Synthesis and Characterization of Covalent Triazine Frameworks Based on 4,4′-(Phenazine-5,10-diyl)dibenzonitrile and Its Application in CO2/CH4 Separation
Abstract
1. Introduction
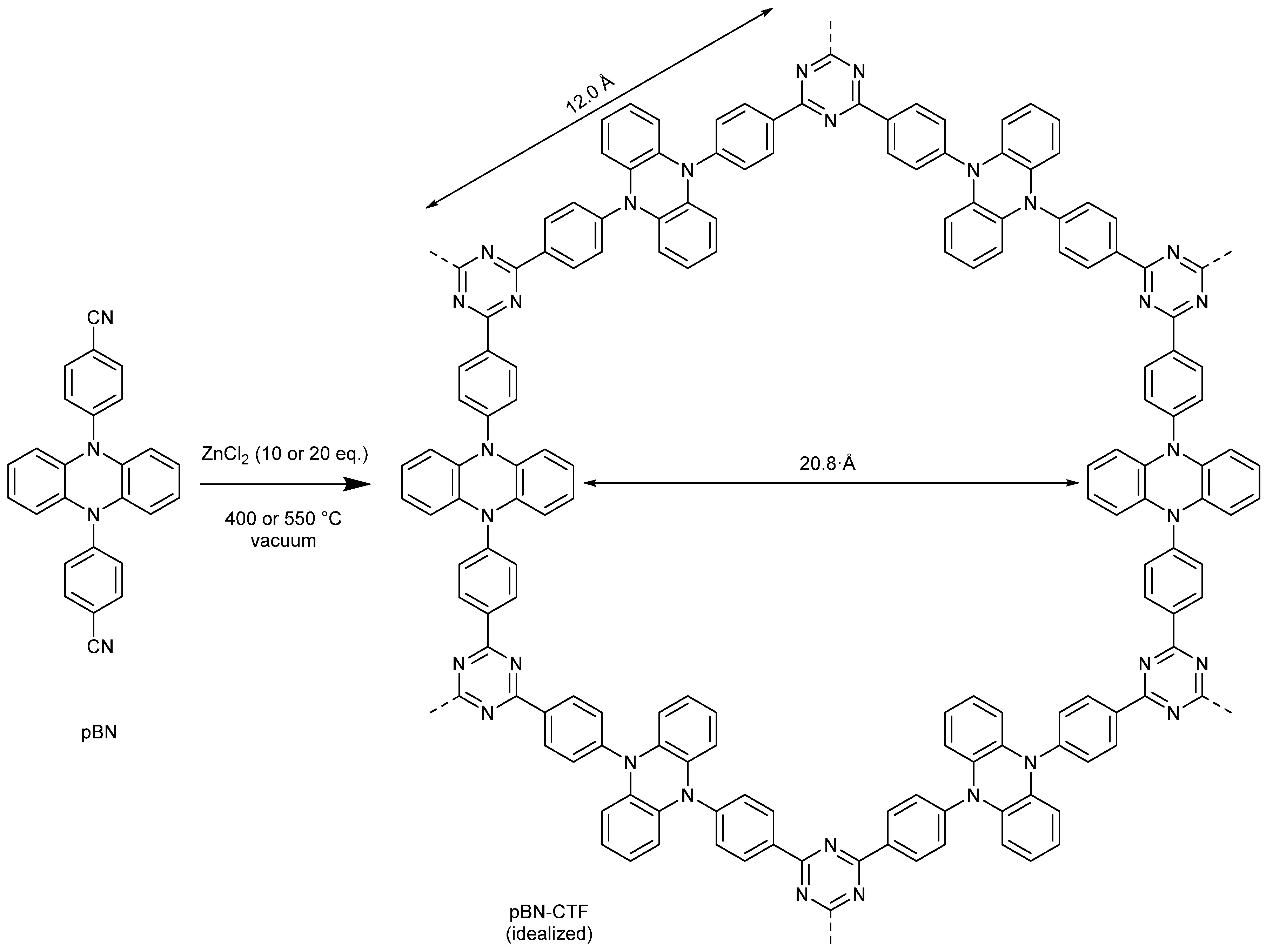
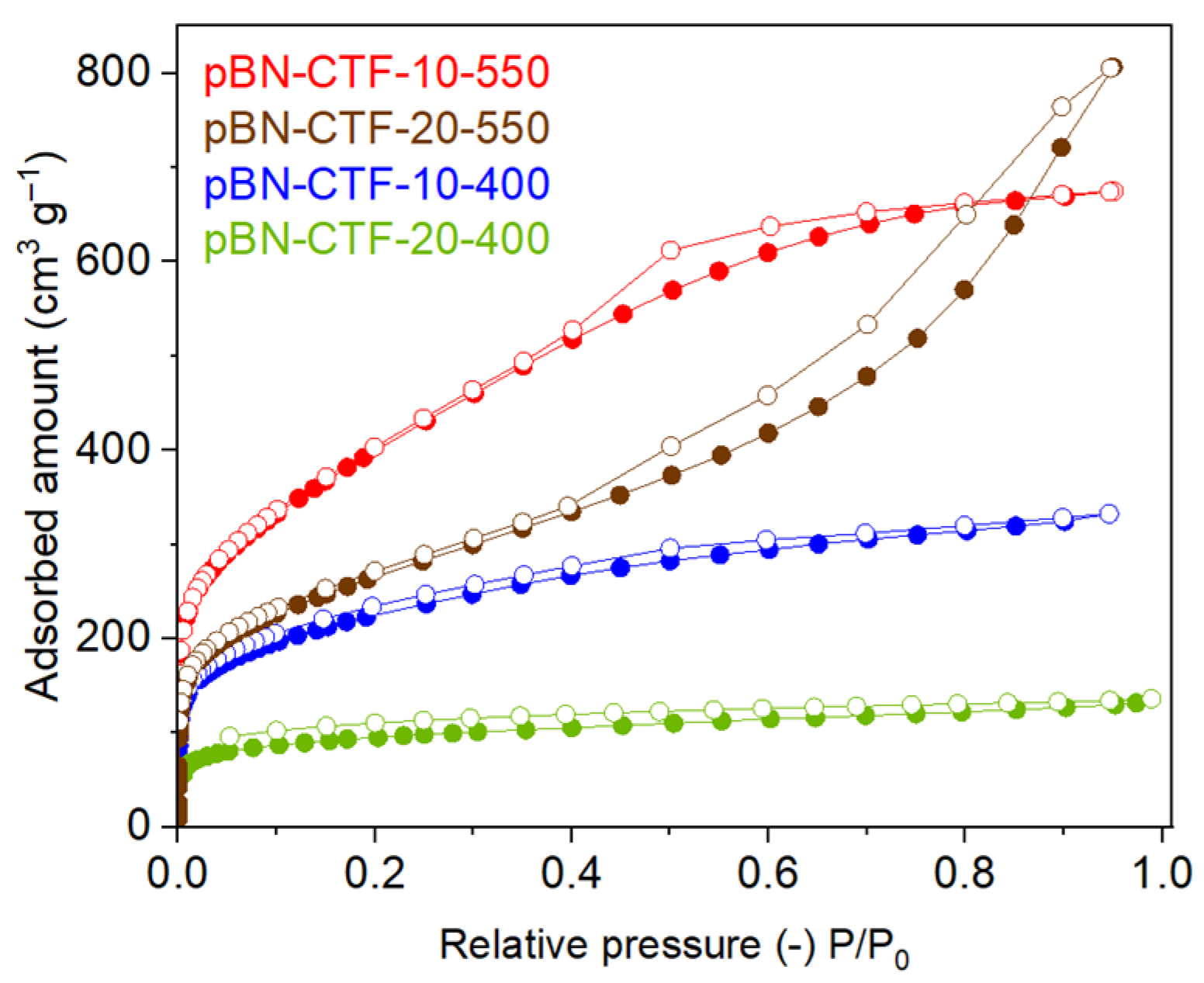
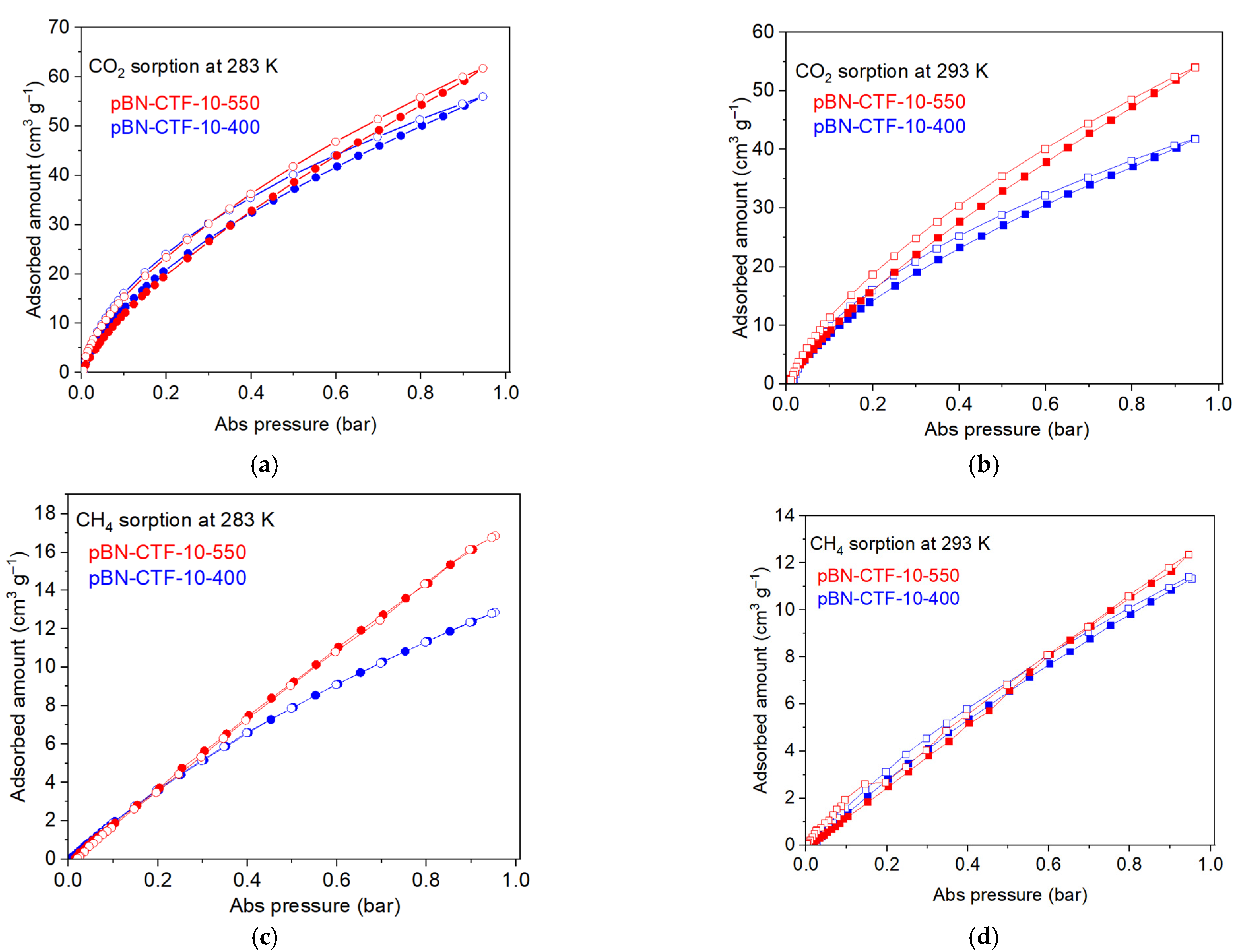
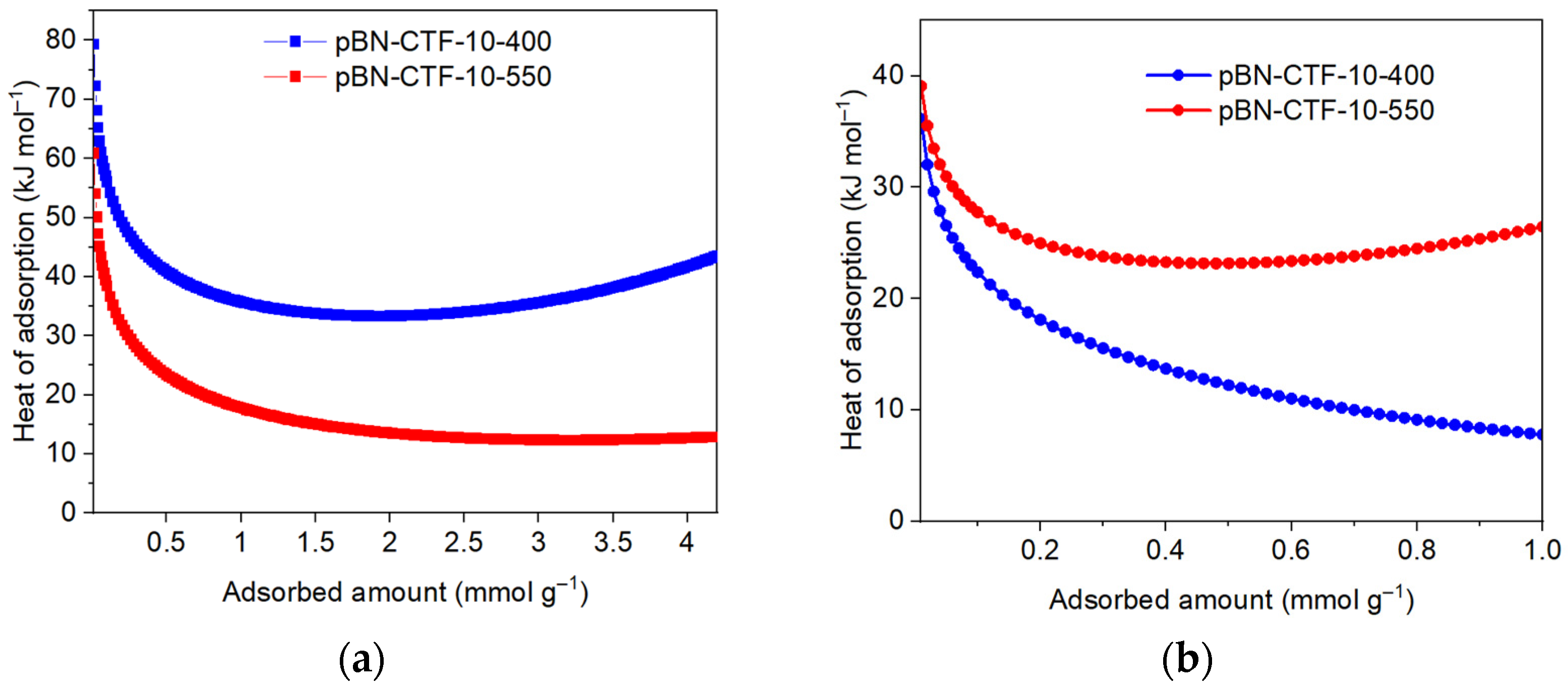
2. Results and Discussion
3. Materials and Methods
3.1. Instrumentation
3.2. Chemicals
3.3. Synthesis of 5,10-Dihydrophenazine
3.4. Synthesis of 4,4′-(Phenazine-5,10-diyl)dibenzonitrile (pBN)
3.5. pBN-CTF Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Bojdys, M.J.; Jeromenok, J.; Thomas, A.; Antonietti, M. Rational Extension of the Family of Layered, Covalent, Triazine-Based Frameworks with Regular Porosity. Adv. Mater. 2010, 22, 2202–2205. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.B.; Clowes, R.; Berardo, B.E.; Jelfs, K.E.; Zwijnenburg, M.A.; Sprick, R.S.; Cooper, A.I. Structurally Diverse Covalent Triazine-Based Framework Materials for Photocatalytic Hydrogen Evolution from Water. Chem. Mater. 2019, 31, 8830–8838. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Forget, A.; Su, D.; Thomas, A.; Antonietti, M. From Microporous Regular Frameworks to Mesoporous Materials with Ultrahigh Surface Area: Dynamic Reorganization of Porous Polymer Networks. J. Am. Chem. Soc. 2008, 130, 13333–13337. [Google Scholar] [CrossRef] [PubMed]
- Kuecken, S.; Acharjya, A.; Zhi, L.; Schwarze, M.; Schomäcker, R.; Thomas, A. Fast tuning of covalent triazine frameworks for photocatalytic hydrogen evolution. Chem. Commun. 2017, 53, 5854–5857. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Li, M.; Yin, Y.; Chen, J.; Zhong, Q.; Du, R.; Liu, S.; He, Y.; Fu, W.; Zeng, F. Advances in the Synthesis of Covalent Triazine Frameworks. ACS Omega 2023, 8, 4527–4542. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, L.; Jin, S.; Tan, B. Covalent triazine frameworks: Synthesis and applications. J. Mater. Chem. A 2019, 7, 5153–5172. [Google Scholar] [CrossRef]
