Mechanochemical Preparation of Biomass-Derived Porous Carbons
Abstract
1. Introduction
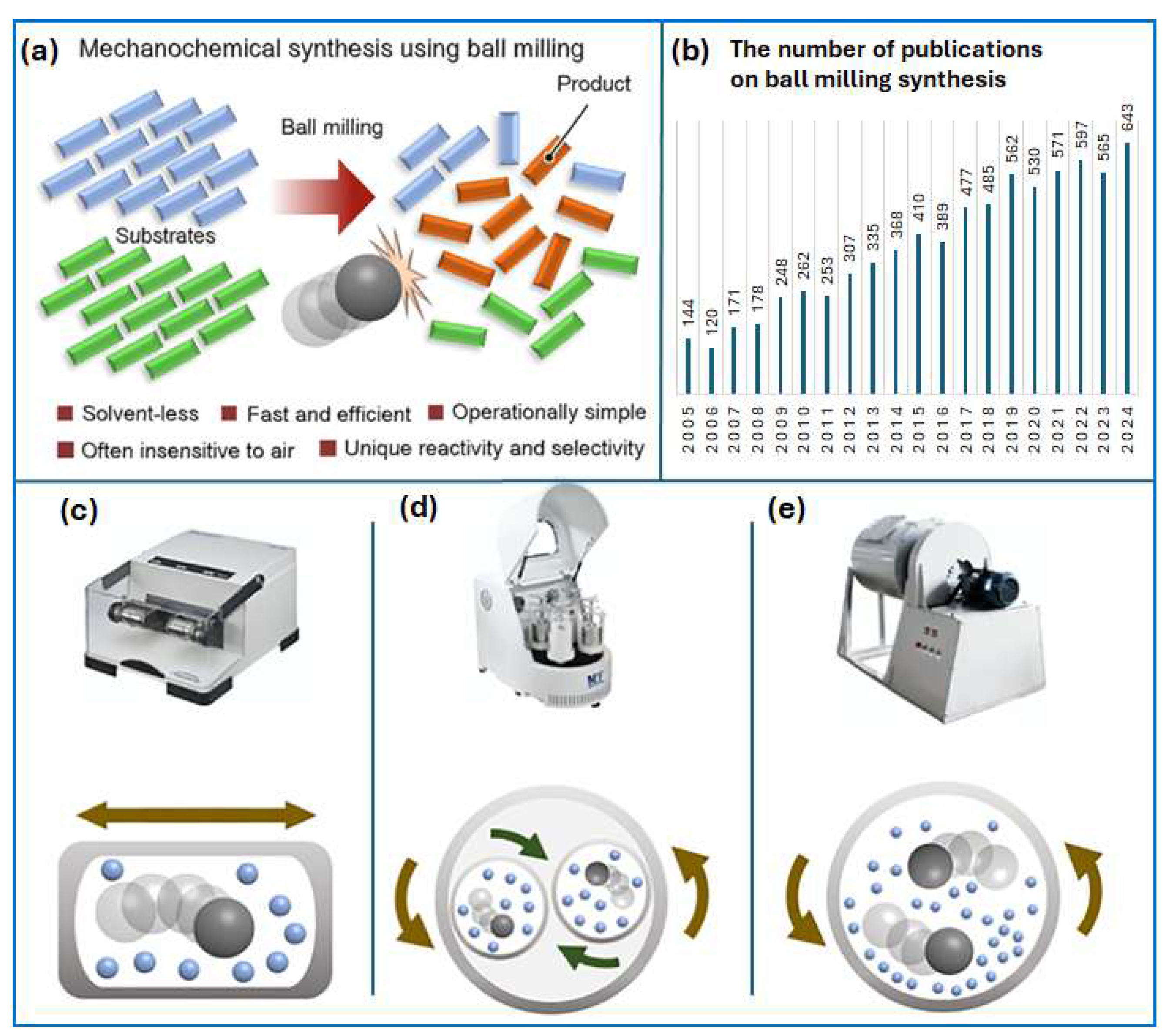
2. Mechanochemical Synthesis
2.1. History of Mechanochemistry
2.2. Fundamentals of Mechanochemistry
2.3. The Importance of Controlling the Mechanochemical Process
2.4. Scalability of a Mechanochemical Process
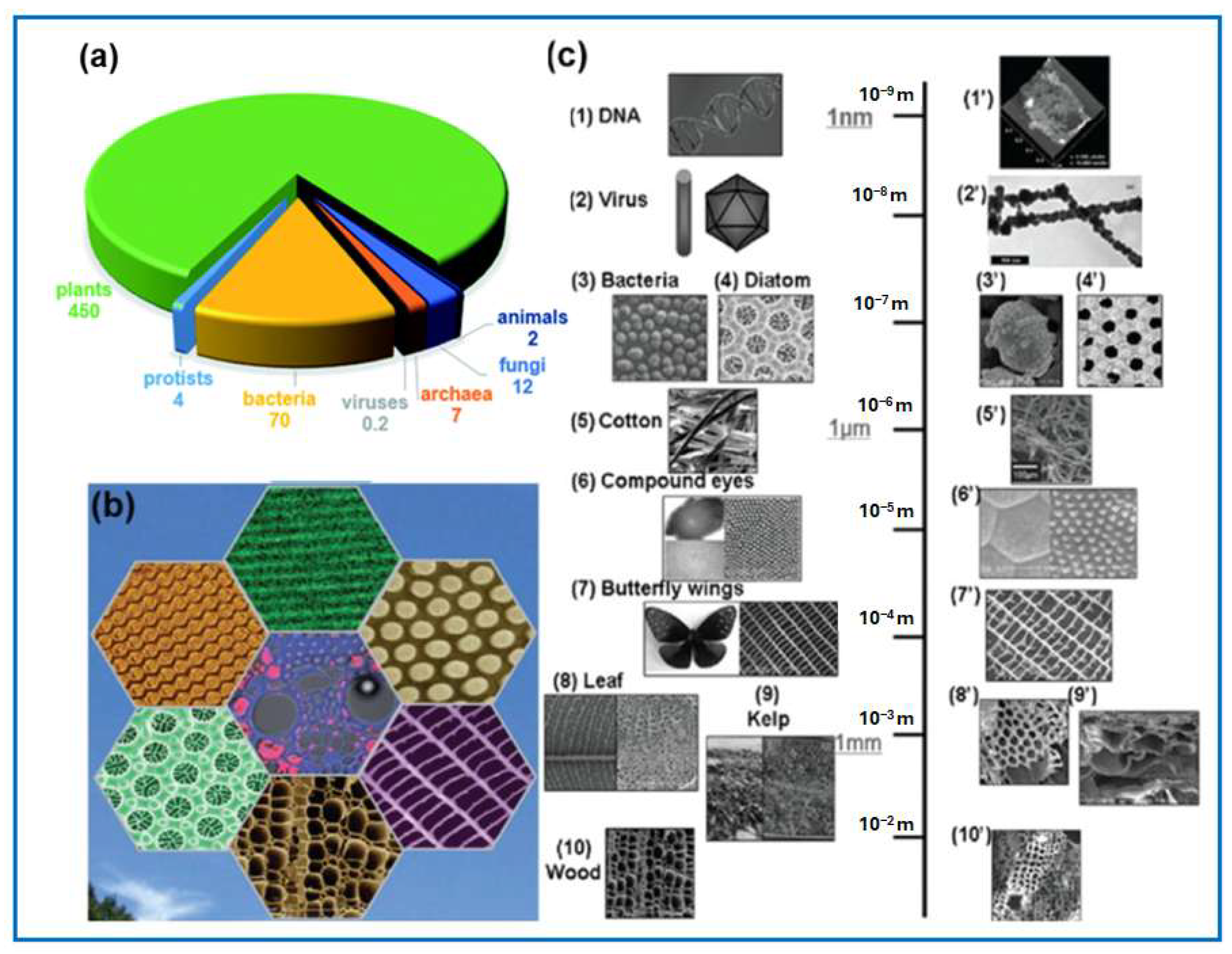
3. Biomass
3.1. Main Types of Biomass
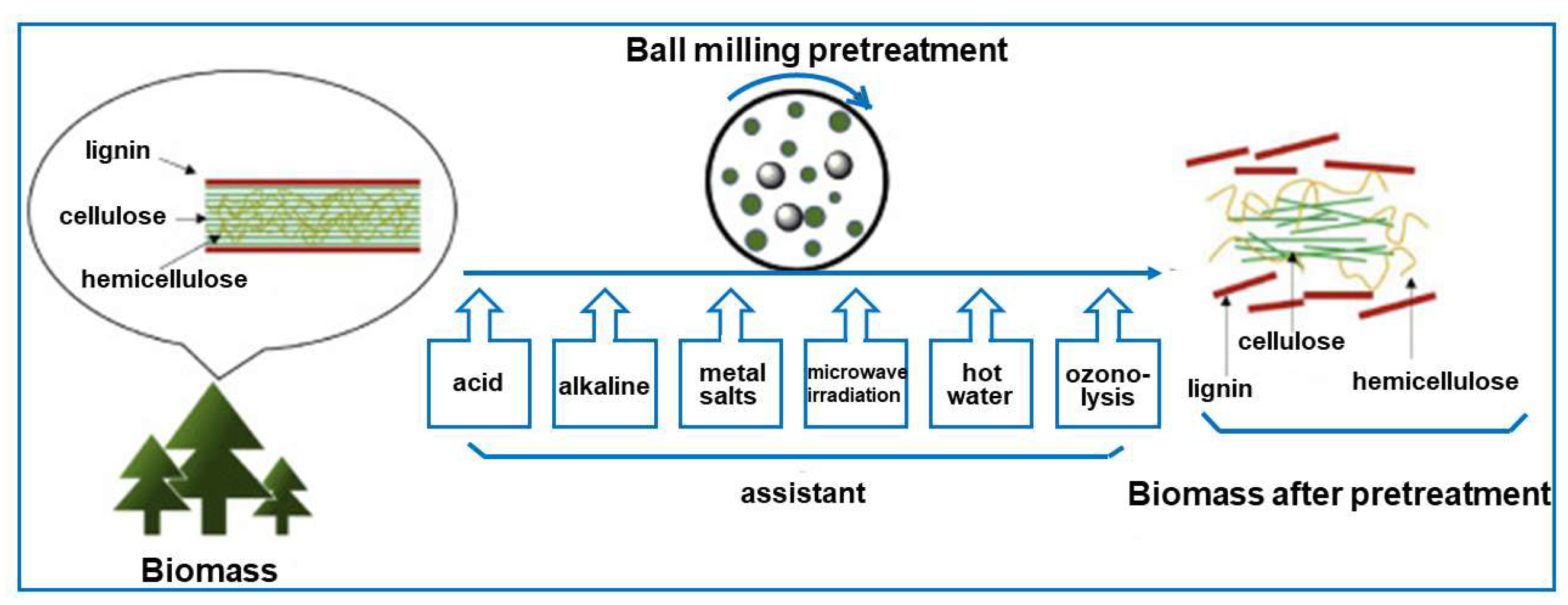
3.2. Biomass Pretreatment
4. Conversion of Biomass Waste into Porous Carbons
5. Mechanochemical Synthesis of Biomass-Derived Carbons
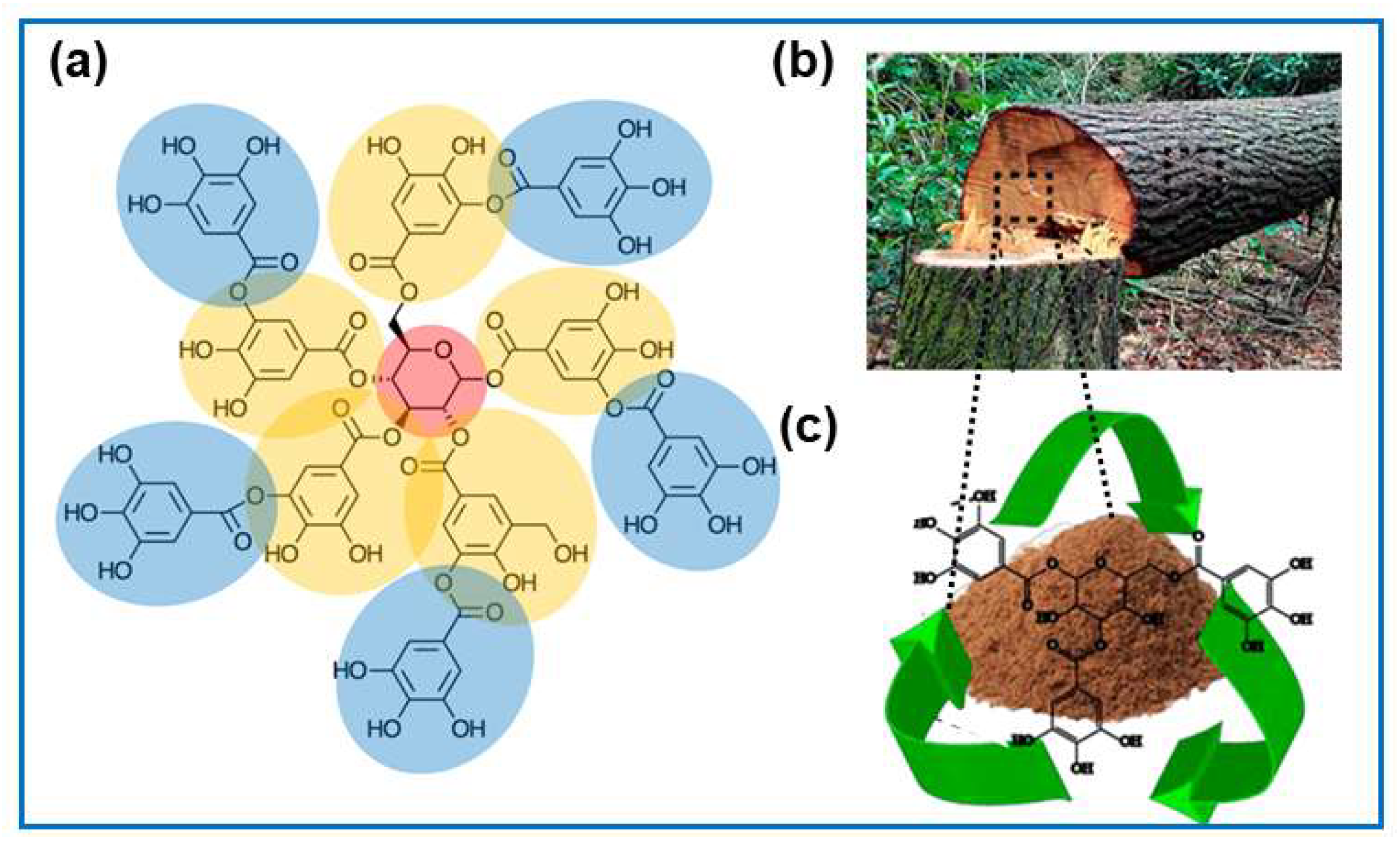
5.1. Tannin-Derived Porous Carbons
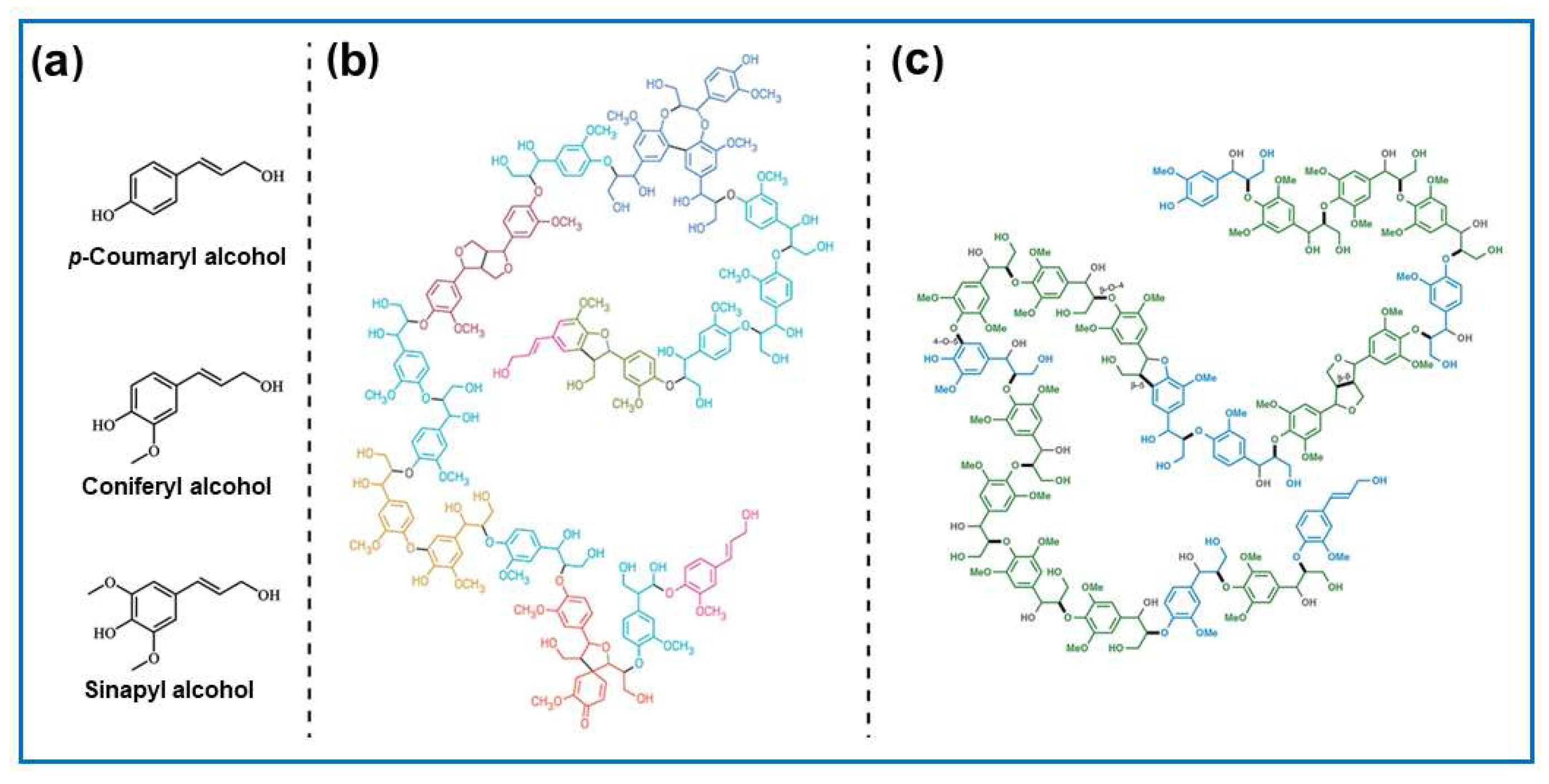
5.2. Lignocellulosic Biomass-Derived Carbons
5.3. Biomass Waste-Derived Carbons
6. Applications of Biomass-Derived Carbons
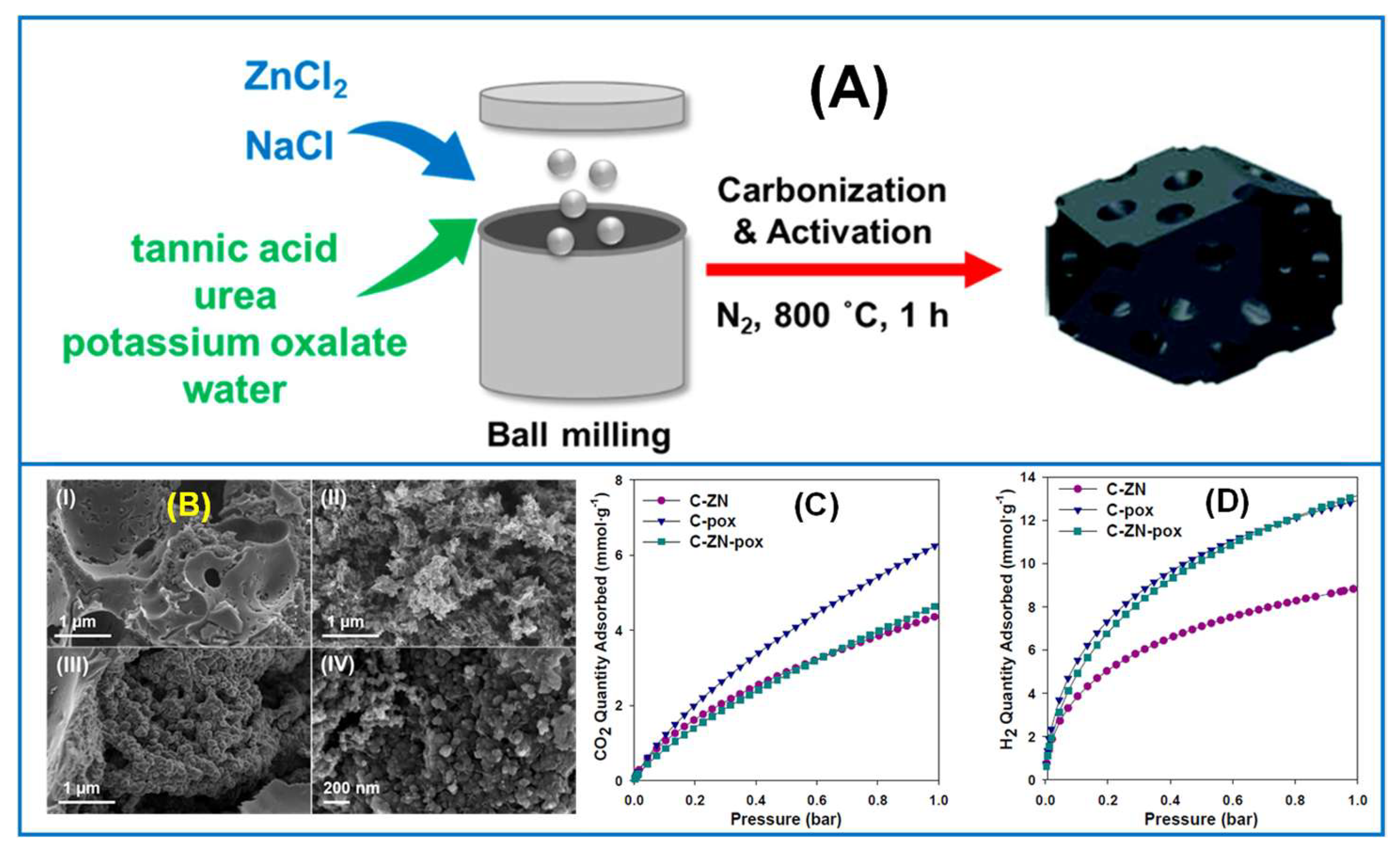
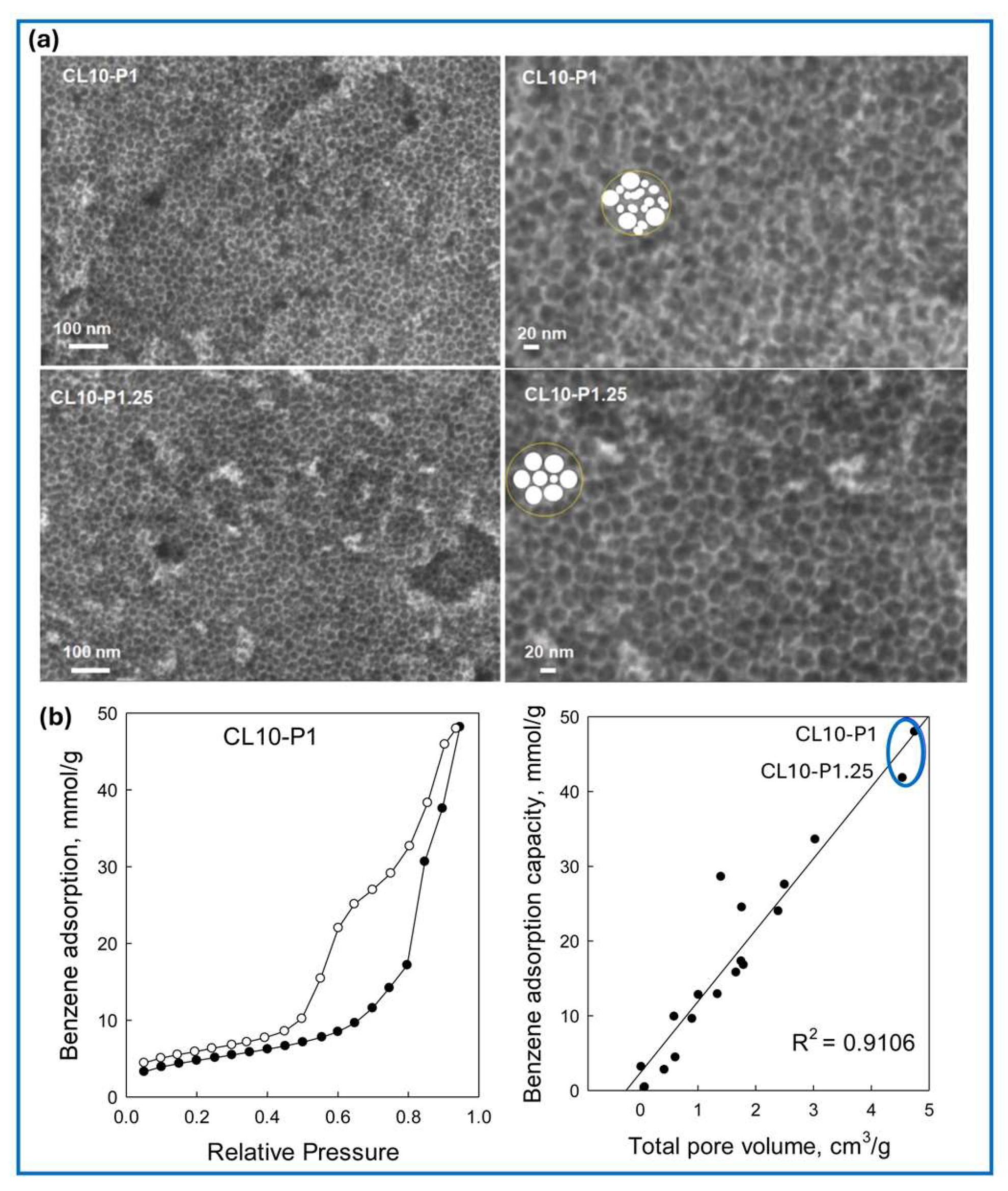
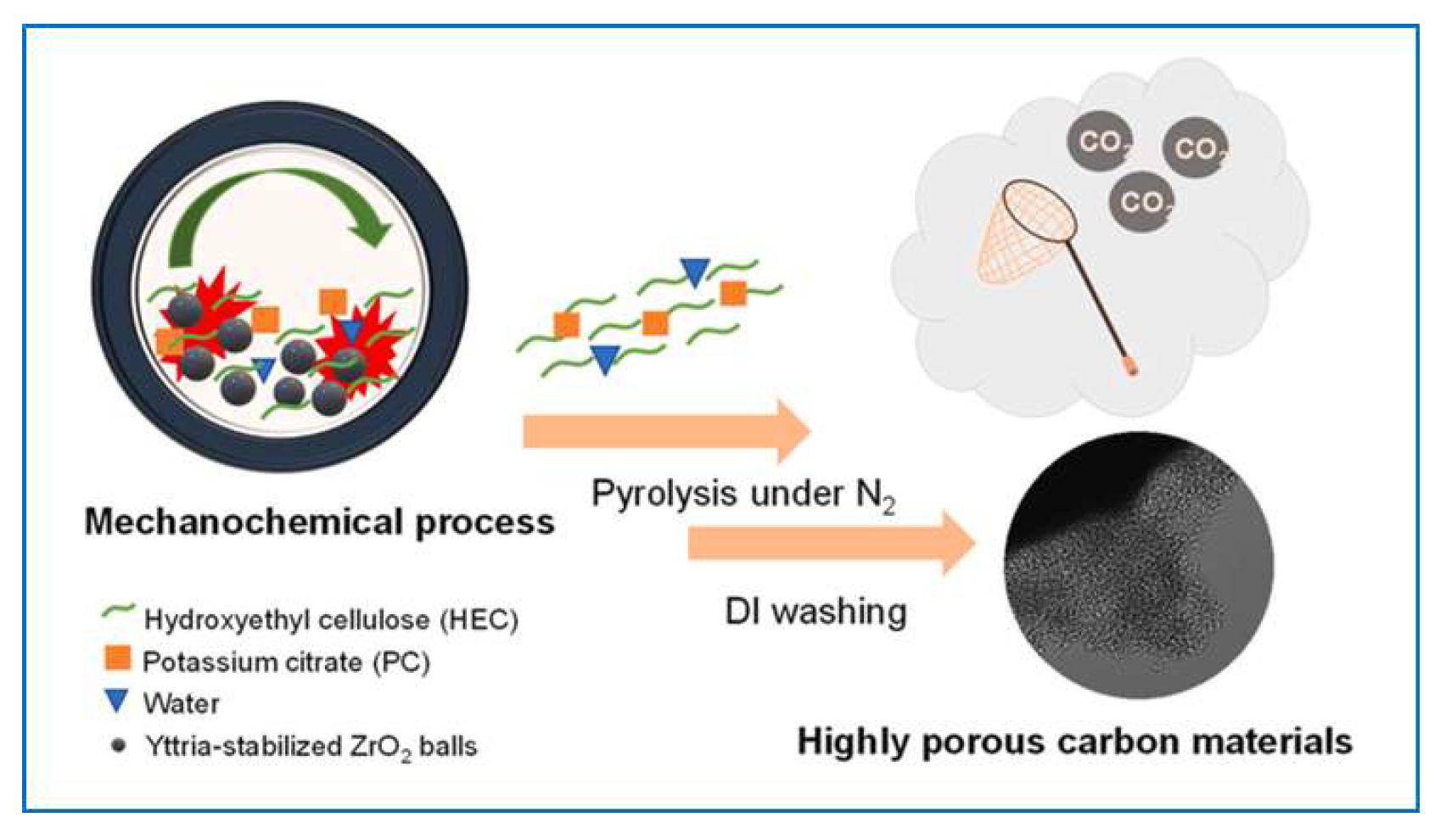
6.1. Gas Adsorption on Mechanochemically Synthesized Carbons
6.2. Catalysis
6.3. Electrochemical and Energy-Related Applications
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kubota, K.; Ito, H. Mechanochemical Cross-Coupling Reactions. Trends Chem. 2020, 2, 1066–1081. [Google Scholar] [CrossRef]
- Zhao, L.-Y.; Dong, X.-L.; Lu, A.-H. Mechanochemical Synthesis of Porous Carbons and Their Applications in Catalysis. Chempluschem 2020, 85, 866–875. [Google Scholar] [CrossRef]
- Amrute, A.P.; Łodziana, Z.; Schreyer, H.; Weidenthaler, C.; Schüth, F. High-Surface-Area Corundum by Mechanochemically Induced Phase Transformation of Boehmite. Science 2019, 366, 485–489. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for New and Cleaner Synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.L.; Cao, Q.; Browne, D.L. Mechanochemistry as an Emerging Tool for Molecular Synthesis: What Can It Offer? Chem. Sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Xiong, X.; Fu, J.; Yang, J.; Qiu, M.; Qi, X.; Tsang, D.C.W. Recent Advances in Mechanochemical Production of Chemicals and Carbon Materials from Sustainable Biomass Resources. Renew. Sustain. Energy Rev. 2020, 130, 109944. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy Storage Applications of Activated Carbons: Supercapacitors and Hydrogen Storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef]
- Pavlenko, V.; Khosravi H, S.; Żółtowska, S.; Haruna, A.B.; Zahid, M.; Mansurov, Z.; Supiyeva, Z.; Galal, A.; Ozoemena, K.I.; Abbas, Q.; et al. A Comprehensive Review of Template-Assisted Porous Carbons: Modern Preparation Methods and Advanced Applications. Mater. Sci. Eng. R Rep. 2022, 149, 100682. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Jastrzębski, K.; Kula, P. Emerging Technology for a Green, Sustainable Energy-Promising Materials for Hydrogen Storage, from Nanotubes to Graphene—A Review. Materials 2021, 14, 2499. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Phuriragpitikhon, J.; Jaroniec, M. Recent Advances in the Development and Applications of Biomass-Derived Carbons with Uniform Porosity. J. Mater. Chem. A 2020, 8, 18464–18491. [Google Scholar] [CrossRef]
- Cheng, B.; Wan, L.; Du, C.; Ye, H.; Tian, Z.; Xie, M. Reduced Graphene Composited N-Containing Polymer Engineered by Ball Milling as Electrode for High-Volumetric-Performance Supercapacitor. J. Energy Storage 2024, 99, 113259. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and Sustainable Manufacture of Chemicals from Biomass: State of the Art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Adams, P.; Bridgwater, T.; Lea-Langton, A.; Ross, A.; Watson, I. Chapter 8—Biomass Conversion Technologies. In Greenhouse Gas Balances of Bioenergy Systems; Thornley, P., Adams, P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 107–139. ISBN 978-0-08-101036-5. [Google Scholar]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic Biomass Pyrolysis Mechanism: A State-of-the-Art Review. Prog. Energy Combust Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Chaubey, A.K.; Pratap, T.; Preetiva, B.; Patel, M.; Singsit, J.S.; Pittman, C.U., Jr.; Mohan, D. Definitive Review of Nanobiochar. ACS Omega 2024, 9, 12331–12379. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical Synthesis of Advanced Nanomaterials for Catalytic Applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef]
- Takacs, L. The Historical Development of Mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef]
- Agricola, G. De Re Metallica; Hoover, H.C.; Hoover, L.H., Translators; Dover Publications, Inc.: Garden City, NY, USA, 1912. [Google Scholar]
- Bacon, F.; Rawley, W. Opuscula Varia Posthuma, Philosophica, Civilia, et Theologica (1663); Daniel, E.R., Pulleyn, I.O., Eds.; Apud Johannem Ravesteinium: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Horie, K.; Máximo, B.; Fox, R.B.; He, J.; Hess, M.; Kahovec, J.; Kitayama, T.; Kubisa, P.; Maréchal, E.; Mormann, W.; et al. Definitions of terms relating to reactions of polymers and to functional polymeric materials (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76, 889–906. [Google Scholar] [CrossRef]
- Michalchuk, A.A.L.; Tumanov, I.A.; Drebushchak, V.A.; Boldyreva, E. V Advances in Elucidating Mechanochemical Complexities via Implementation of a Simple Organic System. Faraday Discuss. 2014, 170, 311–335. [Google Scholar] [CrossRef]
- Noorduin, W.L.; Izumi, T.; Millemaggi, A.; Leeman, M.; Meekes, H.; Van Enckevort, W.J.P.; Kellogg, R.M.; Kaptein, B.; Vlieg, E.; Blackmond, D.G. Emergence of a Single Solid Chiral State from a Nearly Racemic Amino Acid Derivative. J. Am. Chem. Soc. 2008, 130, 1158–1159. [Google Scholar] [CrossRef]
- Kumar, N.; Biswas, K. Fabrication of Novel Cryomill for Synthesis of High Purity Metallic Nanoparticles. Rev. Sci. Instrum. 2015, 86, 083903. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of Mechanochemistry: From Nanoparticles to Technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Stolle, A.; Schmidt, R.; Jacob, K. Scale-up of Organic Reactions in Ball Mills: Process Intensification with Regard to Energy Efficiency and Economy of Scale. Faraday Discuss. 2014, 170, 267–286. [Google Scholar] [CrossRef]
- Crawford, D.E.; Miskimmin, C.K.G.; Albadarin, A.B.; Walker, G.; James, S.L. Organic Synthesis by Twin Screw Extrusion (TSE): Continuous, Scalable and Solvent-Free. Green Chem. 2017, 19, 1507–1518. [Google Scholar] [CrossRef]
- Hajiali, F.; Jin, T.; Yang, G.; Santos, M.; Lam, E.; Moores, A. Mechanochemical Transformations of Biomass into Functional Materials. ChemSusChem 2022, 15, e202102535. [Google Scholar] [CrossRef]
- Lomovskiy, I.; Bychkov, A.; Lomovsky, O.; Skripkina, T. Mechanochemical and Size Reduction Machines for Biorefining. Molecules 2020, 25, 5345. [Google Scholar] [CrossRef]
