Abstract
Ceria is an important redox catalyst due to the facile Ce3+/Ce4+ switching at its surface. Therefore, in situ determination of the oxidation state of surface cerium cations is of significant interest. Infrared spectroscopy of probe molecules such as CO holds great potential for this purpose. However, the ability of CO to reduce Ce4+ cations is an important drawback as it alters the initial cerium speciation. Dinitrogen (N2), due to its chemical inertness, presents an attractive alternative. We recently demonstrated that low-temperature 15N2 adsorption on stoichiometric ceria leads to the formation of complexes with Ce4+ cations on the (110) and (100) planes (bands at 2257 and 2252 cm−1, respectively), while the (111) plane is inert. Here, we report results on the low-temperature 15N2 adsorption on reduced ceria nanoshapes (cubes, polyhedra, and rods). A main band at 2255 cm−1, with a weak shoulder at 2254 cm−1, was observed. We attributed these bands to 15N2 adsorbed on Ce3+ sites located on edges and corners as well as on {100} facets. In conclusion, 15N2 adsorbs on the most acidic surface Ce3+ sites and enables their distinction from Ce4+ cations.
1. Introduction
Ceria (CeO2) and ceria-based materials are highly versatile, finding applications across various fields including catalysis [1,2], sensors [3], chemical mechanical polishing [4], and advancing biomedicine [5,6]. This versatility stems largely from ceria’s ability to reversibly switch between Ce4+ and Ce3+ oxidation states, and to accommodate oxygen vacancies without disrupting its stable fluorite lattice structure [7]. As a result, CeO2 can readily release or absorb oxygen under varying conditions, making it particularly effective in redox catalysis. A similar mechanism underpins its use in solid oxide fuel cells, where ceria facilitates oxygen ion transport between electrodes [2,7]. Additionally, ceria nanoparticles serve as biological nanozymes, mimicking enzymatic antioxidant activity through redox cycling of Ce3+/Ce4+ [5,6]. Ceria is also increasingly used in organic synthesis [8], where its catalytic behavior is closely linked to both redox and acid-base surface properties. Therefore, a key challenge in the research of ceria lies in accurately determining the oxidation and coordination state of surface cerium cations under working conditions. However, despite extensive investigation, significant inconsistencies remain in the literature.
Many investigations have been carried out using polycrystalline ceria, which exposes a complex mix of surface facets and structural defects, making it difficult to attribute specific behaviors to individual surface features. To overcome this, recent efforts have focused on nanostructured ceria with well-defined crystallographic planes [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25], offering model systems that have advanced our understanding of the relationship between surface structure and properties.
The facet-dependent reactivity of ceria is particularly evident in CO oxidation, where the (100) and (110) surfaces are significantly more active than the more stable (111) plane [10,12,13,20,26]. Similar facet-dependent trends are observed in H2 oxidation [14,15], soot combustion [27], the water–gas shift (WGS) reaction [16], and reverse WGS reactions [28]. Facet selectivity also governs alcohol conversions; for instance, 2-propanol undergoes dehydrogenation to acetone on {100} facets, whereas dehydration to propene is favored on the (111) surface [22].
Designing efficient CeO2-based catalysts requires a deep understanding of surface redox and acid–base properties, which can be probed using advanced characterization techniques applied to well-defined nanostructures. Infrared (IR) spectroscopy is particularly powerful for investigating ceria’s surface chemistry at the molecular level. It allows for the direct observation of surface hydroxyls, adsorbed oxygen species, and common contaminants such as carbonates and nitrates [29,30]. Furthermore, the presence of Ce3+ can be tracked via its characteristic spin-orbital f–f transition, typically observed in the 2150–2110 cm−1 range [23,31,32,33,34,35,36,37,38,39].
IR spectroscopy of probe molecules is a powerful method for characterizing oxide surfaces. Among these, carbon monoxide (CO) is the most widely used probe [29], but its application to ceria is not without drawbacks. CO can reduce Ce4+ to Ce3+, forming surface carbonates or hydrogen carbonates [40,41,42], which alter the original surface composition, and carbonate-like features have been detected even after low-temperature (173 K) CO adsorption [21,43]. Moreover, the Ce3+-related electronic transition bands overlap with the CO stretching modes, leading to potential misassignments [17,31,44,45,46], unless isotopic labeling is employed [47].
Dinitrogen (N2) has emerged as a promising alternative to CO as an infrared probe for studying the surface acidity on oxides [48,49,50,51,52,53,54,55,56,57,58,59]. Its chemical inertness makes it especially attractive for probing ceria surfaces, as it neither oxidizes nor reduces cerium cations, nor does it form surface-bound anions such as carbonates. Thus, N2 preserves the original cerium speciation. Furthermore, as a very weak base, N2 is unlikely to induce significant surface perturbations.
Although N2 is IR-inactive in the gas phase due to its centrosymmetric structure, adsorption lowers its symmetry, rendering the ν(N–N) stretching mode IR-active. The reference frequency for adsorbed N2 is proposed at ~2324 cm−1, slightly red-shifted from the gas-phase Raman value at 2331 cm−1 [49]. When N2 interacts with low-valent metal cations such as Cu+ [55] or Ni+ [58], it forms σ and π bonds that weaken the N≡N bond, leading to a red shift in ν(N–N). In contrast, adsorption onto d0 cations like Ce4+ occurs via electrostatic interaction [49]. This results in polarization of the molecule and, due to the Stark effect, the N-N stretching frequency is blue shifted. The extent of the shift reflects the polarizing power of the surface cation. Similar is the situation with the Ce3+ ions where no back π-donation is expected [25]. Given the weak nature of the interaction in these cases, measurements typically require cryogenic temperatures. It is also worth noting that IR intensity does not directly correlate with the concentration of adsorbed species, as the extinction coefficient depends on polarization. In cases of symmetric adsorption, the IR signal may be nearly undetectable—a phenomenon proposed for Cu–ZSM-5 [55].
Unlike CO, the IR signature of adsorbed N2 does not interfere with the gas-phase vibration. However, the 14N2 band can overlap with CO2 gas-phase absorption centered at 2349 cm−1. To circumvent this issue, we employed the 15N2 isotopologue, whose Raman reference frequency is at 2252 cm−1.
In this study, we investigate the potential of using 15N2 as an IR probe for surface characterization of reduced ceria nanoparticles (cubes, polyhedra, and rods). To the best of our knowledge, N2 has not yet been applied to this system. To strengthen the assignments, we studied the dosed adsorption of 15N2, and the co-adsorption of 15N2 with CO and O2. Although the main techniques used were FTIR, TPR, and DFT calculations, the samples were also characterized by XRD, HRTEM, Raman spectroscopy, and XPS. This work completes our previous investigations on 15N2 adsorption on stoichiometric ceria [59], thus providing a comprehensive view of N2 interaction with CeO2. It is important to note that detecting both Ce3+ and Ce4+ sites is essential for ceria-based systems because their importance in catalysis stems mainly from their ability to easily switch between the two cerium oxidation states.
2. Results
2.1. Basic Characteristics of the Samples
The following three ceria samples with different morphologies were studied: nanocubes (CeO2-NC), nanopolyhedra (CeO2-NP), and nanorods (CeO2-NR). The same materials were used in previous works [24,25,59] and characterized by HRTEM, XRD, TPR, nitrogen adsorption, IR spectroscopy, etc. The key physicochemical characteristics are summarized in Table 1.

Table 1.
Basic characteristics of the ceria samples studied.
2.1.1. Initial Characterization
The XRD patterns of all three samples confirmed their cubic CeO2 fluorite structure (space group Fmm). HRTEM images showed that the CeO2-NC sample consisted of cubic crystals, predominantly terminated by {100} facets. The {110} and {111} facets were also exposed but to a lesser extent. As expected, the CeO2-NP sample was characterized by a preferential exposure of the {111} and to a lesser extent the {110} facets at the expense of the {100} facets [24,27,60]. Only a slight reduction in crystallite size and surface area was observed after calcination. In the case of CeO2-NR, a high proportion of {110} facets was observed, along with some {111} and {100} facets. The nanorods exhibited the smallest average crystallite size, the highest specific surface area, and the largest void volume between particles. In summary, all three low-index facets were present in each sample, albeit in different proportions. As shown in Table 1, the dominant facets of the CeO2-NC, CeO2-NP, and CeO2-NR were {100}, {111}, and {110}, respectively.
Raman spectroscopy is a widely used technique for characterizing ceria-based systems. This is because Raman spectra are sensitive to some important parameters such as particle size and the presence of defects. The most notable feature in the Raman spectra of stoichiometric ceria is the intense F2g band around 460 ± 5 cm−1. Decreasing the crystallite size of CeO2 below ca. 50 nm leads to broadening, asymmetry, and red shift of the F2g band [61]. The Raman spectra of our samples are shown in Figure S1 (see Supplementary Materials) and are consistent with these findings. All spectra were dominated by a single band ranging from 462.5 to 457.5 cm−1. The lowest frequency was detected in the CeO2-NR sample, which had the highest surface area. As expected, the band appeared broad and slightly asymmetric.
Other important features in the Raman spectra of ceria are the D bands. The so-called D2 band, which is observed around 550 cm−1, is associated with reduced Ce3+ cations [61,62] and also appears with ceria doped with other trivalent cations [62]. This band was not discernible in our spectra, which confirms the lack of Ce3+ sites in the calcined samples. Another D band (D1) was observed around 600 cm−1 and is associated with stoichiometric defects. This band is usually detected with ceria, having a high specific surface area [61,62,63]. Indeed, the D1/F2g intensity ratio was highest in the CeO2-NR sample, which is consistent with its high dispersion.