- Lee, J.-S.M.; Cooper, A.I. Advances in Conjugated Microporous Polymers. Chem. Rev. 2020, 120, 2171–2214. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Jena, H.S.; Leus, K.; van der Voort, P. Covalent triazine frameworks—A sustainable perspective. Green Chem. 2020, 22, 1038–1071. [Google Scholar] [CrossRef]
- Aggarwal, S.; Awasthi, S.K. Emerging trends in the development and applications of triazine-based covalent organic polymers: A comprehensive review. Dalton Trans. 2024, 53, 11601–11643. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Esquivel, D.; Dey, S.; Fernández-Terán, R.; Goto, Y.; Inagaki, S.; Van Der Voort, P.; Janiak, C. A photoluminescent covalent triazine framework: CO2 adsorption, light-driven hydrogen evolution and sensing of nitroaromatics. J. Mater. Chem. A 2016, 4, 13450–13457. [Google Scholar] [CrossRef]
- Gao, Q.; Li, X.; Ning, G.-H.; Leng, K.; Tian, B.; Liu, C.; Tang, W.; Xu, H.-S.; Loh, K.P. Highly photoluminescent two-dimensional imine-based covalent organic frameworks for chemical sensing. Chem. Commun. 2018, 54, 2349–2352. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Bhunia, A.; Esquivel, D.; Janiak, C. Covalent triazine-based frameworks (CTFs) from triptycene and fluorene motifs for CO2 adsorption. J. Mater. Chem. A 2016, 4, 6259–6263. [Google Scholar] [CrossRef]
- Wessely, I.D.; Schade, A.M.; Dey, S.; Bhunia, A.; Nuhnen, A.; Janiak, C.; Bräse, S. Covalent Triazine Frameworks Based on the First Pseudo-Octahedral Hexanitrile Monomer via Nitrile Trimerization: Synthesis, Porosity, and CO2 Gas Sorption Properties. Materials 2021, 14, 3214. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, H.; Liu, D.; Wang, C.; Li, J.; Zhong, C. Covalent Triazine-Based Frameworks with Ultramicropores and High Nitrogen Contents for Highly Selective CO2 Capture. Environ. Sci. Technol. 2016, 50, 4869–4876. [Google Scholar] [CrossRef] [PubMed]
