Effects of Germination on the Nutritional Profile of Five Distinct Pea Varieties
Abstract
1. Introduction
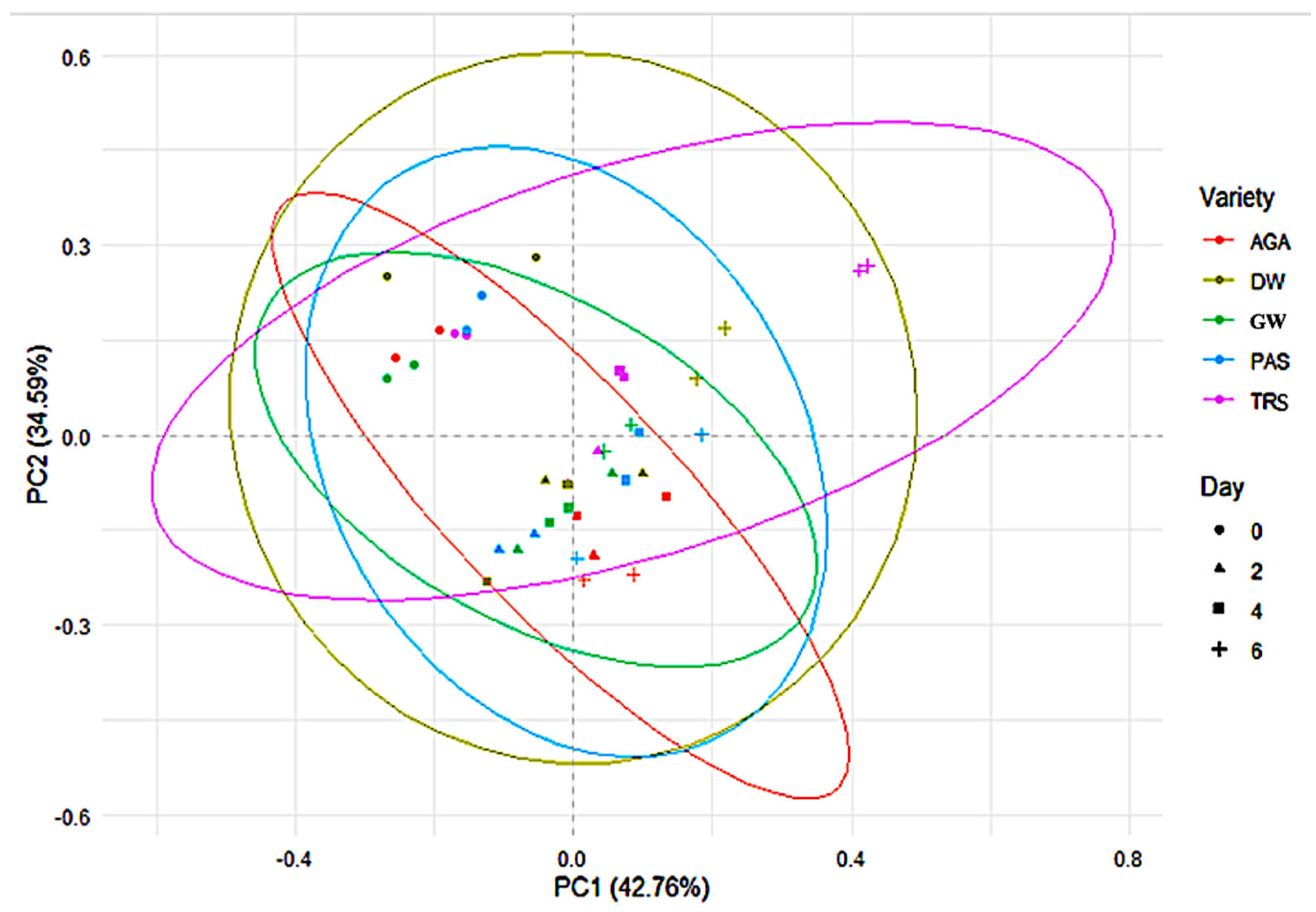
2. Results
2.1. Proximate Composition
2.1.1. Effect of Variables on Composition
2.1.2. Nutrient Composition
2.2. B Vitamin Concentrations
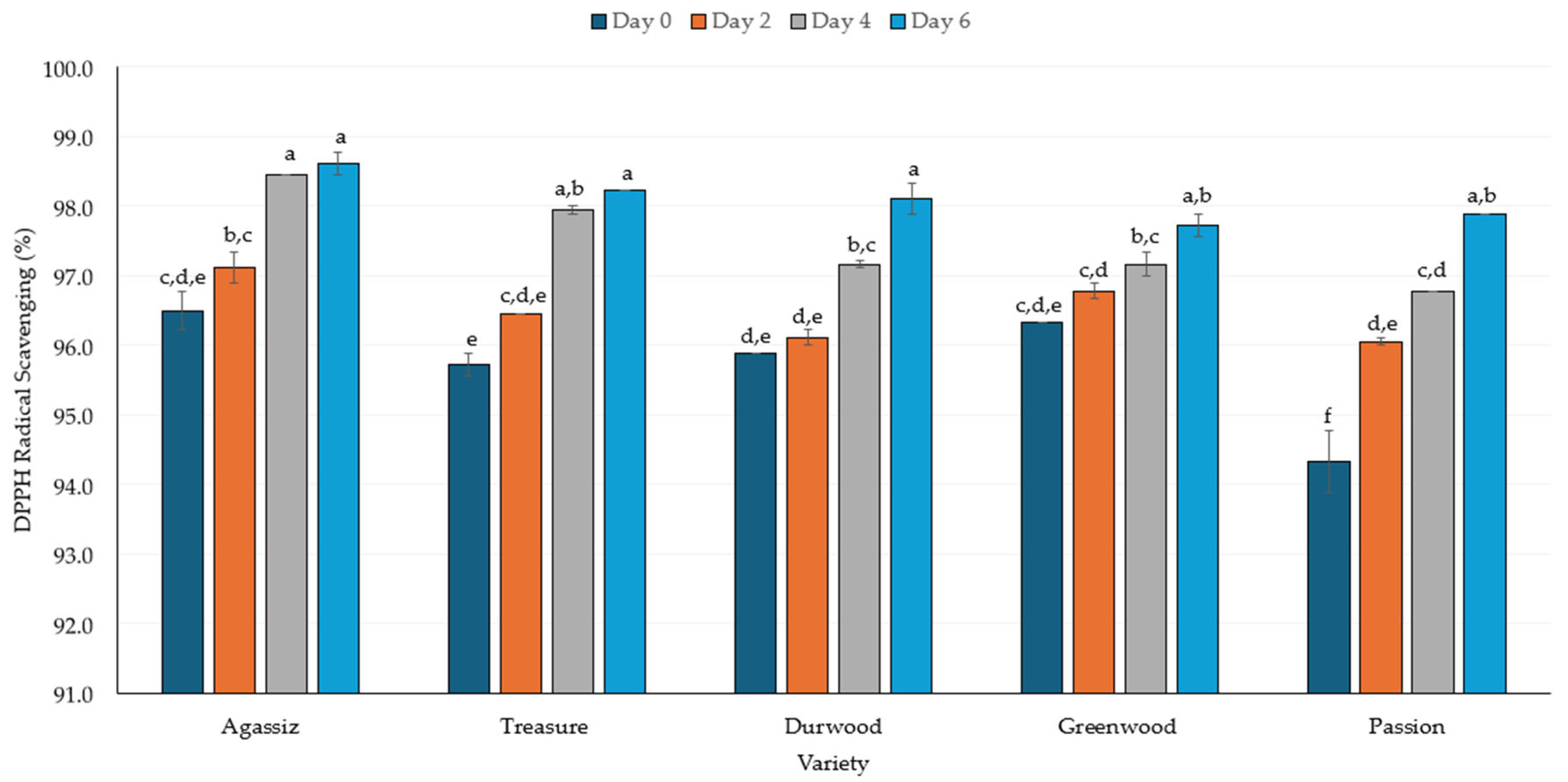
2.3. DPPH Radical Scavenging
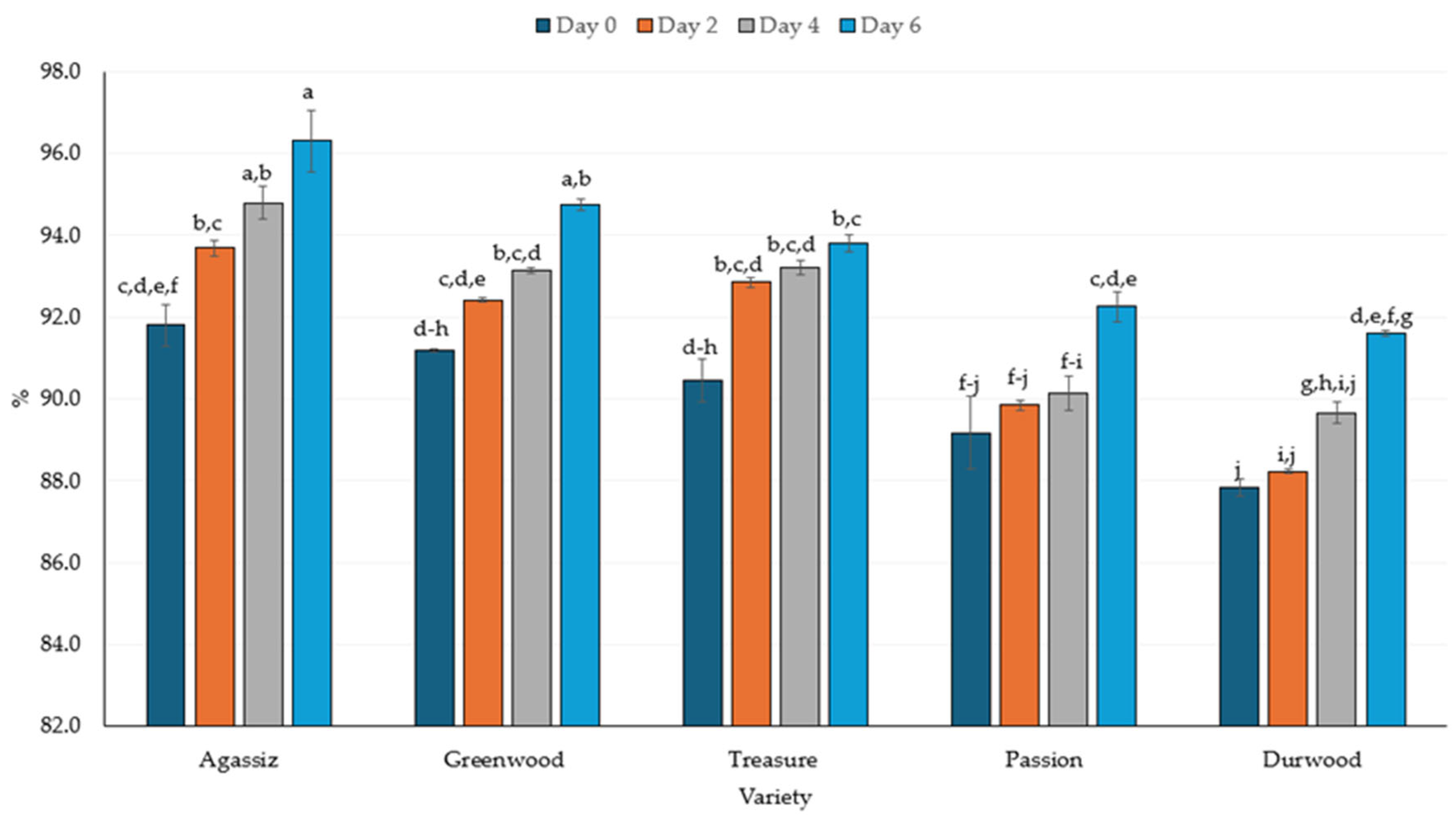
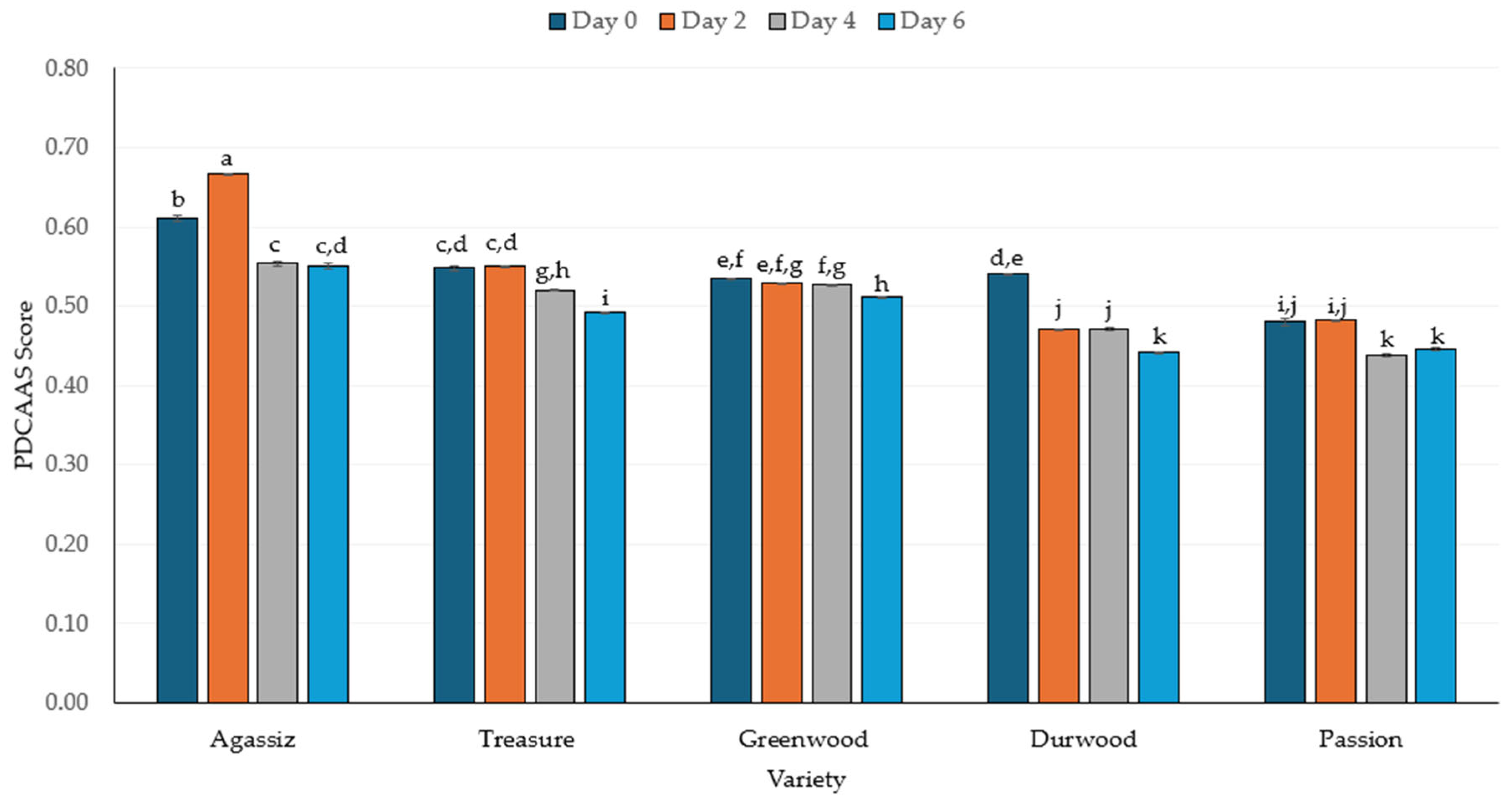
2.4. Protein Digestibility and In Vitro Protein-Corrected Amino Acid Score (IV-PDCAAS)
3. Discussion
3.1. General Composition
3.2. B Vitamins
3.3. Antioxidant Activity
3.4. Protein Digestibility and PDCAAS
4. Materials and Methods
4.1. Pea Germination
4.2. Composition Analysis
4.3. In Vitro Protein Digestibility Corrected Amino Acid Score (IV-PDCAAS)
4.4. Determination of B Vitamins by HPLC
4.5. Antioxidant Activity
4.6. Experimental and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGA | Agassiz |
| DW | Durwood |
| GW | Greenwood |
| PAS | Passion |
| TRS | Treasure |
| IVPD | In vitro protein digestibility |
| IV-PDCAAS | In vitro protein digestibility corrected amino acid score |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| PCA | Principal Component Analysis |
| TDF | Total dietary fiber |
| IDF | Insoluble dietary fiber |
| SDF | Soluble dietary fiber |
References
- Food and Agriculture Organization of the United Nations. Pea Production—UN FAO. Production: Crops and Livestock Products. 2025. Available online: https://archive.ourworldindata.org/20250718-111234/grapher/pea-production.html (accessed on 18 July 2025).
- Azarpazhooh, E.; Boye, J.I. Composition of Processed Dry Beans and Pulses. In Dry Beans and Pulses: Production, Processing and Nutrition; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 101–128. [Google Scholar] [CrossRef]
- Nelson, A.M.; Roemmich, J.N. Effect of source on trust of pulse nutrition information and perceived likelihood of following dietary guidance. Psychol. Health 2023, 40, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Jayasinghe, M.A.; Senadheera, S.A.; Ranaweera, K.K.D.S. Determination of macronutrient compositions in selected, frequently consumed cereals, cereal-based foods, legumes, and pulses prepared according to common culinary methods in Sri Lanka. J. Food Sci. Technol. 2020, 57, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Soncin Alfaro, G.; McGee, R.; Kiszonas, A. Influence of genotype and environment on field pea composition and milling traits. J. Sci. Food Agric. 2025, 105, 4884–4892. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, N. Studies on functional, thermal and pasting properties of flours from different chickpea (Cicer arietinum L.) cultivars. Food Chem. 2005, 91, 403–411. [Google Scholar] [CrossRef]
- Sun, G.; Ni, P.; Lam, E.; Hrapovic, S.; Bing, D.; Yu, B.; Ai, Y. Exploring the functional attributes and in vitro starch and protein digestibility of pea flours having a wide range of amylose content. Food Chem. 2023, 405, 134938. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hatcher, D.W.; Gawalko, E. Effect of variety and processing on nutrients and certain anti-nutrients in field peas (Pisum sativum). Food Chem. 2008, 111, 132–138. [Google Scholar] [CrossRef]
- El-Safy, F.S.; Salem, R.H.A.; Mukhtar, E.Y.Y. The impact of soaking and germination on chemical composition, carbohydrate fractions, digestibility, antinutritional factors, and mineral content of some legumes and cereals grain seeds. Alex. Sci. Exch. J. 2013, 34, 499–513. [Google Scholar]
