Coordination Polymers Bearing Angular 4,4′-Oxybis[N-(pyridin-3-ylmethyl)benzamide] and Isomeric Dicarboxylate Ligands: Synthesis, Structures and Properties
Abstract
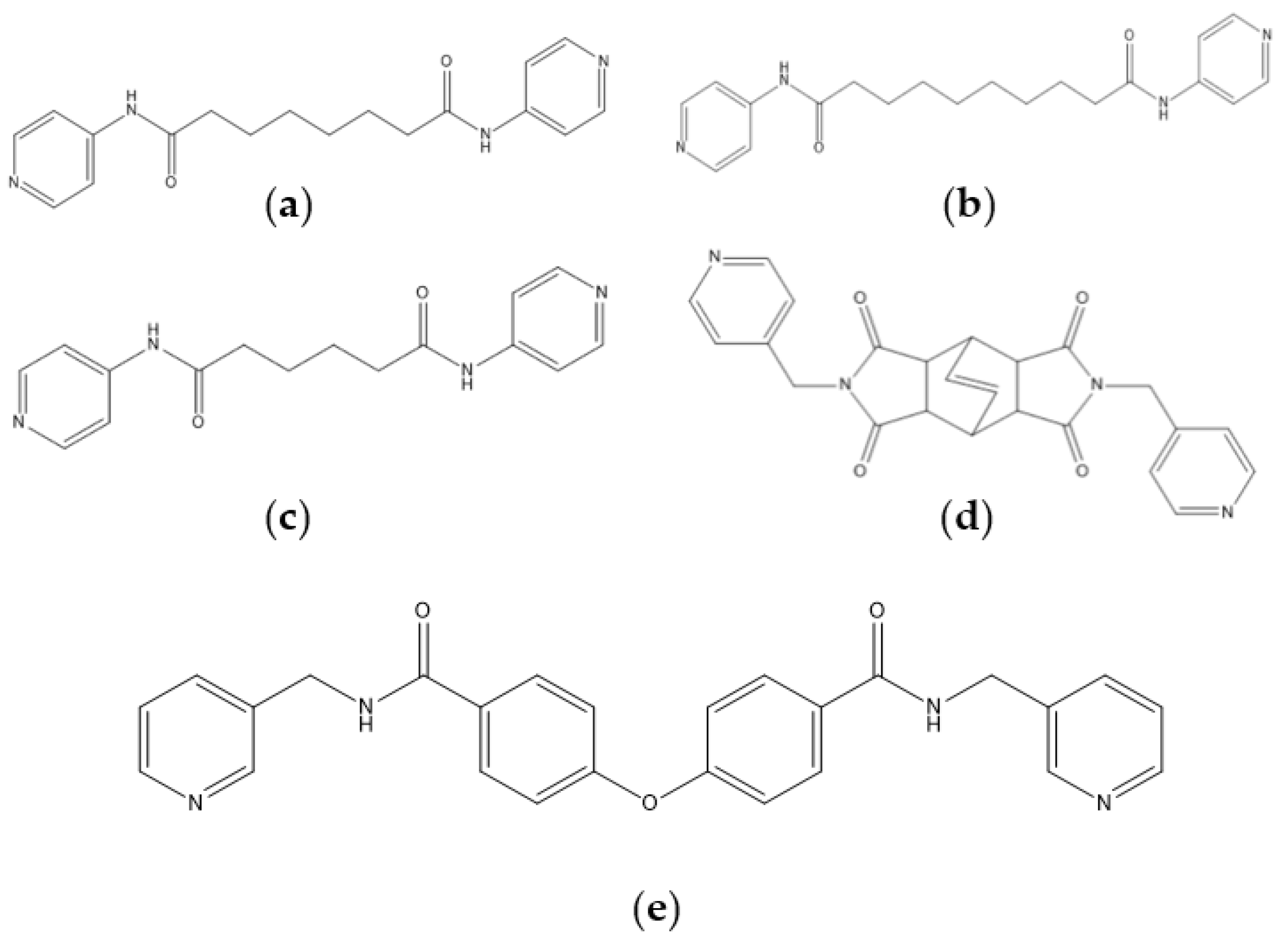
1. Introduction
2. Results and Discussion
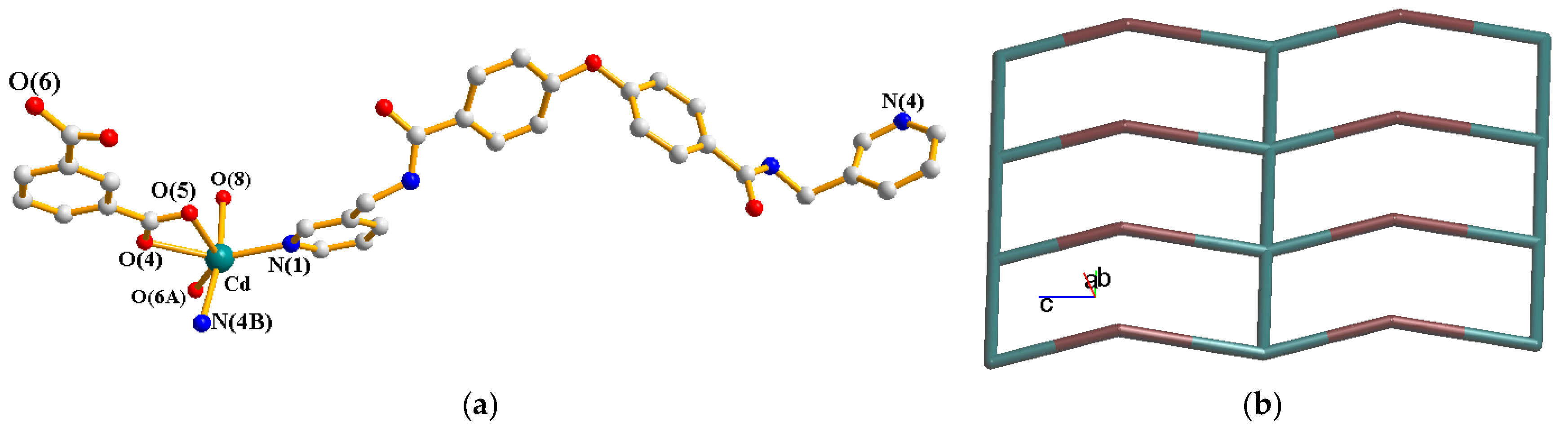
2.1. Structure of {[Cd(L)(1,3,-BDC)(H2O)]∙2H2O}n, 1
2.2. Structure of {[Cd(L)(1,4-HBDC)(1,4-BDC)0.5]∙2H2O}n, 2
2.3. Structure of {[Cu2(L)2(1,3-BDC)2]∙1.5H2O}n, 3
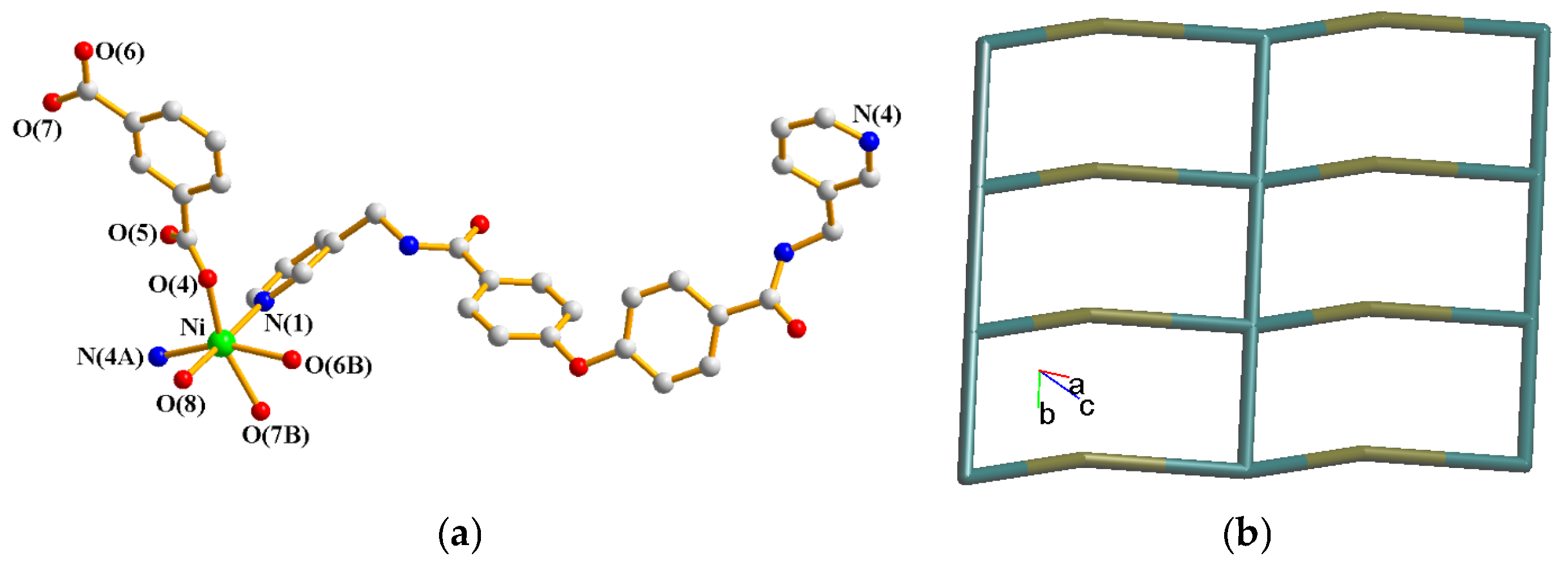
2.4. Structure of {[Ni(L)(1,3-BDC)(H2O)]∙2H2O}n, 4
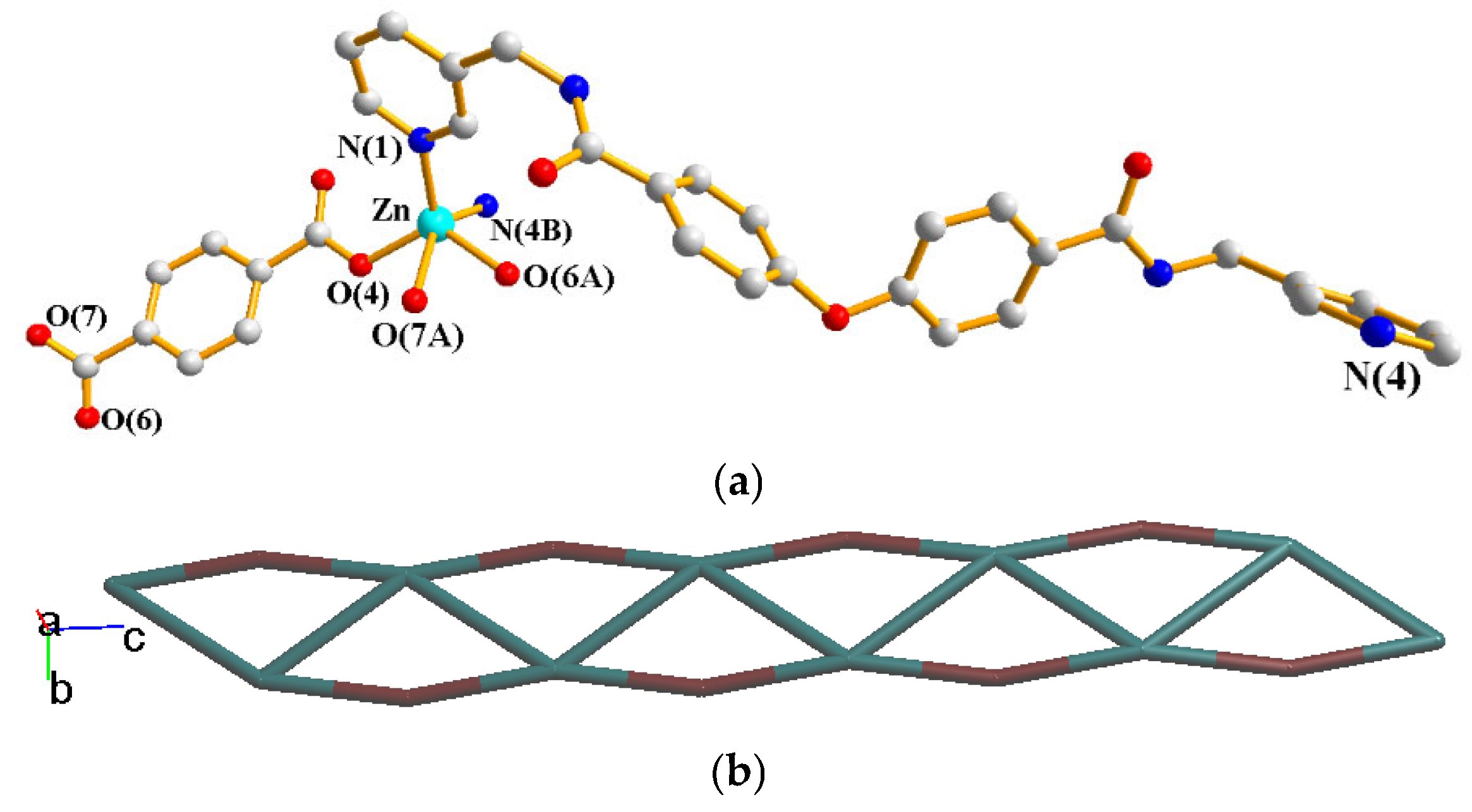
2.5. Structure of {[Zn(L)(1,3-BDC)]∙4H2O}n, 5
2.6. Structure of {[Zn(L)(1,4-BDC)]∙2H2O}n, 6
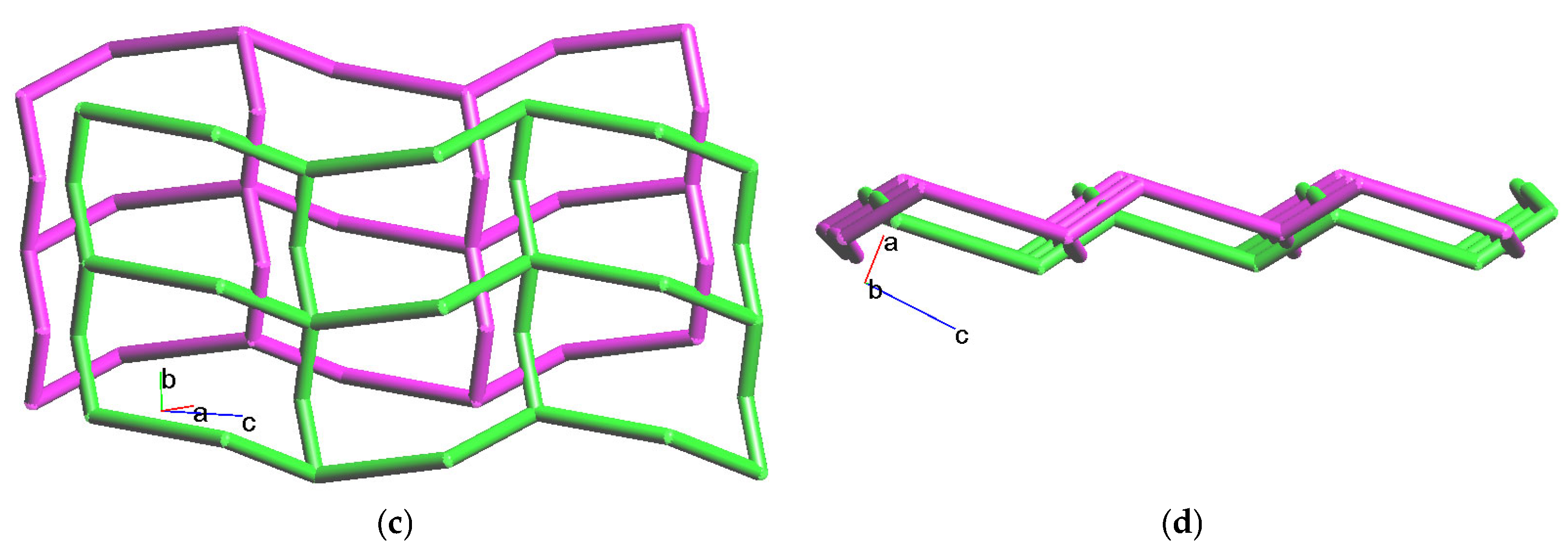
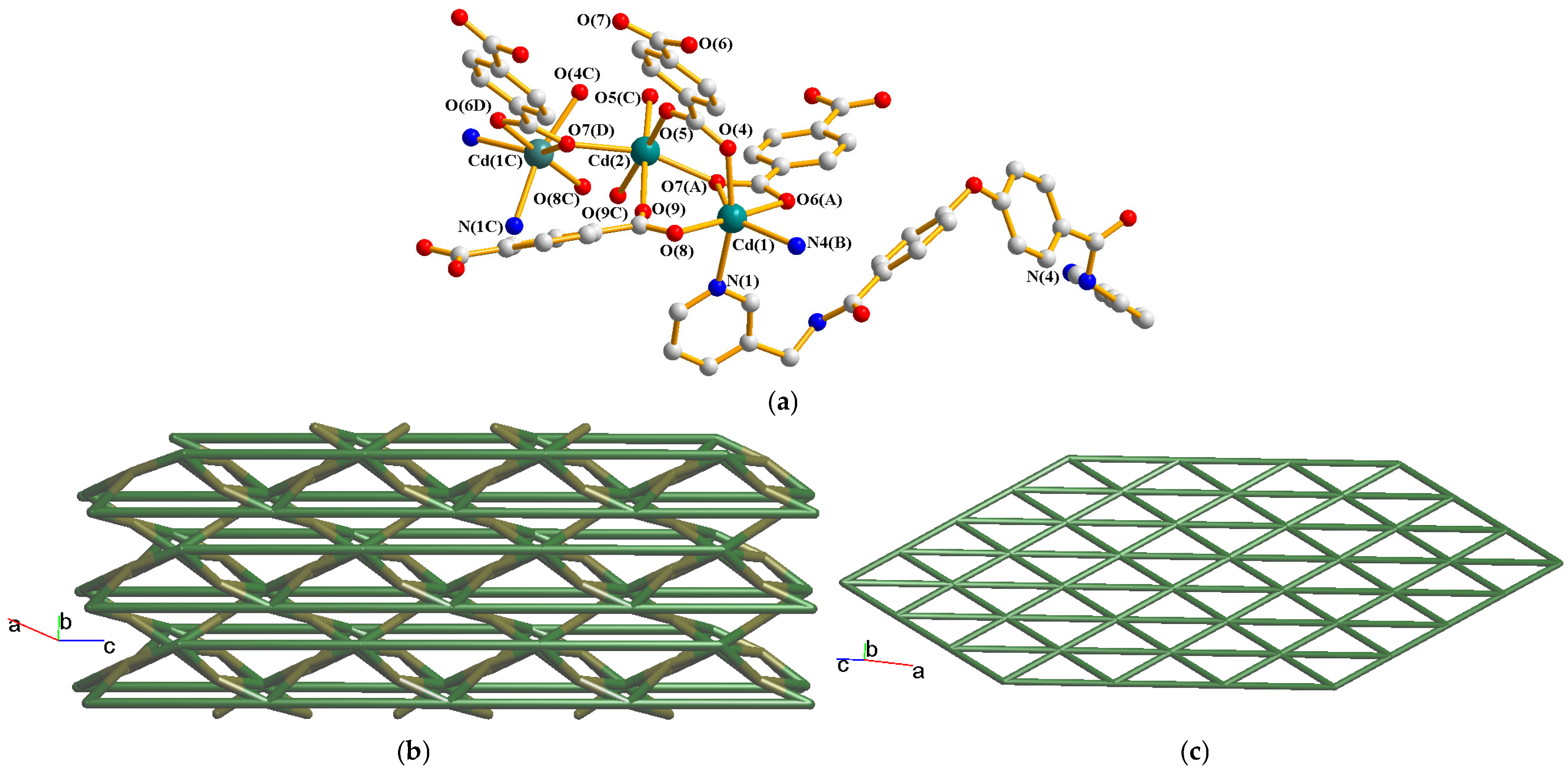
2.7. Structure of [Cd3(L)2(1,4-BDC)3]n, 7
2.8. Ligand Conformations and Coordination Modes
2.9. Powder X-Ray Analysis
2.10. Thermal Properties
2.11. Luminescent Properties
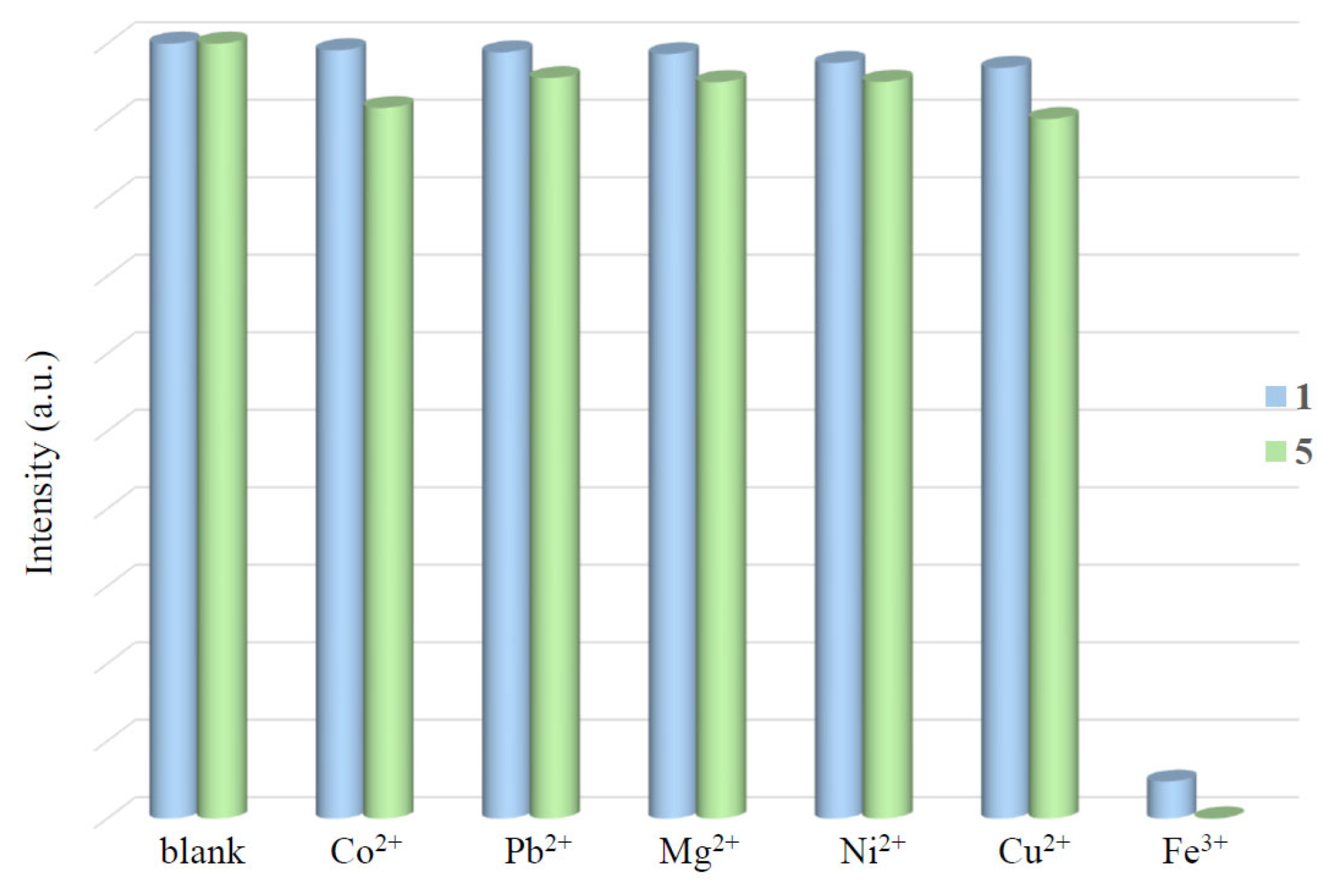
2.12. Metal Ion Detection
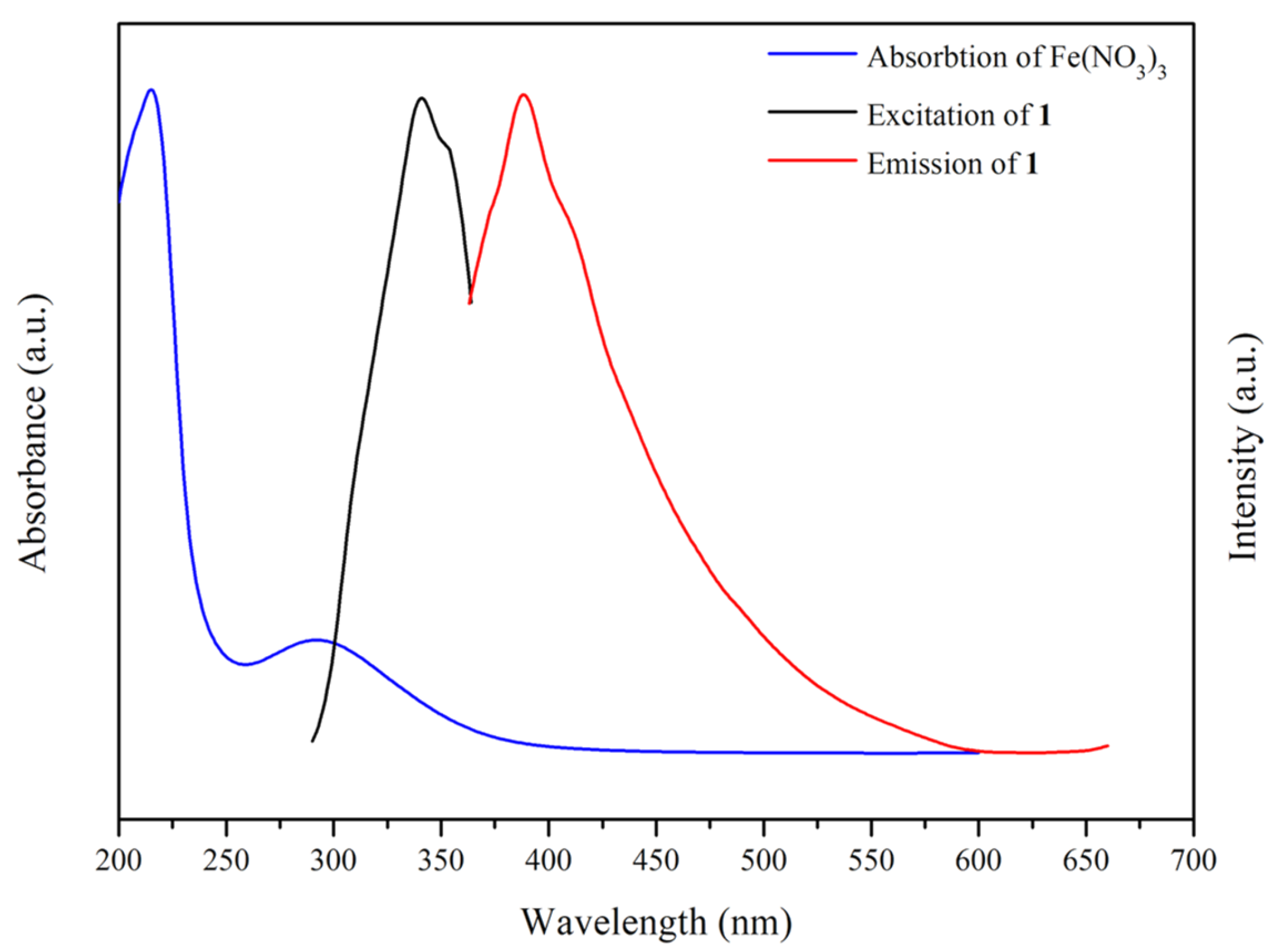
2.13. Luminescent Quenching Mechanism
3. Experimental Section
3.1. General Procedures
3.2. Materials
3.3. Preparations
3.3.1. {[Cd(L)(1,3-BDC)(H2O)]∙2H2O}n, 1
3.3.2. {[Cd(L)(1,4-HBDC)(1,4-BDC)0.5]∙2H2O}n, 2
3.3.3. {[Cu2(L)2(1,3-BDC)2]∙1.5H2O}n, 3
3.3.4. {[Ni(L)(1,3-BDC)(H2O)]∙2H2O}n, 4
3.3.5. {[Zn(L)(1,3-BDC)]∙4H2O}n, 5
3.3.6. {[Zn(L)(1,4-BDC)]∙2H2O}n, 6
3.3.7. [Cd3(L)2(1,4-BDC)3]n, 7
3.4. X-Ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Martinez, J.G.; Kitagawa, S.; Öhrström, L.; Keeffe, M.O.; Suh, M.P.