Synthesis of Novel Bioactive Lipophilic Hydroxyalkyl Esters and Diesters Based on Hydroxyphenylacetic Acids
Abstract
1. Introduction
2. Results and Discussions
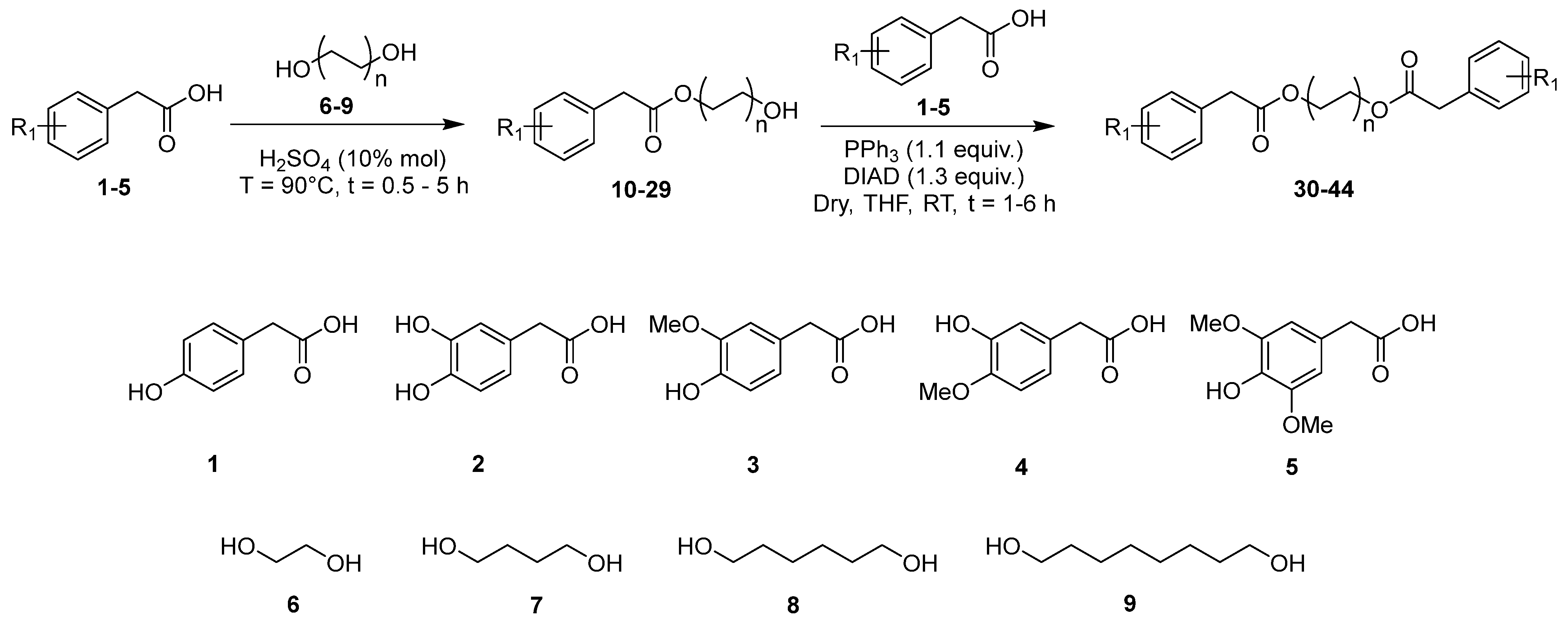
2.1. Synthetic Procedures
2.2. Synthesis of Hydroxyalkyl Esters 10–29
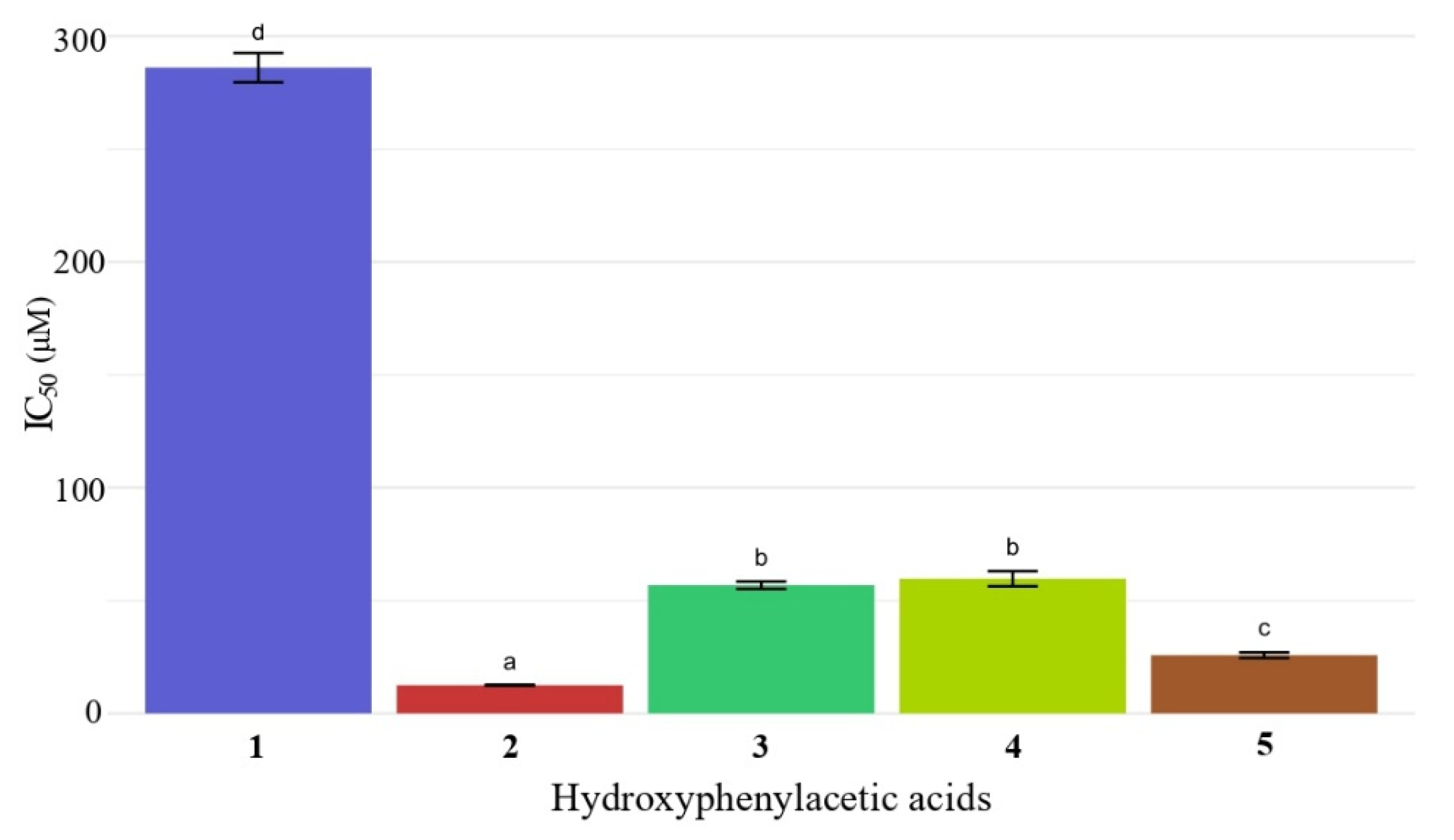
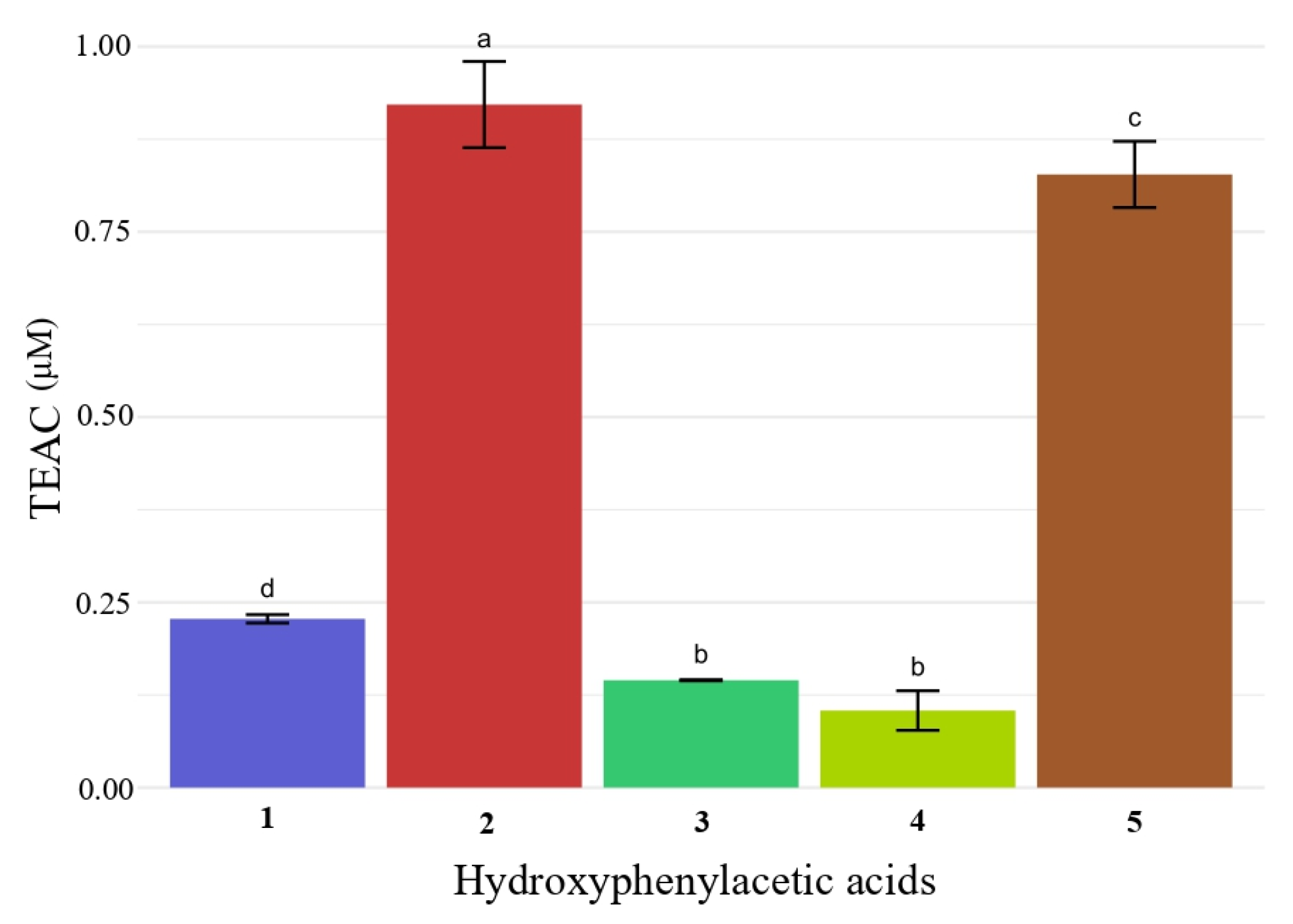
2.3. Antioxidant Activity of Hydroxyphenylacetic Acids 1–5 and of Hydroxyalkyl Esters 10–29 by DPPH and ABTS Assays
2.4. Synthesis of Butyl Diarylacetates 30–44
2.5. Antioxidant Activity of Butyl Diarylacetates by DPPH and ABTS Assays
2.6. Qualitative Evaluation of the Bactericidal Properties of Hydroxyphenylacetic Acids 1–5 and Butyl Diarylacetates 31, 36, 37, and 38 Against Staphylococcus aureus and Escherichia coli
3. Material and Methods
3.1. Reagents and Instruments
3.2. Synthesis of Hydroxyalkyl Esters 10–29 by Fischer Esterification
- 2-Hydroxyethyl 2-(4-hydroxyphenyl) acetate 10. White solid, mp = 72–73 °C. FT-IR (neat): 3379, 3176, 1699, 1219, 1171, 531 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 7.11 (d, J = 8.6 Hz, 2H, Ph-H), 6.74 (d, J = 8.6 Hz, 2H, Ph-H), 4.87 (bs, 2H, -OH), 4.17–4.14 (m, 2H, -CH2-O), 3.75–3.72 (m, 2H, -CH2-O), 3.58 (s, 2H, -COCH2-); 13C NMR (100.6 MHz) (CD3OD) δ = 172.8 (C), 156.1 (C), 130.0 (CH), 124.9 (C), 114.9 (CH), 65.8 (CH2), 59.6 (CH2), 39.6 (CH2). HRMS: m/z [M + H]+ calcd. for C10H13O4: 197.0814; found: 197.0805.
