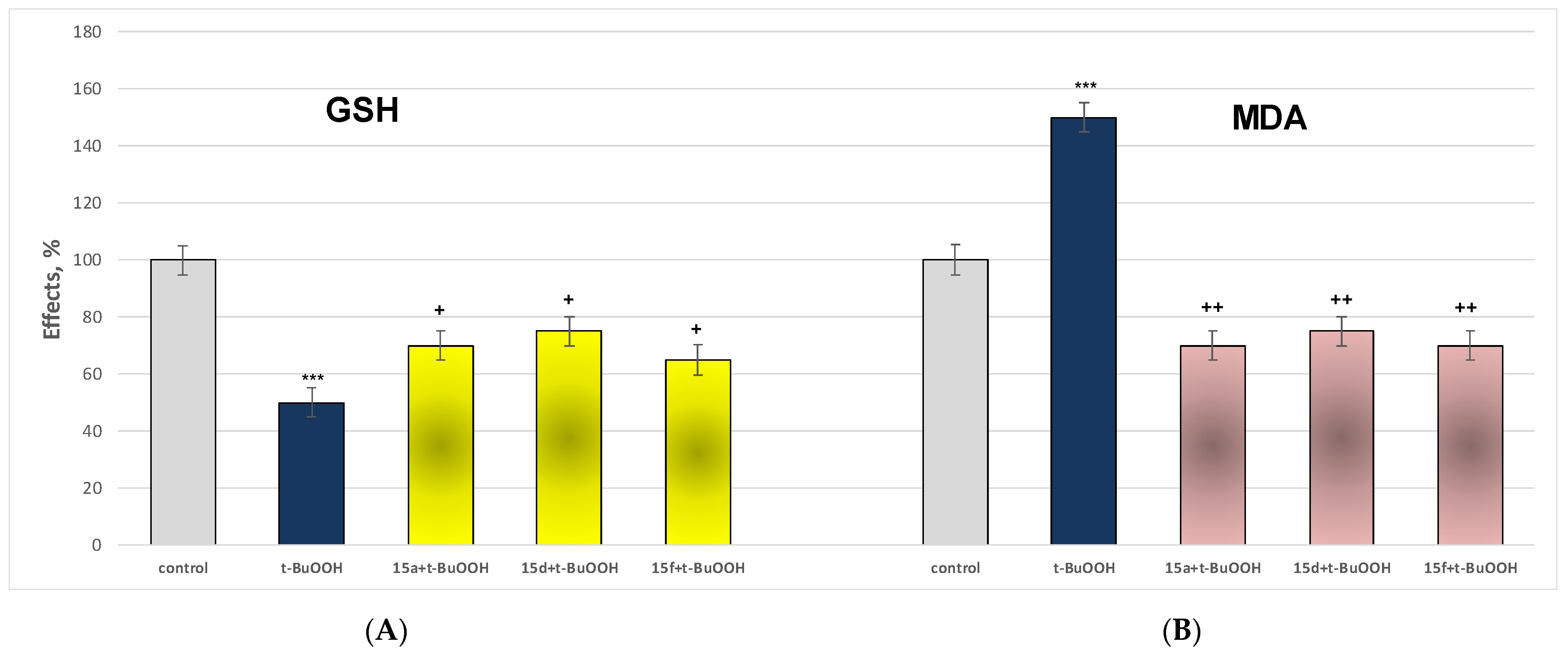
Effect of the Most Active and Least Toxic Substances in a Model of t-BuOOH-Induced Oxidative Stress on Parameters Characterizing the Functional-Metabolic Profile of Brain Mitochondria
Effect of Most Active and Least Toxic Substances in a Model of Non-Enzyme-Induced Lipid Peroxidation (Fe2+/AA) on Parameters Characterizing the Functional Metabolic Profile of Brain Microsomes
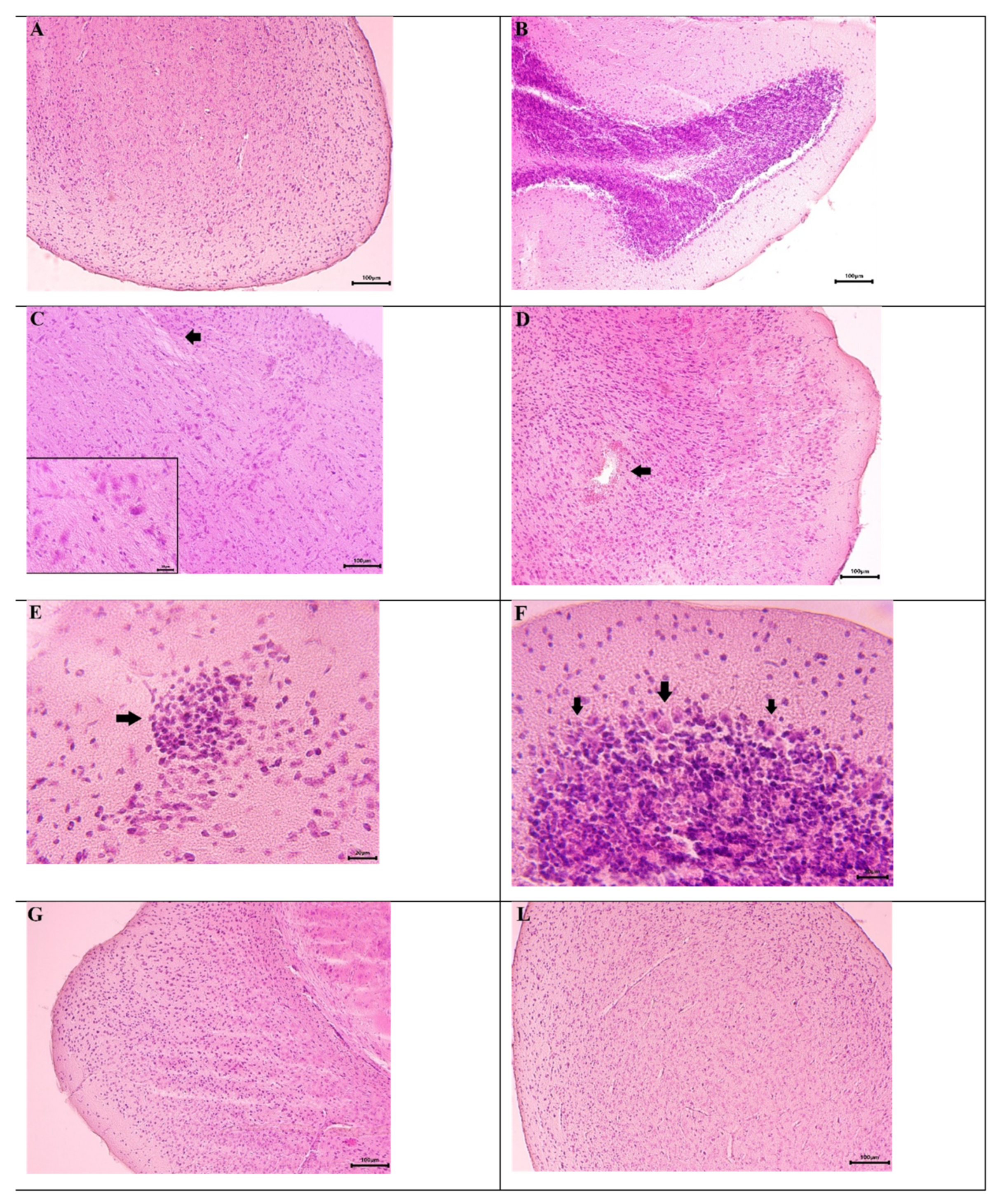
2.3. Histopathological Analysis Results
3. Discussion
3.1. Molecular Docking Studies
3.2. Pharmacological Evaluations
3.2.1. Neurotoxicity Assessment
3.2.2. Effects of the Target New Compounds 13, 15, 15a–15g on Human Recombinant MAOA/MAOB Enzyme
3.2.3. Neuroprotection Effects
3.2.4. Effect of Most Active and Least Toxic Substances in a Model of 6-OHDA-Induced Neurotoxicity on Parameters Characterizing the Functional-Metabolic Profile of Brain Synaptosomes
3.2.5. Effect of the Most Active and Least Toxic Substances in a Model of t-BuOOH-Induced Oxidative Stress on Parameters Characterizing the Functional-Metabolic Profile of Brain Mitochondria
3.2.6. Effect of Most Active and Least Toxic Substances in a Model of Non-Enzyme-Induced Lipid Peroxidation (Fe2+/AA) on Parameters Characterizing the Functional-Metabolic Profile of Brain Microsomes
4. Materials and Methods
4.1. Chemistry
4.1.1. Paal-Knorr Cyclization for Synthesis of the Target N-Pyrrolyl Carboxylic Acid
4.1.2. Synthesis of the Intermediate N-Pyrrolyl Carboxylic Ester
4.1.3. Synthesis of the Initial Hydrazide
4.1.4. General Synthesis of the New Hydrazones 15a–15g
4.2. Molecular Docking Simulations
4.3. Animals
4.4. Preparation of Rat Brain Synaptosomes and Mitochondria
4.4.1. Establishing a Dopamine Model of Neurotoxicity
4.4.2. MTT Assay to Assess Synaptosomal Viability
4.4.3. Determination of Reduced Glutathione (GSH) in Isolated Brain Synaptosomes by the Method of Robyt [51]
4.5. Tert-Butyl Hydroperoxide-Induced Oxidative Stress
4.5.1. Determination of Malondialdehyde (MDA) Production in Brain Mitochondria [53]
4.5.2. Determination of GSH Level in Brain Mitochondria [53]
4.6. Isolation of Brain Microsomes [54]
4.7. Iron/Ascorbate-Induced Lipid Peroxidation (LPO)
Determination of MDA in Brain Microsomes [55]