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers Design, Analysis and Application; The Royal Society of Chemistry: London, UK, 2009. [Google Scholar]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Safarifard, V.; Morsali, A. Influence of an amine group on the highly efficient reversible adsorption of iodine in two novel isoreticular interpenetrated pillared-layer microporous metal–organic frameworks. CrystEngComm 2014, 16, 8660–8663. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Chen, Z.-L.; Wang, S.-W.; Chen, J.-D. Coordination Polymers Constructed from Polycarboxylic Acids and Semi-Rigid Bis-pyridyl-bis-imide Ligands: Synthesis, Structures and Iodine Adsorption. Inorganics 2025, 13, 69. [Google Scholar] [CrossRef]
- Mondal, S.; Dastidar, P. Mixed Ligand Coordination Polymers for Metallogelation and Iodine Adsorption. Cryst. Growth Des. 2019, 19, 470–478. [Google Scholar] [CrossRef]
- Liu, J.H.; Qi, Y.J.; Zhao, D.; Li, H.H.; Zheng, S.T. Heterometallic Organic Frameworks Built from Trinuclear Indium and Cuprous Halide Clusters: Ligand-Oriented Assemblies and Iodine Adsorption Behavior. Inorg. Chem. 2019, 58, 516–523. [Google Scholar] [CrossRef]
- Huang, W.-C.; Chen, W.-H.; Chen, C.-L.; Liao, T.-T.; Chen, Y.-W.; Chen, J.-D. Formation of entangled Co(II) coordination polymers based on bis-pyridyl-bis-amide and angular dicarboxylate ligands: A structural comparison. CrystEngComm 2023, 25, 5575–5587. [Google Scholar] [CrossRef]
- Craciun, N.; Melnic, E.; Siminel, A.V.; Costriucova, N.V.; Chisca, D.; Fonari, M.S. Cd(II)-Based Coordination Polymers and Supramolecular Complexes Containing Dianiline Chromophores: Synthesis, Crystal Structures, and Photoluminescence Properties. Inorganics 2025, 13, 90. [Google Scholar] [CrossRef]