- 4-Hydroxybutyl 2-(4-hydroxyphenyl)acetate 11. Pale yellow oil. FT-IR (neat): 3323, 2948, 1708, 1514, 1223, 1033 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 7.09 (d, J = 8.6 Hz, 2H, Ph-H), 6.74 (d, J = 8.6 Hz, 2H, Ph-H), 4.88 (bs, 2H, -OH), 4.11 (t, J = 6.5 Hz, 2H, -CH2-O), 3.56 (t, J = 6.4 Hz, 2H, -CH2-O), 3.53 (s, 2H, -COCH2-), 1.73–1.66 (m, 2H, CH2-CH2-CH2), 1.60–1.53 (m, 2H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.7 (C), 156.2 (C), 129.9 (CH), 125.0 (C), 114.9 (CH), 64.3 (CH2), 61.0 (CH2), 39.8 (CH2), 28.6 (CH2), 24.9 (CH2); HRMS: m/z [M + H]+ calcd. for C12H17O4: 225.1127; found: 225.1116.
- 6-Hydroxyhexyl 2-(4-hydroxyphenyl)acetate 12. White solid, mp = 67–68 °C. FT-IR (neat): 3440, 3141, 1713, 1220, 1170, 987 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 7.09 (d, J = 8.6 Hz, 2H, Ph-H), 6.74 (d, J = 8.6 Hz, 2H, Ph-H), 4.88 (s, 2H, -OH), 4.09 (t, J = 6.6 Hz, 2H, -CH2-O), 3.56–3.53 (m, 4H), 1.67–1.60 (m, 2H, R-CH2-R), 1.56–1.49 (m, 2H, CH2-CH2-CH2), 1.41–1.31 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.8 (C), 156.2 (C), 129.9 (CH), 125.1 (C), 114.9 (CH), 64.4 (CH2), 61.4 (CH2), 39.9 (CH2), 32.1 (CH2), 28.3 (CH2), 25.4 (CH2), 25.1 (CH2); HRMS: m/z [M + H]+ calcd. for C14H21O4: 253.1440; found: 253.1428.
- 8-Hydroxyoctyl 2-(4-hydroxyphenyl)acetate 13. White wax. FT-IR (neat): 3325, 2927, 2853, 1728, 1173, 1028 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 7.09 (d, J = 8.4 Hz, 2H, Ph-H), 6.74 (d, J = 8.4 Hz, 2H, Ph-H), 4.88 (s, 2H, -OH), 4.08 (t, J = 6.6 Hz, 2H, -CH2-O), 3.56 (t, J = 6.6 Hz, 2H, -CH2-O), 3.52 (s, 2H, -COCH2-), 1.63–1.60 (m, 2H, CH2-CH2-CH2), 1.57–1.50 (m, 2H, CH2-CH2-CH2), 1.36–1.27 (m, 8H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.8 (C), 156.2 (C), 129.9 (CH), 125.1 (C), 114.9 (CH), 64.5 (CH2), 61.6 (CH2), 39.9 (CH2), 32.2 (CH2), 29.1 (CH2), 28.9 (CH2), 28.3 (CH2), 25.5 (CH2), 25.4 (CH2); HRMS: m/z [M + H]+ calcd. for C16H25O4: 281.1753; found: 281.1738.
- 2-Hydroxyethyl 2-(3,4-dihydroxyphenyl)acetate 14. Pale pink solid, mp = 80–81 °C. FT-IR (neat): 3420, 3221, 1698, 1447, 1115 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.74 (d, J = 2.1 Hz, 1H, Ph-H), 6.71 (d, J = 8.1 Hz, 1H, Ph-H), 6.60 (dd, J1 = 8.1 Hz, J2 = 2.1 Hz, 1H, Ph-H), 4.88 (s, 3H, -OH), 4.17–4.14 (m, 2H, -CH2-O), 3.75–3.73 (m, 2H, -CH2-O), 3.52 (s, 2H, -COCH2-); 13C NMR (100.6 MHz) (CD3OD) δ = 172.8 (C), 144.9 (C), 144.1 (C), 125.5 (C), 120.3 (CH), 116.0 (CH), 114.9 (CH), 65.8 (CH2), 59.6 (CH2), 39.8 (CH2); HRMS: m/z [M + H]+ calcd. for C10H13O5: 213.0763; found: 213.0753.
- 4-Hydroxybutyl 2-(3,4-dihydroxyphenyl)acetate 15. Yellow oil. FT-IR (neat): 3329, 2947, 2362, 1708, 1519, 1285 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.73–6.70 (m, 2H, Ph-H), 6.59 (dd, J1 = 8.0 Hz, J2 = 2.1 Hz, 1H, Ph-H), 4.88 (s, 3H, -OH), 4.1 (t, J = 6.5 Hz, 2H, -CH2-O), 3.56 (t, J = 6.5 Hz, 2H, -CH2-O), 3.47 (s, 2H, -COCH2-), 1.74–1.67 (m, 2H, CH2-CH2-CH2), 1.60–1.53 (m, 2H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.7 (C), 144.9 (C), 144.0 (C), 125.6 (C), 120.2 (CH), 115.9 (CH), 114.9 (CH), 64.3 (CH2), 61.0 (CH2), 40.1 (CH2), 28.6 (CH2), 24.9 (CH2); HRMS: m/z [M + H]+ calcd. for C12H17O5: 241.1076; found: 241.1065.
- 6-Hydroxyhexyl 2-(3,4-dihydroxyphenyl)acetate 16. Orange oil. FT-IR (neat): 3363, 2935, 1709, 1519, 1284 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.72–6.70 (m, 2H, Ph-H), 6.60–6.58 (m, 1H, Ph-H), 4.87 (s, 3H, -OH), 4.09 (t, J = 6.5 Hz, 2H, -CH2-O), 3.54 (t, J = 6.5 Hz, 2H, -CH2-O), 3.46 (s, 2H, COCH2-), 1.64–1.61 (m, 2H, CH2-CH2-CH2), 1.53–1.51 (m, 2H, CH2-CH2-CH2), 1.37–1.31 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.8 (C), 144.9 (C), 144.0 (C), 125.7 (C), 120.2 (CH), 115.9 (CH), 114.9 (CH), 64.4 (CH2), 61.5 (CH2), 40.2 (CH2), 32.1 (CH2), 28.3 (CH2), 25.4 (CH2), 25.1 (CH2); HRMS: m/z [M + H]+ calcd. for C14H21O5: 269.1389; found: 269.1375.
- 8-Hydroxyoctyl 2-(3,4-dihydroxyphenyl)acetate 17. Purified with semi-preparative RP-HPLC, Teknokroma column Mediterranea SEA18 5mm (25 × 0.78 cm) using CH3CN/H2O 50/50 v/v as mobile phase with flow rate 4 mL/min. Pale yellow wax. FT-IR (neat): 3387, 2924, 2852, 1707, 1354, 1194 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.73–6.70 (m, 2H, Ph-H), 6.59 (dd, J1 = 8.0 Hz, J2 = 2.0 Hz, 1H, Ph-H), 4.86 (bs, 3H, -OH), 4.08 (t, J = 6.5 Hz, 2H, -CH2-O), 3.56 (t, J = 6.5 Hz, 2H, -CH2-O), 3.46 (s, 2H, COCH2-), 1.63–1.50 (m, 4H, CH2-CH2-CH2), 1.32 (m, 8H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.8 (C), 144.9 (C), 144.0 (C), 125.7 (C), 120.2 (CH), 115.9 (CH), 114.9 (CH), 64.5 (CH2), 61.6 (CH2), 40.2 (CH2), 32.2 (CH2), 29.0 (CH2), 28.9 (CH2), 28.3 (CH2), 25.5 (CH2), 25.4 (CH2); HRMS: m/z [M + H]+ calcd. for C16H25O5: 297.1702; found: 297.1687.