4.8. Determination of Human Recombinant MAOA/B Enzyme Activity
4.9. Histopathological Analysis
4.10. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zafar, S.; Yaddanapudi, S.S. Parkinson Disease. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2023. [Google Scholar]
- Sensi, S.L.; Russo, M.; Tiraboschi, P. Biomarkers of diagnosis, prognosis, pathogenesis, response to therapy: Convergence or divergence? Lessons from Alzheimer’s disease and synucleinopathies. Handb. Clin. Neurol. 2023, 192, 187–218. [Google Scholar]
- Fereshtehnejad, S.M.; Postuma, R.B. Subtypes of Parkinson’s disease: What do they tell us about disease progression? Curr. Neurol. Neurosci. Rep. 2017, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Thenganatt, M.A.; Jankovic, J. Parkinson disease subtypes. JAMA Neurol. 2014, 71, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.M.; Zeighami, Y.; Dagher, A.; Postuma, R.B. Clinical criteria for subtyping Parkinson’s disease: Biomarkers and longitudinal progression. Brain 2017, 140, 1959–1976. [Google Scholar] [CrossRef]
- Lawton, M.; Ben-Shlomo, Y.; May, M.T.; Baig, F.; Barber, T.R.; Klein, J.C.; Swallow, D.M.A.; Malek, N.; Grosset, K.A.; Bajaj, N.; et al. Developing and validating Parkinson’s disease subtypes and their motor and cognitive progression. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1279–1287. [Google Scholar] [CrossRef]
- van Rooden, S.M.; Colas, F.; Martínez-Martín, P.; Visser, M.; Verbaan, D.; Marinus, J.; Chaudhuri, R.K.; Kok, J.N.; van Hilten, J.J. Clinical subtypes of Parkinson’s disease. Mov. Disord. 2011, 26, 51–58. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.H.; Katzenschlager, R.; Lim, S.Y.; Barton, B.; de Bie, R.M.; Seppi, K.; Coelho, M.; Sampaio, C. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2018, 33, 1248–1266. [Google Scholar] [CrossRef] [PubMed]
- Mateev, E.; Angelov, B.; Kondeva-Burdina, M.; Valkova, I.; Georgieva, M.; Zlatkov, A. Design, synthesis, biological evaluation and molecular docking of pyrrole-based compounds as antioxidant and MAO-B inhibitory agents. Farmacia 2022, 70, 344–354. [Google Scholar] [CrossRef]