- Sharma, S.; Sahni, P. Germination behaviour, techno-functional characteristics, antinutrients, antioxidant activity, and mineral profile of lucerne as influenced by germination regimes. J. Food Meas. Char. 2021, 15, 1796–1809. [Google Scholar] [CrossRef]
- Allai, F.M.; Azad, Z.R.A.A.; Gul, K.; Dar, B.N. Whole grains: A review on the amino acid profile, mineral content, physicochemical, bioactive composition, and health benefits. Int. J. Food Sci. Technol. 2022, 57, 1849–1865. [Google Scholar] [CrossRef]
- Guzmán-Ortiz, F.A.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Mora-Escobedo, R.; Rojas-León, A.; Rodríguez-Marín, M.L.; Falfán-Cortés, R.N.; Román-Gutiérrez, A.D. Enzyme activity during germination of different cereals: A review. Food Rev. Int. 2019, 35, 177–200. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, E.; Reddy, P.R. The effect of germination and cooking on the in vitro digestibility of starch in some legumes. Nutr. Rep. Int. 1981, 23, 799–804. [Google Scholar]
- Vidal-Valverde, C.; Frias, J. Changes in carbohydrates during germination of lentils. Z. Für Lebensm.-Unters. Und -Forsch. 1992, 194, 461–464. [Google Scholar] [CrossRef]
- Donkor, O.N.; Stojanovska, L.; Ginn, P.; Ashton, J.; Vasiljevic, T. Germinated grains—Sources of bioactive compounds. Food Chem. 2012, 135, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jin, Z.; Simsek, S.; Hall, C.; Rao, J.; Chen, B. Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea. Food Chem. 2019, 295, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Lu, H.; Shu, Q.; Zhang, Y.; Chen, Q. New perspectives on physiological, biochemical and bioactive components during germination of edible seeds: A review. Trends Food Sci. Technol. 2022, 123, 187–197. [Google Scholar] [CrossRef]
- Kylen, A.M.; McCready, R.M. Nutrients in seeds and sprouts of alfalfa, lentils, mung beans, and soybeans. J. Food Sci. 1975, 40, 1008–1009. [Google Scholar] [CrossRef]
- Sierra, I.; Vidal-Valverde, C. Kinetics of free and glycosylated B6 vitamers, thiamin, and riboflavin during germination of pea seeds. J. Sci. Food Agric. 1999, 79, 307–310. [Google Scholar] [CrossRef]
- Urbano, G.; Aranda, P.; Vílchez, A.; Aranda, C.; Cabrera, L.; Porres, J.M.; López-Jurado, M. Effects of germination on the composition and nutritive value of proteins in Pisum sativum L. Food Chem. 2005, 93, 671–679. [Google Scholar] [CrossRef]
- Chinma, C.E.; Abu, J.O.; Adedeji, O.E.; Aburime, L.C.; Joseph, D.G.; Agunloye, G.F.; Adebo, J.A.; Oyeyinka, S.A.; Njobeh, P.B.; Adebo, O.A. Nutritional composition, bioactivity, starch characteristics, thermal and microstructural properties of germinated pigeon pea flour. Food Biosci. 2022, 49, 101900. [Google Scholar] [CrossRef]
- Paja̧k, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.; Prodanov, M.; Sierra, I.; Vidal-Valverde, C. Effect of light on carbohydrates and hydrosoluble vitamins of lentils during soaking. J. Food Prot. 1995, 58, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Urbano, G.; Lopez-Jurado, M.; Hernandez, J.; Fernandez, M.; Moren, M.-C.; Frias, J.; Diaz-Pollan, C.; Prodanov, M.; Vidal-Valverde, C. Nutritional assessment of raw, heated, and germinated lentils. Food Chem. 1995, 43, 1871–1877. [Google Scholar] [CrossRef]
- Vidal-Valverde, C.; Frias, J.; Estrella, I.; Gorospe, M.J.; Ruiz, R.; Bacon, J. Nutritional changes in legumes after germination. Food Chem. 1994, 42, 2291–2295. [Google Scholar] [CrossRef]
- Wu, N.N.; Li, R.; Li, Z.J.; Tan, B. Effect of germination in the form of paddy rice and brown rice on their phytic acid, GABA, γ-oryzanol, phenolics, flavonoids, and antioxidant capacity. Food Res. Int. 2022, 159, 111603. [Google Scholar] [CrossRef] [PubMed]
