Recent Advances on Biomass-Derived Carbon Materials-Based Electrochemical Sensors
Abstract
1. Introduction
2. Preparation of Biomass-Derived Materials
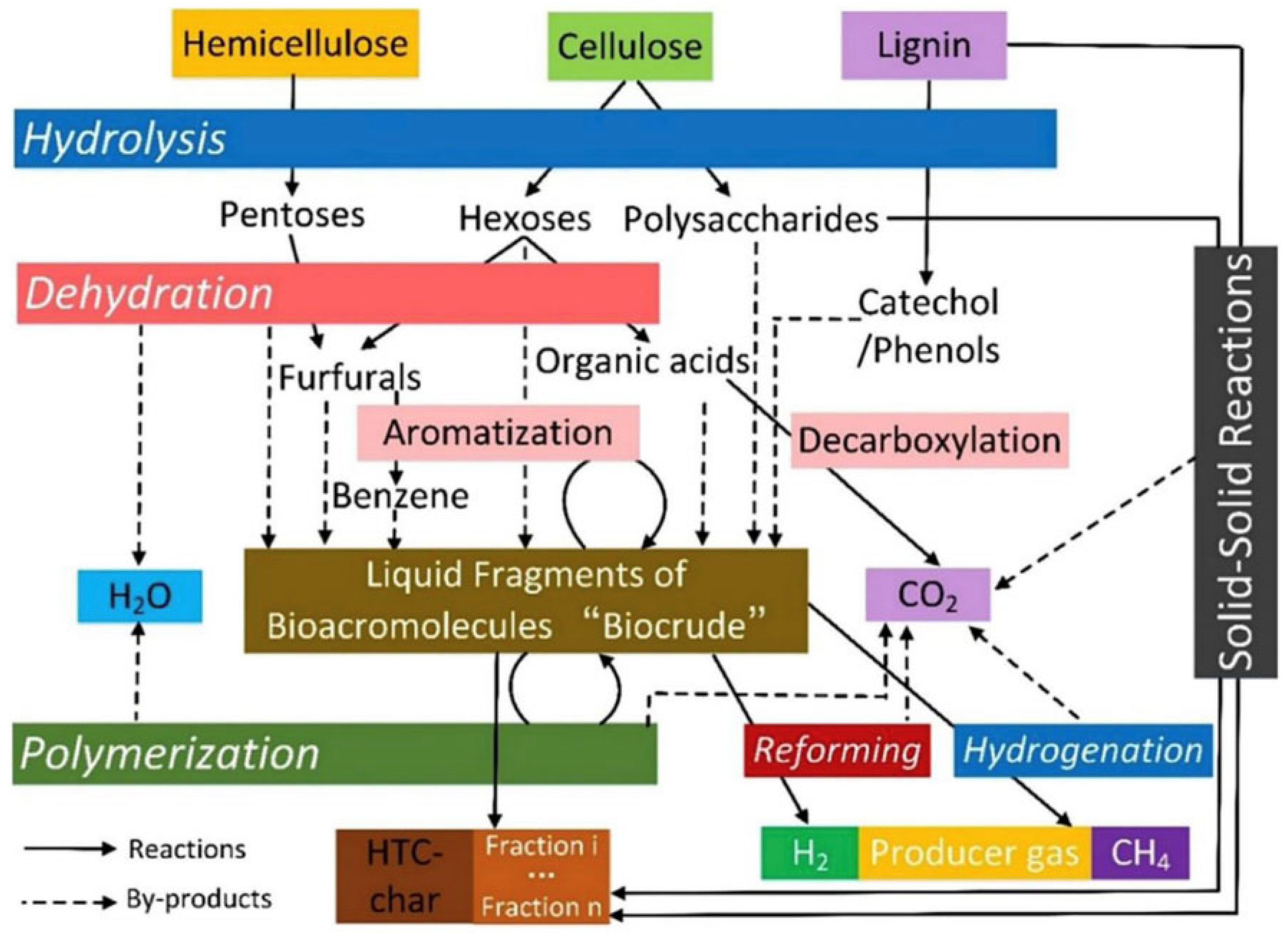
2.1. Pyrolysis Carbonization Method
2.2. Hydrothermal Carbonization
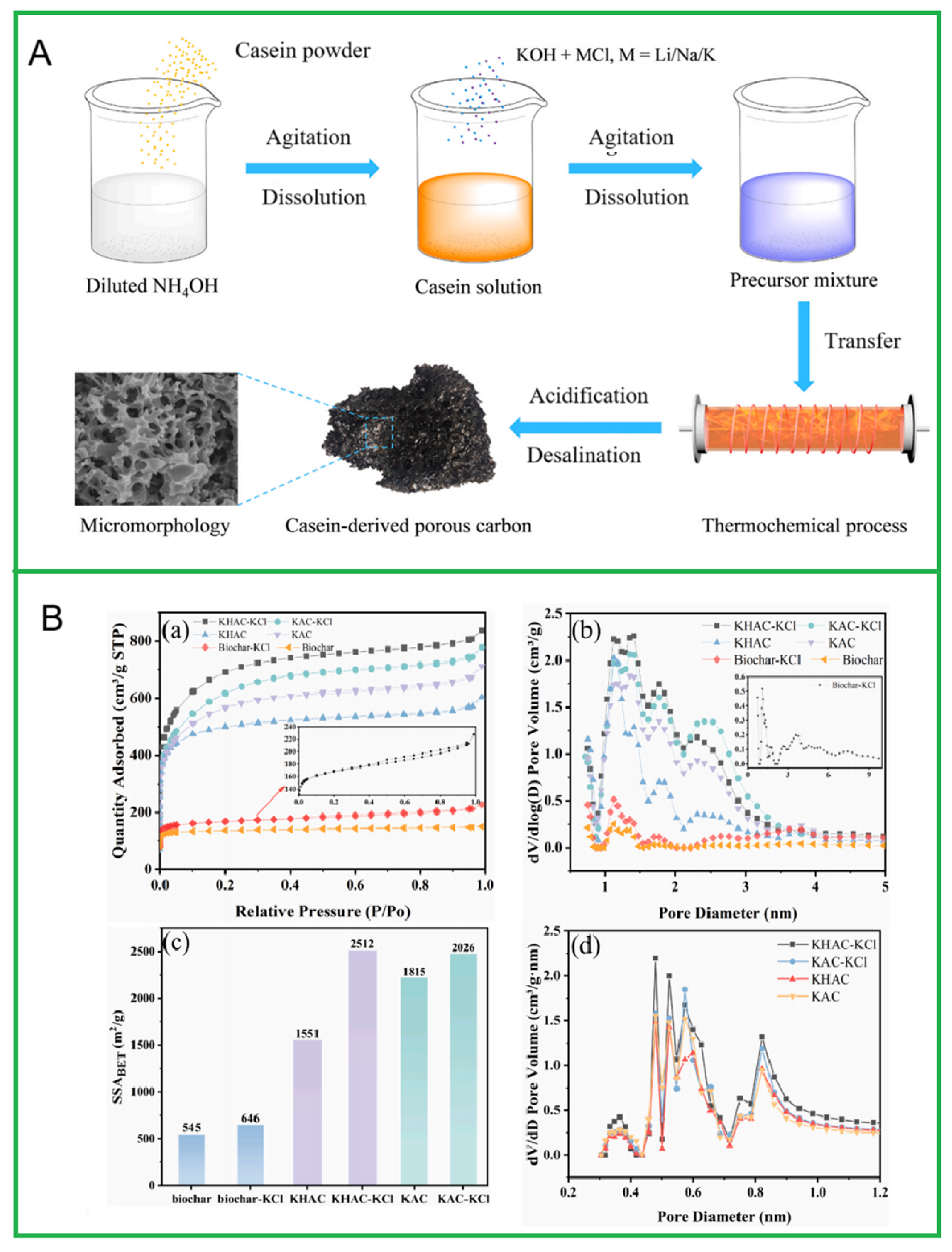
2.3. Molten Salt Carbonization Method
2.4. Other Carbonization Methods
3. Application of BDCM-Based Materials in Electrochemical Sensors
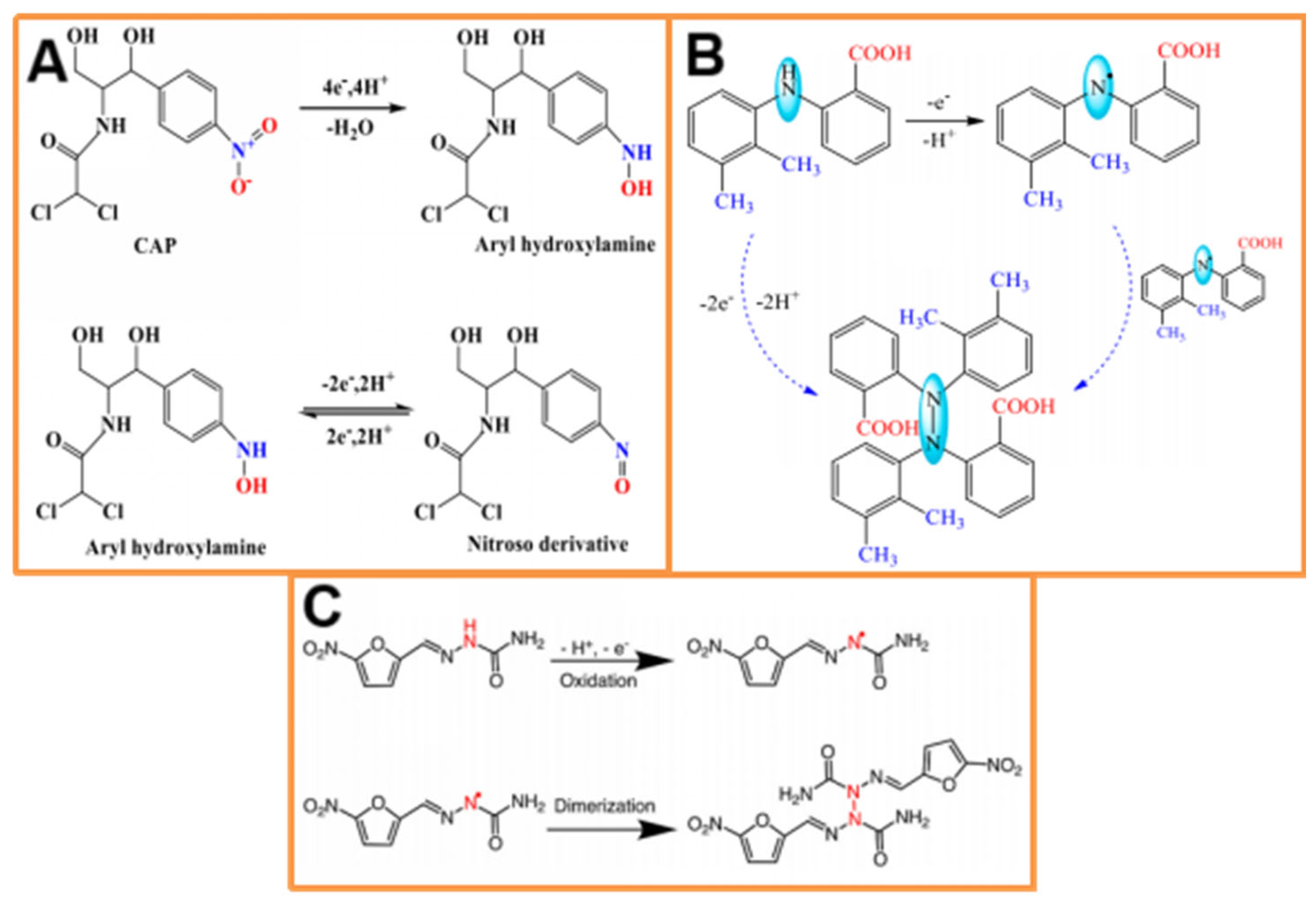
3.1. Application in Environmental Pollutant Analysis
3.1.1. Application in Organic Pollutant Analysis
3.1.2. Application in Heavy Metal Analysis
3.1.3. Application in Environmental Microplastic Analysis
3.2. Application in Drug Analysis
3.3. Application in Biomolecular Analysis
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakker, E.; Telting-Diaz, M. Electrochemical sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef]
- Hao, X.; Song, W.; Wang, Y.; Qin, J.; Jiang, Z. Recent advancements in electrochemical sensors based on MOFs and their derivatives. Small 2025, 21, 2408624. [Google Scholar] [CrossRef]
- Gibi, C.; Liu, C.H.; Barton, S.C.; Anandan, S.; Wu, J.J. Carbon materials for electrochemical sensing application-a mini review. J. Taiwan Inst. Chem. Eng. 2024, 154, 105071. [Google Scholar] [CrossRef]
- Li, H.R.; Xu, H.; Li, S.S.; Wu, X.F. Heterojunction composite-based electrochemical sensors for hazardous substances detection in environmental and biological system. Coord. Chem. Rev. 2025, 540, 216787. [Google Scholar] [CrossRef]
- Wei, Y.; Lu, X.; Zhang, S.; Cao, L.; Zhao, B.; Chen, Y.; Sun, Y.; Liu, W.; Zhu, N. Micro-sonobot-based decontamination for the renewable sweat glucose sensors. Sens. Actuators B Chem. 2025, 441, 137998. [Google Scholar] [CrossRef]
- Yu, K.N.; Wang, R.Z.; Liu, D.D. A review of rapid detections for emerging contaminants in ground water. Rock Miner. Anal. 2023, 42, 1063–1077. [Google Scholar]
- Wang, L.Y.; Chang, S.J.; Chen, C.J.; Liu, J.T. Metal-organic frameworks for electrochemical glucose sensors: Progress and challenges. Coord. Chem. Rev. 2025, 543, 216907. [Google Scholar] [CrossRef]
- Abedeen, M.Z.; Sharma, M.; Kushwaha, H.S.; Gupta, R. Sensitive enzyme-free electrochemical sensors for the detection of pesticide residues in food and water. Trends Anal. Chem. 2024, 176, 117729. [Google Scholar] [CrossRef]
- Jiang, Y.; Sima, Y.; Liu, L.; Zhou, C.; Shi, S.; Wan, K.; Chen, A.; Tang, N.; He, Q.; Liu, J. Research progress on portable electrochemical sensors for detection of mycotoxins in food and environmental samples. Chem. Eng. J. 2024, 485, 149860. [Google Scholar] [CrossRef]
- He, Q.; Wang, B.; Liang, J.; Liu, J.; Liang, B.; Li, G.; Long, Y.; Zhang, G.; Liu, H. Research on the construction of portable electrochemical sensors for environmental compounds quality monitoring. Mater. Today Adv. 2023, 17, 100340. [Google Scholar] [CrossRef]
- Loganathan, H.; Dhinasekaran, D.; Rajendran, A.R. Recent advances in MXene-based electrochemical sensors for healthcare applications. TrAC Trends Anal. Chem. 2025, 189, 118276. [Google Scholar] [CrossRef]
- Khizar, S.; Zine, N.; Sigaud, M.; Elaissari, A.; Errachid, A. Electrochemical Sensors for Enhanced and Rapid Detection of Illicit Drugs. Electroanalysis 2025, 37, e12034. [Google Scholar] [CrossRef]
- Zouaoui, F.; Menassol, G.; Ducros, C.; Mailley, P.; Thomas, Y. Electrochemical sensors based on amorphous carbon electrode: A review. Microchem. J. 2025, 209, 112650. [Google Scholar] [CrossRef]
- He, J.; Xu, X.; Li, M.; Zhou, S.; Zhou, W. Recent advances in perovskite oxides for non-enzymatic electrochemical sensors: A review. Anal. Chim. Acta 2023, 1251, 341007. [Google Scholar] [CrossRef]
- Baycan, F.; Saglikoglu, G. Fabrication of AuNPs/PHCQE/GPE electrochemical sensor for the determination of isoniazid. Microchem. J. 2025, 209, 112662. [Google Scholar] [CrossRef]
- Kumar, P.; Ali, S.; Ahmad, K.; Raza, W.; Khan, R.A. Construction of a hydrazine electrochemical sensor using Ag@ ZIF as the electrode material. RSC Adv. 2025, 15, 3089–3097. [Google Scholar] [CrossRef]
- Jiao, Y.; Yu, X. Recent advances in wearable electrochemical sensors for in situ detection of biochemical markers. Sci. China Mater. 2025, 68, 755–774. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Sher, M.; Ameer, S.; Slaughter, G. Laser-Induced Graphene Sensor for the Electrochemical Detection of Acetaminophen. ChemistrySelect 2025, 10, e02914. [Google Scholar] [CrossRef]
- Seker, S.; Surucu, O.; Economou, A.; Wang, J. “On-plant” wearable electrochemical sensor for atmospheric lead monitoring. Talanta 2025, 287, 127654. [Google Scholar] [CrossRef]
- Guan, G.; Lin, Z.; Qian, J.; Wang, F.; Qu, L.; Zou, B. Research Progress on the Application of Nanoenzyme Electrochemical Sensors for Detecting Zearalenone in Food. Nanomaterials 2025, 15, 712. [Google Scholar] [CrossRef]
- Ebadi, S.; Ghanbari, K.; Zahedi-Tabrizi, M. Development of an electrochemical sensor based on Ni-Bio-MOF and a molecular imprinted polymer for determination of diclofenac: Electrochemical and DFT investigations. RSC Adv. 2025, 15, 16983–16998. [Google Scholar] [CrossRef]