- Kuga, S.; Wu, M. Mechanochemistry of Cellulose. Cellulose 2019, 26, 215–225. [Google Scholar] [CrossRef]
- Tursi, A. A Review on Biomass: Importance, Chemistry, Classification, and Conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Zhong, Y.; Shen, T.; Wang, X.; Xia, X.; Tu, J. Natural Biomass-Derived Carbons for Electrochemical Energy Storage. Mater. Res. Bull. 2017, 88, 234–241. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, Y.; Song, N.; Li, X. Biomass-Derived Renewable Carbon Materials for Electrochemical Energy Storage. Mater. Res. Lett. 2017, 5, 69–88. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The Biomass Distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yu, S.-H.; Wang, K.; Liu, L.; Xu, X.-W. Functional Carbonaceous Materials from Hydrothermal Carbonization of Biomass: An Effective Chemical Process. Dalton Trans. 2008, 40, 5414–5423. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Balu, A.M.; van der Waal, J.C.; Luque, R. Biomass-Derived Porous Carbon Materials: Synthesis and Catalytic Applications. ChemCatChem 2015, 7, 1608–1629. [Google Scholar] [CrossRef]
- Hemingway, R.W. Practical Polyphenolics: From Structure to Molecular Recognition and Physiological Action By Edwin Haslam (University of Sheffield). Cambridge University Press, New York, NY. 1998. Xv + 422 Pp. 17 × 24.5 Cm. $100.00. J. Nat. Prod. 1998, 61, 1454–1455. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Jiao, Y.; Katahira, R.; Ciesielski, P.N.; Himmel, M.; Zhu, H. Heavy Metal-Free Tannin from Bark for Sustainable Energy Storage. Nano Lett. 2017, 17, 7897–7907. [Google Scholar] [CrossRef]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in Nutrient Dynamics of Forest Ecosystems—A Review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Lemieux, J.; Bélanger, D.; Santato, C. Toward Biosourced Materials for Electrochemical Energy Storage: The Case of Tannins. ACS Sustain. Chem. Eng. 2021, 9, 6079–6086. [Google Scholar] [CrossRef]
- Shen, X.; Xin, Y.; Liu, H.; Han, B. Product-Oriented Direct Cleavage of Chemical Linkages in Lignin. ChemSusChem 2020, 13, 4367–4381. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin Depolymerisation Strategies: Towards Valuable Chemicals and Fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Cravotto, G.; Manzoli, M.; Tabasso, S. Sono- and Mechanochemical Technologies in the Catalytic Conversion of Biomass. Chem. Soc. Rev. 2021, 50, 1785–1812. [Google Scholar] [CrossRef]
- Ali, W.A.; Richards, S.E.; Alzard, R.H. Unlocking the Potential of Ball Milling for Nanomaterial Synthesis: An Overview. J. Ind. Eng. Chem. 2025, 149, 63–69. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Subhedar, A.; Bhadauria, S.; Ahankari, S.; Kargarzadeh, H. Nanocellulose in Biomedical and Biosensing Applications: A Review. Int. J. Biol. Macromol. 2021, 166, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Lasrado, D.; Ahankari, S.; Kar, K. Nanocellulose-Based Polymer Composites for Energy Applications—A Review. J. Appl. Polym. Sci. 2020, 137, 48959. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant. Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Kumar, A.; Rapoport, A.; Kunze, G.; Kumar, S.; Singh, D.; Singh, B. Multifarious Pretreatment Strategies for the Lignocellulosic Substrates for the Generation of Renewable and Sustainable Biofuels: A Review. Renew. Energy 2020, 160, 1228–1252. [Google Scholar] [CrossRef]
- Hu, G.; Yu, W. Effect of Hemicellulose from Rice Bran on Low Fat Meatballs Chemical and Functional Properties. Food Chem. 2015, 186, 239–243. [Google Scholar] [CrossRef]
- Rath, S.S.; Sahoo, H. A Review on the Application of Starch as Depressant in Iron Ore Flotation. Miner. Process. Extr. Metall. Rev. 2022, 43, 122–135. [Google Scholar] [CrossRef]
- Le Corre, D.; Bras, J.; Dufresne, A. Starch Nanoparticles: A Review. Biomacromolecules 2010, 11, 1139–1153. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment Technologies for an Efficient Bioethanol Production Process Based on Enzymatic Hydrolysis: A Review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Putro, J.N.; Soetaredjo, F.E.; Lin, S.-Y.; Ju, Y.-H.; Ismadji, S. Pretreatment and Conversion of Lignocellulose Biomass into Valuable Chemicals. RSC Adv. 2016, 6, 46834–46852. [Google Scholar] [CrossRef]
- Barakat, A.; Monlau, F.; Solhy, A.; Carrere, H. Mechanical Dissociation and Fragmentation of Lignocellulosic Biomass: Effect of Initial Moisture, Biochemical and Structural Proprieties on Energy Requirement. Appl. Energy 2015, 142, 240–246. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Fujimoto, S.; Hirata, S.; Hassan, M.A. Ball Milling Pretreatment of Oil Palm Biomass for Enhancing Enzymatic Hydrolysis. Appl. Biochem. Biotechnol. 2014, 173, 1778–1789. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.; Kim, J.; Mitchell, R.J.; Lee, J.H. Environmentally Friendly Pretreatment of Plant Biomass by Planetary and Attrition Milling. Bioresour. Technol. 2013, 144, 50–56. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, P.; Ye, J.; Wu, Y.; Liu, J.; Fang, W.; Xu, D.; Wang, B.; Yan, L.; Zeng, G. Comparison of Various Pretreatments for Ethanol Production Enhancement from Solid Residue after Rumen Fluid Digestion of Rice Straw. Bioresour. Technol. 2018, 247, 147–156. [Google Scholar] [CrossRef]
- Jiang, L.; Zheng, A.; Meng, J.; Wang, X.; Zhao, Z.; Li, H. A Comparative Investigation of Fast Pyrolysis with Enzymatic Hydrolysis for Fermentable Sugars Production from Cellulose. Bioresour. Technol. 2019, 274, 281–286. [Google Scholar] [CrossRef]
- Buaban, B.; Inoue, H.; Yano, S.; Tanapongpipat, S.; Ruanglek, V.; Champreda, V.; Pichyangkura, R.; Rengpipat, S.; Eurwilaichitr, L. Bioethanol Production from Ball Milled Bagasse Using an On-Site Produced Fungal Enzyme Cocktail and Xylose-Fermenting Pichia Stipitis. J. Biosci. Bioeng. 2010, 110, 18–25. [Google Scholar] [CrossRef]
- Real Pérez, C.; Alcalá González, M.D.; Romero Sarria, F.; Hidalgo López, M.d.C.; Córdoba Gallego, J.M. Investigating the Room- and Cryo-Milling Impact in Lignocellulosic Biomass and Its Consequence over Pyrolysis and Oxidative Treatments. J. Clean Prod. 2024, 437, 140761. [Google Scholar] [CrossRef]
- Avolio, R.; Bonadies, I.; Capitani, D.; Errico, M.E.; Gentile, G.; Avella, M. A Multitechnique Approach to Assess the Effect of Ball Milling on Cellulose. Carbohydr. Polym. 2012, 87, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kwon, J.H.; Kim, T.H.; Choi, W. Il Impact of Planetary Ball Mills on Corn Stover Characteristics and Enzymatic Digestibility Depending on Grinding Ball Properties. Bioresour. Technol. 2017, 241, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Singh, A.; Ashogbon, A.O.; Bobade, H. Ball-Milling: A Sustainable and Green Approach for Starch Modification. Int. J. Biol. Macromol. 2023, 237, 124069. [Google Scholar] [CrossRef] [PubMed]