- 2-Hydroxyethyl 2-(4-hydroxy-3-methoxyphenyl)acetate 18. Orange oil. FT-IR (neat): 3420, 2942, 1719, 1516, 1275, 1154 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.89 (d, J = 1.6 Hz, 1H, Ph-H), 6.76–6.71 (m, 2H, Ph-H), 4.87 (s, 2H, -OH), 4.18–4.16 (m, 2H, -CH2-O), 3.85 (s, 3H, -OCH3), 3.76–3.73 (m, 2H, -CH2-O), 3.59 (s, 2H, COCH2-); 13C NMR (100.6 MHz) (CD3OD) δ = 172.7 (C), 147.5 (C), 145.3 (C), 125.5 (C), 121.6 (CH), 114.7 (CH), 112.6 (CH), 65.9 (CH2), 59.6 (CH2), 55.0 (CH3), 39.9 (CH2); HRMS: m/z [M + H]+ calcd. for C11H15O5: 227.0919; found: 227.0908.
- 4-Hydroxybutyl 2-(4-hydroxy-3-methoxyphenyl)acetate 19. Colorless oil. FT-IR (neat): 3423, 2940, 1719, 1516, 1273, 1153 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.87 (d, J = 1.9 Hz, 1H, Ph-H), 6.76–6.69 (m, 2H, Ph-H), 4.87 (s, 2H, -OH), 4.12 (t, J = 6.4 Hz, 2H, -CH2-O), 3.85 (s, 3H, -OCH3), 3.56 (t, J = 6.4 Hz, 2H, -CH2-O), 3.55 (s, 2H, COCH2-), 1.74–1.67 (m, 2H, CH2-CH2-CH2), 1.61–1.53 (m, 2H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.7 (C), 147.5 (C), 145.3 (C), 125.6 (C), 121.5 (CH), 114.8 (CH), 112.5 (CH), 64.4 (CH2), 61.0 (CH2), 55.0 (CH3), 40.2 (CH2), 28.6 (CH2), 24.9 (CH2); HRMS: m/z [M + H]+ calcd. for C13H19O5: 255.1232; found: 255.1222.
- 6-Hydroxyhexyl 2-(4-hydroxy-3-methoxyphenyl)acetate 20. Colorless oil. FT-IR (neat): 3356, 2934, 1715, 1514, 1271, 1149 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.86 (d, J = 1.9 Hz, 1H, Ph-H), 6.76–6.69 (m, 2H, Ph-H), 4.87 (s, 2H, -OH), 4.10 (t, J = 6.5 Hz, 2H, -CH2-O), 3.85 (s, 3H, -OCH3), 3.56–3.52 (m, 4H), 1.67–1.60 (m, 2H, CH2-CH2-CH2), 1.55–1.48 (m, 2H, CH2-CH2-CH2), 1.40–1.31 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.7 (C), 147.5 (C), 145.3 (C), 125.6 (C), 121.5 (CH), 114.8 (CH), 112.5 (CH), 64.5 (CH2), 61.4 (CH2), 55.0 (CH3), 40.3 (CH2), 32.1 (CH2), 28.3 (CH2), 25.4 (CH2), 25.1 (CH2); HRMS: m/z [M + H]+ calcd. for C15H23O5: 283.1545; found: 283.1532.
- 8-Hydroxyoctyl 2-(4-hydroxy-3-methoxyphenyl)acetate 21. Pale yellow oil. FT-IR (neat): 3391, 2929, 2854, 1724, 1516, 1275, 1152 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.86 (d, J = 1.4 Hz, 1H, Ph-H), 6.76–6.69 (m, 2H, Ph-H), 4.85 (s, 2H, -OH), 4.09 (t, J = 6.5 Hz, 2H, -CH2-O), 3.85 (s, 3H, -OCH3), 3.55 (t, J = 6.7 Hz, 2H, -CH2-O), 3.53 (s, 2H, COCH2-), 1.63–1.58 (m, 2H, CH2-CH2-CH2), 1.55–1.50 (m, 2H, CH2-CH2-CH2), 1.31 (bs, 8H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.7 (C), 147.5 (C), 145.3 (C), 125.6 (C), 121.5 (CH), 114.8 (CH), 112.5 (CH), 64.5 (CH2), 61.6 (CH2), 55.0 (CH3), 40.3 (CH2), 32.2 (CH2), 29.1 (CH2), 28.9 (CH2), 28.3 (CH2), 25.5 (CH2), 25.4 (CH2); HRMS: m/z [M + H]+ calcd. for C17H27O5: 311.1858; found: 311.1841.
- 2-Hydroxyethyl 2-(3-hydroxy-4-methoxyphenyl)acetate 22. Pale pink solid, mp = 63–64 °C. FT-IR (neat): 3387, 3246, 1697, 1509, 1216 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.86 (d, J = 8.2 Hz, 1H, Ph-H), 6.77 (bs, 1H, Ph-H), 6.72 (dd, J1 = 8.2 Hz, J2 = 1.6 Hz, 1H, Ph-H), 4.89 (s, 2H, -OH), 4.16 (t, J = 4.9 Hz, 2H, -CH2-O), 3.84 (s, 3H, -OCH3), 3.74 (t, J = 4.9 Hz, 2H, -CH2-O), 3.55 (s, 2H, COCH2-); 13C NMR (100.6 MHz) (CD3OD) δ = 172.5 (C), 146.8 (C), 146.1 (C), 127.0 (C), 120.2 (CH), 116.0 (CH), 111.4 (CH), 65.9 (CH2), 59.6 (CH2), 55.0 (CH3), 39.8 (CH2); HRMS: m/z [M + H]+ calcd. for C11H15O5: 227.0919; found: 227.0908.
- 4-Hydroxybutyl 2-(3-hydroxy-4-methoxyphenyl)acetate 23. Light yellow oil. FT-IR (neat): 3397, 2939, 1715, 1511, 1274 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.86 (d, J = 8.1 Hz, 1H, Ph-H), 6.76 (s, 1H, Ph-H), 6.71 (d, J = 8.1 Hz, 1H, Ph-H), 4.87 (s, 2H, -OH), 4.12 (t, J = 6.3 Hz, 2H, -CH2-O), 3.84 (s, 3H, -OCH3), 3.56 (t, J = 6.3 Hz. 2H, -CH2-O), 3.51 (s, 2H, COCH2-), 1.74–1.67 (m, 2H, CH2-CH2-CH2), 1.60–1.53 (m, 2H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.5 (C), 146.8 (C), 146.2 (C), 127.1 (C), 120.1 (CH), 115.9 (CH), 111.4 (CH), 64.4 (CH2), 61.0 (CH2), 55.0 (CH3), 40.1 (CH2), 28.6 (CH2), 24.9 (CH2); HRMS: m/z [M + H]+ calcd. for C13H19O5: 255.1232; found: 255.1221.
- 6-Hydroxyhexyl 2-(3-hydroxy-4-methoxyphenyl)acetate 24. Pale yellow oil. FT-IR (neat): 3402, 2933, 2857, 1717, 1511, 1273 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.86 (d, J = 8.2 Hz, 1H, Ph-H), 6.76 (bs, 1H, Ph-H), 6.71 (d, J = 8.2 Hz, 1H, Ph-H), 4.87 (s, 2H, -OH), 4.09 (t, J = 6.4 Hz, 2H, -CH2-O), 3.84 (s, 3H, -OCH3), 3.54 (t, J = 6.4 Hz, 2H, -CH2-O), 3.50 (s, 2H, COCH2-), 1.65–1.62 (m, 2H, CH2-CH2-CH2), 1.53–1.51 (m, 2H, CH2-CH2-CH2), 1.36 (bs, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.5, 146.8, 146.2, 127.1, 120.1 (CH), 115.9 (CH), 111.3 (CH), 64.4 (CH2), 61.4 (CH2), 55.0 (CH3), 40.2 (CH2), 32.1 (CH2), 28.3 (CH2), 25.4 (CH2), 25.1 (CH2); HRMS: m/z [M + H]+ calcd. for C15H23O5: 283.1545; found: 283.1532.
- 8-Hydroxyoctyl 2-(3-hydroxy-4-methoxyphenyl)acetate 25. Light yellow oil. FT-IR (neat): 3400, 2929, 2855, 1725, 1512, 1275 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.86 (d, J = 8.2 Hz, 1H, Ph-H), 6.76 (d, J = 2.0 Hz, 1H, Ph-H), 6.71 (dd, J1 = 8.2 Hz, J2 = 2.0 Hz, 1H, Ph-H), 4.85 (s, 2H, -OH), 4.09 (t, J = 6.5 Hz, 2H, -CH2-O), 3.84 (s, 3H, -OCH3), 3.55 (t, J = 6.5 Hz, 2H, -CH2-O), 3.50 (s, 2H, COCH2-), 1.63–1.60 (m, 2H, CH2-CH2-CH2), 1.55–1.50 (m, 2H, CH2-CH2-CH2), 1.32 (bs, 8H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.6 (C), 146.8 (C), 146.2 (C), 127.1 (C), 120.1 (CH), 115.9 (CH), 111.4 (CH), 64.5 (CH2), 61.6 (CH2), 55.0 (CH3), 40.2 (CH2), 32.2 (CH2), 29.0 (CH2), 28.8 (CH2), 28.3 (CH2), 25.5 (CH2), 25.4 (CH2); HRMS: m/z [M + H]+ calcd. for C17H27O5: 311.1858; found: 311.1841.
- 2-Hydroxyethyl 2-(4-hydroxy-3,5-dimethoxyphenyl)acetate 26. Pale yellow solid, mp = 72–73 °C. FT-IR (neat): 3482, 3358, 2938, 1697, 1515, 1447 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.59 (s, 2H, Ph-H), 4.87 (s, 2H, -OH), 4.19–4.16 (m, 2H), 3.84 (s, 6H, -OCH3), 3.76–3.74 (m, 2H), 3.60 (s, 2H, COCH2-); 13C NMR (100.6 MHz) (CD3OD) δ = 172.6 (C), 147.8 (C), 134.3 (C), 124.7 (C), 106.3 (CH), 65.9 (CH2), 59.6 (CH2), 55.3 (CH3), 40.3 (CH2); HRMS: m/z [M + H]+ calcd. for C12H17O6: 257.1025; found: 257.1011.