The samples were also characterized by XPS [24]. The results showed a very low concentration of Ce3+ sites (ca. 5 % for all samples), which indicates that most of the cerium cations were in an oxidized state in the calcined samples. Furthermore, we believe that the low amount of Ce3+ sites detected by XPS was due to sample reduction during analysis, as recently demonstrated by [64].
2.1.2. Temperature-Programmed Reduction
Consistent with previous studies [14,21,23,27,38,44,45,46,61,62,63], the H2-TPR profiles of our ceria samples were bimodal, showing distinct low-temperature (680–863 K) and high-temperature (1132–1135 K) peaks (Figure 1, patterns a–c). The low-temperature peaks depend on the sample morphology and are typically associated with a reduction of surface Ce4+ cations, though some reports suggest a contribution from subsurface layers as well [27,65]. The high-temperature peak, nearly identical across all samples, is attributed to a reduction of the bulk.
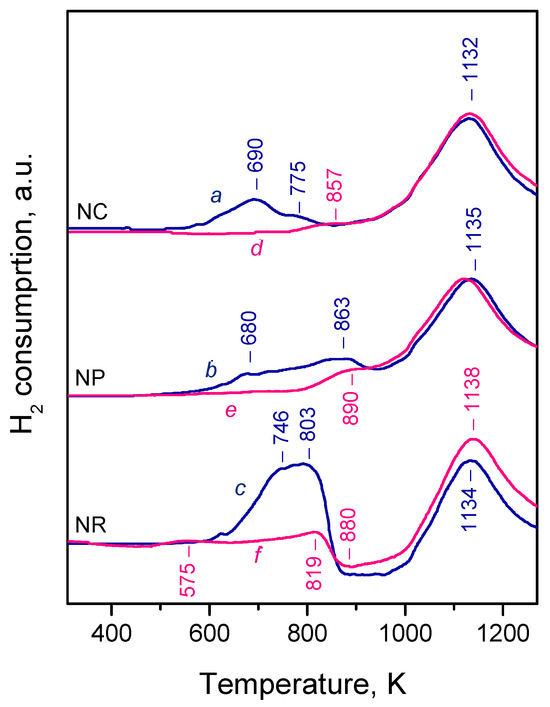
Figure 1.
TPR profiles of CeO2-NC (a,d), CeO2-NP (b,e), and CeO2-NR samples (c,f). Patterns a–c: pre-oxidized samples; patterns d–f: samples pre-reduced at 773 K (see text for details).
Among the three samples, CeO2-NC exhibited the most easily reducible surface, with a dominant low-temperature peak at 690 K, evidently corresponding to the highly exposed {100} facets. A similar, though less intense, peak was observed with CeO2-NP, consistent with its lower {100} facet exposure. Additionally, a peak at 863 K was detected with this sample and could be related to the {111} facets, as recently reported [64]. This aligns with findings that oxygen vacancy formation energy is highest for the (111) plane [66].
The TPR profile of CeO2-NR is more complex. In addition to reduction peaks at 746 and 803 K, a broad negative feature appeared between 850 and 1020 K and is attributed to the desorption of dissolved hydrogen [23,61,62]. Therefore, reciprocal component(s) due to hydrogen dissolution should contribute to the positive peaks at a lower temperature. The high hydrogen uptake observed for CeO2-NR likely resulted from its high surface area and/or significant exposure of {110} facets. While hydrogen dissolution/desorption cannot be excluded in the other samples, the signals were not clearly discernible.
The amounts of hydrogen consumed during the reduction of the samples up to 773 K and those corresponding to the low-temperature peaks are presented in Table S1 (see Supplementary Materials).
To probe the redox behaviour further, TPR was performed on samples that had been pre-reduced up to 773 K. The aim was to assess whether any surface or subsurface Ce4+ sites remained. For CeO2-NC (Figure 1, pattern d), the initial low-temperature peak nearly disappeared, with only a minor feature around 857 K remaining, possibly due to Ce4+ on {111} facets. A similar observation was made for CeO2-NP (Figure 1, pattern e). Only a weak feature around 890 K was registered in the low-temperature region and can be associated with residual Ce4+ cations on the {111} facets.
CeO2-NR again showed a distinct behavior. A new peak appeared at 575 K, absent in the oxidized sample, and was attributed to hydrogen dissolution. This process is known to require the presence of Ce3+ sites and oxygen vacancies [67], which justify the appearance of these peaks with the reduced sample at a relatively low temperature. However, this peak was relatively weak and did not account for the more intense negative peak around 880 K. Therefore, a second positive peak at 819 K was also ascribed to hydrogen dissolution. Accordingly, in the oxidized sample, the first peak at 746 K can be assigned to Ce4+ reduction, and the second (803 K) to hydrogen dissolution.
Despite differing experimental conditions (higher H2 pressure, longer reduction time, and static conditions in IR experiments), we show in the discussion that the TPR results suggest that, under the IR reduction protocol, nearly all surface Ce4+ sites on CeO2-NC and CeO2-NR were reduced. A small fraction of non-reduced Ce4+ sites remained on CeO2-NP.
2.1.3. Background IR Spectra
In situ FTIR spectra of oxidized and reduced samples recorded at 100 K are compared in Figure 2, focusing on the OH stretching and the Ce3+ electronic transition regions. Note that, compared with room temperature, hydroxyl bands appeared at slightly higher wavenumbers (by 3–4 cm−1) [24] and the different components of the Ce3+ electronic band were well resolved [23].
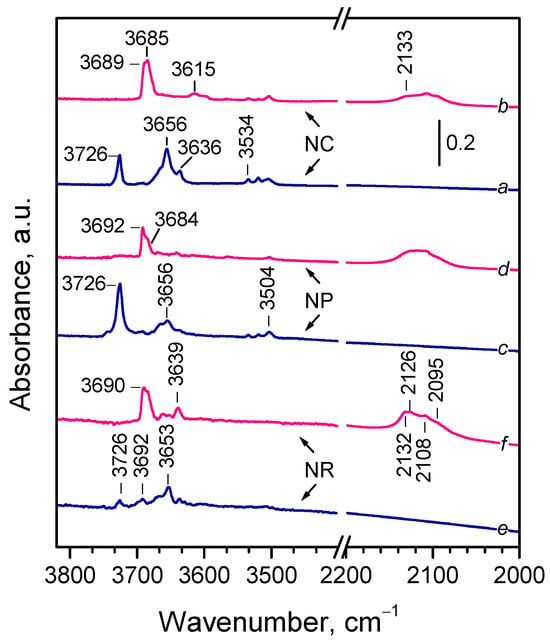
Figure 2.
Background FTIR spectra of CeO2-NC, CeO2-NP, and CeO2-NR samples. Spectra are recorded at 100 K in the presence of 2 mbar He with oxidized (a,c,e) and reduced (b,d,f) samples.
The infrared spectra of all oxidized samples (Figure 2, spectra a,c,e) contained three groups of OH bands. A sharp band at 3726 cm−1 was assigned to terminal Ce4+–OH groups [23,24,32,33,35,38,68,69,70,71]. This band was weak with the CeO2-NR sample. A series of bands between 3668 and 3636 cm−1, and a weak band at 3692 cm−1 (only for CeO2-NR), were attributed to bridging hydroxyls [23,24,32,33,35,38,44,67,68,69,70,71]. Additionally, low-intensity bands in the 3534–3504 cm−1 range were assigned to an oxyhydroxide phase [23,24,32,33,38,68,70,71]. Although spectral details varied, all samples showed features typical of oxidized ceria. Notably, these OH groups were resistant to evacuation at 773 K, suggesting they are associated with defect sites (e.g., edges, corners) rather than flat crystal planes.
Bands due to residual carbonates [32,35] were detected in the 1574–1290 cm−1 region with the CeO2-NC and CeO2-NR samples, but were nearly absent with CeO2-NP, in agreement with previous findings [71]. No Ce3+ electronic transition bands were detected with the oxidized samples, indicating the absence of reduced Ce3+ sites, also supported by their pale yellow color.
The hydroxyl spectra of the reduced samples (Figure 2, spectra b,d,f) were simpler, dominated by bands between 3692 and 3684 cm−1, typical of bridging hydroxyls on reduced ceria [23,32,33,35,38,44,66,68,69,72,73]. CeO2-NR additionally displayed weak bands at 3662 and 3639 cm−1. No terminal OH groups were detected and the oxyhydroxide-related bands were significantly weakened.
All reduced samples exhibited composite Ce3+ electronic bands with at least four well resolved components at 2133, 2126, 2108–2106, and 2094 cm−1 with varying intensities. A magnified view is shown in Figure S2. Notably, the 2133 cm−1 component was most intense with the CeO2-NR sample.
2.2. FTIR Study of 15N2 Adsorption
2.2.1. General Observations
As previously reported [59], adsorption of 15N2 on the oxidized ceria gave rise to two distinct IR bands centered at 2257 and 2252–2251.5 cm−1 (Figure 3A, spectra a,c,e). The relative intensities of these bands varied among the samples and reflected differences in the exposure of specific crystal facets. The band at 2252 cm−1 was assigned to 15N2 adsorbed on Ce4+ sites on the {100} facets, while the 2257 cm−1 band was related to Ce4+ sites on the {110} facets. The contribution of species formed on edge sites to the latter band was also considered. The Ce4+ sites from the {111} facets were too weakly electrophilic to bind 15N2.
