CdZnS Nanowire Decorated with Graphene for Efficient Photocatalytic Hydrogen Evolution
Abstract
1. Introduction
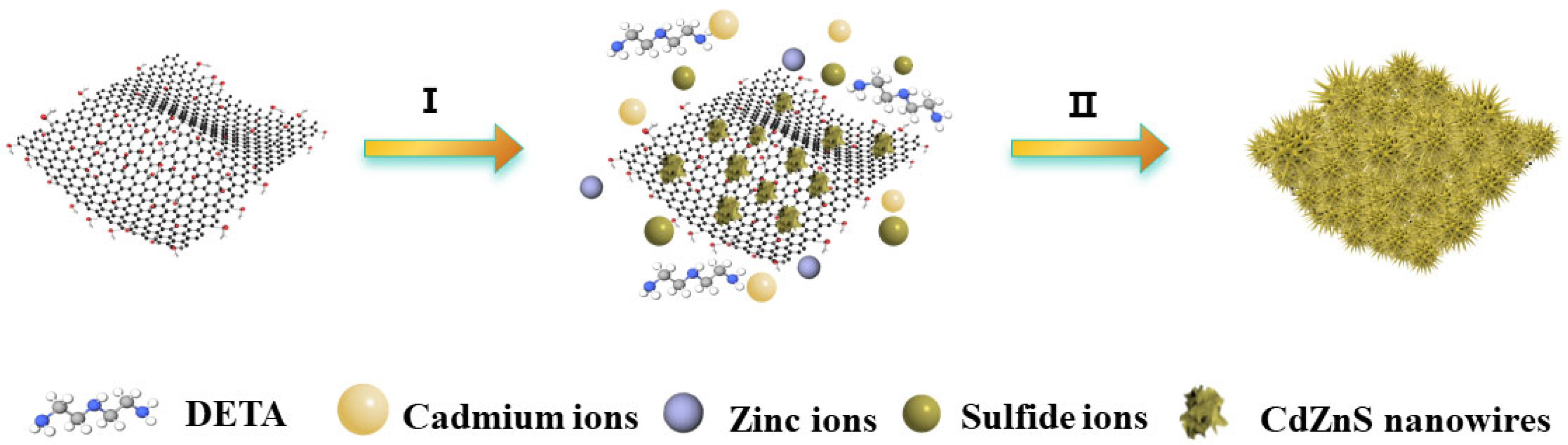
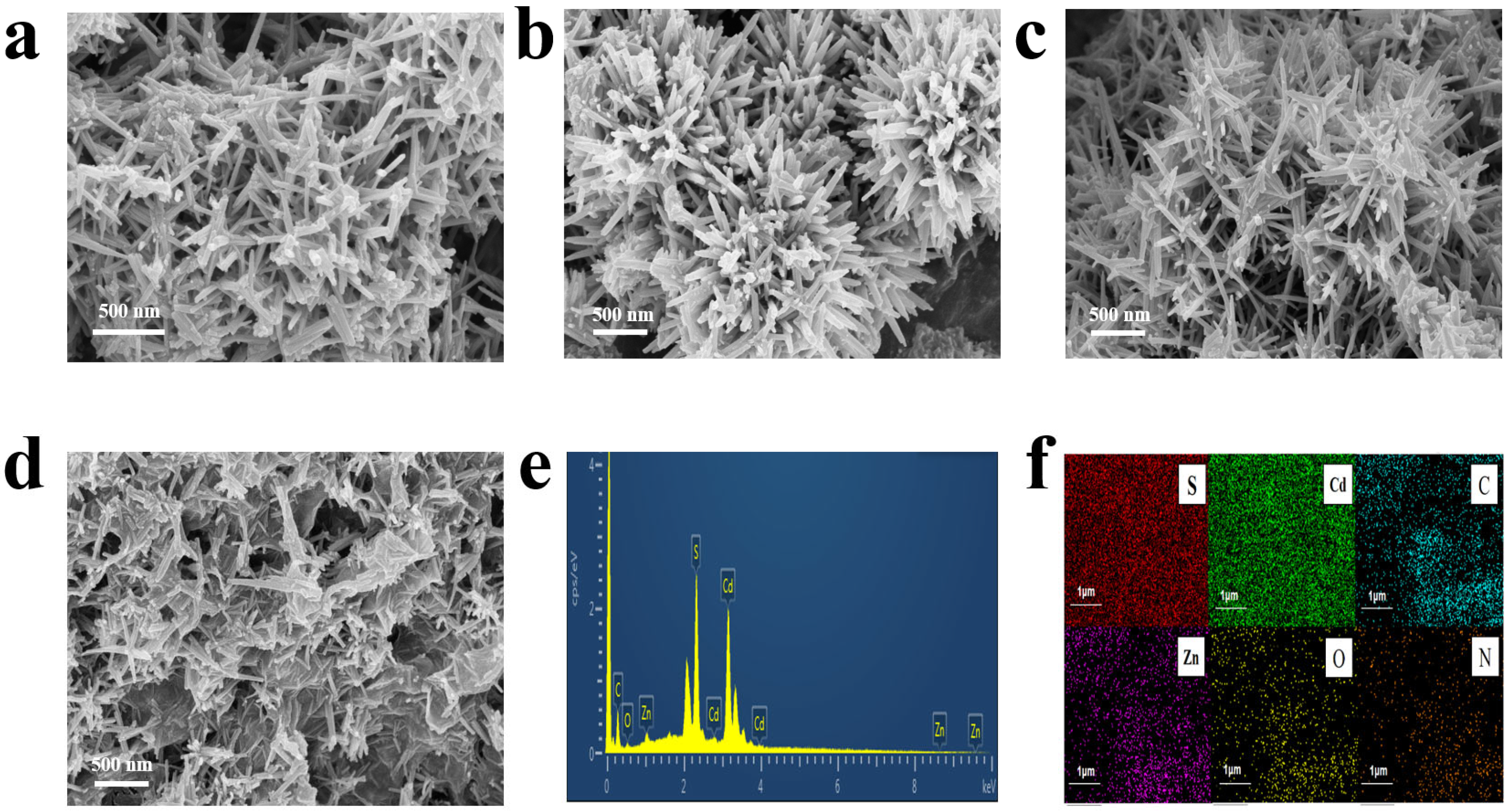
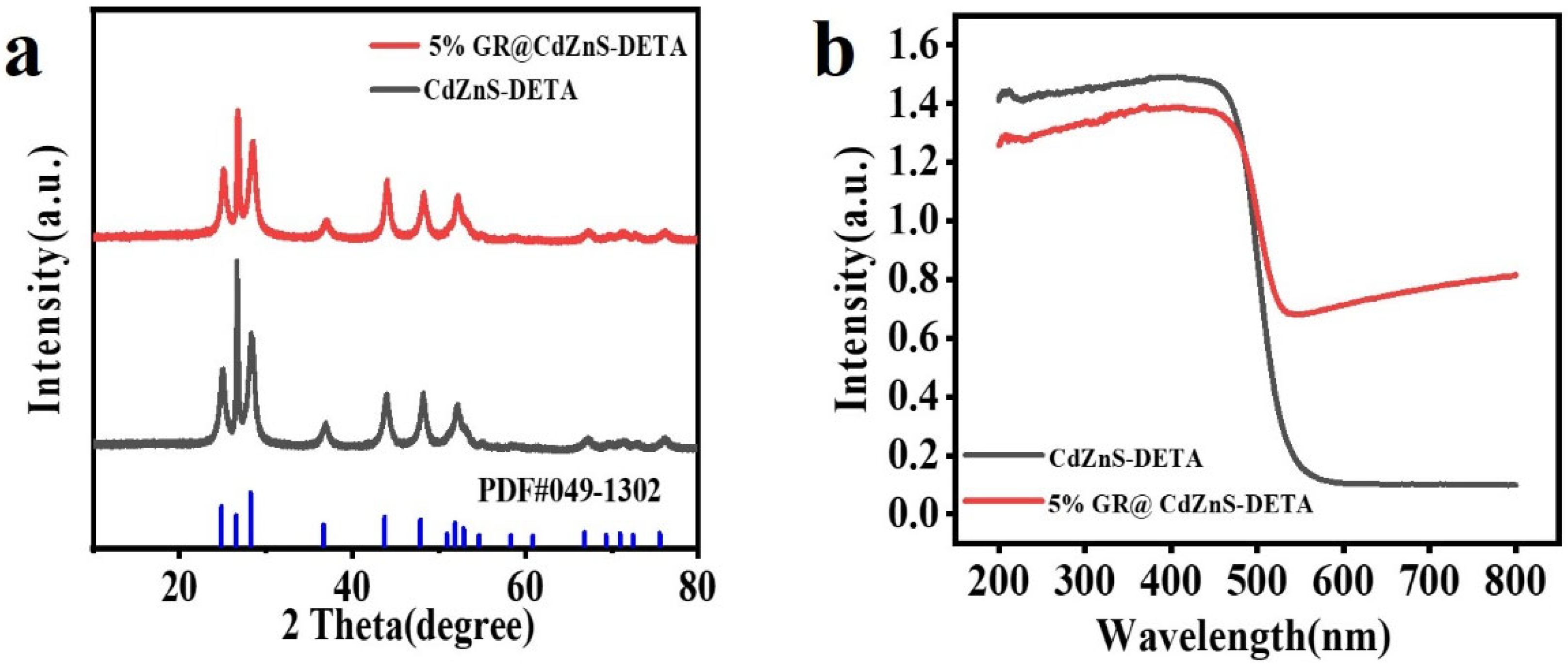
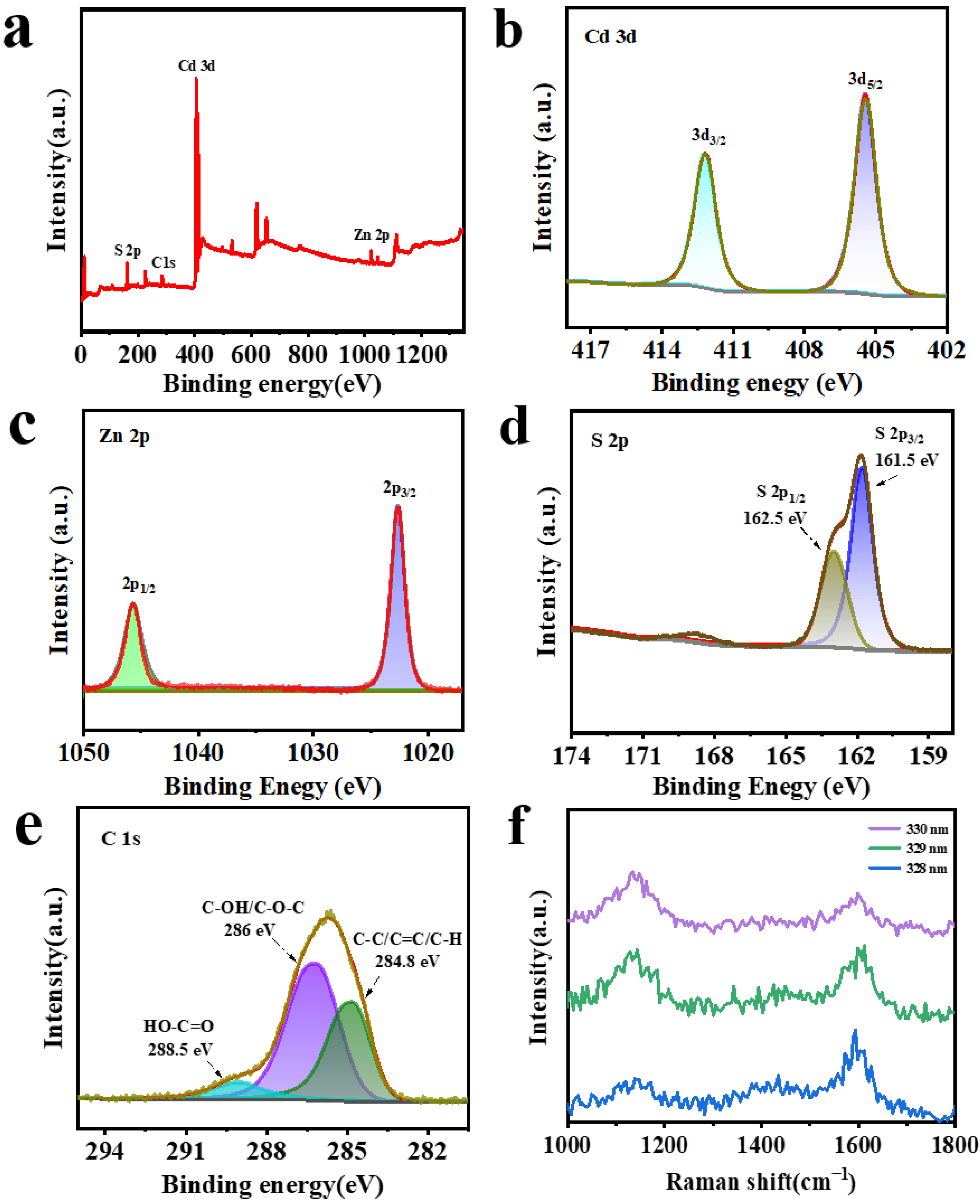
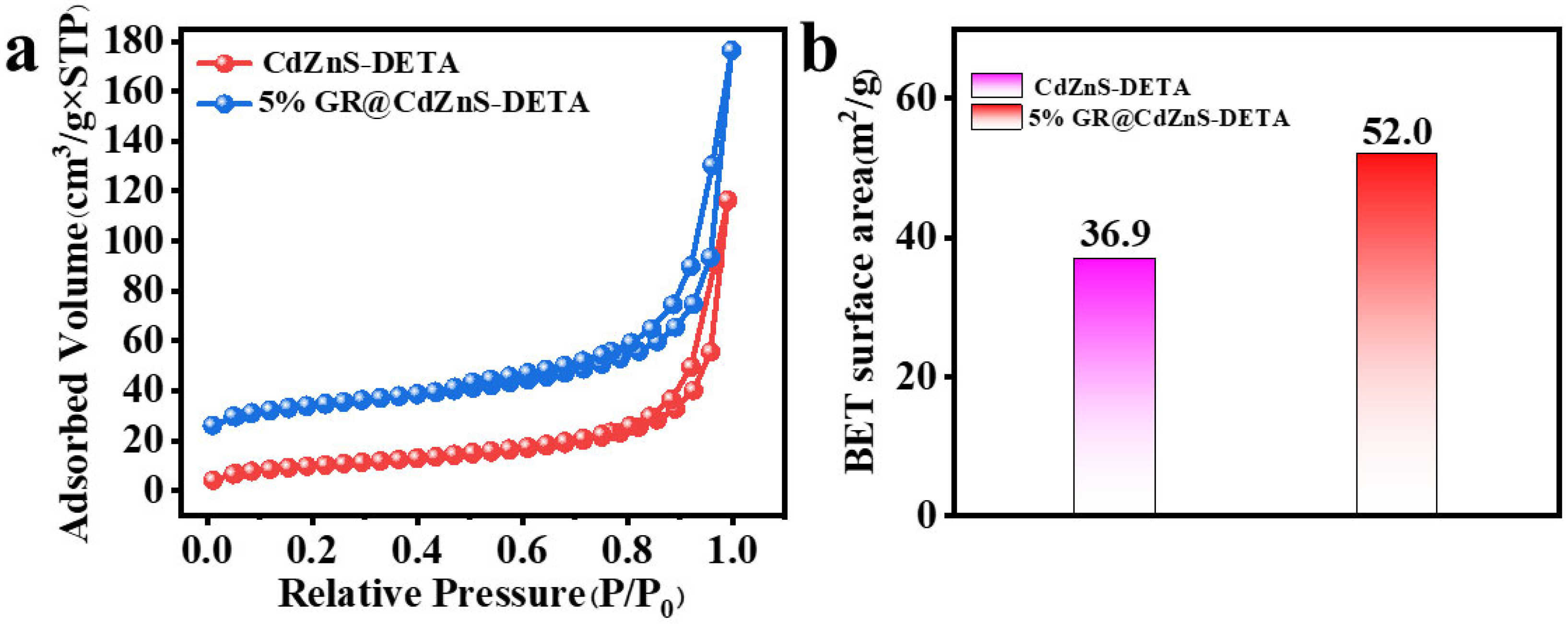
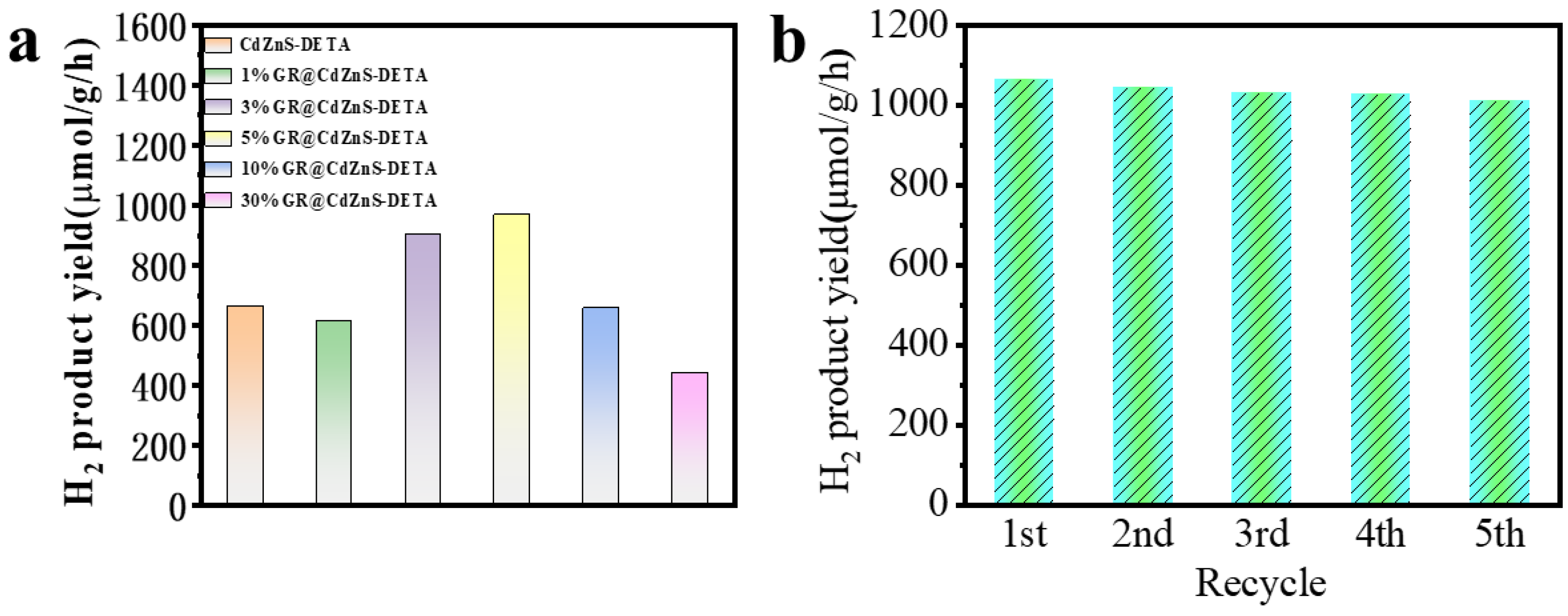
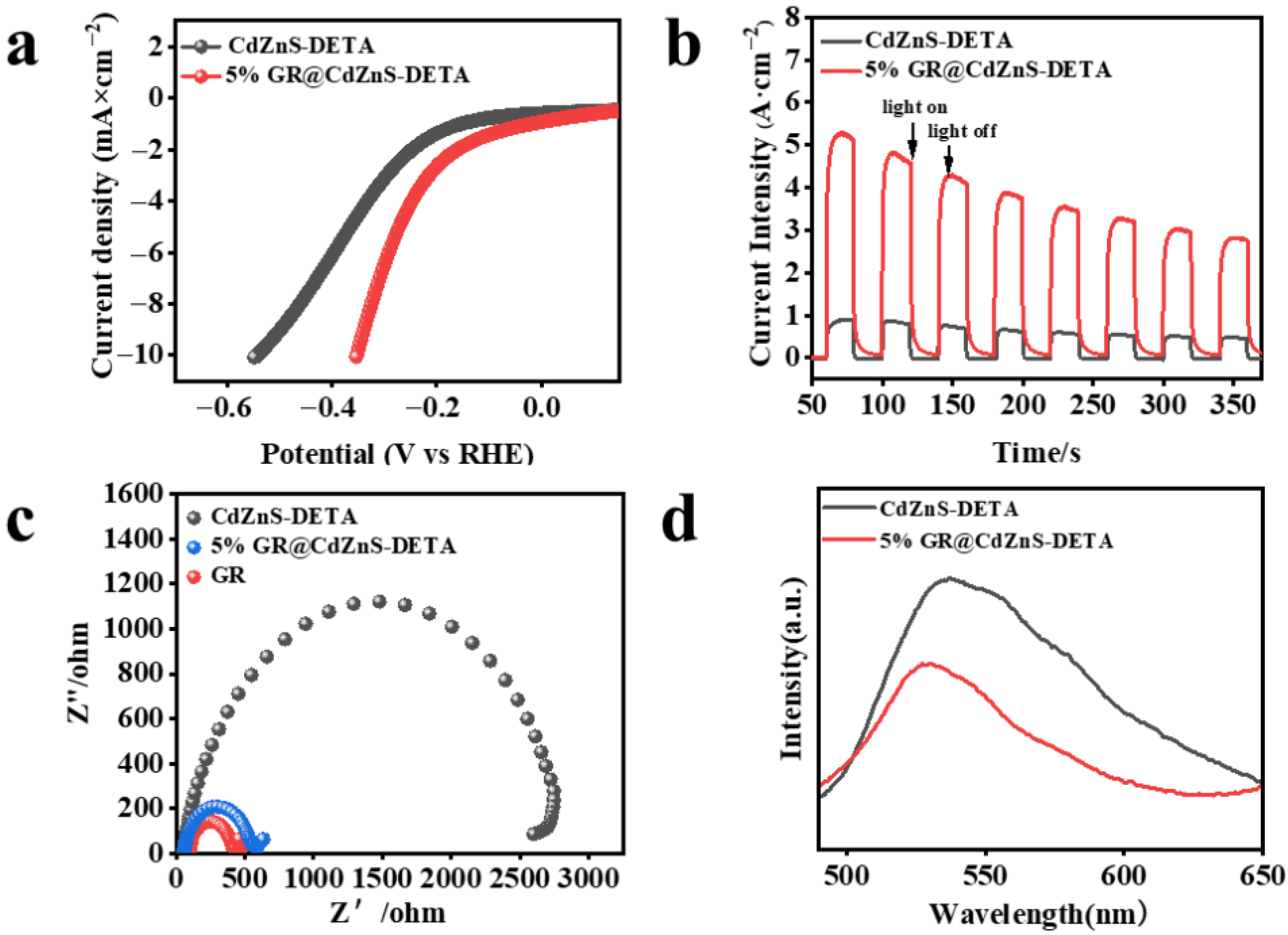
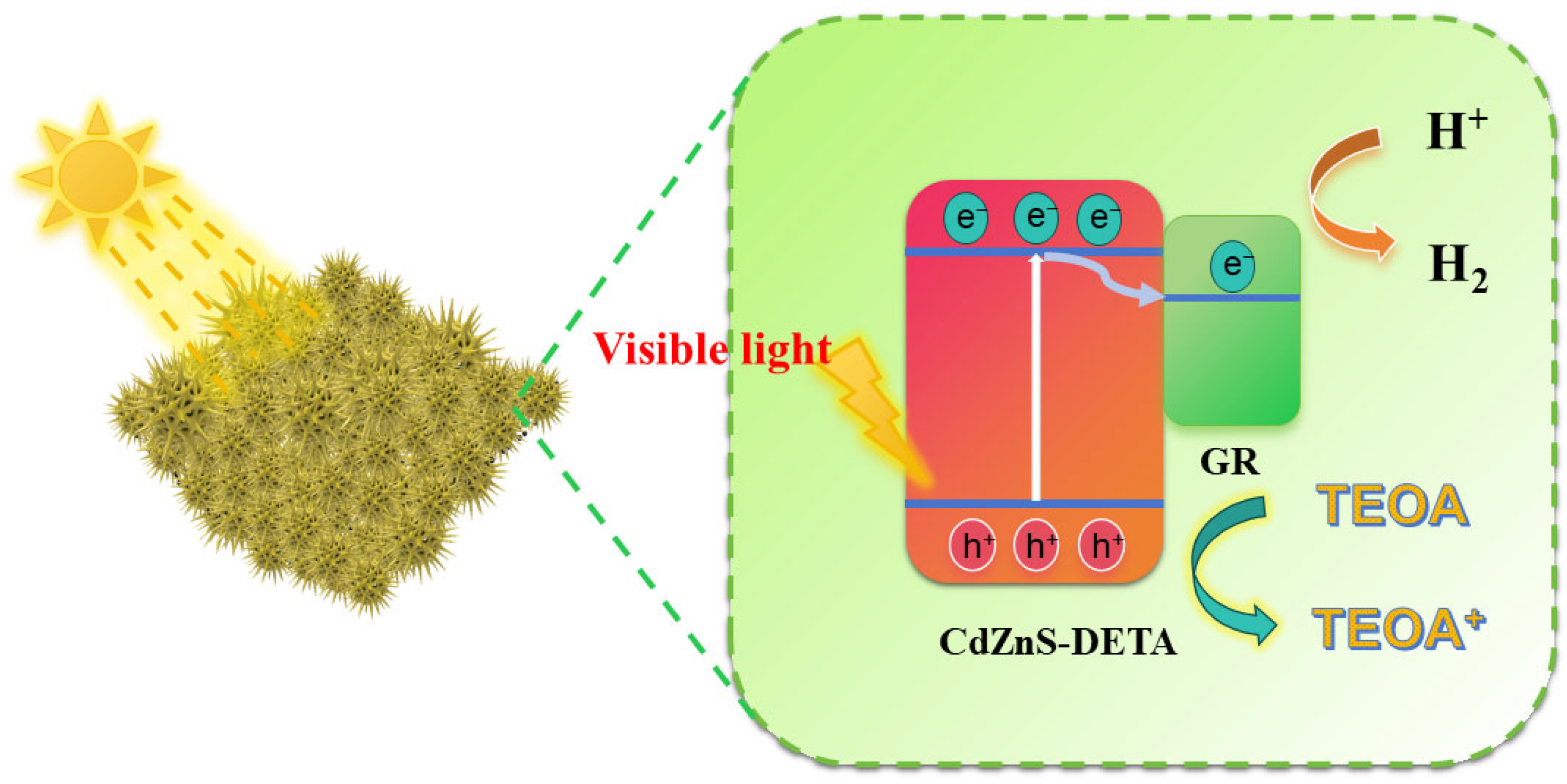
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Synthesis of GR@CdZnS
3.3. Photocatalytic Activity Evaluation H2 Evolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Z.; Jin, W.; Sun, Y.H.; Li, X.; Mao, L.; Cai, X.Y.; Lou, Z.Z. Interface charge separation in Cu2CoSnS4/ZnIn2S4 heterojunction for boosting photocatalytic hydrogen production. Chin. J. Struct. Chem. 2023, 42, 9. [Google Scholar] [CrossRef]
- He, H.; Wang, Z.; Zhang, J.; Shao, C.; Dai, K.; Fan, K. Interface Chemical Bond Enhanced Ions Intercalated Carbon Nitride/CdSe-Diethylenetriamine S-Scheme Heterojunction for Photocatalytic H2O2 Synthesis in Pure Water. Adv. Funct. Mater. 2024, 34, 2315426. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.Y.; Ma, S.Q.; Zhang, Y.J.; Wang, L.Z.; Zhu, G.Q.; Huang, W.; Wang, S.C. Induced dipole moments in amorphous ZnCdS catalysts facilitate photocatalytic H2 evolution. Nat. Commun. 2024, 15, 11. [Google Scholar] [CrossRef]