- Monson, P.A. Understanding Adsorption/Desorption Hysteresis for Fluids in Mesoporous Materials Using Simple Molecular Models and Classical Density Functional Theory. Microporous Mesoporous Mater. 2012, 160, 47–66. [Google Scholar] [CrossRef]
- Balzer, C.; Waag, A.M.; Gehret, S.; Reichenauer, G.; Putz, F.; Hüsing, N.; Paris, O.; Bernstein, N.; Gor, G.Y.; Neimark, A. V Adsorption-Induced Deformation of Hierarchically Structured Mesoporous Silica—Effect of Pore-Level Anisotropy. Langmuir 2017, 33, 5592–5602. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F.; Lopez-Gonzalez, J.D.D.; Berenguer, C. Activated Carbons from Almond Shells—I: Preparation and Characterization by Nitrogen Adsorption. Carbon 1982, 20, 513–518. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Martín-Martínez, J.M.; Molina-Sabio, M.; Pérez-Lledó, I.; Prado-Burguete, C. A Comparison of the Porous Texture of Two CO2 Activated Botanic Materials. Carbon 1985, 23, 19–24. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Molina-Sabio, M.; González, M.T. The Use of Steam and CO2 as Activating Agents in the Preparation of Activated Carbons. Carbon 1995, 33, 15–23. [Google Scholar] [CrossRef]
- Bader, N.; Ouederni, A. Optimization of Biomass-Based Carbon Materials for Hydrogen Storage. J. Energy Storage 2016, 5, 77–84. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Rodríguez-Reinoso, F. Chemical versus Physical Activation of Coconut Shell: A Comparative Study. Microporous Mesoporous Mater. 2012, 152, 163–171. [Google Scholar] [CrossRef]
- Ello, A.S.; de Souza, L.K.C.; Trokourey, A.; Jaroniec, M. Coconut Shell-Based Microporous Carbons for CO2 Capture. Microporous Mesoporous Mater. 2013, 180, 280–283. [Google Scholar] [CrossRef]
- Saini, V.K.; Pinto, M.; Pires, J. Natural Clay Binder Based Extrudates of Mesoporous Materials: Improved Materials for Selective Adsorption of Natural and Biogas Components. Green Chem. 2011, 13, 1251–1259. [Google Scholar] [CrossRef]
- Ello, A.S.; de Souza, L.K.C.; Trokourey, A.; Jaroniec, M. Development of Microporous Carbons for CO2 Capture by KOH Activation of African Palm Shells. J. CO2 Util. 2013, 2, 35–38. [Google Scholar] [CrossRef]
- Md Arshad, S.H.; Ngadi, N.; Aziz, A.A.; Amin, N.S.; Jusoh, M.; Wong, S. Preparation of Activated Carbon from Empty Fruit Bunch for Hydrogen Storage. J. Energy Storage 2016, 8, 257–261. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Cruz Junior, O.F.; Sreńscek-Nazzal, J. Design of Highly Microporous Activated Carbons Based on Walnut Shell Biomass for H2 and CO2 Storage. Carbon 2023, 201, 633–647. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, L.; Kong, W.; Yin, H.; Hu, W.; Bi, X.; Yang, Z.; Chen, W.; Hu, N. Hierarchically Bread-Derived Carbon Foam Decorated with Defect Engineering in Reduced Graphene Oxide/Carbon Particle Nanocomposites as Electrode Materials for Solid-State Supercapacitors. J. Energy Storage 2023, 68, 107616. [Google Scholar] [CrossRef]
- Muhammad, R.; Nah, Y.-C.; Oh, H. Spider Silk-Derived Nanoporous Activated Carbon Fiber for CO2 Capture and CH4 and H2 Storage. J. CO2 Util. 2023, 69, 102401. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-Derived Carbon: Synthesis and Applications in Energy Storage and Conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, B.; Lin, X.; Xie, Z. Biomass-Derived Hierarchical Porous Carbons: Boosting the Energy Density of Supercapacitors via an Ionothermal Approach. J. Mater. Chem. A 2017, 5, 13009–13018. [Google Scholar] [CrossRef]
- Szczesniak, B.; Borysiuk, S.; Choma, J.; Jaroniec, M. Mechanochemical Synthesis of Highly Porous Materials. Mater. Horiz. 2020, 7, 1457–1473. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.; Yang, S.; Schott, J.A.; Liu, X.; Mahurin, S.M.; Huang, C.; Zhang, Y.; Fulvio, P.F.; Chisholm, M.F.; et al. Solid-State Synthesis of Ordered Mesoporous Carbon Catalysts via a Mechanochemical Assembly through Coordination Cross-Linking. Nat. Commun. 2017, 8, 15020. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Izquierdo, M.T.; Medjahdi, G.; Ghanbaja, J.; Celzard, A.; Fierro, V. Ordered Mesoporous Carbons Obtained by Soft-Templating of Tannin in Mild Conditions. Microporous Mesoporous Mater. 2018, 270, 127–139. [Google Scholar] [CrossRef]
- Schlienger, S.; Graff, A.-L.; Celzard, A.; Parmentier, J. Direct Synthesis of Ordered Mesoporous Polymer and Carbon Materials by a Biosourced Precursor. Green Chem. 2012, 14, 313–316. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Parmentier, J.; Pasc, A.; Celzard, A. Easy and Eco-Friendly Synthesis of Ordered Mesoporous Carbons by Self-Assembly of Tannin with a Block Copolymer. Green Chem. 2016, 18, 3265–3271. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Głowniak, S.; Woźniak, J.; Popiel, S.; Choma, J.; Jaroniec, M. Ordered Mesoporous Carbons with Well-Dispersed Nickel or Platinum Nanoparticles for Room Temperature Hydrogen Adsorption. Molecules 2023, 28, 6551. [Google Scholar] [CrossRef]
- Castro-Gutiérrez, J.; Sanchez-Sanchez, A.; Ghanbaja, J.; Díez, N.; Sevilla, M.; Celzard, A.; Fierro, V. Synthesis of Perfectly Ordered Mesoporous Carbons by Water-Assisted Mechanochemical Self-Assembly of Tannin. Green Chem. 2018, 20, 5123–5132. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Zhang, P.; Yang, S.; Zhan, W.; Dai, S. Mechanochemical Synthesis of Ruthenium Cluster@Ordered Mesoporous Carbon Catalysts by Synergetic Dual Templates. Chem. Eur. J. 2019, 25, 8494–8498. [Google Scholar] [CrossRef]
- Shan, W.; Zhang, P.; Yang, S.; Zhu, H.; Wu, P.; Xing, H.; Dai, S. Sustainable Synthesis of Alkaline Metal Oxide-Mesoporous Carbons via Mechanochemical Coordination Self-Assembly. J. Mater. Chem. A 2017, 5, 23446–23452. [Google Scholar] [CrossRef]
- Zhao, J.; Shan, W.; Zhang, P.; Dai, S. Solvent-Free and Mechanochemical Synthesis of N-Doped Mesoporous Carbon from Tannin and Related Gas Sorption Property. Chem. Eng. J. 2020, 381, 122579. [Google Scholar] [CrossRef]
- Tiruye, G.A.; Muñoz-Torrero, D.; Berthold, T.; Palma, J.; Antonietti, M.; Fechler, N.; Marcilla, R. Functional Porous Carbon Nanospheres from Sustainable Precursors for High Performance Supercapacitors. J. Mater. Chem. A 2017, 5, 16263–16272. [Google Scholar] [CrossRef]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Highly Porous Carbons Synthesized from Tannic Acid via a Combined Mechanochemical Salt-Templating and Mild Activation Strategy. Molecules 2021, 26, 1826. [Google Scholar] [CrossRef]