- Georgieva, M.; Mateev, E.; Valkova, I.; Kuteva, H.; Tzankova, D.; Stefanova, D.; Yordanov, Y.; Lybomirova, K.; Zlatkov, A.; Tzankova, V.; et al. Neurotoxicity, Neuroprotection, In Vitro MAOA/MAOB Inhibitory Activity Assessment, Molecular Docking, and Permeability Assay Studies of Newly Synthesized Hydrazones Containing a Pyrrole Ring. Molecules 2024, 29, 4338. [Google Scholar] [CrossRef]
- Kondeva-Burdina, M.; Mateev, E.; Angelov, B.; Tzankova, V.; Georgieva, M. In Silico Evaluation and In Vitro Determination of Neuroprotective and MAO-B Inhibitory Effects of Pyrrole-Based Hydrazones: A Therapeutic Approach to Parkinson’s Disease. Molecules 2022, 27, 8485. [Google Scholar] [CrossRef] [PubMed]
- Mateev, E.; Karatchobanov, V.; Dedja, M.; Diamantakos, K.; Mateeva, A.; Muhammed, M.T.; Irfan, A.; Kondeva-Burdina, M.; Valkova, I.; Georgieva, M.; et al. Novel Pyrrole Derivatives as Multi-Target Agents for the Treatment of Alzheimer’s Disease: Microwave-Assisted Synthesis, In Silico Studies and Biological Evaluation. Pharmaceuticals 2024, 17, 1171. [Google Scholar] [CrossRef]
- Amarnath, V.; Anthony, D.C.; Amarnath, K.; Valentine, W.M.; Wetterau, L.A.; Graham, D.G. Intermediates in the Paal-Knorr synthesis of pyrroles. J. Org. Chem. 1991, 56, 6924–6931. [Google Scholar] [CrossRef]
- Török, B.; Schäfer, C.; Kokel, A. Chapter 3.8—Ring transformations by heterogeneous catalysis. In Heterogeneous Catalysis in Sustainable Synthesis, 1st ed.; Török, B., Schäfer, C., Kokel, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 491–542. [Google Scholar]
- Bijev, A.; Georgieva, M. Pyrrole-based hydrazones synthesized and evaluated in vitro as potential tuberculostatics. Lett. Drug Des. Discov. 2010, 7, 430–437. [Google Scholar] [CrossRef]
- Georgieva, M.; Bijev, A.; Prodanova, P. Synthesis and comparative study of tuberculostatic activity of pyrrole-based hydra-zones related to structural variations. Pharmacia 2010, 52, 3–14. [Google Scholar]
- Russel, J.S.; Pelkey, E.T.; Greger, J.G. Chapter 5.2—Five-Membered Ring Systems: Pyrroles and Benzo Analogs. In Progress in Heterocyclic Chemistry; Gribble, G.W., Joule, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 23, pp. 155–194. [Google Scholar]
- Yasmin, N.; Ray, J.K. A New Facile Approach to Isoindole and Pyrrole Derivatives. Synlett 2010, 6, 924–930. [Google Scholar] [CrossRef]