- Zheng, M.; Wu, P.; Li, L.; Yu, F.; Ma, J. Adsorption/desorption behavior of ciprofloxacin on aged biodegradable plastic PLA under different exposure conditions. J. Environ. Chem. Eng. 2023, 11, 109256. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wang, Y.C.; Wang, H.J.; Zhong, D.C.; Lu, T.B. Enhancing photocatalytic performance of metal-organic frameworks for CO2 reduction by a bimetallic strategy. Chin. Chem. Lett. 2022, 33, 2065–2068. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Yan, Y.-Q.; Wei, Y.; Wang, J.; Li, A.; Huang, W.-Y.; Zhang, J.-L.; Yang, K.; Lu, K.-Q. Decorating ZnIn2S4 with earth-abundant Co9S8 and Ni2P dual cocatalysts for boosting photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2024, 78, 452–459. [Google Scholar] [CrossRef]
- Li, M.; Van Der Veer, M.; Yang, X.; Weng, B.; Shen, L.; Huang, H.; Dong, X.; Wang, G.; Roeffaers, M.B.J.; Yang, M.-Q. Twin boundary defect engineering in Au cocatalyst to promote alcohol splitting for coproduction of H2 and fine chemicals. J. Colloid Interface Sci. 2024, 657, 819–829. [Google Scholar] [CrossRef]
- Pan, Z.M.; Zhang, G.G.; Wang, X.C. Polymeric Carbon Nitride/Reduced Graphene Oxide/Fe2O3: All-Solid-State Z-Scheme System for Photocatalytic Overall Water Splitting. Angew. Chem.-Int. Ed. 2019, 58, 7102–7106. [Google Scholar] [CrossRef]
- Jin, Y.X.; Zheng, D.D.; Fang, Z.P.; Pan, Z.M.; Wang, S.B.; Hou, Y.D.; Savateev, O.; Zhang, Y.F.; Zhang, G.G. Salt-melt synthesis of poly(heptazine imide) in binary alkali metal bromides for enhanced visible-light photocatalytic hydrogen production. Interdiscip. Mater. 2024, 3, 389–399. [Google Scholar] [CrossRef]
