Effect of pH on the Efficiency of Pyrogallol, Gallic Acid, and Alkyl Gallates in Trapping Methylglyoxal
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of pH in Trapping of MGO by Pyrogallol, Gallic Acid, and Gallate Esters
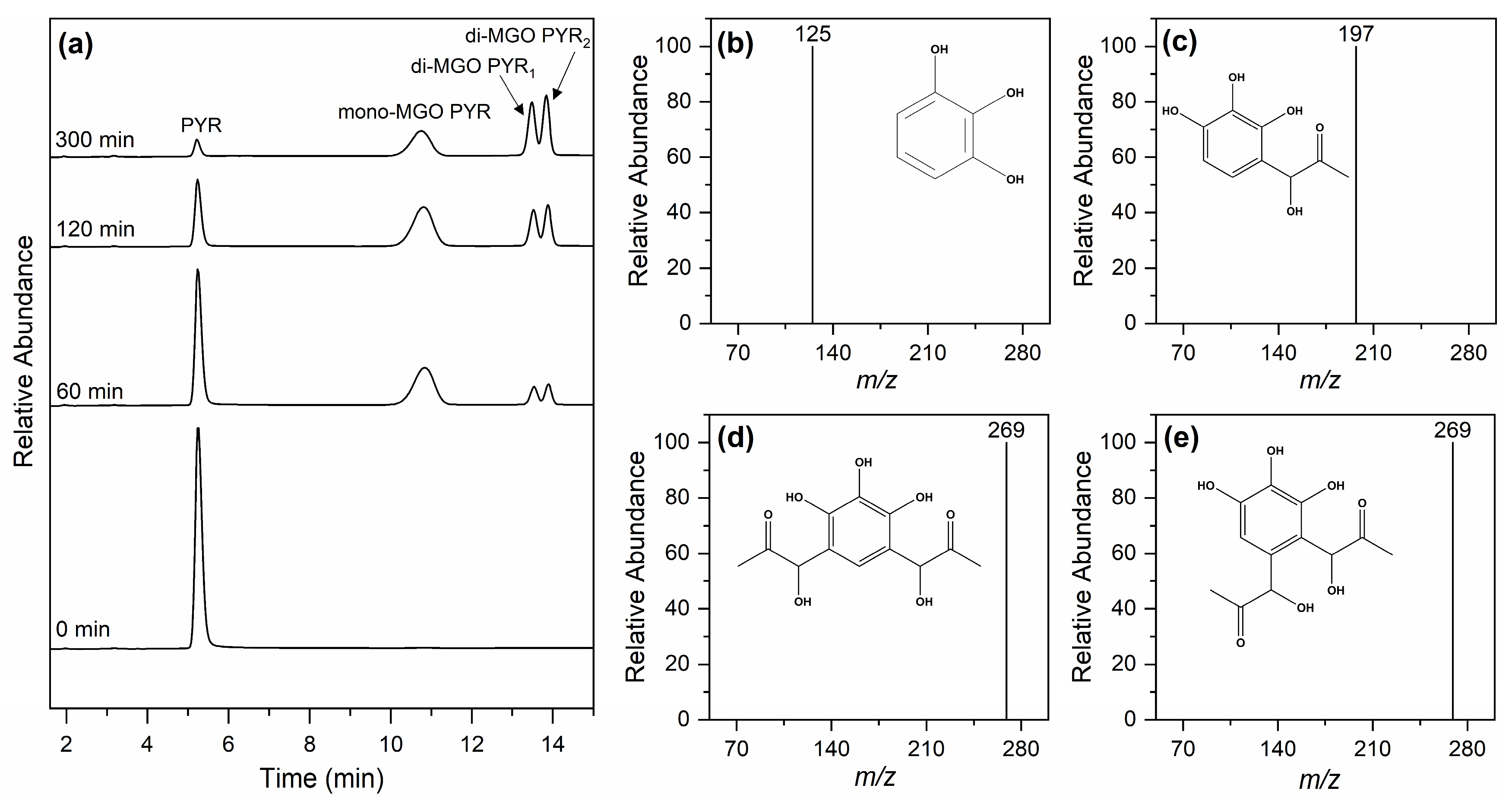
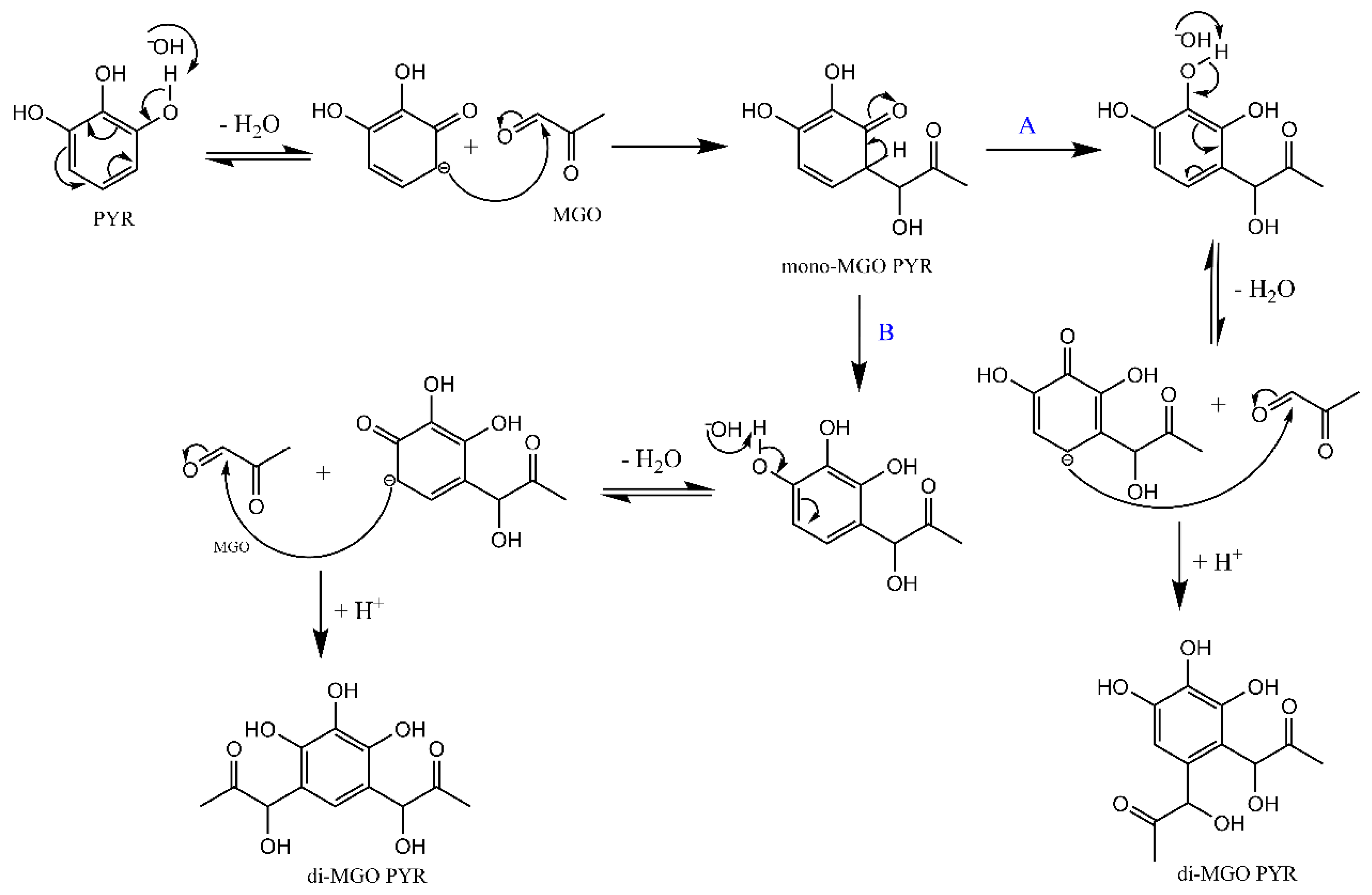
2.2. MGO Adducts of Pyrogallol at pH 6.5 to 8.0 by LC-MS Analysis
3. Materials and Methods
3.1. Materials
3.2. Trapping of MGO by the Phenolic Compounds
3.3. HPLC-DAD Analysis of MGO
3.4. LC-MS Analysis of Reaction Products
3.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MGO | Methylglyoxal |
| AGEs | Advanced Glycation End Products |
| RAGE | Receptor for AGEs |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| HPLC | High Performance Liquid Chromatography |
| DAD | Diode Array Detector |
| mono-MGO PYR | Mono-MGO conjugated adduct of pyrogallol |
| di-MGO PYR | Di-MGO conjugated adduct of pyrogallol |
| DETAPAC | Diethylenetriaminepentaacetic acid |
| ANOVA | Analysis of Variance |
| HSD | Honestly Significant Difference |
References
- Degen, J.; Hellwig, M.; Henle, T. 1,2-Dicarbonyl compounds in commonly consumed foods. J. Agric. Food Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ho, C.T. Flavour chemistry of methylglyoxal and glyoxal. Chem. Soc. Rev. 2012, 41, 4140–4149. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Guo, H.; Ou, J.; Liu, P.; Huang, C.; Wang, M.; Simal-Gandara, J.; Battino, M.; Jafari, S.M.; Zou, L.; et al. Benefits, deleterious effects and mitigation of methylglyoxal in foods: A critical review. Trends Food Sci. Technol. 2021, 107, 201–212. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Gensberger-Reigl, S.; Henle, T.; Pischetsrieder, M. Food-derived 1,2-dicarbonyl compounds and their role in diseases. Semin. Cancer Biol. 2018, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Dolores, M.; Pía, M.; Maza, D.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-cervantes, M.H.; Bastos, D.H.M.; Medrano, A.; et al. Dietary Advanced Glycation End Products and Their Role in Health and Disease. Adv. Nutr. 2015, 15, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Diel, P.; Eisenbrand, G.; Grune, T.; Henle, T.; Humpf, H.; Joost, H.; Marko, D.; Raupbach, J.; Roth, A.; et al. Critical Reviews in Toxicology Dietary glycation compounds—Implications for human health. Crit. Rev. Toxicol. 2024, 54, 485–617. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hu, G.Z.X.; Jie, J.W.; Fang, L. High correlation of methylglyoxal with acrylamide formation in glucose/asparagine Maillard reaction model. Eur. Food Res. Technol. 2008, 226, 1301–1307. [Google Scholar] [CrossRef]