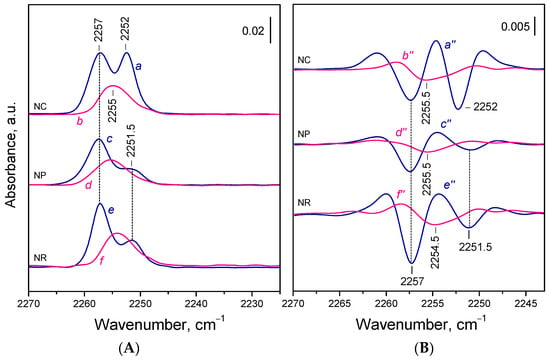
Figure 3.
(A) FTIR spectra of 15N2 (0.5 mbar) adsorbed at 100 K on CeO2-NC (a,b), CeO2-NP (c,d), and CeO2-NR (e,f). Oxidized samples (a,c,e) and reduced samples (b,d,f). (B) Second derivatives of the spectra presented in panel A.
When 15N2 was adsorbed on the reduced samples, one principal band at ca. 2255 cm−1 appeared in all three cases (Figure 3A, spectra b,d,f). This band was absent from oxidized samples and was therefore assigned to 15N2 species formed on Ce3+ sites. Its intensity was 40–60% of the total intensity observed in oxidized samples. No bands typical of 15N2 adsorbed on stoichiometric samples were detected, which confirms the full surface reduction of the Ce4+ cations on the {100} and {110} facets.
2.2.2. Adsorption of 15N2 on Reduced Ceria Nanocubes (CeO2-NC)
Let us now examine the adsorption of 15N2 on the reduced CeO2-NC sample in more detail. Exposure of the sample to 0.5 mbar of 15N2 at 100 K resulted in the appearance of a band centered around 2255 cm−1, accompanied by a weak shoulder near 2248 cm−1 (Figure 4A, spectrum a). Upon evacuation, the shoulder disappeared rapidly, and the main band decreased in intensity. The shoulder at 2248 cm−1 is attributed to 15N2 weakly polarized by surface OH groups. In the hydroxyl stretching region, only minor changes were observed and difference spectra revealed a red shift of an OH band at ca. 3685 cm−1 by approximately 13 cm−1, indicating a very weak interaction, consistent with the negligible acidity of the OH groups of Ce3+ cations. Consequently, the 2248 cm−1 band was easily removed by evacuation. As will be discussed later, 15N2 adsorbed on basic sites might also contribute to this band.
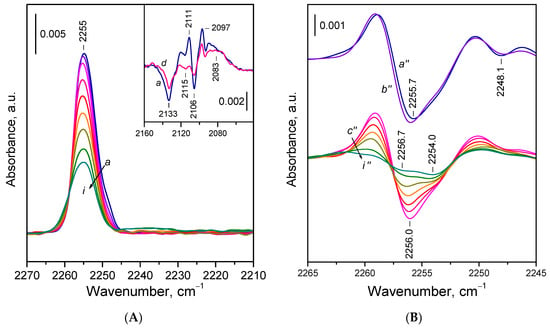
Figure 4.
(A) FTIR spectra of 15N2 adsorbed at 100 K on reduced CeO2-NC sample. Equilibrium 15N2 pressure of 0.5 mbar (a) and evolution of the spectra under dynamic vacuum (b–i). The inset shows the changes in the Ce3+ electronic band caused by 15N2 adsorption. (B) Second derivatives of the spectra presented in Panel A.
Second derivative analysis (Figure 4B, spectra c׳׳–i׳׳) shows that the band remaining after destruction of the OH 15N2 species is composite, comprising two components centered at ca. 2256 and 2254 cm−1. The higher frequency component shifted slightly towards 2257 cm−1 as coverage decreased, while the maximum of the lower frequency component remained unchanged. The component at 2254 cm−1 was more resistant to evacuation, indicating more stable species.
Interestingly, the stability contradicts the expected trend in which stronger Ce3+–15N2 interactions (i.e., more stable adducts) would result in a higher frequency band. A possible explanation is that the 2254 cm−1 band corresponds to species in which the N2 molecule is bound by both ends, but predominantly through one nitrogen atom. This binding mode leads to lower polarization of the N2 molecule but enhances overall stability. However, based on the DFT studies (see below), we propose an alternative assignment of the band, namely to 15N2 bridging two Ce3+ sites by one 15N atom.
Close inspection of the spectra recorded during the final stages of evacuation (Figure 4A, spectra h,i) reveals the emergence of a faint shoulder around 2259 cm−1 and a broad absorption between 2245 and 2230 cm−1. These features are associated with minor oxygen uptake and partial reoxidation of few Ce3+ sites. The component at 2259 cm−1 is attributed to 15N2 on Ce4+ sites, while the bands between 2245 and 2230 cm−1 are attributed to the first overtone of superoxide O2− species. These assignments were confirmed by the addition of small amounts of O2 to the system (details not reported here).
Adsorption of 15N2 also induced changes in the Ce3+ 2F5/2 → 2F7/2 electronic transition band (Figure 4A). Difference spectra showed two well-defined minima at 2133 and 2106 cm−1, alongside a broad positive band peaking around 2083 cm−1. Two sharp maxima at 2111 and 2097 cm−1 were also present, though they may have resulted from an overlap between a broad positive band at ~2104 cm−1 and a sharp negative band at 2106 cm−1. This interpretation is supported by spectra registered with the CeO2-NR sample (see below). Upon evacuation, the spectra gradually reverted to the original state.
2.2.3. Adsorption of 15N2 on Reduced Ceria Nanopolyhedra (CeO2-NP)
The FTIR spectra of 15N2 adsorbed on the reduced CeO2-NP sample are presented in Figure 5. Similar to CeO2-NC, a single dominant band appeared near 2255 cm−1, composed of two components. Compared with CeO2-NC, the lower frequency component was less pronounced and appeared at slightly lower wavenumbers. The changes in the Ce3+ electronic transition band upon 15N2 adsorption are comparable to those observed for CeO2-NC (Figure 5A).
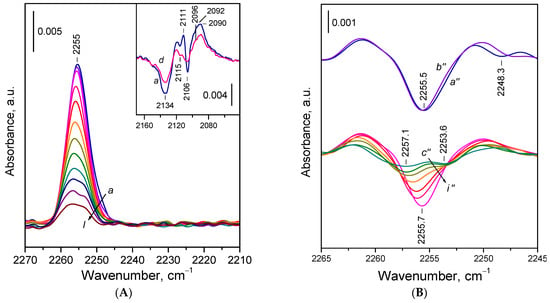
Figure 5.
(A) FTIR spectra of 15N2 adsorbed at 100 K on reduced CeO2-NP. Equilibrium 15N2 pressure of 0.5 mbar (a) and evolution of the spectra under dynamic vacuum (b–l). The inset shows the changes in the Ce3+ electronic band caused by 15N2 adsorption. (B) Second derivatives of the spectra presented in Panel A.
2.2.4. Adsorption of 15N2 and 14N2 on Reduced Ceria Nanorods (CeO2-NR)
The general spectral pattern of 15N2 adsorption on reduced CeO2-NR (Figure 6) was similar to that observed for the other samples. A notable difference was the much more pronounced negative band at 2134–2133 cm−1. In addition, the component at ca 2254 cm−1 was less intense compared with the CeO2-NC sample.
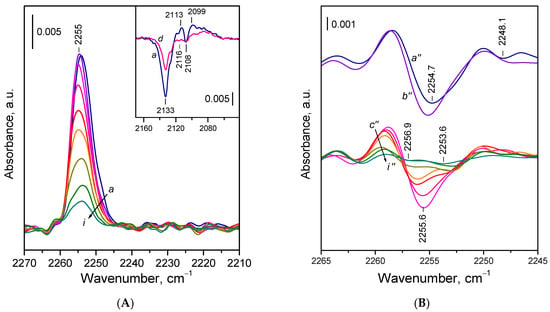
Figure 6.
(A) FTIR spectra of 15N2 adsorbed at 100 K on reduced CeO2-NR. Equilibrium 15N2 pressure of 0.5 mbar (a) and evolution of the spectra under dynamic vacuum (b–i). The inset shows the changes in the Ce3+ electronic band caused by 15N2 adsorption. (B) Second derivatives of the spectra presented in Panel A.
To check for the eventual existence of IR-invisible adsorbed dinitrogen, we studied successive adsorption of small doses of 15N2. The respective IR spectra are shown in Figure S3, while the dependence of the integral intensity of the 15N2 stretching band on the added amount of dinitrogen is depicted on Figure 7A. Inspection of Figure 7A shows that the 2255 cm−1 band began to develop after the first doses of added 15N2, and the dependence at low coverage was nearly linear. This clearly indicates the absence of a stronger IR-invisible adsorption form because, in such a case, the 2255 cm−1 band should have begun to develop with some delay. However, the results do not exclude the existence of a weakly bound invisible 15N2.
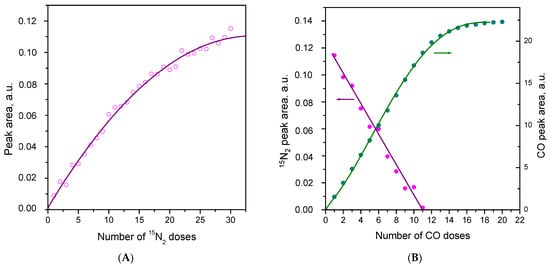
Figure 7.
