Recovery of Natural Pyrazines and Alcohols from Fusel Oils Using an Innovative Extraction Installation
Abstract
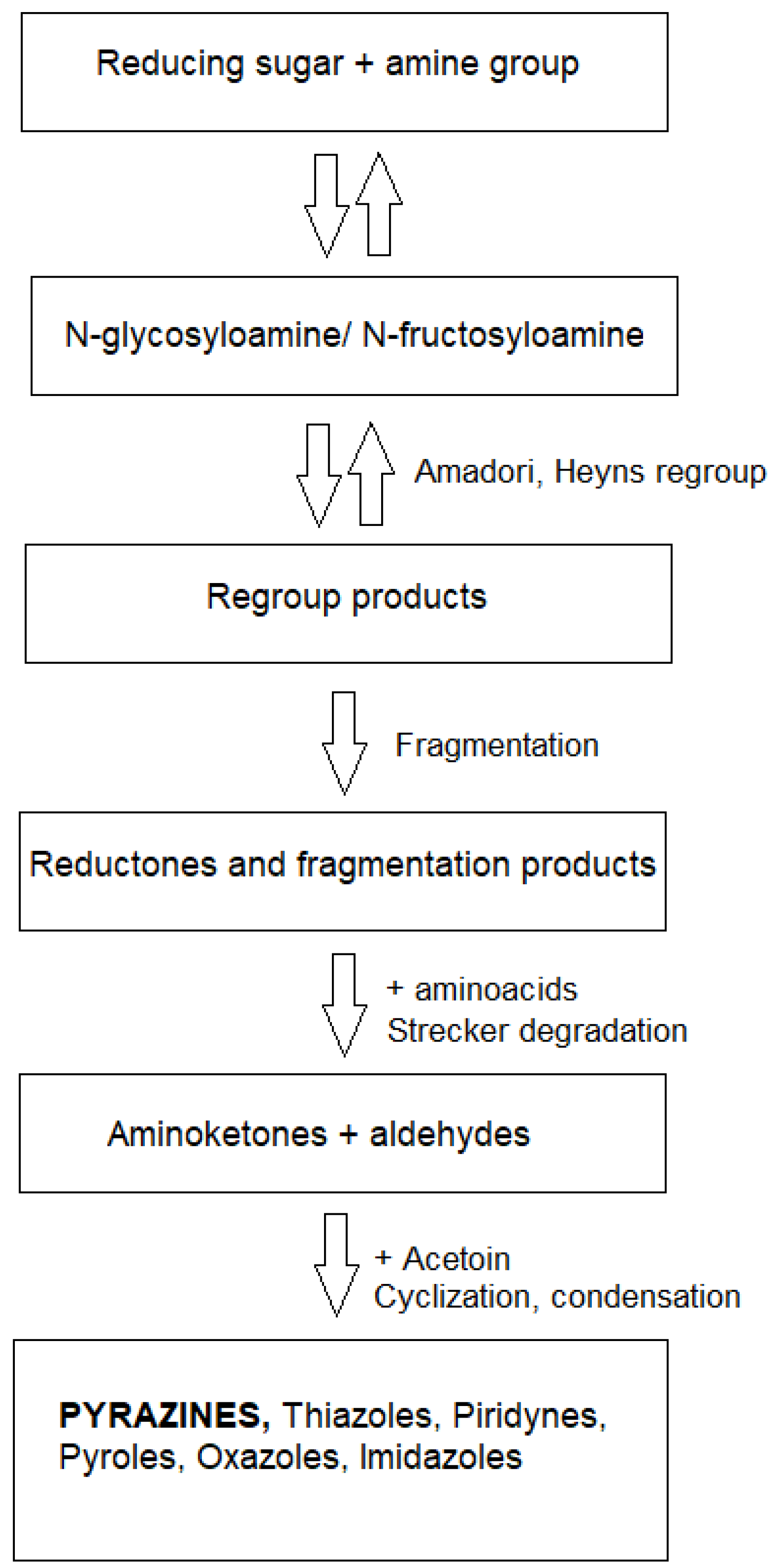
1. Introduction
2. Results and Discussion
2.1. Determination of the Chemical Composition of Fusel Oil
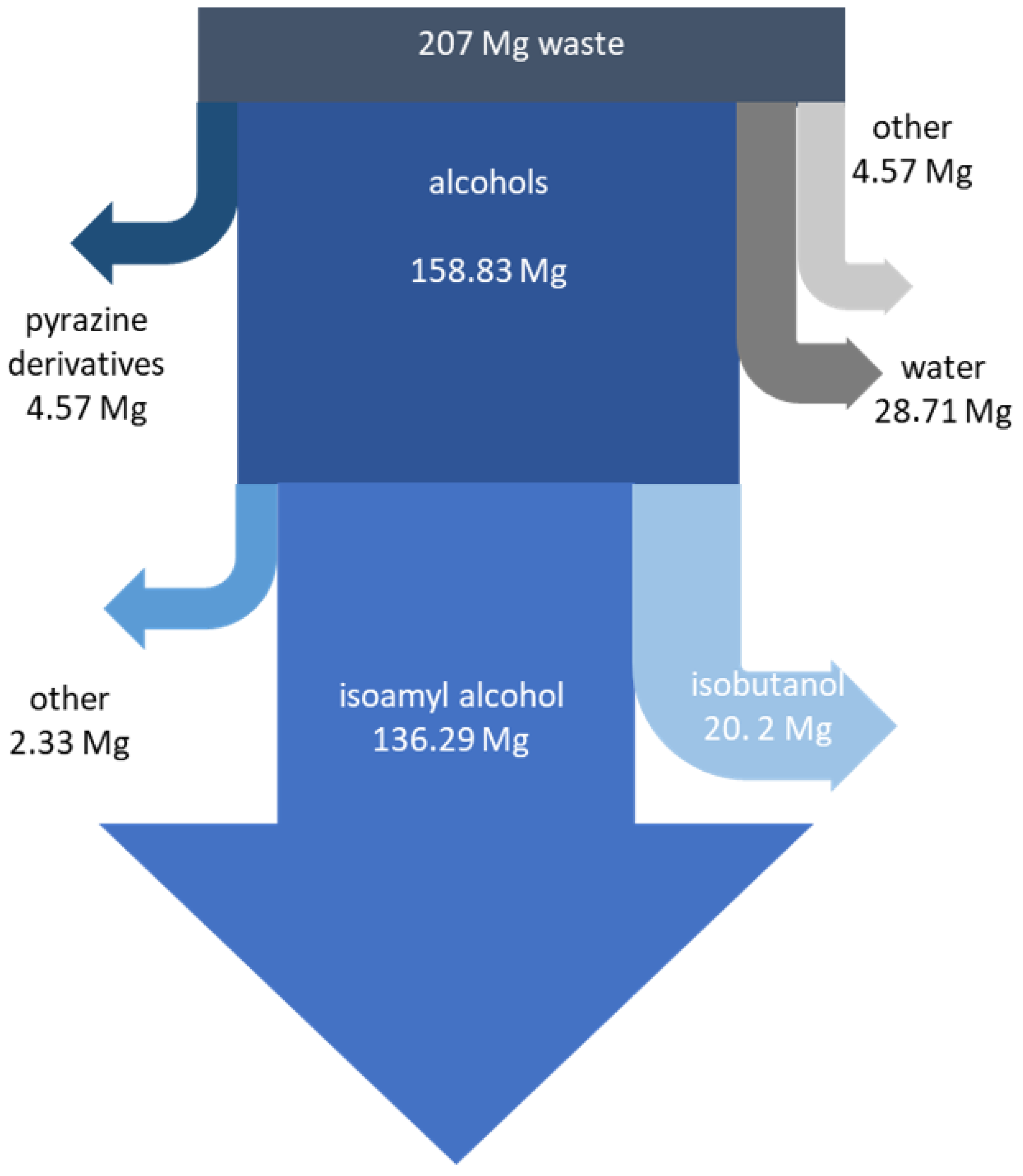
2.2. Recovery of Valuable Components from Fusel Oils
3. Materials and Methods
3.1. Chemical Reagents
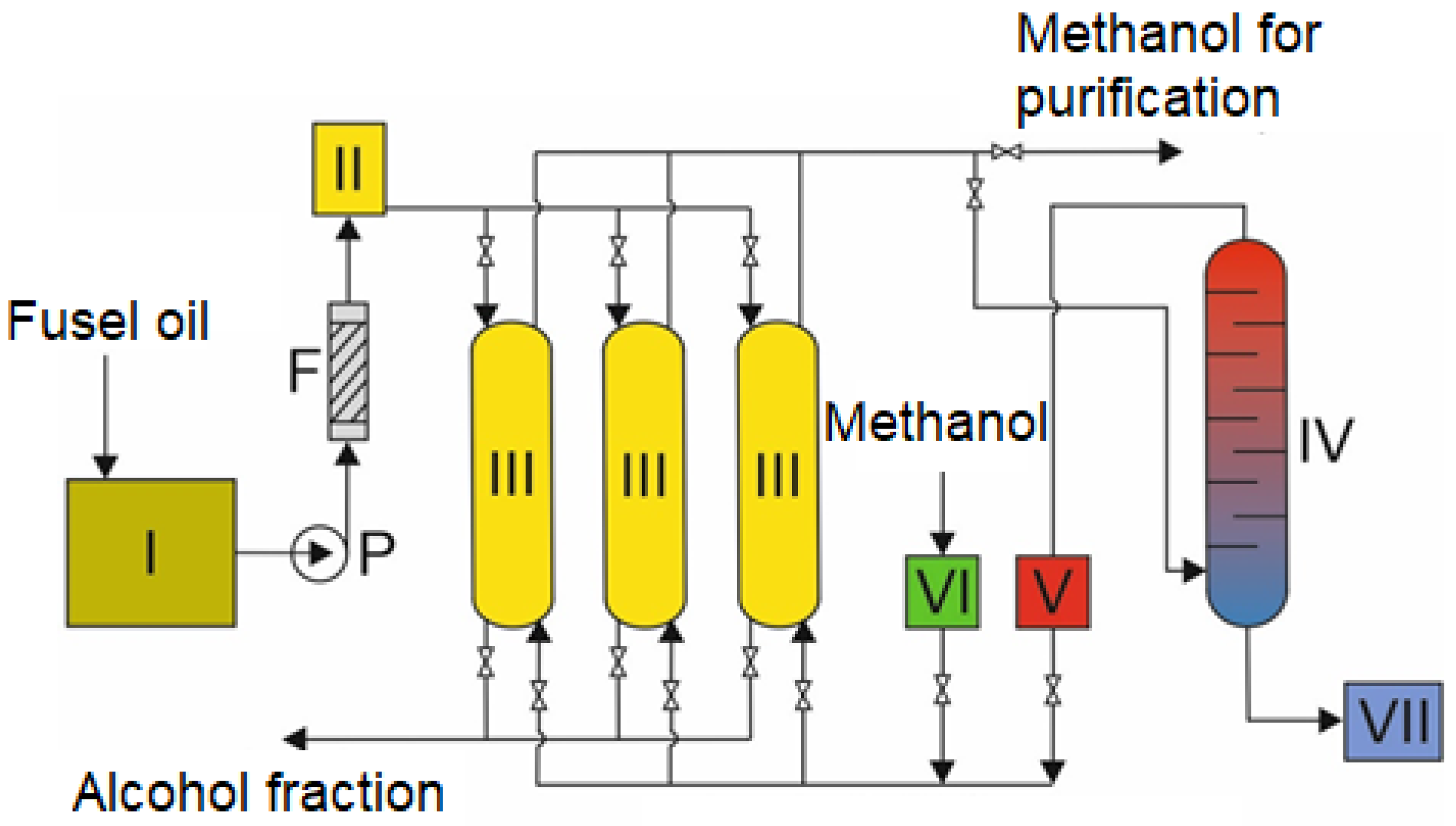
3.2. Characteristics of the Installation for Recovery of Pyrazines from Fusel Oils
3.3. Fractional Distillation of a Water-Alcohol Mixture
3.4. Chromatographic Analysis of Fusel Oils and Extracts Containing Pyrazines
3.5. Water Content Analysis
4. Limitations and Future Perspective
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Directive, E. Directive 2008/98/EC of the European parliament and of the Council of 19 November 2008 on waste and repealing certain Directives. Off. J. Eur. Union L. 2008, 312, 3e30. [Google Scholar]
- Mymrin, V.; Pedroso, D.E.; Pedroso, C.; Alekseev, K.; Avanci, M.A.; Winter, E.; Cechin, L.; Rolim, P.H.B.; Iarozinski, A.; Catai, R.E. Environmentally clean composites with hazardous aluminum anodizing sludge, concrete waste, and lime production waste. J. Clean. Prod. 2018, 174, 380–388. [Google Scholar] [CrossRef]
