Fabrication of Antibacterial Poly(ethylene terephthalate)/Graphene Nanocomposite Fibers by In Situ Polymerization for Fruit Preservation
Abstract
1. Introduction
2. Results
2.1. Preparation of Graphene/PET Nanocomposite Fibers
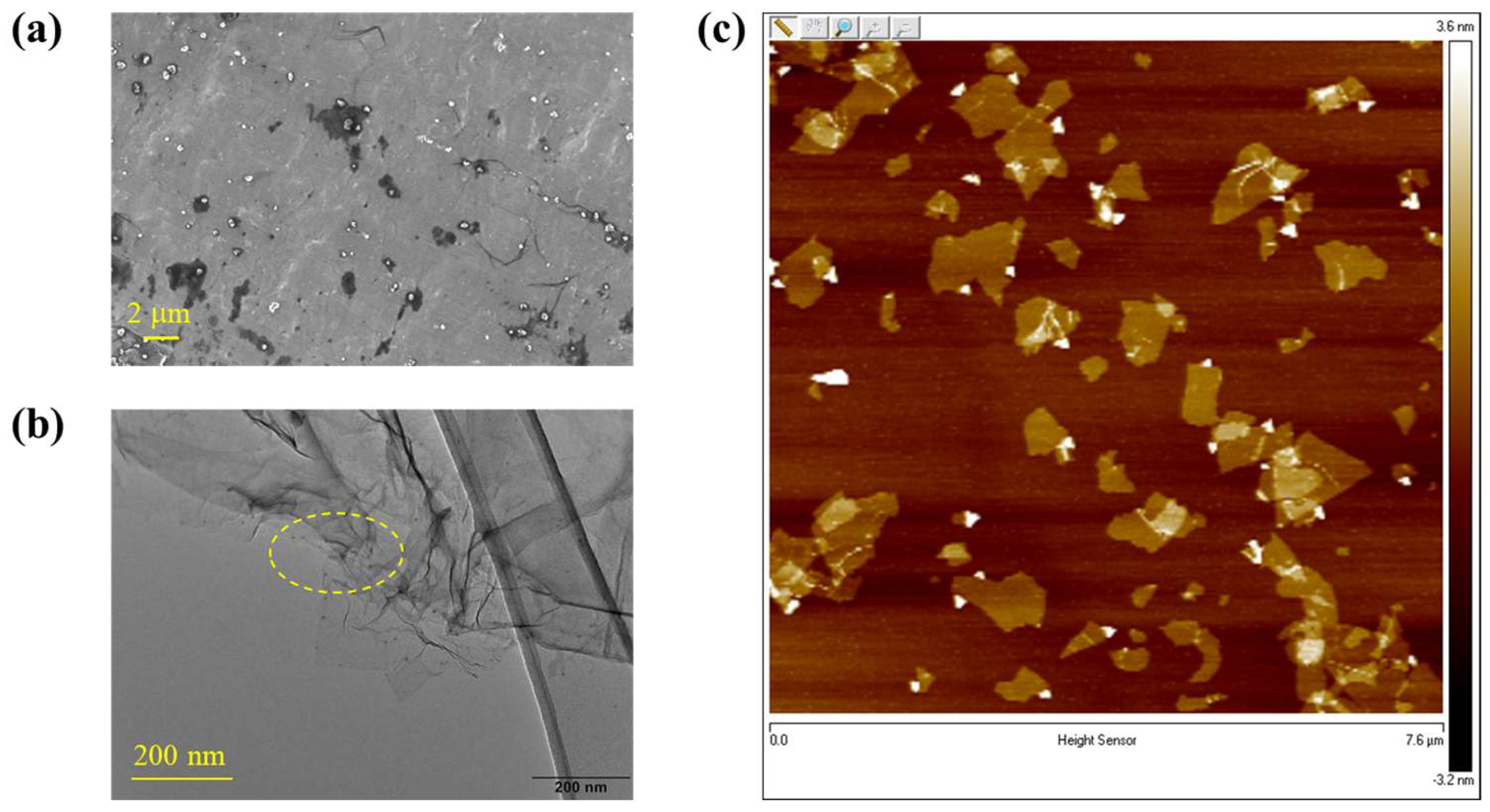
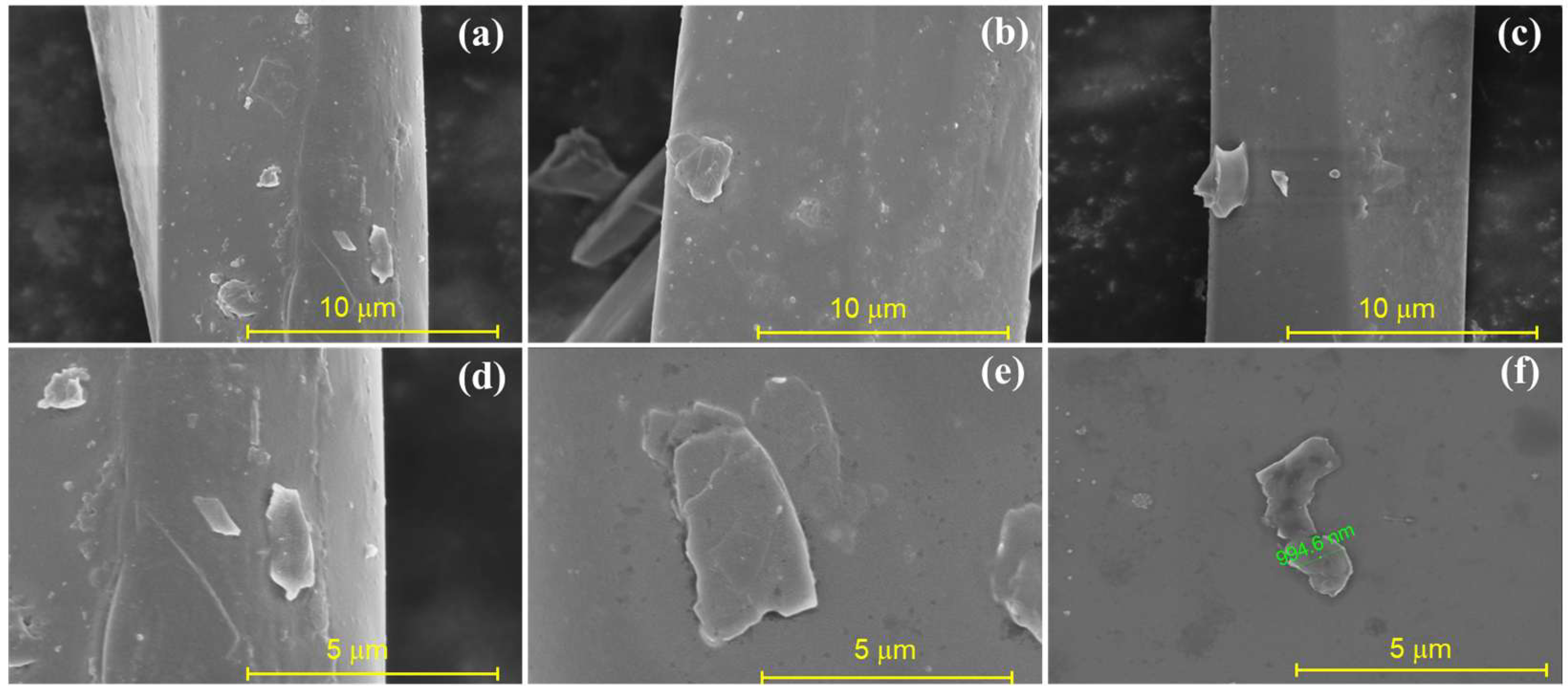
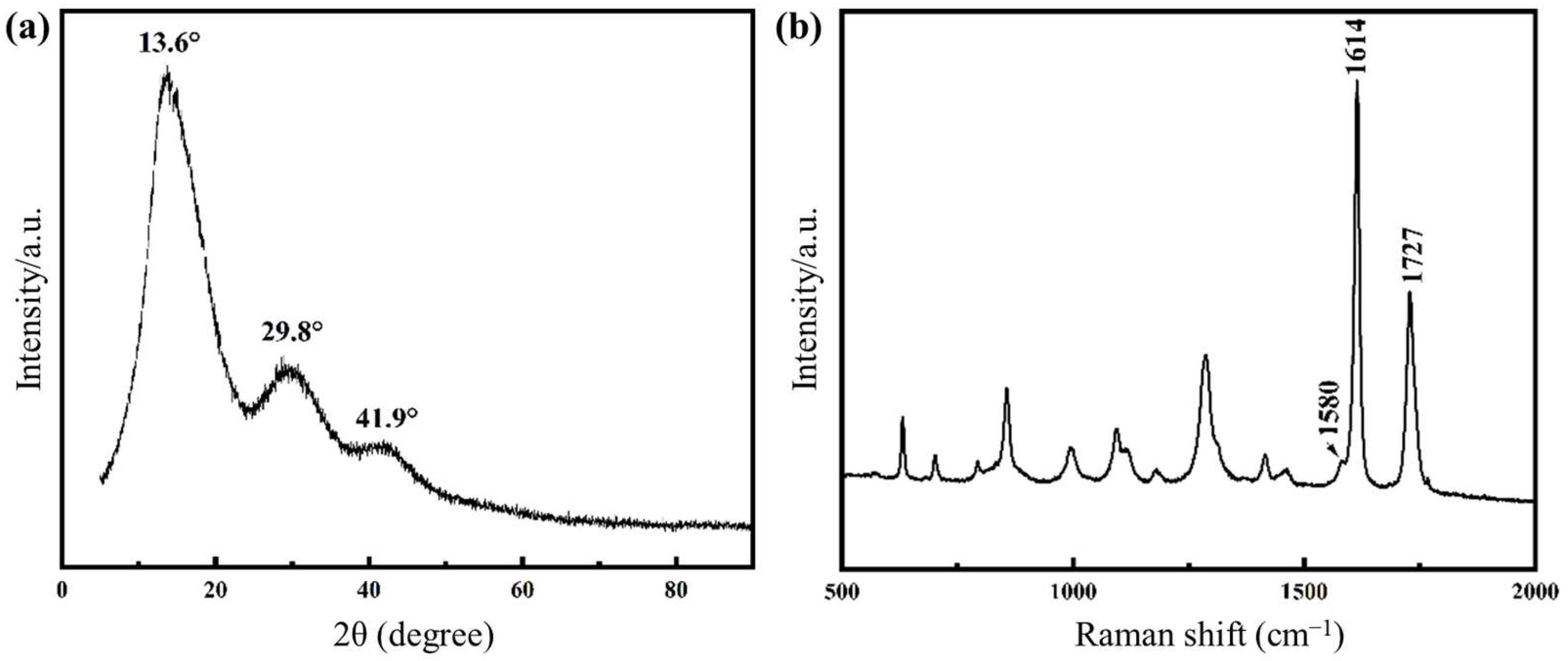
2.2. Structure and Morphology of the Nanocomposite Fibers
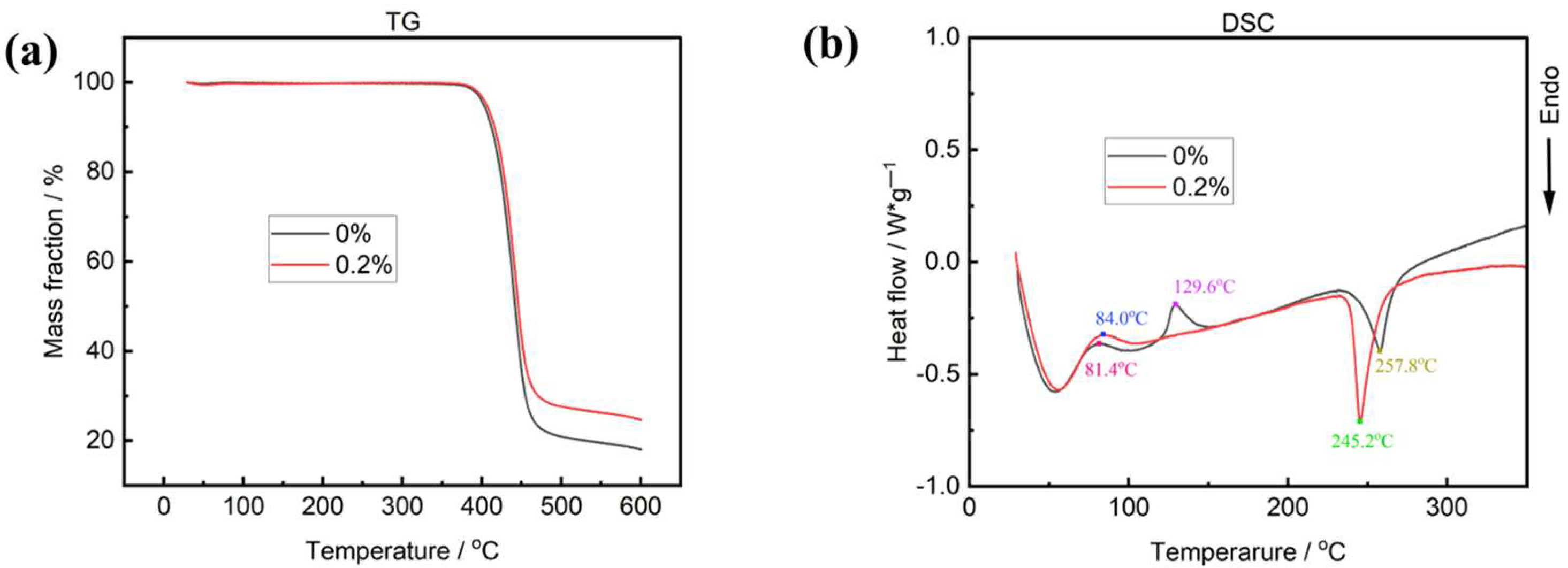
2.3. Thermal Analysis of the Nanocomposite Fibers
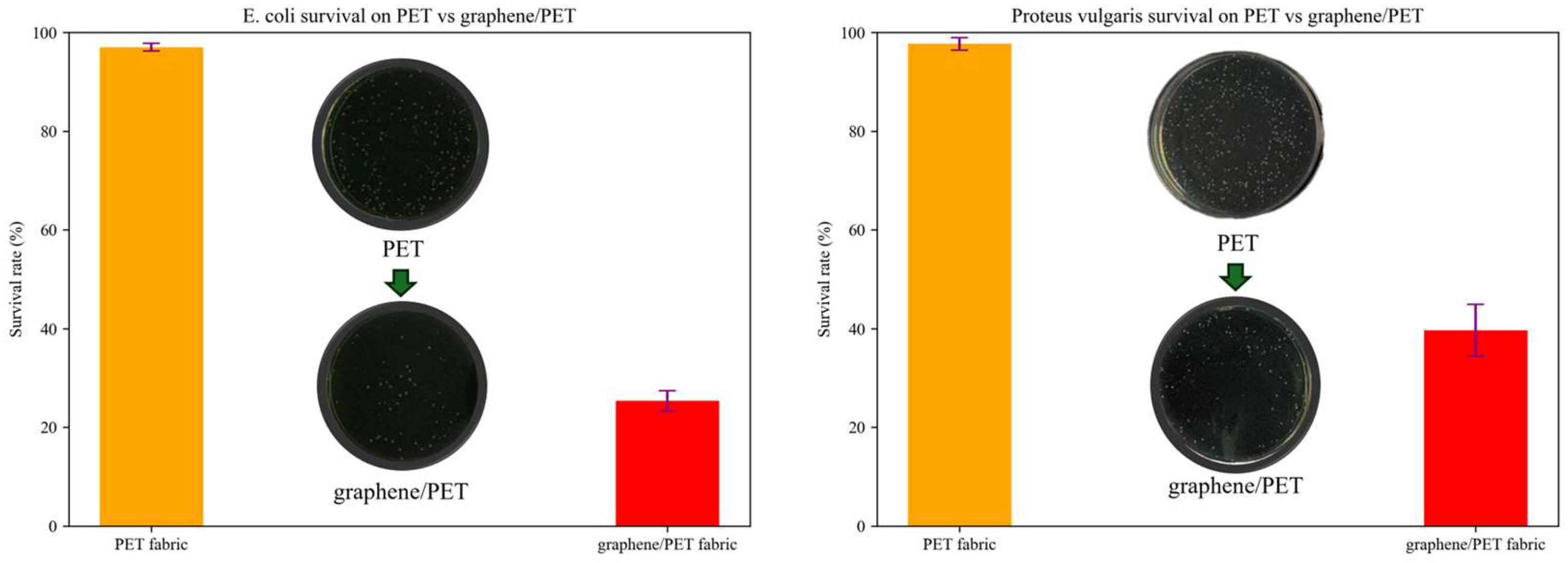
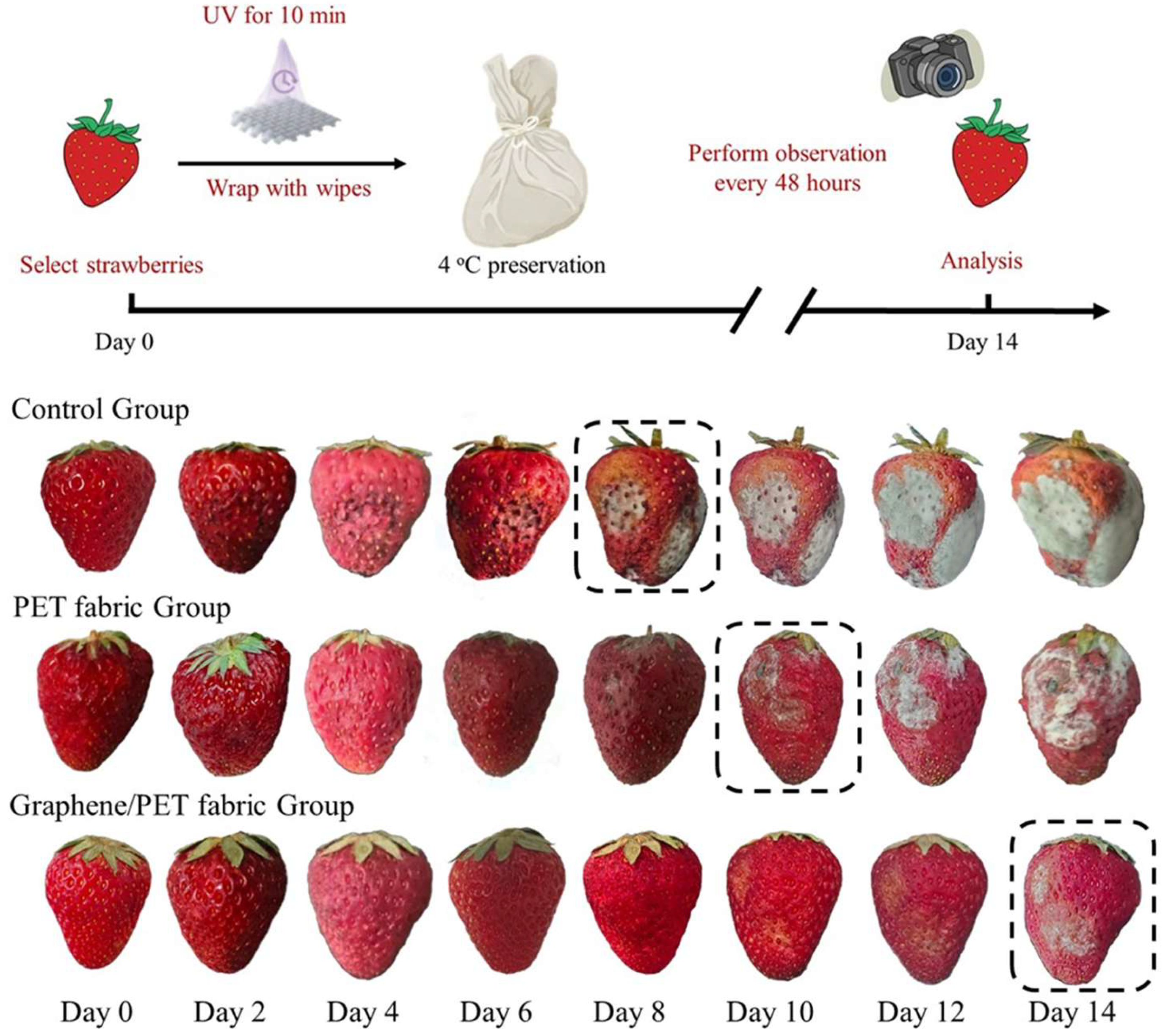
2.4. Antibacterial Activity and Fruit Preservation Using the Nanocomposite Fabric
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Graphene/PET Nanocomposites
3.3. Characterization Methods
3.4. In Vitro Antibacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent Progress in Graphene/Polymer Nanocomposites. Adv. Mater. 2020, 33, 2001105. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Cheong, I.W. Recent Studies on Dispersion of Graphene–Polymer Composites. Polymers 2021, 13, 2375. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Abdala, A.A.; Mittal, V.; Seifert, S.; Herring, A.M.; Liberatore, M.W. Processable conductive graphene/polyethylene nanocomposites: Effects of graphene dispersion and polyethylene blending with oxidized polyethylene on rheology and microstructure. Polymer 2016, 98, 143–155. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, K.H.; Parvez, M.M.H.; Irizarry, N.; Uddin, M.N. Polymer Nanocomposites with Optimized Nanoparticle Dispersion and Enhanced Functionalities for Industrial Applications. Processes 2025, 13, 994. [Google Scholar] [CrossRef]
- Wang, H.; Fu, Z.; Zhao, X.; Li, Y.; Li, J. Reactive Nanoparticles Compatibilized Immiscible Polymer Blends: Synthesis of Reactive SiO2 with Long Poly(methyl methacrylate) Chains and the in Situ Formation of Janus SiO2 Nanoparticles Anchored Exclusively at the Interface. ACS Appl. Mater. Interfaces 2017, 9, 14358–14370. [Google Scholar] [CrossRef] [PubMed]
- Tarannum, F.; Muthaiah, R.; Danayat, S.; Foley, K.; Annam, R.S.; Walters, K.B.; Garg, J. Chemically Edge-Carboxylated Graphene Enhances the Thermal Conductivity of Polyetherimide–Graphene Nanocomposites. ACS Appl. Mater. Interfaces 2022, 14, 14753–14763. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