- Tian, G.; Chen, J.; Yang, D.; Liang, C.; Zhao, Q.; Liu, Y.; Lu, W.; Qi, D. Self-healable and stretchable electrochemical sensor for sweat glucose detection. Talanta 2025, 295, 128428. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, W.; Sunarso, J.; Xu, X.; Zhong, Y.; Shao, Z.; Chen, X.; Zhu, H. 3D ordered macroporous SmCoO3 perovskite for highly active and selective hydrogen peroxide detection. Electrochim. Acta 2018, 260, 372–383. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, M.; Lu, D.; Wang, Y.; Li, H.; Sun, W.; Long, J.; Jeerapan, I.; Marty, J.L.; Zhu, Z. Nonenzymatic Glucose Electrochemical Sensor Based on Pd-Cu Bimetallic Aerogels. Talanta 2025, 287, 127641. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.D.; He, Y.; Qi, X.R.; Jin, T. Rapid detection of fentanyl by electrochemical sensor based on Fe/Ni binary metal-organic frameworks/multi-walled carbon nanotubes. Chin. J. Anal. Chem. 2024, 53, 1152–1162. [Google Scholar]
- Liu, W.; Li, H.; Huang, D.; Tan, X.; Zhao, M.; Cheng, Q.; Yi, M.; Ding, Q.; Ren, Y.; Liu, G. Engineering α-MoO3/TiO2 heterostructures derived from MOFs/MXene hybrids for high-performance triethylamine sensor. Chem. Eng. J. 2024, 483, 149340. [Google Scholar] [CrossRef]
- Gao, P.; Hussain, M.Z.; Zhou, Z.; Warnan, J.; Elsner, M.; Fischer, R.A. Zr-based metalloporphyrin MOF probe for electrochemical detection of parathion-methyl. Biosens. Bioelectron. 2024, 261, 116515. [Google Scholar] [CrossRef]
- Hai, M.; Zhang, W.; Jia, N. Determination of trace lead and copper in seawater by inductively coupled plasma-mass spectrometry with coconut shell biochar enrichment. Rock Miner. Anal. 2024, 43, 281–288. [Google Scholar]
- Ramachandran, T.; Khan, R.; Ghosh, A.; Hussien, M.; Kumar, Y.A.; Reddy, N.P.; Moniruzzaman, M. Sustainable carbon electrode materials from biomass for redox flow batteries. Biomass Bioenergy 2025, 198, 107846. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube-titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of humidity on gas sensing performance of carbon nanotube gas sensors operated at room temperature. IEEE Sens. J. 2020, 21, 5763–5770. [Google Scholar] [CrossRef]
- Mathew, A.T.; Bhat, V.S.; Supriya, S.; Hegde, G. TEMPO mediated electrocatalytic oxidation of pyridyl carbinol using palladium nanoparticles dispersed on biomass derived porous nanoparticles. Electrochim. Acta 2020, 354, 136624. [Google Scholar] [CrossRef]
- Kasataka, K.; Umemoto, S.; Ikeda, A. Potential use of biochar as a catalyst and an adsorbent in plastic pyrolysis process. Waste Biomass Valoriz. 2025. [Google Scholar] [CrossRef]
- Chen, X.; Ma, M.; Wu, J.; Chen, Y.; Xu, S.; Li, D.; Sang, W. Reuse of spent lithium cobaltate as an efficient catalyst for the CO2 gasification of biochar. Biomass Convers. Biorefinery 2025, 15, 18551–18565. [Google Scholar] [CrossRef]
- Khan, N.; Gupta, A.; Ahamad, S.; Hussain, M.K.; Khan, M.U.; Siddiqui, Z.N. Functionalized biochar catalysts: Advancing green chemistry in synthesis of O-and N-heterocycles. Environ. Res. 2025, 284, 122136. [Google Scholar] [CrossRef]
- Qi, T.; Liu, S.; Sun, L.; Feng, X.; Zhao, B.; An, S.; Xing, L.; Jing, Q.; Jiang, H.; Li, Q.; et al. Efficient CO2 capture by a defect-driven O/N co-doped ultramicroporous carbon derived from plastics and biomass solid wastes. Sep. Purif. Technol. 2025, 364, 132585. [Google Scholar] [CrossRef]
- Faggiano, A.; Cicatelli, A.; Guarino, F.; Castiglione, S.; Proto, A.; Fiorentino, A.; Motta, O. Optimizing CO2 capture: Effects of chemical functionalization on woodchip BDCM adsorption performance. J. Environ. Manag. 2025, 380, 125059. [Google Scholar] [CrossRef]
- Zhang, P.; Luo, J.; Huo, L.; Zhao, L.; Yao, Z. Straw-derived porous biochar by ball milling for CO2 capture: Adsorption performance and enhanced mechanisms. Ind. Crops Prod. 2025, 229, 121000. [Google Scholar] [CrossRef]
- Kim, C.; Talapaneni, S.N.; Dai, L. Porous carbon materials for CO2 capture, storage and electrochemical conversion. Mater. Rep. Energy 2023, 3, 100199. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, M.; Zhang, K.; Liu, W.; Wang, J.; Ren, L.; Wang, F. Effect mechanisms of microwave on CO2 adsorption with cellulosic and non-cellulosic BDCM. Sep. Purif. Technol. 2025, 373, 133610. [Google Scholar] [CrossRef]
- Li, G.F.; Xu, W.Z.; Wei, M.H.; Yan, Z.; You, J.; Jiang, H.; Huang, J. Preparation of 3D porous biochar adsorbent and its adsorption behavior for phenanthrene. Ecol. Environ. Sci. 2024, 33, 261–271. [Google Scholar]
- Priya, S.; Salmataj, S.A.; Anusha, B.; Bhat, P. Synthesis, characterisation of agricultural biomass derived activated carbon and its applications. Mater. Res. Express 2025, 12, 012001. [Google Scholar] [CrossRef]