- Studziński, W.; Gackowska, A.; Dadzibóg, M. Management of selected waste generated during cable production. Environ. Sci. Pollut. Res. 2024, 31, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.L.B.; Cunha, G.N.; Santos, P.; Meireles, M.A.; Martínez, J. Fusel oil: Water adsorption and enzymatic synthesis of acetate esters in supercritical CO2. J. Supercrit. Fluids 2018, 142, 22–31. [Google Scholar] [CrossRef]
- Ser Schaft, V.P. Approaches to production of natural flavours. In Flavour Development, Analysis and Perception in Food and Beverages; Woodhead Publishing: Cambridge, UK, 2015; pp. 235–248. [Google Scholar] [CrossRef]
- Dörmő, N.; Bélafi-Bakó, K.; Bartha, L.; Ehrenstein, U.; Gubicza, L. Manufacture of an environmental-safe biolubricant from fusel oil by enzymatic esterification in solvent-free system. Biochem. Eng. J. 2004, 21, 229–234. [Google Scholar] [CrossRef]
- Özgülsün, A.; Karaosmanôglu, F.; Tüter, M. Esterification reaction of oleic acid with a fusel oil fraction for production of lubricating oil. J. Am. Oil Chem. Soc. 2000, 77, 105–110. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, R.; Yang, F.; Xie, Y.; Guo, Y.; Yao, W.; Zhou, W. Control strategies of pyrazines generation from Maillard reaction. Trends Food Sci. Technol. 2021, 112, 795–807. [Google Scholar] [CrossRef]