- Sofi, S.A.; Singh, J.; Muzaffar, K.; Mir, S.A. Effect of germination time on physico-chemical, functional, pasting, rheology and electrophoretic characteristics of chickpea flour. Food Meas. 2020, 14, 2380–2392. [Google Scholar] [CrossRef]
- Wang, N.; Daun, J.K. Effect of variety and crude protein content on nutrients and certain antinutrients in field peas (Pisum sativum L.). J. Sci. Food Agric. 2004, 84, 1021–1029. [Google Scholar] [CrossRef]
- de Almeida Costa, G.E.D.; Queiroz-Monici, K.D.S.; Reis, S.; de Oliveira, A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem. 2006, 94, 327–330. [Google Scholar] [CrossRef]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). British J. Nut. 2012, 108, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ul-Haq, M.; Ahmad, S.; Amarowicz, R. Compositional studies of some pea (Pisum sativum L.) seed cultivars commonly consumed in Pakistan. Italian J. Food Sci. 2013, 25, 295–302. [Google Scholar]
- Ray, H.; Bett, K.; Tar’an, B.; Vandenberg, A.; Thavarajah, D.; Warkentin, T. Mineral micronutrient content of cultivars of field pea, chickpea, common bean, and lentil grown in Saskatchewan, Canada. Crop Sci. 2015, 54, 1698–1708. [Google Scholar] [CrossRef]
- Wang, N.; Hatcher, D.; Warkentin, T.; Toews, R. Effect of cultivar and environment on physicochemical and cooking characteristics of field pea (Pisum sativum). Food Chem. 2012, 118, 109–115. [Google Scholar] [CrossRef]
- Ghavidel, R.; Prakash, J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT-Food Sci. Technol. 2007, 40, 1292–1299. [Google Scholar] [CrossRef]
- Borade, V.P.; Kadam, S.S.; Salunkhe, D.K. Changes in phytate phosphorus and minerals during germination and cooking of horse gram and moth bean. Plant Food Hum Nutr. 1984, 34, 151–157. [Google Scholar] [CrossRef]
- Alonso, R.; Grant, G.; Dewey, P.; Marzo, F. Nutritional assessment in vitro and in vivo of raw and extruded peas (Pisum sativum L.). 2000. J. Agric. Food Chem. 2000, 48, 2286–2290. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; McCurdy, A.; Baik, B.K. Textural property of 6 legume curds in relation to their protein constituents. J. Food Sci. 2002, 67, 1725–1730. [Google Scholar] [CrossRef]
- Guleria, S.; Dua, S.; Chongtham, N. Analysis of variability in different genotypes of pea (Pisum sativum L.) on the basis of protein markers. Legume Res. 2009, 32, 265–269. [Google Scholar]
- Mohammed, Y.; Chen, C.; Walia, M.; Torrion, J.; McVay, K.; Lamb, P.; Miller, P.; Eckhoff, J.; Miller, J.; Khan, Q. Dry pea (Pisum sativum L.) protein, starch, and ash concentrations as affected by cultivar and environment. Can. J. Plant Sci. 2018, 98, 1188–1198. [Google Scholar] [CrossRef]
- Hsu, D.; Leung, H.K.; Finney, P.L.; Morad, M.M. Effect of germination on nutritive value and baking properties of dry peas, lentils, and faba beans. J. Food Sci. 1980, 45, 87–92. [Google Scholar] [CrossRef]
- Rumiyati, R.; James, A.J.; Jayasena, V. Effect of germination on the nutritional and protein profile of Australian sweet lupin (Lupinus angustifolius L.). Food Nut. Sci. 2012, 3, 621–626. [Google Scholar] [CrossRef]
- Masood, T.; Ullah, H.; Khyber, S.; Masood, T.; Shah, H.U.; Zeb, A. Effect of sprouting time on proximate composition and ascorbic acid level of mung bean (Vigna radiata L.) and chickpea (Cicer arietinum L.) seeds. J. Anim. Plant Sci. 2014, 24, 850–859. [Google Scholar]
- Fouad, A.A.; Rehab, F.M.A. Effect of germination time on proximate analysis, bioactive compounds and antioxidant activity of lentil (Lens culinaris medik.) sprouts. Acta Sci. Pol.-Technol. Aliment. 2015, 14, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Ghumman, A.; Kaur, A.; Singh, N. Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocoll. 2016, 61, 843–850. [Google Scholar] [CrossRef]