- Yang, X.; Tian, X.; Xue, Y.; Wang, C. Application of iron-modified BDCM in the fields of adsorption and degradation of antibiotics. J. Environ. Manag. 2025, 380, 124875. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Zhuang, S.; Wang, J. Adsorption of heavy metals by biochar in aqueous solution: A review. Sci. Total Environ. 2025, 968, 178898. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Shi, X.; Liu, Y.; Zhao, C.; Pan, H.; Liu, X.; Huang, S. Effects of bagasse-derived biochar aging on its performance for levofloxacin adsorption. Inorg. Chem. Commun. 2025, 174, 113966. [Google Scholar] [CrossRef]
- Blankenship, L.S.; Mokaya, R. Cigarette butt-derived carbons have ultra-high surface area and unprecedented hydrogen storage capacity. Energy Environ. Sci. 2017, 10, 2552–2562. [Google Scholar] [CrossRef]
- Han, G.; Qi, F.; Sun, Y.; Zhou, J.; Wang, Y.; Sui, S.; Feng, B.; Jia, Y.; Tian, X.; Wang, X.; et al. Biomass derived honeycomb porous carbon combined with metal oxide for broadband microwave absorption. Compos. Commun. 2025, 55, 102303. [Google Scholar] [CrossRef]
- Wei, H.; Chen, S.; Chen, Z.; Tang, L.; Xue, J.; Wang, C.; Wang, Z.; Li, Y. Hetero-interface engineering of biomass carbon foam for broadband microwave absorption and thermal insulation properties. Carbon 2025, 241, 120385. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, X.; Zhu, H.; Li, D.; Nie, Y.; Gao, B.; Xiang, G. Corn silk-derived biomass carbon materials for low-frequency microwave absorption and energy storage. Nanoscale 2025, 17, 6030–6038. [Google Scholar] [CrossRef]
- Yu, J.L.; Lu, M.X.; He, M.M.; Wei, Y.Z.; Cai, L.Q.; Pan, Z.D.; Bei, Y.M.; Li, X.C. Study on the performance and mechanism of hexavalent chromium removal from water by BC and ATP supported nano-zero-valent iron. Acta Sci. Circumst. 2024, 44, 127–136. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Huang, R.; Yang, C.; Zhang, M.; Xie, P.; Xiong, J.; Lu, X. Lanthanum-modified sludge biochar for geothermal water fluoride removal. Materials 2025, 18, 1421. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Hossain, M.F.; Khan, M.T.; Zulfiqar, R.; Zhou, Y. Microplastic removal from water using biomass-based carbon: A review of recent advances. Chem. Asian J. 2025, e00346. [Google Scholar] [CrossRef]
- Clouse, D.E.; He, J.; Wang, D.; Davis, V.A. Tailored activated carbons from biomass components for aqueous PFAS adsorption. Mater. Lett. 2025, 384, 138050. [Google Scholar] [CrossRef]
- Fang, J.; Wang, D.; Wilkin, R.; Su, C. Realistic and field scale applications of biochar for water remediation: A literature review. J. Environ. Manag. 2025, 385, 125524. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fang, K.; Chen, Q.; Xu, J.; Wong, C.-P. Integrated paper electrodes derived from cotton stalks for high-performance flexible supercapacitors. Nano Energy 2018, 53, 337–344. [Google Scholar] [CrossRef]
- Astuti, F.; Susanto, J.K.; Arifah, L.R.; Yuwana, L.; Ramadhan, M.R.; Lailiyah, Q.; Darminto, D. Carbon material derived from biomass: A study of structural and electrochemical performances for dual-carbon sodium-ion batteries. Phys. Scr. 2025, 100, 055955. [Google Scholar] [CrossRef]
- Sahoo, R.; Venkatachalam, S.; Sundara, R. Influence of electrolyte on the electrochemical performance of the biomass-derived hard carbon for potassium ion batteries. Batter. Supercaps 2025, 8, e202400682. [Google Scholar] [CrossRef]
- Sun, T.; Gao, C.; Tan, X.; Zhang, N.; Xie, G.; Shi, Z.; Yang, Z.; Wang, T.; Wu, Y. High-value utilization of corn plants derived biomass carbon materials for potassium ion storage. Sustain. Mater. Technol. 2025, 44, e01359. [Google Scholar] [CrossRef]
- Cui, J.; Li, W.; Su, P.; Song, X.; Ye, W.; Zhang, Y.; Chen, Z. Repair surface defects on biomass derived hard carbon anodes with N-doped soft carbon to boost performance for sodium-ion batteries. Adv. Energy Mater. 2025, 2502082. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Xia, Y.; Wang, W.; Song, H.; Liu, S. High-performance nitrogen-doped porous carbon materials derived from waste biomass for supercapacitors. Chem. Phys. 2025, 596, 112745. [Google Scholar] [CrossRef]
- Egun, I.L.; Akinwolemiwa, B.; He, H.; Ma, M.; Chen, Z.; Chen, G.Z.; Hu, D. Molten base carbonisation and activation of non-lignin-rich biomass into hierarchically porous carbon with surface-rich functionalities for supercapacitor electrodes. Chem. Eng. J. 2025, 509, 161386. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Z.; Zhang, C.; Hao, T.; Wang, T.; Deng, D.; Sun, Z.; Wang, Y.; Xu, C.; Zeng, J.; et al. Monolithic carbon derived from biomass via zinc-assisted pyrolysis for lithium–sulfur batteries. Green Chem. 2025, 27, 3326–3334. [Google Scholar] [CrossRef]
- Bhat, V.S.; Supriya, S.; Hegde, G. Review-biomass derived carbon materials for electrochemical sensors. J. Electrochem. Soc. 2019, 167, 037526. [Google Scholar] [CrossRef]
- Chou, C.M.; Dai, Y.D.; Yuan, C.; Shen, Y.H. Preparation of an electrochemical sensor utilizing graphene-like biochar for the detection of tetracycline. Environ. Res. 2023, 236, 116785. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, M.V.S.; Carvalho, S.W.M.M.; Gevaerd, A.; Silva, J.O.; Santos, E.; Carregosa, I.S.; Wisniewski, A.; Marcolino-Junior, L.H.; Bergamini, M.F.; Sussuchi, E.M. Electrochemical sensor based on biochar and reduced graphene oxide nanocomposite for carbendazim determination. Talanta 2020, 220, 121334. [Google Scholar] [CrossRef]