- Halder, A.; Bhattacharya, B.; Haque, F.; Ghoshal, D. Structural Diversity in Six Mixed Ligand Zn(II) Metal−Organic Frameworks Constructed by Rigid and Flexible Dicarboxylates and Different N,N′ Donor Ligands. Cryst. Growth Des. 2017, 17, 6613–6624. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Sun, D.; Yan, Z.-H.; Blatov, V.A.; Wang, L.; Sun, D.-F. Syntheses, Topological Structures, and Photoluminescences of Six New Zn(II) Coordination Polymers Based on Mixed Tripodal Imidazole Ligand and Varied Polycarboxylates. Cryst. Growth Des. 2013, 13, 1277–1289. [Google Scholar] [CrossRef]
- Niu, D.; Yang, J.; Guo, J.; Kan, W.-Q.; Song, S.-Y.; Du, P.; Ma, J.-F. Syntheses, Structures, and Photoluminescent Properties of 12 New Metal–Organic Frameworks Constructed by a Flexible Dicarboxylate and Various N-Donor Ligands. Cryst. Growth Des. 2012, 12, 2397–2410. [Google Scholar] [CrossRef]
- Pamei, M.; Puzari, A. Luminescent transition metal–organic frameworks: An emerging sensor for detecting biologically essential metal ions. Nano-Struct. Nano-Objects 2019, 19, 100364. [Google Scholar] [CrossRef]
- Ge, F.-Y.; Sun, G.-H.; Lei, M.; Ren, S.-S.; Zheng, H.-G. Four New Luminescent Metal–Organic Frameworks as Multifunctional Sensors for Detecting Fe3+, Cr2O72− and Nitromethane. Cryst. Growth Des. 2020, 20, 1898–1904. [Google Scholar] [CrossRef]
- Zhang, S.-H.; Zhang, S.-Y.; Li, J.-R.; Huang, Z.-Q.; Jing, Y.; Yue, K.-F.; Wang, Y.-Y. Rational synthesis of an ultra-stable Zn(ii) coordination polymer based on a new tripodal pyrazole ligand for the highly sensitive and selective detection of Fe3+ and Cr2O72− in aqueous media. Dalton Trans. 2020, 49, 11201–11208. [Google Scholar] [CrossRef]
- Shi, Y.-S.; Qiang, Y.; Zhang, J.-W.; Cui, G.-H. Four dual-functional luminescent Zn(ii)-MOFs based on 1,2,4,5-benzenetetracarboxylic acid with pyridylbenzimidazole ligands for detection of iron(iii) ions and acetylacetone. CrystEngComm 2021, 23, 1604–1615. [Google Scholar] [CrossRef]