- 4-Hydroxybutyl 2-(4-hydroxy-3,5-dimethoxyphenyl)acetate 27. Pale yellow solid, mp = 79–80 °C. FT-IR (neat): 3482, 3098, 2960, 1731, 1113 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.57 (s, 2H, Ph-H), 4.87 (s, 2H, -OH), 4.14 (t, J = 6.3 Hz, 2H, -CH2-O), 3.84 (s, 6H, -OCH3), 3.58–3.56 (m, 4H), 1.75–1.68 (m, 2H, CH2-CH2-CH2), 1.61–1.54 (m, 2H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.5 (C), 147.8 (C), 134.3 (C), 124.8 (C), 106.2 (CH), 64.4 (CH2), 61.0 (CH2), 55.3 (CH3), 40.6 (CH2), 28.6 (CH2), 24.9 (CH2); HRMS: m/z [M + H]+ calcd. for C14H21O6: 285.1338; found: 285.1326.
- 6-Hydroxyhexyl 2-(4-hydroxy-3,5-dimethoxyphenyl)acetate 28. Yellow solid, mp = 77–78 °C. FT-IR (neat): 3477, 2936, 1726, 1115 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.57 (s, 2H, Ph-H), 4.87 (s, 2H, -OH), 4.11 (t, J = 6.5 Hz, 2H, -CH2-O), 3.84 (s, 6H, -OCH3), 3.55 (s, 2H, COCH2-), 3.54 (t, J = 6.6 Hz, 2H, -CH2-O), 1.68–1.61 (m, 2H, CH2-CH2-CH2), 1.55–1.48 (m, 2H, CH2-CH2-CH2), 1.40–1.31 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.6 (C), 147.8 (C), 134.3 (C), 124.9 (C), 106.2 (CH), 64.5 (CH2), 61.4 (CH2), 55.4 (CH3), 40.7 (CH2), 32.1 (CH2), 28.3 (CH2), 25.4 (CH2), 25.1 (CH2); HRMS: m/z [M + H]+ calcd. for C16H25O6: 313.1651; found: 313.1635.
- 8-Hydroxyoctyl 2-(4-hydroxy-3,5-dimethoxyphenyl)acetate 29. Yellow solid, mp = 72–73 °C. FT-IR (neat): 3479, 2934, 1729, 1116 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 6.57 (s, 2H, Ph-H), 4.87 (s, 2H, -OH), 4.10 (t, J = 6.5 Hz, 2H, -CH2-O), 3.84 (s, 6H, -OCH3), 3.57–3.53 (m, 4H), 1.64–1.60 (m, 2H, CH2-CH2-CH2), 1.55–1.50 (m, 2H, CH2-CH2-CH2), 1.32 (bs, 8H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.6 (C), 147.8 (C), 134.3 (C), 124.9 (C), 106.2 (CH), 64.5 (CH2), 61.6 (CH2), 55.4 (CH3), 40.7 (CH2), 32.2 (CH2), 29.1 (CH2), 28.9 (CH2), 28.3 (CH2), 25.6 (CH2), 25.4 (CH2); HRMS: m/z [M + H]+ calcd. for C18H29O6: 341.1964; found: 341.1951.
3.3. Synthesis of Butyl Diarylacetates 30–34 and 36–44 by Mitsunobu Reaction
- Butane-1,4-diyl bis(2-(4-hydroxyphenyl)acetate) 30. White solid, mp = 120–121 °C; spectroscopic data are in accordance with those reported in the literature [35].
- 4-(2-(3,4-Dihydroxyphenyl)acetoxy)butyl 2-(4-hydroxyphenyl)acetate 31. Pale brown solid, mp = 124–125 °C. FT-IR (neat): 3394, 2964, 1706, 1610, 1517, 1171 cm−1; 1H NMR (400.13 MHz) (CD3OD) δ = 7.09 (d, J = 8.4 Hz, 2H, Ph-H), 6.75–6.70 (m, 4H, Ph-H), 6.58 (dd, J1 = 8.1 Hz, J2 = 1.9 Hz, 1H, Ph-H), 4.87 (s, 3H, -OH), 4.07 (m, 4H, -CH2-O), 3.52 (s, 2H, COCH2-), 3.46 (s, 2H, COCH2-), 1.64 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CD3OD) δ = 172.71 (C), 172.66 (C), 156.1 (C), 144.9 (C), 144.1 (C), 129.9 (CH), 125.6 (C), 125.0 (C), 120.2 (CH), 115.9 (CH), 114.93 (CH), 114.89 (CH), 63.97 (CH2), 63.93 (CH2), 40.1 (CH2), 39.8 (CH2), 24.88 (CH2), 24.86 (CH2); HRMS: m/z [M + H]+ calcd. for C20H23O7: 375.1444; found: 375.1431.
- 4-(2-(4-Hydroxy-3-methoxyphenyl)acetoxy)butyl 2-(4-hydroxyphenyl)acetate 32. Yellow oil. FT-IR (neat): 3402, 2959, 1709, 1514, 1149 cm−1; 1H NMR (400.13 MHz) (CDCl3) δ = 7.12 (d, J = 8.6 Hz, 2H, Ph-H), 6.87 (d, J = 8.0 Hz, 1H, Ph-H), 6.82–6.74 (m, 4H, Ph-H), 5.79 (bs, 1H, -OH), 5.69 (s, 1H, -OH), 4.12–4.09 (m, 4H, -CH2-O), 3.87 (s, 3H, -OCH3), 3.55 (s, 2H, COCH2-), 3.54 (s, 2H, COCH2-), 1.68–1.65 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CDCl3) δ = 172.3 (C), 172.2 (C), 155.0 (C), 146.5 (C), 144.7 (C), 130.4 (CH), 125.83 (C), 125.77 (C), 122.1 (CH), 115.5 (CH), 114.5 (CH), 111.8 (CH), 64.4 (CH2), 64.3 (CH2), 55.9 (CH3), 41.0 (CH2), 40.5 (CH2), 25.2 (2× CH2); HRMS: m/z [M + H]+ calcd. for C21H25O7: 389.1600; found: 389.1580.
- 4-(2-(3-Hydroxy-4-methoxyphenyl)acetoxy)butyl 2-(4-hydroxyphenyl)acetate 33. Yellow wax. FT-IR (neat): 3394, 2960, 1710, 1512, 1130, 1027 cm−1; 1H NMR (400.13 MHz) (CDCl3) δ = 7.15–7.13 (m, 2H, Ph-H), 6.88 (d, J = 2.0 Hz, 1H, Ph-H), 6.83–6.76 (m, 4H, Ph-H), 5.74 (m, 1H, -OH), 5.56 (m, 1H, -OH), 4.12–4.06 (m, 4H, -CH2-O), 3.89 (s, 3H, -OCH3), 3.55 (s, 2H, COCH2-), 3.52 (s, 2H, COCH2-), 1.66–1.65 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CDCl3) δ = 172.1 (C), 171.9 (C), 154.9 (C), 145.8 (C), 145.5 (C), 130.4 (CH), 127.2 (C), 126.0 (C), 120.9 (CH), 115.52 (CH), 115.49 (CH), 110.8 (CH), 64.35 (CH2), 64.32 (CH2), 56.0 (CH3), 40.8 (CH2), 40.6 (CH2), 25.22 (CH2), 25.17 (CH2); HRMS: m/z [M + H]+ calcd. for C21H25O7: 389.1600; found: 389.1577.
- 4-(2-(4-Hydroxy-3,5-dimethoxyphenyl)acetoxy)butyl 2-(4-hydroxyphenyl)acetate 34. Colorless oil. FT-IR (neat): 3360, 2939, 1726, 1714, 1117 cm−1; 1H NMR (400.13 MHz) (CDCl3) δ = 7.12 (d, J = 8.5 Hz, 2H, Ph-H), 6.76 (d, J = 8.5 Hz, 2H, Ph-H), 6.53 (s, 2H, Ph-H), 5.69 (bs, 1H, -OH), 5.54 (s, 1H, -OH), 4.10 (m, 4H, -CH2-O), 3.88 (s, 6H, -OCH3), 3.54 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CDCl3) δ = 172.1 (C), 171.9 (C), 155.0 (C), 147.0 (C), 133.9 (C), 130.4 (CH), 125.8 (C), 125.0 (C), 115.5 (CH), 106.1 (CH), 64.4 (CH2), 64.3 (CH2), 56.3 (CH3), 41.5 (CH2), 40.5 (CH2), 25.2 (2x CH2); HRMS: m/z [M + H]+ calcd. for C22H27O8: 419.1706; found: 419.1681.
- 4-(2-(3,4-Dihydroxyphenyl)acetoxy)butyl 2-(4-hydroxy-3-methoxyphenyl)acetate 36. Yellow wax. FT-IR (neat): 3339, 2939, 1713, 1515, 1148 cm−1; 1H NMR (400.13 MHz) (CDCl3) δ = 6.86 (d, J = 8.0 Hz, 1H, Ph-H), 6.80–6.75 (m, 3H, Ph-H), 6.65–6.64 (m, 2H, Ph-H), 6.16 (bs, 1H, -OH), 5.84 (bs, 1H, -OH), 4.13–4.08 (m, 4H, -CH2-O), 3.84 (s, 3H, -OCH3), 3.56 (s, 2H, COCH2-), 3.47 (s, 2H, COCH2-), 1.68–1.66 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CDCl3) δ = 172.8 (C), 172.6 (C), 146.6 (C), 144.7 (C), 143.8 (C), 143.4 (C), 126.1 (C), 125.6 (C), 122.1 (CH), 121.6 (CH), 116.2 (CH), 115.3 (CH), 114.6 (CH), 111.9 (CH), 64.8 (CH2), 64.5 (CH2), 55.9 (CH3), 41.1 (CH2), 40.8 (CH2), 25.2 (CH2), 25.0 (CH2); HRMS: m/z [M + H]+ calcd. for C21H25O8: 405.1549; found: 405.1529.