(A) Dependence of the integral intensity of the 15N2 band at 2255 cm−1 on the amount of 15N2 added to CeO2-NP at 100 K. (B) Dependencies of the integral intensities of the 15N2 and CO bands on the amount of CO added at 100 K to the system 0.5 mbar 15N2/CeO2-NR.
It is also to be noted that no negative band at 2133 cm−1 appeared after adsorption of the first several doses of 15N2 (Figure S3). This band started to develop at high coverages and was of significant intensity only under some 15N2 equilibrium pressure. Simultaneously a negative OH band developed at 3685 cm−1. These observations imply that the 2133 cm−1 band or at least part of it is associated with hydroxylated Ce3+ sites.
To obtain more information on this issue, we studied the gradual displacement of adsorbed N2 by CO. The FTIR spectra are shown in Figure S4, and the dependencies of the 15N2 and carbonyl band intensities on the amount of CO added into the system are presented in Figure 7B. As can be seen, the intensity of the 15N2 band decreased linearly with the amount of added CO and it disappeared completely before the carbonyl band fully developed. Thus, the results practically exclude the possibility of the existence of an invisible 15N2 with an adsorption strength comparable to that of the 2255 cm−1 adsorption form. For more details, see the discussion section.
It should also be noted that, after replacing all adsorbed 15N2, a carbonyl band around 2147 cm−1 developed. This band has been attributed to CO complexes on the reduced {110} facets [25]. Thus, the result indicates that N2 is not adsorbed on these facets.
We also considered the possibility of the existence of some 15N2 bands that lie in the region of the Ce3+ electronic transition and are masked by it. These bands would presumably be due to molecules that are almost parallel to the surface. If such bands exist, they should appear around 2160 cm−1 when 14N2 is adsorbed. However, the experiment with 14N2 revealed the appearance of only one band around 2133 cm−1, which clearly indicates the absence of any 15N2 bands in the 2140–2090 cm−1 region.
Finally, we studied the low-temperature co-adsorption of 15N2 and O2. As already described, the adsorption of 15N2 results in the appearance of a bandwith two components at 2256 and 2254 cm−1 (See Figure S5, spectrum a, and the corresponding second derivative a”). Dosing oxygen to the system led to the erosion of these two bands and the appearance of (i) bands at 2258.5 and 2251.7 cm−1, typical of 15N2 adsorbed on Ce4+ sites, and (ii) weak bands at 2241.5 and 2232.5 cm−1, due to the first overtone of the O–O stretching modes of adsorbed superoxide (O2−) species [23]. It is important to note that at the end of the process the bands characteristic of 15N2 adsorbed on reduced Ce3+ sites totally disappeared, which indicates the full oxidation of the Ce3+ adsorption sites to Ce4+ (Figure S5, spectrum b and the corresponding second derivative b’’).
In conclusion, two types of dinitrogen complexes of Ce3+ cations consistently appeared across all reduced samples, though with varying intensity ratios. Some Ce3+ sites were too weakly acidic to adsorb 15N2, even at 100 K.
2.3. DFT Modeling of N2 Adsorption on Ceria
To further understand the adsorption of N2 on reduced ceria, we performed DFT calculations using surface and nanoparticle models. In some configurations, the nitrogen molecule was positioned perpendicular to the surface and remained bound via one of its nitrogen atoms to Ce3+ cations. Other configurations show the nitrogen molecule reorienting to a near-parallel position relative to the surface, binding via both nitrogen atoms to adjacent cerium cations.
Table 2 summarizes the calculated binding energies of dinitrogen molecule in the studied model ceria systems, as well as the vibrational frequencies for 15N2 adsorbed via one nitrogen atom at Ce3+ sites. The N–N stretching modes for the adsorption where both nitrogen atoms are coordinated were expected to be IR-inactive. Interestingly, the binding energies for the surface parallel configurations on CeO2(100) and CeO2(110) planes were 0.10 to 0.20 eV higher in absolute value compared with those of the linear complexes. This increase can be attributed to the bonding mode and additional dispersion interactions present in these structures.

Table 2.
Calculated binding energies (BE) of 15N2 molecule in the studied model ceria systems and vibrational frequencies for 15N2 adsorbed via one of the nitrogen atoms at Ce3+ centers.
The calculated 15N2 vibrational frequencies for the complexes bound by one N-atom were as follows: (i) 2249 cm−1 for linear coordination to a Ce3+ cation in the nanoparticle model; (ii) 2247 cm−1 for adsorption at the edge of a stepped CeO2(111) surface; and (iii) 2241 cm−1 for bridge coordination to two Ce3+ cations in the CeO2(100) surface model (Figure 8).
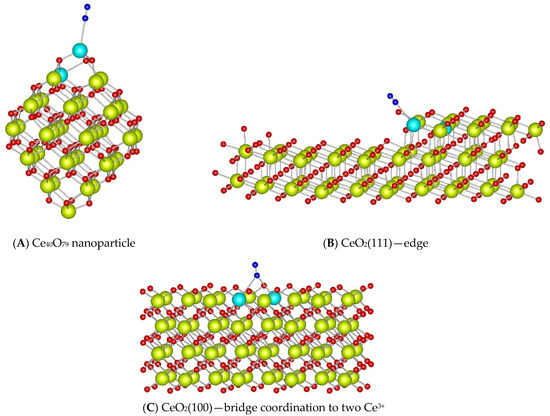
Figure 8.
Complexes of N2 ligand adsorbed to Ce3+ cations located at the surface reduced by creating an O vacancy in Ce40O80 nanoparticle (A), CeO2(111) stepped (B), and CeO2(100) (C) surface models. Color coding: Ce4+—yellow, Ce3+—light blue, O—red, N—blue.
These calculated frequencies help interpret the experimental FTIR data. The observed band at 2256–2255 cm−1 across all samples likely corresponds to 15N2 adsorbed at exposed Ce3+ sites, such as at corners or edges, with calculated frequencies of 2249–2247 cm−1. The second experimental band at 2254–2253 cm−1 is consistent with bridge-coordinated 15N2 on the CeO2(100) plane, which aligns with the calculated frequency of 2241 cm−1. Other surface sites, where the nitrogen molecule is bound through both nitrogen atoms, likely do not contribute to the observed IR spectra due to IR inactivity.
3. Discussion
This study presents a comprehensive analysis of 15N2 adsorption on reduced ceria, combining experimental techniques (primarily in situ FTIR spectroscopy and TPR) with DFT modeling. The results are compared with those obtained for stoichiometric (oxidized) ceria. Below, we discuss the spectral features of adsorbed dinitrogen, the potential active sites for its adsorption on reduced ceria and the feasibility of using N2 as a probe molecule for ceria-containing systems. As this is a pioneering study, some questions remain open.
3.1. Spectral Features of Adsorbed Dinitrogen
In their seminal work, Zecchina et al. [49] discussed the reference frequency for adsorbed dinitrogen. They selected a Raman value of 2324 cm−1, corresponding to ca. 2248 cm−1 for 15N2. When the N2 molecule is “on-top” adsorbed on a cationic site that is an electrostatic acid, it is polarized and the N–N stretching mode is activated in the IR spectra. This also leads to an increase in the N–N stretching frequency due to the Stark effect. The extinction coefficient also increases with polarization.
The adsorption causes frequency shift and the band intensity correlates with the polarization force of the cation. For example, in alkali metal exchanged MOR zeolites, the ν(NN) shift increases in the sequence Cs+ < Rb+ < K+ < Na+ < Li+, ranging from 5 cm−1 (Cs+) to 16 cm−1 (Li+) [49]. A moderate increase in extinction coefficient with the polarizing force of the cation is also observed [49,74]. Notably, the interaction of N2 with Ce3+ and Ce4+ cations as well as with OH groups is also essentially electrostatic.
15N2 also interacts with OH groups. Since these groups usually have a lower polarizing ability than cations, the observed stretching frequency of adsorbed dinitrogen is also lower. Another possibility is for 15N2 to interact with basic sites [75]. In this case, the stretching frequency of the adsorbed molecule is also low. The interaction of 15N2 with hydroxyls gives rise to a weak band around 2248 cm−1, with a possible contribution of 15N2 adsorbed on basic sites.
However, the adsorption configuration may differ. Small diatomic molecules can bridge two cations in two ways: via one atom or via both atoms. The former configuration has already been reported for CO and N2 on oxidized ceria [24,59,76], as well as for CO on reduced ceria [25]. The results of the present study suggest that this adsorption mode is also likely for N2 on reduced ceria. In this case, the N2 stretching frequency depends on the electrostatic field but does not directly reflect the Lewis acidity, as the probe interacts with two cations simultaneously.
Another potential configuration involves the N2 molecule lying parallel to the surface and bridging two cations equally. In this idealized arrangement, the molecule is not polarized in the direction parallel to the surface and is thus IR-inactive. Small symmetry violations can lead to the appearance of IR bands, which will however be of negligible intensity. The same applies to cases in which N2 bridges two cations situated on two different planes.
3.2. Reduction of Ceria
The most important feature of ceria is its ability to reversibly lose oxygen under reducing conditions, simultaneously converting Ce4+ ions to Ce3+ without disrupting the robust crystal structure. This process yields materials with the general formula CeO2−x (0 < x < 0.28) [1]. Reduction can also occur in vacuum, but only at high temperatures or upon irradiation. Conversely, in an oxidizing environment, e.g., at ambient conditions, these cations are readily reoxidized back to Ce4+.