- Cai, C.; Fu, N.; Zhou, Z.; Wu, M.; Yang, Z.; Liu, R. Mechanochemical Synthesis of Tannic Acid-Fe Coordination Compound and Its Derived Porous Carbon for CO2 Adsorption. Energy Fuels 2018, 32, 10779–10785. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Osuchowski, Ł.; Choma, J.; Jaroniec, M. Record-High Benzene Adsorption Capacity of Colloid-Imprinted Mesoporous Carbons Synthesized from Biomass by Ball Milling. Sep. Purif. Technol. 2025, 360, 131097. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Hu, S. Adsorption of Benzene, Toluene, Ethylbenzene and p-Xylene by NaOCl-Oxidized Carbon Nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 83–91. [Google Scholar] [CrossRef]
- Wibowo, N.; Setyadhi, L.; Wibowo, D.; Setiawan, J.; Ismadji, S. Adsorption of Benzene and Toluene from Aqueous Solutions onto Activated Carbon and Its Acid and Heat Treated Forms: Influence of Surface Chemistry on Adsorption. J. Hazard. Mater. 2007, 146, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dou, B.; Zhang, Z.; Wang, J.; Liu, H.; Hao, Z. Adsorption of Benzene, Cyclohexane and Hexane on Ordered Mesoporous Carbon. J. Environ. Sci. 2015, 30, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, J.; Nasri, N.S.; Ahmad Zaini, M.A.; Hamza, U.D.; Ani, F.N. Adsorption of Benzene and Toluene onto KOH Activated Coconut Shell Based Carbon Treated with NH3. Int. Biodeterior Biodegrad. 2015, 102, 245–255. [Google Scholar] [CrossRef]
- Khan, A.; Szulejko, J.E.; Samaddar, P.; Kim, K.-H.; Liu, B.; Maitlo, H.A.; Yang, X.; Ok, Y.S. The Potential of Biochar as Sorptive Media for Removal of Hazardous Benzene in Air. Chem. Eng. J. 2019, 361, 1576–1585. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Osuchowski, Ł.; Choma, J.; Jaroniec, M. Highly Porous Carbons Obtained by Activation of Polypyrrole/Reduced Graphene Oxide as Effective Adsorbents for CO2, H2 and C6H6. J. Porous Mater. 2018, 25, 621–627. [Google Scholar] [CrossRef]
- Osuchowski, Ł.; Szczęśniak, B.; Choma, J.; Jaroniec, M. High Benzene Adsorption Capacity of Micro-Mesoporous Carbon Spheres Prepared from XAD-4 Resin Beads with Pores Protected Effectively by Silica. J. Mater. Sci. 2019, 54, 13892–13900. [Google Scholar] [CrossRef]
- Shen, X.; Ou, R.; Lu, Y.; Yuan, A.; Liu, J.; Gu, J.; Hu, X.; Yang, Z.; Yang, F. Record-High Capture of Volatile Benzene and Toluene Enabled by Activator Implant-Optimized Banana Peel-Derived Engineering Carbonaceous Adsorbents. Environ. Int. 2020, 143, 105774. [Google Scholar] [CrossRef]
- Wang, C.; Yin, H.; Tian, P.; Sun, X.; Pan, X.; Chen, K.; Chen, W.-J.; Wu, Q.-H.; Luo, S. Remarkable Adsorption Performance of MOF-199 Derived Porous Carbons for Benzene Vapor. Environ. Res. 2020, 184, 109323. [Google Scholar] [CrossRef] [PubMed]
- Gwardiak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Benzene Adsorption on Synthesized and Commercial Metal–Organic Frameworks. J. Porous Mater. 2019, 26, 775–783. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Ultrahigh Benzene Adsorption Capacity of Graphene-MOF Composite Fabricated via MOF Crystallization in 3D Mesoporous Graphene. Microporous Mesoporous Mater. 2019, 279, 387–394. [Google Scholar] [CrossRef]
- Moroni, M.; Roldan-Molina, E.; Vismara, R.; Galli, S.; Navarro, J.A.R. Impact of Pore Flexibility in Imine-Linked Covalent Organic Frameworks on Benzene and Cyclohexane Adsorption. ACS Appl. Mater. Interfaces 2022, 14, 40890–40901. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Sustainable Mechanochemical Synthesis of Catalytically Graphitized Mesoporous Carbons. Microporous Mesoporous Mater. 2024, 372, 113117. [Google Scholar] [CrossRef]
- Ohta, Y.; Nishi, S.; Haga, T.; Tsubouchi, T.; Hasegawa, R.; Konishi, M.; Nagano, Y.; Tsuruwaka, Y.; Shimane, Y.; Mori, K.; et al. Screening and Phylogenetic Analysis of Deep-Sea Bacteria Capable of Metabolizing Lignin-Derived Aromatic Compounds. Open J. Mar. Sci. 2012, 2, 177–187. [Google Scholar] [CrossRef]
- Babeł, K.; Jurewicz, K. KOH Activated Lignin Based Nanostructured Carbon Exhibiting High Hydrogen Electrosorption. Carbon 2008, 46, 1948–1956. [Google Scholar] [CrossRef]
- Gao, S.; Chen, Y.; Fan, H.; Wei, X.; Hu, C.; Luo, H.; Qu, L. Large Scale Production of Biomass-Derived N-Doped Porous Carbon Spheres for Oxygen Reduction and Supercapacitors. J. Mater. Chem. A 2014, 2, 3317–3324. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, X.; Wang, C.; Zhong, L.; Fu, F.; Zhu, J.; Zhang, Z.; Qin, Y.; Yang, D.; Xu, C.C. Lignin Derived Carbon Materials: Current Status and Future Trends. Carbon Res. 2022, 1, 14. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, L.; Wang, Z.; Tian, Y.; Qu, Y.; Wang, Y.; Li, J.; Liu, H. Spherical Microporous/Mesoporous Activated Carbon from Pulping Black Liquor. J. Chem. Technol. Biotechnol. 2011, 86, 1177–1183. [Google Scholar] [CrossRef]
- Rodriguez-Mirasol, J.; Cordero, T.; Rodriguez, J.J. Activated Carbons from Carbon Dioxide Partial Gasification of Eucalyptus Kraft Lignin. Energy Fuels 1993, 7, 133–138. [Google Scholar] [CrossRef]
- Schneidermann, C.; Jäckel, N.; Oswald, S.; Giebeler, L.; Presser, V.; Borchardt, L. Solvent-Free Mechanochemical Synthesis of Nitrogen-Doped Nanoporous Carbon for Electrochemical Energy Storage. ChemSusChem 2017, 10, 2416–2424. [Google Scholar] [CrossRef]
- Lu, H.; Sun, X.; Gaddam, R.R.; Kumar, N.A.; Zhao, X.S. Electrocapacitive Properties of Nitrogen-Containing Porous Carbon Derived from Cellulose. J. Power Sources 2017, 360, 634–641. [Google Scholar] [CrossRef]
- Wang, H.; Sun, F.; Qu, Z.; Wang, K.; Wang, L.; Pi, X.; Gao, J.; Zhao, G. Oxygen Functional Group Modification of Cellulose-Derived Hard Carbon for Enhanced Sodium Ion Storage. ACS Sustain. Chem. Eng. 2019, 7, 18554–18565. [Google Scholar] [CrossRef]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Hydrothermal Carbonization of Abundant Renewable Natural Organic Chemicals for High-Performance Supercapacitor Electrodes. Adv. Energy Mater. 2011, 1, 356–361. [Google Scholar] [CrossRef]
- Gui, Z.; Zhu, H.; Gillette, E.; Han, X.; Rubloff, G.W.; Hu, L.; Lee, S.B. Natural Cellulose Fiber as Substrate for Supercapacitor. ACS Nano 2013, 7, 6037–6046. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, J.; Duan, Y.; Yu, A.; Berry, R.M.; Tam, K.C. Conductive Cellulose Nanocrystals with High Cycling Stability for Supercapacitor Applications. J. Mater. Chem. A 2014, 2, 19268–19274. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Lu, H.; Li, H.; Zhao, X.S. Cellulose-Derived Hierarchical Porous Carbon for High-Performance Flexible Supercapacitors. Carbon 2018, 140, 139–147. [Google Scholar] [CrossRef]