- Rani, P.; Gauri; Husain, A.; Bhasin, K.K.; Kumar, K. A Doubly Interpenetrated Cu(II) Metal–Organic Framework for Selective Molecular Recognition of Nitroaromatics. Cryst. Growth Des. 2020, 20, 7141–7151. [Google Scholar] [CrossRef]
- Chen, J.-K.; Yang, S.-M.; Li, B.-H.; Lin, C.-H.; Lee, S. Fluorescence Quenching Investigation of Methyl Red Adsorption on Aluminum-Based Metal–Organic Frameworks. Langmuir 2018, 34, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Biradha, K. Luminescent Coordination Polymers of Naphthalene Based Diamide with Rigid and Flexible Dicarboxylates: Sensing of Nitro Explosives, Fe (III) Ion, and Dyes. Cryst. Growth Des. 2018, 18, 3683–3692. [Google Scholar] [CrossRef]
- Das, P.; Mandal, S.K. A dual-functionalized, luminescent and highly crystalline covalent organic framework: Molecular decoding strategies for VOCs and ultrafast TNP sensing. J. Mater. Chem. A 2018, 6, 16246–16256. [Google Scholar] [CrossRef]
- Bruker, A.X.S. APEX2, V2008.6; SAD ABS V2008/1, SAINT+ V7.60A, SHELXTL V6.14; Bruker AXS Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]





| Ligand Conformation | Coordination Mode | |
|---|---|---|
| 1 | 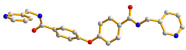 syn–anti | 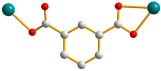 μ2-κ2O,O′;κO″ |
| 2 | 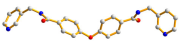 syn–syn | 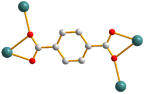 μ4-κ2O,O′;κO′;κ2O″,O‴; κO‴ 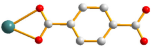 κ2O,O′ |
| 3 |  anti–anti 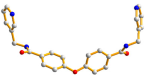 syn–anti | 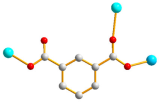 μ3-κO;κO′;κO″ |
| 4 | 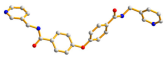 syn–anti | 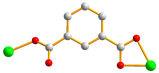 μ2-κ2O,O′;κO″ |
| 5 | 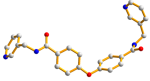 anti–anti |  μ2-κO;κO′ |