Our results on the characterization of the non-reduced samples support this understanding. Neither IR spectroscopy (Figure 2) nor Raman spectroscopy (Figure S1) detected Ce3+ sites. A small amount of Ce3+ cations was detected by XPS and, as noted earlier, they likely originated from a slight reduction during the measurement process [64]. In contrast, the presence of Ce3+ in all reduced samples was unambiguously confirmed by an IR band observed at 2133–2095 cm−1, which corresponds to the 2F5/2 → 2F7/2, the spin-orbital electronic transition of Ce3+. Another indication of the reduction was the bluish color of the reduced samples.
It is well established that the reduction of pure ceria with H2 occurs in two stages. Initially, surface (and probably some subsurface) Ce4+ cations are reduced at temperatures below 900 K [14,21,23,27,38,44,45,46,61,62,63]. At higher temperatures, reduction of the bulk begins. While the temperature required for bulk reduction is largely independent of sample morphology, surface reduction is influenced by the crystallite habitus, as different surface structures exhibit varying reducibility. In any case, the two-stage reduction allows obtaining samples in which only the surface sites are reduced.
For particles larger than 5 nm, surface-only reduction has a weak impact on the overall stoichiometry, which remains close to CeO2. For instance, in the CeO2-NP sample with a specific surface area of 29 m2 g−1, assuming an idealized morphology enclosed by {111} facets (with a surface cerium cation density of ~3.83 atoms nm−2), about 3.2% of the cerium cations were located at the surface. Upon their full reduction, the stoichiometry changed to CeO1.99. The lower value we obtained suggests that some subsurface reduction also took place, most probably for the (111) plane. The effect of surface reduction on the stoichiometry was more pronounced in the case of the CeO2-NR sample, which had a significantly higher specific surface area (110 m2 g−1).
The data in Table S1, derived from TPR measurements, indicate that the stoichiometry of the reduced sample is consistent with expectations based on surface reduction, and even suggest some degree of subsurface reduction. Given that the reduction for the IR experiments was conducted under more aggressive conditions (100 mbar H2 for 30 min), we can reasonably conclude that all surface Ce4+ cations were converted to Ce3+. Indeed, adsorption of 15N2 on the reduced sample revealed no bands attributable to Ce4+ sites on the {100} and {110} facets or on edge sites. However, we cannot completely rule out the presence of a small number of residual Ce4+ cations on the {111} facets.
3.3. N2 Adsorption Sites on Reduced Ceria
A general observation in this study is that the intensity of the bands of 15N2 adsorbed on reduced samples was consistently lower than that on their oxidized counterparts. Furthermore, the observed bands were positioned between those found with oxidized ceria. Since the molar extinction coefficient for the 15N2 stretching modes decreases only moderately with decreasing wavenumber, these findings suggest a decrease in the number of N2 adsorption sites after sample reduction. As the total number of surface-exposed cations does not decrease upon reduction, this implies that some Ce3+ cations are too weakly acidic to form adducts with dinitrogen.
Several types of adsorption sites can be considered on reduced ceria: (i) OH groups (ii) basic sites; (iii) surface Ce3+ cations on low-index planes such as (100), (110), and (111); (iv) Ce3+ cations at defects, including edges and corners (especially relevant in nanomaterials); and (iv) residual to reduction Ce4+ sites.
Three of these types are excluded from further discussion. First are the OH groups, which only interact with 15N2 at relatively high pressures and the adducts can be distinguished by the low wavenumber band at 2248 cm−1. Second are the basic sites, evidenced on some oxides by CO adsorption [77]. If they adsorb N2, the complexes formed should be monitored by a component of the weak band around 2248 cm−1. Third, non-reduced Ce4+ sites, where experimental evidence indicates that all Ce4+ cations on the {100} and {110} facets are reduced. Moreover, CO adsorption studies suggest that the remaining Ce4+ cations on {111} facets, if any, are of a negligible amount. Thus, the focus shifts to Ce3+ sites on regular planes and at defect sites.
First we underline that, to explicitly attribute some IR bands to definite surface planes, experiments with monocrystals are required. However, the use of shape-controlled nanoparticles combined with DFT calculations allows making some reasonable conclusions.
Reduction affects the Lewis acidity of cations through different mechanisms. Lowering the oxidation state of an isolated cation typically leads to reduced polarizing force due to the decrease in the positive charge and increase in the cationic radius. However, in oxide systems, cations are coordinated by oxygen anions, and reduction results in a lower coordination number. This should enhance the Lewis acidity. The resulting acidity depends on the interplay between these opposing effects. Based on our analysis of CO adsorption studies [25], we previously concluded that ceria reduction leads to (i) a sharp decrease in acidity for cations on {110} facets; (ii) a slight decrease on {100} facets; and (iii) a slight increase on {111} facets.
Cations at edges and defects are highly undercoordinated and remain the most active adsorption sites, despite some loss in acidity after reduction. Based on these considerations and on the DFT results, we assign the main 15N2 band at 2255 cm−1 to dinitrogen adsorbed at defect sites, primarily on crystal edges.
It was reported that the reduction of cations on the CeO2(111) plane leads to a slight increase in their Lewis acidity [25]. Thus, although the oxidized plane is inert towards adsorption, it is possible that the reduced facets adsorb N2. However, we do not support such assumption due to two reasons: (i) DFT calculations indicate low stability of Ce3+-N2 adducts on reduced CeO2(111) terrace and (ii) the 2255 cm−1 band is particularly weak in the CeO2-NP sample, which predominantly exposes the {111} facets.
The shoulder band at 2254 cm−1 is most pronounced for nanocubes, indicating it can be associated with the (100) facets. This aligns with DFT predictions of N2 bridging two Ce3+ cations with one nitrogen atom. This also explains the higher stability and lower frequency of the band at 2254 cm−1 compared with the main band at 2256 cm−1. Although the polarizing force is weaker, N2 is bound to two cations simultaneously, resulting in stronger binding. The relatively low band intensity can be explained by both a lower extinction coefficient (consistent with the lower frequency) and the adsorption geometry; if one N2 molecule bridges two cations, the number of adsorbed molecules is effectively halved relative to the number of available sites.
In summary, the specific arrangement of Ce3+ cations at the surface, including the exposure of {100}, {110}, and {111} facets, influences both the strength and the geometry of dinitrogen adsorption.
3.4. Potential Use of N2 as an IR Probe Molecule for Testing Surface of Ceria and Related Materials
The present results indicate that N2 has both advantages and limitations as a probe for ceria surfaces. The main limitation is its inability to interact with very weak Lewis acid sites. Therefore, a complete assessment of the Lewis acidity should combine N2 with another probe molecule, such as CO or pyridine.
Nonetheless, N2 offers important advantages. Its chemical inertness ensures that it does not form charged species upon adsorption, preserving the original surface speciation. Additionally, due to the weak nature of its interaction with the surface and the fact that no geminal species are formed, band positions are nearly independent of surface coverage. Consequently, differences in band maxima greater than 1 cm−1 can be considered significant. Thus, N2 can provide clear evidence for the presence of acidic Ce4+ and/or Ce3+ cations on surfaces subjected to different pre-treatments.
3.5. Future Directions
Since this is a pioneering study for the use of N2 as an IR probe for the surface of ceria and related materials, it leaves some open questions. Future works could focus on extending the reported findings here to more complex catalytic systems, including modified ceria (e.g., sulfated samples), doped ceria (e.g., with transition metals), mixed oxides (e.g., ceria-zirconia), and ceria with adsorbed species (e.g., peroxides and/or O2−). Additionally, investigations with single crystals could be essential to validate the conclusions drawn here.
4. Materials and Methods
4.1. Synthesis of the Samples
Two nanoscale ceria samples with distinct morphologies—cubes and rods—were synthesized using a hydrothermal method [9,18]. An aqueous solution of 5 g of Ce(NO3)3·6H2O (Fluka, Buchs, Switzerland, 99% purity) in 85 mL of water was added dropwise to 150 mL of a 36 wt.% NaOH solution (Merck, Darmstadt, Germany, 99% purity) under vigorous stirring. After forming a suspension, stirring continued for an additional 30 min before transferring the mixture to an autoclave.
To obtain nanocubes (designated CeO2-NC) and nanorods (CeO2-NR), the autoclave was maintained at 453 K and 373 K, respectively, for 24 h. Following the hydrothermal process, the suspensions were centrifuged, the precipitates were thoroughly rinsed with deionized water, dried at 393 K, and then calcined at 673 K for 2 h.
Nanopolyhedra (CeO2-NP) were prepared by calcining a portion of the CeO2-NC sample at 923 K for 1 h. It is well-documented that annealing ceria nanocubes at temperatures exceeding 873 K induces surface reconstruction resulting in the formation of {111} facets [24,27,60].
4.2. Characterization Techniques
FTIR spectra were recorded in situ using a Nicolet 6700 FTIR spectrometer equipped with a liquid nitrogen-cooled MCT-A detector (Thermo Scientific, Waltham, MA, USA), with 64 scans collected at a spectral resolution of 2 cm−1. Self-supporting pellets (~10 mg cm−2) were prepared by pressing powdered samples and analyzed in a custom-built glass IR cell allowing measurements from ambient temperature down to 100 K. The IR cell was connected to a vacuum-adsorption system, maintaining a residual pressure below 10−3 Pa. The samples were initially activated by heating in 100 mbar of O2 at 773 K for 30 min, followed by evacuation at the same temperature for an additional 30 min. These samples will be referred to further on as oxidized samples. To obtain reduced materials, the oxidized samples were subjected to reduction in 100 mbar of H2 for 30 min, followed by evacuation for 30 min, all this at 773 K. Finally, adsorption of 15N2 was performed at 100 K and an initial equilibrium pressure of 0.5 mbar, followed by evacuation. To ensure efficient thermal conductivity, helium (2 mbar) was introduced into the IR cell prior to the addition of 15N2.