- Weng, Z.; Lin, Y.; Guo, S.; Zhang, X.; Guo, Q.; Luo, Y.; Ou, X.; Ma, J.; Zhou, Y.; Jiang, J.; et al. Site Engineering of Covalent Organic Frameworks for Regulating Peroxymonosulfate Activation to Generate Singlet Oxygen with 100 % Selectivity. Angew. Chem. Int. Ed. 2023, 135, e202310934. [Google Scholar] [CrossRef]
- Fu, W.; Fan, J.J.; Xiang, Q.J. Ag2S quantum dots decorated on porous cubic-CdS nanosheets-assembled flowers for photocatalytic CO2 Reduction. Chin. J. Struct. Chem. 2022, 41, 2206039–2206047. [Google Scholar]
- Zheng, X.L.; Yang, Y.Q.; Song, Y.M.; Ma, Z.X.; Gao, Q.Z.; Liu, Y.H.; Li, J.; Wu, X.; Wang, X.B.; Mao, W.H.; et al. Recent advances in photocatalytic hydrogen evolution of AgIn5S8-based photocatalysts. Interdiscip. Mater. 2023, 2, 669–688. [Google Scholar] [CrossRef]
- Yan, Y.Q.; Wu, Y.Z.; Wu, Y.H.; Weng, Z.L.; Liu, S.J.; Liu, Z.G.; Lu, K.Q.; Han, B. Recent advances of CeO2-based composite materials for photocatalytic applications. ChemSusChem 2024, 17, e202301778. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, C.; Shen, L. Polar materials for photocatalytic applications: A critical review. Interdiscip. Mater. 2024, 3, 530–564. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Zhang, J.; Ruzimuradov, O.; Dai, K.; Low, J. Tunable Interfacial Charge Transfer in a 2D–2D Composite for Efficient Visible-Light-Driven CO2 Conversion. Adv. Mater. 2023, 35, 2300643. [Google Scholar] [CrossRef]
- Li, S.; Dong, K.; Cai, M.; Li, X.; Chen, X. A plasmonic S-scheme Au/MIL-101(Fe)/BiOBr photocatalyst for efficient synchronous decontamination of Cr(VI) and norfloxacin antibiotic. eScience 2024, 4, 100208. [Google Scholar] [CrossRef]