| 6 |  anti–anti | 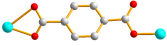 μ2-κ2O,O′;κO″ |
| 7 | 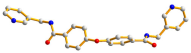 syn–anti |  μ4-κO;κO′;κO″;κO‴  μ4-κO;κO′;κ2O″,O‴; κO‴ |
| Complex | Weight Loss of Solvent °C (Calc/Found),% | Weight Loss of Ligand °C (Calc/Found),% |
|---|---|---|
| 1 | 3 H2O 90–130 (7.38/7.02) | L + 1,3-BDC2− 325–800 (82.60/78.28) |
| 2 | 1 H2O ~220 (2.65/2.16) | L + 1,4-BDC2− 275–800 (82.60/82.50) |
| 3 | 1.5 H2O ~230 (1.99/3.31) | L + 1,3-BDC2− 245–800 (88.66/89.94) |
| 4 | 3 H2O 80–180 (7.60/7.55) | L + 1,3-BDC2− 335–800 (82.28/84.44) |
| 5 | 4 H2O ~170 (9.11/9.72) | L + 1,3-BDC2− 310–795 (72.13/81.35) |
| 6 | 2 H2O ~335 (4.87/5.11) | L + 1,4-BDC2− 345–800 (84.90/85.80) |
| 7 | L + 1,4-BDC2− 345–800 (80.24/76.99) |
| Compound | Excitation λex (nm) | Emission λem (nm) | Complex | Excitation λex (nm) | Emission λem (nm) |
|---|---|---|---|---|---|
| 1,3-H2BDC | 280,310 | 355 | 1 | 340 | 388 |
| 1,4-H2BDC | 280,330 | 385 | 2 | 330 | 385 |
| L | 340 | 398 | 5 | 341 | 422 |
| 6 | 335 | 435 | |||
| 7 | 321 | 421 |
| Complex | 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|---|
| Formula | C34H32CdN4O10 | C38H33CdN4O11 | C68H55Cu2N8O15.5 | C34H32NiN4O10 | ||
| Formula weight | 769.03 | 834.08 | 1359.28 | 715.34 | ||
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic | ||
| Space group | Pī | Pī | Pī | Pī | ||
| a, Å | 9.3402(5) | 9.2561(5) | 11.8074(2) | 9.3094(2) | ||
| b, Å | 10.1891(6) | 9.9219(5) | 16.1994(3) | 10.0075(1) | ||
| c, Å | 18.4381(11) | 22.6929(11) | 16.9383(3) | 18.1094(3) | ||
| α, ° | 81.322(3) | 82.3318(13) | 73.9272(12) | 79.3537(8) | ||
| β, ° | 77.049(3) | 80.9252(15) | 78.3137(12) | 78.0679(7) | ||
| γ, ° | 70.622(3) | 62.7198(13) | 78.6938(12) | 73.4443(7) | ||
