Formation and Characterization of Xylitol-Modified Glycidyl Methacrylate-co-Ethyl Methacrylate Matrices for Controlled Release of Antimicrobial Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. The Synthesis of P(EMA)-co-(GMA)]-Xyl and the Preparation of Drug-Loaded Gels for Microbiological and API Release Studies
2.2. An Influence of API-Loaded [P(EMA)-co-(GMA)]-Xyl/AGR Gels on Bacterial Growth
| Microbial Strain | API | Bacteria Growth Inhibition Zone (mm) | |||
|---|---|---|---|---|---|
| A (0.1%) | B (0.5%) | C (1.0%) | D (2.0%) | ||
| Escherichia coli DH5α | BAC | Not observed | |||
| BG | 16.5 ± 0.7 (16.5 ± 2.1) | 17.5 ± 0.7 (18.5 ± 2.1) | 19.5 ± 0.7 (18.0 ± 1.4) | 20.5 ± 2.1 (20.0 ± 1.4) | |
| FUR | 20.5 ± 0.7 (18.5 ± 0.7) | 21.5 ± 0.7 (19.0 ± 1.4) | 20.5 ± 2.1 (18.0 ± 1.4) | 21.0 ± 1.4 (19.0 ± 1.4) | |
| GV | 10.0 ± 1.4 (8.5 ± 0.7) | 11.5 ± 0.7 * (6.5 ± 0.7) | 12.5 ± 0.7 * (6.0 ± 1.4) | 13.0 ± 1.4 * (7.0 ± 1.4) | |
| Pseudomonas aeruginosa ATCC 9027 | BAC | Not observed | |||
| BG | 10.0 ± 1.4 (8.5 ± 0.7) | 11.5 ± 0.7 (8.0 ± 1.4) | 13.0 ± 0.0 (9.0 ± 1.4) | 13.0 ± 1.4 (8.5 ± 2.1) | |
| FUR | Not observed | ||||
| GV | 7.5 ± 0.7 (8.5 ± 2.1) | 9.0 ± 1.4 (8.0 ± 1.4) | 9.5 ± 0.7 (9.5 ± 0.7) | 10 ± 1.4 (9.0 ± 1.4) | |
| Staphylococcus epidermidis ATCC 12228 | BAC | 7.5 ± 0.7 (9.0 ± 1.4) | 15.0 ± 1.4 ** (12.0 ± 1.4) | 17.0 ± 1.4 ** (14.5 ± 0.7) | 19.5 ± 2.1 ** (17.5 ± 2.1) ** |
| BG | 14.0 ± 1.4 (8.5 ± 0.7) | 17.0 ± 1.4 (12.5 ± 0.7) | 19.0 ± 1.4 (16.0 ± 1.4) ** | 20.5 ± 2.1 ** (16.5 ± 2.1) ** | |
| FUR | 13.5 ± 0.7 (13.0 ± 1.4) | 15.5 ± 0.7 (15.5 ± 0.7) | 16.5 ± 2.1 (15.5 ± 2.1) | 17.5 ± 0.7 (18.0 ± 1.4) | |
| GV | 16.0 ± 0.0 (15.5 ± 0.7) | 16.5 ± 0.7 (15.5 ± 2.1) | 17.5 ± 2.1 (16.5 ± 0.7) | 19.0 ± 1.4 (17.0 ± 1.4) | |
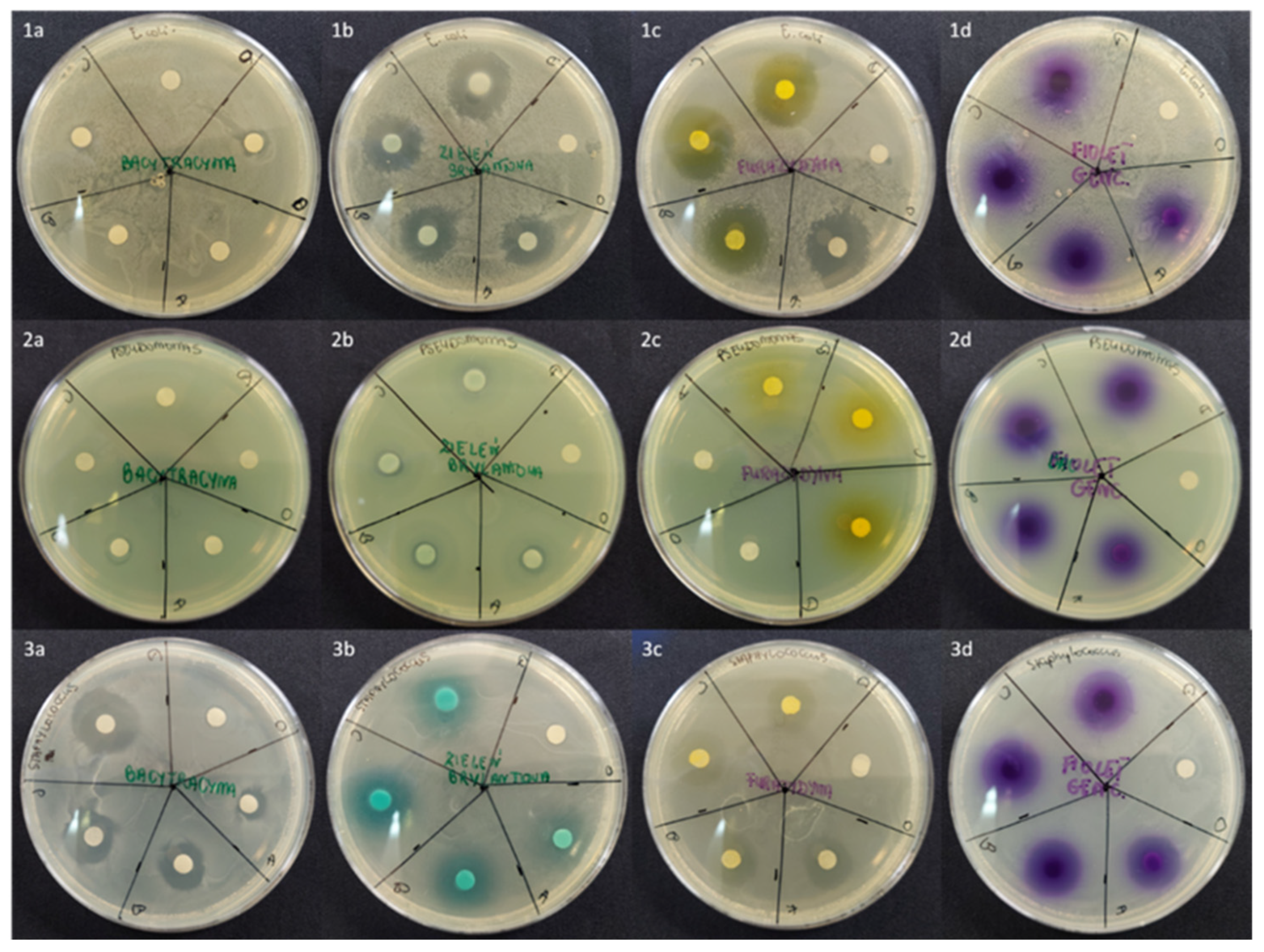
2.3. Hydrophilic Properties of [P(EMA)-co-(GMA)]-Xyl Cooligomer
2.4. BG Degradation Studies in Relation to Light, Temperature, and Solvent
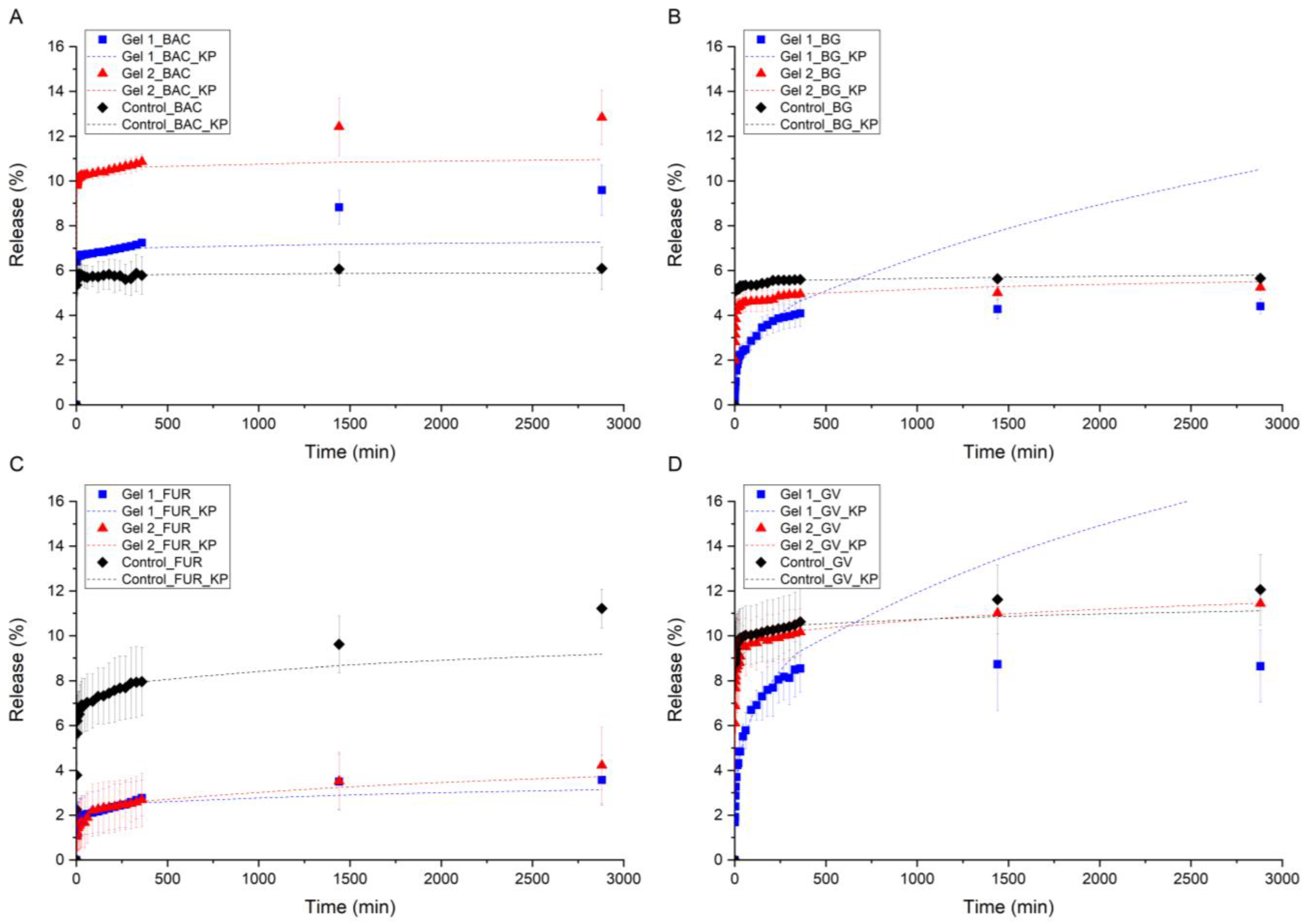
2.5. In Vitro API Release Study
2.6. Kinetic Models and Statistical Analysis of API Release Study
| API | R2 | The Strongest Effect | GEL 1 vs. GEL 2 | Comment | |
|---|---|---|---|---|---|
| Marginal * | Conditional ** | ||||
| BAC | 0.964 | 0.977 | Gel 2 > Gel 1 > control | Yes | High, rising release |
| BG | 0.930 | 0.959 | Gel 1 < Gel 2 < control | Yes | Gel 1 inhibits more strongly than Gel 2 |
| FUR | 0.847 | 0.864 | Gel 1 ≈ Gel 2 < control | Slightly | Long-lasting effect of Gels 1 and 2 |
| GV | 0.817 | 0.860 | Gel 1 < Gel 2 < control | Yes | Gel 1 durable and strong, Gel 2 weaker |
3. Materials and Methods
3.1. Materials
3.2. Methods
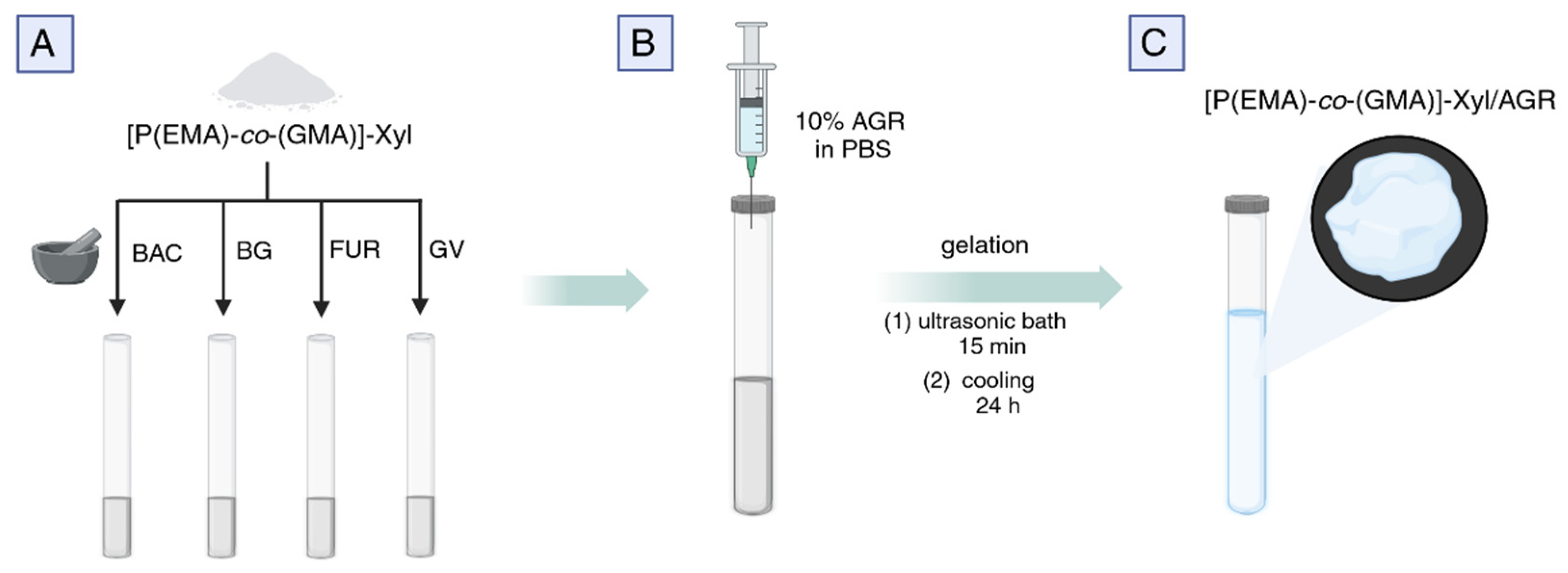
3.2.1. Drug-Loaded [P(EMA)-co-(GMA)]-Xyl Gel Preparation
| AGR (mg) | PBS (mL) | [P(EMA)-co-(GMA)]-Xyl (mg) | API | |||||