- Liu, X.; Quan, W. Progress on the Synthesis Pathways and Pharmacological Effects of Naturally Occurring Pyrazines. Molecules 2024, 29, 3597. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef]
- Alexandri, M.; Kachrimanidou, V.; Papapostolou, H.; Papadaki, A.; Kopsahelis, N. Sustainable Food Systems: The Case of Functional Compounds towards the Development of Clean Label Food Products. Foods 2022, 11, 2796. [Google Scholar] [CrossRef]
- Martirosyan, D.; Miller, E. Bioactive compounds: The key to functional foods. Bioact. Compd. Health Dis. 2018, 1, 36–39. [Google Scholar] [CrossRef]
- De Azevedo, D.Q.; Campioni, B.M.; Pedroz Lima, F.A.; Medina-Franco, J.L.; Castilho, R.O.; Maltarollo, V.G. A critical assessment of bioactive compounds databases. Future Med. Chem. 2024, 16, 1029–1051. [Google Scholar] [CrossRef]
- Yu, Z.; Su, Y.; Zhang, Y.; Zhu, P.; Mei, Z.; Zhou, X.; Yu, H. Potential use of ultrasound to promote fermentation, maturation, and properties of fermented foods: A review. Food Chem. 2021, 357, 129805. [Google Scholar] [CrossRef]
- Massa, T.B.; Cardozo-Filho, L.; da Silva, C. Fusel oil reaction in pressurized water: Characterization and antimicrobial activity. 