- 2-(2-(3,4-Dihydroxyphenyl)acetoxy)ethyl 2-(3-hydroxy-4-methoxyphenyl)acetate 37. Orange wax. FT-IR (neat): 3391, 1706, 1510, 1131, 762 cm−1; 1H NMR (400.13 MHz) (CDCl3) δ = 6.87 (d, J = 1.9 Hz, 1H, Ph-H), 6.81–6.76 (m, 3H, Ph-H), 6.65 (dd, J1 = 8.0 Hz, J2 = 2.0 Hz, 1H, Ph-H), 6.61 (bs, 1H, Ph-H), 6.07 (bs, 1H, -OH), 5.91 (bs, 1H, -OH), 4.11–4.09 (m, 4H, -CH2-O), 3.86 (s, 3H, -OCH3), 3.54 (s, 2H, COCH2-), 3.49 (s, 2H, COCH2-), 1.68–1.66 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CDCl3) δ = 172.7 (C), 172.6 (C), 145.9 (C), 145.5 (C), 143.8 (C), 143.4 (C), 126.9 (C), 126.1 (C), 121.6 (CH), 121.0 (CH), 116.2 (CH), 115.6 (CH), 115.3 (CH), 110.9 (CH), 64.8 (CH2), 64.6 (CH2), 56.0 (CH3), 40.9 (CH2), 40.8 (CH2), 25.3 (CH2), 25.0 (CH2); HRMS: m/z [M + H]+ calcd. for C21H25O8: 405.1549; found: 405.1530.
- 4-(2-(3,4-Dihydroxyphenyl)acetoxy)butyl 2-(4-hydroxy-3,5-dimethoxyphenyl)acetate 38. White solid, mp = 74–75 °C. FT-IR (neat): 3512, 3425, 1717, 1699, 1513, 1105 cm−1; 1H NMR (400.13 MHz) (CDCl3) δ = 6.78–6.60 (m, 3H, Ph-H), 6.52 (s, 2H, Ph-H), 6.01 (bs, 1H), 5.63 (bs, 1H), 4.13 (t, J = 6.2 Hz, 2H, -CH2-O), 4.09 (t, J = 5.9 Hz, 2H, -CH2-O), 3.85 (s, 6H, -OCH3), 3.56 (s, 2H, COCH2-), 3.47 (s, 2H, COCH2-), 1.68–1.67 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CDCl3) δ = 172.6 (C), 172.3 (C), 147.0 (C), 143.8 (C), 143.4 (C), 133.9 (C), 126.1 (C), 124.8 (C), 121.6 (CH), 116.1 (CH), 115.1 (CH), 106.1 (CH), 64.8 (CH2), 64.4 (CH2), 56.3 (CH3), 41.6 (CH2), 40.9 (CH2), 25.3 (CH2), 25.1 (CH2); HRMS: m/z [M + H]+ calcd. for C22H27O9: 435.1655; found: 435.1641.
- Butane-1,4-diyl bis(2-(4-hydroxy-3-methoxyphenyl)acetate) 39. White solid, mp = 118–119 °C. FT-IR (neat): 3460, 1720, 1512, 1138, 1036 cm−1; 1H NMR (400.13 MHz) (CDCl3) δ = 6.88–6.76 (m, 6 H, Ph-H), 5.67 (bs, 1H, -OH), 4.12–4.09 (m, 4H, -CH2-O), 3.88 (s, 6H, -OCH3), 3.55 (s, 4H, COCH2-), 1.69–1.66 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CDCl3) δ = 171.9 (C), 146.5 (C), 144.8 (C), 125.8 (C), 122.1 (CH), 114.4 (CH), 111.8 (CH), 64.3 (CH2), 55.9 (CH2), 41. (CH2), 25.2 (CH2); HRMS: m/z [M + H]+ calcd. for C22H27O8: 419.1706; found: 419.1683.
- 4-(2-(4-Hydroxy-3-methoxyphenyl)acetoxy)butyl 2-(3-hydroxy-4-methoxyphenyl)acetate 40. Yellow wax. FT-IR (neat): 3458, 1720, 1512, 1131, 1027 cm−1; 1H NMR (400.13 MHz) (CDCl3) δ = 6.88–6.75 (m, 6H, Ph-H), 5.78–5.72 (m, 2H, -OH), 4.11–4.10 (m, 4H, -CH2-O), 3.88 (s, 3H, -OCH3), 3.87 (s, 3H, -OCH3), 3.55 (s, 2H, COCH2-), 3.52 (s, 2H, COCH2-), 1.69–1.66 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CDCl3) δ = 172.0 (C), 171.8 (C), 146.5 (C), 145.8 (C), 145.6 (C), 144.8 (C), 127.2 (C), 125.8 (C), 122.1 (CH), 120.8 (CH), 115.5 (CH), 114.4 (CH), 111.8 (CH), 110.8 (CH), 64.33 (CH2), 64.26 (CH2), 56.0 (CH3), 55.9 (CH3), 41.0 (CH2) 40.8 (CH2), 25.23 (CH2), 25.21 (CH2); HRMS: m/z [M + H]+ calcd. for C22H27O8: 419.1706; found: 419.1684.
- 4-(2-(4-Hydroxy-3,5-dimethoxyphenyl)acetoxy)butyl 2-(4-hydroxy-3-methoxyphenyl)acetate 41. Yellow wax. FT-IR (neat): 3417, 1721, 1613, 1516, 1115 cm−1; 1H NMR (400.13 MHz) (CDCl3) δ = 6.86 (d, J = 8.0 Hz, 1H, Ph-H), 6.81 (d, J = 1.7 Hz, 1H, Ph-H), 6.76 dd, (J1 = 8.0 Hz, J2 = 1.7 Hz, 1H, Ph-H), 6.52 (s, 2H, Ph-H), 5.67 (bs, 1H, -OH), 5.54 (bs, 1H, -OH), 4.11–4.10 (m, 4H, -CH2-O), 3.88 (s, 9H, -OCH3), 3.54 (s, 2H, COCH2-), 3.53 (s, 2H, COCH2-), 1.69–1.67 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CDCl3) δ = 171.9 (C), 171.7 (C), 147.0 (C), 146.5 (C), 144.8 (C), 133.9 (C), 125.7 (C), 124.5 (C), 122.1 (CH), 114.4 (CH), 111.8 (CH), 106.1 (CH), 64.3 (CH2), 64.2 (CH2), 56.3 (CH3), 55.9 (CH3), 41.4 (CH2), 41.0 (CH2), 25.23 (CH2), 25.20 (CH2); HRMS: m/z [M + H]+ calcd. for C23H29O9: 449.112; found: 449.1788.
- Butane-1,4-diyl bis(2-(3-hydroxy-4-methoxyphenyl)acetate) 42. Yellow wax. FT-IR (neat): 3391, 1736, 1589, 1510, 1126 cm−1; 1H NMR (400.13 MHz) (CDCl3) δ = 6.87 (1.9 Hz, 2H, Ph-H), 6.82–6.75 (m, 4H, Ph-H), 5.80 (bs, 2H, -OH), 4.11–4.08 (m, 4H, -CH2-O), 3.87 (s, 6H, -OCH3), 3.53 (s, 4H, COCH2-), 1.68–1.66 (m, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CDCl3) δ = 171.8 (C), 145.8 (C), 145.6 (C), 127.2 (C), 120.8 (CH), 115.6 (CH), 110.8 (CH), 64.3 (CH2), 56.0 (CH3), 40.8 (CH2), 25.2 (CH2); HRMS: m/z [M + H]+ calcd. for C22H27O8: 419.1706; found: 419.1683.
- 4-(2-(4-Hydroxy-3,5-dimethoxyphenyl)acetoxy)butyl 2-(3-hydroxy-4-methoxyphenyl)acetate 43. Pale yellow solid, mp = 42–43 °C. FT-IR (neat): 3447, 1715, 1514, 1213, 1115 cm−1; 1H NMR (400.13 MHz) (CDCl3) δ = 6.86 (d, J = 2.0 Hz, 1H, Ph-H), 6.81–6.79 (m, 1H, Ph-H), 6.75 (dd, J1 = 8.2 Hz, J2 = 2.0 Hz, 1H, Ph-H), 6.52 (s, 2H, Ph-H), 5.75 (bs, 1H, -OH), 5.55 (bs, 1H, -OH), 4.11–4.10 (m, 4H, -CH2-O), 3.88 (s, 6H, -OCH3), 3.87 (s, 3H, -OCH3), 3.54 (s, 2H, COCH2-), 3.51 (s, 2H, COCH2-), 1.68 (m, 4 H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CDCl3) δ = 171.8 (C), 171.5 (C), 147.0 (C), 145.8 (C), 145.6 (C), 133.9 (C), 127.2 (C), 124.9 (C), 120.8 (CH), 115.5 (CH), 110.8 (CH), 106.0 (CH), 64.4 (CH2), 64.2 (CH2), 56.3 (CH3), 56.0 (CH3), 41.4 (CH2), 40.7 (CH2), 25.24 (CH2), 25.20 (CH2); HRMS: m/z [M + H]+ calcd. for C23H29O9: 449.1812; found: 449.1788.
- Butane-1,4-diyl bis(2-(4-hydroxy-3,5-dimethoxyphenyl)acetate) 44. White solid, mp = 129–130 °C. FT-IR (neat): 3423, 1733, 1615, 1521, 1468 cm−1; 1H NMR (400.13 MHz) (CDCl3) δ = 6.51 (s, 4H, Ph-H), 5.54 (bs, 2H, -OH), 4.10 (bs, 4H, -CH2-O), 3.86 (s, 12H, -OCH3), 3.52 (s, 4H, COCH2-), 1.68 (bs, 4H, CH2-CH2-CH2); 13C NMR (100.6 MHz) (CDCl3) δ = 171.7 (C), 147.0 (C), 133.9 (C), 124.9 (C), 106.0 (CH), 64.3 (CH2), 56.3 (CH3), 41.4 (CH2), 25.2 (CH2); HRMS: m/z [M + H]+ calcd. for C24H31O10: 479.1917; found: 479.1896.