|---|---|---|---|---|---|---|---|---|
| Conc. (%) | BAC (mg) | BG (mg) | FUR (mg) | GV (mg) | ||||
| 50.0 | 0.45 | 50.0 Gel 2 0.00 Control | Microbiological studies | 0.1 | 10.1 | 10.1 | 10.1 | 10.1 |
| 0.5 | 50.5 | 50.5 | 50.5 | 50.5 | ||||
| 1.0 | 101.0 | 101.0 | 101.0 | 101.0 | ||||
| 2.0 | 202.0 | 202.0 | 202.0 | 202.0 | ||||
| 100.0 Gel 1 | Release studies | - | 11.5 | 7.5 | 7.5 | 3.75 | ||
| 50.0 Gel 2 | - | 10.78 | 7.0 | 7.0 | 3.5 | |||
| 0.00 Control | ||||||||
3.2.2. Antibacterial Properties of [P(EMA)-co-(GMA)]-Xyl-Based Gel
3.2.3. Contact Angle
3.2.4. Rheological Studies of Gel
3.2.5. BG Degradation Studies
3.2.6. In Vitro Release Studies of APIs
3.2.7. Kinetic Models
| Exponent (n) | Overall Solute Diffusion Mechanism |
|---|---|
| ≤0.45 (0.43) | Fickian diffusion |
| 0.45 (0.43) < n < 0.89 (0.85) | Anomalous (non-Fickian) diffusion |
| 0.89 (0.85) < n < 1 | Case-II transport |
| n > 1 | Super case II transport |
3.2.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lintzeri, D.; Karimian, N.; Blume-Peytavi, U.; Kottner, J. Epidermal thickness in healthy humans: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, J.; Chandan, N.; Lio, P.; Shi, V. The Skin Barrier and Moisturization: Function, Disruption, and Mechanisms of Repair. Ski. Pharmacol. Physiol. 2023, 36, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Duan, Z.; Chen, X.; Li, M. The immune function of dermal fibroblasts in skin defence against pathogens. Exp. Dermatol. 2023, 32, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Tampucci, S.; Tofani, G.; Chetoni, P.; Di Gangi, M.; Mezzetta, A.; Paganini, V.; Burgalassi, S.; Pomelli, C.S.; Monti, D. Sporopollenin Microcapsule: Sunscreen Delivery System with Photoprotective Properties. Pharmaceutics 2022, 14, 2041. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Burgess, D.J. Perspectives on Physicochemical and In Vitro Profiling of Ophthalmic Ointments. Pharm. Res. 2018, 35, 234. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.M.; Mijaljica, D.; Townley, J.P.; Spada, F.; Harrison, I.P. Vehicles for Drug Delivery and Cosmetic Moisturizers: Review and Comparison. Pharmaceutics 2021, 13, 2012. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Y.; Lv, B.; Wang, B.; Choi, S.; Bai, N.; Liu, Z.; Chen, X.D.; Cheng, Y. Thermoresponsive Phase Change Oleogel Microcapsules for Coencapsulation of Hydrophilic and Hydrophobic Actives. ACS Appl. Mater. Interfaces 2024, 16, 68348–68357. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Lu, S.-H.; Gao, R.; Kuo, C.-H.; Chung, W.-H.; Lien, W.-C.; Wu, C.-C.; Diao, Y.; Wang, H.-M.D.; Nayak, A.K. A Novel Biocompatible Herbal Extract-Loaded Hydrogel for Acne Treatment and Repair. Oxidative Med. Cell Longev. 