- Chen, L.H.; Wells, C.E.; Fordham, J.R. Germinated seeds for human consumption. J. Food Sci. 1975, 40, 1290–1294. [Google Scholar] [CrossRef]
- Urbano, G.; Aranda, P.; Gómez-Villalva, E.; Frejnagel, S.; Porres, J.M.; Frías, J.; Vidal-Valverde, C.; López-Jurado, M. Nutritional evaluation of pea (Pisum sativum L.) protein diets after mild hydrothermal treatment and with and without added phytase. J. Agric. Food Chem. 2003, 51, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Frias, J.; Granito, M.; Vidal-Valverde, C. Fermented pigeon pea (Cajanus cajan) ingredients in pasta products. J. Agric. Food Chem. 2006, 54, 6685–6691. [Google Scholar] [CrossRef] [PubMed]
- Gemede, H.F.; Ratta, N. Antinutritional factors in plant foods: Potential health benefits and adverse effects. Int. J. Nutr. Food Sci. 2014, 3, 284–289. [Google Scholar] [CrossRef]
- Yoshida, H.; Tomiyama, Y.; Saiki, M.; Mizushina, Y. Tocopherol content and fatty acid distribution of peas (Pisum sativum L.). J. Am. Oil Chem. Soc. 2007, 84, 1031–1038. [Google Scholar] [CrossRef]
- Hahm, T.; Park, S.; Lo, Y.M. Effects of germination on chemical composition and functional properties of sesame (Sesamum indicum L.) seeds. Biores. Technol. 2009, 100, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, F.; Caceres, P.; Martínez-Villaluenga, C.; Rosell, C.; Frias, J. Effects of germination on the nutritive value and bioactive compounds of brown rice breads. Food Chem. 2015, 173, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulou, D.; Grigorakis, K.; Stasini, M.; Alexis, M.; Iliadis, K. Differences in chemical composition of field pea (Pisum sativum L.) cultivars: Effects of cultivation area and year. Food Chem. 2007, 103, 847–852. [Google Scholar] [CrossRef]
- Hood-Niefer, S.D.; Warkentin, T.D.; Chibbar, R.N.; Vandenberg, A.; Tyler, R.T. Effect of genotype and environment on the concentrations of starch and protein in, and the physicochemical properties of starch from, field pea and faba bean. J. Sci. Food Agric. 2012, 92, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Uriyo, M.G. Changes in enzyme activities during germination of cowpeas (Vigna unguiculata, cv. California blackeye). Food Chem. 2001, 73, 7–10. [Google Scholar] [CrossRef]
- Ghavidel, R.; Prakash, J.; Davoodi, M. Assessment of enzymatic changes in some legume seeds during germination. Agro. Food Ind. Tech. 2011, 22, 45–47. [Google Scholar]
- Olaerts, H.; Roye, C.; Derde, L.J.; Sinnaeve, G.; Meza, W.R.; Bodson, B.; Courtin, C.M. Impact of preharvest sprouting of wheat (Triticum aestivum) in the field on starch, protein, and arabinoxylan properties. J. Agric. Food Chem. 2016, 64, 8324–8332. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Garg, N.; Garg, S.; Kumar, A. Starch phosphorylase: Role in starch metabolism and biotechnological applications. Crit. Rev. Biotechnol. 2009, 29, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.; Zhou, Y. In vitro and in vivo hydrolysis of legume starches by α-amylase and resistant starch formation in legumes—A review. Carbohydr. Poly. 2003, 54, 401–417. [Google Scholar] [CrossRef]
- Gao, L.; Wu, Y.; Wan, C.; Wang, P.; Yang, P.; Gao, X.; Eeckhout, M.; Gao, J. Structural and physicochemical properties of pea starch affected by germination treatment. Food Hydrocoll. 2022, 124, 107303. [Google Scholar] [CrossRef]
- Black, R.; Brouwer, J.; Meares, C.; Iyer, L. Variation in physico-chemical properties of field peas (Pisum sativum). Food Res. Int. 1998, 31, 81–86. [Google Scholar] [CrossRef]
