Phytochemical Profile, Toxicological Screening, Antitumor Activity, and Immunomodulatory Response of Saline Extract from Euphorbia hirta L. Leaves
Abstract
1. Introduction
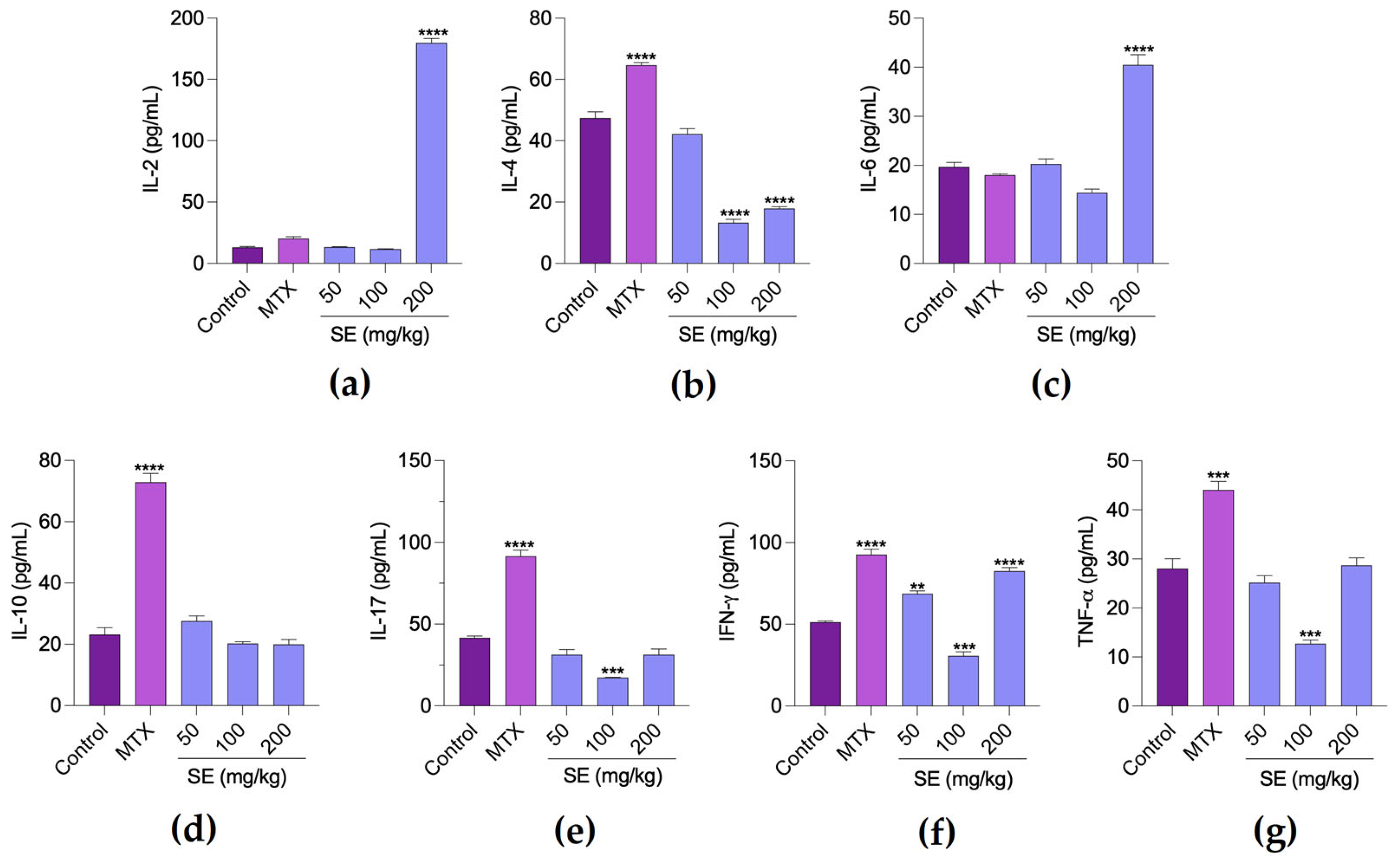
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extract Preparation
4.2. Phytochemical Characterization
4.2.1. Thin Layer Chromatography
4.2.2. Total Phenol Content
4.2.3. Total Flavonoid Content
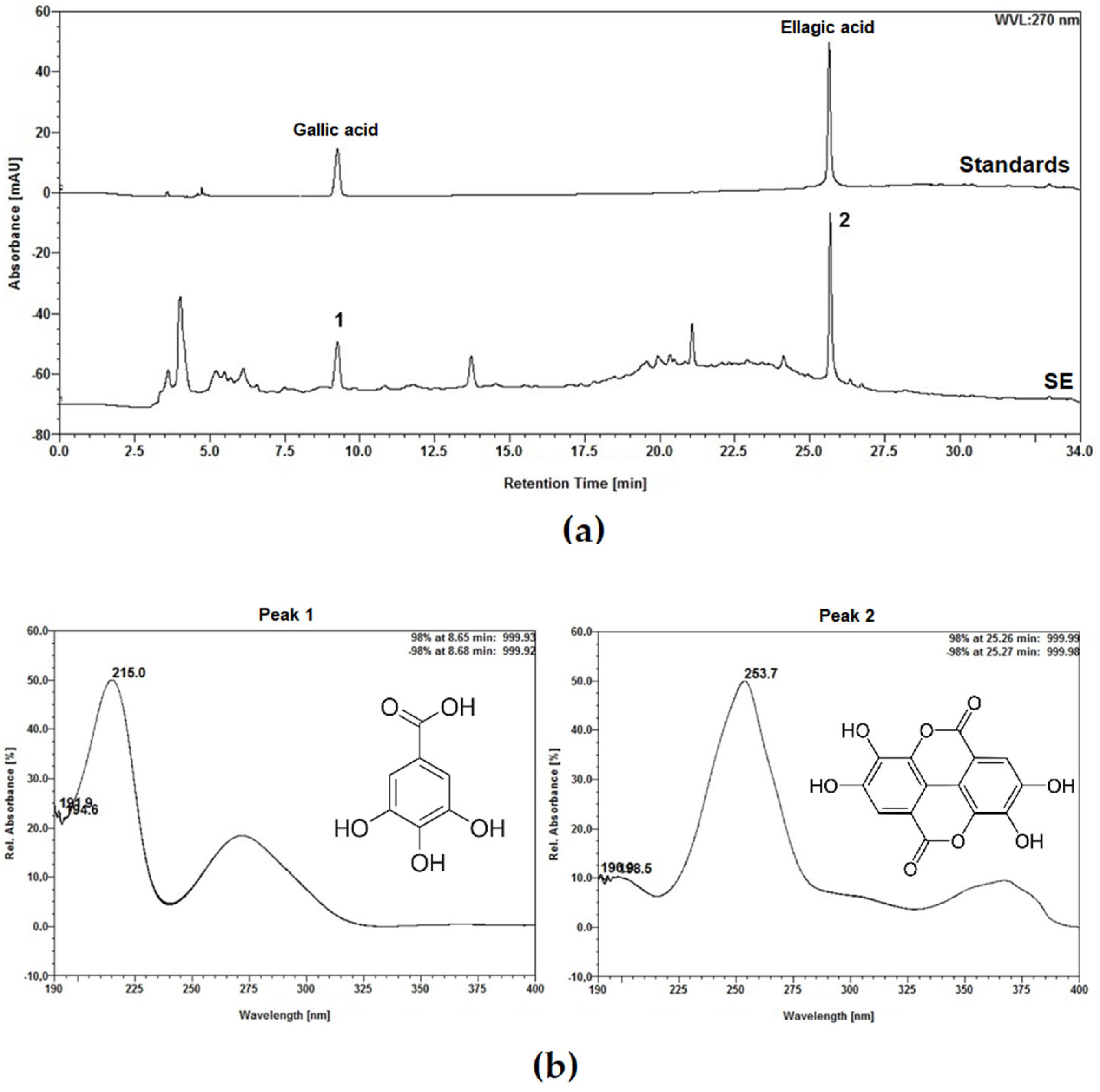
4.2.4. Analysis by High-Performance Liquid Chromatography (HPLC)
4.2.5. Protein Content and Hemagglutinating Activity Assay
4.3. Animals
4.4. Acute Toxicity Assessment
4.4.1. Evaluation of Animal Weight, Water and Food Consumption, and Organ Weight
4.4.2. Evaluation of Hematological and Biochemical Parameters
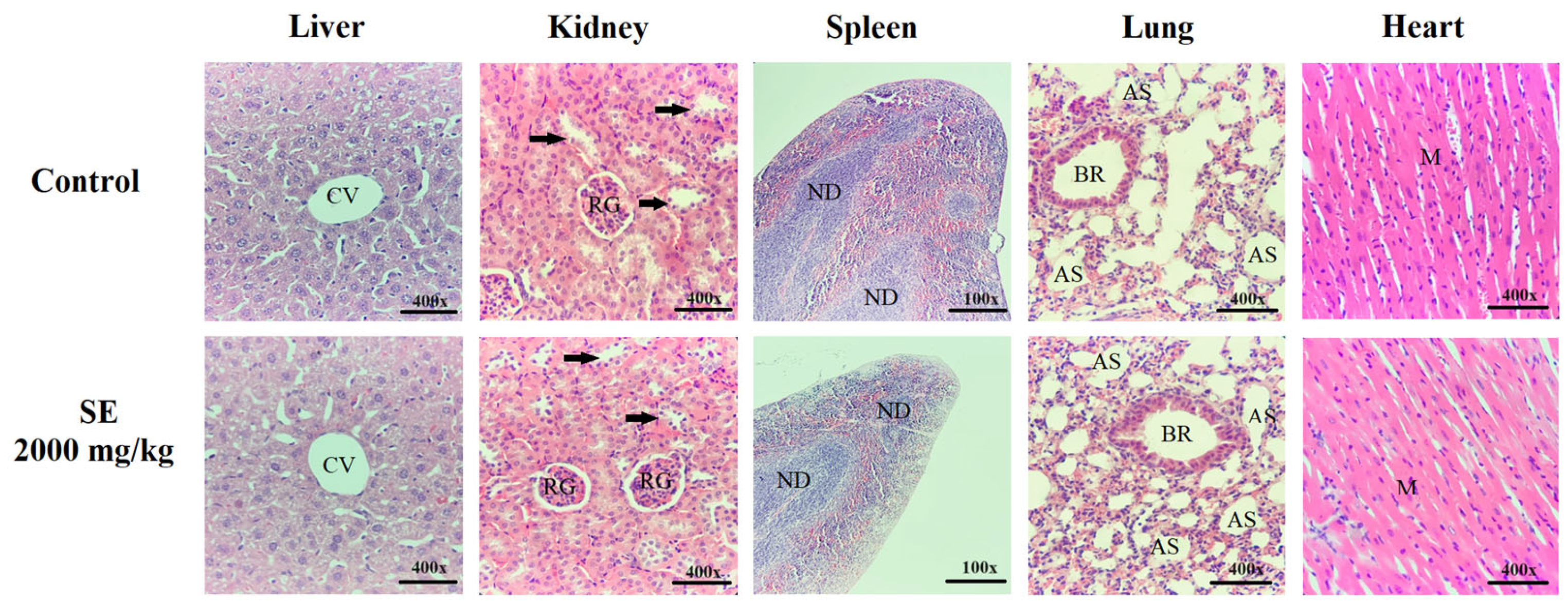
4.4.3. Histopathological Analysis
4.5. Genotoxicity Assessment
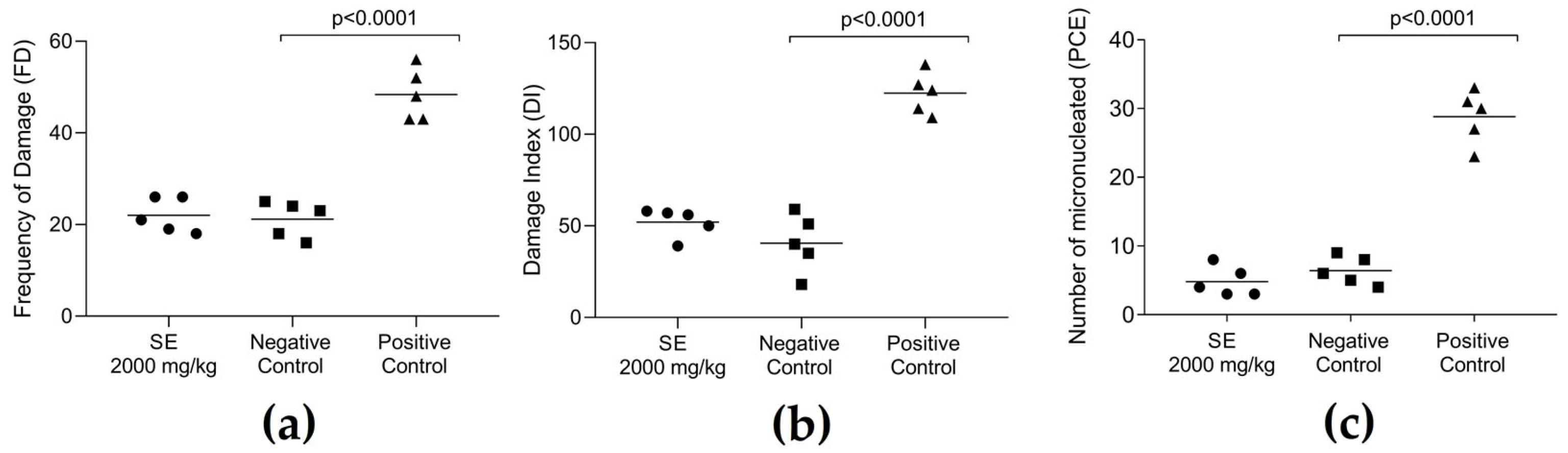
4.5.1. Comet Assay
4.5.2. Micronucleus Test
4.6. Evaluation of Antitumor Activity
4.6.1. In Vitro Evaluation of Cytotoxicity to Sarcoma 180 Cells by MTT Assay
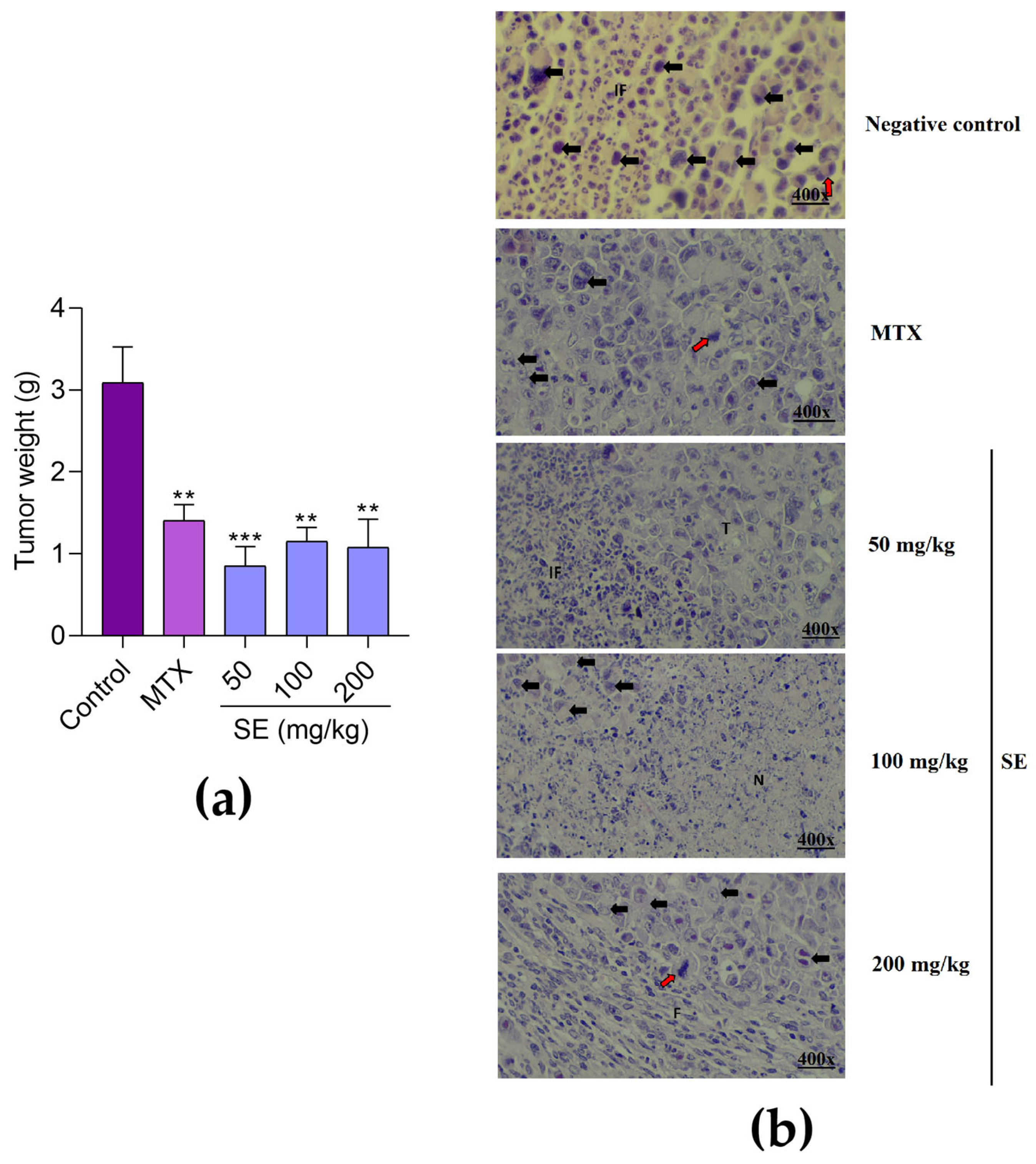
4.6.2. In Vivo Antitumor Evaluation
4.6.3. Evaluation of Cytokine Levels in Tumor Microenvironment
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ostroverkhova, D.; Przytycka, T.M.; Panchenko, A.R. Cancer driver mutations: Predictions and reality. Trends Mol. Med. 2023, 29, 554–566. [Google Scholar] [CrossRef]
- Garces, A.H.I.; Porta, N.; Graham, T.A.; Banerji, U. Clinical trial designs for evaluating and exploiting cancer evolution. Cancer Treat. Rev. 2023, 118, 102583. [Google Scholar] [CrossRef]
- Finley, L.W.S. What is cancer metabolism? Cell 2023, 186, 1670–1688. [Google Scholar] [CrossRef]
- Nematullah, M.; Hasmatullah; Agnihotri, A.; Kumar, S.; Husain, A.; Rahman, A. Evaluation of therapeutics’ drug monitoring during cancer chemotherapy: A review. Intell. Pharm. 2023, 1, 157–161. [Google Scholar] [CrossRef]
- Zeien, J.; Qiu, W.; Triay, M.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kaye, A.D. Clinical implications of chemotherapeutic agent organ toxicity on perioperative care. Biomed. Pharmacother. 2022, 146, 112503. [Google Scholar] [CrossRef]
- Elgemeie, G.H.; Mohamed-Ezzat, R.A. Natural products in chemotherapy of cancers. In New Strategies Targeting Cancer Metabolism; Elgemeie, G.H., Mohamed-Ezzat, R.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 507–546. [Google Scholar]
- Budi, H.S.; Farhood, B. Tumor microenvironment remodeling in oral cancer: Application of plant-derived natural products and nanomaterials. Environ. Res. 2023, 233, 116432. [Google Scholar] [CrossRef]
- Motyka, S.; Jafernik, K.; Ekiert, H.; Sharifi-Rad, J.; Calina, D.; Al-Omari, B.; Szopa, A.; Cho, W.C. Podophyllotoxin and its derivatives: Potential anticancer agents of natural origin in cancer chemotherapy. Biomed. Pharmacother. 2023, 158, 114145. [Google Scholar] [CrossRef]