2021, 2021, 5598291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, L.; Xiang, S.; Jiang, C.; Wu, W.; Ruan, S.; Du, Q.; Chen, T.; Xue, Y.; Chen, H.; et al. Formulation and Characterization of a 3D-Printed Cryptotanshinone-Loaded Niosomal Hydrogel for Topical Therapy of Acne. Aaps Pharmscitech 2020, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Chandran, R.; Tohit, E.R.M.; Stanslas, J.; Salim, N.; Mahmood, T.M.T. Investigation and Optimization of Hydrogel Microneedles for Transdermal Delivery of Caffeine. Tissue Eng. Part C Methods 2022, 28, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zheng, Y.; Jin, J.; Wu, X.; Xu, K.; Dai, M.; Niu, Q.; Zheng, H.; He, X.; Shen, J. Immunoregulation in Diabetic Wound Repair with a Photoenhanced Glycyrrhizic Acid Hydrogel Scaffold. Adv. Mater. 2022, 34, e2200521. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Shi, Z.; Yue, K.; Huang, X.; Xu, Y.; Gao, C.; Yao, Z.; Zhang, Y.S.; Wang, J. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 2021, 124, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cui, Z.; Liu, B.; Xiang, J.; Qiu, D.; Tian, Y.; Qu, X.; Yang, Z. An Injectable Strong Hydrogel for Bone Reconstruction. Adv. Healthc. Mater. 2019, 8, 1900709. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Yin, Y.; Liu, J.; Li, P.; Zhao, Y.; Bai, D.; Zhao, H.; Han, X.; Chen, Q. High-Strength and Injectable Supramolecular Hydrogel Self-Assembled by Monomeric Nucleoside for Tooth-Extraction Wound Healing. Adv. Mater. 2022, 34, 2108300. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Gu, Y.; Qin, L.; Chen, L.; Chen, X.; Cui, W.; Li, F.; Xiang, N.; He, X. Injectable thermosensitive hydrogel-based drug delivery system for local cancer therapy. Colloids Surf. B Biointerfaces 2021, 200, 111581. [Google Scholar] [CrossRef] [PubMed]
- Demirci, T.; Hasköylü, M.E.; Eroğlu, M.S.; Hemberger, J.; Öner, E.T. Levan-based hydrogels for controlled release of Amphotericin B for dermal local antifungal therapy of Candidiasis. Eur. J. Pharm. Sci. 2020, 145, 105255. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Li, T.; Yang, W.; Li, D.; Ji, S.; Ding, L. Amphotericin B-conjugated polypeptide hydrogels as a novel innovative strategy for fungal infections. R. Soc. Open Sci. 2018, 5, 171814. [Google Scholar] [CrossRef] [PubMed]
- Mazurkeviciute, A.; Ramanauskiene, K.; Ivaskiene, M.; Grigonis, A.; Briedis, V. Topical antifungal bigels: Formulation, characterization and evaluation. Acta Pharm. 2018, 68, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, Z.; Abubaker, M.A.; Ding, L.; Zhang, J.; Yang, S.; Fan, Z. Antibacterial polyvinyl alcohol/bacterial cellulose/nano-silver hydrogels that effectively promote wound healing. Mater. Sci. Eng. C 2021, 126, 112171. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Tan, T.; Guo, Z.; Ji, Y.; Hu, J.; Zhang, Y. Gram-selective antibacterial peptide hydrogels. Biomater. Sci. 2022, 10, 3831–3844. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhong, X.; Zhang, Y.; Bao, B.; Liu, L.; Bao, H.; Bao, C.; Cheng, X.; Zhu, L.; Lin, Q. Hyaluronic acid-based antibacterial hydrogels constructed by a hybrid crosslinking strategy for pacemaker pocket infection prevention. Carbohydr. Polym. 2020, 245, 116525. [Google Scholar] [CrossRef] [PubMed]