- Jang, H.W.; Jiang, Y.; Hengel, M.; Shibamoto, T. Formation of 4 (5)-Methylimidazole and Its Precursors, α—Dicarbonyl Compounds, in Maillard Model Systems. J. Agric. Food Chem. 2013, 61, 6865–6872. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Morales, M.A.; Rojas, A. Polyphenols and AGEs/RAGE axis. Trends and challenges. Food Res. Int. 2020, 129, 108843. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Liu, H.; Wang, J.; Sun, B. Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: A comprehensive review. Food Res. Int. 2020, 130, 108933. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Feng, Y.; Tan, J.; Zhou, C.; Xu, J.; Chen, Y.; Xiao, J.; He, Y.; Wang, C.; Zhou, M.; et al. Inhibition of advance glycation end products formation, gastrointestinal digestion, absorption and toxicity: A comprehensive review. Int. J. Biol. Macromol. 2023, 249, 125814. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, G.; Neophytou, C.M.; Vasincu, A.; Gregoriou, Y.; Hadjipakkou, H.; Pinakoulaki, E.; Christodoulou, M.C.; Ioannou, G.D.; Stavrou, I.J.; Christou, A.; et al. Anti-cancer activity and phenolic content of extracts derived from cypriot carob (Ceratonia siliqua L.) pods using different solvents. Molecules 2021, 26, 5017. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Shao, X.; Bai, N.; Lo, C.Y.; Yang, C.S.; Ho, C.T. Tea polyphenol (-)-epigallocatechin-3-gallate: A new trapping agent of reactive dicarbonyl species. Chem. Res. Toxicol. 2007, 20, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Bai, N.; He, K.; Ho, C.T.; Yang, C.S.; Sang, S. Apple polyphenols, phloretin and phloridzin: New trapping agents of reactive dicarbonyl species. Chem. Res. Toxicol. 2008, 21, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Shao, X.; Chen, H.; Ho, C.T.; Sang, S. Genistein inhibits advanced glycation end product formation by trapping methylglyoxal. Chem. Res. Toxicol. 2011, 24, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, H.; Sang, S. Trapping Methylglyoxal by Genistein and Its Metabolites in Mice. Chem. Res. Toxicol. 