- Sfragano, P.S.; Laschi, S.; Renai, L.; Fichera, M.; Del Bubba, M.; Palchetti, I. Electrochemical sensors based on sewage sludge–derived biochar for the analysis of anthocyanins in berry fruits. Anal. Bioanal. Chem. 2022, 414, 6295–6307. [Google Scholar] [CrossRef]
- Wang, R.; Lan, X.; Zhou, T.; Qian, X.; Qu, B.; Lv, P.; Wang, Y. Detection of chloramphenicol in dairy products based on biogas residue biochar based electrochemical sensor. J. Food Compos. Anal. 2024, 125, 105824. [Google Scholar] [CrossRef]
- Gemeiner, P.; Sarakhman, O.; Hatala, M.; Ház, A.; Roupcová, P.; Mackuľak, T.; Barek, J.; Švorc, Ľ. A new generation of fully-printed electrochemical sensors based on BDCM/ethylcellulose-modified carbon electrodes: Fabrication, characterization and practical applications. Electrochim. Acta 2024, 487, 144161. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Y.; Kan, X.; Chen, M.; Dai, J.; Zhang, Y.; Pang, P.; Ma, W.; Zhang, J. An electrochemical sensor for detection of lead (II) ions using biochar of spent coffee grounds modified by TiO2 nanoparticles. Molecules 2024, 29, 5704. [Google Scholar] [CrossRef]
- Dong, S.; Yang, Z.; Liu, B.; Zhang, J.; Xu, P.; Xiang, M.; Lu, T. (Pd, Au, Ag) nanoparticles decorated well-ordered macroporous carbon for electrochemical sensing applications. J. Electroanal. Chem. 2021, 897, 115562. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Y.; Liu, T.; Wang, G.T.; Sun, M.M.; Jiang, Y.Y.; He, H.; Wang, Y.Y.; Zou, P.; Wang, X.X.; et al. A dual-template imprinted polymer electrochemical sensor based on AuNPs and nitrogen-doped graphene oxide quantum dots coated on NiS2/biomass carbon for simultaneous determination of dopamine and chlorpromazine. Chem. Eng. J. 2020, 389, 124417. [Google Scholar] [CrossRef]
- Kong, Z.; Zhang, H.; Zhou, T.; Xie, L.; Wang, B.; Jiang, X. Biomass-derived functional materials: Preparation, functionalization, and applications in adsorption and catalytic separation of carbon dioxide and other atmospheric pollutants. Sep. Purif. Technol. 2024, 354, 129099. [Google Scholar] [CrossRef]
- Xiong, C.; Zheng, C.; Jiang, X.; Xiao, X.; Wei, H.; Zhou, Q.; Ni, Y. Recent progress of green biomass based composite materials applied in supercapacitors, sensors, and electrocatalysis. J. Energy Storage 2023, 72, 108633. [Google Scholar] [CrossRef]
- Hou, B.; Wang, L.; Yang, X.; Li, Y.; Wu, Z.; Pan, J.; Wang, L. Application of bimetallic cerium-based biochar adsorbent for efficient removal of Cr (VI): Effective regulation of Fe doping and straw-based biochar incorporation. Sep. Purif. Technol. 2025, 364, 132506. [Google Scholar] [CrossRef]
- Irfan, J.A.; Charulatha, G.; Mary, L.M.; Ramanamoorthy, S.; Karthikeyan, C.; Saravanakumar, N.K.; Manikandan, G.; Jayaprakash, J.; Palani, K.N. Biomass-derived biochar for electrochemical energy storage and conversion systems: Opportunities and challenges. Future Batter. 2025, 6, 100077. [Google Scholar]
- Liu, W.-J.; Li, W.-W.; Jiang, H.; Yu, H.-Q. Fates of chemical elements in biomass during its pyrolysis. Chem. Rev. 2017, 117, 6367–6398. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, H.; Wang, Y.; Laurindo, M.B.J.; Zhao, J.; Hasebe, Y.; Zhang, Z. Crab gill-derived nanorod-like carbons as bifunctional electrochemical sensors for detection of hydrogen peroxide and glucose. Ionics 2024, 30, 3541–3552. [Google Scholar] [CrossRef]
- Chang, W.; Zhu, Y.; Ma, Y.; Zheng, Z.; Wang, C. Silk derived Fe/N-doping porous carbon nanosheets for chloramphenicol electrochemical detection. Curr. Anal. Chem. 2022, 18, 1017–1028. [Google Scholar] [CrossRef]
- Jin, B.; Liu, S.; Jin, D. Azalea Petal-derived porous carbon-thionine based ratiometric electrochemical sensor for the simultaneous determination of ascorbic acid and uric acid. Russ. J. Electrochem. 2023, 59, 1151–1161. [Google Scholar]
- Nguyen, D.T.; Hoang, T.K.; Tran, T.D.; Nguyen, M.H.; Trinh, K.T.; Khuong, D.A.; Tsubota, T.; Pham, T.D. Adsorption characteristics of individual and binary mixture of ciprofloxacin antibiotic and lead (II) on synthesized bamboo-biochar. Environ. Res. 2025, 273, 121225. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, Z.; Luo, C.; Zhang, J.; Cao, X.; Liu, J.; Mu, J. Honeycomb-like activated carbon with microporous nanosheets structure prepared from waste biomass cork for highly efficient dye wastewater treatment. J. Hazard. Mater. 2021, 416, 125896. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Wu, Q.; Zong, T.; Xin, X.; Xie, J.; Yang, J. Use of rice straw nano-biochar to slow down water infiltration and reduce nitrogen leaching in a clayey soil. Sci. Total Environ. 2024, 948, 174956. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, Y.; Jin, L.; Yang, B. Properties of biochars derived from different straw at 500 ℃ pyrolytic temperature: Implications for their use to improving acidic soil water retention. Agric. Water Manag. 2024, 301, 108953. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Cai, W.; Luo, Z.; Zhou, J.; Wang, Q. A review on the selection of raw materials and reactors for biomass fast pyrolysis in China. Fuel Process. Technol. 2021, 221, 106919. [Google Scholar] [CrossRef]