- Zheng, Q.; Hua, R. CuCl-catalyzed cycloaddition of 1,3-butadiynes with primary amines: An atom-economic process for synthesis of 1,2,5-trisubsituted pyrroles. Tetrahedron Lett. 2010, 51, 4512–4514. [Google Scholar] [CrossRef]
- Kramer, S.; Madsen, J.L.H.; Rottländer, M.; Skrydstrup, T. Access to 2,5-diamidopyrroles and 2,5-diamidofurans by Au(I)-catalyzed double hydroamination or hydration of 1,3-diynes. Org. Lett. 2010, 12, 2758–2761. [Google Scholar] [CrossRef]
- Demir, A.S.; Emrullahoğlua, M.; Buran, K. Gold(I)/Zn(II) catalyzed tandem hydroamination/annulation reaction of 4-yne-nitriles. Chem. Commun. 2010, 46, 8032–8034. [Google Scholar] [CrossRef]
- Huo, J.; He, G.; Chen, W.; Hu, X.; Deng, Q.; Chen, D. A minireview of hydroamination catalysis: Alkene and alkyne substrate selective, metal complex design. BMC Chem. 2019, 13, 89. [Google Scholar] [CrossRef]
- Chen, C.; Xi, C.; Lai, C.; Wang, R.; Hong, X. Coupling Reactions of 1,4-Dicuprio-1,3-dienes: Formation of Carbocycles. Eur. J. Org. Chem. 2004, 2004, 647–650. [Google Scholar] [CrossRef]
- Tzankova, D.; Vladimirova, S.; Aluani, D.; Yordanov, Y.; Peikova, L.; Georgieva, M. Synthesis, in vitro safety and antioxidant activity of new pyrrole hydrazones. Acta Pharm. 2020, 70, 303–324. [Google Scholar] [CrossRef]
- Feinstein, W.P.; Brylinski, M. Calculating an optimal box size for ligand docking and virtual screening against experimental and predicted binding pockets. J. Cheminform. 2015, 7, 18. [Google Scholar] [CrossRef]
- Binde, C.D.; Tvete, I.F.; Gåsemyr, J.; Natvig, B.; Klemp, M. A multiple treatment comparison meta-analysis of monoamine oxidase type B inhibitors for Parkinson’s disease. Br. J. Clin. Pharmacol. 2018, 84, 1917–1927. [Google Scholar] [CrossRef]
- Binde, C.D.; Tvete, I.F.; Gåsemyr, J.I.; Natvig, B.; Klemp, M. Comparative effectiveness of dopamine agonists and monoamine oxidase type-B inhibitors for Parkinson’s disease: A multiple treatment comparison meta-analysis. Eur. J. Clin. Pharmacol. 2020, 76, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Tvete, I.F.; Klemp, M. Parkinson’s disease, treatment choice and survival over time. Clin. Park. Relat. Disord. 2022, 6, 100136. [Google Scholar] [CrossRef] [PubMed]