- Huang, P.; Wu, M.; Kuga, S.; Wang, D.; Wu, D.; Huang, Y. One-Step Dispersion of Cellulose Nanofibers by Mechanochemical Esterification in an Organic Solvent. ChemSusChem 2012, 5, 2319–2322. [Google Scholar] [CrossRef]
- Onsri, P.; Dubadi, R.; Chuenchom, L.; Dechtrirat, D.; Jaroniec, M. Highly Porous Carbons Prepared via Water-Assisted Mechanochemical Treatment of Cellulose-Based Materials Followed by Carbonization and Mild Activation. Microporous Mesoporous Mater. 2024, 364, 112869. [Google Scholar] [CrossRef]
- Lin, X.; Liang, Y.; Lu, Z.; Lou, H.; Zhang, X.; Liu, S.; Zheng, B.; Liu, R.; Fu, R.; Wu, D. Mechanochemistry: A Green, Activation-Free and Top-Down Strategy to High-Surface-Area Carbon Materials. ACS Sustain. Chem. Eng. 2017, 5, 8535–8540. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Xu, L. Solvent-Free Mechanochemical Preparation of Hierarchically Porous Carbon for Supercapacitor and Oxygen Reduction Reaction. Chem. Eur. J. 2018, 24, 18097–18105. [Google Scholar] [CrossRef] [PubMed]
- Qanytah; Syamsu, K.; Fahma, F.; Pari, G. Characterization of Ball-Milled Sago Pith Waste-Based Activated Carbon Treated with KOH and KMnO4 as Activating Agent. IOP Conf. Ser. Mater. Sci. Eng. 2020, 935, 012043. [Google Scholar] [CrossRef]
- Jiang, B.; Cao, L.; Yuan, Q.; Ma, Z.; Huang, Z.; Lin, Z.; Zhang, P. Biomass Straw-Derived Porous Carbon Synthesized for Supercapacitor by Ball Milling. Mater. 2022, 15, 924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Y.; Guo, L.; Sun, X.; Huang, N. Ball-Milling Effect on Biomass-Derived Nanocarbon Catalysts for the Oxygen Reduction Reaction. ChemistrySelect 2021, 6, 6019–6028. [Google Scholar] [CrossRef]
- Guo, C.; Liao, W.; Li, Z.; Sun, L.; Chen, C. Easy Conversion of Protein-Rich Enoki Mushroom Biomass to a Nitrogen-Doped Carbon Nanomaterial as a Promising Metal-Free Catalyst for Oxygen Reduction Reaction. Nanoscale 2015, 7, 15990–15998. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Rouissi, T.; Verma, M.; Surampalli, R.Y.; Valero, J.R. A Green Method for Production of Nanobiochar by Ball Milling- Optimization and Characterization. J. Clean Prod. 2017, 164, 1394–1405. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, X.; Xiang, W.; Zhang, J.; Cao, C.; Wang, H.; Hu, X.; Gao, B. Ball Milling Biochar with Ammonia Hydroxide or Hydrogen Peroxide Enhances Its Adsorption of Phenyl Volatile Organic Compounds (VOCs). J. Hazard. Mater. 2021, 403, 123540. [Google Scholar] [CrossRef]
- Lesbayev, B.; Auyelkhankyzy, M.; Ustayeva, G.; Yeleuov, M.; Rakhymzhan, N.; Maltay, A.; Maral, Y. Recent Advances: Biomass-Derived Porous Carbon Materials. S. Afr. J. Chem. Eng. 2023, 43, 327–336. [Google Scholar] [CrossRef]
- Balahmar, N.; Mitchell, A.C.; Mokaya, R. Generalized Mechanochemical Synthesis of Biomass-Derived Sustainable Carbons for High Performance CO2 Storage. Adv. Energy Mater. 2015, 5, 1500867. [Google Scholar] [CrossRef]
- Liu, D.; Shao, L.; Zhan, P.; Zhang, L.; Wu, Z.; Wang, J.; Ma, X.; Huang, J. Insights into Mechanochemical Assisted Preparation of Lignin-Based N-Doped Porous Carbon with Tunable Porosity and Ultrahigh Surface Oxygen Content for Efficient CO2 Capture. Sep. Purif. Technol. 2024, 347, 127657. [Google Scholar] [CrossRef]
- Rajendiran, R.; Nallal, M.; Park, K.H.; Li, O.L.; Kim, H.-J.; Prabakar, K. Mechanochemical Assisted Synthesis of Heteroatoms Inherited Highly Porous Carbon from Biomass for Electrochemical Capacitor and Oxygen Reduction Reaction Electrocatalysis. Electrochim. Acta 2019, 317, 1–9. [Google Scholar] [CrossRef]
- Yue, M.; Lu, H.; Liu, H. Study on the Preparation of Biomass-Derived Porous Carbon and Enhanced Carbon Capture Performance via MOF-Assisted Granulation. Langmuir 2025, 41, 12078–12088. [Google Scholar] [CrossRef]
- Zou, R.; Wang, B.; Zhu, L.; Yan, L.; Shi, F.; Sun, Y.; Shao, B.; Zhang, S.; Sun, W. Biomass derived porous carbon supported nano-Co3O4 composite for high-performance supercapacitors. Diam. Relat. Mater. 2022, 126, 109060. [Google Scholar] [CrossRef]
- Cai, T.; Wang, H.; Jin, C.; Sun, Q.; Nie, Y. Fabrication of nitrogen-doped porous electrically conductive carbon aerogel from waste cabbage for supercapacitors and oil/water separation. J. Mater. Sci. Mater. Electron. 2018, 29, 4334–4344. [Google Scholar] [CrossRef]
- Zang, L.; Bu, Z.; Sun, L.; Zhang, Y. Hollow carbon fiber sponges from crude catkins: An ultralow cost absorbent for oils and organic solvents. RSC Adv. 2016, 6, 48715–48719. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, S.; Fu, Y.; Ma, W.; Cheng, Y.; Zhang, H. Experimental study on thermal runaway behaviors of 18650 li-ion battery under enclosed and ventilated conditions. Fire Saf. J. 2021, 125, 103417. [Google Scholar] [CrossRef]
- Yang, H.; Sun, J.; Zhang, Y.; Xue, Q.; Xia, S. Preparation of hydrophobic carbon aerogel using cellulose extracted from luffa sponge for adsorption of diesel oil. Ceram. Int. 2021, 47, 33827–33834. [Google Scholar] [CrossRef]
- Lei, E.; Sun, J.; Gan, W.; Wu, Z.; Xu, Z.; Xu, L.; Ma, C.; Li, W.; Liu, S. N-doped cellulose-based carbon aerogels with a honeycomb-like structure for high-performance supercapacitors. J. Energy Storage 2021, 38, 102414. [Google Scholar] [CrossRef]
- Li, C.; Sun, F.; Lin, Y. Refining cocoon to prepare (N, S, and Fe) ternary-doped porous carbon aerogel as efficient catalyst for the oxygen reduction reaction in alkaline medium. J. Power Sour. 2018, 384, 48–57. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Zhu, F.; You, L.; Shen, X.; Li, S. Removal of organic solvents/oils using carbon aerogels derived from waste durian shell. J. Taiwan Inst. Chem. Eng. 2017, 78, 351–358. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Li, J.; Wang, X. Cotton derived carbonaceous aerogels for the efficient removal of organic pollutants and heavy metal ions. J. Mater. Chem. A 2015, 3, 6073–6081. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Wang, Y.; You, L.; Shen, X.; Li, S. An environmentally friendly carbon aerogels derived from waste pomelo peels for the removal of organic pollutants/oils. Microporous Mesoporous Mater. 2017, 241, 285–292. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Samad, Y.A.; Polychronopoulou, K.; Liao, K. Lightweight and highly conductive aerogel-like carbon from sugarcane with superior mechanical and EMI shielding properties. ACS Sustain. Chem. Eng. 2015, 3, 1419–1427. [Google Scholar] [CrossRef]
- Chen, Z.; Zhuo, H.; Hu, Y.; Lai, H.; Liu, L.; Zhong, L.; Peng, X. Wood-derived lightweight and elastic carbon aerogel for pressure sensing and energy storage. Adv. Funct. Mater. 2020, 30, 1910292. [Google Scholar] [CrossRef]