For the in situ FTIR experiments, the following gases and adsorbates were used: 15N2 (Sigma-Aldrich, Darmstadt, Germany, 98 at. %), O2 (Messer, Clifford, Germany, 99.999%), H2 (Messer, >99.999%), and He (Messer, 99.999%). Prior to use, both 5N2 and He were further purified by passing through a liquid nitrogen trap.
Temperature-programmed reduction experiments were performed using ChemBET TPR/TPD (Quantachrome Instruments Co., Ltd., Boynton Beach, FL, USA) apparatus equipped with a thermal conductivity detector and using a 5% H2/Ar mixture (Messer) at a flow rate of 20 mL min−1 as a reducer. Before the experiments, the samples were heated to 723 K in a flowing stream of 5% O2/He (Messer) and then cooled down to room temperature. TPR curves were recorded while reducing the samples and increasing the temperature from 300 to 1273 K at a ramp rate of 10 K min−1. For separate TPR runs, the samples were first reduced to 773 K for 1 h and then cooled in an Ar flow before the TPR run.
Raman spectra were obtained with a Renishaw inVia™ confocal Raman microscope (Renishaw, Wotton-under-Edge, UK) using. The wavelength of the laser was 532 nm.
The high-resolution transmission electron microscopy patterns were obtained with a JEOL 2100 transmission electron microscope (JEOL Ltd., Tokyo, Japan) at an accelerating voltage of 200 kV.
Powder X-ray diffraction (XRD) analysis was performed on a Bruker D8 Advance diffractometer (Bruker AXS, Berlin, Germany) exploring a CuKα radiation.
The textural characteristics of the samples were determined by low-temperature nitrogen adsorption using a static volumetric Quantachrome NOVA 1200e apparatus (Quantachrome Instruments Co., Ltd., Boynton Beach, FL, USA).
4.3. DFT Studies
The periodic plane wave density functional theory (DFT) calculations were carried out using VASP code [78,79,80]. We used PW91 exchange–correlation functional [81] in combination with the empirical correction for dispersion interaction (PW91+D2) [82]. For the proper localization of the Ce4f electrons in the modeled systems containing Ce3+ cations, we employed on-site Coulombic correction with U = 4 eV [83,84]. We set the kinetic energy cut-off to 415 eV. The description of the core valence–electron interactions was performed employing the projector-augmented wave scheme [85]. The geometry of the models was relaxed until the maximum forces on each atom became lower than 0.02 eV/Å. The Brillouin zone consists of the Γ point only.
The binding energy of N2 (BE) to Ce4+ or Ce3+ cations was calculated as follows:
where E(N2/CeO2−x) is the electronic energy of the adsorption complex of N2 to a cerium cation from the corresponding reduced ceria system, E(CeO2−x) is the energy of the ceria system, and E(N2) is the energy of the N2 molecule in the gas phase. Thus, the negative values of BE correspond to an attractive interaction. The calculated frequencies for 15N2 were scaled by 0.9668, as described previously [59], to compensate for the difference between the experimental Raman frequency, 2331 cm−1 [86], and the calculated value of isolated 14N2, 2411 cm−1.
BE = E(N2/CeO2−x) − E(CeO2−x) − E(N2)
We employed four models of ceria to study the N2 adsorption on reduced ceria systems, which are based on the structures reported earlier [24]—slab models of CeO2(111) surface with a step and regular surfaces of CeO2(100) and CeO2(110), as well as a Ce40O80 nanoparticle model. From those models we produced reduced structures by the removal of one or two oxygen atoms, thus reducing two or four Ce4+ to Ce3+ cations.
5. Conclusions
The low-temperature (100 K) adsorption of 15N2 on reduced ceria leads to the formation of the following surface species:
- Species characterized by an IR band at 2256–2255 cm−1. We attribute this band to 15N2 coordinated linearly to a corner or edge Ce3+ site on the ceria surface.
- Species characterized by an IR band at 2254–2253 cm−1. We assign this band to 15N2 bridging two Ce3+ cations on the CeO2{100} facets by one nitrogen atom.
- 15N2 polarized by surface OH groups of ceria. This adsorption form is only observed in the presence of gas-phase 15N2 and appears at 2248 cm−1 in the IR spectra. The contribution of 15N2 adsorbed on basic sites to this band is not excluded.
- Due to their very weak acidity, the Ce3+ sites on the regular CeO2{110} and CeO2{111} facets are considered not to form detectable complexes with 15N2.
Therefore, IR spectroscopy of 15N2 adsorbed at low temperature can be used to identify and monitor acidic Ce3+ sites and to distinguish them from Ce4+ cations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30153100/s1. Figure S1. Raman spectra of the samples; Figure S2. FTIR spectra of the Ce3+ 2F5/2 → 2F7/2 electronic transition band; Figure S3. FTIR spectra of doses of 15N2 successively adsorbed on reduced ceria nanorods; Figure S4. IR spectral changes occluding after dosing CO to the 15N2-CeO2-NR system; Figure S5. IR spectral changes occurring after dosing O2 to the 15N2-CeO2-NR system. Table S1. Consumption of H2 during reduction of ceria samples.
Author Contributions
Conceptualization, K.I.H., methodology, M.Y.M. and G.N.V.; investigation, K.K.C., B.S.K., N.L.D., E.Z.I., and H.A.A.; writing—original draft preparation, H.A.A. and K.I.H.; writing—review and editing, K.K.C., M.Y.M., G.N.V., H.A.A., and K.I.H.; visualization, K.K.C., B.S.K., N.L.D., and E.Z.I.; funding acquisition, K.I.H. All authors have read and agreed to the published version of the manuscript.
Funding
The experimental and partly the theoretical parts of this work were financially supported by the Bulgarian Science Fund (Project number KΠ-06-ДB-1/2021).
Data Availability Statement
Data will be made available upon request.
Acknowledgments
B.S.K. is grateful to the European Union, NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No BG-RRP-2.004-0008, for the support. Computational resources at the Discoverer supercomputer were provided by Discoverer PetaSC and EuroHPC JU.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
References
- Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.T.R.; Shirinfar, B.; Majeed, S.; Najam-ul-Haq, M.; Hussain, D.; Iqbal, T.; Ahmed, N. Synthesis, Design and sensing applications of nanostructured ceria-based materials. Analyst 2018, 143, 5610–5628. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, R.; Tan, B.; Wang, F.; Yan, M.; Zhao, X.; Zhao, J. Research progress on the application of ceria nanoparticles as abrasives in dielectric layer CMP and post cleaning: Structure, morphology, doping, and mechanism. Colloids Surf. A 2023, 679, 132551. [Google Scholar] [CrossRef]
- Li, H.; Xia, P.; Pan, S.; Qi, Z.; Fu, C.; Yu, Z.; Kong, W.; Chang, Y.; Wang, K.; Wu, D.; et al. The advances of ceria nanoparticles for biomedical applications in orthopaedics. Int. J. Nanomed. 2020, 15, 7199–7214. [Google Scholar] [CrossRef] [PubMed]
- Rozhin, P.; Melchionna, M.; Fornasiero, P.; Marchesan, S. Nanostructured ceria: Biomolecular templates and (bio)applications. Nanomaterials 2021, 11, 2259. [Google Scholar] [CrossRef] [PubMed]
- Aneggi, E.; Boaro, M.; Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Insights into the redox properties of ceria-based oxides and their implications in catalysis. J. Alloys Compd. 2006, 408–412, 1096–1102. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Wu, Z. In situ spectroscopic insights into the redox and acid-base properties of ceria catalysts. Chin. J. Catal. 2021, 42, 2122–2140. [Google Scholar] [CrossRef]
- Mai, H.X.; Sun, L.D.; Zhang, Y.W.; Si, R.; Feng, W.; Zhang, H.P.; Liu, H.C.; Yan, C.K. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J. Phys. Chem. B 2005, 109, 24380–24388. [Google Scholar] [CrossRef] [PubMed]
- Tana; Zhang, M.; Li, J.; Li, H.; Li, Y.; Shen, W. Morphology-dependent redox and catalytic properties of CeO2 nanostructures: Nanowires, nanorods and nanoparticles. Catal. Today 2009, 148, 179–183. [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M.; Overbury, S.H. Probing defect sites on CeO2 nanocrystals with well-defined surface planes by Raman spectroscopy and O2 adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, M.; Overbury, S.H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes. J. Catal. 2012, 285, 61–73. [Google Scholar] [CrossRef]