3.4. Evaluation of the LogP
3.5. Evaluation of the In Vitro Antioxidant Activity
3.5.1. DPPH Assay
3.5.2. ABTS Assay
3.6. Statistical Analysis
3.7. Qualitative Evaluation of the Bactericidal Properties Against Staphylococcus aureus and Escherichia coli
3.7.1. Bacterial Stains
3.7.2. Growth Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in plants: Structure, biosynthesis, abiotic stress regulation, and practical applications. Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Velotti, F. Natural polyphenols as immunomodulators to rescue immune response homeostasis: Quercetin as a research model against severe COVID-19. Molecules 2021, 26, 5803. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant polyphenols and their potential benefits on cardiovascular health: A review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.V.; Al-Taher, F.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Health-improving effects of polyphenols on the human intestinal microbiota: A review. Int. J. Mol. Sci. 2025, 26, 1335. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Hussain, Y.; Santarcangelo, C.; Baldi, A.; Di Minno, A.; Khan, H.; Xiao, J.; Daglia, M. Natural polyphenols for the preservation of meat and dairy products. Molecules 2022, 27, 1906. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Campo, M.; Cassiani, C.; Fochetti, A.; Ieri, F.; Lombardi, A.; Urciuoli, S.; Vignolini, P.; Villanova, N.; Vita, C. Polyphenol-rich extracts from agroindustrial waste and byproducts: Results and perspectives according to the green chemistry and circular economy. J. Agric. Food Chem. 2024, 72, 12871. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kim, J.Y.; Lee, Y.S. Phenolic compounds in active packaging and edible films/coatings: Natural bioactive molecules and novel packaging ingredients. Molecules 2022, 27, 7513. [Google Scholar] [CrossRef] [PubMed]
- Luzi, F.; Pannucci, E.; Clemente, M.; Grande, E.; Urciuoli, S.; Romani, A.; Torre, L.; Puglia, D.; Bernini, R.; Santi, L. Hydroxytyrosol and oleuropein-enriched extracts obtained from olive oil wastes and by-products as active antioxidant ingredients for poly-(vinyl alcohol)-based films. Molecules 2021, 26, 2104. [Google Scholar] [CrossRef] [PubMed]
- Afonso, T.B.; Bonifácio-Lopes, T.; Costa, E.M.; Pintado, M.E. Phenolic compounds from by-products for functional textiles. Materials 2023, 16, 7248. [Google Scholar] [CrossRef] [PubMed]
- Ciupei, D.; Colişar, A.; Leopold, L.; Stanila, A.; Diaconeasa, Z.M. Polyphenols: From classification to therapeutic potential and bioavailability. Foods 2024, 13, 4131. [Google Scholar] [CrossRef] [PubMed]
- Ficco, D.B.M.; Petroni, K.; Mistura, L.; D’Addezio, L. Polyphenols in cereals: State of the art of available information and its potential use in epidemiological studies. Nutrients 2024, 16, 2155. [Google Scholar] [CrossRef] [PubMed]
- Valanciene, E.; Jonuskiene, I.; Syrpas, M.; Augustiniene, E.; Matulis, P.; Simonavicius, A.; Malys, N. Advances and prospects of phenolic acids production, biorefinery and analysis. Biomolecules 2020, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Xu, A.C.; Krul, C.; Venema, K.; Liu, Y.; Niu, Y.; Lu, J.; Bensoussan, L.; Seeram, N.P.; Heber, D. Of the major phenolic acids formed during human microbial fermentation of tea, citrus, and soy flavonoid supplements, only 3,4-dihydroxyphenylacetic acid has antiproliferative activity. J. Nutr. 2006, 136, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Skrbek, S.; Rüfer, C.E.; Marko, D.; Esselen, M. Quercetin and its microbial degradation product 3,4-dihydroxyphenylacetic acid generate hydrogen peroxide modulating their stability under in vitro conditions. J. Food Nutr. Res. 2009, 48, 129–140. [Google Scholar]
- Gonzalez-Sarrias, A.; Núñez-Sánchez, M.A.; Tomas-Barberan, F.A.; Espín, J.C. Neuroprotective effects of bioavailable polyphenol-derived metabolites against oxidative stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Lingyi, L.; Cheng, J.; Ying, Z. Lipophilic phenolic compounds (Lipo-PCs): Emerging antioxidants applied in lipid systems. RSC Adv. 2014, 4, 2879. [Google Scholar]
- Bovicelli, P.; Bernini, R.; Antonioletti, R.; Mincione, E. Selective halogenation of flavanones. Tetrahedron Lett. 2002, 43, 5563–5567. [Google Scholar] [CrossRef]
- Appendino, G.; Minassi, A.; Daddario, N.; Bianchi, F.; Tron, G.C. Chemoselective esterification of phenolic acids and alcohols. Org. Lett. 2002, 4, 3839. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Mateos, R.; Traka, M.H.; Bacon, J.R.; Bongaerts, R.; Sarria, B.; Bravo, L.; Kroon, P.A. Hydroxytyrosyl ethyl ether exhibits stronger intestinal anticarcinogenic potency and effects on transcript profiles compared to hydroxytyrosol. Food Chem. 2013, 138, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Floris, B.; Galloni, P.; Conte, V.; Sabuzi, F. Tailored functionalization of natural phenols to improve biological activity. Biomolecules 2021, 11, 1325. [Google Scholar] [CrossRef] [PubMed]
- Aissa, I.; Sghair, R.M.; Bouaziz, M.; Laouini, D.; Sayadi, S.; Gargouri, Y. Synthesis of lipophilic tyrosyl esters derivatives and assessment of their antimicrobial and antileishmania activities. Lipids Health Dis. 2012, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Trujillo, M.; Pereira-Caro, G.; Madrona, A.; Cert, A.; Espartero, J.L. New lipophilic tyrosyl esters. Comparative antioxidant evaluation with hydroxytyrosyl esters. J. Agric. Food Chem. 2008, 56, 10960–10966. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Crisante, F.; Barontini, M.; Tofani, D.; Balducci, V.; Gambacorta, A. Synthesis and structure/antioxidant activity relationship of novel catecholic antioxidants structurally analogues to hydroxytyrosol and its lipophilic esters. J. Agric. Food Chem. 2012, 60, 7408–7416. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Carastro, I.; Santoni, F.; Clemente, M. Synthesis of lipophilic esters of tyrosol, homovanillyl alcohol and hydroxytyrosol. Antioxidants 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Prevete, G.; Scipioni, E.; Donati, E.; Villanova, N.; Fochetti, A.; Lilla, L.; Borocci, S.; Bernini, R.; Mazzonna, M. Impact of pharmacokinetic enhancement strategies on the antimicrobial and antioxidant activities of hydroxytyrosol. RSC Adv. 2025, 15, 344–3464. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, S.S.; Chandrasekar, V.; Belur, P.D. Synthesis and characterization of 3,4-dihydroxyphenyl acetic acid esters and study of their efficacy in bulk fish oil. Food Chem. 2024, 441, 138380. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Buceta, E.; Carrero, P.; Doyagüez, E.G.; Madrona, A.; Quesada, E.; Camarasa, M.J.; Perez-Perez, M.J.; Leyssen, P.; Paeshuyse, J.; Neyts, J.; et al. Linear and branched alkyl-esters and amides of gallic acid and other (mono-, di-and tri-) hydroxy benzoyl derivatives as promising anti-HCV inhibitors. Eur. J. Med. Chem. 2015, 92, 656–671. [Google Scholar] [CrossRef] [PubMed]
- Laguna, O.; Durand, E.; Barea, B.; Dauget, S.; Fine, F.; Villeneuve, P.; Lecomte, J. Synthesis and evaluation of antioxidant activities of novel hydroxyalkyl esters and bis-aryl esters based on sinapic and caffeic acids. J. Agric. Food Chem. 2020, 68, 9308–9318. [Google Scholar] [CrossRef] [PubMed]
- Roma, E.; Mattoni, E.; Lupattelli, P.; Moeini, S.S.; Gasperi, T.; Bernini, R.; Incerpi, S.; Tofani, D. New dihydroxytyrosyl esters from dicarboxylic acids: Synthesis and evaluation of the antioxidant activity in vitro (ABTS) and in cell-cultures (DCF Assay). Molecules 2020, 25, 3135. [Google Scholar] [CrossRef] [PubMed]
- Pagoni, A.; Vassiliou, S. A novel and efficient synthesis of 3,4-dihydroxyphenylacetic ester and amide derivatives/conjugates and assessment of their antioxidant activity. Nat. Prod. Res. 2017, 32, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://patentscope.wipo.int/search/en/result.jsf?inchikey=NOTCMYLBWIIRSS-UHFFFAOYSA-N (accessed on 9 June 2025).
- Faiza; Hardacre, B.; Scott, C.; Winroth, S.; Ishida, H. Synthesis of bio-based and intrinsically flame-retardant benzoxazine containing dynamic ester bond that quantitatively satisfies all twelve principles of green chemistry. ACS Sustain. Chem. Eng. 2024, 12, 13525–13534. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [PubMed]
- Rana, S.; Rayhan, M.A.; Emon, S.H.; Islam, T.; Rathry, K.; Hasan, M.; Mansur, M.I.; Srijon, B.C.; Islam, S.; Ray, A.; et al. Antioxidant activity of Schiff base ligands using the DPPH scavenging assay: An updated review. RSC Adv. 2024, 14, 33094–33123. [Google Scholar] [CrossRef] [PubMed]
- Barontini, M.; Bernini, R.; Carastro, R.; Gentili, P.; Romani, A. Synthesis and DPPH radical scavenging activity of novel compounds obtained from tyrosol and cinnamic acid derivatives. New J. Chem. 2014, 38, 809. [Google Scholar] [CrossRef]
- Moazzen, A.; Oztinen, N.; Ak-Sakalli, E.; Koşar, M. Structure-antiradical activity relationships of 25 natural antioxidant phenolic compounds from different classes. Heliyon 2022, 8, 10467. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.A.; Unakal, C.G. Staphylococcus aureus Infection; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef] [PubMed]
- Sora, V.M.; Meroni, G.; Martino, P.A.; Soggiu, A.; Bonizzi, L.; Zecconi, A. Extraintestinal pathogenic Escherichia coli: Virulence factors and antibiotic resistance. Pathogens 2021, 10, 1355. [Google Scholar] [CrossRef] [PubMed]
- Rahal, E.A.; Kazzi, N.; Nassar, F.J.; Matar, G.M. E. coli O157:H7-Clinical aspects and novel treatment approaches. Front. Cell. Infect. Microbiol. 2012, 2, 138. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-T.; Luo, D.; Huang, J.-N.; Wang, L.-L.; Zhang, F.-G.; Xi, T.; Lu, Y.-Y. Antibacterial Constituents from Antarctic Fungus, Aspergillus sydowii SP-1. Nat. Prod. Res. 2017, 32, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Maldonado, A.F.; Schieber, A.; Ganzle, M.G. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N. The potential of systems biology to discover antibacterial mechanisms of plant phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Zieniuk, B.; Wołoszynowska, M.; Białecka-Florjańczyk, E.; Fabiszewska, A. Synthesis of industrially useful phenolic compounds esters by means of biocatalysts obtained along with waste fish oil utilization. Sustainability 2020, 12, 5804. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- RStudio: Integrated Development Environment for R; RStudio: Boston, MA, USA. 2015. Available online: https://www.rstudio.com/ (accessed on 3 March 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. _dplyr: A Grammar of Data Manipulation_. R Package Version 1.1.4. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 3 March 2025).