- Titirici, M.M.; Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef]
- Cheng, G.; Li, H.-J.; Fang, J.-H.; Wang, X.-Q.; Li, J.-Q.; Chang, J.-F.; Teng, N.; Wang, J.-T. Hydrothermal synthesis and structural design of zero-to three-dimensional biomass-derived carbon nanomaterials. Carbon Lett. 2025, 35, 441–467. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titirici, M.M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef]
- Yang, L.; Huang, K.; Li, X.; Zhang, Y.; Yin, P.; Sun, X. Fluorescence switching sensor for sensitive detection of curcumin/Mn2+ using biomass carbon quantum dots: A combination of experimental and theoretical insights. J. Environ. Chem. Eng. 2024, 12, 114533. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, C.; Duan, X.; Bao, L.; Wang, G.; Fu, W. A portable smartphone platform based on fluorescent carbon quantum dots derived from biowaste for on-site detection of permanganate. New J. Chem. 2024, 48, 12626–12632. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, H.; Du, S.; Wang, D.; Liu, Q. Biomass carbon quantum dots: Mimicking peroxidase-like activity and sensitive fluorometric and colorimetric detection of dopamine hydrochloride in meat products. Food Biosci. 2024, 61, 104719. [Google Scholar] [CrossRef]
- Silva, T.; Silva, A.D.; Silva, A.A.S.; da Silva, R.C.; Moreira, R.P.L.; Silva, T.A. One-pot hydrothermal biochar obtained from malt bagasse waste as an electrode-modifying material towards the stripping voltammetric sensing of lead. Electroanalysis 2024, 36, e202300425. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Ab Rahim, M.H.; Park, J.-W.; Kim, H.-J. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Chen, W.H.; Biswas, P.P.; Zhang, C.; Kwon, E.E.; Chang, J.-S. Achieving carbon credits through biomass torrefaction and hydrothermal carbonization: A review. Renew. Sustain. Energy Rev. 2025, 208, 115056. [Google Scholar] [CrossRef]
- Wang, H.; Meng, H.; Olowoyo, J.O.; Zeng, Y.; Zheng, Y. Advancements in lignin valorization for energy storage applications: Sustainable technologies for lignin extraction and hydrothermal carbonization. Nanomaterials 2025, 15, 309. [Google Scholar] [CrossRef]
- Wang, F.; Qi, X.; Zhang, H.; Yang, Z. Innovative molten salt iechniques for biomass valorization: Transforming biomass into advanced carbon materials. Carbon 2025, 234, 119999. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Lu, C.; Li, S.; Zhu, M. Molten salt technique for the synthesis of carbon-based materials for supercapacitors. Green Chem. 2023, 25, 10209–10234. [Google Scholar] [CrossRef]
- He, M.; Han, J.; Cheng, Y.; Qi, H. Filter paper-derived hierarchical porous carbon by a simple molten salt strategy in air for high-performance supercapacitors. ACS Appl. Energy Mater. 2024, 7, 9816–9826. [Google Scholar] [CrossRef]
- Liu, X.; Antonietti, M. Molten salt activation for synthesis of porous carbon nanostructures and carbon sheets. Carbon 2014, 69, 460–466. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Xie, X.; Li, C.; Liu, D. Pyrolysis of rice husk in molten lithium chloride: Biochar structure evolution and CO2 adsorption. J. Energy Inst. 2024, 113, 101526. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, F.; Yuan, Z.; Li, Y.; Sun, P.; Lu, X.; Chong, F. Casein-derived nitrogen and phosphorus co-doped porous carbons via a thermochemical process of molten salt and caustic potash for supercapacitors. J. Power Sources 2024, 612, 234708. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, X.; Diao, R.; Qi, F.; Ma, P. Structural regulation of ultra-microporous biomass-derived carbon materials induced by molten salt synergistic activation and its application in CO2 capture. Chem. Eng. J. 2024, 486, 150227. [Google Scholar] [CrossRef]
- Egun, I.L.; He, H.; Hu, D.; Chen, G.Z. Molten salt carbonization and activation of biomass to functional biocarbon. Adv. Sustain. Syst. 2022, 6, 2200294. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, H.; Li, C.; Chen, D.; Sun, C.; Yang, Z. A simple and recyclable molten-salt route to prepare superthin biocarbon sheets based on the high water-absorbent agaric for efficient lithium storage. Carbon 2020, 157, 286–294. [Google Scholar] [CrossRef]
- Pang, Z.; Li, G.; Xiong, X.; Ji, L.; Xu, Q.; Zou, X.; Lu, X. Molten salt synthesis of porous carbon and its application in supercapacitors: A review. J. Energy Chem. 2021, 61, 622–640. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Factors affecting the carbon yield and adsorption capability of the mangosteen peel activated carbon prepared by microwave assisted K2CO3 activation. Chem. Eng. J. 2012, 180, 66–74. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Zheng, T.; Li, N.; Wang, P.; Abulikemu, G. Modification of bamboo-based activated carbon using microwave radiation and its effects on the adsorption of methylene blue. Appl. Surf. Sci. 2010, 256, 3309–3315. [Google Scholar] [CrossRef]