- Binde, C.D.; Tvete, I.F.; Klemp, M. Time until Need for Levodopa among New Users of Dopamine Agonists or MAO-B Inhibitors. Park. Dis. 2021, 2021, 9952743. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, S.; Alfayez, L.; Alkhudhair, M. Parkinson’s Disease: Current Treatment Modalities and Emerging Therapies. Cureus 2024, 16, e75647. [Google Scholar] [CrossRef]
- Kedia, S.; Fertan, E.; Wu, Y.; Zhang, Y.P.; Meisl, G.; Lam, J.Y.; Wiseman, F.K.; McEwan, W.A.; Quaegebeur, A.; Spillantini, M.G.; et al. SynPull: An advanced method for studying neurodegeneration-related aggregates in synaptosomes using super-resolution microscopy. Cell Chem. Biol. 2025, 32, 338–351.e4. [Google Scholar] [CrossRef]
- Klemmensen, M.M.; Borrowman, S.H.; Pearce, C.; Pyles, B.; Chandra, B. Mitochondrial dysfunction in neurodegenerative disorders. Neurotherapeutics 2024, 21, e00292. [Google Scholar] [CrossRef]
- Garcia-Ruiz, P.J.; Martinez Castrillo, J.C.; Alonso-Canovas, A.; Barcenas, A.H.; Vela, L.; Alonso, P.S.; Mata, M.; Gonzalez, N.O.; Fernandez, I.M. Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: A multicentre study. J. Neurol. Neurosurg. Psychiatry 2014, 85, 840–844. [Google Scholar] [CrossRef]
- Rabinak, C.A.; Nirenberg, M.J. Dopamine agonist withdrawal syndrome in Parkinson disease. Arch. Neurol. 2010, 67, 58–63. [Google Scholar] [CrossRef]
- Pondal, M.; Marras, C.; Miyasaki, J.; Moro, E.; Armstrong, M.J.; Strafella, A.P.; Shah, B.B.; Fox, S.; Prashanth, L.K.; Phielipp, N.; et al. Clinical features of dopamine agonist withdrawal syndrome in a movement disorders clinic. J. Neurol. Neurosurg. Psychiatry 2013, 84, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Turcano, P.; Mielke, M.M.; Bower, J.H.; Parisi, J.E.; Cutsforth-Gregory, J.K.; Ahlskog, J.E.; Savica, R. Levodopa-induced dyskinesia in Parkinson disease: A population-based cohort study. Neurology 2018, 91, e2238–e2243. [Google Scholar] [CrossRef]
- Fowler, C.J.; Benedetti, M.S. The metabolism of dopamine by both forms of monoamine oxidase in the rat brain and its inhibition by cimoxatone. J. Neurochem. 1983, 40, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Brannan, T.; Prikhojan, A.; Martinez-Tica, J.; Yahr, M.D. In vivo comparison of the effects of inhibition of MAO-A versus MAO-B on striatal L-DOPA and dopamine metabolism. J. Neural. Transm. Park Dis. Dement. Sect. 1995, 10, 79–89. [Google Scholar] [CrossRef]