- Vazhayal, L.; Wilson, P.; Prabhakaran, K. Waste to wealth: Lightweight, mechanically strong and conductive carbon aerogels from waste tissue paper for electromagnetic shielding and CO2 adsorption. Chem. Eng. J. 2020, 381, 122628. [Google Scholar] [CrossRef]
- . Foorginezhad, S.; Weiland, F.; Chen, Y.; Hussain, S.; Ji, X. Review and analysis of porous adsorbents for effective CO2 capture. Renew. Sustain. Energy Rev. 2025, 215, 115589. [Google Scholar] [CrossRef]
- Nelson, K.M.; Mahurin, S.M.; Mayes, R.T.; Williamson, B.; Teague, C.M.; Binder, A.J.; Baggetto, L.; Veith, G.M.; Dai, S. Preparation and CO2 Adsorption Properties of Soft-Templated Mesoporous Carbons Derived from Chestnut Tannin Precursors. Microporous Mesoporous Mater. 2016, 222, 94–103. [Google Scholar] [CrossRef]
- Dubadi, R.; Jaroniec, M. One-Pot Mechanochemical Synthesis of Carbons with High Microporosity and Ordered Mesopores for CO2 Uptake at Ambient Conditions. Nanomaterials 2023, 13, 2262. [Google Scholar] [CrossRef]
- Saning, A.; Dubadi, R.; Chuenchom, L.; Dechtrirat, D.; Jaroniec, M. Microporous Carbons Obtained via Solvent-Free Mechanochemical Processing, Carbonization and Activation with Potassium Citrate and Zinc Chloride for CO2 Adsorption. Separations 2023, 10, 304. [Google Scholar] [CrossRef]
- Xing, H.; Zhiyuan, L.; Kai, W.; Yi, L. Fabrication of Three Dimensional Interconnected Porous Carbons from Branched Anodic Aluminum Oxide Template. Electrochem. Commun. 2011, 13, 1082–1085. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Sun, M.; Ma, W.; Dong, Y.; Xie, Z.; Kong, L.; Zhao, Z. Scaled and Green Production of C-Doped BN Derived from Biomass Waste for Highly Efficient Oxidative Dehydrogenation of Propane to Propylene. Renew. Energy 2025, 243, 122553. [Google Scholar] [CrossRef]
- Liu, J.; Xie, L.; Wang, Z.; Mao, S.; Gong, Y.; Wang, Y. Biomass-Derived Ordered Mesoporous Carbon Nano-Ellipsoid Encapsulated Metal Nanoparticles inside: Ideal Nanoreactors for Shape-Selective Catalysis. Chem. Commun. 2020, 56, 229–232. [Google Scholar] [CrossRef] [PubMed]
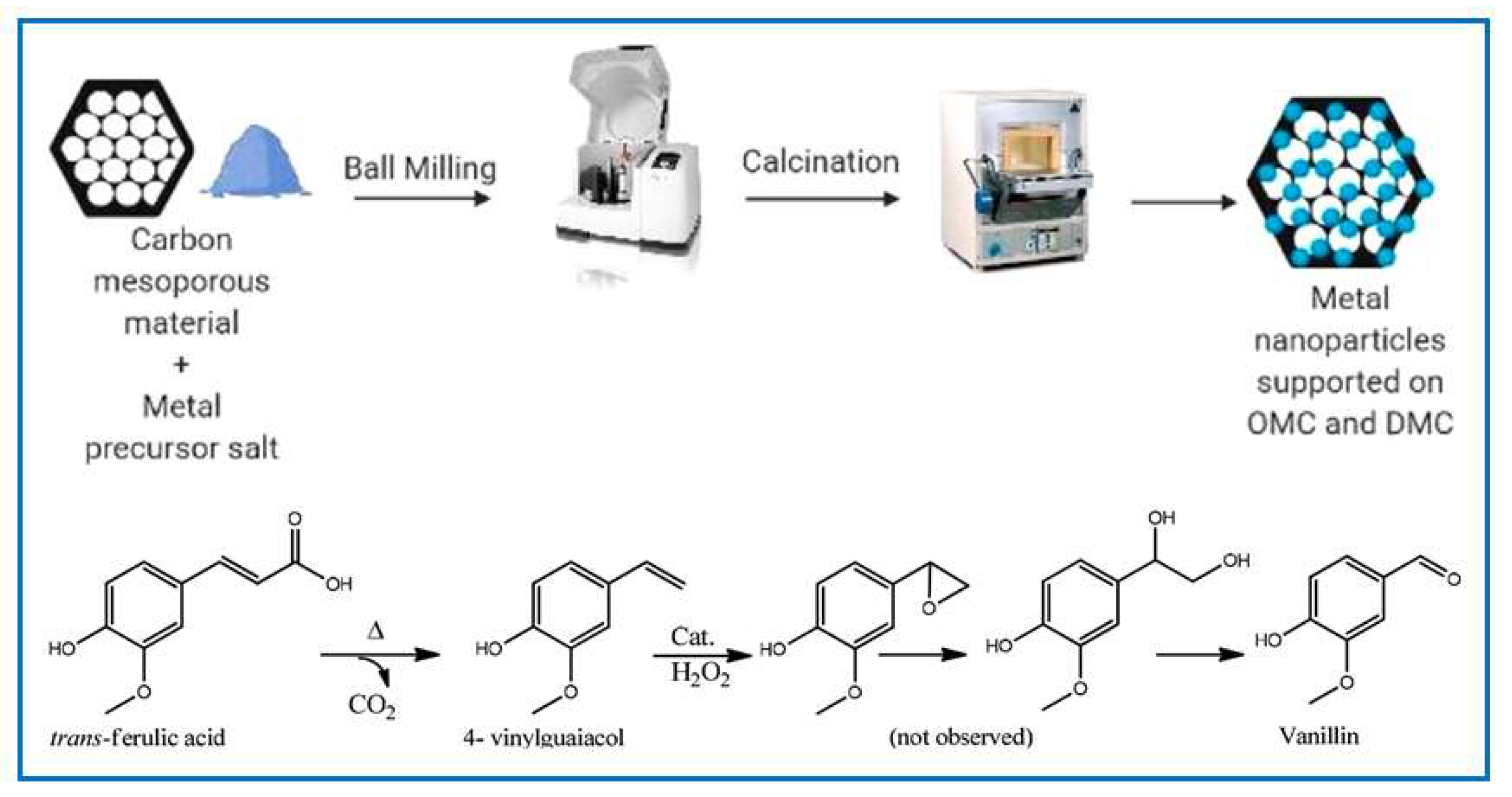
- Lázaro, N.; Castro-Gutiérrez, J.; Ramírez-Vidal, P.; Celzard, A.; Fierro, V.; AlGarni, T.S.; Pineda, A.; Luque, R. Mechanochemical Functionalization of Mesoporous Carbons for the Catalytic Transformation of Trans-Ferulic Acid into Vanillin. ACS Sustain. Chem. Eng. 2021, 9, 4704–4710. [Google Scholar] [CrossRef]
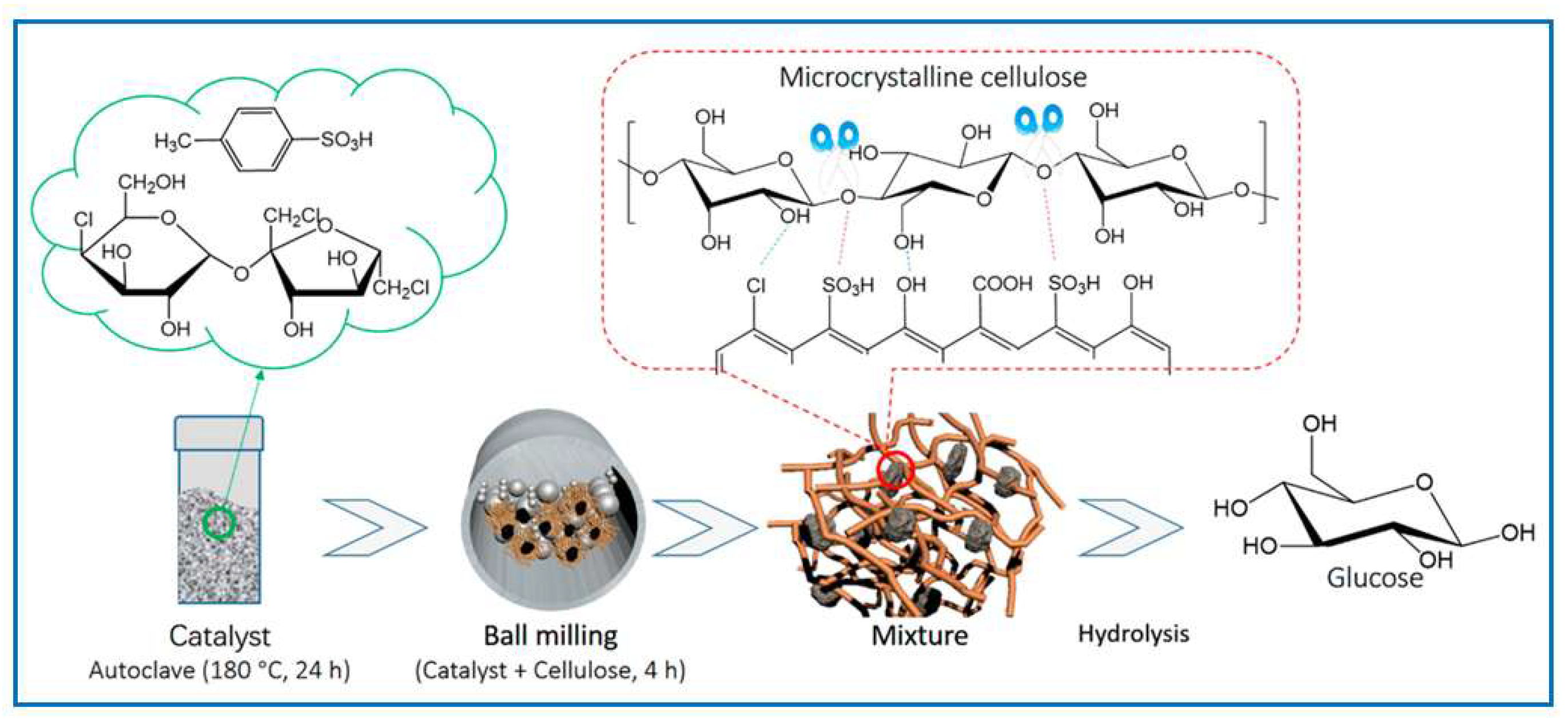
- Qiu, M.; Bai, C.; Yan, L.; Shen, F.; Qi, X. Efficient Mechanochemical-Assisted Production of Glucose from Cellulose in Aqueous Solutions by Carbonaceous Solid Acid Catalysts. ACS Sustain. Chem. Eng. 2018, 6, 13826–13833. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.; Li, H.; Cao, Q.; Jin, L.; Li, P. Porous Graphitic Carbon Microtubes Derived from Willow Catkins as a Substrate of MnO2 for Supercapacitors. J. Power Sources 2017, 344, 176–184. [Google Scholar] [CrossRef]
- Guan, L.; Pan, L.; Peng, T.; Gao, C.; Zhao, W.; Yang, Z.; Hu, H.; Wu, M. Synthesis of Biomass-Derived Nitrogen-Doped Porous Carbon Nanosheests for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 8405–8412. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.; Liu, C.; Li, D.; Kim, H.-K.; Harris, C.; Lao, C.; Abdelkader, A.; Xi, K. Facile Mechanochemical Synthesis of Non-Stoichiometric Silica-Carbon Composite for Enhanced Lithium Storage Properties. J. Alloys Compd 2019, 801, 658–665. [Google Scholar] [CrossRef]
- Tian, W.; Gao, Q.; Tan, Y.; Yang, K.; Zhu, L.; Yang, C.; Zhang, H. Bio-inspired beehive-like hierarchical nanoporous carbon derived from bamboobased industrial by-product as a high performance supercapacitor electrode material. J. Mater. Chem. A 2015, 3, 5656–5664. [Google Scholar] [CrossRef]
- Wang, R.; Wang, P.; Yan, X.; Lang, J.; Peng, C.; Xue, Q. Promising porous carbon derived from celtuce leaves with outstanding supercapacitance and CO2 capture performance. ACS Appl. Mater. Interface. 2012, 4, 5800–5806. [Google Scholar] [CrossRef]
- Olivares-Marin, M.; Fernandez, J.A.; Lazaro, M.J.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V.; Stoeckli, F.; Centeno, T.A. Cherry stones as precursor of activated carbons for supercapacitors. Mater. Chem. Phys. 2009, 114, 323–327. [Google Scholar] [CrossRef]
- Valente Nabais, J.M.; Teixeira, J.G.; Almeida, I. Development of easy made low cost bindless monolithic electrodes from biomass with controlled properties to be used as electrochemical capacitors. Bioresour. Technol. 2011, 102, 2781–2787. [Google Scholar] [CrossRef]
- Bhattacharjya, D.; Yu, J.S. Activated carbon made from cow dung as electrode material for electrochemical double layer capacitor. J. Power Sources 2014, 262, 224–231. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, L.Z.; Zhou, M.Q.; Guan, H.; Qiao, S.; Antonietti, M.; Titirici, M.M. Nitrogen-containing hydrothermal carbons with superior performance in supercapacitors. Adv. Mater. 2010, 22, 5202–5206. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Pi, Y.T.; Lu, L.M.; Xu, S.H.; Ren, T.Z. Hierarchical porous active carbon from fallen leaves by synergy of K2CO3 and their supercapacitor performance. J. Power Sources 2015, 299, 519–528. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Hu, C.C.; Wang, C.C. Effects of pore structure and electrolyte onthe capacitive characteristicsof steamand KOH-activated carbons for supercapacitors. J. Power Sources 2005, 144, 302–309. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Huang, Y.; Wang, W. A fish scale based hierarchical lamellar porous carbon material obtained using a natural template for high performance electrochemical capacitors. J. Mater. Chem. 2010, 20, 4773–4775. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Yang, F.; Yang, X. Promising carbons for supercapacitors derived from fungi. Adv. Mater. 2011, 23, 2745–2748. [Google Scholar] [CrossRef]
- Long, C.; Chen, X.; Jiang, L.; Zhi, L.; Fan, Z. Porous layer-stacking carbon derived from in-built template in biomass for high volumetric performance supercapacitors. Nano Energy 2015, 12, 141–151. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, J.; Hao, L.; Rong Xue, R.; Sun, G.; Yi, B. High rate performance activated carbons prepared from ginkgo shells for electrochemical supercapacitors. Carbon 2013, 56, 146–154. [Google Scholar] [CrossRef]
- Falco, C.; Sieben, J.M.; Brun, N.; Sevilla, M.; van der Mauelen, T.; Morallón, E.; Cazorla-Amorós, D.; Titirici, M.M. Hydrothermal carbons from hemicellulose-derived aqueous hydrolysis products as electrode materials for supercapacitors. ChemSusChem 2013, 6, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Kohandehghan, A.; Zhi Li, Z.; Cui, K.; Tan, X.; Stephenson, T.J.; Cecil, K.; King’ondu, C.K.; Holt, C.M.B.; et al. Interconnected carbon nanosheets derived from hemp for ultrafast supercapacitors with high energy. ACS Nano 2013, 7, 5131–5141. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Sun, F.; Xu, Y.; Qiu, L.; Liu, C.; Wang, S.; Yan, F. Human hair-derived carbon flakes for electrochemical supercapacitors. Energy Environ. Sci. 2014, 7, 379–386. [Google Scholar] [CrossRef]