- Hufenus, R.; Yan, Y.; Dauner, M.; Kikutani, T. Melt-Spun Fibers for Textile Applications. Materials 2020, 13, 4298. [Google Scholar] [CrossRef]
- Scarano, D.; Cesano, F. Graphene and Other 2D Layered Nanomaterials and Hybrid Structures: Synthesis, Properties and Applications. Materials 2021, 14, 7108. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, C.; Wang, B.; Chen, Y.; Wang, H. In situ polymerization and characterization of graphite nanoplatelet/poly(ethylene terephthalate) nanocomposites for construction of melt-spun fibers. RSC Adv. 2017, 7, 33477–33485. [Google Scholar] [CrossRef]
- Leon-Boigues, L.; Flores, A.; Gomez-Fatou, M.A.; Vega, J.F.; Ellis, G.J.; Salavagione, H.J. PET/Graphene Nanocomposite Fibers Obtained by Dry-Jet Wet-Spinning for Conductive Textiles. Polymers 2023, 15, 1245. [Google Scholar] [CrossRef]
- Gungordu Er, S.; Edirisinghe, M.; Tabish, T.A. Graphene-Based Nanocomposites as Antibacterial, Antiviral and Antifungal Agents. Adv. Healthc. Mater. 2023, 12, e2201523. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Luceno-Sanchez, J.A. Antibacterial Activity of Polymer Nanocomposites Incorporating Graphene and Its Derivatives: A State of Art. Polymers 2021, 13, 2105. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, J.; Zhou, H.; Zhou, S.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Wang, H. Biodegradable intelligent film for food preservation and real-time visual detection of food freshness. Food Hydrocoll. 2022, 129, 107665. [Google Scholar] [CrossRef]
- Wang, J.; Lv, S.; Gui, X.; Yu, Y. Degradable rGO-MoS2-Fe2O3 based carboxymethyl cellulose packaging films for fruit preservation. Soft Mater. 2024, 22, 139–148. [Google Scholar] [CrossRef]
- Guo, J.; Khan, M.R.; Ahmad, N.; Zhang, W. Enhancing fruit preservation with sodium alginate films incorporating propolis extract and graphene oxide. Int. J. Biol. Macromol. 2025, 288, 138778. [Google Scholar] [CrossRef]
- Wu, L.; Lv, S.; Wei, D.; Zhang, S.; Zhang, S.; Li, Z.; Liu, L.; He, T. Structure and properties of starch/chitosan food packaging film containing ultra-low dosage GO with barrier and antibacterial. Food Hydrocoll. 2023, 137, 108329. [Google Scholar] [CrossRef]
- Lan, W.; Wang, S.; Chen, M.; Sameen, D.E.; Lee, K.; Liu, Y. Developing poly(vinyl alcohol)/chitosan films incorporate with d-limonene: Study of structural, antibacterial, and fruit preservation properties. Int. J. Biol. Macromol. 2020, 145, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, S.; Koh, J. Physicochemical and optical activity of chitosan based ternary nanocomposites for food packaging applications. J. Mol. Struct. 2024, 1311, 138210. [Google Scholar] [CrossRef]
- Tiwari, A.; Tiwari, A.; Kumar, S.; Singh, S.; Dutta, P.K. Chapter 18—Perspectives for polymer-based antimicrobial films in food packaging applications. In Nanobiotechnology for Food Processing and Packaging; Academic Press: Cambridge, MA, USA, 2024; pp. 323–366. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, Y.; Li, Y.; Peng, J.; Wang, G.; Ong, W.J.; Li, N. MXenes: An emergent materials for packaging platforms and looking beyond. Nano Sel. 2022, 3, 1123–1147. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Fabrication of polylactic acid/carbon nanotubes/chitosan composite fibers by electrospinning for strawberry preservation. Int. J. Biol. Macromol. 2019, 121, 1329–1336. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, Y.; Liu, Y.; Li, X.; Wu, S. Application of chitosan in fruit preservation: A review. Food Chem.: X 2024, 23, 101589. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.A.; Materon, L.; Parsons, J.G.; Alcoutlabi, M. Synthesis, Characterization, and Antibacterial Activity of Graphene Oxide/Zinc Hydroxide Nanocomposites. Appl. Sci. 2024, 14, 6274. [Google Scholar] [CrossRef]
- Xing, L.; Wang, Y.; Wang, S.; Zhang, Y.; Mao, S.; Wang, G.; Liu, J.; Huang, L.; Li, H.; Belfiore, L.A.; et al. Effects of Modified Graphene Oxide on Thermal and Crystallization Properties of PET. Polymers 2018, 10, 613. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Vickery, W.M.; Lee, K.; Lee, S.M.; Orlando, J.D.; Sydlik, S.A. Plastic Composites from Repurposed Poly(ethylene terephthalate) Wasted Functionalized Graphene Oxide through Dynamic Depolymerization. ACS Appl. Nano Mater. 2024, 7, 3691–3701. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L. Crystallization of Poly(ethylene terephthalate): A Review. Polymers 2024, 16, 1975. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. In situ Polymerization Approach to Graphene-Reinforced Nylon-6 Composites. Macromolecules 2010, 43, 6716–6723. [Google Scholar] [CrossRef]
