Photochemically-Enabled Umpolung Conversion of 2-Acyloxybenzaldehydes into 2-Hydroxybenzofuranones
Abstract
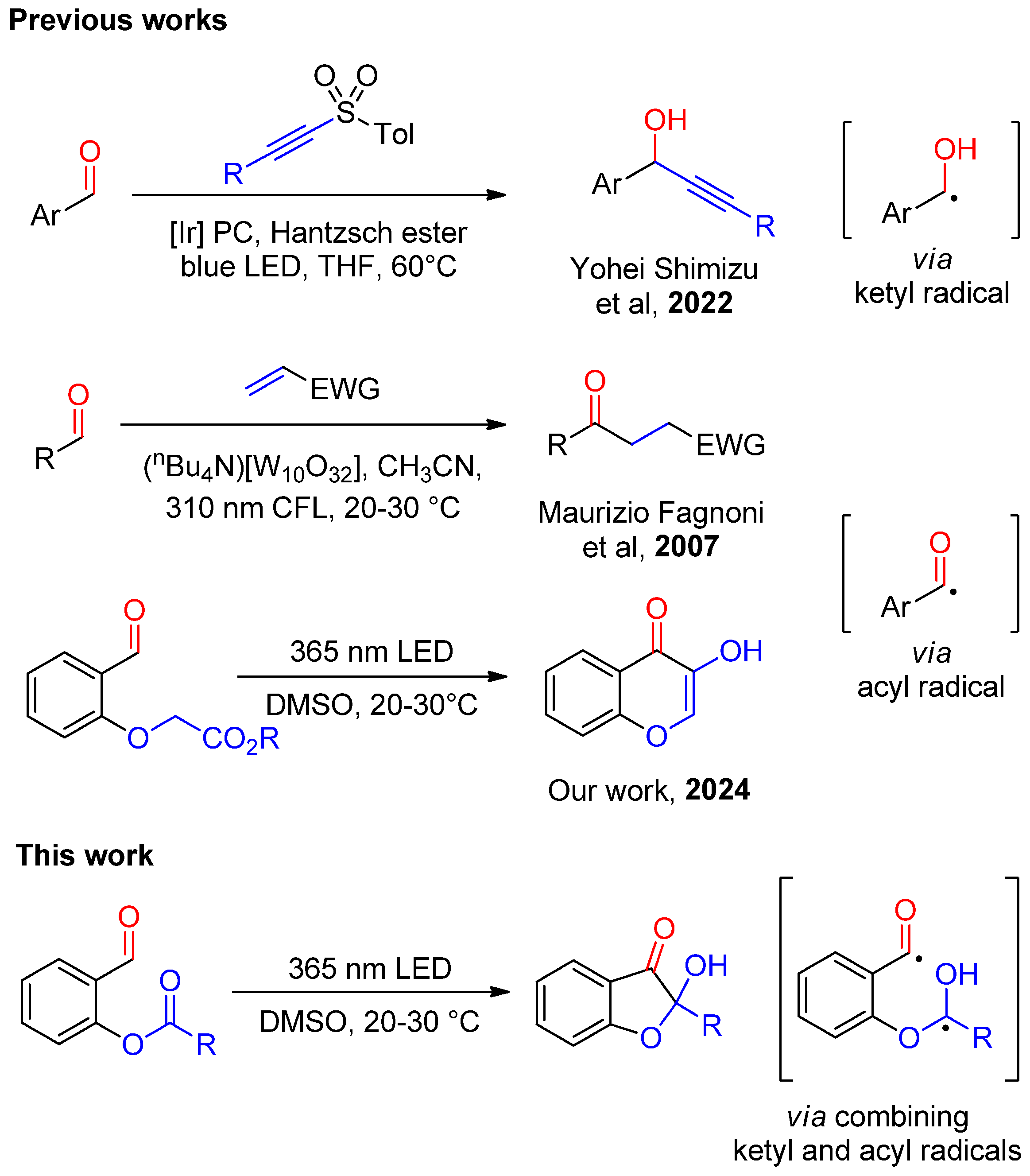
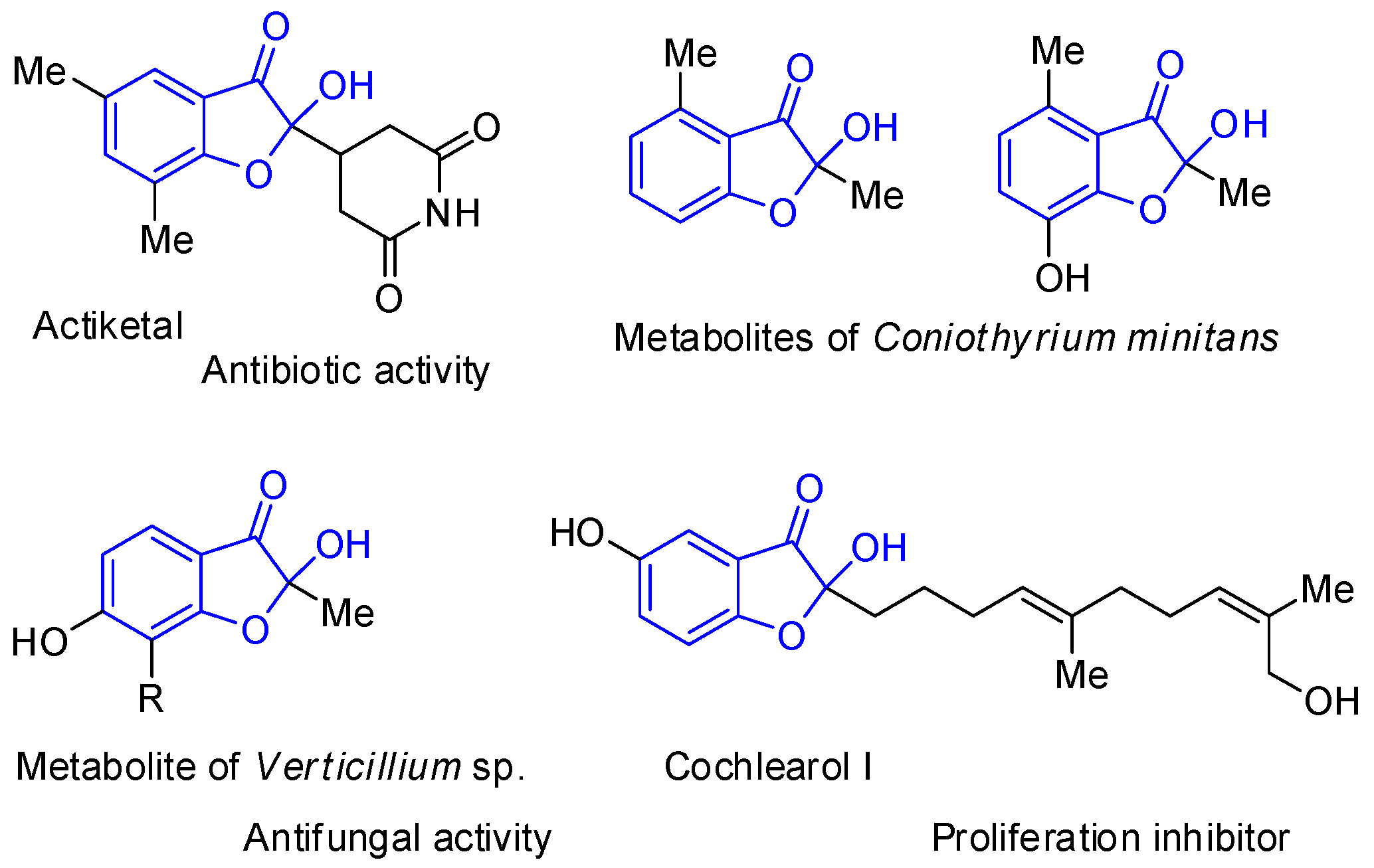
1. Introduction
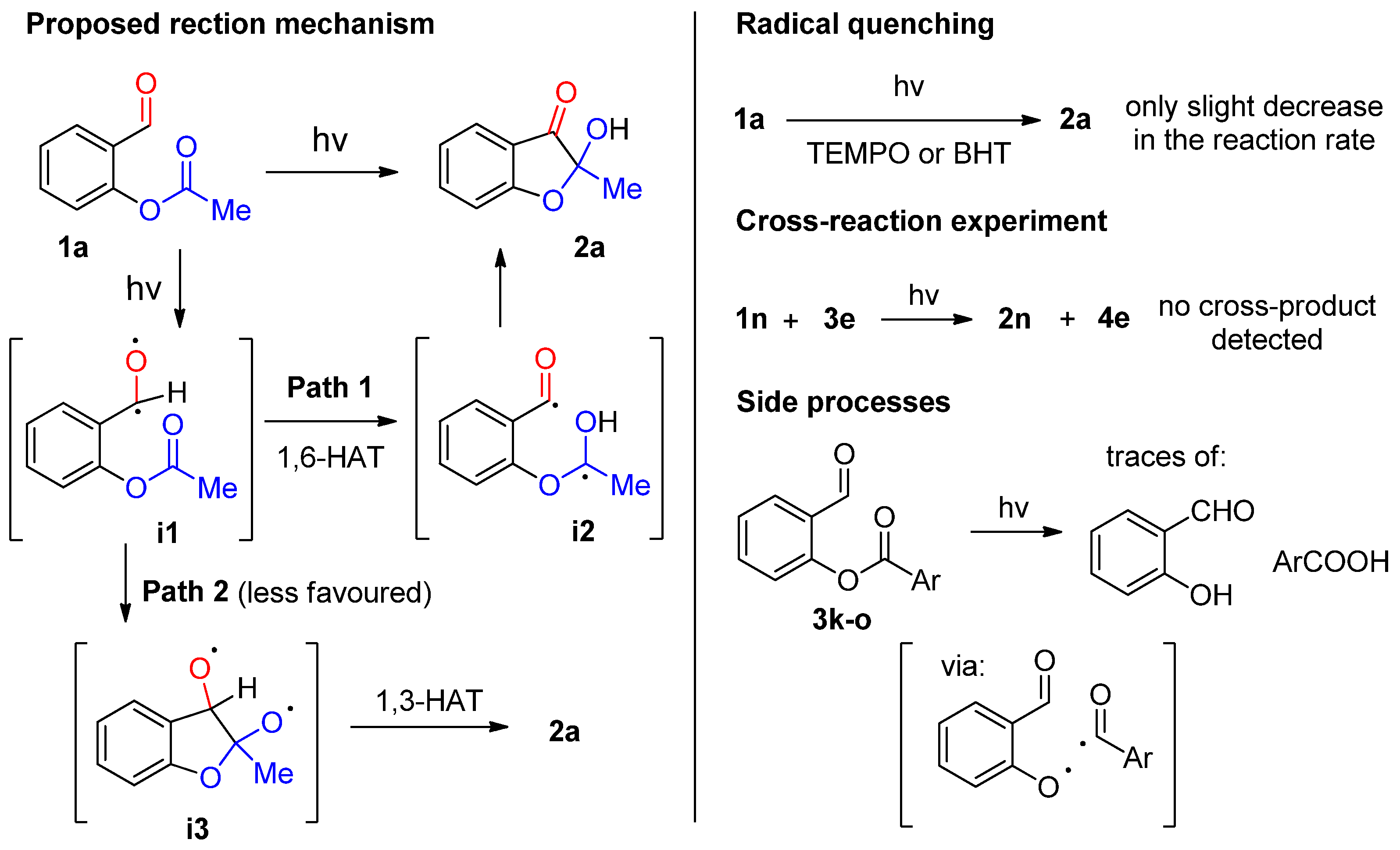
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Starting Compounds 1 and 3
3.2.1. Synthesis of the Starting Compound 1
- 2-Formylphenyl acetate (1a). Yield, 1.36 g (83%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.08 (1H, s), 7.91 (1H, dd, J = 7.6, 1.6 Hz), 7.76 (1H, td, J = 7.7, 1.6 Hz), 7.50 (1H, t, J = 7.5 Hz), 7.31 (1H, d, J = 8.1 Hz), 2.35 (3H, s). The spectral properties corresponded to the literature data [52].