| V, Å3 | 1607.61(16) | 1824.65(16) | 3014.74(10) | 1567.95(5) | ||
| Z | 2 | 2 | 2 | 2 | ||
| Dcalc, Mg/m3 | 1.589 | 1.518 | 1.497 | 1.515 | ||
| F(000) | 784 | 850 | 1402 | 744 | ||
| µ (Mo Kα), mm−1 | 0.746 | 0.666 | 0.785 | 0.687 | ||
| Range (2θ) for data collection, deg | 2.126 to 28.499 | 2.315 to 25.091 | 1.590 to 28.294 | 2.142 to 28.331 | ||
| Independent reflection | 8049[R(int) = 0.0210] | 6465[R(int) = 0.0725] | 14,951[R(int) = 0.0380] | 7808[R(int) = 0.0216] | ||
| Data/restraint/parameter | 8049/0/450 | 6465/0/649 | 14,951/0/842 | 7808/0/442 | ||
| quality-of-fit indicator c | 1.035 | 1.057 | 1.023 | 1.025 | ||
| Final R indices [I > 2σ(I)] a,b | R1 = 0.0259,wR2 = 0.0629 | R1 = 0.0531,wR2 = 0.1280 | R1 = 0.0462,wR2 = 0.1016 | R1 = 0.0301,wR2 = 0.0733 | ||
| R indices (all data) | R1 = 0.0283,wR2 = 0.0645 | R1 = 0.0718,wR2 = 0.1426 | R1 = 0.0840,wR2 = 0.1161 | R1 = 0.0377,wR2 = 0.0770 | ||
| Complex | 5 | 6 | 7 | |||
| Formula | C34H34ZnN4O11 | C34H30ZnN4O9 | C76H56Cd3N8O18 | |||
| Formula weight | 740.02 | 703.99 | 1706.48 | |||
| Crystal system | Monoclinic | Monoclinic | Monoclinic | |||
| Space group | P21/n | P21/c | C2/c | |||
| a, Å | 13.2504(4) | 14.6873(2) | 41.100(2) | |||
| b, Å | 9.5834(3) | 12.5880(2) | 9.7849(4) | |||
| c, Å | 26.5625(9) | 17.7902(3) | 18.1394(7) | |||
| α, ° | 90 | 90 | 90 | |||
| β, ° | 95.3579(6) | 97.0769(10) | 113.9416(11) | |||
| γ, ° | 90 | 90 | 90 | |||
| V, Å3 | 3358.27(19) | 3264.06(9) | 6667.3(5) | |||
| Z | 4 | 4 | 4 | |||
| Dcalc, Mg/m3 | 1.464 | 1.433 | 1.700 | |||
| F(000) | 1536 | 1456 | 3424 | |||
| µ (Mo Kα), mm−1 | 0.799 | 0.814 | 1.031 | |||
| Range (2θ) for data collection, deg | 1.540 to 28.393 | 1.397 to 25.999 | 2.151 to 26.051 | |||