- Ramos, D.B.M.; Araújo, M.T.M.F.; Araújo, T.C.L.; Neto, O.G.S.; Silva, M.G.; Silva, Y.A.; Torres, D.J.L.; Patriota, L.L.S.; Melo, C.M.L.; Lorena, V.M.B.; et al. Evaluation of antitumor activity and toxicity of Schinus terebinthifolia leaf extract and lectin (SteLL) in sarcoma 180-bearing mice. J. Ethnopharmacol. 2019, 233, 148–157. [Google Scholar] [CrossRef]
- Konozy, E.H.E.; Osman, M.E.M. Plant lectin: A promising future anti-tumor drug. Biochimie 2022, 202, 136–145. [Google Scholar] [CrossRef]
- Okem, A.; Henstra, C.; Lambert, M.; Hayeshi, R. A review of the pharmacodynamic effect of chemo-herbal drug combinations therapy for cancer treatment. Med. Drug Discov. 2023, 17, 100147. [Google Scholar] [CrossRef]
- Ugwah-Oguejiofor, C.J.; Okoli, C.O.; Ugwah, M.O.; Umaru, M.L.; Ogbulie, C.S.; Mshelia, H.E.; Umar, M.; Njan, A.A. Acute and sub-acute toxicity of aqueous extract of aerial parts of Caralluma dalzielii N. E. Brown in mice and rats. Heliyon 2019, 5, e01179. [Google Scholar] [CrossRef]
- Nalimu, F.; Oloro, J.; Peter, E.L.; Ogwang, P.E. Acute and sub-acute oral toxicity of aqueous whole leaf and green rind extracts of Aloe vera in Wistar rats. BMC Complement. Med. Ther. 2022, 22, 16. [Google Scholar] [CrossRef]
- Bardoloi, A.; Soren, A.D. Genotoxicity induced by medicinal plants. Bull. Natl. Res. Cent. 2022, 46, 119. [Google Scholar] [CrossRef]
- Cayona, R.; Creencia, E. Phytochemicals of Euphorbia hirta L. and their inhibitory potential against SARS-CoV-2 main protease. Front. Mol. Biosci. 2022, 8, 801401. [Google Scholar] [CrossRef]
- Uddin, M.S.; Billah, M.M.; Nahar, Z. Pharmacological actions of Euphorbia hirta: A review. Int. J. Hortic. Sci. 2019, 1, 84–89. [Google Scholar] [CrossRef]
- Tripathi, A.N.; Sati, S.C.; Kumar, P. Euphorbia hirta Linn.—An invasive plant: A review of its traditional uses, phytochemistry and pharmacological properties. System 2021, 17, 20–22. [Google Scholar]
- Khursheed, A.; Jain, V.; Wani, A.R. Euphorbia hirta as a gold mine of high value phytochemicals: A comprehensive review of its pharmacological activities and possible role against SARS-CoV-2. Biomed. Res. Ther. 2022, 9, 4930–4949. [Google Scholar] [CrossRef]
- Sharma, N.; Samarakoon, K.W.; Gyawali, R.; Park, Y.H.; Lee, S.J.; Oh, S.J.; Lee, T.H.; Jeong, D.K. Evaluation of the antioxidant, anti-inflammatory, and anticancer activities of Euphorbia hirta ethanolic extract. Molecules 2014, 19, 14567–14581. [Google Scholar] [CrossRef]
- Kwan, Y.P.; Saito, T.; Ibrahim, D.; Al-Hassan, F.M.S.; Oon, C.E.; Chen, Y.; Jothy, S.L.; Kanwar, J.R.; Sasidharan, S. Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharm. Biol. 2016, 54, 1223–1236. [Google Scholar] [PubMed]
- Kalaivani, S.; Jayanthi, S.; Revathi, K.; Chandrasekaran, R. Phytochemical profile of Euphorbia hirta plant extract and its in vitro anticancer activity against the liver cancer HepG2 cells. Vegetos 2024, 37, 528–535. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Deepak, M.; Praveen, T.K.; Lijini, K.R.; Salman, M.; Sadheeshnakumari, S.; Balachandran, I. Metabolite profiling and anti-cancer activity of two medicinally important Euphorbia species. Med. Omics 2023, 7, 100018. [Google Scholar] [CrossRef]