- 2-Formyl-4-methylphenyl acetate (1b). Yield, 1.60 g (90%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.04 (1H, s), 7.70 (1H, d, J = 1.7 Hz), 7.55 (1H, dd, J = 8.2, 1.7 Hz), 7.18 (1H, d, J = 8.2 Hz), 2.38 (3H, s), 2.33 (3H, s). The spectral properties corresponded to the literature data [53].
- 4-Ethyl-2-formylphenyl acetate (1c). Yield, 1.84 g (96%); yellowish viscous oil. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.05 (1H, s), 7.73 (1H, d, J = 2.0 Hz), 7.59 (1H, dd, J = 8.2, 2.0 Hz), 7.21 (1H, d, J = 8.2 Hz), 2.69 (2H, q, J = 7.6 Hz), 2.33 (3H, s), 1.21 (3H, t, J = 7.6 Hz). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 190.0, 169.4, 149.2, 142.2, 135.0, 129.7, 127.6, 123.6, 27.3, 20.6, 15.3. HRMS (ESI) m/z: 193.0854 found (calcd for C11H13O3+, [M + H]+ 193.0859).
- 5-(Tert-butyl)-2-formylphenyl acetate (1d). Yield, 2.14 g (97%); yellowish viscous oil. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.00 (1H, s), 7.84 (1H, d, J = 8.1 Hz), 7.52–7.53 (1H, m), 7.28 (1H, d, J = 1.8 Hz), 2.34 (3H, s), 1.30 (9H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 189.5, 169.3, 159.6, 151.0, 130.9, 125.6, 123.5, 120.5, 35.2, 30.5, 20.7. HRMS (ESI) m/z: 221.1171 found (calcd for C13H17O3+, [M + H]+ 221.1172).
- 2,4-Di-tert-butyl-6-formylphenyl acetate (1e). Yield, 2.24 g (81%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 9.92 (1H, s), 7.82 (1H, d, J = 2.4 Hz), 7.71 (1H, d, J = 2.4 Hz), 2.38 (3H, s), 1.34 (18H, m). The spectral properties corresponded to the literature data [54].
- 3-Formyl-[1,1′-biphenyl]-4-yl acetate (1f). Yield, 2.28 g (95%); white solid; m.p., 118–120 °C. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.14 (1H, s), 8.18 (1H, d, J = 2.0 Hz), 8.04 (1H, dd, J = 8.3, 2.0 Hz), 7.74 (2H, d, J = 7.5 Hz), 7.51 (2H, t, J = 7.5 Hz), 7.43 (1H, t, J = 7.3 Hz), 7.40 (1H, d, J = 8.3 Hz), 2.37 (3H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 190.2, 169.2, 150.2, 138.5, 138.2, 133.5, 129.4, 129.1, 128.2, 128.0, 126.7, 124.4, 20.7. HRMS (ESI) m/z: 241.0860 found (calcd for C15H13O3+, [M + H]+ 241.0859).
- 4-Fluoro-2-formylphenyl acetate (1g). Yield, 1.70 g (93%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.05 (1H, d, J = 1.9 Hz), 7.68 (1H, dd, J = 8.4, 3.2 Hz), 7.63 (1H, td, J = 8.4, 3.2 Hz), 7.39 (1H, dd, J = 8.9, 4.5 Hz), 2.35 (3H, s). The spectral properties corresponded to the literature data [55].
- 4-Chloro-2-formylphenyl acetate (1h). Yield, 1.95 g (97%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.04 (1H, s), 7.92 (1H, d, J = 2.6 Hz), 7.82 (1H, dd, J = 8.6, 2.6 Hz), 7.38 (1H, d, J = 8.6 Hz), 2.35 (3H, s). The spectral properties corresponded to the literature data [56].
- 3-Chloro-2-formylphenyl acetate (1i). Yield, 1.57g (79%), yellowish viscous oil. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.30 (1H, s), 7.72 (1H, t, J = 8.1 Hz), 7.57 (1H, dd, J = 8.0, 0.8 Hz), 7.28 (1H, d, J = 8.0 Hz), 2.31 (3H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 188.6, 168.9, 150.8, 136.6, 135.6, 128.6, 124.8, 123.4, 20.6. HRMS (ESI) m/z: 199.0156 found (calcd for C9H8ClO3+, [M + H]+ 199.0156).
- 5-Bromo-2-formylphenyl acetate (1j). Yield, 2.16 g (89%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.06 (1H, s), 7.84 (1H, d, J = 8.3 Hz), 7.72 (1H, d, J = 8.3 Hz), 7.67 (1H, d, J = 1.1 Hz), 2.35 (3H, s). The spectral properties corresponded to the literature data [57].
- 2-Formyl-4-methoxyphenyl acetate (1k). Yield, 1.57 g (81%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.04 (1H, s), 7.38 (1H, d, J = 3.2 Hz), 7.30–7.31 (1H, m), 7.23 (1H, d, J = 8.8 Hz), 3.83 (3H, s), 2.33 (3H, s). The spectral properties corresponded to the literature data [58].
- 2-Formyl-6-methoxyphenyl acetate (1l). Yield, 1.55 g (80%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.10 (1H, s), 7.47–7.50 (1H, m), 7.44 (2H, d, J = 4.8 Hz), 3.84 (3H, s), 2.35 (3H, s). The spectral properties corresponded to the literature data [59].
- 5-Ethoxy-2-formylphenyl acetate (1m). Yield, 1.92 g (92%); yellow solid; m.p., 58–60 °C. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 9.90 (1H, s), 7.84 (1H, d, J = 8.6 Hz), 7.02 (1H, dd, J = 8.6, 2.2 Hz), 6.85 (1H, d, J = 2.2 Hz), 4.15 (2H, q, J = 6.9 Hz), 2.33 (3H, s), 1.35 (3H, t, J = 6.9 Hz). 13C NMR (201 MHz, DMSO-d6) δ, ppm: 188.3, 168.9, 164.1, 152.9, 132.9, 121.3, 112.7, 109.4, 64.2, 20.6, 14.3. HRMS (ESI) m/z: 209.0808 found (calcd for C11H13O4+, [M + H]+ 209.0808).
- Methyl 4-acetoxy-3-formylbenzoate (1n). Yield, 1.89 g (85%); yellow solid; m.p., 88–90 °C. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.14 (1H, s), 8.45 (1H, d, J = 1.2 Hz), 8.28 (1H, dd, J = 8.4, 1.2 Hz), 7.49 (1H, d, J = 8.4 Hz), 3.90 (3H, s), 2.38 (3H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 189.6, 168.8, 164.8, 154.3, 135.8, 131.7, 128.0, 127.9, 124.6, 52.5, 20.7. HRMS (ESI) m/z: 223.0602 found (calcd for C11H11O5+, [M + H]+ 223.0601).