- Qiao, Z.-A.; Wu, Z.; Dai, S. Shape-controlled ceria-based nanostructures for catalysis applications. ChemSusChem 2013, 6, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Désaunay, J.; Bonura, G.; Chiodo, V.; Freni, S.; Couzinié, J.-P.; Bourgon, J.; Ringuedé, A.; Labat, F.; Adamo, C.; Cassir, M. Surface-dependent oxidation of H2 on CeO2 surfaces. J. Catal. 2013, 297, 193–201. [Google Scholar] [CrossRef]
- Agarwal, S.; Lefferts, L.; Mojet, B.L. Ceria nanocatalysts: Shape dependent reactivity and formation of OH. ChemCatChem 2013, 5, 479–489. [Google Scholar] [CrossRef]
- Agarwal, S.; Lefferts, L.; Mojet, B.L.; Ligthart, D.A.J.M.; Hensen, E.J.M.; Mitchell, D.R.G.; Erasmus, W.J.; Anderson, B.G.; Olivier, E.J.; Neethling, J.H.; et al. Exposed surfaces on shape-controlled ceria nanoparticles revealed through AC-TEM and water–gas shift reactivity. ChemSusChem 2013, 6, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Zhu, N.; Hensen, E.J.M.; Mojet, B.L.; Lefferts, L. Surface-dependence of defect chemistry of nanostructured ceria. J. Phys. Chem. C 2015, 119, 12423–12433. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Djinovic, P.; Tchernychova, E.; Tkachenko, O.P.; Kustov, L.M.; Pintar, A. Nanoshaped CuO/CeO2 materials: Effect of the exposed ceria surfaces on catalytic activity in N2O decomposition reaction. ACS Catal. 2015, 5, 5357–5365. [Google Scholar] [CrossRef]
- Matei-Rutkovska, F.; Postole, G.; Rotaru, C.G.; Florea, M.; Pârvulescu, V.I.; Gelin, P. Synthesis of ceria nanopowders by microwave-assisted hydrothermal method for dry reforming of methane. Int. J. Hydrogen Energy 2016, 41, 2512–2525. [Google Scholar] [CrossRef]
- Bezkrovnyi, O.S.; Kraszkiewicz, P.; Ptak, M.; Kepinski, L. Thermally induced reconstruction of ceria nanocubes into zigzag {111}-nanofacetted structures and its influence on catalytic activity in CO oxidation. Catal. Commun. 2018, 117, 94–98. [Google Scholar] [CrossRef]
- Cao, T.; You, R.; Li, Z.; Zhang, X.; Li, D.; Chen, S.; Zhang, Z.; Huang, W. Morphology-dependent CeO2 catalysis in acetylene semihydrogenation reaction. Appl. Surf. Sci. 2020, 501, 144120. [Google Scholar] [CrossRef]
- Sudduth, B.; Yun, D.; Sun, J.; Wang, Y. Facet-dependent selectivity of CeO2 nanoparticles in 2-propanol conversion. J. Catal. 2021, 404, 96–108. [Google Scholar] [CrossRef]
- Chakarova, K.; Drenchev, N.; Mihaylov, M.; Hadjiivanov, K.I. Interaction of O2 with reduced ceria nanoparticles at 100–400 K: Fast oxidation of Ce3+ ions and dissolved H2. Catalysts 2024, 14, 45. [Google Scholar] [CrossRef]
- Chakarova, K.; Zdravkova, V.; Karapenchev, B.; Nihtianova, D.; Ivanova, E.; Aleksandrov, H.; Koleva, I.; Panayotov, D.; Mihaylov, M.; Vayssilov, G.; et al. Evolution of Ce4+ Lewis acidity during dehydroxylation of ceria nanoparticles with different morphology: An integrated FTIR, DFT and HRTEM study. J. Catal. 2024, 433, 115463. [Google Scholar] [CrossRef]
- Chakarova, K.K.; Karapenchev, B.S.; Drenchev, M.; Ivanova, E.Z.; Aleksandrov, H.A.; Panayotov, D.A.; Mihaylov, M.Y.; Vayssilov, G.N.; Hadjiivanov, K.I. FTIR study of low-temperature CO adsorption on reduced ceria nanoparticles with different morphology: A comparison with oxidized samples. J. Catal. 2025, 443, 115986. [Google Scholar] [CrossRef]
- Aneggi, E.; Llorca, J.; Boaro, M.; Trovarelli, A. Surface-structure sensitivity of CO oxidation over polycrystalline ceria powders. J. Catal. 2005, 234, 88–95. [Google Scholar] [CrossRef]
- Aneggi, E.; Wiater, D.; Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-dependent activity of ceria in soot combustion. ACS Catal. 2014, 4, 172–181. [Google Scholar] [CrossRef]
- Kovacevic, M.; Mojet, B.L.; van Ommen, J.G.; Lefferts, L. Effects of morphology of cerium oxide catalysts for reverse water gas shift reaction. Catal. Lett. 2016, 146, 770–777. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Vayssilov, G.N. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. Adv. Catal. 2002, 47, 307–511. [Google Scholar] [CrossRef]
- Davydov, A.A. Infrared Spectroscopy of Adsorbed Species on the Surface of Transition Metal Oxides; J. Wiley & Sons: Chichester, UK, 1990. [Google Scholar]
- Bozon-Verduraz, F.; Bensalem, A. IR studies of cerium dioxide: Influence of impurities and defects. J. Chem. Soc. Faraday Trans. 1994, 90, 653–657. [Google Scholar] [CrossRef]
- Binet, C.; Badri, A.; Lavalley, J.-C. A spectroscopic characterization of the reduction of ceria from electronic transitions of intrinsic point defects. J. Phys. Chem. 1994, 98, 6392–6398. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.-C. IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Vayssilov, G.N.; Mihaylov, M.; Petkov, P.S.; Hadjiivanov, K.I.; Neyman, K.M. Reassignment of the vibrational spectra of carbonates, formates, and related surface species on ceria: A combined density functional and infrared spectroscopy investigation. J. Phys. Chem. C 2011, 115, 23435–23454. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Ivanova, E.Z.; Aleksandrov, H.A.; Petkov, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. FTIR and density functional study of NO interaction with reduced ceria: Identification of N3− and NO2− as new intermediates in NO conversion. Appl. Catal. B 2015, 176–177, 107–119. [Google Scholar] [CrossRef]
- Wu, W.; Savereide, L.M.; Notestein, J.; Weitz, E. In-situ IR spectroscopy as a probe of oxidation/reduction of Ce in nanostructured CeO2. Appl. Surf. Sci. 2018, 445, 548–554. [Google Scholar] [CrossRef]
- Grünbacher, M.; Schlicker, L.; Bekheet, M.F.; Gurlo, A.; Klötzer, B.; Penner, S. H2 reduction of Gd- and Sm-doped ceria compared to pure CeO2 at high temperatures: Effect on structure, oxygen nonstoichiometry, hydrogen solubility and hydroxyl chemistry. Phys. Chem. Chem. Phys. 2018, 20, 22099–22113. [Google Scholar] [CrossRef] [PubMed]
- Panayotov, D.; Zdravkova, V.; Lagunov, O.; Andonova, S.; Spassova, I.; Nihtianova, D.; Atanasova, G.; Drenchev, N.; Ivanova, E.; Mihaylov, M.; et al. Capturing CO2 by ceria and ceria-zirconia nanomaterials of different origin. Phys. Chem. Chem. Phys. 2023, 25, 17154–17175. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Bollini, P. On the utility of Ce3+ spin-orbit transitions in the interpretation of rate data in ceria catalysis: Theory, validation, and application. J. Phys. Chem. C 2023, 127, 234–247. [Google Scholar] [CrossRef]
- Bensalem, D.; Bozon-Verduraz, F.; Delamar, M.; Bugli, G. Preparation and characterization of highly dispersed silica-supported ceria. Appl. Catal. A 1995, 121, 81–93. [Google Scholar] [CrossRef]
- Vassileva, E.; Varimezova, B.; Hadjiivanov, K. Column solid-phase extraction of heavy metal ions on a high surface area CeO2 as a pre-concentration method for trace determination. Anal. Chim. Acta 1996, 336, 141–150. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Confer, M.P.; Li, J.; Wang, R. In-situ DRIFTS study of chemically etched CeO2 nanorods supported transition metal oxide catalysts. Mol. Catal. 2021, 509, 111629. [Google Scholar] [CrossRef]
- Chen, S.; Cao, T.; Gao, Y.; Li, D.; Xiong, F.; Huang, W. Probing surface structures of CeO2, TiO2, and Cu2O nanocrystals with CO and CO2 chemisorption. J. Phys. Chem. C 2016, 120, 21472–21485. [Google Scholar] [CrossRef]
- Laachir, A.; Perrichon, V.; Badri, A.; Lamotte, J.; Catherine, E.; Lavalley, J.C.; El Fallah, J.; Hilaire, L.; Le Normand, F.; Quéméré, E.; et al. Reduction of CeO2 by hydrogen: Magnetic susceptibility and Fourier-transform infrared, ultraviolet and X-ray photoelectron spectroscopy measurements. J. Chem. Soc. Faraday Trans. 1991, 87, 1601–1609. [Google Scholar] [CrossRef]
- Jacobs, G.; Williams, L.; Graham, U.; Thomas, G.A.; Sparks, D.E.; Davis, B.H. Low temperature water–gas shift: In situ DRIFTS-reaction study of ceria surface area on the evolution of formates on Pt/CeO2 fuel processing catalysts for fuel cell applications. Appl. Catal. A 2003, 252, 107–118. [Google Scholar] [CrossRef]
- Zepeda, T.A.; Hernandez-Maldonado, J.A.; Fuentes, G.A.; Fierro-Gonzalez, J.C.; Goomez, S.A. Spectroscopic insights into gold-cerium oxidation states influencing catalytic activity and selectivity in CeO2-supported gold during CO-PROX reaction. Mol. Catal. 2024, 552, 113693. [Google Scholar] [CrossRef]