- Bügel, S.; Hähnel, M.; Kunde, T.; de Sousa Amadeu, N.; Sun, Y.; Spieß, A.; Beglau, T.H.Y.; Schmidt, B.M.; Janiak, C. Synthesis and Characterization of a Crystalline Imine-Based Covalent Organic Framework with Triazine Node and Biphenyl Linker and Its Fluorinated Derivate for CO2/CH4 Separation. Materials 2022, 15, 2807. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Bügel, S.; Sorribas, S.; Nuhnen, A.; Bhunia, A.; Coronas, J.; Janiak, C. Synthesis and Characterization of Covalent Triazine Framework CTF-1@Polysulfone Mixed Matrix Membranes and Their Gas Separation Studies. Front. Chem. 2019, 7, 693. [Google Scholar] [CrossRef] [PubMed]
- Bügel, S.; Spieß, A.; Janiak, C. Covalent triazine framework CTF-fluorene as porous filler material in mixed matrix membranes for CO2/CH4 separation. Microporous Mesoporous Mater. 2021, 316, 110941. [Google Scholar] [CrossRef]
- Bügel, S.; Hoang, Q.-D.; Spieß, A.; Sun, Y.; Xing, S.; Janiak, C. Biphenyl-Based Covalent Triazine Framework/Matrimid® Mixed-Matrix Membranes for CO2/CH4 Separation. Membranes 2021, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, Covalent Triazine-Based Frameworks Prepared by Ionothermal Synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef] [PubMed]
- Buyukcakir, O.; Je, S.H.; Talapaneni, S.N.; Kim, D.; Coskun, A. Charged Covalent Triazine Frameworks for CO2 Capture and Conversion. ACS Appl. Mater. Interfaces 2017, 9, 7209–7216. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Liu, D.; Huang, W.; Liu, J.; Yang, R. Synthesis of covalent triazine-based frameworks with high CO2 adsorption and selectivity. Polym. Chem. 2015, 6, 7410–7417. [Google Scholar] [CrossRef]