- Mahdavi, B.; Zare, H.; Qorbani, M.; Atabati, H.; Kakhki, M.R.V.; Raoofi, A.; Ebrahimi, V. Euphorbia granulata Forssk: Evaluation of antioxidant activity, cytotoxicity, and apoptosis induction in breast cancer cells. S. Afr. J. Anim. Sci. 2022, 150, 576–582. [Google Scholar] [CrossRef]
- Rozimamat, R.; Kehrimen, N.; Aisa, H.A. New compound from Euphorbia alatavica Boiss. Nat. Prod. Res. 2019, 33, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Mekam, P.N.; Martini, S.; Nguefack, J.; Tagliazucchi, D.; Stefani, E. Phenolic compounds profile of water and ethanol extracts of Euphorbia hirta L. leaves showing antioxidant and antifungal properties. S. Afr. J. Anim. Sci. 2019, 127, 319–332. [Google Scholar] [CrossRef]
- Kumar, P.; Kale, R.K.; Baquer, N.Z. Antioxidant effects of quercetin in streptozotocin-induced diabetic rats. Indian J. Biochem. Biophys. 2011, 48, 241–246. [Google Scholar]
- Wu, Y.; Qu, W.; Geng, D.; Liang, J.Y.; Luo, Y.L. Phenols and flavonoids from the aerial part of Euphorbia hirta. Chin. J. Nat. Med. 2012, 10, 40–42. [Google Scholar] [CrossRef]
- Das, K.; Asdaq, S.M.B.; Khan, M.S.; Amrutha, S.; Alamri, A.; Alhomrani, M.; Alsanie, W.F.; Bhaskar, A.; Shree, G.C.; Harshitha, P. Phytochemical investigation and evaluation of in vitro anti-inflammatory activity of Euphorbia hirta ethanol leaf and root extracts: A comparative study. J. King Saud Univ. Sci. 2022, 34, 102261. [Google Scholar] [CrossRef]
- Lima, K.G.; Krause, G.C.; Schuster, A.D.; Catarina, A.V.; Basso, B.S.; Mesquita, F.C.; Pedrazza, L.; Marczak, E.S.; Martha, B.A.; Nunes, F.B.; et al. Gallic acid reduces cell growth by induction of apoptosis and reduction of IL-8 in HepG2 cells. Biomed. Pharmacother. 2016, 84, 1282–1290. [Google Scholar] [CrossRef]
- Lone, S.H.; Rehman, S.U.; Bhat, K.A. Synthesis of gallic-acid-1-phenyl-1H-[1,2,3] triazol-4-yl methyl esters as effective antioxidants. Drug Res. 2017, 11, 111–118. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Mesaik, A.M.; Muhammad, A.; Chukwuma, C.I.; Manhas, N.; Singh, P.; Aremu, O.S.; Islam, M.S. Flowers of Clerodendrum volubile exacerbate immunomodulation by suppressing phagocytic oxidative burst and modulation of COX-2 activity. Biomed. Pharmacother. 2016, 83, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Erukainure, O.L.; Sanni, O.; Islam, M.S. Clerodendrum volubile: Phenolics and applications to health. In Polyphenols: Mechanisms of Action in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 53–68. [Google Scholar]
- Molehin, O.R.; Oloyede, O.I.; Idowu, K.A.; Adeyanju, A.A.; Olowoyeye, A.O.; Tubi, O.I.; Komolafe, O.E.; Gold, A.S. White butterfly (Clerodendrum volubile) leaf extract protects against carbon tetrachloride-induced hepatotoxicity in rats. Biomed. Pharmacother. 2017, 96, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.R.F.; Patriota, L.L.S.; Marinho, A.O.; Costa, J.A.; Ribeiro, B.G.; Santos, V.B.S.; Napoleão, D.C.; Cavalcanti, J.V.F.L.; Vieira, L.D.; Pereira, M.C.; et al. Subacute symptoms of depression and anxiety in stress-exposed mice: Role of Schinus terebinthifolia Raddi leaf lectin (SteLL). J. Ethnopharmacol. 2025, 341, 119343. [Google Scholar] [CrossRef]