- Kapanya, A.; Somsunan, R.; Molloy, R.; Jiranusornkul, S.; Leewattanapasuk, W.; Jongpaiboonkit, L.; Kong, Y. Synthesis of polymeric hydrogels incorporating chlorhexidine gluconate as antibacterial wound dressings. J. Biomater. Sci. Polym. Ed. 2020, 31, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Shen, X.; Xiao, D.; Rong, L.; Mao, Z.; Wang, B.; Sui, X.; Zhao, M.; Feng, X. Antibacterial thyme oil-loaded zwitterionic emulsion hydrogels. J. Mater. Chem. B 2022, 10, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, Z.; Zhu, J.; Wen, Y.; Zhao, F.; Lei, L.; Phan-Thien, N.; Khoo, B.C.; Li, J. Thermoresponsive Hydrogel Induced by Dual Supramolecular Assemblies and Its Controlled Release Property for Enhanced Anticancer Drug Delivery. Biomacromolecules 2020, 21, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dai, X.; Sui, Y.; Li, R.; Zhang, C. Thermally induced and physically cross-linked hydrogel doped with graphene oxide for controlled release. Soft Matter 2021, 17, 3664–3671. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Yao, H.; Fan, D.; Zhou, L.; Wei, S. Hyaluronic acid thiol modified injectable hydrogel: Synthesis, characterization, drug release, cellular drug uptake and anticancer activity. Carbohydr. Polym. 2021, 254, 117286. [Google Scholar] [CrossRef] [PubMed]
- Rezk, A.I.; Obiweluozor, F.O.; Choukrani, G.; Park, C.H.; Kim, C.S. Drug release and kinetic models of anticancer drug (BTZ) from a pH-responsive alginate polydopamine hydrogel: Towards cancer chemotherapy. Int. J. Biol. Macromol. 2019, 141, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.; Mann, J.K.; English, E.; Harper, D.P.; Carrier, D.J.; Rials, T.G.; Labbé, N.; Chmely, S.C. Sustainable Hydrogels Based on Lignin-Methacrylate Copolymers with Enhanced Water Retention and Tunable Material Properties. Biomacromolecules 2018, 19, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, J.; Zhang, C.; Hu, W.; Wang, L.; et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: An Effective Strategy for Tissue Engineering. Stem Cell Rev. Rep. 2019, 15, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, N.; Rezaei, A.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Luo, H.; RamaKrishna, S.; Berto, F. Recent Advances on Bioprinted Gelatin Methacrylate-Based Hydrogels for Tissue Repair. Tissue Eng. Part A 2021, 27, 679–702. [Google Scholar] [CrossRef] [PubMed]