- Mukhtar, A.; Mellon, N.B.; Bustam, M.A.; Saqib, S.; Lee, S.-P.; Kareem, F.A.A.; Ullah, S. Impact of amine functionality on the selective CO2/CH4 adsorption behavior of porous covalent triazine adsorbent. J. Nat. Gas Eng. 2020, 83, 103582. [Google Scholar] [CrossRef]
- Wang, G.; Leus, K.; Jena, H.S.; Krishnaraj, C.; Zhao, S.; Depauw, H.; Tahir, N.; Liu, Y.-Y.; Van Der Voort, P. A fluorine-containing hydrophobic covalent triazine framework with excellent selective CO2 capture performance. J. Mater. Chem. A 2018, 6, 6370–6375. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, K.X.; Teng, B.; Zhang, T.; Han, Y. A perfluorinated covalent triazine-based framework for highly selective and water–tolerant CO2 capture. Energy Environ. Sci. 2013, 6, 3684–3692. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Chemical functionalization strategies for carbon dioxide capture in microporous organic polymers. Polym. Int. 2013, 62, 345–352. [Google Scholar] [CrossRef]
- Flaig, R.W.; Osborn Popp, T.M.; Fracaroli, A.M.; Kapustin, E.A.; Kalmutzki, M.J.; Altamimi, R.M.; Fathieh, F.; Reimer, J.A.; Yaghi, O.M. The Chemistry of CO2 Capture in an Amine-Functionalized Metal–Organic Framework under Dry and Humid Conditions. J. Am. Chem. Soc. 2017, 139, 12125–12128. [Google Scholar] [CrossRef] [PubMed]
- Gunasekar, G.H.; Park, K.; Ganesan, V.; Lee, K.; Kim, N.-K.; Jung, K.-D.; Yoon, S. A Covalent Triazine Framework, Functionalized with Ir/N-Heterocyclic Carbene Sites, for the Efficient Hydrogenation of CO2 to Formate. Chem. Mater. 2017, 29, 6740–6748. [Google Scholar] [CrossRef]
- Jia, J.; Chen, Z.; Belmabkhout, Y.; Adil, K.; Bhatt, P.M.; Solovyeva, V.A.; Shekhah, O.; Eddaoudi, M. Carbonization of covalent triazine-based frameworks via ionic liquid induction. J. Mater. Chem. A 2018, 6, 15564–15568. [Google Scholar] [CrossRef]