- Miljković, R.; Marinković, E.; Prodić, I.; Kovačević, A.; Protić-Rosić, I.; Vasić, M.; Lukić, I.; Gavrović-Jankulović, M.; Stojanović, M. Ameliorative Effect of Banana Lectin in TNBS-Induced Colitis in C57BL/6 Mice Relies on the Promotion of Antioxidative Mechanisms in the Colon. Biomolecules 2025, 15, 476. [Google Scholar] [CrossRef]
- Konozy, E.H.E.; Osman, M.E.M.; Dirar, A.I.; Ghartey-Kwansah, G. Plant lectins: A new antimicrobial frontier. Biomed. Pharmacother. 2022, 155, 113735. [Google Scholar] [CrossRef]
- Brito, J.S.; Marinho, A.O.; Coelho, L.C.B.B.; Oliveira, A.M.; Paiva, P.M.G.; Patriota, L.L.S.; Napoleão, T.H. Toxicity and antitumor activity of the water-soluble lectin from Moringa oleifera Lam. seeds (WSMoL) in sarcoma 180-bearing mice. Toxicon 2023, 234, 107306. [Google Scholar] [CrossRef]
- Rahman, M.S.; Rana, S.; Islam, A.A. Antithrombotic and anti-inflammatory activities of leaf methanolic extract of Euphorbia hirta Lin. Int. J. Altern. Complement. Med. 2019, 12, 154–162. [Google Scholar]
- Rajeh, M.A.B.; Kwan, Y.P.; Zakaria, Z.; Latha, L.Y.; Jothy, S.L.; Sasidharan, S. Acute toxicity impacts of Euphorbia hirta L. extract on behavior, organs body weight index and histopathology of organs of the mice and Artemia salina. Pharmacogn. Res. 2012, 4, 170. [Google Scholar] [CrossRef]
- Akomas, S.C.; Ijioma, S.N.; Emelike, C.U. Effects of Euphorbia hirta on hematological and biochemical indices in albino rats. Am. J. Hyg. 2015, 4, 1–5. [Google Scholar]
- Sousa, M.A.; Sales-Campos, H.; Sousa, R.C.M.; Oliveira, C.A.F.; Calixto, L.A. Evaluation of the Gallic Acid effects on Hepatic Steatosis in Obese Swiss Mice. Nutr. Hosp. 2020, 37, 439–443. [Google Scholar]
- Majid, A.; Nishat, S.; Ali, M.M.; Hussain, A. Anti-obesity effect of gallic acid isolated from Terminalia bellerica in Wistar rats. J. Pharm. Pharmacol. 2019, 71, 915–922. [Google Scholar]
- Okla, M.; Kang, I.; Kim, D.M.; Gourineni, V.; Shay, N.; Gu, L.; Chung, S. Ellagic acid modulates lipid accumulation in primary human adipocytes and human hepatoma Huh7 cells via discrete mechanisms. J. Nutr. Biochem. 2015, 26, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Zanwar, A.A.; Hegde, M.V.; Bodhankar, S.L. Gallic acid prevents high-fat diet-induced dyslipidaemia and oxidative stress in rats. Br. J. Nutr. 2014, 112, 603–609. [Google Scholar]
- Choubey, S.; Varughese, L.R.; Kumar, V.; Beniwal, V. Insights into molecular interactions of gallic acid derivatives as multi-targeted anticancer agents. Med. Chem. Res. 2015, 24, 1106–1121. [Google Scholar]
- Ghaddar, A.A.; Hage-Sleiman, R.; Kobeissi, A.H. Gastric antisecretory and cytoprotective effects of the hydroalcoholic extract of Euphorbia hirta leaves in rats. J. Ethnopharmacol. 2022, 279, 114458. [Google Scholar]