- Sevilla, M.; Gu, W.; Falco, C.; Titirici, M.M.; Fuertes, A.B.; Yushin, G. Hydrothermal synthesis of microalgae-derived microporous carbons for electrochemical capacitors. J. Power Sources 2014, 267, 26–32. [Google Scholar] [CrossRef]
- Farma, R.; Deraman, M.; Awitdrus, A.; Talib, I.A.; Taer, E.; Basri, N.H.; Manjunatha, J.G.; Ishak, M.M.; Dollah, B.N.M.; Hashmi, S.A. Preparation of highly porous binderless activated carbon electrodes from fibres of oil palm empty fruit bunches for application in supercapacitors. Bioresour. Technol. 2013, 132, 254–261. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Tak, J.K.; Holt, C.M.B.; Tan, X.; Xu, Z.; Shalchi Amirkhiz, B.; Harfield, D.; Anyia, A.; Stephenson, T.; et al. Supercapacitors based on carbons with tuned porosity derived from paper pulp mill sludge biowaste. Carbon 2013, 57, 317–328. [Google Scholar] [CrossRef]
- He, X.; Ling, P.; Qiu, J.; Yu, M.; Zhang, X.; Yu, C.; Zheng, M. Efficient preparation of biomass-based mesoporous carbons for supercapacitors with both high energy density and high power density. J. Power Sources 2013, 240, 109–113. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, H.; Huang, Y.; Wang, W.; Wei, S. Hierarchical porous carbon obtained from animal bone and evaluation in electric double-layer capacitors. Carbon 2011, 49, 838–843. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Amaresh, S.; Lee, S.N.; Sun, X.; Aravindan, V.; Lee, Y.G.; Lee, Y.S. Construction of high-energy-density supercapacitors from pine-cone-derived high-surface-area carbons. ChemSusChem 2014, 7, 1435–1442. [Google Scholar] [CrossRef]
- He, X.; Ling, P.; Yu, M.; Wang, X.; Zhang, X.; Zheng, M. Rice husk-derived porous carbons with high capacitance by ZnCl2 activation for supercapacitors. Electrochimica Acta 2013, 105, 635–641. [Google Scholar] [CrossRef]
- Taer, E.; Deraman, M.; Talib, I.A.; Awitdrus, A.; Hashmi, S.A.; Umar, A.A. Preparation of a highly porous binderless activated carbon monolith from rubber wood sawdust by a multi-step activation process for application in supercapacitors. Int. J. Electrochem. Sci. 2011, 6, 3301–3315. [Google Scholar] [CrossRef]
- Feng, H.; Zheng, M.; Dong, H.; Xiao, Y.; Hu, H.; Sun, Z.; Long, C.; Yijin Cai, Y.; Zhao, X.; Zhang, H.; et al. Three-dimensional honeycomb-like hierarchically structured carbon for high-performance supercapacitors derived from highash-content sewage sludge. J. Mater. Chem. A 2015, 3, 15225–15234. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Idrees, F.; Ma, X. Hierarchical porous nitrogen-doped carbon nanosheets derived fromsilk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, W.; Zhuo, S.; Zhou, J.; Li, F.; Qiao, S.Z.; Lu, G.Q. Preparation of capacitor’s electrode from sunflower seed shell. Bioresour. Technol. 2011, 102, 1118–1123. [Google Scholar] [CrossRef]
- Kalpana, D.; Cho, S.H.; Lee, S.B.; Lee, Y.S.; Misra, R.; Renganathan, N.G. Recycled waste paper – a new source of raw material for electric double-layer capacitors. J. Power Sources 2009, 190, 587–591. [Google Scholar] [CrossRef]
- Peng, C.; Yan, X.B.; Wang, R.T.; Lang, J.W.; Ou, Y.J.; Xue, Q.J. Promising activated carbons derived from waste tea-leaves and their application in high performance supercapacitors electrodes. Electrochim. Acta 2013, 87, 401–408. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q. Water bamboo-derived porous carbons as electrode materials for supercapacitors. New J. Chem. 2015, 39, 3859–3864. [Google Scholar] [CrossRef]
- Wu, X.L.; Wen, T.; Guo, H.L.; Yang, S.; Wang, X.; Xu, A.W. Biomass-derived sponge-like carbonaceous hydrogels and aerogels for supercapacitors. ACS Nano 2013, 7, 3589–3597. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, N.; Lei, S.; Yan, R.; Tian, X.; Wang, J.; Song, Y.; Xu, D.; Guo, Q.; Liu, L. Promising biomass-based activated carbons derived from willow catkins for high performance supercapacitors. Electrochim. Acta 2015, 166, 1–11. [Google Scholar] [CrossRef]
- Sun, H.; He, W.; Zong, C.; Lu, L. Template-free synthesis of renewable macroporous carbon via yeast cells for high-performance supercapacitor electrode materials. ACS Appl. Mater. Interface 2013, 5, 2261–2268. [Google Scholar] [CrossRef]
- Arfelis, S.; Martín-Perales, A.I.; Nguyen, R.; Pérez, A.; Cherubin, I.; Len, C.; Malpartida, I.; Bala, A.; Fullana-i-Palmer, P. Linking Mechanochemistry with the Green Chemistry Principles: Review Article. Heliyon 2024, 10, e34655. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zeng, L.; Li, X.; Chen, N.; Bai, S.; He, H.; Wang, Q.; Zhang, C. A Review on Mechanochemistry: Approaching Advanced Energy Materials with Greener Force. Adv. Mater. 2022, 34, 2108327. [Google Scholar] [CrossRef]
- Cuccu, F.; De Luca, L.; Delogu, F.; Colacino, E.; Solin, N.; Mocci, R.; Porcheddu, A. Mechanochemistry: New Tools to Navigate the Uncharted Territory of “Impossible” Reactions. ChemSusChem 2022, 15, e202200362. [Google Scholar] [CrossRef]



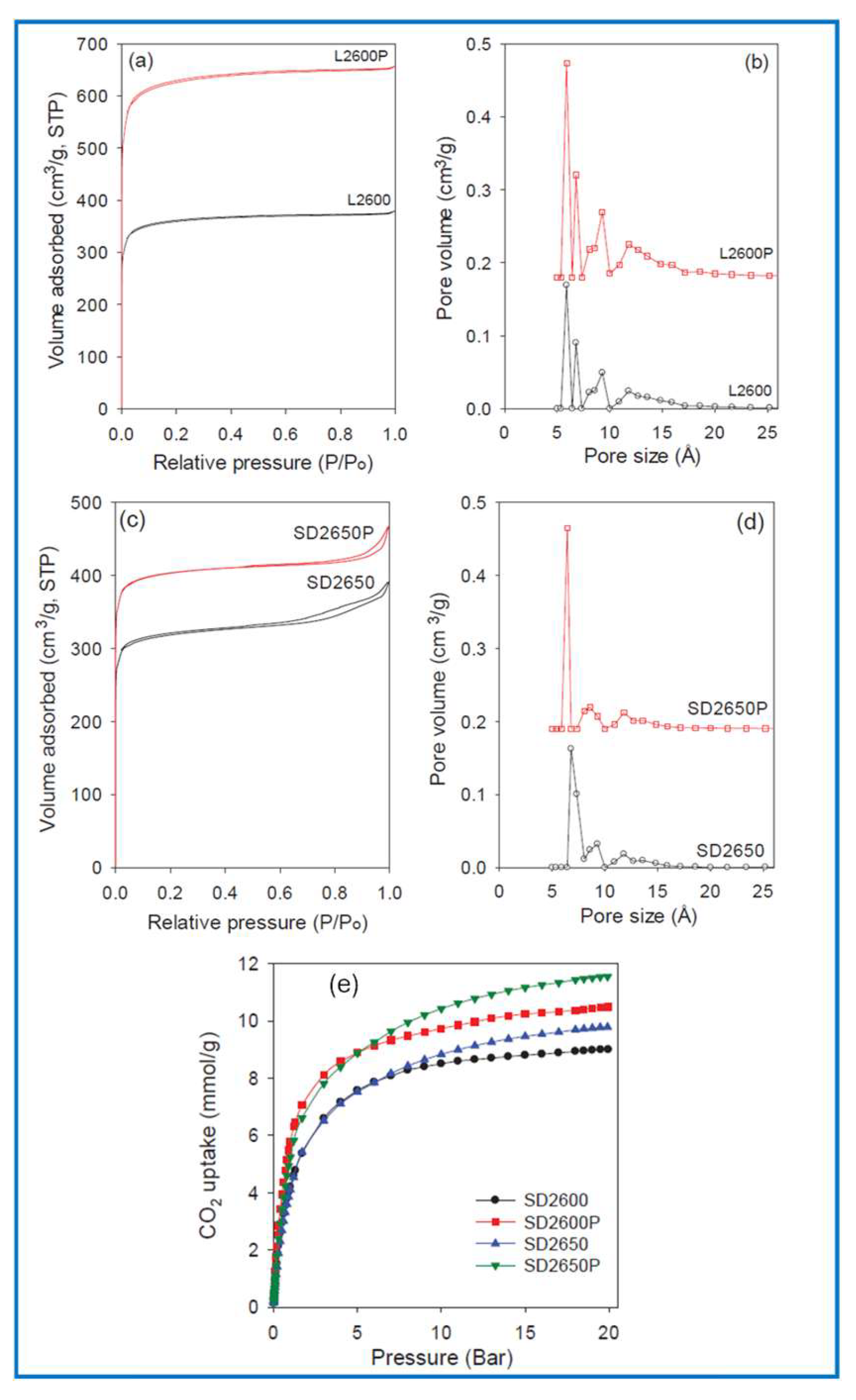
| Sample | VSP (cm3g1) * | SBET (m2g1) † | Vmi (cm3g1) ǂ | Smi (m2g1) § | wKJS (nm) # |
|---|---|---|---|---|---|
| C@Tannin-Zn | 0.23 | 514 | 0.20 | 469 | – |
| C@Tannin-F127 | 0.36 | 395 | 0.11 | 245 | – |
| OMC@F1270.4-800 | 0.59 | 773 | 0.19 | 475 | 7.3 |
| OMC@F1270.6-800 | 0.76 | 1057 | 0.24 | 601 | 7.8 |
| OMC@F1270.8-800 | 0.58 | 621 | 0.12 | 293 | 6.9 |
| OMC@F1271.0-450 | 0.66 | 547 | 0.08 | 180 | 8.6 |
| OMC@F1271.0-600 | 0.67 | 869 | 0.17 | 412 | 8.2 |
| OMC@F1271.0-800 | 0.69 | 734 | 0.16 | 390 | 7.8 |
| Ni-OMC@F1270.8-450 | 0.96 | 996 | 0.19 | 464 | 6.9 |
| Ni-OMC@F1270.8-600 | 0.73 | 769 | 0.15 | 356 | 7.8 |
| Ni-OMC@F1270.8-800 | 0.52 | 558 | 0.14 | 355 | 9.2 |
| OMC@F380.8-800 | 0.49 | 722 | 0.16 | 381 | 5.3 |
| OMC@F680.8-800 | 0.58 | 770 | 0.15 | 350 | 5.3 |
| OMC@F870.8-800 | 0.62 | 765 | 0.17 | 412 | 5.9 |
| OMC@F880.8-800 | 0.61 | 733 | 0.13 | 316 | 5.7 |
| OMC@P650.8-800 | 0.60 | 851 | 0.15 | 340 | 4.2 |
| OMC@P850.8-800 | 0.66 | 770 | 0.17 | 405 | 6.6 |
| OMC@P1030.8-800 | 0.76 | 825 | 0.19 | 466 | 7.5 |
| OMC@P1230.8-800 | 0.73 | 811 | 0.13 | 310 | 5.4 |
| OMC@Bj781-800 | 0.89 | 695 | 0.16 | 382 | 17 |
| OMC@TritonX1000.8-800 | 0.50 | 782 | 0.17 | 407 | 5.0 |
| OMC@(F127 + Ph3P)0.8-800 | 0.57 | 496 | 0.10 | 244 | 10.4 |
| No. | Raw Material | Type of Carbon Material | SSA, m2 g−1 | Application | Ref. |