2016, 29, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xia, Q.; Lu, Y.; Zheng, T.; Sang, S.; Lv, L. Influence of Quercetin and Its Methylglyoxal Adducts on the Formation of α-Dicarbonyl Compounds in a Lysine/Glucose Model System. J. Agric. Food Chem. 2017, 65, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Poojary, M.M.; Andersen, M.L.; Lund, M.N. Trapping of Carbonyl Compounds by Epicatechin: Reaction Kinetics and Identification of Epicatechin Adducts in Stored UHT Milk. J. Agric. Food Chem. 2020, 68, 7718–7726. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Liang, Y.; Si, B.; Chang, C.; Lu, Y.; Lv, L. Capture of single or multiple reactive carbonyl species by mangiferin under high temperatures. Food Chem. 2024, 460, 140712. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Du, R.; Zhao, X.; Xu, Y.; Xiang, Q.; Wu, H.; Lu, Y.; Lv, L. Scavenging Glyoxal and Methylglyoxal by Synephrine Alone or in Combination with Neohesperidin at High Temperatures. J. Agric. Food Chem. 2024, 72, 5828–5841. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Chen, H.; Zhu, Y.; Sedighi, R.; Ho, C.T.; Sang, S. Essential structural requirements and additive effects for flavonoids to scavenge methylglyoxal. J. Agric. Food Chem. 2014, 62, 3202–3210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Poojary, M.M.; Andersen, M.L.; Lund, M.N. The effect of molecular structure of polyphenols on the kinetics of the trapping reactions with methylglyoxal. Food Chem. 2020, 319, 126500. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Poojary, M.M.; Andersen, M.L.; Lund, M.N. Effect of pH on the reaction between naringenin and methylglyoxal: A kinetic study. Food Chem. 2019, 298, 125086. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Gökmen, V. Kinetic evaluation of the reaction between methylglyoxal and certain scavenging compounds and determination of their in vitro dicarbonyl scavenging activity. Food Res. Int. 2019, 121, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.Y.; Hsiao, W.T.; Chen, X.Y. Efficiency of trapping methylglyoxal by phenols and phenolic acids. J. Food Sci. 2011, 76, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tao, F.; Hou, Y.; Lu, Y.; Zheng, T.; Sang, S.; Lv, L. Dual effects of propyl gallate and its methylglyoxal adduct on carbonyl stress and oxidative stress. Food Chem. 2018, 265, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Zhang, H.Y.; Shen, L. Proton dissociation is important to understanding structure-activity relationships of gallic acid antioxidants. Bioorganic Med. Chem. Lett. 2006, 16, 4095–4098. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huo, X.; Wang, S.; Yin, Z. The inhibitory effects of natural antioxidants on protein glycation as well as aggregation induced by methylglyoxal and underlying mechanisms. Colloids Surfaces B Biointerfaces 2022, 212, 112360. [Google Scholar] [CrossRef] [PubMed]