- Stoughton-Ens, M.; Hatcher, D.; Wang, N.; Warkentin, T. Influence of genotype and environment on the dietary fiber content of field pea (Pisum sativum L.) grown in Canada. Food Res. Int. 2010, 43, 547–552. [Google Scholar] [CrossRef]
- Wang, M.; Chen, X.; Dong, L.; Nan, X.; Ji, W.; Wang, S.; Sun, W.; Zhou, Q. Modification of pea dietary fiber by ultrafine grinding and hypoglycemic effect in diabetes mellitus mice. J. Food Sci. 2021, 86, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Boye, J.; Hu, X. Nutritional quality and techno-functional changes in raw, germinated and fermented yellow field pea (Pisum sativum L.) upon pasteurization. LWT 2018, 92, 147–154. [Google Scholar] [CrossRef]
- Ibagon, J.A.; Lee, S.A.; Nyachoti, C.M.; Stein, H.H. Influence of particle size and origin of field peas on apparent ileal digestibility of starch and amino acids and standardized ileal digestibility of amino acids when fed to growing pigs. Transl. Anim. Sci. 2024, 8, txae008. [Google Scholar] [CrossRef] [PubMed]
- Uppal, V.; Bains, K. Effect of germination periods and hydrothermal treatments on in vitro protein and starch digestibility of germinated legumes. J. Food Sci. Technol. 2012, 49, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Martín-Cabrejas, M.A.; Ariza, N.; Esteban, R.; Mollá, E.; Waldron, K.; López-Andréu, F.J. Effect of germination on the carbohydrate composition of the dietary fiber of peas (Pisum sativum L.). J. Agric. Food Chem. 2003, 51, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Avezum, L.; Madode, E.Y.; Mestres, C.; Achir, N.; Delpech, C.; Chapron, M.; Gibert, O.; Rajjou, L.; Rondet, E. New insights into the rapid germination process of lentil and cowpea seeds: High thiamine and folate, and low α-galactoside content. Food Chem. 2024, 439, 138027. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Arcot, J.; Shrestha, A. Folate retention in selected processed legumes. Food Chem. 2000, 68, 295–298. [Google Scholar] [CrossRef]
- Sen Gupta, D.; Thavarajah, D.; Knutson, P.; Thavarajah, P.; McGee, R.J.; Coyne, C.J.; Kumar, S. Lentils (Lens culinaris L.), a rich source of folates. J. Agric. Food Chem. 2013, 61, 7794–7799. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.B.; Ashokkumar, K.; Diapari, M.; Ambrose, S.J.; Zhang, H.X.; Tar’an, B.; Bett, K.E.; Vandenberg, A.; Warkentin, T.; Purves, R.W. Genetic diversity of folate profiles in seeds of common bean, lentil, chickpea and pea. J. Food Comp. Anal. 2015, 42, 134–140. [Google Scholar] [CrossRef]
- Pinheiro, S.S.; Anunciação, P.C.; de Morais Cardoso, L.; Della Lucia, C.M.; de Carvalho, C.W.P.; Queiroz, W.A.W.; Sant’Ana, H.M.P. Stability of B vitamins, vitamin E, xanthophylls and flavonoids during germination and maceration of sorghum (Sorghum bicolor L.). Food Chem. 2021, 345, 128775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; De Silva, D.; Dissanayaka, D.; Warkentin, T.; Vandenberg, A. Validated B vitamin quantification from lentils by selected reaction monitoring mass spectrometry. Food Chem. 2021, 359, 129810. [Google Scholar] [CrossRef] [PubMed]
- Shohag, M.J.I.; Wei, Y.; Yang, X. Changes of folate and other potential health-promoting phytochemicals in legume seeds as affected by germination. J. Agric. Food Chem. 2012, 60, 9137–9143. [Google Scholar] [CrossRef] [PubMed]
- Coffigniez, F.; Rychlik, M.; Mestres, C.; Striegel, L.; Bohuon, P.; Briffaz, A. Modelling folates reaction kinetics during cowpea seed germination in comparison with soaking. Food Chem. 2021, 340, 127960. [Google Scholar] [CrossRef] [PubMed]