- Macková, H.; Hlídková, H.; Kaberova, Z.; Proks, V.; Kučka, J.; Patsula, V.; Vetrik, M.; Janoušková, O.; Podhorská, B.; Pop-Georgievski, O.; et al. Thiolated poly(2-hydroxyethyl methacrylate) hydrogels as a degradable biocompatible scaffold for tissue engineering. Mater. Sci. Eng. C 2021, 131, 112500. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.R.U.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis For Wound Healing Applications. Int. J. Nanomed. 2019, 14, 9603–9617. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dong, L.; Kong, B.; Huang, Y.; Zhong, S.; Connon, C.; Tan, J.; Yang, S.; Sun, W.; Mi, S. Effects of Gelatin Methacrylate Hydrogel on Corneal Repair and Regeneration in Rats. Transl. Vis. Sci. Technol. 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Rahali, K.; Ben Messaoud, G.; Kahn, C.J.; Sanchez-Gonzalez, L.; Kaci, M.; Cleymand, F.; Fleutot, S.; Linder, M.; Desobry, S.; Arab-Tehrany, E. Synthesis and Characterization of Nanofunctionalized Gelatin Methacrylate Hydrogels. Int. J. Mol. Sci. 2017, 18, 2675. [Google Scholar] [CrossRef] [PubMed]
- Chyzy, A.; Pawelski, D.; Vivcharenko, V.; Przekora, A.; Bratychak, M.; Astakhova, O.; Breczko, J.; Drozdzal, P.; Plonska-Brzezinska, M.E. Microwave-Assisted Synthesis of Modified Glycidyl Methacrylate–Ethyl Methacrylate Oligomers, Their Physico-Chemical and Biological Characteristics. Molecules 2022, 27, 337. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, L.; Zhou, X. Bacitracin-Ag Nanoclusters as a Novel Antibacterial Agent Combats Shigella flexneri by Disrupting Cell Membrane and Inhibiting Biofilm Formation. Nanomaterials 2021, 11, 2928. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.; Singh, S.L.; Khan, A.U. Exploring antibiofilm potential of bacitracin against streptococcus mutans. Microb. Pathog. 2020, 149, 104279. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Schnell, L.; Müller, B.; Müller, M.; Papatheodorou, P.; Barth, H. The Antibiotic Bacitracin Protects Human Intestinal Epithelial Cells and Stem Cell-Derived Intestinal Organoids from Clostridium difficile Toxin TcdB. Stem Cells Int. 2019, 2019, 4149762. [Google Scholar] [CrossRef] [PubMed]
- Arezzo, A.; Mistrangelo, M.; Bonino, M.A.; Salusso, P.; Forcignanò, E.; Vettoretto, N.; Botteri, E.; Cillara, N.; Ottonello, R.; Testa, V.; et al. Oral neomycin and bacitracin are effective in preventing surgical site infections in elective colorectal surgery: A multicentre, randomized, parallel, single-blinded trial (COLORAL-1). Updates Surg. 2021, 73, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Noguchi, S.; Tabata, K.; Matsumoto, Y. Topical gentian violet for cutaneous infection and nasal carriage with MRSA. Int. J. Dermatol. 