- Ping, K.Y.; Darah, I.; Chen, Y.; Sasidharan, S. Cytotoxicity and genotoxicity assessment of Euphorbia hirta in MCF-7 cell line model using comet assay. Asian Pac. J. Trop. Biomed. 2013, 3, 692–696. [Google Scholar] [CrossRef]
- Anitha, P.; Geegi, P.G.; Yogeswari, J.; Anthoni, S.A. In vitro anticancer activity of ethanolic extract of Euphorbia hirta (L.). Sci. Technol. Art. Res. J. 2014, 3, 8–13. [Google Scholar] [CrossRef]
- Liu, Y.; Murakami, N.; Ji, H.; Abreu, P.; Zhang, S. Antimalarial flavonol glycosides from Euphorbia hirta. Pharm. Biol. 2007, 45, 278–281. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Cornelio, K.B. Triterpenes from Euphorbia hirta and their cytotoxicity. Chin. J. Nat. Med. 2013, 11, 528–533. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef]
- Chen, H.S.; Bai, M.H.; Zhang, T.; Li, G.D.; Liu, M. Ellagic acid induces cell cycle arrest and apoptosis through TGF-β/Smad3 signaling pathway in human breast cancer MCF-7 cells. Int. J. Oncol. 2015, 46, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Sánchez, M.Á.; Karmokar, A.; González-Sarrías, A.; García-Villalba, R.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Brown, K.; Espín, J.C. In vivo relevant mixed urolithins and ellagic acid inhibit phenotypic and molecular colon cancer stem cell features: A new potentiality for ellagitannin metabolites against cancer. Food Chem. Toxicol. 2016, 92, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R905–R931. [Google Scholar] [CrossRef]
- Nishi, M.; Chang, H.S.; Ishikawa, F.; Uchiyama, T.J. Antitumor activity of Panax ginseng-fruit extract (PG-FE) on Sarcoma-180 bearing mice is based on its cytokine productive effect. J. Tradit. Med. 2004, 21, 221–230. [Google Scholar]
- Cheng, D.H.; Liu, Y.; Wang, L. Antitumor effects of ethanol extract from Ventilago leiocarpa Benth on Sarcoma 180 tumor-bearing mice and possible immune mechanism. Chin. J. Integr. Med. 2021, 27, 905–911. [Google Scholar] [CrossRef]
- Amorim, P.K.; Matoso, T.M.D.; Barros, M.C.; Guedes, C.C.S.; Freitas, A.F.S.; Souza, T.G.S.; Chagas, C.A.; Alves, M.M.M.; Carvalho, F.A.A.; Albuquerque, L.P.; et al. Antioxidant and Antileishmanial Activities, and Evaluation of Acute Oral Toxicity and Genotoxicity of Bixa orellana Leaf Extracts. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2024, 94, 811–821. [Google Scholar] [CrossRef]
- Li, H.B.; Cheng, K.W.; Wong, C.C.; Fan, K.W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Procópio, T.F.; Patriota, L.L.S.; Moura, M.C.; Silva, P.M.; Oliveira, A.P.S.; Carvalho, L.V.N.; Lima, T.A.; Soares, T.; Silva, T.D.; Coelho, L.C.B.B.; et al. CasuL: A new lectin isolated from Calliandra surinamensis leaf pinnulae with cytotoxicity to cancer cells, antimicrobial activity and antibiofilm effect. Int. J. Biol. Macromol. 2017, 98, 419–429. [Google Scholar] [CrossRef]
- OECD (Organization for Economic Cooperation and Development). Guideline for Testing of Chemicals: Acute Oral Toxicity–Acute Toxic Class Method. Guideline 423; OECD (Organization for Economic Cooperation and Development): Paris, France, 2001. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1998, 175, 184–191. [Google Scholar] [CrossRef]