- Ahmad, V.; Ansari, M.O. Antimicrobial Activity of Graphene-Based Nanocomposites: Synthesis, Characterization, and Their Applications for Human Welfare. Nanomaterials 2022, 12, 4002. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Handy, R.D.; Upton, M.; Besinis, A. Review of Antimicrobial Nanocoatings in Medicine and Dentistry: Mechanisms of Action, Biocompatibility Performance, Safety, and Benefits Compared to Antibiotics. ACS Nano 2023, 17, 7064–7092. [Google Scholar] [CrossRef]
- Chong, Y.; Ge, C.; Fang, G.; Tian, X.; Ma, X.; Wen, T.; Wamer, W.G.; Chen, C.; Chai, Z.; Yin, J.-J. Crossover between Anti- and Pro-oxidant Activities of Graphene Quantum Dots in the Absence or Presence of Light. ACS Nano 2016, 10, 8690–8699. [Google Scholar] [CrossRef]
- Guo, S.; Raya, J.; Ji, D.; Nishina, Y.; Ménard-Moyon, C.; Bianco, A. Is carboxylation an efficient method for graphene oxide functionalization? Nanoscale Adv. 2020, 2, 4085–4092. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Chen, Q.; Han, A.; Liu, M.; Zhong, W.; Shao, X.; Jiang, Y.; Lin, J.; Luo, Z.; Yang, J.; et al. Fabrication of Antibacterial Poly(ethylene terephthalate)/Graphene Nanocomposite Fibers by In Situ Polymerization for Fruit Preservation. Molecules 2025, 30, 3109. https://doi.org/10.3390/molecules30153109