3 Biotech 2023, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Van Boekel, M.A.J.S. Formation of flavour compounds in the Maillard reaction. Biotechnol. Adv. 2006, 24, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.; Rosa, M.F.; Aires-Barros, M.R.; Cabral, J.M.S. Enzymatic esterification of ethanol and oleic acid A. kinetic study. J. Mol. Catal. B Enzym. 2001, 11, 999–1005. [Google Scholar] [CrossRef]
- Nemestóthy, N.; Gubicza, L.; Fehér, E.; Bélafi-Bakó, K. Biotechnological Utilisation of Fusel Oil AFood Industry By-Product AKinetic Model on Enzymatic Esterification of i-Amyl Alcohol Oleic Acid by Candida antarctica Lipase, B. Food Technol. Biotechnol. 2008, 46, 44–50. [Google Scholar]
- Çiftçi, B.; Karagöz, M.; Aydin, M.; Çelik, M.B. The effect of fusel oil and waste biodiesel fuel blends on a CI engine performance, emissions, and combustion characteristics. J. Therm. Anal. Calorim. 2024, 149, 7783–7796. [Google Scholar] [CrossRef]
- Simsek, S.; Ozdalyan, B. Improvements to the Composition of Fusel Oil and Analysis of the Effects of Fusel Oil–Gasoline Blends on a Spark-Ignited (SI) Engine’s Performance and Emissions. Energies 2018, 11, 625. [Google Scholar] [CrossRef]
- Simsek, S.; Ozdalyan, B.; Saygın, H. Improvement of the properties of sugar factory fusel oil waste and investigation of its effect on the performance and emissions of spark ignition engine. Bioresour. Technol. 2019, 14, 440–452. [Google Scholar] [CrossRef]