- Eiji, Y.; Haruyoshi, H.; Masaki, T.; Motoyoshi, K.; Setsuko, A. The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides with triethylenemelamine. Mutat. Res. 1992, 278, 127–130. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 1997, 21, A-3B. [Google Scholar] [CrossRef]


| Parameter | Treatment | |
|---|---|---|
| Control | SE (2000 mg/kg) | |
| Body weight gain (g) | 2.40 ± 0.24 | 0.50 ± 0.87 * |
| Food consumption (%) | 15.26 ± 0.83 | 14.00 ± 0.57 |
| Water consumption (mL × 100) | 23.00 ± 0.39 | 22.00 ± 0.87 |
| Parameter | Treatment | |
|---|---|---|
| Control | SE (2000 mg/kg) | |
| Erythrocytes (106/mm3) | 5.77 ± 0.49 | 5.41 ± 0.53 |
| Hematocrit (%) | 38.76 ± 2.56 | 39.24 ± 3.03 |
| Hemoglobin (g/dL) | 14.89 ± 0.33 | 14.20 ± 0.27 |
| Mean Corpuscular Volume (%) | 48.11 ± 3.49 | 47.11 ± 3.71 |
| Mean Corpuscular Hemoglobin (%) | 17.39 ± 0.27 | 18.06 ± 0.32 |
| Mean Corpuscular Hemoglobin Concentration (%) | 37.18 ± 2.87 | 36.83 ± 3.04 |
| Platelets (103/mm3) | 907.66 ± 77.19 | 926.70 ± 81.92 |
| Leukocytes (103/mm3) | 6.23 ± 0.64 | 6.39 ± 0.40 |
| Segmented Leukocytes (%) | 70.70 ± 4.32 | 71.14 ± 4.57 |
| Lymphocytes (%) | 25.89 ± 0.30 | 25.13 ± 0.38 |
| Monocytes (%) | 3.10 ± 0.32 | 3.15 ± 0.36 |
| Basophil (%) | 0.10 ± 0.03 | 0.12 ± 0.03 |
| Eosinophil (%) | 1.56 ± 0.18 | 1.50 ± 0.14 |
| Parameter | Treatment | |
|---|---|---|
| Control | SE (2000 mg/kg) | |
| Albumin (g/dL) | 35.10 ± 3.02 | 37.12 ± 3.33 |
| Alanine aminotransferase (U/L) | 69.77 ± 3.46 | 69.08 ± 4.15 |
| Aspartate aminotransferase (U/L) | 96.13 ± 4.47 | 94.69 ± 5.13 |
| Alkaline phosphatase (U/L) | 14.11 ± 0.64 | 14.34 ± 0.50 |
| Bilirubin (mg/dL) | 0.46 ± 0.12 | 0.48 ± 0.09 |
| Gamma-glutamyl transferase (U/L) | 13.56 ± 0.40 | 14.09 ± 0.54 |
| Total protein (g/dL) | 72.56 ± 4.10 | 73.89 ± 5.19 |
| Blood urea (mg/dL) | 0.38 ± 0.06 | 0.40 ± 0.09 |
| Creatinine (mg/dL) | 4.21 ± 0.54 | 4.09 ± 0.37 |
| Total cholesterol (mg/dL) | 73.30 ± 4.14 | 73.60 ± 4.55 |
| Triglycerides (mg/dL) | 97.11 ± 4.10 | 81.45 ± 4.12 * |
| High-density lipoprotein-cholesterol (mg/dL) | 40.10 ± 2.10 | 41.13 ± 3.50 |
| Low-density lipoprotein-cholesterol (mg/dL) | 29.80 ± 2.01 | 21.09 ± 2.09 * |
| Very low density lipoprotein-cholesterol(mg/dL) | 16.02 ± 1.16 | 12.60 ± 1.02 * |
| Relative Organ Weight (%) | Treatment | |
|---|---|---|
| Control | SE (2000 mg/kg) | |
| Heart | 0.52 ± 0.03 | 0.44 ± 0.04 |
| Kidney | 1.38 ± 0.04 | 1.32 ± 0.07 |
| Liver | 5.79 ± 0.24 | 5.67 ± 0.29 |
| Lung | 0.85 ± 0.02 | 0.78 ± 0.05 |
| Spleen | 0.59 ± 0.03 | 0.57 ± 0.03 |
| Parameter | Groups | |||||
|---|---|---|---|---|---|---|
| Sham | Negative Control | MTX | SE (50 mg/kg) | SE (100 mg/kg) | SE (200 mg/kg) | |
| Body weight gain (g) | 3.40 ± 0.48 | 4.00 ± 1.30 | 3.80 ± 0.66 | 1.20 ± 0.73 | −0.83 ± 1.24 * | −0.66 ± 0.84 * |
| Food consumption (%)—day 1–7 | 18.64 ± 1.78 | 18.28 ± 0.77 | 19.27 ± 0.61 | 19.18 ± 0.54 | 17.88 ± 0.87 | 17.46 ± 0.87 |