2000, 39, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Saji, M.; Taguchi, S.; Uchiyama, K.; Osono, E.; Hayama, N.; Ohkuni, H. Efficacy of gentian violet in the eradication of methicillin-resistant Staphylococcus aureus from skin lesions. J. Hosp. Infect. 1995, 31, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.W.; Agostini, G.; Olomu, O.; Runco, D.; Jung, J.Y.; Chole, R.A. Gentian Violet and Ferric Ammonium Citrate Disrupt Pseudomonas Aeruginosa Biofilms. Laryngoscope 2008, 118, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, J.; Granik, V.; Belik, A.; Koksharova, O.; Khmel, I. Effect of nitrofurans and NO generators on biofilm formation by Pseudomonas aeruginosa PAO1 and Burkholderia cenocepacia 370. Res. Microbiol. 2009, 160, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.W. The Use of Brilliant Green in the Treatment of Chronic Ulcers of the Skin. N. Engl. J. Med. 1948, 239, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Sessoms, A.R.; Banwart, G.J. Effect of Brilliant Green on the Motility of Salmonellae and Other Selected Microorganisms. Appl. Microbiol. 1969, 18, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.R.; Banwart, G.J. Effect of Various Concentrations of Brilliant Green and Bile Salts on Salmonellae and Other Microorganisms. Appl. Microbiol. 1965, 13, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Ito, K. Dispersion Polymerization. In Polymer Particles, Advances in Polymer Science; Okubo, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 175, pp. 299–328. ISBN 978-3-540-22923-0. [Google Scholar]
- Deng, M.; Li, M.; Zhao, Y.; Jiang, Z.; Guo, X. A novel one-pot strategy to prepare β-cyclodextrin functionalized capillary monoliths for enantioseparation of basic drugs. Talanta 2018, 189, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, D.; Borah, M.; Mandal, M.; Karak, N. Swelling induced mechanically tough starch–agar based hydrogel as a control release drug vehicle for wound dressing applications. J. Mater. Chem. B 2023, 11, 2927–2936. [Google Scholar] [CrossRef] [PubMed]
- Brelle, L.; Faÿ, F.; Ozturk, T.; Didier, N.; Renard, E.; Langlois, V. Hydrogel Based on Polyhydroxyalkanoate Sulfonate: Control of the Swelling Rate by the Ionic Group Content. Biomacromolecules 2023, 24, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Joiner, J.B.; Prasher, A.; Young, I.C.; Kim, J.; Shrivastava, R.; Maturavongsadit, P.; Benhabbour, S.R. Effects of Drug Physicochemical Properties on In-Situ Forming Implant Polymer Degradation and Drug Release Kinetics. Pharmaceutics 2022, 14, 1188. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, S.A.; Tamaddon, L.; Karkhane, R.; Riazi-Esfahani, M.; Dorkoosh, F.A.; Rafiee-Tehrani, M. Design and development of intraocular polymeric implant systems for long-term controlled-release of clindamycin phosphate for toxoplasmic retinochoroiditis. Adv. Biomed. Res. 2015, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Brazel, C.S. Analysis of burst release of proxyphylline from poly(vinyl alcohol) hydrogels. Chem. Eng. Commun. 2003, 190, 519–532. [Google Scholar] [CrossRef]