- Alenezi, R.A.; Erdiwansyah; Mamat, R.; Norkhizan, A.M.; Najafi, G. The effect of fusel-biodiesel blends on the emissions and performance of a single cylinder diesel engine. Fuel 2020, 279, 118438. [Google Scholar] [CrossRef]
- Pasquet, P.L.; Bertagnolli, C.; Villain-Gambier, M.; Trébouet, D. Investigation of phenolic compounds recovery from brewery wastewater with coupled membrane adsorption process. J. Environ. Chem. Eng. 2024, 12, 112478. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart advanced solvents for bioactive compounds recovery from agri-food by-products: A review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Lara-Montano, O.D.; Melendez-Hernandez, P.A.; Bautista-Ortega, R.Y.; Hernandez, S.; Amaya-Delgado, L.; Hernandez-Escoto, H. Experimental Study on the Extractive Distillation Based Purification of second Generation Bioethanol. Chem. Eng. Trans. 2019, 74, 67–72. [Google Scholar] [CrossRef]
- Quiroz-Ramírez, J.J.; Sánchez-Ramírez, E.; Segovia-Hernández, J.G. Energy, exergy and techno-economic analysis for biobutanol production: A multi-objective optimization approach based on economic and environmental criteria. Clean. Techn. Environ. Policy 2018, 20, 1663–1684. [Google Scholar] [CrossRef]