- Graves, S.; Piepho, H.; Dorai-Raj, S.; Selzer, L. _multcompView: Visualizations of Paired Comparisons_. R Package Version 0.1-10. 2024. Available online: https://CRAN.R-project.org/package=multcompView (accessed on 3 March 2025).
- Wiegand, I.; Hilpert, K.; Hancock, R. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
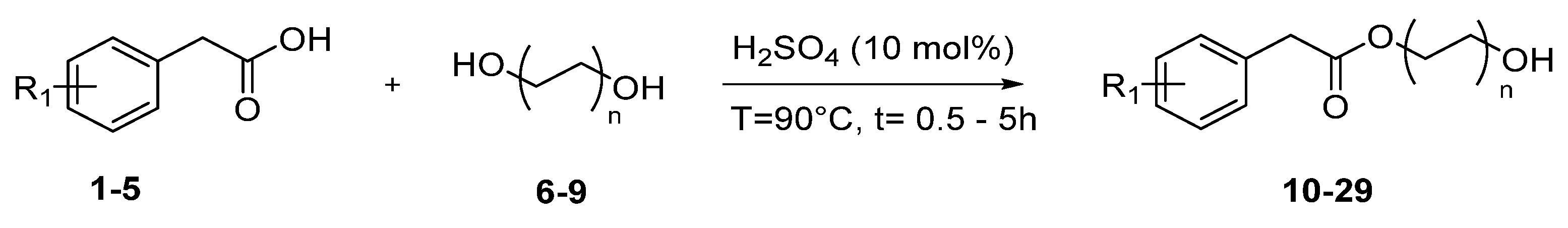

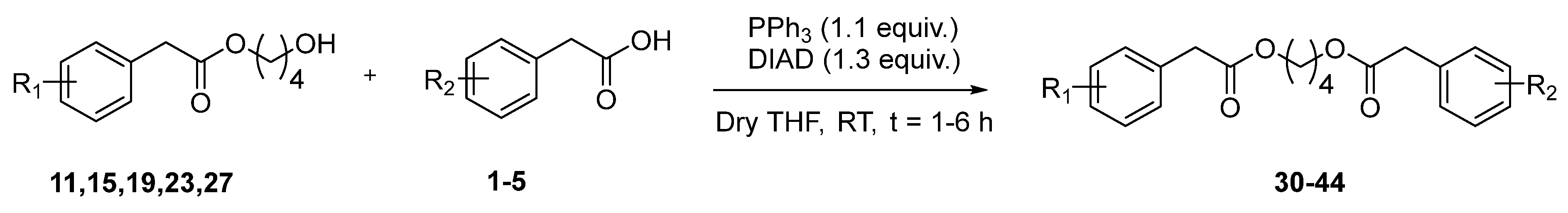

| Entry | Acid | Diol | Time (h) | Ester | Yield (%) a | LogP b |
|---|---|---|---|---|---|---|
| 1 | 1 | 6 | 5 | 10 | 78 | 0.89 |
| 2 | 1 | 7 | 1 | 11 | 85 | 1.45 |
| 3 | 1 | 8 | 2 | 12 | 88 | 2.29 |
| 4 | 1 | 9 | 1.5 | 13 | 72 | 3.12 |
| 5 | 2 | 6 | 4 | 14 | 79 | 0.50 |
| 6 | 2 | 7 | 1 | 15 | 69 | 1.06 |
| 7 | 2 | 8 | 2 | 16 | 76 | 1.90 |
| 8 | 2 | 9 | 1 | 17 | 46 | 2.73 |
| 9 | 3 | 6 | 2 | 18 | 82 | 0.77 |
| 10 | 3 | 7 | 1 | 19 | 96 | 1.32 |
| 11 | 3 | 8 | 1 | 20 | 65 | 2.16 |
| 12 | 3 | 9 | 1 | 21 | 60 | 2.99 |
| 13 | 4 | 6 | 5 | 22 | 67 | 0.77 |
| 14 | 4 | 7 | 3 | 23 | 80 | 1.32 |
| 15 | 4 | 8 | 1 | 24 | 81 | 2.16 |
| 16 | 4 | 9 | 1 | 25 | 65 | 2.99 |
| 17 | 5 | 6 | 3 | 26 | 94 | 0.64 |
| 18 | 5 | 7 | 1.5 | 27 | 86 | 1.20 |
| 19 | 5 | 8 | 4 | 28 | 79 | 2.03 |
| 20 | 5 | 9 | 0.5 | 29 | 65 | 2.87 |
| Entry | Compound | IC50 (μM) a | TEAC (μM) a |
|---|---|---|---|
| 1 | 1 | >200 | <0.05 |
| 2 | 10 | >200 | <0.05 |
| 3 | 11 | >200 | <0.05 |
| 4 | 12 | >200 | <0.05 |
| 5 | 13 | >200 | <0.05 |
| 6 | 2 | 12.5 ± 0.2 | 0.92 ± 0.05 |
| 7 | 14 | 13.0 ± 0.4 | 0.88 ± 0.07 |
| 8 | 15 | 13.1 ± 0.5 | 0.85 ± 0.10 |
| 9 | 16 | 17.8 ± 0.4 | 0.63 ± 0.17 |
| 10 | 17 | 16.0 ± 0.1 | 0.52 ± 0.01 |
| 11 | 3 | 56.8 ± 1.6 | 0.14 ± 0.01 |
| 12 | 18 | 39.5 ± 0.4 | 0.16 ± 0.01 |
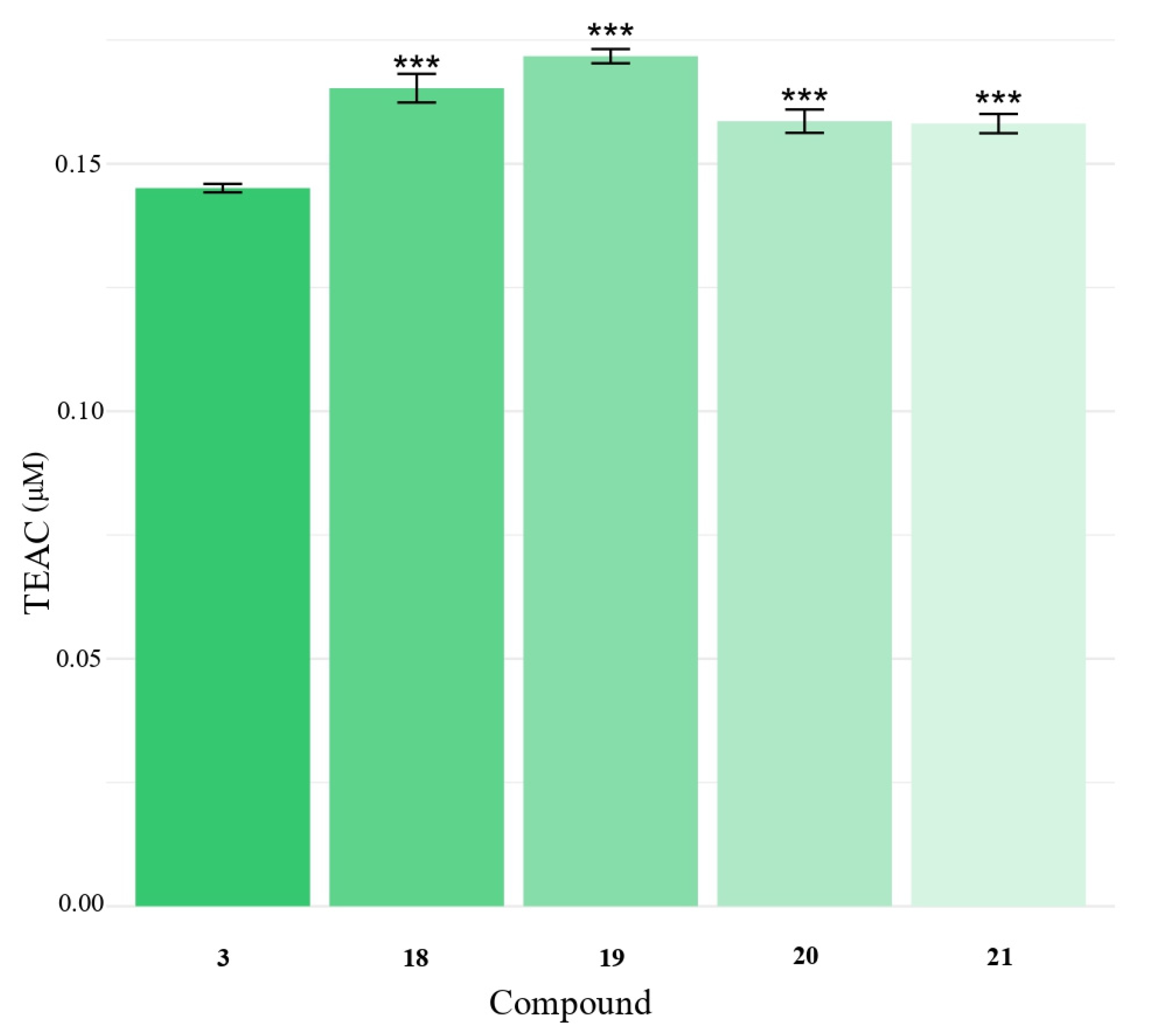
| 13 | 19 | 40.3 ± 1.5 | 0.17 ± 0.01 |
| 14 | 20 | 51.4 ± 1.1 | 0.15 ± 0.02 |
| 15 | 21 | 51.2 ± 0.7 | 0.15 ± 0.02 |
| 16 | 4 | 59.7 ± 3.3 | 0.10 ± 0.02 |
| 17 | 22 | 41.2 ± 0.2 | 0.09 ± 0.01 |
| 18 | 23 | 48.9 ± 2.6 | 0.13 ± 0.01 |
| 19 | 24 | 49.7 ± 1.4 | 0.09 ± 0.01 |
| 20 | 25 | 50.6 ± 0.4 | 0.09 ± 0.01 |
| 21 | 5 | 25.8 ± 1.2 | 0.42 ± 0.04 |
| 22 | 26 | 24.2 ± 1.0 | 0.25 ± 0.09 |
| 23 | 27 | 24.3 ± 0.9 | 0.28 ± 0.01 |
| 24 | 28 | 26.1 ± 0.4 | 0.24 ± 0.01 |
| 25 | 29 | 28.2 ± 1.5 | 0.36 ± 0.06 |
| Entry | Ester | Acid | Time (h) | Diester | Yield (%) a | LogP b |
|---|---|---|---|---|---|---|
| 1 | 11 | 1 | 4 | 30 | 56 | 3.13 |
| 2 | 11 | 2 | 2.5 | 31 | 59 | 2.74 |
| 3 | 11 | 3 | 2 | 32 | 60 | 3.01 |
| 4 | 11 | 4 | 5 | 33 | 53 | 3.01 |
| 5 | 11 | 5 | 1 | 34 | 55 | 2.88 |
| 6 | 15 | 2 | 5 | 35 | Not isolated | Not calculated |