- Keskin, S.; van Heest, T.M.; Sholl, D.S. Can Metal–Organic Framework Materials Play a Useful Role in Large-Scale Carbon Dioxide Separations? ChemSusChem 2010, 3, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, S. Recent Advancements in the Synthesis of Covalent Triazine Frameworks for Energy and Environmental Applications. Polymers 2019, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Antonietti, M. General, Metal-free Synthesis of Carbon Nanofiber Assemblies from Plant Oils. Angew. Chem. Int. Ed. 2021, 60, 24257–24265. [Google Scholar] [CrossRef] [PubMed]
- Katekomol, P.; Roeser, J.; Bojdys, M.; Weber, J.; Thomas, A. Covalent Triazine Frameworks Prepared from 1,3,5-Tricyanobenzene. Chem. Mater. 2013, 25, 1542–1548. [Google Scholar] [CrossRef]
- Kuecken, S.; Schmidt, J.; Zhi, L.; Thomas, A. Conversion of amorphous polymer networks to covalent organic frameworks under ionothermal conditions: A facile synthesis route for covalent triazine frameworks. J. Mater. Chem. A 2015, 3, 24422–24427. [Google Scholar] [CrossRef]
- Ren, S.; Bojdys, M.J.; Dawson, R.; Laybourn, A.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. Porous, Fluorescent, Covalent Triazine-Based Frameworks Via Room-Temperature and Microwave-Assisted Synthesis. Adv. Mater. 2012, 24, 2357–2361. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Vasylyeva, V.; Janiak, C. From a Supramolecular Tetranitrile to a Porous Covalent Triazine-Based Framework with High Gas Uptake Capacities. Chem. Commun. 2013, 49, 3961–3963. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, S.; Xiao, Y.-X.; Dietrich, D.; Giesen, B.; Barthel, J.; Ying, J.; Yang, X.-Y.; Janiak, C. Nickel nanoparticles supported on a covalent triazine framework as electrocatalyst for oxygen evolution reaction and oxygen reduction reactions. Beilstein J. Nanotechnol. 