- Hefni, M.; Öhrvik, V.; Tabekha, M.; Witthöft, C. Folate content in foods commonly consumed in Egypt. Food Chem. 2010, 121, 540–545. [Google Scholar] [CrossRef]
- Frias, J.; Miranda, M.L.; Doblado, R.; Vidal-Valverde, C. Effect of germination and fermentation on the antioxidant vitamin content and antioxidant capacity of Lupinus albus L. var. Multolupa. Food Chem. 2005, 92, 211–220. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, T.; Zhou, Y.; Tang, W.; Xiao, Y.; Meng, Z.; Fang, X. Relationships between antioxidant compounds and antioxidant activities of tartary buckwheat during germination. J. Food Sci. Technol. 2015, 52, 2458–2463. [Google Scholar] [CrossRef] [PubMed]
- Tarr, J.; Arditti, J. Niacin biosynthesis in seedlings of Zea mays. Plant Physiol. 1982, 69, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Amorós, M.L.; Hernández, T.; Estrella, I. Effect of germination on legume phenolic compounds and their antioxidant activity. J. Food Compos. Anal. 2006, 19, 277–283. [Google Scholar] [CrossRef]
- Tarasevičienė, Ž.; Viršilė, A.; Danilčenko, H.; Duchovskis, P.; Paulauskienė, A.; Gajewski, M. Effects of germination time on the antioxidant properties of edible seeds. CyTA-J. Food 2018, 17, 447–454. [Google Scholar] [CrossRef]
- Borges-Martínez, E.; Gallardo-Velázquez, T.; Cardador-Martínez, A.; Moguel-Concha, D.; Osorio-Revilla, G.; Ruiz-Ruiz, J.; Jiménez-Martínez, C. Phenolic compounds profile and antioxidant activity of pea (Pisum sativum L.) and black bean (Phaseolus vulgaris L.) sprouts. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Uchegbu, N.; Ishiwu, C. Germinated Pigeon Pea (Cajanus cajan): A novel diet for lowering oxidative stress and hyperglycemia. Food Sci. Nutr. 2016, 4, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, A.; Singh, B. Characterization of in vitro antioxidant activity, bioactive components, and nutrient digestibility in pigeon pea (Cajanus cajan) as influenced by germination time and temperature. J. Food Biochem. 2019, 43, e12706. [Google Scholar] [CrossRef] [PubMed]
- Gujral, H.S.; Angurala, M.; Sharma, P.; Singh, J. Phenolic content and antioxidant activity of germinated and cooked pulses. Int. J. Food Prop. 2011, 14, 1366–1374. [Google Scholar] [CrossRef]
- Gumus, Z.P.; Moulahoum, H.; Tok, K.; Kocadag Kocazorbaz, E.; Zihnioglu, F. Activity-guided purification and identification of endogenous bioactive peptides from barley sprouts (Hordeum vulgare L.) with diabetes treatment potential. Int. J. Food Sci. Technol. 2023, 58, 3285–3292. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Huang, X.; Song, L.; Li, N.; Cheng, Y.; Shen, Y.; Li, Q.; Li, T.; Hai, D. Changes in physio-biochemical metabolism, phenolics and antioxidant capacity of different Chinese pea varieties during germination. Int. J. Food Sci. Technol. 2023, 58, 167–180. [Google Scholar] [CrossRef]
- Setia, R.; Dai, Z.; Nickerson, M.T.; Sopiwnyk, E.; Malcolmson, L.; Ai, Y. Impacts of short-term germination on the chemical compositions, technological characteristics and nutritional quality of yellow pea and faba bean flours. Food Res. Int. 2019, 122, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, B.; Li, Y.; Fei, C.; Xiong, Y.; Li, L.; Liu, Y.; Tong, L.; Huang, Y.; Wang, F. Effect of germination on the digestion of legume proteins. Foods 2024, 13, 2655. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A. The Effect of Germination on the Physicochemical, Functional, and Nutritional Properties of Yellow Pea, Red Lentil and Green Lentil Flours. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2023. Available online: https://harvest.usask.ca/bitstream/10388/14591/1/QURESHI-THESIS-2023.pdf (accessed on 23 June 2025).