- Hoang, A.T.; Kumar, S.; Lichtfouse, E.; Cheng, C.K.; Varma, R.S.; Senthilkumar, N.; Nguyen, P.Q.P.; Nguyen, X.P. Remediation of heavy metal polluted waters using activated carbon from lignocellulosic biomass: An update of recent trends. Chemosphere 2022, 302, 134825. [Google Scholar] [CrossRef]
- Fu, W.; Guo, X.; Pan, M.; Song, J.; Xi, B. Preparation of catkin fiber biochar and its adsorption properties for Cr(VI) in dye wastewater. Fang Zhi Xue Bao 2022, 43, 8–15. [Google Scholar]
- Meng, L.; Yek, P.N.Y.; Foong, S.Y.; Liew, R.K.; Ge, S.; Lam, S.S. Single-mode microwave pyrolysis of engineered biochar from shrimp shell waste for landfill wastewater treatment. J. Ind. Eng. Chem. 2025. [Google Scholar] [CrossRef]
- Dong, H.; Belver, C.; Chen, S.; Li, G.; He, Q.; Guo, J.; Zhao, F.; Gao, G.; Guan, J.; Bedia, J. Activation of peroxydisulfate by phosphoric acid-modified microwave biochar for tetracycline removal in water: Mechanistic insights. J. Water Process Eng. 2025, 75, 107966. [Google Scholar] [CrossRef]
- Li, Y.; Luo, L.; Kong, Y.; Li, Y.; Wang, Q.; Wang, M.; Li, Y.; Davenport, A.; Li, B. Recent advances in molecularly imprinted polymer-based electrochemical sensors. Biosens. Bioelectron. 2024, 249, 116018. [Google Scholar] [CrossRef]
- Liang, M.; Liu, Y.; Lu, S.; Wang, Y.; Gao, C.; Fan, K.; Liu, H. Two-dimensional conductive MOFs toward electrochemical sensors for environmental pollutants. Trends Anal. Chem. 2024, 177, 117800. [Google Scholar] [CrossRef]
- Sha, T.; Li, X.; Liu, J.; Sun, M.; Wang, N.; Bo, X.; Guo, Y.; Hu, Z.; Zhou, M. Biomass waste derived carbon nanoballs aggregation networks-based aerogels as electrode material for electrochemical sensing. Sens. Actuators B Chem. 2018, 277, 195–204. [Google Scholar] [CrossRef]
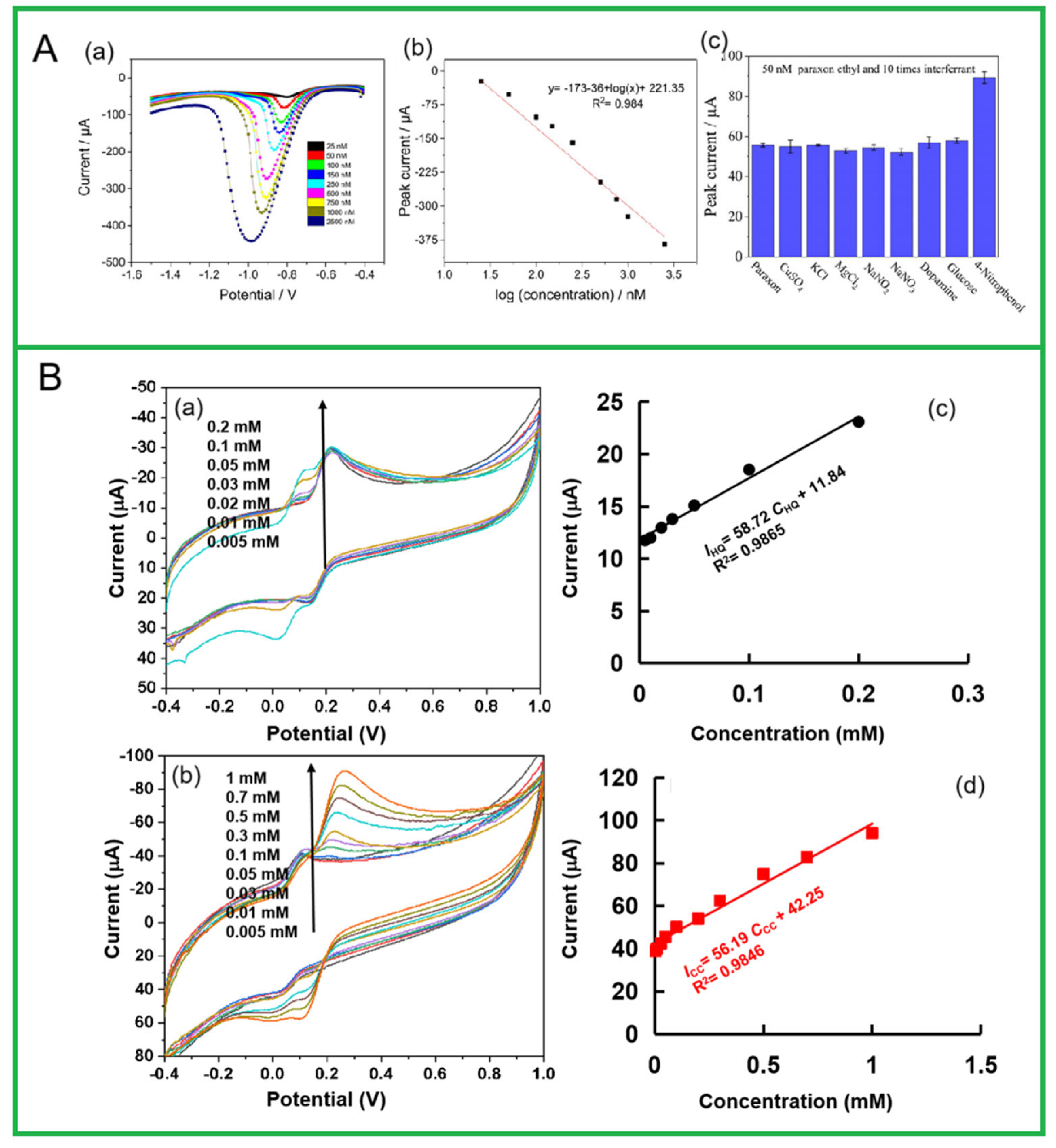
- Adiraju, A.; Brahem, A.; Lu, T.; Al-Hamry, A.; Zhou, Y.; Wei, L.; Jalasutram, A.; Tegenkamp, C.; Halouani, K.; Kanoun, O. Electrochemical enrichment of biochar coal modified on carbon electrodes for the detection of nitrite and paraxon ethyl pesticide. J. Compos. Sci. 2024, 8, 217. [Google Scholar] [CrossRef]
- Madhu, R.; Karuppiah, C.; Chen, S.M.; Veerakumar, P.; Liu, S.-B. Electrochemical detection of 4-nitrophenol based on biomass derived activated carbons. Anal. Methods 2014, 6, 5274–5280. [Google Scholar] [CrossRef]
- Xu, Y.; Lei, W.; Zhang, Y.; Fan, H.; Hao, Q.; Gao, S. Bamboo fungus-derived porous nitrogen-doped carbon for the fast, sensitive determination of bisphenol A. J. Electrochem. Soc. 2016, 164, B3043. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, Y.; Mário, B.J.L.; Wang, S. Cellulose-derived hierarchical porous carbon based electrochemical sensor for simultaneous detection of catechol and hydroquinone. Ionics 2024, 30, 1089–1100. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, J.; Lin, J.; Wang, X.; Chen, K.; Huang, X.; Huang, J.; Wang, C.; Hu, Z.; Xu, H. Preparation of biochar derived from lychee shell and its application in simultaneous detection of catechol and hydroquinone. J. Appl. Polym. Sci. 2025, 142, e56894. [Google Scholar] [CrossRef]
- Mahfoz, W.; Shah, S.S.; Al-Betar, A.R.; Aziz, A. Date leaves-derived submicron/nano carbon-modified glassy carbon electrode for highly sensitive and simultaneous detection of 1-naphthol and 2-naphthol. J. Electrochem. Soc. 2024, 171, 047505. [Google Scholar] [CrossRef]
- Malode, S.J.; Nelogal, P.S.; Al-Otaibi, J.S.; Alodhayb, A.N.; Shetti, N.P. Improved electrochemical detection of 2, 4, 6-trichlorophenol using Cu-doped biochar derived from green pea peels. J. Mol. Struct. 2025, 1343, 142866. [Google Scholar] [CrossRef]
- Pan, Z.; Gong, T.; Liang, P. Heavy metal exposure and cardiovascular disease. Circ. Res. 2024, 134, 1160–1178. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, J.; Bi, Y.; Ma, C.; Bai, J.; Hu, Z.; Zhou, M. Biomass derived worm-like nitrogen-doped-carbon framework for trace determination of toxic heavy metal lead (Ⅱ). Anal. Chim. Acta 2020, 1116, 16–26. [Google Scholar] [CrossRef]
- Valenga, M.G.P.; Gevaerd, A.; Marcolino-Junior, L.H.; Bergamini, M.F. Biochar from sugarcane bagasse: Synthesis, characterization, and application in an electrochemical sensor for copper(Ⅱ) determination. Biomass Bioenergy 2024, 184, 107206. [Google Scholar] [CrossRef]
- Bressi, V.; Celesti, C.; Ferlazzo, A.; Len, T.; Moulaee, K.; Neri, G.; Luque, R.; Espro, C. Waste-derived carbon nanodots for fluorimetric and simultaneous electrochemical detection of heavy metals in water. Environ. Sci. Nano 2024, 11, 1245–1258. [Google Scholar] [CrossRef]