| Food consumption (%)—day 8–14 | 17.12 ± 1.17 | 16.46 ± 1.07 | 17.77 ± 0.84 | 14.89 ± 0.63 | 15.44 ± 1.19 | 14.34 ± 0.82 |
| Water consumption (100 × mL)—day 1–7 | 27.70 ± 1.80 | 25.48 ± 0.85 | 29.37 ± 1.49 | 26.21 ± 1.86 | 25.5 ± 1.47 | 26.70 ± 1.71 |
| Water consumption (100 × mL)—day 8–14 | 26.80 ± 2.10 | 23.94 ± 2.13 | 28.46 ± 2.28 | 22.10 ± 1.39 | 25.24 ± 2.54 | 22.52 ± 1.84 |
| Relative Organ Weight (%) | Treatment | |||||
|---|---|---|---|---|---|---|
| Sham | Negative Control | MTX | SE (50 mg/kg) | SE (100 mg/kg) | SE (200 mg/kg) | |
| Heart | 0.47 ± 0.02 | 0.41 ± 0.02 | 0.42 ± 0.02 | 0.47 ± 0.02 | 0.43 ± 0.03 | 0.41 ± 0.01 |
| Kidney | 1.24 ± 0.04 | 1.28 ± 0.07 | 1.18 ± 0.05 | 1.28 ± 0.08 | 1.23 ± 0.03 | 1.09 ± 0.05 |
| Liver | 4.92 ± 0.26 | 5.70 ± 0.44 | 6.08 ± 0.45 | 5.08 ± 0.24 | 5.23 ± 0.39 | 4.84 ± 0.17 |
| Lung | 0.75 ± 0.01 | 0.70 ± 0.03 | 0.69 ± 0.04 | 0.76 ± 0.03 | 0.77 ± 0.08 | 0.77 ± 0.06 |
| Spleen | 0.43 ± 0.03 | 0.82 ± 0.08 * | 1.00 ± 0.08 ** | 0.83 ± 0.09 * | 0.85 ± 0.11 * | 0.70 ± 0.07 * |
| Parameter | Treatment | |||||
|---|---|---|---|---|---|---|
| Sham | Negative Control | MTX | SE (50 mg/kg) | SE (100 mg/kg) | SE (200 mg/kg) | |
| Erythrocytes (106/mm3) | 8.60 ± 0.15 | 7.60 ± 0.29 | 7.00 ± 0.33 ** | 8.60 ± 0.23 | 8.85 ± 0.25 | 8.41 ± 0.26 |
| Hematocrit (%) | 45.10 ± 0.75 | 39.83 ± 1.90 | 37.60 ± 1.74 ** | 45.33 ± 1.40 | 46.60 ± 1.35 | 43.33 ± 1.07 |
| Hemoglobin (g/dL) | 15.26 ± 0.20 | 13.32 ± 0.58 | 11.94 ± 0.62 *** | 14.82 ± 0.44 | 15.06 ± 0.43 | 14.29 ± 0.37 |
| Mean Corpuscular Volume (%) | 52.81 ± 0.53 | 53.61 ± 1.40 | 54.89 ± 0.91 | 52.71 ± 0.83 | 52.67 ± 0.43 | 51.38 ± 0.51 |
| Mean Corpuscular Hemoglobin (%) | 17.75 ± 0.12 | 17.36 ± 0.47 | 17.39 ± 0.18 | 17.22 ± 0.13 | 17.02 ± 0.10 | 16.91 ± 0.22 |
| Mean Corpuscular Hemoglobin Concentration (%) | 33.63 ± 0.22 | 32.38 ± 0.25 | 31.17 ± 0.46 ** | 32.70 ± 0.43 | 32.32 ± 0.25 | 32.73 ± 0.28 |
| Platelets (103/mm3) | 851.40 ± 80.0 | 735.33 ± 84. | 818.20 ± 138.7 | 987.50 ± 230.2 | 970.00 ± 152.0 | 1160.00 ± 300.0 |
| Leukocytes (103/mm3) | 5.07 ± 0.42 | 13.88 ± 2.24 * | 21.58 ± 2.22 **** | 11.12 ± 2.09 | 11.38 ± 2.18 | 9.84 ± 1.37 |
| Segmented Leukocytes (%) | 21.60 ± 2.10 | 61.00 ± 4.82 *** | 66.80 ± 5.63 **** | 49.33 ± 7.54 * | 53.60 ± 8.18 ** | 66.43 ± 1.04 *** |
| Lymphocytes (%) | 76.20 ± 2.27 | 36.33 ± 4.61 *** | 31.60 ± 5.46 *** | 46.33 ± 8.16 * | 43.20 ± 8.51 ** | 27.87 ± 1.88 **** |
| Monocytes (%) | 1.44 ± 0.67 | 1.60 ± 0.24 | 1.92 ± 0.71 | 2.40 ± 0.51 | 2.92 ± 0.71 | 3.55 ± 0.80 |
| Basophil (%) | 0.60 ± 0.89 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.55 ± 0.32 | 0.48 ± 0.12 | 0.22 ± 0.16 |
| Eosinophil (%) | 0.33 ± 0.21 | 1.00 ± 0.42 | 0.66 ± 0.81 | 0.66 ± 0.33 | 0.60 ± 0.32 | 0.16 ± 0.17 |
| Parameter | Treatment | |||||
|---|---|---|---|---|---|---|
| Sham | Negative Control | MTX | SE (50 mg/kg) | SE (100 mg/kg) | SE (200 mg/kg) | |
| Albumin (g/dL) | 36.59 ± 2.32 | 36.74 ± 2.41 | 32.14 ± 4.12 * | 35.07 ± 3.02 | 35.15 ± 3.27 | 36.70 ± 0.44 |
| Alanine aminotransferase (U/L) | 67.51 ± 3.36 | 67.28 ± 4.35 | 102.52 ± 9.78 * | 68.42 ± 5.15 | 66.25 ± 4.38 | 66.62 ± 5.48 |