- Bhoumick, M.C.; Paul, S.; Roy, S.; Harvey, B.G.; Mitra, S. Recovery of Isoamyl Alcohol by Graphene Oxide Immobilized Membrane and Air-Sparged Membrane Distillation. Membranes 2024, 14, 49. [Google Scholar] [CrossRef]
- Kirdi, R.; Ben Akacha, N.; Messaoudi, Y.; Gargouri, M. Enhanced synthesis of isoamyl acetate using liquid-gas biphasic system by the transesterification reaction of isoamyl alcohol obtained from fusel oil. Biotechnol. Bioprocess Eng. 2017, 22, 413–422. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, B.; Fan, S.; Liu, J.; Qin, Y.; Jian, S.; Wang, Y.; Xiao, Z. Membrane Distillation of Butanol from Aqueous Solution with Polytetrafluoroethylene Membrane. Chem. Eng. Technol. 2020, 43, 1160–1166. [Google Scholar] [CrossRef]
- Ndaba, B.; Chiyanzu, I.; Marx, S. N-Butanol derived from biochemical and chemical routes: A review. Biotechnol. Rep. 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Osada, Y.; Shibamoto, T. Antioxidative activity of volatile extracts from Maillard model systems. Food Chem. 2006, 98, 522–528. [Google Scholar] [CrossRef]
- Choudhary, D.; Garg, S.; Kaur, M.; Sohal, H.S.; Malhi, D.S.; Kaur, L.; Verma, M.; Sharma, A.; Mutreja, V. Advances in the Synthesis and Bio-Applications of Pyrazine Derivatives: A Review. Polycycl. Aromat. Compd. 2023, 43, 4512–4578. [Google Scholar] [CrossRef]