Wu J, Chen Q, Han A, Liu M, Zhong W, Shao X, Jiang Y, Lin J, Luo Z, Yang J, et al. Fabrication of Antibacterial Poly(ethylene terephthalate)/Graphene Nanocomposite Fibers by In Situ Polymerization for Fruit Preservation. Molecules. 2025; 30(15):3109. https://doi.org/10.3390/molecules30153109
Chicago/Turabian StyleWu, Jiarui, Qinhan Chen, Aobin Han, Min Liu, Wenhuan Zhong, Xiaojue Shao, Yan Jiang, Jing Lin, Zhenyang Luo, Jie Yang, and et al. 2025. "Fabrication of Antibacterial Poly(ethylene terephthalate)/Graphene Nanocomposite Fibers by In Situ Polymerization for Fruit Preservation" Molecules 30, no. 15: 3109. https://doi.org/10.3390/molecules30153109
APA StyleWu, J., Chen, Q., Han, A., Liu, M., Zhong, W., Shao, X., Jiang, Y., Lin, J., Luo, Z., Yang, J., & Li, G. (2025). Fabrication of Antibacterial Poly(ethylene terephthalate)/Graphene Nanocomposite Fibers by In Situ Polymerization for Fruit Preservation. Molecules, 30(15), 3109. https://doi.org/10.3390/molecules30153109