3.2.2. Synthesis of the Starting Compounds 3a–3d, 3h–3l, and 3p
- 2-Formylphenyl 2-methoxyacetate (3a). Yield, 1.16 g (60%); white oil. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.08 (1H, s), 7.94 (1H, dd, J = 7.6, 1.7 Hz), 7.76–7.79 (1H, m), 7.50–7.55 (1H, m), 7.35 (1H, d, J = 8.1 Hz), 4.45 (2H, s), 3.42 (3H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 190.1, 169.0, 150.3, 135.8, 131.5, 127.8, 126.9, 123.7, 68.8, 58.7. HRMS (ESI) m/z: 195.0652 found (calcd for C10H11O4+, [M + H]+ 195.0652).
- 2-Formylphenyl cyclohexanecarboxylate (3b). Yield, 1.59 g (69%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.04 (1H, s), 7.91 (1H, dd, J = 7.7, 1.6 Hz), 7.74–7.77 (1H, m), 7.49 (1H, t, J = 7.5 Hz), 7.27 (1H, d, J = 8.1 Hz), 2.71 (1H, tt, J = 11.1, 3.7 Hz), 1.75 (2H, ddd, J = 13.2, 3.8, 3.7 Hz), 1.60–1.67 (2H, m), 1.51–1.59 (2H, m), 1.24–1.37 (4H, m). The spectral properties corresponded to the literature data [60].
- 2-Formylphenyl 2-(neopentyloxy)acetate (3c). Yield, 1.63 g (65%); white oil. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.08 (1H, s), 7.93 (1H, dd, J = 7.6, 1.5 Hz), 7.77 (1H, td, J = 7.7, 1.4 Hz), 7.52 (1H, t, J = 7.5 Hz), 7.35 (1H, d, J = 8.1 Hz), 4.51 (2H, s), 3.28 (2H, s), 0.91 (9H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 190.1, 169.3, 150.5, 135.7, 131.2, 127.9, 126.8, 123.7, 81.2, 67.9, 31.8, 26.5. HRMS (ESI) m/z: 251.1280 found (calcd for C14H19O4+, [M + H]+ 251.1278).
- 2-Formylphenyl 5-phenylpentanoate (3d). Yield, 1.97 g (70%); white oil. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.05 (1H, s), 7.91 (1H, dd, J = 7.6, 1.6 Hz), 7.75 (1H, td, J = 7.7, 1.7 Hz), 7.49 (1H, t, J = 7.5 Hz), 7.26–7.31 (3H, m), 7.22 (2H, d, J = 7.1 Hz), 7.18 (1H, t, J = 7.3 Hz), 2.72 (2H, t, J = 6.9 Hz), 2.64 (2H, t, J = 6.9 Hz), 1.66–1.73 (4H, m). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 189.9, 171.7, 151.0, 141.9, 135.7, 131.0, 128.3, 128.2, 127.9, 126.6, 125.7, 123.8, 34.8, 33.0, 30.3, 23.7. HRMS (ESI) m/z: 283.1330 found (calcd for C18H19O3+, [M + H]+ 283.1329).
- 2-Formylphenyl pivalate (3h). Yield, 1.96 g (95%); white oil. 1H NMR (300 MHz, DMSO-d6) δ, ppm: 10.03 (1H, s) 7.92 (1H, dd, J = 7.7, 1.5 Hz) 7.76 (1H, td, J = 7.7, 1.7 Hz) 7.50 (1H, t, J = 7.6 Hz) 7.27 (1H, d, J = 8.2 Hz) 1.35 (9H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 189.4, 176.2, 151.3, 135.7, 130.9, 128.1, 126.6, 123.6, 38.8, 26.7. HRMS (ESI) m/z: 207.1018 found (calcd for C12H15O3+, [M + H]+ 207.1016).
- 2-Formylphenyl cyclopentanecarboxylate (3i). Yield, 2.04 g (88%); white oil. 1H NMR (800 MHz, DMSO-d6) δ, ppm 10.06 (1H, s), 7.91 (1H, dd, J = 7.6, 1.4 Hz), 7.74–7.77 (1H, m), 7.49 (1H, t, J = 7.5 Hz), 7.28 (1H, d, J = 8.0 Hz), 2.69 (2H, d, J = 7.4 Hz), 2.26–2.32 (1H, m), 1.83–1.88 (2H, m), 1.61–1.66 (2H, m), 1.52–1.56 (2H, m), 1.24–1.28 (2H, m). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 189.7, 171.3, 151.1, 135.7, 130.8, 128.0, 126.6, 123.7, 35.6, 31.9, 24.6. HRMS (ESI) m/z: 233.1169 found (calcd for C14H17O3+, [M + H]+ 233.1172).
- 2-Formylphenyl cyclopentanecarboxylate (3j). Yield, 1.42 g (65%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.05 (1H, s), 7.91 (1H, dd, J = 7.6, 1.6 Hz), 7.74–7.76 (1H, m), 7.49 (1H, t, J = 7.5 Hz), 7.30 (1H, d, J = 8.1 Hz), 3.14 (1H, quin, J = 7.9 Hz), 1.92–2.02 (4H, m), 1.61–1.70 (4H, m). The spectral properties corresponded to the literature data [61].
- 2-Formylphenyl benzoate (3k). Yield, 1.24 g (55%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.10 (1H, s), 8.17 (2H, dd, J = 8.3, 1.2 Hz), 7.99 (1H, dd, J = 7.7, 1.6 Hz), 7.80–7.83 (1H, m), 7.76–7.79 (1H, m), 7.62–7.65 (2H, m), 7.57 (1H, t, J = 7.5 Hz), 7.48 (1H, d, J = 8.0 Hz). The spectral properties corresponded to the literature data [62].
- 2-Formylphenyl 4-(2-methoxyethoxy)benzoate (3l). Yield, 1.38 g (46%); white oil. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.09 (1H, s), 8.11 (2H, m), 7.97 (1H, dd, J = 7.7, 1.6 Hz), 7.80 (1H, td, J = 7.7, 1.7 Hz), 7.54 (1H, t, J = 7.5 Hz), 7.45 (1H, d, J = 8.1 Hz), 7.15 (2H, m), 4.23–4.26 (2H, m), 3.70–3.72 (2H, m), 3.33 (3H, s). 13C NMR (201 MHz, DMSO-d6) δ, ppm: 189.5, 164.1, 163.1, 151.2, 135.6, 132.2, 131.0, 128.1, 126.6, 123.9, 120.6, 114.7, 70.1, 67.4, 58.2. HRMS (ESI) m/z: 301.1071 found (calcd for C17H17O5+, [M + H]+ 301.1071).
- Ethyl (2-formylphenyl) succinate (3p). Yield, 2.25 g (90%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.09 (1H, s), 7.90 (1H, dd, J = 7.7, 1.5 Hz), 7.77 (1H, td, J = 7.7, 1.6 Hz), 7.49 (1H, t, J = 7.5 Hz), 7.28 (1H, d, J = 8.1 Hz), 4.10 (2H, q, J = 7.1 Hz), 2.95 (2H, t, J = 6.6 Hz), 2.71 (2H, t, J = 6.6 Hz), 1.19 (3H, t, J = 7.1 Hz). The spectral properties corresponded to the literature data [63].
3.2.3. Synthesis of the Starting Compounds 3e–3g and 3m–3o
- 2-Formylphenyl isobutyrate (3e). Yield, 1.23 g (64%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.05 (1H, s), 7.92 (1H, dd, J = 7.6, 1.6 Hz), 7.74–7.77 (1H, m), 7.50 (1H, t, J = 7.5 Hz), 7.29 (1H, d, J = 8.1 Hz), 2.92 (1H, spt, J = 7.0 Hz), 1.28 (6H, d, J = 7.0 Hz). The spectral properties corresponded to the literature data [61].
- Tert-butyl (2-formylphenyl) carbonate (3f). Yield, 1.18 g (58%); white oil. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.04 (1H, s), 7.92 (1H, dd, J = 7.7, 1.6 Hz), 7.73–7.78 (1H, m), 7.47–7.53 (1H, m), 7.30 (1H, d, J = 8.1 Hz), 3.48–3.61 (1H, m), 2.37–2.43 (2H, m), 2.28–2.33 (2H, m), 1.99–2.04 (1H, m), 1.88–1.94 (1H, m). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 189.8, 173.1, 151.0, 135.6, 131.2, 128.0, 126.6, 123.7, 37.1, 24.6, 17.8. HRMS (ESI) m/z: 205.0854 found (calcd for C12H13O3+, [M + H]+ 205.0859).
- Bis(2-formylphenyl) glutarate (3g). Yield, 1.36 g (40%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.09 (2H, s), 7.93 (2H, dd, J = 7.7, 1.7 Hz), 7.76 (2H, td, J = 7.7, 1.7 Hz), 7.51 (2H, td, J = 7.5, 0.5 Hz), 7.30–7.36 (2H, m), 2.86 (4H, t, J = 7.4 Hz), 2.07 (2H, quin, J = 7.4 Hz). The spectral properties corresponded to the literature data [64].
- 2-Formylphenyl nicotinate (3m). Yield, 1.10 g (48%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.12 (1H, s), 9.27–9.31 (1H, m), 8.93 (1H, dd, J = 4.8, 1.6 Hz), 8.50 (1H, dt, J = 7.9, 1.9 Hz), 8.01 (1H, dd, J = 7.7, 1.6 Hz), 7.83 (1H, td, J = 7.7, 1.7 Hz), 7.67–7.69 (1H, m), 7.59 (1H, t, J = 7.5 Hz), 7.52 (1H, d, J = 8.1 Hz). The spectral properties corresponded to the literature data [65].