- Fayek, N.M.; Xiao, J.; Farag, M.A. A multifunctional study of naturally occurring pyrazines in biological systems; formation mechanisms, metabolism, food applications and functional properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 5322–5338. [Google Scholar] [CrossRef] [PubMed]
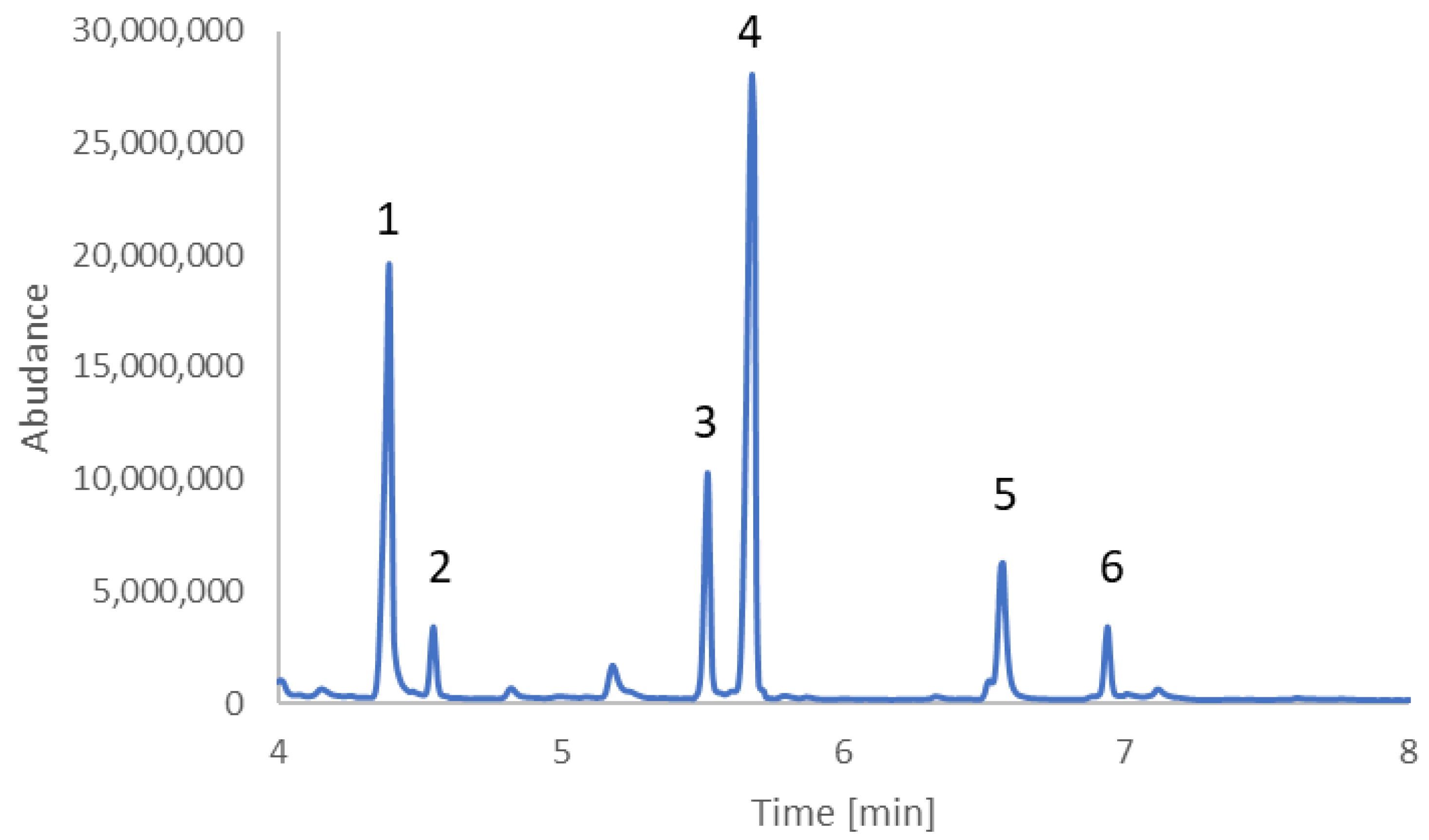
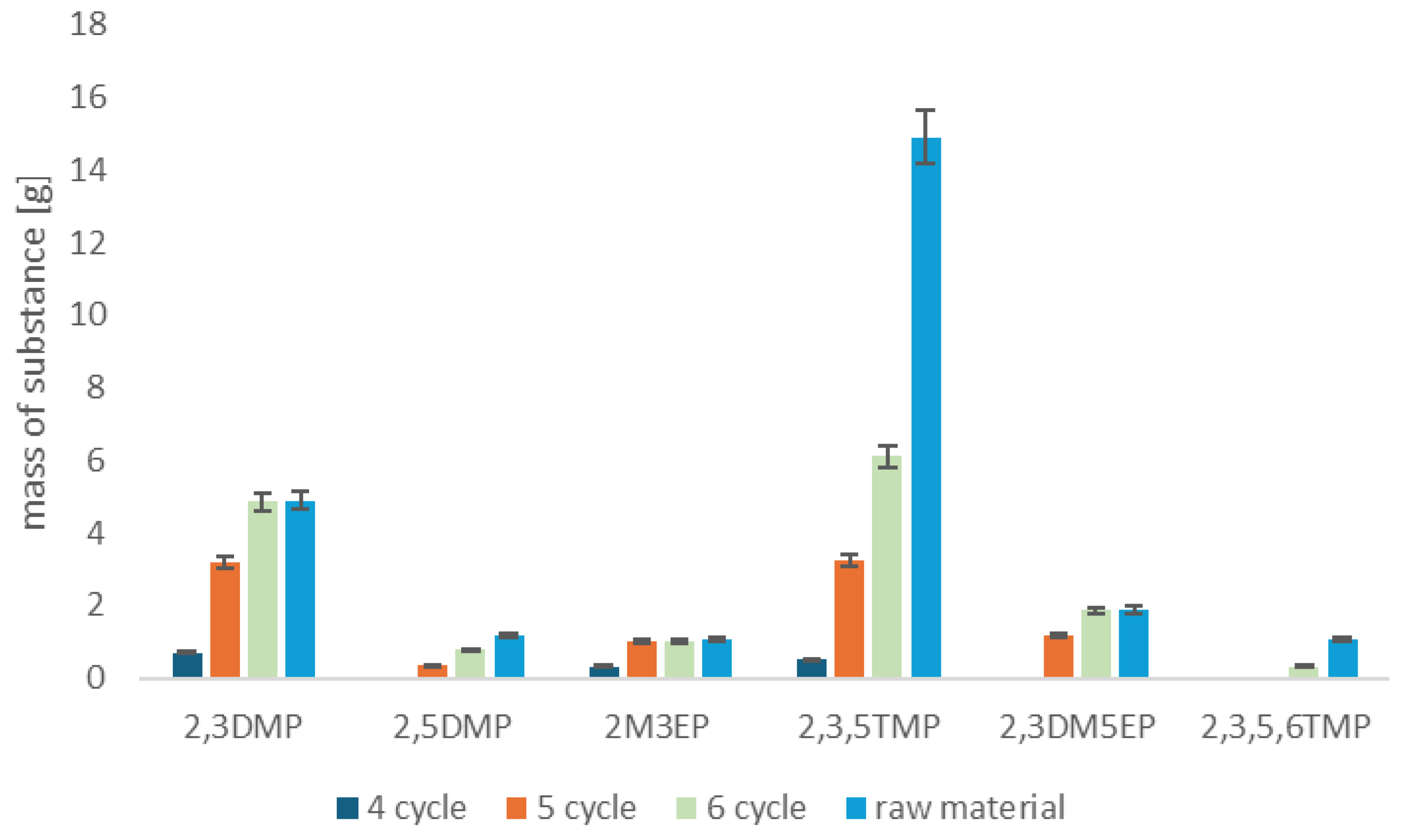

| Chemical Composition | Percentage Content [%] | m/z |
|---|---|---|
| water | 13.87 | - |
| 1-propanol | 0.96 | 31 (100), 27 (12), 60 (10) |
| 1-butanol | 0.17 | 41 (70), 43 (52), 56 (100) |
| izobutanol | 9.76 | 31 (52), 41 (65), 43 (100) |
| Isoamyl alcohol | 65.84 | 42 (100), 55 (66), 70 (51) |
| 2,3DMP | 0.49 | 67 (100), 108 (70), 109 (20) |
| 2,5DMP | 0.12 | 40 (40), 42 (100), 108 (60) |
| 2M3EP | 0.11 | 67 (83), 94 (73), 121 (100), 122 (86) |
| 2,3,5TMP | 1.49 | 42 (100), 81 (33), 122 (50) |
| 2,3DM5EP | 0.19 | 42 (28), 135 (100), 136 (75) |
| 2,3,5,6TMP | 0.11 | 54 (100), 95 (15), 136 (64), 137 (15) |
| other compounds | 6.9 | - |
| LOD | LOQ | R2 | RSD | Linear Range | Calibration Equation | |
|---|---|---|---|---|---|---|
| µg L−1 | [%] | [mg/L] | ||||
| 2,3DMP | 1.4 | 4.2 | 0.9961 | 5.1 | 0.5–50 | y = 1.247x + 0.023 |
| 2,5DMP | 2.3 | 6.9 | 0.9777 | 5.3 | 0.5–50 | y = 0.998x + 0.041 |
| 2M3EP | 0.8 | 2.4 | 0.9962 | 5.6 | 0.5–50 | y = 1.156x + 0.015 |
| 2,3,5TMP | 0.47 | 1.41 | 0.9987 | 4.2 | 0.5–50 | y = 1.289x + 0.008 |
