Recent Research Advances in HER2-Positive Breast Cancer Concerning Targeted Therapy Drugs
Abstract
1. Introduction
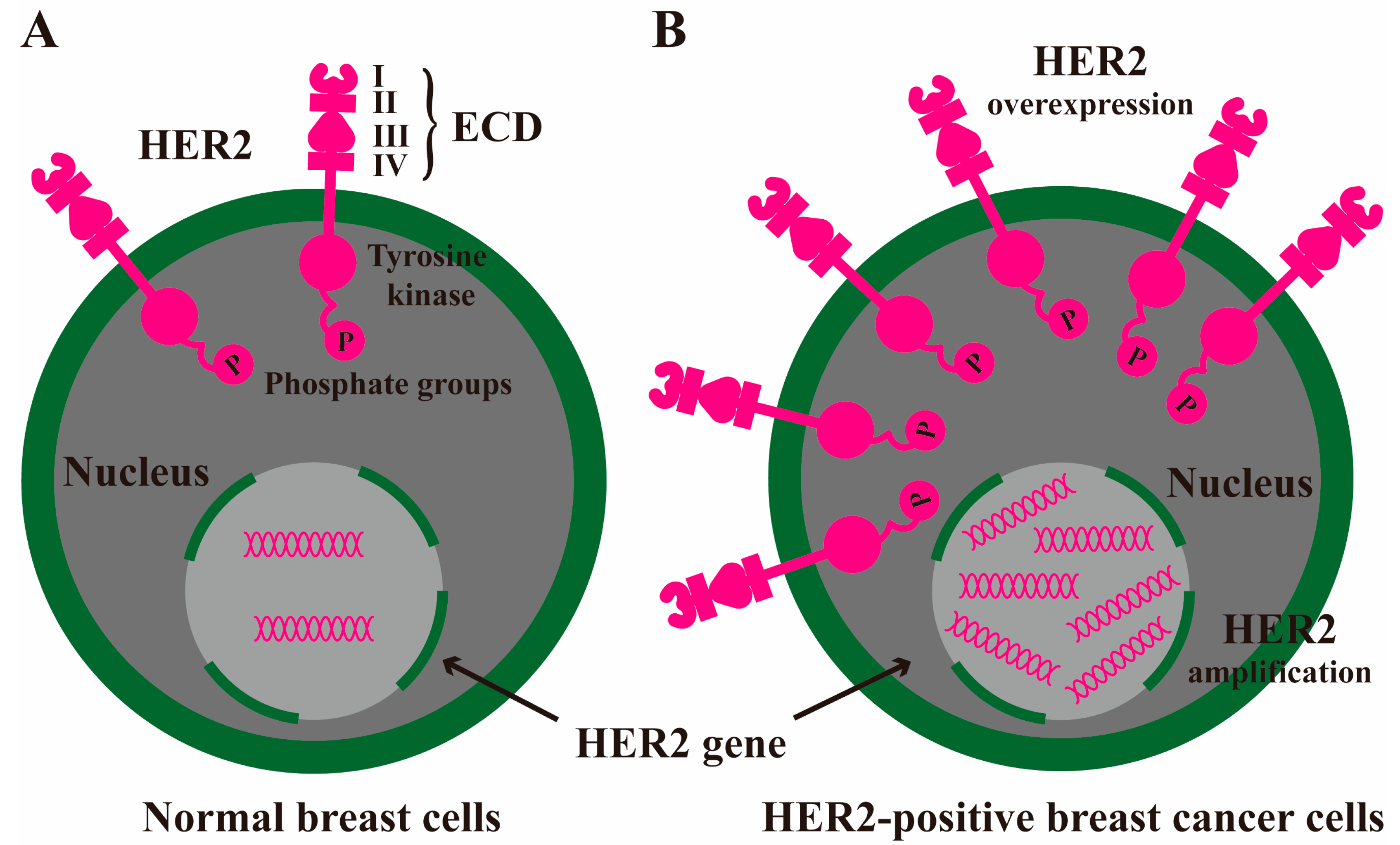
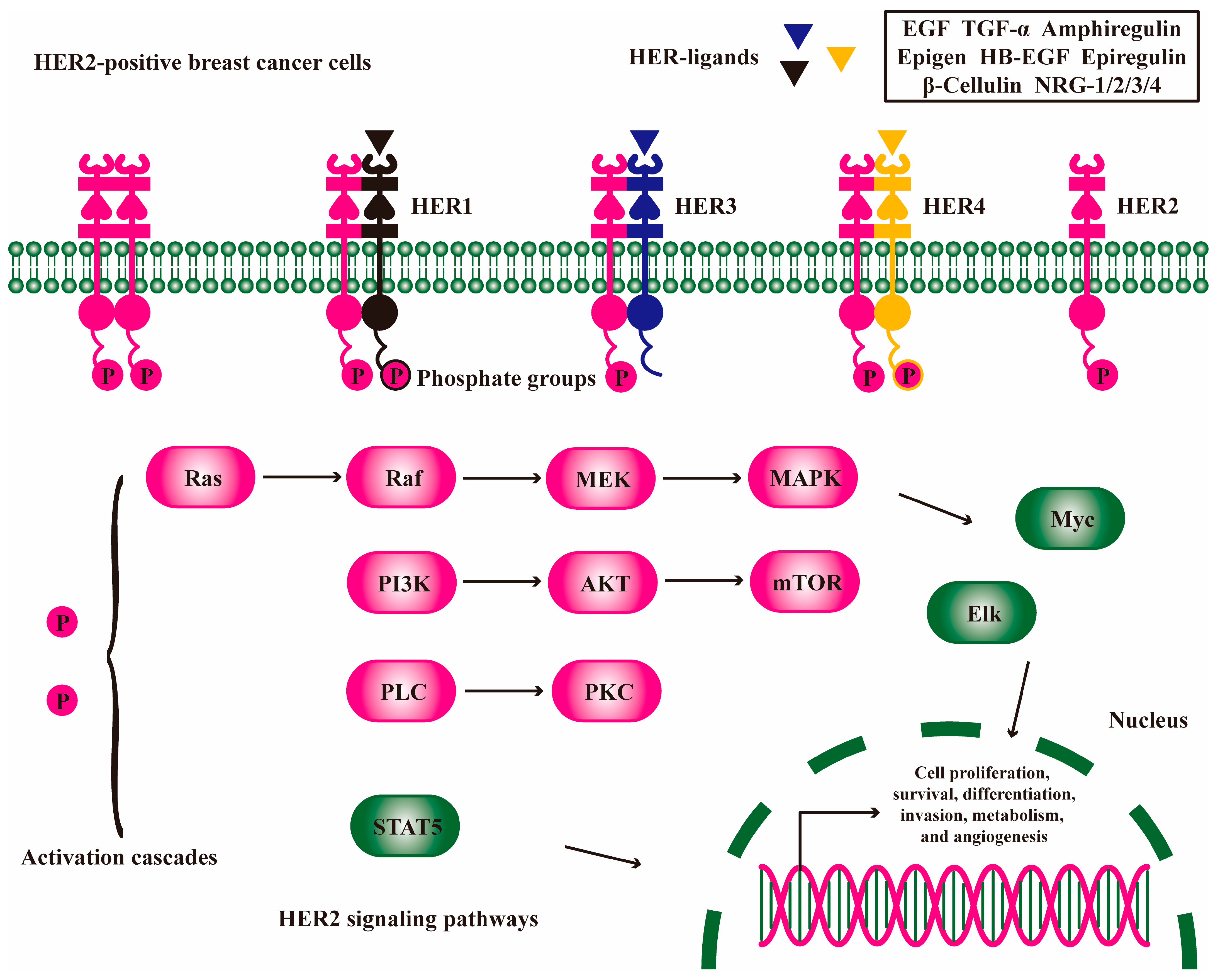
2. HER2 Signaling Pathway to Exacerbate Breast Cancer
3. mAb Drugs
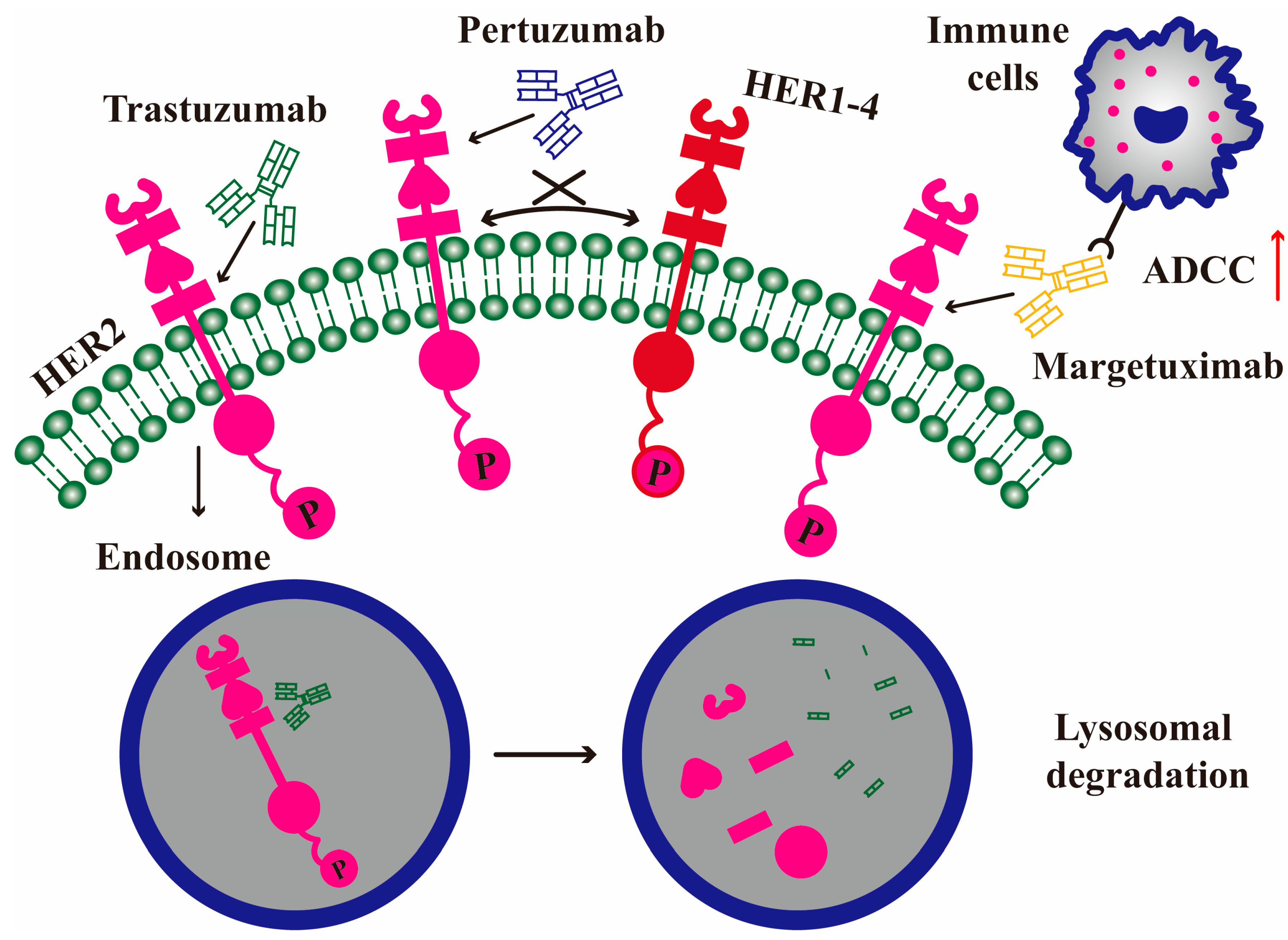
3.1. Trastuzumab
3.2. Pertuzumab
3.3. Margetuximab
| MAb Drugs | Phase | Action Mode | Refs. |