- Nam, M.-H.; Sa, M.; Ju, Y.H.; Park, M.G.; Lee, C.J. Revisiting the Role of Astrocytic MAOB in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 4453. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Drahota, Z.; Krivakova, P.; Cervinkova, Z.; Kmonickova, E.; Lotkova, H.; Kucera, O.; Houstek, J. Tert-butyl hydroperoxide selectively inhibits mitochondrial respiratory-chain enzymes in isolated rat hepatocytes. Physiol. Res. 2005, 54, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Öllinger, K.; Brunk, U.T. Cellular injury induced by oxidative stress is mediated through lysosomal damage. Free. Radic. Biol. Med. 1995, 19, 565–574. [Google Scholar] [CrossRef]
- O’Donnell, V.; Burkitt, M.J. Mitochondrial metabolism of a hydroperoxide to free radicals in human endothelial cells: An electron spin resonance spin-trapping investigation. Biochem. J. 1994, 304, 707–713. [Google Scholar] [CrossRef]
- Zaidi, S.I.; Agarwal, R.; Eichler, G.; Rihter, B.D.; Kenney, M.E.; Mukhtar, H. Photodynamic effects of new silicon phthalocyanines: In vitro studies utilizing rat hepatic microsomes and human erythrocyte ghosts as model membrane sources. Photochem. Photobiol. 1993, 58, 204–210. [Google Scholar] [CrossRef]
- Bijev, A. New heterocyclic hydrazones in the search for antitubercular agents: Synthesis and in vitro evaluations. Lett. Drug Des. Dis. 2006, 3, 506–512. [Google Scholar] [CrossRef]
- Mateev, E.; Valkova, I.; Angelov, B.; Georgieva, M.; Zlatkov, A. Validation through re-docking, cross-docking and ligand enrichment in various well-resoluted mao-b receptors. Int. J. Pharmaceut. Sci. Res. 2022, 13, 1099–1107. [Google Scholar]
- Taupin, P.; Zini, S.; Cesselin, F.; Ben-Ari, Y.; Roisin, M.P. Subcellular fractionation on Percoll gradient of mossy fiber synaptosomes: Morphological and biochemical characterization in control and degranulated rat hippocampus. J. Neurochem. 1994, 62, 1586–1595. [Google Scholar] [CrossRef]