- 2-Formylphenyl isonicotinate (3n). Yield, 1.25 g (55%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.10 (1H, s), 8.91 (2H, dd, J = 4.6, 1.3 Hz), 8.04 (2H, dd, J = 4.4, 1.6 Hz), 8.02 (1H, dd, J = 7.7, 1.6 Hz), 7.83 (1H, td, J = 7.8, 1.7 Hz), 7.60 (1H, t, J = 7.5 Hz), 7.52 (1H, d, J = 8.0 Hz). The spectral properties corresponded to the literature data [66].
- 2-Formylphenyl furan-2-carboxylate (3o). Yield, 1.12 g (52%). 1H NMR (800 MHz, DMSO-d6) δ, ppm: 10.10 (1H, s), 8.06–8.18 (1H, m), 7.98 (1H, dd, J = 7.6, 1.6 Hz), 7.80 (1H, td, J = 7.8, 1.7 Hz), 7.63 (1H, d, J = 3.5 Hz), 7.56 (1H, t, J = 7.5 Hz), 7.47 (1H, d, J = 8.1 Hz), 6.83 (1H, dd, J = 3.5, 1.7 Hz). The spectral properties corresponded to the literature data [65].
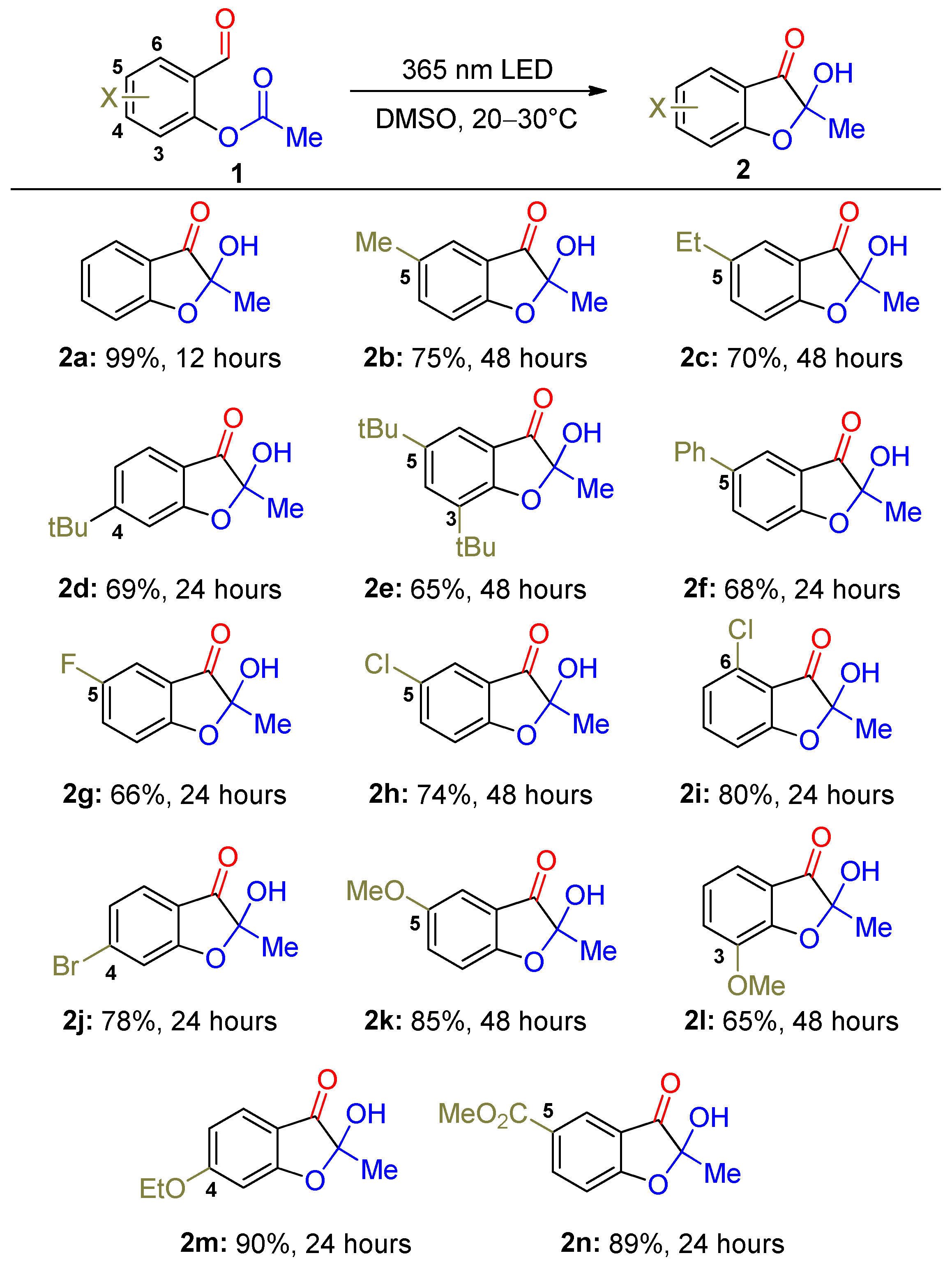
3.3. Photoinduced Synthesis of the 2-Hydroxy-Benzofuran-3(2H)-Ones 2 and 4
- 2-Hydroxy-2-methylbenzofuran-3(2H)-one (2a). Yield, 162 mg (99%). Reaction time, ~12 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.72–7.74 (1H, m), 7.69 (1H, s), 7.62 (1H, dd, J = 7.6, 0.8 Hz), 7.14 (1H, d, J = 8.3 Hz), 7.12 (1H, t, J = 7.4 Hz), 1.45 (3H, s). The spectral properties corresponded to the literature data [39].
- 2-Hydroxy-2,5-dimethylbenzofuran-3(2H)-one (2b). Yield, 134 mg (75%); yellow solid; m.p., 100–102 °C. Reaction time, ~48 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm m: 7.63 (1H, s), 7.55 (1H, dd, J = 8.4, 1.6 Hz), 7.41 (1H, s), 7.04 (1H, d, J = 8.4 Hz), 2.30 (3H, s), 1.43 (3H, s). 13C NMR (201 MHz, DMSO-d6) δ, ppm: 199.2, 167.8, 140.0, 131.0, 123.8, 118.3, 112.9, 104.3, 21.8, 20.0. HRMS (ESI) m/z: 179.0704 found (calcd for C10H11O3+, [M + H]+ 179.0703).
- 5-Ethyl-2-hydroxy-2-methylbenzofuran-3(2H)-one (2c). Yield, 134 mg (70%). Reaction time, ~48 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.62 (1H, s), 7.60 (1H, dd, J = 8.5, 2.0 Hz), 7.43 (1H, d, J = 1.4 Hz), 7.06 (1H, d, J = 8.4 Hz), 2.61 (2H, q, J = 7.6 Hz), 1.44 (3H, s), 1.17 (3H, t, J = 7.6 Hz). The spectral properties corresponded to the literature data [39].
- 6-(Tert-butyl)-2-hydroxy-2-methylbenzofuran-3(2H)-one (2d). Yield, 152 mg (69%); white solid; m.p., 48–50 °C. Reaction time, ~24 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.60 (1H, s), 7.54 (1H, d, J = 8.1 Hz), 7.19 (1H, d, J = 8.1 Hz), 7.11 (1H, s), 1.44 (3H, s), 1.30 (10H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 198.5, 169.9, 163.5, 124.1, 119.6, 116.0, 109.7, 104.4, 35.7, 30.6, 21.7. HRMS (ESI) m/z: 221.1172 found (calcd for C13H17O3+, [M + H]+ 221.1172).
- 5,7-Di-tert-butyl-2-hydroxy-2-methylbenzofuran-3(2H)-one (2e). Yield, 180 mg (65%); yellow solid; m.p., 138–140 °C. Reaction time, ~48 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.61 (1H, d, J = 2.1 Hz), 7.60 (1H, s), 7.37 (1H, d, J = 2.1 Hz), 1.45 (3H, s), 1.38 (9H, s), 1.29 (9H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 199.9, 166.4, 144.0, 134.7, 132.3, 118.3, 117.5, 103.8, 34.3, 34.0, 31.1, 28.9, 21.8. HRMS (ESI) m/z: 277.1803 found (calcd for C17H25O3+, [M + H]+ 277.1798).
- 2-Hydroxy-2-methyl-5-phenylbenzofuran-3(2H)-one (2f). Yield, 163 mg (68%); yellow solid; m.p., 121–123 °C. Reaction time, ~24 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 8.06 (1H, dd, J = 8.6, 2.0 Hz), 7.85 (1H, d, J = 2.0 Hz), 7.78 (1H, s), 7.66–7.70 (2H, m), 7.46 (2H, t, J = 7.7 Hz), 7.35–7.39 (1H, m), 7.25 (1H, d, J = 8.6 Hz), 1.50 (3H, s). 13C NMR (201 MHz, DMSO-d6) δ, ppm: 199.1, 168.9, 138.8, 137.8, 134.2, 129.0, 127.4, 126.5, 121.9, 119.1, 113.8, 104.9, 21.7. HRMS (ESI) m/z: 241.0859 found (calcd for C15H13O3+, [M + H]+ 241.0859).