- Yang, Q.; Luo, Y.; Wang, H.; Li, J.; Gao, X.; Gao, J.; Feng, B. Effects of germination on the physicochemical, nutritional and in vitro digestion characteristics of flours from waxy and nonwaxy proso millet, common buckwheat and pea. Innov. Food Sci. Emerg. Techn. 2012, 67, 102586. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.; Hu, X. In vitro digestibility, protein composition and techno-functional properties of Saskatchewan grown yellow field peas (Pisum sativum L.) as affected by processing. Food Res. Int. 2017, 92, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Nitride, C.; Vegarud, G.E.; Comi, I.; Devold, T.G.; Røseth, A.; Marti, A.; Iametti, S.; Mamone, G.; Picariello, G.; Alfieri, F.; et al. Effect of sprouting on the proteome of chickpea flour and on its digestibility by ex vivo gastro-duodenal digestion complemented with jejunal brush border membrane enzymes. Food Res. Int. 2022, 154, 111012. [Google Scholar] [CrossRef] [PubMed]
- Ohanenye, I.C.; Tsopmo, A.; Ejike, C.E.C.; Udenigwe, C.C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends Food Sci. Technol. 2020, 101, 213–222. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Wang, A.; Wang, J.; Wu, X.; Wu, Y.; Fu, Y.; Sun, H. Insights into interactions between food polyphenols and proteins: An updated overview. J. Food Process. Preserv. 2022, 46, e16597. [Google Scholar] [CrossRef]
- Savelkoul, F.H.M.G.; Van Der Poel, A.F.B.; Tamminga, S. The presence and inactivation of trypsin inhibitors, tannins, lectins and amylase inhibitors in legume seeds during germination. A review. Plant Food Hum. Nutr. 1992, 42, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Nnanna, I.A.; Phillips, R.D. Changes in oligosaccharide content, enzyme activities and dry matter during controlled germination of cowpeas (Vigna unguiculata). J. Food Sci. 1988, 53, 1782–1786. [Google Scholar] [CrossRef]
- Bera, I.; O’Sullivan, M.; Flynn, D.; Shields, D.C. Relationship between protein digestibility and the proteolysis of legume proteins during seed germination. Molecules 2023, 28, 3204. [Google Scholar] [CrossRef] [PubMed]
- Cereals and Grains Association. Moisture—Air Oven Methods. In AACC Approved Methods of Analysis, 11th ed.; Method 44-15.02; Cereals and Grains Association: St. Paul, MN, USA, 2016. [Google Scholar]
- Cereals and Grains Association. Crude Protein—Combustion Method. In AACC Approved Methods of Analysis, 11th ed.; Method 46-30.01; Cereals and Grains Association: St. Paul, MN, USA, 2016. [Google Scholar]
- Cereals and Grains Association. Total Starch Assay Procedure (Megazyme Amyloglucosidase/alpha-Amylase Method). In AACC Approved Methods of Analysis, 11th ed.; Method 76-13.01; Cereals and Grains Association: St. Paul, MN, USA, 2016. [Google Scholar]
- Cereals and Grains Association. Ash—Basic Method. In AACC Approved Methods of Analysis, 11th ed.; Method 08-01.01; Cereals and Grains Association: St. Paul, MN, USA, 2016. [Google Scholar]
- American Oil Chemists Society. Oil in Seed Meals and Cakes. In Official Methods and Recommended Practices of the AOCS, 7th ed.; AOCS Official Method Ba 3-38; American Oil Chemists Society: Urbana. IL, USA, 2022. [Google Scholar]
- Association of Official Analytical Chemists. Total, Soluble, and Insoluble Dietary Fibre in Foods. In Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Latimer, G., Eds.; AOAC Official Method 991.43; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Anonymous. Protein Digestibility Assay Procedure. Animal-Safe Accurate Protein Quality Score (ASAP-Quality Score Method) for Determination of the Protein Digestibility Amino Acid Score (PDCAAS). Available online: www.megazyme.com (accessed on 4 March 2024).
- Agyenim-Boateng, K.G.; Zhang, S.; Islam, M.S.; Gu, Y.; Li, B.; Azam, M.; Abdelghany, A.M.; Qi, J.; Ghosh, S.; Shaibu, A.S.; et al. Profiling of naturally occurring folates in a diverse soybean germplasm by HPLC-MS/MS. Food Chem. 2022, 384, 132520. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Janaswamy, S. Biodegradable, UV-blocking, and antioxidant films from lignocellulosic fibers of spent coffee grounds. Int. J. Biol. Macromol. 2023, 253, 126798. [Google Scholar] [CrossRef] [PubMed]
- Abdollahikhamene, H. Effect of Six-Day Germination on Chemical Composition, Functional, and Nutritional Properties of Pea Varieties. Electron. Theses Diss. 2025, 1528. Available online: https://openprairie.sdstate.edu/etd2/1528 (accessed on 1 June 2025).


| Measurement | p Value | ||
|---|---|---|---|
| Variety | Day | Variety × Day | |
| Moisture (%) | <0.05 | <0.05 | <0.05 |
| Protein (%) | <0.05 | <0.05 | 0.29 |
| Fat (%) | <0.05 | 0.06 | <0.05 |
| Total starch (%) | <0.05 | 0.85 | <0.05 |
| Insoluble dietary fiber (%) | <0.05 | <0.05 | <0.05 |
| Soluble dietary fiber (%) | 0.12 | <0.05 | 0.23 |
| Ash (%) | <0.05 | <0.05 | <0.05 |
| Day | ||||
|---|---|---|---|---|
| Variety | 0 | 2 | 4 | 6 |
| Moisture (%) * | ||||
| Agassiz | 9.5 ± 0.1 hi | 10.1 ± 0.1 g | 12.3± 0.2 e | 14.0 ± 0.1 d |
| Durwood | 9.6 ± 0.1 ghi | 9.1 ± 0.0 gh | 13.7 ± 0.2 d | 19.1 ± 0.1 a |
| Greenwood | 9.6 ± 0.1 ghi | 10.1 ± 0.1 g | 13.4 ± 0.1 d | 16.5 ± 0.3 b |
| Passion | 8.2 ± 0.0 j | 10.9 ± 0.1 f | 15.6 ± 0.1 c | 15.4 ± 0.1 c |