| Aspartate aminotransferase (U/L) | 95.43 ± 6.14 | 94.43 ± 5.59 | 155.79 ± 12.51 * | 94.14 ± 7.33 | 90.44 ± 8.12 | 92.85 ± 5.04 |
| Alkaline phosphatase (U/L) | 13.86 ± 0.71 | 14.26 ± 0.60 | 17.43 ± 0.80 * | 14.01 ± 0.70 | 14.09 ± 0.86 | 14.09 ± 0.32 |
| Bilirubin (mg/dL) | 0.73 ± 0.19 | 0.59 ± 0.15 | 0.65 ± 0.18 | 0.57 ± 0.14 | 0.69 ± 0.23 | 0.42 ± 0.41 |
| Gamma-glutamyl transferase (U/L) | 14.95 ± 0.93 | 15.75 ± 0.84 | 15.33 ± 0.95 | 15.49 ± 1.02 | 15.56 ± 1.09 | 14.14 ± 0.53 |
| Total protein (g/dL) | 75.42 ± 6.42 | 75.23 ± 6.41 | 51.54 ± 5.45 * | 73.06 ± 5.18 | 74.60 ± 5.15 | 75.46 ± 5.35 |
| Blood urea (mg/dL) | 0.32 ± 0.07 | 0.29 ± 0.06 | 0.65 ± 0.08 * | 0.37 ± 0.08 | 0.31 ± 0.09 | 0.48 ± 0.08 |
| Creatinine (mg/dL) | 0.47 ± 0.09 | 0.42 ± 0.04 | 2.04 ± 0.40 * | 0.49 ± 0.07 | 0.52 ± 0.08 | 0.44 ± 0.15 |
| Total cholesterol (mg/dL) | 96.58 ± 7.45 | 96.77 ± 5.67 | 90.55 ± 6.13 | 94.33 ± 6.15 | 85.35 ± 5.16 * | 77.49 ± 7.54 * |
| Triglycerides (mg/dL) | 99.27 ± 10.31 | 95.51 ± 9.69 | 91.42 ± 8.10 | 88.13 ± 9.13 | 80.22 ± 9.18 * | 74.88 ± 6.65 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.A.d.; Marinho, A.d.O.; Alves, R.R.d.V.; de Barros, M.C.; Nova, I.C.V.; de Oliveira, S.A.; Alves, J.V.d.O.; Silva, V.F.; Ferreira, M.R.A.; de Oliveira, A.M.; et al. Phytochemical Profile, Toxicological Screening, Antitumor Activity, and Immunomodulatory Response of Saline Extract from Euphorbia hirta L. Leaves. Molecules 2025, 30, 3105. https://doi.org/10.3390/molecules30153105
Costa JAd, Marinho AdO, Alves RRdV, de Barros MC, Nova ICV, de Oliveira SA, Alves JVdO, Silva VF, Ferreira MRA, de Oliveira AM, et al. Phytochemical Profile, Toxicological Screening, Antitumor Activity, and Immunomodulatory Response of Saline Extract from Euphorbia hirta L. Leaves. Molecules. 2025; 30(15):3105. https://doi.org/10.3390/molecules30153105
Chicago/Turabian StyleCosta, Jainaldo Alves da, Amanda de Oliveira Marinho, Robson Raion de Vasconcelos Alves, Matheus Cavalcanti de Barros, Isabella Coimbra Vila Nova, Sheilla Andrade de Oliveira, João Victor de Oliveira Alves, Vitória Figueiredo Silva, Magda Rhayanny Assunção Ferreira, Alisson Macário de Oliveira, and et al. 2025. "Phytochemical Profile, Toxicological Screening, Antitumor Activity, and Immunomodulatory Response of Saline Extract from Euphorbia hirta L. Leaves" Molecules 30, no. 15: 3105. https://doi.org/10.3390/molecules30153105
APA StyleCosta, J. A. d., Marinho, A. d. O., Alves, R. R. d. V., de Barros, M. C., Nova, I. C. V., de Oliveira, S. A., Alves, J. V. d. O., Silva, V. F., Ferreira, M. R. A., de Oliveira, A. M., Soares, L. A. L., Maia, C. S., Tenório, F. d. C. Â. M., Lorena, V. M. B. d., Sá, R. A., Napoleão, T. H., Patriota, L. L. d. S., Macedo, M. L. R., & Paiva, P. M. G. (2025). Phytochemical Profile, Toxicological Screening, Antitumor Activity, and Immunomodulatory Response of Saline Extract from Euphorbia hirta L. Leaves. Molecules, 30(15), 3105. https://doi.org/10.3390/molecules30153105