- 5-Fluoro-2-hydroxy-2-methylbenzofuran-3(2H)-one (2g). Yield, 120 mg (66%). Reaction time, ~24 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.78 (1H, s), 7.63 (1H, td, J = 9.0, 2.9 Hz), 7.47 (1H, dd, J = 7.0, 2.9 Hz), 7.20 (1H, dd, J = 9.0, 3.7 Hz), 1.46 (3H, s). The spectral properties corresponded to the literature data [39].
- 5-Chloro-2-hydroxy-2-methylbenzofuran-3(2H)-one (2h). Yield, 147 mg (74%); yellow solid; m.p., 90–92 °C. Reaction time, ~48 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.84 (1H, s), 7.77 (1H, dd, J = 8.8, 2.4 Hz), 7.69 (1H, d, J = 2.4 Hz), 7.21 (1H, d, J = 8.8 Hz), 1.47 (3H, s). 13C NMR (201 MHz, DMSO-d6) δ, ppm: 198.3, 167.9, 138.6, 125.8, 123.8, 119.9, 115.3, 105.3, 21.6. HRMS (ESI) m/z: 199.0154 found (calcd for C9H8ClO3+, [M + H]+ 199.0156).
- 4-Chloro-2-hydroxy-2-methylbenzofuran-3(2H)-one (2i). Yield, 159 mg (80%); yellow solid; m.p., 80–82 °C. Reaction time, ~24 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.82 (1H, s), 7.70 (1H, t, J = 8.1 Hz), 7.14 (1H, d, J = 7.8 Hz), 7.12 (1H, d, J = 8.3 Hz), 1.47 (3H, s). 13C NMR (201 MHz, DMSO-d6) δ, ppm: 196.4, 170.2, 139.6, 130.5, 122.5, 115.5, 112.1, 104.7, 21.6. HRMS (ESI) m/z: 199.0157 found (calcd for C9H8ClO3+, [M + H]+ 199.0156).
- 6-Bromo-2-hydroxy-2-methylbenzofuran-3(2H)-one (2j). Yield, 190 mg (78%); white solid; m.p., 96–98 °C. Reaction time, ~24 h. 1H NMR (700 MHz, DMSO-d6) δ, ppm: 7.85 (1H, s), 7.57 (1H, dd, J = 8.1, 0.3 Hz), 7.50 (1H, d, J = 1.3 Hz), 7.30 (1H, dd, J = 8.1, 1.3 Hz), 1.47 (3H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 198.1, 169.6, 132.6, 126.1, 125.2, 117.9, 116.5, 105.3, 21.6. HRMS (ESI) m/z: 242.9648 found (calcd for C9H8BrO3+, [M + H]+ 242.9651).
- 2-Hydroxy-5-methoxy-2-methylbenzofuran-3(2H)-one (2k). Yield, 165 mg (85%); light green solid; m.p., 100–102 °C. Reaction time, ~48 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.63 (1H, s), 7.35 (1H, dd, J = 8.9, 2.8 Hz), 7.09 (1H, d, J = 8.9 Hz), 7.08 (1H, d, J = 2.8 Hz), 3.77 (3H, s), 1.43 (3H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 199.5, 164.6, 154.3, 128.1, 118.4, 114.3, 105.2, 104.7, 55.8, 21.8. HRMS (ESI) m/z: 195.0652 found (calcd for C10H11O4+, [M + H]+ 195.0652).
- 2-Hydroxy-7-methoxy-2-methylbenzofuran-3(2H)-one (2l). Yield, 126 mg (65%); yellow solid; m.p., 100–102 °C. Reaction time, ~48 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.72 (1H, s), 7.35 (1H, d, J = 7.8 Hz), 7.16 (1H, d, J = 7.7 Hz), 7.05 (1H, t, J = 7.8 Hz), 3.87 (3H, s), 1.45 (3H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 199.4, 159.6, 146.0, 122.2, 119.9, 119.4, 115.3, 104.6, 55.9, 21.7. HRMS (ESI) m/z: 195.0652 found (calcd for C10H11O4+, [M + H]+ 195.0652).
- 6-Ethoxy-2-hydroxy-2-methylbenzofuran-3(2H)-one (2m). Yield, 194 mg (90%); yellow solid; m.p., 78–80 °C. Reaction time, ~24 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.63 (1H, s), 7.50 (1H, d, J = 8.4 Hz), 6.65 (1H, d, J = 1.9 Hz), 6.62–6.65 (1H, m), 4.14 (2H, q, J = 7.0 Hz), 1.43 (3H, s), 1.35 (3H, t, J = 7.0 Hz). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 196.5, 171.9, 167.8, 125.8, 111.3, 111.2, 105.1, 96.8, 64.3, 21.9, 14.3. HRMS (ESI) m/z: 209.0813 found (calcd for C11H13O4+, [M + H]+ 209.0808).
- Methyl 2-hydroxy-2-methyl-3-oxo-2,3-dihydrobenzofuran-5-carboxylate (2n). Yield, 198 mg (89%); yellow solid; m.p., 88–90 °C. Reaction time, ~24 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 8.29 (1H, dd, J = 8.7, 1.8 Hz), 8.13 (1H, d, J = 1.8 Hz), 7.98 (1H, s), 7.28 (1H, d, J = 8.7 Hz), 3.86 (3H, s), 1.50 (3H, s). 13C NMR (201 MHz, DMSO-d6) δ, ppm: 198.3, 172.0, 165.1, 139.5, 126.1, 123.3, 118.8, 113.8, 106.0, 52.3, 21.5. HRMS (ESI) m/z: 223.0597 found (calcd for C11H11O5+, [M + H]+ 223.0601).
- 2-Hydroxy-2-(methoxymethyl)benzofuran-3(2H)-one (4a). Yield, 165 mg (85%); yellow solid; m.p., 98–100 °C. Reaction time, ~24 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.92 (1H, s), 7.72 (1H, ddd, J = 8.4, 7.2, 1.4 Hz), 7.59 (1H, dd, J = 7.6, 0.9 Hz), 7.17 (1H, d, J = 8.3 Hz), 7.08–7.12 (1H, m), 3.66–3.70 (1H, m), 3.61–3.65 (1H, m), 3.16 (3H, s). 13C NMR (201 MHz, DMSO-d6) δ, ppm: 198.6, 170.5, 138.9, 124.1, 121.7, 119.7, 113.0, 103.6, 73.4, 59.0. HRMS (ESI) m/z: 195.0650 found (calcd for C10H11O4+, [M + H]+ 195.0652).
- 2-Cyclohexyl-2-hydroxybenzofuran-3(2H)-one (4b). Yield, 139 mg (60%); yellow solid; m.p., 73–75 °C. Reaction time, ~24 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.70–7.72 (1H, m), 7.58 (1H, dd, J = 7.6, 0.8 Hz), 7.56 (1H, s), 7.15 (1H, d, J = 8.3 Hz), 7.09 (1H, t, J = 7.4 Hz), 1.91 (1H, d, J = 12.8 Hz), 1.78–1.81 (1H, m), 1.69–1.72 (1H, m), 1.57–1.63 (2H, m), 1.43 (1H, d, J = 13.0 Hz), 1.03–1.17 (5H, m). 13C NMR (201 MHz, DMSO-d6) δ, ppm: 200.1, 170.3, 138.9, 123.9, 121.6, 120.1, 112.9, 106.7, 43.2, 25.8, 25.6, 25.4, 25.2, 25.1. HRMS (ESI) m/z: 233.1173 found (calcd for C14H17O3+, [M + H]+ 233.1172).
- 2-Hydroxy-2-((neopentyloxy)methyl)benzofuran-3(2H)-one (4c). Yield, 155 mg (62%); white solid; m.p., 63–65 °C. Reaction time, ~12 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.88 (1H, s), 7.70 (1H, ddd, J = 8.3, 7.2, 1.4 Hz), 7.59 (1H, dd, J = 7.6, 0.8 Hz), 7.16 (1H, d, J = 8.3 Hz), 7.08 (1H, t, J = 7.4 Hz), 3.72–3.75 (1H, m), 3.67–3.70 (1H, m), 3.04 (1H, d, J = 8.6 Hz), 2.93 (1H, d, J = 8.6 Hz), 0.56 (9H, s). 13C NMR (201 MHz, DMSO-d6) δ, ppm: 199.0, 170.5, 138.7, 123.7, 121.5, 120.1, 112.7, 104.1, 81.3, 72.4, 31.6, 26.0. HRMS (ESI) m/z: 251.1279 found (calcd for C14H19O4+, [M + H]+ 251.1278).
- 2-Hydroxy-2-(4-phenylbutyl)benzofuran-3(2H)-one (4d). Yield, 212 mg (75%); yellow solid; m.p., 78–80 °C. Reaction time, ~24 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.72 (1H, ddd, J = 8.3, 7.2, 1.4 Hz), 7.64 (1H, s), 7.60 (1H, dd, J = 7.6, 0.8 Hz), 7.24 (2H, t, J = 7.6 Hz), 7.12–7.16 (4H, m), 7.11 (1H, t, J = 7.4 Hz), 1.82–1.87 (1H, m), 1.79 (1H, ddd, J = 14.0, 11.1, 5.2 Hz), 1.50–1.55 (2H, m), 1.36–1.40 (1H, m), 1.15–1.24 (2H, m), 0.73–0.88 (1H, m). 13C NMR (201 MHz, DMSO-d6) δ, ppm: 199.5, 169.9, 142.0, 139.0, 128.2, 128.1, 125.6, 124.3, 121.7, 119.3, 113.1, 105.6, 35.0, 34.9, 30.9, 21.7. HRMS (ESI) m/z: 283.1322 found (calcd for C18H19O3+, [M + H]+ 283.1329).