- Sharma, R.; Rana, D.S.; Gupta, N.; Thakur, S.; Thakur, K.K.; Singh, D. Parthenium hysterophorus derived nanostructures as an efficient carbocatalyst for the electrochemical sensing of mercury(Ⅱ) ions. Chemosphere 2024, 354, 141591. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, F.A.; Bashir, A.; Qureashi, A.; Nazir, I.; Fatima, K.; Pandith, A.H.; Bhat, M.A. SnS2 decorated biochar: A robust platform for the photocatalytic degradation and electrochemical sensing of pollutants. New J. Chem. 2024, 48, 7111–7124. [Google Scholar] [CrossRef]
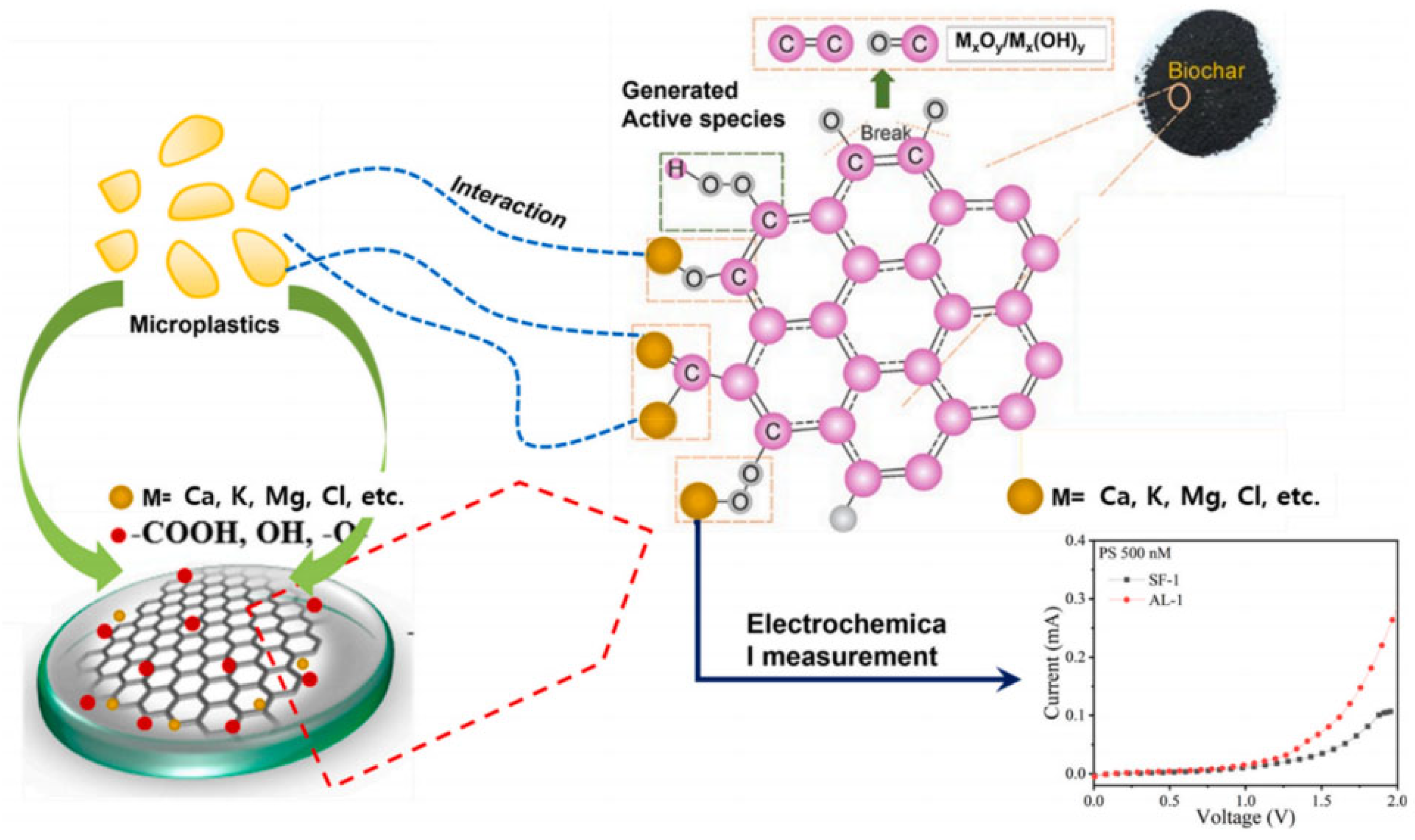
- Nguyen, H.H.T.; Kim, E.; Imran, M.; Choi, Y.-H.; Kwak, D.-H.; Ameen, S. Microplastic contaminants detection in aquatic environment by hydrophobic cerium oxide nanoparticles. Chemosphere 2024, 357, 141961. [Google Scholar] [CrossRef]
- Kim, S.A.; Kim, E.B.; Imran, M.; Shahzad, K.; Moon, D.H.; Akhtar, M.S.; Ameen, S.; Park, S.H. Naturally manufactured biochar materials based sensor electrode for the electrochemical detection of polystyrene microplastics. Chemosphere 2024, 351, 141151. [Google Scholar] [CrossRef]
- Ateia, M.; Wei, H.; Andreescu, S. Sensors for emerging water contaminants: Overcoming roadblocks to innovation. Environ. Sci. Technol. 2024, 58, 2636–2651. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.M.; Jeon, Y.; Lee, J.; Oh, J.; Antink, W.H.; Kim, D.; Piao, Y. Novel two-step activation of biomass-derived carbon for highly sensitive electrochemical determination of acetaminophen. Sens. Actuators B Chem. 2018, 259, 50–58. [Google Scholar] [CrossRef]
- Prakasam, S.; Maharajan, N.; Chinnathambi, S. Dual Sensing of Dopamine and Acetaminophen Mediated by Biomass Derived Phosphorous Rich Carbon. ChemistrySelect 2024, 9, e202401920. [Google Scholar] [CrossRef]
- Yalikun, N.; Mamat, X.; Li, Y.; Hu, X.; Wang, P.; Hu, G. N, S, P-triple doped porous carbon as an improved electrochemical sensor for metronidazole determination. J. Electrochem. Soc. 2019, 166, B1131. [Google Scholar] [CrossRef]
- Veerakumar, P.; Sangili, A.; Chen, S.M.; Pandikumar, A.; Lin, K.-C. Fabrication of platinum-rhenium nanoparticle-decorated porous carbons: Voltammetric sensing of furazolidone. ACS Sustain. Chem. Eng. 2020, 8, 3591–3605. [Google Scholar] [CrossRef]
- Malode, S.J.; Joshi, M.; Shetti, N.P.; Alshehri, M.A. Biowaste-derived carbon nanomaterial-based sensor for the electrochemical analysis of mefenamic acid in the presence of CTAB. Mater. Today Commun. 2024, 39, 108723. [Google Scholar] [CrossRef]
- Liu, C.; Lv, L.; Sun, Y.; Di, X. Specific temperature-modulated crab shell-derived porous carbon as a typical recycling material for nitrofurazone electrochemical sensor. Microporous Mesoporous Mater. 2024, 374, 113143. [Google Scholar] [CrossRef]
- Ramadhass, K.D.; Ganesan, M.; Chen, T.W.; Chen, S.-M.; Ali, M.A.; Habila, M.A.; El-Marghany, A.; Sheikh, M. 3D honey-comb like nitrogen self-doped porous carbon networks for high-performance electrochemical detection of antibiotic drug furazolidone. J. Electrochem. Soc. 2021, 168, 047503. [Google Scholar] [CrossRef]
- Cheng, H.; Weng, W.; Xie, H.; Liu, J.; Luo, G.; Huang, S.; Sun, W.; Li, G. Au-Pt@biomass porous carbon composite modified electrode for sensitive electrochemical detection of baicalein. Microchem. J. 2020, 154, 104602. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; He, L. Sensitive voltammetric determination of baicalein at thermally reduced graphene oxide modified glassy carbon electrode. Electroanalysis 2013, 25, 2136–2144. [Google Scholar] [CrossRef]
- Ai, Y.; Liu, J.; Yan, L.; Li, G.; Wang, X.; Sun, W. Banana peel derived biomass carbon: Multi-walled carbon nanotube composite modified electrode for sensitive voltammetric detection of baicalein. J. Chin. Chem. Soc. 2022, 69, 359–365. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Liu, X.; Xiong, Y.; Han, C.; Zhang, Y.; Bo, X.; Guo, L. Novel bamboo leaf shaped CuO nanorod@ hollow carbon fibers derived from plant biomass for efficient and nonenzymatic glucose detection. Analyst 2015, 140, 6412–6420. [Google Scholar] [CrossRef]
- Qu, P.; Gong, Z.; Cheng, H.; Xiong, W.; Wu, X.; Pei, P.; Zhao, R.; Zeng, Y.; Zhu, Z. Nanoflower-like CoS-decorated 3D porous carbon skeleton derived from rose for a high performance nonenzymatic glucose sensor. RSC Adv. 2015, 5, 106661–106667. [Google Scholar] [CrossRef]
- Shan, B.; Ji, Y.; Zhong, Y.; Chen, L.; Li, S.; Zhang, J.; Chen, L.; Liu, X.; Chen, Y.; Yan, N.; et al. Nitrogen-containing three-dimensional biomass porous carbon materials as an efficient enzymatic biosensing platform for glucose sensing. RSC Adv. 2019, 9, 25647–25654. [Google Scholar] [CrossRef]
- Padmapriya, A.; Thiyagarajan, P.; Devendiran, M.; Kalaivani, R.A.; Shanmugharaj, A.M. Electrochemical sensor based on N, P-doped carbon quantum dots derived from the banana flower bract (Musa acuminata) biomass extract for selective and picomolar detection of dopamine. J. Electroanal. Chem. 2023, 943, 117609. [Google Scholar] [CrossRef]
- Valenga, M.G.P.; Didek, L.K.; Gevaerd, A.; Marcolino-Junior, L.H.; Bergamini, M.F. An eco-friendly alternative for voltammetric determination of creatinine in urine sample using copper(Ⅱ) immobilized on biochar. Microchem. J. 2024, 200, 110489. [Google Scholar] [CrossRef]