- Su, B.; Kong, Y.; Wang, S.; Zuo, S.; Lin, W.; Fang, Y.; Hou, Y.; Zhang, G.; Zhang, H.; Wang, X. Hydroxyl-Bonded Ru on Metallic TiN Surface Catalyzing CO2 Reduction with H2O by Infrared Light. J. Am. Chem. Soc. 2023, 145, 27415–27423. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wu, Y.; Wang, J.; Wu, Y.-H.; Weng, Z.; Huang, W.-Y.; Yang, K.; Zhang, J.-L.; Li, Q.; Lu, K.-Q.; et al. Rationally designed dual cocatalysts on ZnIn2S4 nanoflowers for photoredox coupling of benzyl alcohol oxidation with H2 evolution. J. Mater. Chem. A 2024, 12, 18986–18992. [Google Scholar] [CrossRef]
- Weng, Z.; Lin, Y.; Han, B.; Zhang, X.; Guo, Q.; Luo, Y.; Ou, X.; Zhou, Y.; Jiang, J. Donor-acceptor engineered g-C3N4 enabling peroxymonosulfate photocatalytic conversion to 1O2 with nearly 100% selectivity. J. Hazard. Mater. 2023, 448, 130869. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.W.; Guo, L.J. A novel method for the preparation of a highly stable and active CdS photocatalyst with a special surface nanostructure. J. Phys. Chem. B 2006, 110, 11139–11145. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, X.H.; Yu, L.; Wang, Y.R.; Ning, J.Q.; Xu, S.J.; Lou, X.W. Carbon-Coated CdS Petalous Nanostructures with Enhanced Photostability and Photocatalytic Activity. Angew. Chem.-Int. Ed. 2013, 52, 5636–5639. [Google Scholar] [CrossRef]