|---|---|---|---|---|---|
| 1. | Abundant radish | N-O-P co-doped carbon aerogel | 1649 | For high-performance supercapacitors | [138] |
| 2. | Cabbage leaf waste | Carbon aerogels | 536 | For supercapacitors and oil/water separation | [139] |
| 3. | Catkins | Hollow carbon fiber sponge | 438 | Absorbent for oils and organic solvents | [140] |
| 4. | Cellulose | A N-doped carbon aerogel | 1196 | For high-performance supercapacitors | [141] |
| 5. | Cellulose | Carbon aerogels | - | For adsorption of diesel oil | [142] |
| 6. | Chitosan | N self-doped carbon aerogel | 1480 | For high-performance supercapacitors | [143] |
| 7. | Cocoon | Carbon aerogels | 714 | As efficient catalyst for oxygen reduction reaction in alkaline medium | [144] |
| 8. | Durian shell | Carbon aerogels | 735 | For removal of organic pollutants | [145] |
| 9. | Natural cotton waste | Carbon aerogels | 1160 | Adsorbents for wastewater clean-up | [146] |
| 10. | Pomelo peel | Carbon aerogels | 760 | Absorbent for removal of organic pollutants/oils | [147] |
| 11. | Sugarcane | Aerogel-like carbon | 390 | Sensor, energy conversion and storage, and EMI shielding | [148] |
| 12. | Wood | Carbon aerogels | 1124 | For pressure sensing and energy storage | [149] |
| 13. | Waste tissue paper | Carbon aerogels | 1384 | Adsorbent, catalyst supports, and energy storage devices | [150] |
| Mechanochemically prepared carbons | |||||
| 14 | Enoki mushroom | N-doped porous carbon containing nanotubes | 305 | For oxygen reduction reaction | [130] |
| 15 | Flowers | Hierarchical porous carbon | 2148 | For supercapacitors | [126] |
| 16 | Lignin | Porous carbon | 2224 | For CO2 capture | [134] |
| 17 | Lignin | N-doped porous carbon | 2030 | For CO2 capture | [135] |
| 18 | Lotus roots | Porous carbon | 1400 | For electrochemical capacitor and oxygen reduction reaction electrocatalysis | [136] |
| 19 | Pine wood | Nanobiochar | 47 | For removal of organic pollutants | [131] |
| 20 | Rice straw | N-doped porous carbon | 1026 | For CO2 capture | [137] |
| 21 | Sago pith | Porous carbon | 497 | For methylene blue adsorption | [127] |
| 22 | Sawdust | Porous carbon | 1313 | For efficient CO2 capture | [134] |
| 23 | Tannic acid | Porous carbon | 1801 | For CO2 capture | [95] |
| 24 | Tannic acid | Highly porous carbon | 3060 | For efficient H2 and CO2 adsorption | [94] |
| 25 | Tannins | Highly mesoporous carbon | 1218 | For efficient benzene adsorption | [96] |
| 26 | Tannins | Ordered mesoporous carbon | 1000 | For hydrogenation of bulk molecules | [84] |
| 27 | Tobacco straw | Porous carbon | 1293 | For supercapacitors | [128] |
| 28 | Yuba | Porous carbon | 832 | For oxygen reduction reaction | [129] |
| Biomass Resources | Activation Method | Specific Surface Area (cm2 g−1) | Pore Volume (cm3 g−1) | Electrolyte | Highest Specific Capacitance (F g−1) | Ref. |
|---|---|---|---|---|---|---|
| Bamboo waste | HTC/KOH activation | 1472 | – | KOH (6 M) | 301 | [163] |
| Celtuce leaves | KOH activation | 3404 | 1.88 | KOH (2 M) | 421 | [164] |
| Cherry stone | KOH activation | 1300 | – | H2SO4 (2 M) | 230 | [165] |
| Coffee endocarp | Physical activation | 1050 | 0.5 | H2SO4 (1 M) | 176 | [166] |
| Cow dung | KOH activation | 1984 | 0.91 | Organic | 124 | [167] |
| D-glucosamine | HTC | 598 | 0.34 | H2SO4 (1 M) | 300 | [168] |
| Fallen flowers | KHCO3 activation | 2148 | – | KOH (6 M) | 303 | [126] |
| Fallen leaves | KOH/K2CO3 activation | 2869 | 1.598 | KOH (6 M) | 242 | [169] |
| Firwoods | Physical activation | 1016 | 0.747 | NaNO3 (1 M) | 105 | [170] |
| Fish scale | KOH activation | 2273 | 2.74 | KOH (7 M) | 168 | [171] |
| Fungi | HTC | 80.08 | 0.496 | KOH (6 M) | 196 | [172] |
| Fungus | HTC/KOH activation | 1103 | 0.54 | KOH (6 M) | 360 | [173] |
| Ginkgo shells | KOH activation | 1775 | – | KOH (6 M) | 178 | [174] |
| Hemicellulose | HTC/KOH activation | 2300 | ∼1 | H2SO4 (0.5 M) | 300 | [175] |
| Hemp | HTC/KOH activation | 2287 | 1.45 | Liquid ionic | 142 | [176] |
| Human hair | KOH activation | 1306 | 0.9 | KOH (6 M) | 340 | [177] |
| Lignin | K2CO3 activation | 3041 | 2.13 | Liquid ionic | 192 | [116] |
| Lignin | K2CO3 activation | 3041 | 2.13 | Li2SO4 (1 M) | 177 | [116] |
| Lotus roots | K2CO3 activation | 1400 | 0.81 | K2CO3 | 273 | [136] |
| Microalgae | HTC/KOH activation | 2190 | 0.94 | LiCl (6 M) | 200 | [178] |
| Oil palm | Physical activation | 1704 | 0.89 | – | 150 | [179] |
| Paper pulp | HTC/KOH activation | 2980 | 1.75 | Organic | 166 | [180] |
| Peanut shell | Microwave/ZnCl2 activation | 1634 | 1.39 | KOH (6 M) | 245 | [181] |
| Pine nut shells | KOH activation | 2093 | 1.05 | KOH (6 M) | 324 | [161] |
| Pig bone | KOH activation | 2157 | 0.77 | KOH (7 M) | 185 | [182] |
| Pinecone | KOH activation | 3950 | 2.395 | Organic | 198 | [183] |
| Pistachio shell | Physical activation | 1009 | 0.667 | NaNO3 (1 M) | 80 | [170] |
| Rice husk | Microwave/ZnCl2 activation | 1442 | 0.71 | KOH (6 M) | 243 | [184] |
| Rubber wood sawdust | Physical activation | 913 | 0.61 | H2SO4 (1 M) | 138 | [185] |
| Sewage sludge | KOH activation | 2839 | 2.65 | Na2SO4 (1 M) | 379 | [186] |
| Silk | KOH activation | 2494 | 2.28 | Liquid ionic | 242 | [187] |
| Sunflower seed | KOH activation | 2585 | 1.41 | 30 wt.% KOH | 311 | [188] |
| Tobacco straw | ZnO activation | 1293 | 1.43 | KOH (6 M) | 221 | [128] |
| Waste paper | KOH activation | 416 | 0.225 | KOH (6 M) | 160 | [189] |
| Waste tea leaves | KOH activation | 2841 | 1.366 | KOH (2 M) | 330 | [190] |
| Water bamboo | KOH activation | 2352 | 1.11 | KOH (6 M) | 268 | [191] |
| Watermelon | HTC | – | – | KOH (6 M) | 333 | [192] |
| Willow catkins | KOH activation | 1586 | 0.78 | KOH (6 M) | 253 | [193] |
| Wood saw dust | HTC/KOH activation | 2967 | 1.35 | TEABF4 (1 M) | 236 | [119] |
| Yeast cell | KOH activation | – | – | KOH (1 M) | 330 | [194] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choma, J.; Szczęśniak, B.; Jaroniec, M. Mechanochemical Preparation of Biomass-Derived Porous Carbons. Molecules 2025, 30, 3125. https://doi.org/10.3390/molecules30153125
Choma J, Szczęśniak B, Jaroniec M. Mechanochemical Preparation of Biomass-Derived Porous Carbons. Molecules. 2025; 30(15):3125. https://doi.org/10.3390/molecules30153125
Chicago/Turabian StyleChoma, Jerzy, Barbara Szczęśniak, and Mietek Jaroniec. 2025. "Mechanochemical Preparation of Biomass-Derived Porous Carbons" Molecules 30, no. 15: 3125. https://doi.org/10.3390/molecules30153125
APA StyleChoma, J., Szczęśniak, B., & Jaroniec, M. (2025). Mechanochemical Preparation of Biomass-Derived Porous Carbons. Molecules, 30(15), 3125. https://doi.org/10.3390/molecules30153125