- Valenga, M.G.P.; Martins, G.; Martins, T.A.C.; Didek, L.K.; Gevaerd, A.; Marcolino-Junior, L.H.; Bergamini, M.F. Biochar: An environmentally friendly platform for construction of a SARS-CoV-2 electrochemical immunosensor. Sci. Total Environ. 2023, 858, 159797. [Google Scholar] [CrossRef]
- Sobhan, A.; Jia, F.; Kelso, L.C.; Biswas, S.K.; Muthukumarappan, K.; Cao, C.; Wei, L.; Li, Y. A novel activated biochar-based immunosensor for rapid detection of E. coli O157: H7. Biosensors 2022, 12, 908. [Google Scholar] [CrossRef]
- Zhong, L.; Du, X.; Jiang, Y.; Wen, J.; Wang, X.; Shuoti, W.; Peng, R.; Liao, M.; Ou, J.; Yang, Y.; et al. N-doped graphene quantum dots and gold co-modified 3D printed electrode for sensitive detection of dopamine. Microchem. J. 2025, 212, 113432. [Google Scholar] [CrossRef]
- Papavasileiou, A.V.; Děkanovský, L.; Chacko, L.; Wu, B.; Luxa, J.; Regner, J.; Paštika, J.; Koňáková, D.; Sofer, Z. Unraveling the versatility of carbon black–polylactic acid (CB/PLA) 3D-printed electrodes via sustainable electrochemical activation. Small Methods 2025, 2402214. [Google Scholar] [CrossRef]

| Methods | Temperature (°C) | Method Characteristics | Prepared Materials Characteristics | Applications | Refs. |
|---|---|---|---|---|---|
| Pyrolysis carbonization | 400~600 | Simple operation, easy to control, easy industrialized, high energy consumption | Abundant functional groups, low graphitization degree | Adsorption | [77,82,83] |
| 700~1200 | High graphitization degree, high conductivity | Capacitors, electrocatalysis, electrochemical sensing, CO2 capture | [78,79] | ||
| Hydrothermal carbonization | 100~300 | Low synthesis temperature, rapid reaction, low energy consumption | Abundant functional groups, low graphitization degree; the morphology is mostly carbon quantum dots or carbon particles. | Fluorescence sensing, electrocatalysis | [89,90,91,92] |
| Molten salt carbonization | 900~1200 | High thermal efficiency, uniform temperature distribution, reduced energy consumption, enhanced reaction rates | High graphitization degree, large specific surface area | Capacitors, electrocatalysis, electrochemical sensing, CO2 capture | [99,100,101] |
| Modified Electrodes | Detection Method | Analyte | Linear Range (μmol/L) | LOD (μmol/L) | Refs. |
|---|---|---|---|---|---|
| CQD/GCE | I-t | N2H4 | / | 39.7 | [114] |
| AS-BioC/SPCE | SWV | Ethyl paraxon | 0.025~2.5 | 1.63 × 10−3 | [115] |
| AC900/GCE | LSV | 4-nitrophenol | 1~500 | 0.16 | [116] |
| NDC/GCE | DPV | Bisphenol A | 1.0~50.0 | 1.068 | [117] |
| CLC/GCE | CV | HQ | 0.5~3000 | 0.47 | [118] |
| CC | 1.0~3000 | 0.40 | |||
| DLSNC/GCE | CV | 1-NP | 1.0~25 | 0.64 | [120] |
| 2-NP | 1.0~25 | 0.61 | |||
| LSC-THP/CS/GCE | DPV | HQ | 10~2000 | 1.23 | [119] |
| CC | 10~2000 | 0.44 | |||
| HC/CPE | DPAdSV | Pb2+ | 0.50~7.06 | 0.055 | [92] |
| WNCF/BFGCE | DPAdSV | Pb2+ | 0.5~100 μg/L | 0.2 μg/L | [123] |
| BDCM+CPE | DPAdSV | Cu2+ | 1.0~15.0 | 1.09 | [124] |
| CNDS/SPCE | SWASV | Hg2+ Pb2+ Cd2+ Ni2+ | / | 124 ng/L 551 ng/L 453 ng/L 608 ng/L | [125] |
| CGC-600/GCE | DPV | Hg2+ | 10~100 | 6.17 | [126] |
| SnS2@BC/GCE | DPV | Pb2+ Hg2+ | / | 0.28 0.55 | [127] |
| AL-1/GCE | I-V | PS (100 nm) | / | 520 | [129] |
| SF-1/GCE | I-V | PS (100 nm) | / | 440 | [129] |
| Modified Electrodes | Detection Method | Analyte | Linear Range (μmol/L) | LOD (μmol/L) | Refs. |
|---|---|---|---|---|---|
| PNC/PGE | DPV | Chloramphenicol | 1~200 | 0.57 | [78] |
| ZKAKC/GCE | DPV | Acetaminophen | 0.01~20 | 0.004 | [131] |
| PDC/GCE | DPV | Acetaminophen | 0.5~10 | 0.335 | [132] |
| NSP-PC/GCE | LSV | Metronidazole | 0.1~45 50~350 | 0.013 | [133] |
| Pt—Re NP/PAC/GCE | LSV | Furazolidone | 1~299 | 0.075 | [134] |
| RHG/CPE | SWV | Mefenamic acid | 0.001~6000 | 2.13 × 10−3 | [135] |
| C-CS-700/GCE | DPV | Nitrofurazone | 0.4~80 | 0.11 | [136] |
| 3D-PAC/GCE | DPV | Furazolidone | 0.5~290 | 0.5 × 10−3 | [137] |
| Au-Pt@BPC/CILE | DPV | Baicalein | 0.48~2.0 4.0~140.0 | 0.01 | [138] |
| BPBC-MWCNT/GCE | DPV | Baicalein | 0.004~100 | 1.33 × 10−3 | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Deng, Y.; Liu, X.; Wang, B.; Yang, F. Recent Advances on Biomass-Derived Carbon Materials-Based Electrochemical Sensors. Molecules 2025, 30, 3046. https://doi.org/10.3390/molecules30143046
Wang D, Deng Y, Liu X, Wang B, Yang F. Recent Advances on Biomass-Derived Carbon Materials-Based Electrochemical Sensors. Molecules. 2025; 30(14):3046. https://doi.org/10.3390/molecules30143046
Chicago/Turabian StyleWang, Dacheng, Yan Deng, Xiaowei Liu, Baoli Wang, and Feng Yang. 2025. "Recent Advances on Biomass-Derived Carbon Materials-Based Electrochemical Sensors" Molecules 30, no. 14: 3046. https://doi.org/10.3390/molecules30143046
APA StyleWang, D., Deng, Y., Liu, X., Wang, B., & Yang, F. (2025). Recent Advances on Biomass-Derived Carbon Materials-Based Electrochemical Sensors. Molecules, 30(14), 3046. https://doi.org/10.3390/molecules30143046