2020, 11, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Bhunia, A.; Breitzke, H.; Groszewicz, P.B.; Buntkowsky, G.; Janiak, C. Two linkers are better than one: Enhancing CO2 capture and separation with porous covalent triazine-based frameworks from mixed nitrile linkers. J. Mater. Chem. A 2017, 5, 3609–3620. [Google Scholar] [CrossRef]
- Oxley, J.C.; Smith, J.L.; Moran, J.S. Decomposition of Azo- and Hydrazo-Linked Bis Triazines. J. Energy Mater. 2009, 27, 63–93. [Google Scholar] [CrossRef]
- Kuhn, P.; Thomas, A.; Antonietti, M. Toward Tailorable Porous Organic Polymer Networks: A High-Temperature Dynamic Polymerization Scheme Based on Aromatic Nitriles. Macromolecules 2009, 42, 319–326. [Google Scholar] [CrossRef]
- Preis, E.; Dong, W.; Brunklaus, G.; Scherf, U. Microporous, tetraarylethylene-based polymer networks generated in a reductive polyolefination process. J. Mater. Chem. C 2015, 3, 1582–1587. [Google Scholar] [CrossRef]
- Dey, S.; Bhunia, A.; Boldog, I.; Janiak, C. A mixed-linker approach towards improving covalent triazine-based frameworks for CO2 capture and separation. Microporous Mesoporous Mater. 2017, 241, 303–315. [Google Scholar] [CrossRef]
- Bhunia, A.; Boldog, I.; Möller, A.; Janiak, C. Highly stable nanoporous covalent triazine-based frameworks with an adamantane core for carbon dioxide sorption and separation. J. Mater. Chem. A 2013, 1, 14990–14999. [Google Scholar] [CrossRef]
- Tao, L.; Niu, F.; Liu, J.; Wang, T.; Wang, Q. Troger’s base functionalized covalent triazine frameworks for CO2 capture. RSC Adv. 2016, 6, 94365–94372. [Google Scholar] [CrossRef]
- Quantachrome Instruments (1900 Corporate Drive, Boynton Beach, FL 33426 USA) Powder Tech Note 35. Available online: https://wiki.anton-paar.com/en/gas-adsorption-for-surface-area-and-pore-size-analysis/ (accessed on 25 June 2025).