| Treasure | 9.1 ± 0.1 i | 10.1 ± 0.1 gh | 12.6 ± 0.1 e | 18.7 ± 0.6 a |
| Variety | Day | |||
|---|---|---|---|---|
| 0 | 2 | 4 | 6 | |
| Ash (%) * | ||||
| Agassiz | 3.0 ± 0.01 a | 2.8 ± 0.02 b | 2.4 ± 0.01 c | 2.5 ± 0.04 c |
| Durwood | 2.3 ± 0.02 d | 2.1 ± 0.00 fg | 2.0 ± 0.01 hi | 1.8 ± 0.04 ij |
| Greenwood | 2.2 ± 0.01 def | 2.1 ± 0.04 gh | 2.0 ± 0.02 hi | 1.9 ± 0.01 hij |
| Passion | 2.2 ± 0.02 def | 2.0 ± 0.08 h | 1.8 ± 0.01 j | 1.9 ± 0.01 hij |
| Treasure | 2.3 ± 0.06 d | 2.2 ± 0.01 efg | 2.1 ± 0.01 gh | 1.9 ± 0.02 ij |
| Protein (%) * | ||||
| Agassiz | 22.9 ± 0.1 f | 22.4 ± 1.4 f | 24.7 ± 1.8 ef | 26.1 ± 0.6 cde |
| Durwood | 26.0 ± 1.8 de | 28.7 ± 0.4 abcd | 28.7 ± 0.2 abcd | 31.5 ± 0.1 a |
| Greenwood | 24.9 ± 1.5 def | 26.1 ± 0.7 de | 26.8 ± 0.2 cde | 27.2 ± 1.3 bcde |
| Passion | 27.8 ± 0.6 abcde | 27.9 ± 0.5 abcde | 30.9 ± 0.5 ab | 30.1 ± 1.9 abc |
| Treasure | 25.1 ± 0.1 de | 24.3 ± 0.9 cde | 27.4 ± 0.3 bcde | 28.5 ± 0.7 abcde |
| Fat (%) * | ||||
| Agassiz | 1.9 ± 0.0 bc | 1.7 ± 0.2 cd | 2.1 ± 0.1 abc | 2.1 ± 0.2 abc |
| Durwood | 1.4 ± 0.13 cdef | 1.3 ± 0.2 def | 1.5 ± 0.1 cdef | 1.0 ± 0.1 f |
| Greenwood | 2.4 ± 0.2 ab | 2.3 ± 0.2 ab | 2.4 ± 0.1 ab | 2.6 ± 0.1 a |
| Passion | 1.1 ± 0.2 ef | 0.9 ± 0.1 f | 1.0 ± 0.1 f | 1.4 ± 0.3 cdef |
| Treasure | 1.3 ± 0.2 cdef | 1.4 ± 0.16 cdef | 1.6 ± 0.1 cde | 1.0 ± 0.0 f |
| Total Starch (%) * | ||||
| Agassiz | 52.3 ± 2.7 abcd | 54.6 ± 0.5 abc | 55.4 ± 1.4 ab | 59.5 ± 0.2 a |
| Durwood | 45.7 ± 1.7 def | 46.1 ± 3.6 cdef | 46.7 ± 0.8 cdef | 43.6 ± 2.6 ef |
| Greenwood | 48.3 ± 0.2 bcdef | 47.4 ± 1.3 cdef | 48.2 ± 0.5 bcdef | 51.7 ± 2.9 abcde |
| Passion | 50.9 ± 0.1 bcde | 51.4 ± 3.3 abcde | 49.6 ± 0.1 bcde | 40.1 ± 4.2 f |
| Treasure | 51.5 ± 1.4 abcde | 48.2 ± 0.1 bcdef | 49.2 ± 1.5 bcde | 50.5 ± 3.5 bcde |
| Total Dietary Fiber (%) * | ||||
| Agassiz | 19.9 ± 0.3 a | 17.6 ± 1.5 ab | 17.5 ± 1.2 bc | 14.6 ± 0.2 def |
| Durwood | 16.6 ± 0.3 bcd | 15.9 ± 0.4 bcdef | 15.3 ± 0.4 cdef | 14.2 ± 0.5 ef |
| Greenwood | 17.4 ± 0.5 bc | 16.5 ± 0.7 bcd | 16.7 ± 0.6 bcd | 13.8 ± 0.2 f |
| Passion | 17.5 ± 0.2 bc | 16.4 ± 0.2 bcde | 15.9 ± 0.2 bcdef | 15.0 ± 0.1 def |
| Treasure | 17.8 ± 0.3 ab | 15.9 ± 0.2 bcdef | 15.0 ± 0.2 def | 13.9 ± 0.6 f |
| Variety | Day | |||
|---|---|---|---|---|
| 0 | 2 | 4 | 6 | |
| Folate (µg/100 g) * | ||||
| Agassiz | 352 ± 37 bc | 108 ± 5 ghi | 140 ± 22 fgh | 24 ± 16 i |
| Durwood | 518 ± 26 a | 252 ± 13 cde | 162 ± 49 efgh | 136 ± 34 fghi |
| Greenwood | 458 ± 12 a | 231 ± 26 de | 177 ± 1 efg | 70 ± 20 hi |
| Passion | 445 ± 37 ab | 202 ± 4 efg | 217 ± 44 ef | 96 ± 48 ghi |
| Treasure | 440 ± 4 ab | 323 ± 8 cd | 460 ± 14 a | 261 ± 7 cde |
| Niacin (µg/100 g) | ||||
| Agassiz | 538 ± 89 bc | 671 ± 3 bc | 683 ± 122 bc | 609 ± 91 bc |
| Durwood | 699 ± 260 bc | 737 ± 162 bc | 535 ± 152 bc | 950 ± 37 b |
| Greenwood | 456 ± 27 c | 654 ± 140 bc | 583 ± 53 bc | 654 ± 46 bc |
| Passion | 580 ± 42 bc | 545 ± 67 bc | 799 ± 30 bc | 856 ± 199 b |
| Treasure | 714 ± 8 bc | 784 ± 45 bc | 922 ± 22 b | 1434 ± 28 a |
| Pyridoxine (µg/100 g) | ||||
| Agassiz | 1467 ± 44 ab | 187 ± 6 ef | 203 ± 41 ef | 132 ± 3 f |
| Durwood | 1561 ± 88 a | 349 ± 158 de | 374 ± 71 de | 516 ± 25 d |
| Greenwood | 1151 ± 34 c | 258 ± 5 ef | 288 ± 24 ef | 377 ± 8 de |
| Passion | 1298 ± 51 bc | 353 ± 10 de | 271 ± 56 ef | 174 ± 20 ef |
| Treasure | 1402 ± 45 ab | 324 ± 27 def | 362 ± 19 de | 330 ± 1.0 def |
| Riboflavin (µg/100 g) | ||||
| Agassiz | 156 ± 35 abc | 37 ± 2 gh | 71 ± 20 defgh | 54 ± 19 efgh |
| Durwood | 109 ± 50 cdefg | 43 ± 19 gh | 70 ± 15 defgh | 194 ± 15 ab |
| Greenwood | 119 ± 0 cde | 47 ± 6 fgh | 76 ± 1 defgh | 218 ± 2 a |
| Passion | 122 ± 2 cde | 41 ± 8 gh | 83 ± 9 defgh | 115 ± 28 cdef |
| Treasure | 77 ± 4 defgh | 30 ± 6 h | 54 ± 5 efgh | 142 ± 5 bcd |
| Thiamine (µg/100 g) | ||||
| Agassiz | 209 ± 33 c | 251 ± 2 bc | 287 ± 44 bc | 259 ± 20 bc |
| Durwood | 273 ± 61 bc | 278 ± 32 bc | 223 ± 49 bc | 343 ± 23 ab |
| Greenwood | 207 ± 17 c | 249 ± 55 bc | 247 ± 7 bc | 270 ± 18 bc |
| Passion | 279 ± 15 bc | 217 ± 17 bc | 292 ± 17 bc | 247 ± 71 bc |
| Treasure | 241 ± 10 bc | 266 ± 20 bc | 310 ± 2 abc | 432 ± 2 a |
| Measurement | p Value | ||
|---|---|---|---|
| Variety | Day | Variety × Day | |
| In vitro protein digestibility (%) | <0.05 | <0.05 | 0.1 |
| IV-PDCAAS (score 0–1) | <0.05 | <0.05 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdollahikhamene, H.; Pournaki, S.K.; Hall, C. Effects of Germination on the Nutritional Profile of Five Distinct Pea Varieties. Molecules 2025, 30, 3114. https://doi.org/10.3390/molecules30153114
Abdollahikhamene H, Pournaki SK, Hall C. Effects of Germination on the Nutritional Profile of Five Distinct Pea Varieties. Molecules. 2025; 30(15):3114. https://doi.org/10.3390/molecules30153114
Chicago/Turabian StyleAbdollahikhamene, Hojjat, Shirin Kazemzadeh Pournaki, and Clifford Hall. 2025. "Effects of Germination on the Nutritional Profile of Five Distinct Pea Varieties" Molecules 30, no. 15: 3114. https://doi.org/10.3390/molecules30153114
APA StyleAbdollahikhamene, H., Pournaki, S. K., & Hall, C. (2025). Effects of Germination on the Nutritional Profile of Five Distinct Pea Varieties. Molecules, 30(15), 3114. https://doi.org/10.3390/molecules30153114