- Ke, D.N.; Liu, S.L.; Dai, K.; Zhou, J.P.; Zhang, L.; Peng, T.Y. CdS/regenerated cellulose nanocomposite films for highly efficient photocatalytic H2 production under visible light irradiation. J. Phys. Chem. C 2009, 113, 16021–16026. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Wu, S.; Liu, Y.; Sun, Y.; Cao, Y.; Hsu, J.-P.; Shen Wee, A.T.; Jiang, J. An ultra-sensitive electrochemical sensor based on 2D g-C3N4/CuO nanocomposites for dopamine detection. Carbon 2018, 130, 652–663. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Y.; Dong, K.; Chen, X.; Li, S. Floatable S-scheme Bi2WO6/C3N4/carbon fiber cloth composite photocatalyst for efficient water decontamination. Chin. J. Catal. 2023, 52, 239–251. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.S.; Guo, W.; Xia, B.Y. Emerging two-dimensional nanocatalysts for electrocatalytic hydrogen production. Chin. Chem. Lett. 2022, 33, 1831–1840. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Hao, J.-G.; Wei, Y.; Weng, B.; Ge, S.; Yang, K.; Lu, S.; Yang, M.-Q.; Liao, Y. Photocatalytic conversion of diluted CO2 into tunable syngas via modulating transition metal hydroxides. Inorg. Chem. 2023, 63, 795–802. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Li, Y.-H.; Zhang, F.; Qi, M.-Y.; Chen, X.; Tang, Z.-R.; Yamada, Y.M.A.; Anpo, M.; Conte, M.; Xu, Y.-J. Rationally designed transition metal hydroxide nanosheet arrays on graphene for artificial CO2 reduction. Nat. Commun. 2020, 11, 5181. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Chen, J.; Li, L.; Zhao, J.; Zhu, Z. Hierarchical assemblies of CdxZn1−xS complex architectures and their enhanced visible-light photocatalytic activities for H2-production. J. Alloys Compd. 