| Independent reflection | 8416[R(int) = 0.0445] | 6400[R(int) = 0.0572] | 5902[R(int) = 0.0715] | |||
| Data/restraint/parameter | 8416/0/451 | 6400/0/442 | 5902/0/474 | |||
| quality-of-fit indicator c | 1.036 | 1.056 | 1.076 | |||
| Final R indices [I > 2σ(I)] a,b | R1 = 0.0424,wR2 = 0.1018 | R1 = 0.0507,wR2 = 0.0977 | R1 = 0.0378,wR2 = 0.0730 | |||
| R indices (all data) | R1 = 0.0721,wR2 = 0.1134 | R1 = 0.1236,wR2 = 0.1201 | R1 = 0.0737,wR2 = 0.0922 | |||
| Complex | Metal–Ligand Distance | Coordination Geometry and Packing Interactions | Topology |
|---|---|---|---|
| 1 | Cd-N(1) = 2.2697(14) Cd-O(6A) = 2.2733(13) Cd-O(4) =2.3338(13) Cd-O(8) = 2.3393(14) Cd-N(4B) = 2.3427(15) Cd-O(5) = 2.4849(12) | Distorted octahedral N-H---O and O-H---O | 2D net with (64·8·10)(6)-2,4L3 |
| 2 | Cd-N(4A) = 2.277(5) Cd-N(1) = 2.286(6) Cd-O(4) = 2.297(4) Cd-O(5B) = 2.337(4) Cd-O(6) = 2.355(3) Cd-O(7) = 2.434(3) Cd-O(5) = 2.604(4) | Distorted pentagonal bipyramidal N-H---O and O-H---O | 2D net with (42·82·102)(42·84)2(4)2 |
| 3 | Cu(1)-O(7) = 1.9504(17) Cu(1)-O(11) = 1.9616(16) Cu(1)-N(5) = 2.016(2) Cu(1)-N(1) = 2.023(2) Cu(1)-O(8A) = 2.3684(19) Cu(2)-O(14B) = 1.9368(16) Cu(2)-O(9) = 1.9755(15) Cu(2)-N(4C) = 2.011(2) Cu(2)-N(8D) = 2.026(2) Cu(2)-O(13E) = 2.396(2) | Distorted square pyramidal N-H---O and O-H---O | 2D net with (4·5·6)(4·55·63·7)-3,5L66 |
| 4 | Ni-O(4) = 2.0344(10) Ni-N(4A) = 2.0658(12) Ni-N(1) = 2.0895(13) Ni-O(8) = 2.0896(11) Ni-O(7B) = 2.1230(10) Ni-O(6B) = 2.1563(10) | Distorted octahedral N-H---O and O-H---O | 2D net with (64·8·10)(6)-2,4L3 |
| 5 | Zn-O(4) = 1.9293(16) Zn-O(6A) = 1.9419(14) Zn-N(1) = 2.043(2) Zn-N(4B) = 2.111(2) | Distorted tetrahedral N-H---O and O-H---O | 2D net with (84·122)(8)2-2,4L2 |