- 2-Hydroxy-2-isopropylbenzofuran-3(2H)-one (4e). Yield, 173 mg (90%); yellow solid; m.p., 58–60 °C. Reaction time, ~24 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.68–7.74 (1H, m), 7.53–7.64 (2H, m), 7.17 (1H, d, J = 8.3 Hz), 7.10 (1H, t, J = 7.4 Hz), 2.05–2.12 (1H, m), 0.99 (3H, d, J = 6.8 Hz), 0.78 (3H, d, J = 6.8 Hz). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 200.1, 170.4, 139.0, 124.1, 121.6, 120.1, 112.9, 107.0, 33.4, 15.9, 15.5. HRMS (ESI) m/z: 193.0859 found (calcd for C11H13O3+, [M + H]+ 193.0859).
- 2-Cyclobutyl-2-hydroxybenzofuran-3(2H)-one (4f). Yield, 198 mg (97%); yellow solid; m.p., 62–64 °C. Reaction time, ~24 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.73 (1H, ddd, J = 8.4, 7.2, 1.5 Hz), 7.62 (1H, s), 7.59 (1H, dd, J = 7.6, 0.9 Hz), 7.19 (1H, d, J = 8.3 Hz), 7.05–7.13 (1H, m), 2.67–2.76 (1H, m), 2.09–2.15 (1H, m), 1.94–1.99 (1H, m), 1.80–1.87 (2H, m), 1.70–1.76 (2H, m). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 199.4, 170.1, 139.1, 124.2, 121.7, 119.9, 113.1, 105.7, 22.0, 21.0, 17.5. HRMS (ESI) m/z: 205.0858 found (calcd for C12H13O3+, [M + H]+ 205.0859).
- 2,2′-(Propane-1,3-diyl)bis(2-hydroxybenzofuran-3(2H)-one) (4g). Yield, 238 mg (70%); white solid; m.p., 128–130 °C. Reaction time, ~24 h. 1H NMR (800 MHz, DMSO-d6) δ, ppm: 7.69–7.73 (2H, m), 7.65 (2H, d, J = 2.2 Hz), 7.59 (2H, d, J = 7.6 Hz), 7.13 (2H, dd, J = 8.3, 3.4 Hz), 7.10 (2H, t, J = 7.4 Hz), 1.76–1.81 (2H, m), 1.70–1.75 (2H, m), 1.36–1.41 (1H, m), 1.14–1.26 (1H, m). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 199.4, 169.8, 139.1, 124.4, 121.8, 119.2, 113.2, 105.4, 34.9, 15.5. HRMS (ESI) m/z: 341.1018 found (calcd for C19H17O6+, [M + H]+ 341.1020).
- 2-(tert-butyl)-2-hydroxybenzofuran-3(2H)-one (4h). Yield, 134 mg (65%); yellow oil. Reaction time, ~24 h. 1H NMR (300 MHz, DMSO-d6) δ, ppm: 7.66–7.75 (1H, m) 7.61–7.56 (2H, m) 7.15 (1H, d, J = 8.3 Hz) 7.08 (1H, t, J = 7.4 Hz) 0.97 (9H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 200.1, 170.3, 138.9, 124.0, 121.5, 120.5, 112.8, 108.1, 37.3, 23.8. HRMS (ESI) m/z: 207.1019 found (calcd for C12H15O3+, [M + H]+ 207.1016).
3.4. Synthesis of the Heterocyclic Derivatives 5 and 6
3.4.1. Synthesis of the Heterocyclic Derivatives 5
- 4-Methyl-2-(3-methylquinoxalin-2-yl)phenol (5b). Yield, 17 mg (99%); pale yellow solid; m.p., 190–192 °C. 1H NMR (300 MHz, DMSO-d6) δ, ppm: 9.63 (1H, s), 7.96–8.10 (2H, m), 7.71–7.87 (2H, m), 7.07–7.18 (2H, m), 6.88 (1H, d, J = 8.1 Hz), 2.57 (3H, s), 2.27 (3H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 154.1, 154.0, 152.4, 140.6, 140.3, 130.7, 130.7, 129.6, 129.1, 128.7, 128.1, 127.8, 126.1, 115.6, 22.7, 20.0. HRMS (ESI) m/z: 251.1180 found (calcd for C16H15N2O+, [M + H]+ 251.1179).
- 2-(3-Cyclobutylquinoxalin-2-yl)phenol (5d). Yield, 19 mg (97%); yellow oil. 1H NMR (300 MHz, DMSO-d6) δ, ppm: 9.73 (1H, s), 8.11 (1H, dd, J = 7.7, 1.3 Hz), 8.04 (1H, dd, J = 7.7, 1.4 Hz), 7.74–7.88 (2H, m), 7.29–7.38 (1H, m), 7.20–7.28 (1H, m), 6.88–7.03 (2H, m), 3.87 (1H, quin, J = 8.5 Hz), 2.29–2.45 (2H, m), 1.68–2.06 (4H, m). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 158.7, 154.8, 153.3, 140.7, 140.3, 130.3, 130.1, 129.6, 129.1, 128.7, 128.4, 126.2, 119.0, 115.5, 39.0, 27.2, 17.4. HRMS (ESI) m/z: 277.1333 found (calcd for C18H17N2O+, [M + H]+ 277.1335).
- 2-(3-(4-Phenylbutyl)quinoxalin-2-yl)phenol (5f). Yield, 23 mg (93%); yellow oil. 1H NMR (300 MHz, DMSO-d6) δ, ppm: 9.81 (1H, s), 7.96–8.13 (2H, m), 7.72–7.88 (2H, m), 7.30–7.40 (1H, m), 7.17–7.30 (3H, m), 7.03–7.17 (3H, m), 6.88–7.03 (2H, m), 2.91 (2H, t, J = 7.5 Hz), 2.43–2.49 (2H, m), 1.59–1.74 (2H, m), 1.43–1.57 (2H, m). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 157.1, 154.6, 153.9, 142.0, 140.7, 140.3, 130.5, 130.2, 129.7, 129.1, 128.7, 128.2 (4C), 128.2, 126.3, 125.6, 119.2, 115.6, 34.8, 34.5, 30.6, 27.1. HRMS (ESI) m/z: 355.1804 found (calcd for C24H23N2O+, [M + H]+ 355.1805).
3.4.2. Synthesis of the Heterocyclic Derivative 6
- 2-(2-(4-Bromophenyl)-5-methyl-1H-imidazol-4-yl)-4-methylphenol (6b). Yield, 30 mg (65%); pale yellow solid; m.p., 230–232 °C. 1H NMR (300 MHz, DMSO-d6) δ, ppm: 12.89 (1H, br. s.), 12.20 (1H, s), 7.85 (2H, m, J = 8.5 Hz), 7.71 (2H, m, J = 8.5 Hz), 7.30 (1H, s), 6.92 (1H, d, J = 7.9 Hz), 6.76 (1H, d, J = 8.2 Hz), 2.54 (3H, s), 2.26 (3H, s). 13C NMR (75 MHz, DMSO-d6) δ, ppm: 153.5, 140.5, 134.9, 132, 128.3, 128.0, 127.0, 126.5, 126.0, 124.7, 121.5, 118.1, 116.3, 20.4, 12.0. HRMS (ESI) m/z: 329.0280 found (calcd for C16H14BrN2O+, [M + H]+ 329.0284).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machida, K.; Trifonov, L.S.; Ayer, W.A.; Lu, Z.X.; Laroche, A.; Huang, H.C.; Cheng, K.J.; Zantige, J.L. 3(2H)-Benzofuranones and Chromanes from Liquid Cultures of the Mycoparasitic Fungus Coniothyrium Minitans. Phytochemistry 2001, 58, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Ningsih, B.N.S.; Rukachaisirikul, V.; Pansrinun, S.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. New Aromatic Polyketides from the Marine-Derived Fungus Pseudopithomyces Maydicus PSU-AMF350 and Their Antimicrobial Activity. Nat. Prod. Res. 2022, 36, 4982–4989. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-X.; Huang, C.-Y.; Yan, Z.-Y.; Chen, T.; Hong, K.; Long, Y.-H. New furo [3,2-H] isochroman from the Mangrove Endophytic Fungus Aspergillus sp. 085242. Chin. J. Nat. Med. 2020, 18, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Bunbamrung, N.; Intaraudom, C.; Boonyuen, N.; Rachtawee, P.; Laksanacharoen, P.; Pittayakhajonwut, P. Penicisochromans from the Endophytic Fungus Penicillium sp. BCC18034. Phytochem. Lett. 2014, 10, 13–18. [Google Scholar] [CrossRef]
- You, F.; Han, T.; Wu, J.-Z.; Huang, B.-K.; Qin, L.-P. Antifungal Secondary Metabolites from Endophytic Verticillium sp. Biochem. Syst. Ecol. 2009, 37, 162–165. [Google Scholar] [CrossRef]
- Ly, T.N.; Hazama, C.; Shimoyamada, M.; Ando, H.; Kato, K.; Yamauchi, R. Antioxidative Compounds from the Outer Scales of Onion. J. Agric. Food Chem. 2005, 53, 8183–8189. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, T.; Osada, H.; Uzawa, J.; Isono, K. Actiketal, a New Member of the Glutarimide Antibiotics. J. Antibiot. 1991, 44, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Schwitalla, J.W.; Benndorf, R.; Baunach, M.; Steinbeck, C.; Görls, H.; de Beer, Z.W.; Regestein, L.; Beemelmanns, C. Gene Cluster Activation in a Bacterial Symbiont Leads to Halogenated Angucyclic Maduralactomycins and Spirocyclic Actinospirols. Org. Lett. 2020, 22, 2634–2638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Fu, P.; Zhang, Y.; Liu, X.; Ren, F.; Che, Y. Sporulosol, a New Ketal from the Fungus Paraconiothyrium Sporulosum. Molecules 2018, 23, 1263. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Li, M.-K.; Luo, J.-F.; Tu, Z.-C.; Cheng, Y.-X. COX-2 and JAK3 Inhibitory Meroterpenoids from the Mushroom Ganoderma Theaecolum. Tetrahedron 2018, 74, 4259–4265. [Google Scholar] [CrossRef]
- Luo, Q.; Tu, Z.-C.; Yang, Z.-L.; Cheng, Y.-X. Meroterpenoids from the Fruiting Bodies of Ganoderma Theaecolum. Fitoterapia 2018, 125, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Hasegawa, T.; Higashide, E. Tolypomycin, a New Antibiotic. I. J. Antibiot. 1971, 24, 810–816. [Google Scholar] [CrossRef][Green Version]