| 2,3DM5EP | 2.36 | 7.08 | 0.9381 | 5.9 | 0.5–50 | y = 0.876x + 0.052 |
| 2,3,5,6TMP | 0.26 | 0.78 | 0.9987 | 2.7 | 0.5–50 | y = 1.334x + 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Studziński, W.; Podczarski, M.; Piechota, J.; Buziak, M.; Yakovenko, M.; Khokha, Y. Recovery of Natural Pyrazines and Alcohols from Fusel Oils Using an Innovative Extraction Installation. Molecules 2025, 30, 3028. https://doi.org/10.3390/molecules30143028
Studziński W, Podczarski M, Piechota J, Buziak M, Yakovenko M, Khokha Y. Recovery of Natural Pyrazines and Alcohols from Fusel Oils Using an Innovative Extraction Installation. Molecules. 2025; 30(14):3028. https://doi.org/10.3390/molecules30143028
Chicago/Turabian StyleStudziński, Waldemar, Michał Podczarski, Justyna Piechota, Marzena Buziak, Myroslava Yakovenko, and Yurii Khokha. 2025. "Recovery of Natural Pyrazines and Alcohols from Fusel Oils Using an Innovative Extraction Installation" Molecules 30, no. 14: 3028. https://doi.org/10.3390/molecules30143028
APA StyleStudziński, W., Podczarski, M., Piechota, J., Buziak, M., Yakovenko, M., & Khokha, Y. (2025). Recovery of Natural Pyrazines and Alcohols from Fusel Oils Using an Innovative Extraction Installation. Molecules, 30(14), 3028. https://doi.org/10.3390/molecules30143028