- Stokes, A.H.; Freeman, W.M.; Mitchell, S.G.; Burnette, T.A.; Hellmann, G.M.; Vrana, K.E. Induction of GADD45 and GADD153 in neuroblastoma cells by dopamine-induced toxicity. Neurotoxicology 2002, 23, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Mungarro-Menchaca, X.; Ferrera, P.; Morán, J.; Arias, C. β-Amyloid peptide induces ultrastructural changes in synaptosomes and potentiates mitochondrial dysfunction in the presence of ryanodine. J. Neurosci. Res. 2002, 68, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Robyt, J.F.; Ackerman, R.J.; Chittenden, C.G. Reaction of protein disulfide groups with Ellman’s reagent: A case study of the number of sulfhydryl and disulfide groups in Aspergillus oryzae α-amylase, papain, and lysozyme. Arch. Biochem. Biophys. 1971, 147, 262–269. [Google Scholar] [CrossRef]
- Karlsson, J.; Emgard, M.; Brundin, P.; Burkitt, M.J. trans-Resveratrol protects embryonic mesencephalic cells from tert-butyl hydroperoxide: Electron paramagnetic resonance spin trapping evidence for a radical scavenging mechanism. J. Neurochem. 2000, 75, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Shirani, M.; Alizadeh, S.; Mahdavinia, M.; Dehghani, M.A. Th ameliorative effect of quercetin on bisphenol A-induced toxicity in mitochondria isolated from rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 7688–7696. [Google Scholar] [CrossRef]
- Ravindranath, V.; Anandatheerthavarada, H.K. Preparation of brain microsomes with cytochrome P450 activity using calcium aggregation method. Anal. Biochem. 1990, 187, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, D.; Sassi, A.; Dansette, P.M.; Plat, M. A new potent inhibitor of lipid peroxidation in vitro and in vivo, the hepatoprotective drug anisyldithiolthione. Biochem. Biophys. Res. Commun. 1986, 135, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Aguilera, O.M.; Esteban, G.; Bolea, I.; Nikolic, K.; Agbaba, D.; Moraleda, I.; Iriepa, I.; Samadi, A.; Soriano, E.; Unzeta, M.; et al. Design, synthesis, pharmacological evaluation, QSAR analysis, molecular modeling and ADMET of novel donepezil-indolyl hybrids as multipotent cholinesterase/monoamine oxidase inhibitors for the potential treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 21, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Churchill Livingstone (Elsevier Health Sciences): Edinburgh, UK, 2008; pp. 174–186. [Google Scholar]
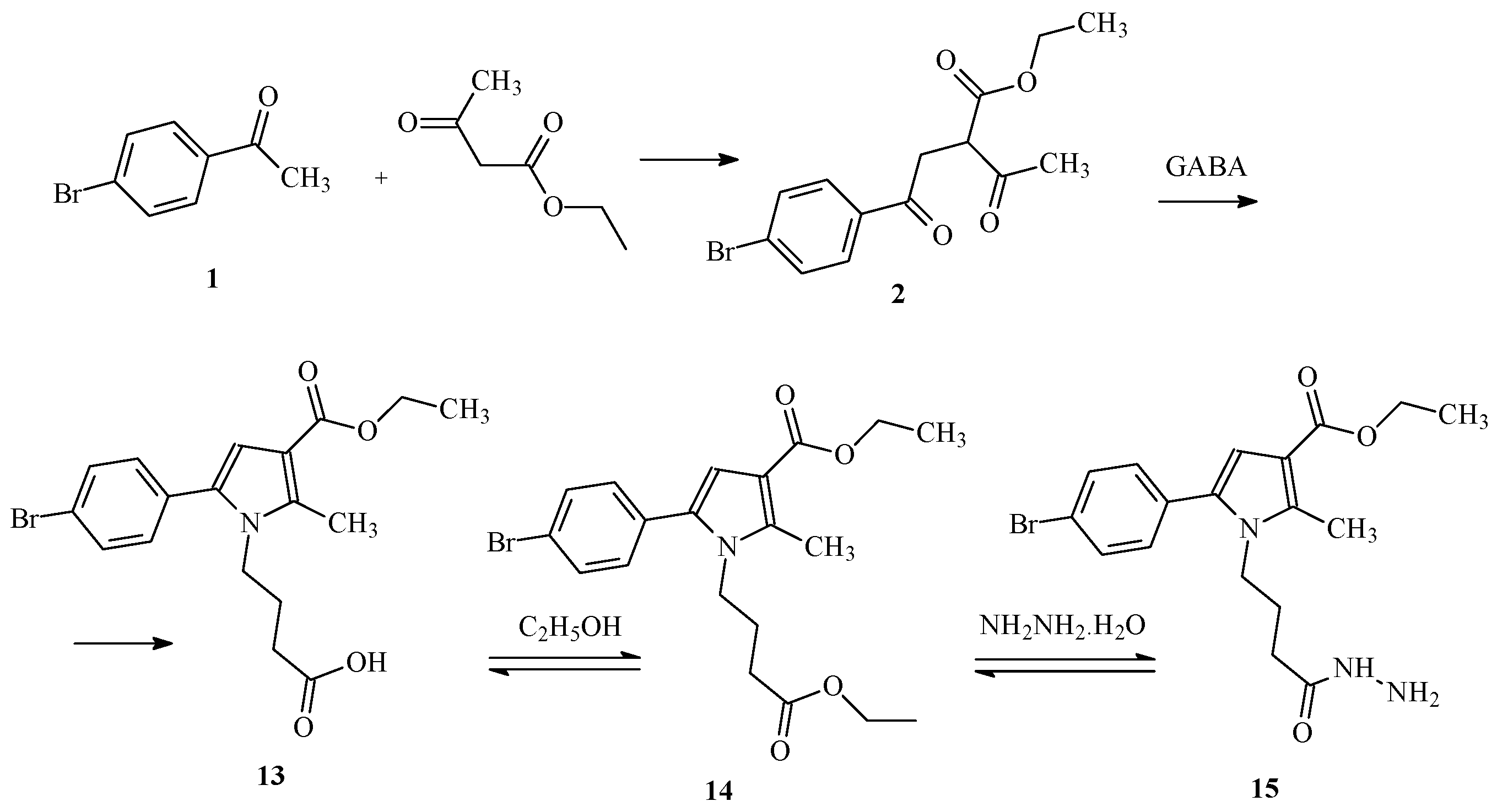
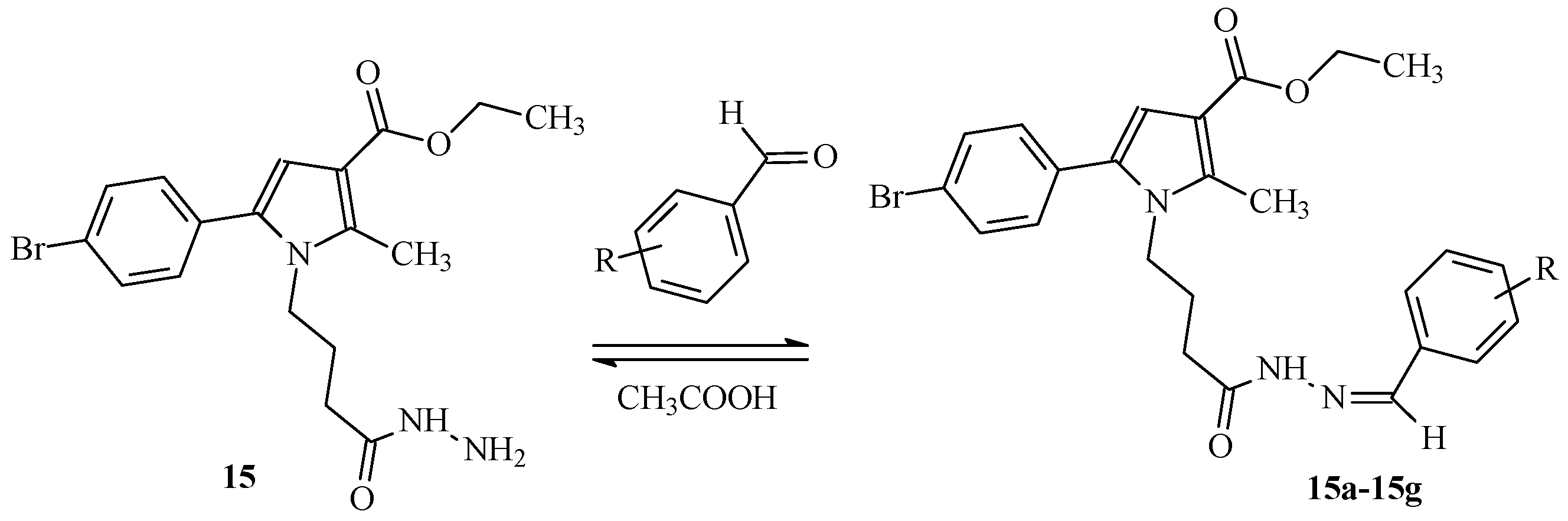
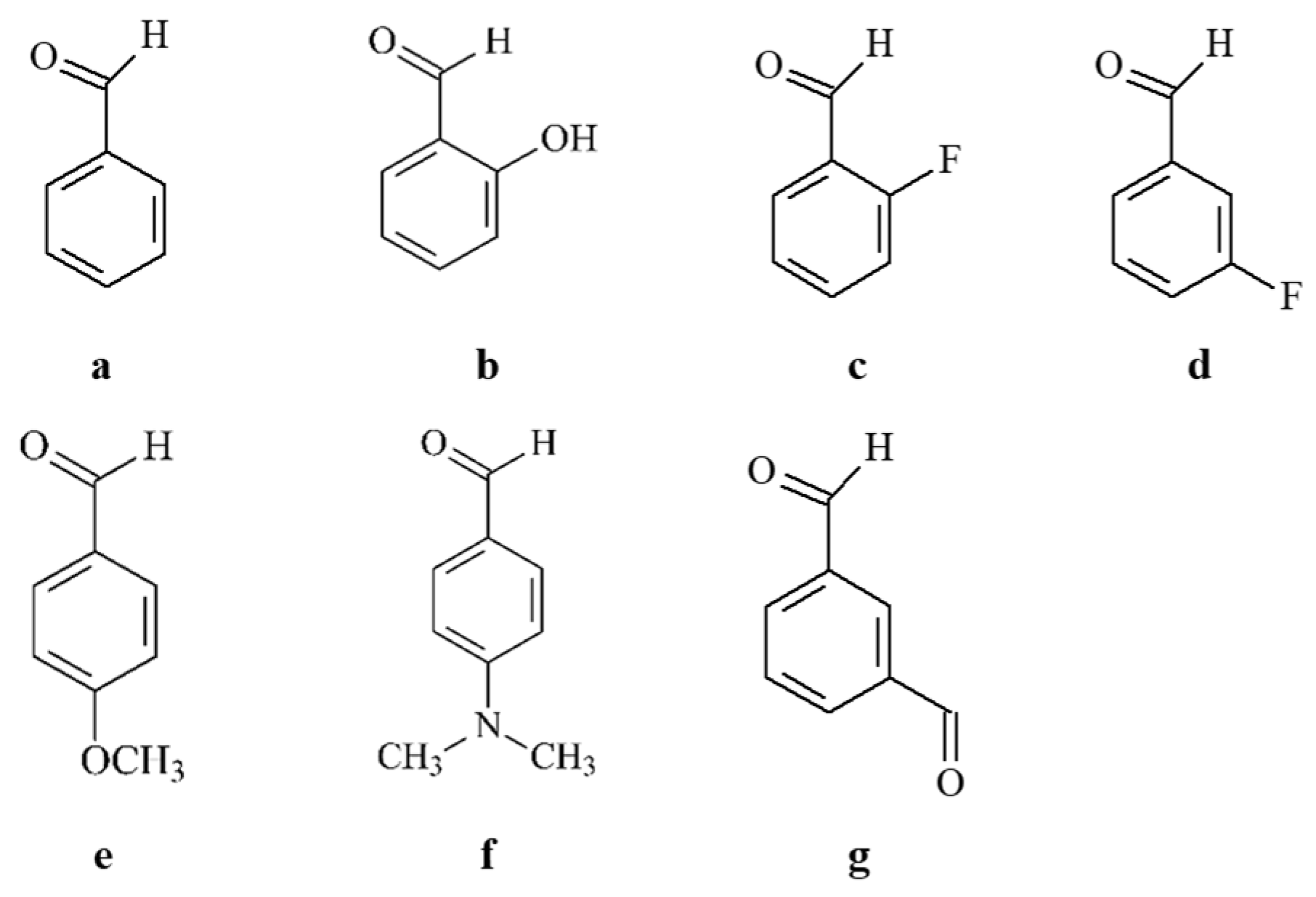