- Daly, H.; Ni, J.; Thompsett, D.; Meunier, F.C. On the usefulness of carbon isotopic exchange for the operando analysis of metal–carbonyl bands by IR over ceria-containing catalysts. J. Catal. 2008, 254, 238–243. [Google Scholar] [CrossRef]
- Sakata, Y.; Kinoshita, N.; Domen, K.; Onishi, T. Infrared studies on dinitrogen and dihydrogen adsorbed over TiO2 at low temperatures. J. Chem. Soc. Faraday Trans. 1987, 83, 2765–2772. [Google Scholar] [CrossRef]
- Geobaldo, F.; Lamberti, C.; Ricchiardi, G.; Bordiga, S.; Zecchina, A.; Turnes Palomino, G.; Otero Arean, C. N2 adsorption at 77 K on H-mordenite and alkali-metal-exchanged mordenites: An IR study. J. Phys. Chem. 1995, 99, 11167–11177. [Google Scholar] [CrossRef]
- Wakabayashi, F.; Kondo, J.N.; Domen, K.; Hirose, C. Direct comparison of N2 and CO as IR-spectroscopic probes of acid sites in H-ZSM-5 zeolite. J. Phys. Chem. 1995, 99, 10573–10580. [Google Scholar] [CrossRef]
- Neyman, K.M.; Strodel, P.; Ruzankin, S.P.; Schlensog, N.; Knözinger, H. N2 and CO molecules as probes of zeolite acidity: An infrared spectroscopy and density functional investigation. Catal. Lett. 1995, 31, 273–285. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Knözinger, H. FTIR spectroscopic evidence of formation of geminal dinitrogen species during the low-temperature N2 adsorption on NaY zeolites. Catal. Lett. 1999, 58, 21–26. [Google Scholar] [CrossRef]
- Zecchina, A.; Otero Arean, C.; Turnes Palomino, G.; Geobaldo, F.; Lamberti, C.; Spoto, G.; Bordiga, S. The vibrational spectroscopy of H2, N2, CO and NO adsorbed on the titanosilicate molecular sieve ETS-10. Phys. Chem. Chem. Phys. 1999, 1, 1649–1657. [Google Scholar] [CrossRef]
- Vayssilov, G.N.; Hu, A.; Birkenheuer, U.; Rösch, N. Dinitrogen as probe molecule of alkali-exchanged zeolites: A density functional study. J. Mol. Catal. A 2000, 162, 135–140. [Google Scholar] [CrossRef]
- Recchia, S.; Dossi, C.; Psaro, R.; Fusi, A.; Ugo, R. Dinitrogen irreversible adsorption on overexchanged Cu-ZSM-5. J. Phys. Chem. B 2002, 106, 13326–13332. [Google Scholar] [CrossRef]
- Larin, D.V.; Vercauteren, D.P.; Lamberti, C.; Bordiga, S.; Zecchina, A. Interaction between probe molecules and zeolites. Part II: Interpretation of the IR spectra of CO and N2 adsorbed in NaY and NaRbY. Phys. Chem. Chem. Phys. 2002, 4, 2424–2433. [Google Scholar] [CrossRef]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Turnes Palomino, G.; Arean, C.O.; Bordiga, S. Computational and experimental studies on the adsorption of CO, N2, and CO2 on Mg-MOF-74. J. Phys. Chem. C 2010, 114, 11185–11191. [Google Scholar] [CrossRef]
- Zdravkova, V.; Mihaylov, M.; Hadjiivanov, K. Coordination of two N2 molecules to one Ni+ site in Ni–ZSM-5: An FTIR spectroscopy study. J. Phys. Chem. C 2024, 116, 12706–12711. [Google Scholar] [CrossRef]
- Chakarova, K.K.; Mihaylov, M.Y.; Karapenchev, B.S.; Koleva, I.Z.; Vayssilov, G.N.; Aleksandrov, H.A.; Hadjiivanov, K.I. N2 as an efficient IR probe molecule for the investigation of ceria-containing materials. Molecules 2024, 29, 3608. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Capdevila-Cortada, M.; Dong, C.; Zhou, Y.; Wang, J.; Yu, X.; Nefedov, A.; Heißler, S.; López, N.; Shen, W.; et al. Surface refaceting mechanism on cubic ceria. J. Phys. Chem. Lett. 2020, 11, 7925–7931. [Google Scholar] [CrossRef] [PubMed]
- Loridant, S. Raman spectroscopy as a powerful tool to characterize ceria-based catalysts. Catal. Today 2021, 373, 98–111. [Google Scholar] [CrossRef]
- Taniguchi, T.; Watanabe, T.; Sugiyama, N.; Subramani, A.K.; Wagata, H.; Matsushita, N.; Yoshimura, M. Identifying defects in ceria-based nanocrystals by UV resonance Raman spectroscopy. J. Phys. Chem. C 2009, 113, 19789–19793. [Google Scholar] [CrossRef]
- Trenque, I.; Magnano, G.C.; Bárta, J.; Chaput, F.; Bolzinger, M.A.; Pitault, I.; Briançon, S.; Masenelli-Varlot, K.; Bugnet, M.; Dujardin, C.; et al. Synthesis routes of CeO2 nanoparticles dedicated to organophosphorus degradation: A benchmark. CrystEngComm 2020, 22, 1725–1737. [Google Scholar] [CrossRef]
- Cardenas, L.; Molinet-Chinaglia, C.; Loridant, S. Unraveling Ce3+ detection at the surface of ceria nanopowders by UPS analysis. Phys. Chem. Chem. Phys. 2022, 24, 22815–22822. [Google Scholar] [CrossRef] [PubMed]
- Hojo, H.; Hirota, K.; Ito, S.; Einaga, S. Reduction mechanism for CeO2 revealed by direct observation of the oxygen vacancy distribution in shape-controlled CeO2. Adv. Mater. Interfaces 2023, 10, 2201954. [Google Scholar] [CrossRef]
- Bruce, L.A.; Hoang, M.; Hughes, A.E.; Turney, T.W. Surface area control during the synthesis and reduction of high area ceria catalyst supports. Appl. Catal. A 1996, 134, 351–362. [Google Scholar] [CrossRef]
- Holgado, J.P.; Munuera, G. XPS/TPR study of the reducibility of M/CeO2 catalysts (M = Pt, Rh): Does junction effect theory apply? Stud. Surf. Sci. Catal. 1995, 96, 109–122. [Google Scholar] [CrossRef]
- Rao, G.R. Influence of metal particles on the reduction properties of ceria-based materials studied by TPR. Bull. Mater. Sci. 1999, 22, 89–94. [Google Scholar] [CrossRef]
- Nolan, M.; Parker, S.C.; Watson, G.W. The electronic structure of oxygen vacancy defects at the low index surfaces of ceria. Surf. Sci. 2005, 595, 223–232. [Google Scholar] [CrossRef]
- Bernal, S.; Calvino, J.J.; Cifredo, G.A.; Gatica, J.M.; Perez Omil, H.; Pintado, J.M. Hydrogen chemisorption on ceria: Influence of the oxide surface area and degree of reduction. J. Chem. Soc. Faraday Trans. 1993, 89, 3499–3505. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Filimonov, V.N. Infrared spectra of surface hydroxyl groups and crystalline structure of oxides. J. Mol. Struct. 1973, 19, 579–589. [Google Scholar] [CrossRef]
- Badri, A.; Binet, C.; Lavalley, J.-C. An FTIR study of surface ceria hydroxy groups during a redox process with H2. J. Chem. Soc. Faraday Trans. 1996, 92, 4669–4673. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, Y.; Tao, F.; Daemen, L.; Foo, G.S.; Nguyen, L.; Zhang, X.; Beste, A.; Ramirez-Cuesta, A.J. Direct neutron spectroscopy observation of cerium hydride species on a cerium oxide catalyst. J. Am. Chem. Soc. 2017, 139, 9721–9727. [Google Scholar] [CrossRef] [PubMed]
- Zverev, S.M.; Smirnov, K.S.; Tsyganenko, A.A. IR spectroscopic study of low-temperature adsorption of molecular nitrogen on the surface of oxides. Kinet. Catal. 1988, 29, 1251–1257. [Google Scholar]
- Chakarova, K.; Strauss, I.; Mihaylov, M.; Drenchev, N.; Hadjiivanov, K. Evolution of acid and basic sites in UiO-66 and UiO-66-NH2 metal-organic frameworks: FTIR study by probe molecules. Microporous Mesoporous Mater. 2019, 281, 110–122. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Yang, C.; Wang, Y.; Wöll, C.; Ganduglia-Pirovano, M.V. Vibrational frequencies of CO bound to all three low-index cerium oxide surfaces: A consistent theoretical description of vacancy-induced changes using density functional theory. J. Chem. Phys. 2023, 159, 034704. [Google Scholar] [CrossRef] [PubMed]
- Babaeva, M.A.; Tsyganenko, A.A. Infrared spectroscopic evidence for the formation of carbonite CO22− ions in CO interaction with basic oxide surfaces. React. Kinet. Catal. Lett. 1987, 34, 9–14. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Vienna Ab Initio Simulation Package (VASP), Version 6.4. A computer program for atomic scale materials modelling. University of Vienna: Vienna, Austria. Available online: https://cmp.univie.ac.at/research/vasp/ (accessed on 19 June 2025).
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Erratum: Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1993, 48, 4978. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.J. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.I.; Aryasetiawan, F.; Lichtenstein, A.I. First-principles calculations of the electronic structure and spectra of strongly correlated systems: The LDA+U method. J. Phys. Condens. Matter 1997, 9, 767–808. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Rasetti, F. Incoherent scattered radiation in diatomic molecules. Phys. Rev. 1929, 34, 367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).