2013, 578, 571–576. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Wen, T.; Zhang, S.; Chang, B.; Guo, Y.; Yang, B. Mesoporous Cd1−xZnxS microspheres with tunable bandgap and high specific surface areas for enhanced visible-light-driven hydrogen generation. J. Colloid Interface Sci. 2016, 467, 97–104. [Google Scholar] [CrossRef]
- Xing, C.J.; Zhang, Y.J.; Yan, W.; Guo, L.J. And structure-controlled solid solution of Cd1-xZnxS photocatalyst for hydrogen production by water splitting. Int. J. Hydrogen Energy 2006, 31, 2018–2024. [Google Scholar] [CrossRef]
- Wang, G.R.; Quan, Y.K.; Yang, K.C.; Jin, Z.L. EDA-assisted synthesis of multifunctional snowflake-Cu2S/CdZnS S-scheme heterojunction for improved the photocatalytic hydrogen evolution. J. Mater. Sci. Technol. 2022, 121, 28–39. [Google Scholar] [CrossRef]
- Li, H.; Tao, S.R.; Wan, S.J.; Qiu, G.G.; Long, Q.; Yu, J.G.; Cao, S.W. S-scheme heterojunction of ZnCdS nanospheres and dibenzothiophene modified graphite carbon nitride for enhanced H2 production. Chin. J. Catal. 2023, 46, 167–176. [Google Scholar] [CrossRef]
- Xue, W.H.; Chang, W.X.; Hu, X.Y.; Fan, J.; Liu, E.Z. 2D mesoporous ultrathin Cd0.5Zn0.5S nanosheet: Fabrication mechanism and application potential for photocatalytic H2 evolution. Chin. J. Catal. 2021, 42, 152–163. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Wei, Y.; Xie, L.; Chen, H.-Q.; Wang, J.; Yang, K.; Zou, L.-X.; Deng, T.; Lu, K.-Q. Decorating CdS with cobaltous hydroxide and graphene dual cocatalyst for photocatalytic hydrogen production coupled selective benzyl alcohol oxidation. Mol. Catal. 2024, 553, 113738. [Google Scholar] [CrossRef]