| IDs | m.p. | Rf | MS Data [M + H]+ (m/z) | Yields% |
|---|---|---|---|---|
| 13 | 93.4–94.8 °C | 0.83 | 395.26 | 88 |
| 15 | 99.8–101.5 °C | 0.56 | 409.30 | 81 |
| 15a | 110.0–110.9 °C | 0.50 | 497.40 | 83 |
| 15b | 119.5–120.3 °C | 0.53 | 513.40 | 87 |
| 15c | 141.9–143.2 °C | 0.60 | 515.40 | 89 |
| 15d | 134.1–135.9 °C | 0.60 | 515.40 | 90 |
| 15e | 133.0–134.4 °C | 0.56 | 527.43 | 73 |
| 15f | 131.7–132.9 °C | 0.58 | 532.36 | 69 |
| 15g | 136.6–137.4 °C | 0.60 | 540.47 | 55 |
| Compounds | IC50 (EC50), (µM ± SD) hMAOA | IC50 (EC50), (µM ± SD) hMAOB | SI |
|---|---|---|---|
| 15a | >200 | 0.692 | >289 |
| 15d | >200 | 0.425 | >471 |
| 15f | 0.821 | 0.710 | 1.16 |
| Selegiline | - | 0.320 ± 0.20 | |
| Chlorgyline | 18.74 ± 0.096 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, M.; Sharkov, M.; Mateev, E.; Tzankova, D.; Popov, G.; Manov, V.; Zlatkov, A.; Simeonova, R.; Kondeva-Burdina, M. Classical Paal-Knorr Cyclization for Synthesis of Pyrrole-Based Aryl Hydrazones and In Vitro/In Vivo Evaluation on Pharmacological Models of Parkinson’s Disease. Molecules 2025, 30, 3154. https://doi.org/10.3390/molecules30153154
Georgieva M, Sharkov M, Mateev E, Tzankova D, Popov G, Manov V, Zlatkov A, Simeonova R, Kondeva-Burdina M. Classical Paal-Knorr Cyclization for Synthesis of Pyrrole-Based Aryl Hydrazones and In Vitro/In Vivo Evaluation on Pharmacological Models of Parkinson’s Disease. Molecules. 2025; 30(15):3154. https://doi.org/10.3390/molecules30153154
Chicago/Turabian StyleGeorgieva, Maya, Martin Sharkov, Emilio Mateev, Diana Tzankova, Georgi Popov, Vasil Manov, Alexander Zlatkov, Rumyana Simeonova, and Magdalena Kondeva-Burdina. 2025. "Classical Paal-Knorr Cyclization for Synthesis of Pyrrole-Based Aryl Hydrazones and In Vitro/In Vivo Evaluation on Pharmacological Models of Parkinson’s Disease" Molecules 30, no. 15: 3154. https://doi.org/10.3390/molecules30153154
APA StyleGeorgieva, M., Sharkov, M., Mateev, E., Tzankova, D., Popov, G., Manov, V., Zlatkov, A., Simeonova, R., & Kondeva-Burdina, M. (2025). Classical Paal-Knorr Cyclization for Synthesis of Pyrrole-Based Aryl Hydrazones and In Vitro/In Vivo Evaluation on Pharmacological Models of Parkinson’s Disease. Molecules, 30(15), 3154. https://doi.org/10.3390/molecules30153154