- Traxler, P.; Schupp, T.; Fuhrer, H.; Richter, W.J. 3-Hydroxyrifamycin S and Further Novel Ansamycins from a Recombinant Strain R-21 of Nocardia Mediterranei. J. Antibiot. 1981, 34, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.J.; Fu, Y.; Yan, G.H.; Bao, G.H.; Xu, C.F.; He, C.H. Isolation and Structure of a New Ansamycin Antibiotic Kanglemycin A from a Nocardia. J. Antibiot. 1988, 41, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.S.; Zhang, B.; Zhang, M.; Guo, Z.K.; Deng, X.Z.; Shi, J.; Li, W.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Rifamorpholines A-E, Potential Antibiotics from Locust-Associated Actinobacteria Amycolatopsis Sp. Hca4. Org. Biomol. Chem. 2017, 15, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Dalisay, D.S.; Chen, J.; Polishchuck, E.A.; Patrick, B.O.; Narula, G.; Ko, M.; Av-Gay, Y.; Li, H.; Magarvey, N.; et al. Aminorifamycins and Sporalactams Produced in Culture by a Micromonospora sp. Isolated from a Northeastern-Pacific Marine Sediment Are Potent Antibiotics. Org. Lett. 2017, 19, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef] [PubMed]
- Bach, T.; Hehn, J.P. Photochemical Reactions as Key Steps in Natural Product Synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 1000–1045. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.; Bracken, C.; Baumann, M. Continuous Flow Photochemistry for the Preparation of Bioactive Molecules. Molecules 2020, 25, 356. [Google Scholar] [CrossRef] [PubMed]
- Dietliker, K.; Braig, A.; Ricci, A. Industrial Applications of Photochemistry: Automotive Coatings and beyond. In Photochemistry; The Royal Society of Chemistry: Cambridge, UK, 2010; pp. 344–368. ISBN 9781847550545. [Google Scholar]
- Moschetta, E.G.; Cook, G.C.; Edwards, L.J.; Ischay, M.A.; Lei, Z.; Buono, F.; Lévesque, F.; Garber, J.A.O.; MacTaggart, M.; Sezen-Edmonds, M.; et al. Photochemistry in Pharmaceutical Development: A Survey of Strategies and Approaches to Industry-Wide Implementation. Org. Process Res. Dev. 2024, 28, 831–846. [Google Scholar] [CrossRef]
- Rehm, T.H. Flow Photochemistry as a Tool in Organic Synthesis. Chemistry 2020, 26, 16952–16974. [Google Scholar] [CrossRef] [PubMed]
- Buglioni, L.; Raymenants, F.; Slattery, A.; Zondag, S.D.A.; Noël, T. Technological Innovations in Photochemistry for Organic Synthesis: Flow Chemistry, High-Throughput Experimentation, Scale-Up, and Photoelectrochemistry. Chem. Rev. 2022, 122, 2752–2906. [Google Scholar] [CrossRef] [PubMed]
- Protti, S.; Fagnoni, M. The Sunny Side of Chemistry: Green Synthesis by Solar Light. Photochem. Photobiol. Sci. 2009, 8, 1499–1516. [Google Scholar] [CrossRef] [PubMed]
- Oelgemöller, M. Solar Photochemical Synthesis: From the Beginnings of Organic Photochemistry to the Solar Manufacturing of Commodity Chemicals. Chem. Rev. 2016, 116, 9664–9682. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.E.; Joyce, K.; Nolan, K.; Oelgemöller, M. Green photochemistry: The use of microemulsions as green media in photooxygenation reactions. Green Chem. 2010, 12, 1544–1547. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Jung, C.; Ortner, J.; Mattay, J.; Zimmermann, E. Green Photochemistry: Solar Photooxygenations with Medium Concentrated Sunlight. Green Chem. 2005, 7, 35–38. [Google Scholar] [CrossRef]
- Oelgemöller, M. Green Photochemical Processes and Technologies for Research & Development, Scale-up and Chemical Production. J. Chin. Chem. Soc. 2014, 61, 743–748. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Jung, C.; Mattay, J. Green Photochemistry: Production of Fine Chemicals with Sunlight. Pure Appl. Chem. 2007, 79, 1939–1947. [Google Scholar] [CrossRef]
- Wang, S.; König, B. Catalytic Generation of Carbanions through Carbonyl Umpolung. Angew. Chem. Int. Ed. Engl. 2021, 60, 21624–21634. [Google Scholar] [CrossRef] [PubMed]
- White, A.M.; Palombi, I.R.; Malins, L.R. Umpolung Strategies for the Functionalization of Peptides and Proteins. Chem. Sci. 2022, 13, 2809–2823. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Enders, D. Radical Umpolung: Efficient Options for the Synthesis of 1,4-Dicarbonyl Compounds. Angew. Chem. Int. Ed. Engl. 2019, 58, 6488–6490. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-J.; Zhang, Y.; Huang, H.-M. Radical Umpolung Chemistry Enabled by Dual Catalysis: Concept and Recent Advances. Catal. Sci. Technol. 2022, 12, 5241–5251. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Wang, Q.; Wang, L. Photochemical-, Electrochemical-, and Photoelectrochemical- Catalyzed Hydrogen Atom Transfer from Aldehydes to Acyl Radicals and Their Transformations. European J. Org. Chem. 2025, 28, e202401206. [Google Scholar] [CrossRef]
- Tanaka, I.; Sawamura, M.; Shimizu, Y. Visible Light-Induced Reductive Alkynylation of Aldehydes by Umpolung Approach. Org. Lett. 2022, 24, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.-X.; Chen, S.-S.; Huang, H.-M. Catalytic Aldehyde-Alkyne Couplings Triggered by Ketyl Radicals. Org. Lett. 2024, 26, 9949–9954. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Ouyang, Y.-J.; Zheng, H.; Liu, H.; Wei, W.-T. Recent Advances in Acyl Radical Enabled Reactions between Aldehydes and Alkenes. Chem. Commun. 2021, 57, 6111–6120. [Google Scholar] [CrossRef] [PubMed]
- Esposti, S.; Dondi, D.; Fagnoni, M.; Albini, A. Acylation of Electrophilic Olefins through Decatungstate-Photocatalyzed Activation of Aldehydes. Angew. Chem. Int. Ed. Engl. 2007, 46, 2531–2534. [Google Scholar] [CrossRef] [PubMed]
- Zhigileva, E.A.; Opryshko, V.E.; Eshtukov-Shcheglov, A.V.; Ivanov, D.S.; Rudik, D.I.; Mikhaylov, A.A.; Ivanov, I.A.; Smirnov, A.Y.; Baranov, M.S. Photochemistry of 2-(2-Formylphenyloxy)acetic Acid Derivatives: Synthesis of Hydroxychromanones and Benzofuranones. Org. Biomol. Chem. 2024, 22, 7848–7853. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Sun, Y.; Ye, Y. Iodine(III)-Mediated Tandem Acetoxylation-Cyclization of O-Acyl Phenols for the Facile Construction of Alpha-Acetoxy Benzofuranones. Org. Lett. 2009, 11, 5174–5177. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.D.; Davidson, W.J.; Elix, J.A.; Leppik, R.A. Annelated Furans. VI. Diels-Alder Addition to 3-Methoxy-2-Vinylbenzofurans. Aust. J. Chem. 1971, 24, 1883. [Google Scholar] [CrossRef]
- Zaitseva, E.R.; Opryshko, V.E.; Ivanov, D.S.; Mikhaylov, A.A.; Smirnov, A.Y.; Baranov, M.S. Synthesis of Chroman-Annulated Cyclopropanols via Photoinduced Intramolecular [2 + 1]-Cycloaddition of 2-Allyloxybenzaldehydes. Org. Biomol. Chem. 2023, 21, 9082–9085. [Google Scholar] [CrossRef] [PubMed]
- Harron, J.; McClelland, R.A.; Thankachan, C.; Tidwell, T.T. Kinetic Study of the Reversible Formation of Cyclic Hemiacetals from 2-(hydroxymethyl)benzaldehyde and 2-(.beta.-Hydroxyethyl)benzaldehyde. J. Org. Chem. 1981, 46, 903–910. [Google Scholar] [CrossRef]
- Dantas, J.A.; Correia, J.T.M.; Paixão, M.W.; Corrêa, A.G. Photochemistry of Carbonyl Compounds: Application in Metal-free Reactions. ChemPhotoChem 2019, 3, 506–520. [Google Scholar] [CrossRef]