- Slabbert, N.P. Ionisation of Some Flavanols and Dihydroflavonols. Tetrahedron 1977, 33, 821–824. [Google Scholar] [CrossRef]
- Makahleh, A.; Saad, B.; Bari, M.F. Synthetic phenolics as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 51–78. [Google Scholar]
- Betterton, E.A.; Hoffmann, M.R. Kinetics, Mechanism, and Thermodynamics of the Reversible Reaction of Methylglyoxal. J. Phys. Chem. 1987, 91, 3011–3020. [Google Scholar] [CrossRef]
- Rezazadeh, S.; Ebrahimi, A.; Nowroozi, A. The effects of structural properties on the methylglyoxal scavenging mechanism of flavonoid aglycones: A quantum mechanical study. Comput. Theor. Chem. 2017, 1118, 26–38. [Google Scholar] [CrossRef]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Nawaz, M.; Naveed, M.; Ullah, I.; Ali, I.; Saqib, M.; Su, Y.; Muhammad, H.; Cheng, K.; Zhou, Q.; Wang, M. The inhibitory effects of endophytic metabolites on glycated proteins under non-communicable disease conditions: A review. Int. J. Biol. Macromol. 2024, 269, 131869. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ho, C.-T. Formation of 2,5-Dimethyl-4-hydroxy-3 (2H)-furanone through Methylglyoxal: A Maillard Reaction Intermediate. J. Agric. Food Chem. 2008, 56, 7405–7409. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjipakkou, H.; Pinakoulaki, E. Effect of pH on the Efficiency of Pyrogallol, Gallic Acid, and Alkyl Gallates in Trapping Methylglyoxal. Molecules 2025, 30, 3086. https://doi.org/10.3390/molecules30153086
Hadjipakkou H, Pinakoulaki E. Effect of pH on the Efficiency of Pyrogallol, Gallic Acid, and Alkyl Gallates in Trapping Methylglyoxal. Molecules. 2025; 30(15):3086. https://doi.org/10.3390/molecules30153086
Chicago/Turabian StyleHadjipakkou, Haria, and Eftychia Pinakoulaki. 2025. "Effect of pH on the Efficiency of Pyrogallol, Gallic Acid, and Alkyl Gallates in Trapping Methylglyoxal" Molecules 30, no. 15: 3086. https://doi.org/10.3390/molecules30153086
APA StyleHadjipakkou, H., & Pinakoulaki, E. (2025). Effect of pH on the Efficiency of Pyrogallol, Gallic Acid, and Alkyl Gallates in Trapping Methylglyoxal. Molecules, 30(15), 3086. https://doi.org/10.3390/molecules30153086