- Rehman, F.; Khan, A.J.; Sama, Z.U.; Alobaid, H.M.; Gilani, M.A.; Safi, S.Z.; Muhammad, N.; Rahim, A.; Ali, A.; Guo, J.; et al. Surface engineered mesoporous silica carriers for the controlled delivery of anticancer drug 5-fluorouracil: Computational approach for the drug-carrier interactions using density functional theory. Front. Pharmacol. 2023, 14, 1146562. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, Ł.; Belniak, P.; Szumiło, M.; Kasperek, R.; Poleszak, E. Release kinetics of sulfadimidine sodium and trimethoprim from tablets containing different excipients prepared by wet granulation method. Curr. Issues Pharm. Med. Sci. 2013, 26, 183–188. [Google Scholar] [CrossRef]
- Swain, S.K.; Patra, N.P.; Sruti, J.; Rao, M.B. Design and Evaluation of Sustained Release Solid Dispersions of Verapamil Hydrochloride. Int. J. Pharm. Sci. Nanotechnol. 2011, 3, 1252–1262. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Kobryń, J.; Witkowska, A.; Raszewski, B.; Zięba, T.; Musiał, W. Modified starch as a new co-excipient in hydrophilic gels for prolonged drug delivery. Sci. Rep. 2025, 15, 19255. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhu, J.; Su, H.; Fang, L.; Tan, T. Preparation a novel pH-sensitive blend hydrogel based on polyaspartic acid and ethylcellulose for controlled release of naproxen sodium. J. Appl. Polym. Sci. 2009, 113, 327–336. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhang, L.; Xu, Z.; Dai, H.; Wu, W. Nanocellulose/Gelatin Composite Cryogels for Controlled Drug Release. ACS Sustain. Chem. Eng. 2019, 7, 6381–6389. [Google Scholar] [CrossRef]
- Laird, N.M.; Ware, J.H. Random-Effects Models for Longitudinal Data. Biometrics 1982, 38, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Vasishth, S.; Broe, M. An Introduction to Linear Mixed Models. In The Foundations of Statistics: A Simulation-Based Approach; Springer: Berlin/Heidelberg, Germany, 2011; pp. 145–159. ISBN 978-3-642-16312-8. [Google Scholar]
- Tiruneh, G.A.; Alemayehu, T.Y.; Allouche, F.K.; Reichert, J.M. Spatial variability modeling of soil fertility for improved nutrient management in Northwest Ethiopia. Arab. J. Geosci. 2021, 14, 2797. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. Accelerated in-vitro release testing methods for extended-release parenteral dosage forms. J. Pharm. Pharmacol. 2012, 64, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Dua, A.; Malik, A. Biocompatible stimuli responsive superabsorbent polymer for controlled release of GHK-Cu peptide for wound dressing application. J. Polym. Res. 2017, 24, 104. [Google Scholar] [CrossRef]