| 7 | 15 | 3 | 2 | 36 | 58 | 2.62 |
| 8 | 15 | 4 | 4.5 | 37 | 45 | 2.62 |
| 9 | 15 | 5 | 2 | 38 | 78 | 2.49 |
| 10 | 19 | 3 | 3 | 39 | 48 | 2.88 |
| 11 | 19 | 4 | 1.5 | 40 | 60 | 2.88 |
| 12 | 19 | 5 | 3 | 41 | 48 | 2.75 |
| 13 | 23 | 4 | 1 | 42 | 65 | 2.88 |
| 14 | 23 | 5 | 6 | 43 | 40 | 2.75 |
| 15 | 27 | 5 | 1.5 | 44 | 71 | 2.63 |
| Entry | Diester | IC50 (μM) a | TEAC (μM) a |
|---|---|---|---|
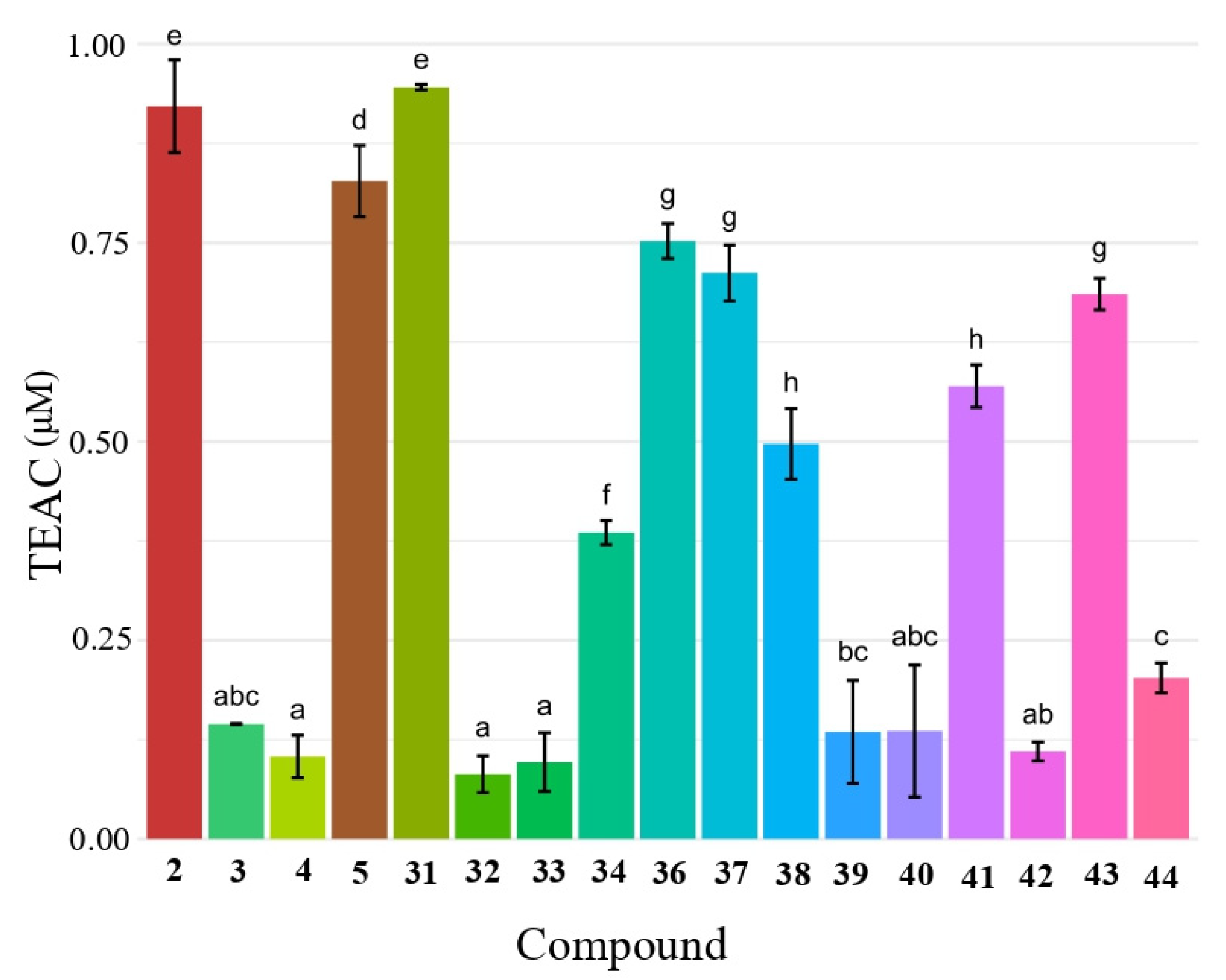
| 1 | 30 | >200 | <0.05 |
| 2 | 31 | 19.5 ± 0.7 | 0.94 ± 0.01 |
| 3 | 32 | 60.8 ± 1.0 | 0.08 ± 0.02 |
| 4 | 33 | 43.1 ± 4.5 | 0.09 ± 0.03 |
| 5 | 34 | 26.9 ± 0.3 | 0.38 ± 0.01 |
| 6 | 36 | 12.6 ± 0.1 | 0.75 ± 0.22 |
| 7 | 37 | 12.2 ± 0.1 | 0.71 ± 0.03 |
| 8 | 38 | 17.5 ± 0.8 | 0.49 ± 0.04 |
| 9 | 39 | 38.2 ± 0.4 | 0.13 ± 0.06 |
| 10 | 40 | 35.3 ± 0.1 | 0.13 ± 0.08 |
| 11 | 41 | 27.3 ± 0.8 | 0.56 ± 0.02 |
| 12 | 42 | 31.3 ± 2.8 | 0.11 ± 0.01 |
| 13 | 43 | 26.4 ± 1.5 | 0.68 ± 0.01 |
| 14 | 44 | 14.3 ± 1.8 | 0.20 ± 0.01 |
| Entry | Compound | MBC (mmol/L) S. aureus ATCC 25923 | MBC (mmol/L) E. coli ATCC 25922 |
|---|---|---|---|
| 1 | 1 | -- a | -- a |
| 2 | 2 | 10 | 10 |
| 3 | 3 | 10 | -- a |
| 4 | 4 | 10 | 10 |
| 5 | 5 | 5.0 | -- a |
| 6 | 31 | 5.0 | 10 |
| 7 | 36 | 5.0 | 10 |
| 8 | 37 | 10 | 10 |
| 9 | 38 | 5.0 | 10 |
| 10 | Gentamicin sulfate b | 0.25 | <0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fochetti, A.; Villanova, N.; Lombardi, A.; Lelli, V.; Gazzilli, Y.; Timperio, A.M.; Fabrizi, G.; Bernini, R. Synthesis of Novel Bioactive Lipophilic Hydroxyalkyl Esters and Diesters Based on Hydroxyphenylacetic Acids. Molecules 2025, 30, 3087. https://doi.org/10.3390/molecules30153087
Fochetti A, Villanova N, Lombardi A, Lelli V, Gazzilli Y, Timperio AM, Fabrizi G, Bernini R. Synthesis of Novel Bioactive Lipophilic Hydroxyalkyl Esters and Diesters Based on Hydroxyphenylacetic Acids. Molecules. 2025; 30(15):3087. https://doi.org/10.3390/molecules30153087
Chicago/Turabian StyleFochetti, Andrea, Noemi Villanova, Andrea Lombardi, Veronica Lelli, Yuri Gazzilli, Anna Maria Timperio, Giancarlo Fabrizi, and Roberta Bernini. 2025. "Synthesis of Novel Bioactive Lipophilic Hydroxyalkyl Esters and Diesters Based on Hydroxyphenylacetic Acids" Molecules 30, no. 15: 3087. https://doi.org/10.3390/molecules30153087
APA StyleFochetti, A., Villanova, N., Lombardi, A., Lelli, V., Gazzilli, Y., Timperio, A. M., Fabrizi, G., & Bernini, R. (2025). Synthesis of Novel Bioactive Lipophilic Hydroxyalkyl Esters and Diesters Based on Hydroxyphenylacetic Acids. Molecules, 30(15), 3087. https://doi.org/10.3390/molecules30153087