| 6 | Zn-O(4) = 1.939(2) Zn-N(1) = 2.033(3) Zn-O(6A) = 2.044(4) Zn-N(4B) = 2.063(3) Zn-O(7A) = 2.421(4) | Distorted square pyramidal N-H---O and O-H---O | 1D net with (43·62·8)(4)-2,4C3 |
| 7 | Cd(1)-O(4) = 2.247(4) Cd(1)-O(6A) = 2.341(3) Cd(1)-O(8) = 2.374(3) Cd(1)-N(4B) = 2.378(4) Cd(1)-N(1) = 2.382(4) Cd(1)-O(7A) = 2.464(3) Cd(2)-O(5) = 2.166(3) Cd(2)-O(5C) = 2.167(3) Cd(2)-O(9C) = 2.272(3) Cd(2)-O(9) = 2.273(3) Cd(2)-O(7A) = 2.402(3) Cd(2)-O(7D) = 2.402(3) | Distorted octahedral N-H---O | 2D net with (36·46·53)-hxl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-H.; Hsieh, Y.-J.; Chen, Y.-H.; Liu, S.-M.; Chen, J.-D. Coordination Polymers Bearing Angular 4,4′-Oxybis[N-(pyridin-3-ylmethyl)benzamide] and Isomeric Dicarboxylate Ligands: Synthesis, Structures and Properties. Molecules 2025, 30, 3283. https://doi.org/10.3390/molecules30153283
Huang Y-H, Hsieh Y-J, Chen Y-H, Liu S-M, Chen J-D. Coordination Polymers Bearing Angular 4,4′-Oxybis[N-(pyridin-3-ylmethyl)benzamide] and Isomeric Dicarboxylate Ligands: Synthesis, Structures and Properties. Molecules. 2025; 30(15):3283. https://doi.org/10.3390/molecules30153283
Chicago/Turabian StyleHuang, Yung-Hao, Yi-Ju Hsieh, Yen-Hsin Chen, Shih-Miao Liu, and Jhy-Der Chen. 2025. "Coordination Polymers Bearing Angular 4,4′-Oxybis[N-(pyridin-3-ylmethyl)benzamide] and Isomeric Dicarboxylate Ligands: Synthesis, Structures and Properties" Molecules 30, no. 15: 3283. https://doi.org/10.3390/molecules30153283
APA StyleHuang, Y.-H., Hsieh, Y.-J., Chen, Y.-H., Liu, S.-M., & Chen, J.-D. (2025). Coordination Polymers Bearing Angular 4,4′-Oxybis[N-(pyridin-3-ylmethyl)benzamide] and Isomeric Dicarboxylate Ligands: Synthesis, Structures and Properties. Molecules, 30(15), 3283. https://doi.org/10.3390/molecules30153283