- Baker, W.R.; Sanders, L.M. Controlled Release Delivery Systems. In Synthetic Membranes: Science, Engineering and Applications; Springer: Dordrecht, The Netherlands, 1986; pp. 581–624. ISBN 978-94-010-8596-0. [Google Scholar]
- Katime, I.; Mendizábal, E. Swelling Properties of New Hydrogels Based on the Dimethyl Amino Ethyl Acrylate Methyl Chloride Quaternary Salt with Acrylic Acid and 2-Methylene Butane-1,4-Dioic Acid Monomers in Aqueous Solutions. Mater. Sci. Appl. 2010, 1, 162–167. [Google Scholar] [CrossRef]
| Mean Contact Angle (°) | Standard Deviation (°) | |
|---|---|---|
| Water | 60.74 | 0.12 |
| Diiodomethane | 18.46 | 0.08 |
| Glycerol | 61.24 | 0.10 |
| Sample | Medium | Temperature | Light | Concentration | ||
|---|---|---|---|---|---|---|
| Water | PBS | 23 °C | 37 °C | (mmol dm−3) | ||
| 1 | + | - | + | - | Daylight | 0.063 |
| 2 | - | + | + | - | Daylight | 0.019 |
| 3 | + | - | - | + | Daylight | 0.069 |
| 4 | - | + | - | + | Daylight | 0.008 |
| 5 | + | - | + | - | Blackout | 0.058 |
| 6 | - | + | + | - | Blackout | 0.037 |
| 7 | + | - | - | + | Blackout | 0.070 |
| 8 | - | + | - | + | Blackout | 0.020 |
| Control | + | - | prepared ex tempore | 0.071 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chyzy, A.; Gnatowski, P.; Piłat, E.; Sienkiewicz, M.; Wozniak, K.; Wojnicka, M.; Brzezinski, K.; Plonska-Brzezinska, M.E. Formation and Characterization of Xylitol-Modified Glycidyl Methacrylate-co-Ethyl Methacrylate Matrices for Controlled Release of Antimicrobial Compounds. Molecules 2025, 30, 3083. https://doi.org/10.3390/molecules30153083
Chyzy A, Gnatowski P, Piłat E, Sienkiewicz M, Wozniak K, Wojnicka M, Brzezinski K, Plonska-Brzezinska ME. Formation and Characterization of Xylitol-Modified Glycidyl Methacrylate-co-Ethyl Methacrylate Matrices for Controlled Release of Antimicrobial Compounds. Molecules. 2025; 30(15):3083. https://doi.org/10.3390/molecules30153083
Chicago/Turabian StyleChyzy, Adam, Przemysław Gnatowski, Edyta Piłat, Maciej Sienkiewicz, Katarzyna Wozniak, Marta Wojnicka, Krzysztof Brzezinski, and Marta E. Plonska-Brzezinska. 2025. "Formation and Characterization of Xylitol-Modified Glycidyl Methacrylate-co-Ethyl Methacrylate Matrices for Controlled Release of Antimicrobial Compounds" Molecules 30, no. 15: 3083. https://doi.org/10.3390/molecules30153083
APA StyleChyzy, A., Gnatowski, P., Piłat, E., Sienkiewicz, M., Wozniak, K., Wojnicka, M., Brzezinski, K., & Plonska-Brzezinska, M. E. (2025). Formation and Characterization of Xylitol-Modified Glycidyl Methacrylate-co-Ethyl Methacrylate Matrices for Controlled Release of Antimicrobial Compounds. Molecules, 30(15), 3083. https://doi.org/10.3390/molecules30153083