- Nechab, M.; Mondal, S.; Bertrand, M.P. 1,n-Hydrogen-Atom Transfer (HAT) Reactions in Which n≠5: An Updated Inventory. Chem. Eur. J. 2014, 20, 16034–16059. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Li, C.-J.; Zeng, H. Photoinduced transition-metal- and external photosensitizer-free intramolecular aryl rearrangement via C(Ar)–O bond cleavage. Chem. Sci. 2020, 11, 5740–5744. [Google Scholar] [CrossRef] [PubMed]
- Zipse, H. Radical Stability—A Theoretical Perspective. In Radicals in Synthesis I. Topics in Current Chemistry; Gansäuer, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 263. [Google Scholar] [CrossRef]
- Škalamera, Đ.; Mlinarić-Majerski, K.; Kleiner, M.K.; Kralj, M.; Oake, J.; Wan, P.; Bohne, C.; Basarić, N. Photochemical Formation of Anthracene Quinone Methide Derivatives. J. Org. Chem. 2017, 82, 6006–6021. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, H.E. The Meta Effect in Organic Photochemistry: Mechanistic and Exploratory Organic Photochemistry. J. Am. Chem. Soc. 1995, 117, 8988–8991. [Google Scholar] [CrossRef]
- Pham, H.; Hunsley, M.; Yang, C.-H.; Wang, H.; Reed, S.M. Demonstration of a Stereospecific Photochemical Meta Effect. Photochem 2022, 2, 69–76. [Google Scholar] [CrossRef]
- Hilborn, J.W.; MacKnight, E.; Pincock, J.A.; Wedge, P.J. Photochemistry of Substituted Benzyl Acetates and Benzyl Pivalates: A Reinvestigation of Substituent Effects. J. Am. Chem. Soc. 1994, 116, 3337–3346. [Google Scholar] [CrossRef]
- Roy, S.; Laha, J.; Reja, A.; Das, D. Allosteric Control of the Catalytic Properties of Dipeptide-Based Supramolecular Assemblies. J. Am. Chem. Soc. 2024, 146, 22522–22529. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.M.; Jursic, B.S. Preparation of Antimicrobial Pyridinohydrazide and Hydrazomethylpyridine-Based Agents. World Intellectual Property Organization. U.S. Patent 15/561,654, 25 March 2016. [Google Scholar]
- Vol’eva, V.B.; Belostotskaya, I.S.; Komissarova, N.L.; Kurkovskaya, L.N. Anionic condensations of 3,5-di-tert-butyl-4(2)-hydroxybenzaldehydes in the presence of weak bases. Rus. J. Org. Chem. 2008, 44, 803–806. [Google Scholar] [CrossRef]
- Karageorgis, G.; Reckzeh, E.S.; Ceballos, J.; Schwalfenberg, M.; Sievers, S.; Ostermann, C.; Pahl, A.; Ziegler, S.; Waldmann, H. Chromopynones are pseudo natural product glucose uptake inhibitors targeting glucose transporters GLUT-1 and -3. Nature Chem. 2018, 10, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Ledeboer, M.W.; Daniels, M.H.; Yu, M.; Harmange, J.-C.P. Pyridazinones as TRPC4 Inhibitors and Their Preparation; World Intellectual Property Organization: Geneva, Switzerland, 2020. [Google Scholar]
- De Assis, F.F.; Huang, X.; Akiyama, M.; Pilli, R.A.; Meggers, E. Visible-Light-Activated Catalytic Enantioselective β-Alkylation of α,β-Unsaturated 2-Acyl Imidazoles Using Hantzsch Esters as Radical Reservoirs. J. Org. Chem. 2018, 83, 10922–10932. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; van Lier, J.E. Molecular iodine in isopropenyl acetate (IPA): A highly efficient catalyst for the acetylation of alcohols, amines and phenols under solvent free conditions. Tetrahedron Lett. 2006, 47, 5345–5349. [Google Scholar] [CrossRef]
- Kleebauer, L.; Zborovsky, L.; Hommernick, K.; Seidel, M.; Weston, J.B.; Süssmuth, R.D. Overcoming AlbD Protease Resistance and Improving Potency: Synthesis and Bioactivity of Antibacterial Albicidin Analogues with Amide Bond Isosteres. Org. Lett. 2021, 23, 7023–7027. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.W.; Zhang, Y.; Zhao, F.; Ai, H.J.; Wu, X.F. Phosphine-catalyzed photo-induced alkoxycarbonylation of alkyl iodides with phenols and 1,4-dioxane through charge-transfer complex. Chin. J. Catal. 2023, 48, 214–223. [Google Scholar] [CrossRef]
- Pattenden, G.; Tankard, M. Acylcobalt salophen complexes. Homolytic decomposition to salicyl aldehyde esters. J. Organomet. Chem. 1993, 460, 237–239. [Google Scholar] [CrossRef]
- Anil, S.M.; Vinayaka, A.C.; Rajeev, N.; Swaroop, T.R.; Mallesha, N.; Rangappa, K.S.; Sadashiva, M.P. Aqueous Chloroplatinic Acid: A Green, Chemoselective and Reusable Catalyst for the Deprotection of Acetals, Ketals, Dioxolanes and Oxathiolanes. ChemistrySelect 2018, 3, 1999–2003. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Saitoh, M.; Kawase, Y. Some fatty acids having an O-heterocycle in their terminal positions. II. ω-(3-Coumarinyl)alkanoic acids and ω-(2-chromonyl)alkanoic acids. J. Heterocycl. Chem. 1991, 28, 125–127. [Google Scholar] [CrossRef]
- Perwin, A.; Mazumdar, N. Synthesis of O-acyl salicylaldehyde derivatives and copolymerization of bis-(2-formylphenyl) fumarate with methyl methacrylate. J. Mol. Struct. 2024, 1304, 137690. [Google Scholar] [CrossRef]
- Zheng, Y.; Song, W.-B.; Xuan, L.-J. Copper-catalyzed oxidative esterification of ortho-formyl phenols without affecting labile formyl group. Tetrahedron Lett. 2015, 56, 4569–4573. [Google Scholar] [CrossRef]
- Akishina, E.A.; Kazak, D.V.; Dikusar, E.A.; Zalesskaya, E.G.; Zhukovskaya, N.A.; Stepin, S.G. Heterocyclic Derivatives of 4-Amino-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one. Russ. J. Gen. Chem. 2020, 90, 1432–1438. [Google Scholar] [CrossRef]
- Wu, X.A.; Ying, P.; Liu, J.Y.; Shen, H.S.; Chen, Y.; He, L. Lithium Chloride–Assisted Selective Hydrolysis of Methyl Esters Under Microwave Irradiation. Synth. Commun. 2009, 39, 3459–3470. [Google Scholar] [CrossRef]


| No. | Solvent | Remaining 1a, % | 2a, % | 2-Acetoxybenzoic Acid, % |
|---|---|---|---|---|
| 1 | CCl4 | 0 | 0 | 90 |
| 2 | DMSO | <1 | 99 | 0 |
| 3 | DMF | <1 | 8 * | 0 |
| 4 | DMAC | <1 | 11 * | 0 |
| 5 | PhMe | 9 | 0 * | 0 |
| 6 | DCM | 9 | 0 | 67 |
| 7 | MeOH | 2 | 0 * | 0 |
| 8 | THF | 0 | 0 * | 0 |
| 9 | acetone | 0 | 0 | 53 |
| 10 | TEA | 0 | 0 * | 0 |
| 11 | (CF3)2CHOH | 92 | 0 | 0 |
| 12 | CH3CN | 0 | 0 | 76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opryshko, V.E.; Krasnova, S.A.; Mikhaylov, A.A.; Bogdanova, Y.A.; Smirnov, A.Y.; Baranov, M.S.; Ivanov, D.S. Photochemically-Enabled Umpolung Conversion of 2-Acyloxybenzaldehydes into 2-Hydroxybenzofuranones. Molecules 2025, 30, 3080. https://doi.org/10.3390/molecules30153080
Opryshko VE, Krasnova SA, Mikhaylov AA, Bogdanova YA, Smirnov AY, Baranov MS, Ivanov DS. Photochemically-Enabled Umpolung Conversion of 2-Acyloxybenzaldehydes into 2-Hydroxybenzofuranones. Molecules. 2025; 30(15):3080. https://doi.org/10.3390/molecules30153080
Chicago/Turabian StyleOpryshko, Victoria E., Svetlana A. Krasnova, Andrey A. Mikhaylov, Yulia A. Bogdanova, Alexander Yu. Smirnov, Mikhail S. Baranov, and Dmitrii S. Ivanov. 2025. "Photochemically-Enabled Umpolung Conversion of 2-Acyloxybenzaldehydes into 2-Hydroxybenzofuranones" Molecules 30, no. 15: 3080. https://doi.org/10.3390/molecules30153080
APA StyleOpryshko, V. E., Krasnova, S. A., Mikhaylov, A. A., Bogdanova, Y. A., Smirnov, A. Y., Baranov, M. S., & Ivanov, D. S. (2025). Photochemically-Enabled Umpolung Conversion of 2-Acyloxybenzaldehydes into 2-Hydroxybenzofuranones. Molecules, 30(15), 3080. https://doi.org/10.3390/molecules30153080