- Lim, H.; Cha, M.C.; Chang, J.Y. Preparation of Microporous Polymers Based on 1,3,5-Triazine Units Showing High CO2 Adsorption Capacity. Macromol. Chem. Phys. 2012, 213, 1385–1390. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yang, J.-P.; Yang, B.-T.; Chen, C.-C.; Lai, L.-L. Introduction of a spiro-linker in triazine-based polymers to enlarge void space and increase IPA adsorbing capacity to 164.7 mg/g at 1000 ppm. J. Taiwan Inst. Chem. Eng. 2022, 140, 104531. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, D.; Huang, D.; Zeng, G.; Xu, P.; Lai, C.; Chen, M.; Cheng, M.; Zhang, C.; Wang, Z. Covalent triazine frameworks for carbon dioxide capture. J. Mater. Chem. A 2019, 7, 22848–22870. [Google Scholar] [CrossRef]
- Özdemir, J.; Mosleh, I.; Abolhassani, M.; Greenlee, L.F.; Beitle, R.R.; Beyzavi, M.H. Covalent Organic Frameworks for the Capture, Fixation, or Reduction of CO2. Front. Energy Res. 2019, 7, 77. [Google Scholar] [CrossRef]
- Hug, S.; Mesch, M.B.; Oh, H.; Popp, N.; Hirscher, M.; Senker, J.; Lotsch, B.V. A fluorene based covalent triazine framework with high CO2 and H2 capture and storage capacities. J. Mater. Chem. A 2014, 2, 5928–5936. [Google Scholar] [CrossRef]
- Lee, Y.J.; Talapaneni, S.N.; Coskun, A. Chemically Activated Covalent Triazine Frameworks with Enhanced Textural Properties for High Capacity Gas Storage. ACS Appl. Mater. Interfaces 2017, 9, 30679–30685. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Leus, K.; Zhao, S.; Van Der Voort, P. Newly Designed Covalent Triazine Framework Based on Novel N-Heteroaromatic Building Blocks for Efficient CO2 and H2 Capture and Storage. ACS Appl. Mater. Interfaces 2018, 10, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Bustam, M.A.; Kari, N.E.; Yeong, Y.F. Ideal Adsorbed Solution Theory (IAST) of Carbon Dioxide and Methane Adsorption Using Magnesium Gallate Metal-Organic Framework (Mg-gallate). Molecules 2023, 28, 3016. [Google Scholar] [CrossRef] [PubMed]
- Nuhnen, A.; Janiak, C. A practical guide to calculate the isosteric heat/enthalpy of adsorption via adsorption isotherms in metal–organic frameworks, MOFs. Dalton Trans. 2020, 49, 10295–10307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jin, Z.; Su, H.; Zhang, J.; Yao, X.; Zhao, H.; Zhu, G. Target synthesis of a novel porous aromatic framework and its highly selective separation of CO2/CH4. Chem. Commun. 2013, 49, 2780–2782. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zou, Z.; Cai, Y.; Qiu, Y.; Zhou, Y.; He, S. An updated study on CH4 isothermal adsorption and isosteric adsorption heat behaviors of variable rank coals. J. Nat. Gas Sci. Eng. 2021, 89, 103899. [Google Scholar] [CrossRef]
- Woschko, D.; Yilmaz, S.; Jansen, C.; Spieß, A.; Oestreich, R.; Matemb Ma Ntep, T.; Janiak, C. Enhanced sorption in an indiumacetylenedicarboxylate metal–organic framework with unexpected chains of cis-μ-OH-connected {InO6} octahedra. Dalton Trans. 2023, 52, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Tuci, G.; Iemhoff, A.; Ba, H.; Luconi, L.; Rossin, A.; Papaefthimiou, V.; Palkovits, R.; Artz, J.; Pham-Huu, C.; Giambastiani, G. Playing with covalent triazine framework tiles for improved CO2 adsorption properties and catalytic performance. Beilstein J. Nanotechnol. 2019, 10, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Tuci, G.; Pilaski, M.; Ba, H.; Rossin, A.; Luconi, L.; Caporali, S.; Pham-Huu, C.; Palkovits, R.; Giambastiani, G. Unraveling Surface Basicity and Bulk Morphology Relationship on Covalent Triazine Frameworks with Unique Catalytic and Gas Adsorption Properties. Adv. Funct. Mater. 2017, 27, 1605672. [Google Scholar] [CrossRef]
- Hug, S.; Stegbauer, L.; Oh, H.; Hirscher, M.; Lotsch, B.V. Nitrogen-Rich Covalent Triazine Frameworks as High-Performance Platforms for Selective Carbon Capture and Storage. Chem. Mater. 2015, 27, 8001–8010. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, C.; Veith, G.M.; Abney, C.W.; Dehaudt, J.; Dai, S. In Situ Doping Strategy for the Preparation of Conjugated Triazine Frameworks Displaying Efficient CO2 Capture Performance. J. Am. Chem. Soc. 2016, 138, 11497–11500. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Liu, C.; Zong, L.; Yu, G.; Cheng, S.; Wang, J.; Weng, Z.; Jian, X. Promoting and Tuning Porosity of Flexible Ether-Linked Phthalazinone-Based Covalent Triazine Frameworks Utilizing Substitution Effect for Effective CO2 Capture. ACS Appl. Mater. Interfaces 2017, 9, 13201–13212. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, K.; Kim, H.; Ganesan, V.; Cho, K.; Jeong, S.K.; Yoon, S. Preparation of covalent triazine frameworks with imidazolium cations embedded in basic sites and their application for CO2 capture. J. Mater. Chem. A 2017, 5, 8576–8582. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Z.; Li, S.; He, X.; Pan, C.; Yan, J.; Yu, G. Functionalized Covalent Triazine Frameworks for Effective CO2 and SO2 Removal. ACS Appl. Mater. Interfaces 2018, 10, 36002–36009. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Liu, C.; Liu, C.; Zhang, S.; Yu, G.; Yang, L.; Yang, F.; Jian, X. Construction of triphenylamine functional phthalazinone-based covalent triazine frameworks for effective CO2 capture. Polym. J. 2018, 151, 65–74. [Google Scholar] [CrossRef]
- Du, J.; Liu, Y.; Krishna, R.; Yu, Y.; Cui, Y.; Wang, S.; Liu, Y.; Song, X.; Liang, Z. Enhancing Gas Sorption and Separation Performance via Bisbenzimidazole Functionalization of Highly Porous Covalent Triazine Frameworks. ACS Appl. Mater. Interfaces 2018, 10, 26678–26686. [Google Scholar] [CrossRef] [PubMed]
- Jena, H.S.; Krishnaraj, C.; Wang, G.; Leus, K.; Schmidt, J.; Chaoui, N.; Van Der Voort, P. Acetylacetone Covalent Triazine Framework: An Efficient Carbon Capture and Storage Material and a Highly Stable Heterogeneous Catalyst. Chem. Mater. 2018, 30, 4102–4111. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, M.; Manna, A.; Krishna, R.; Das, S. Newly designed 1,2,3-triazole functionalized covalent triazine frameworks with exceptionally high uptake capacity for both CO2 and H2. J. Mater. Chem. A 2019, 7, 1055–1068. [Google Scholar] [CrossRef]
- Sánchez, M.I.; Martínez-Costas, J.; Mascareñas, J.L.; Vázquez, M.E. MitoBlue: A Nontoxic and Photostable Blue-Emitting Dye That Selectively Labels Functional Mitochondria. ACS Chem. Biol. 2014, 9, 2742–2747. [Google Scholar] [CrossRef] [PubMed]


| CTF Product (a) | Molar Ratio ZnCl2/Monomer | Temperature (°C) | Yield (%) |
|---|---|---|---|
| pBN-CTF-10-350 | 10 | 350 | 79 |
| pBN-CTF-20-350 | 20 | 350 | 78 |
| pBN-CTF-10-400 | 10 | 400 | 68 |
| pBN-CTF-20-400 | 20 | 400 | 92 |
| pBN-CTF-10-550 | 10 | 550 | 84 |
| pBN-CTF-20-550 | 20 | 550 | 40 |
| CTF Product | SBET (a) (m2 g−1) | Vtot (b) (cm3 g−1) | Vmicro (c) (cm3 g−1) | Vmicro/Vtot (d) | V1nm(CO2) (e) (cm3 g−1) |
|---|---|---|---|---|---|
| pBN-CTF-10-400 | 809 | 0.51 | 0.25 | 0.50 | 0.015 |
| pBN-CTF-20-400 | 348 | 0.19 | 0.15 | 0.79 | 0.009 |
| pBN-CTF-10-550 | 1460 | 1.04 | 0.36 | 0.35 | 0.013 |
| pBN-CTF-20-550 | 950 | 1.25 | 0.19 | 0.31 | 0.010 |
| CTF Product | SBET (195 K) (a) (m2 g−1) | CO2 (cm3 g−1) | CH4 (cm3 g−1) | CO2 Qads 0 (b) (kJ mol−1) | CH4 Qads 0 (b) (kJ mol−1) | IAST Selectivity for 50:50 CO2:CH4 | |||
|---|---|---|---|---|---|---|---|---|---|
| 293 K | 283 K | 195 K | 293 K | 283 K | |||||
| pBN-CTF-10-400 | 524 | 42.8 | 55.9 | 175 | 11.3 | 12.9 | 79 | 36 | 22 |
| pBN-CTF-10-550 | 746 | 54.0 | 61.7 | 320 | 12.3 | 16.9 | 60 | 39 | - (c) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, H.; Oestreich, R.; Küll, V.; Fetzer, M.N.A.; Janiak, C. Synthesis and Characterization of Covalent Triazine Frameworks Based on 4,4′-(Phenazine-5,10-diyl)dibenzonitrile and Its Application in CO2/CH4 Separation. Molecules 2025, 30, 3110. https://doi.org/10.3390/molecules30153110
Othman H, Oestreich R, Küll V, Fetzer MNA, Janiak C. Synthesis and Characterization of Covalent Triazine Frameworks Based on 4,4′-(Phenazine-5,10-diyl)dibenzonitrile and Its Application in CO2/CH4 Separation. Molecules. 2025; 30(15):3110. https://doi.org/10.3390/molecules30153110
Chicago/Turabian StyleOthman, Hanibal, Robert Oestreich, Vivian Küll, Marcus N. A. Fetzer, and Christoph Janiak. 2025. "Synthesis and Characterization of Covalent Triazine Frameworks Based on 4,4′-(Phenazine-5,10-diyl)dibenzonitrile and Its Application in CO2/CH4 Separation" Molecules 30, no. 15: 3110. https://doi.org/10.3390/molecules30153110
APA StyleOthman, H., Oestreich, R., Küll, V., Fetzer, M. N. A., & Janiak, C. (2025). Synthesis and Characterization of Covalent Triazine Frameworks Based on 4,4′-(Phenazine-5,10-diyl)dibenzonitrile and Its Application in CO2/CH4 Separation. Molecules, 30(15), 3110. https://doi.org/10.3390/molecules30153110