- Jiang, J.; Ou-yang, L.; Zhu, L.; Zheng, A.; Zou, J.; Yi, X.; Tang, H. Dependence of electronic structure of g-C 3N4 on the layer number of its nanosheets: A study by Raman spectroscopy coupled with first-principles calculations. Carbon 2014, 80, 213–221. [Google Scholar] [CrossRef]
- Tang, Z.L.; He, W.J.; Wang, Y.L.; Wei, Y.C.; Yu, X.L.; Xiong, J.; Wang, X.; Zhang, X.; Zhao, Z.; Liu, J. Ternary heterojunction in rGO-coated Ag/Cu2O catalysts for boosting selective photocatalytic CO2 reduction into CH4. Appl. Catal. B-Environ. Energy 2022, 311, 14. [Google Scholar] [CrossRef]
- Wu, Y.L.; Li, Y.J.; Zhang, L.J.; Jin, Z.L. NiAl-LDH In-Situ Derived Ni2P and ZnCdS Nanoparticles Ingeniously Constructed S-Scheme Heterojunction for Photocatalytic Hydrogen Evolution. ChemCatChem 2022, 14, 13. [Google Scholar] [CrossRef]
- Tian, J.Z.; Cao, X.F.; Sun, T.; Fan, J.; Miao, H.; Chen, Z.; Li, D.; Liu, E.Z.; Zhu, Y.H. S-scheme Co3(PO4)2/Twinned-Cd0.5Zn0.5S homo-heterojunction for enhanced photocatalytic H2 evolution. Chem. Eng. J. 2023, 471, 13. [Google Scholar] [CrossRef]
- He, K.; Guo, L.J. ZnS/Cd1-xZnxS composite photocatalyst synthesized with excessive strong alkali solution as a hydrothermal solvent: The chemical equilibrium theory of the synthesis process and its influence on photocatalytic performance. Int. J. Hydrogen Energy 2023, 48, 10507–10520. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, W.Y.; Du, B.; Zhou, C.X.; Feng, G.; Cai, J.X.; Wang, T.; Zhang, R.B. Noble metal-free Cd1-xZnxS-Zn1-yCdyS heterostructures for stable and highly effective photocatalytic hydrogen evolution. J. Alloys Compd. 2017, 705, 683–690. [Google Scholar] [CrossRef]
- Su, B.; Zheng, M.; Lin, W.; Lu, X.F.; Luan, D.; Wang, S.; Lou, X.W. S-scheme Co9S8@Cd0.8Zn0.2S-DETA hierarchical nanocages bearing organic CO2 activators for photocatalytic syngas production. Adv. Energy Mater. 2023, 13, 2203290. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Shen, Y.; Liu, Q.; Deng, T.; Lu, K.; Hong, Z. CdZnS Nanowire Decorated with Graphene for Efficient Photocatalytic Hydrogen Evolution. Molecules 2025, 30, 3042. https://doi.org/10.3390/molecules30143042
Wang Z, Shen Y, Liu Q, Deng T, Lu K, Hong Z. CdZnS Nanowire Decorated with Graphene for Efficient Photocatalytic Hydrogen Evolution. Molecules. 2025; 30(14):3042. https://doi.org/10.3390/molecules30143042
Chicago/Turabian StyleWang, Zemeng, Yunsheng Shen, Qingsheng Liu, Tao Deng, Kangqiang Lu, and Zhaoguo Hong. 2025. "CdZnS Nanowire Decorated with Graphene for Efficient Photocatalytic Hydrogen Evolution" Molecules 30, no. 14: 3042. https://doi.org/10.3390/molecules30143042
APA StyleWang, Z., Shen, Y., Liu, Q., Deng, T., Lu, K., & Hong, Z. (2025). CdZnS Nanowire Decorated with Graphene for Efficient Photocatalytic Hydrogen Evolution. Molecules, 30(14), 3042. https://doi.org/10.3390/molecules30143042