|---|---|---|---|
| Trastuzumab (Herceptin) | Approved | Binding to ECD IV, blocking HER2 dimerization, HER2 endocytosis; ADCC | [31,32,33,34,35] |
| Anqutuo Herzuma Trazimera Tuznue | Approved | Trastuzumab biosimilars | [36,37,38,39] |
| Pertuzumab (Perjeta) | Approved | Binding to ECD II, blocking HER2 dimerization; ADCC | [45,46,47] |
| TQB-2440 QL1209 HLX11 | Approved Applied for approval (NCT04629846) Phase I (NCT04411550) | Pertuzumab biosimilars | [49,50,51] |
| Margetuximab (Margenza) | Approved | Binding to ECD IV, blocking HER2 dimerization; enhancing Fc-CD16A and reducing Fc-CD32B to improve ADCC | [52,53] |
4. TKI Drugs
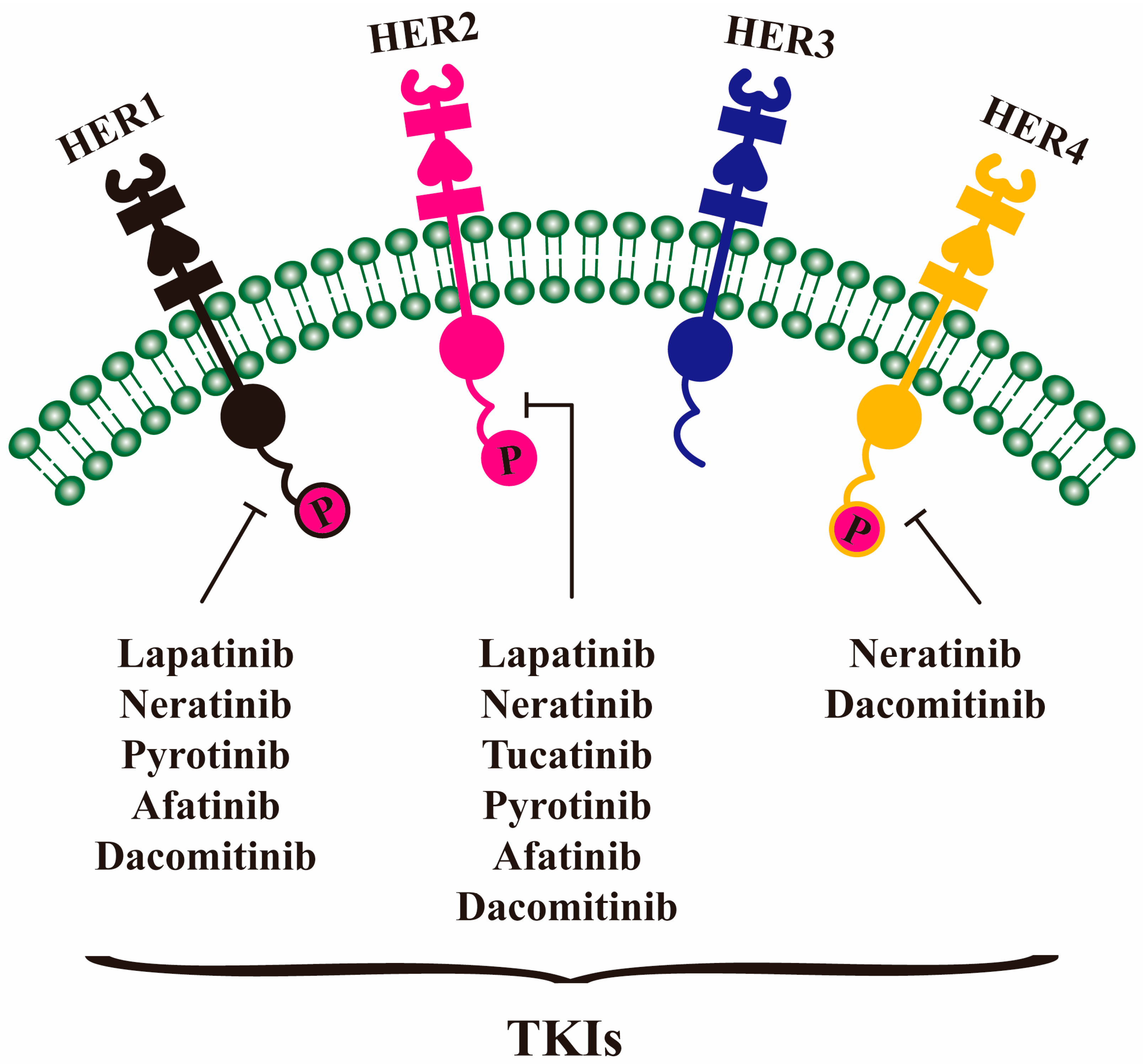
4.1. Lapatinib
4.2. Neratinib
4.3. Tucatinib
4.4. Pyrotinib
4.5. Afatinib and Dacomitinib
| TKI Drugs | Phase | Targets | Refs. |
|---|---|---|---|
| Lapatinib | Approved | HER1, HER2 | [55,56,57,58] |
| Neratinib | Approved | HER1, HER2, HER4 | [61,62,63,64,65] |
| Tucatinib | Approved | HER2 | [67,68] |
| Pyrotinib | Approved | HER1, HER2 | [70,71,72,73] |
| Afatinib | Phase III (NCT01125566) | HER1, HER2 | [74] |
| Dacomitinib | Preclinical | HER1, HER2, HER4 | [75] |
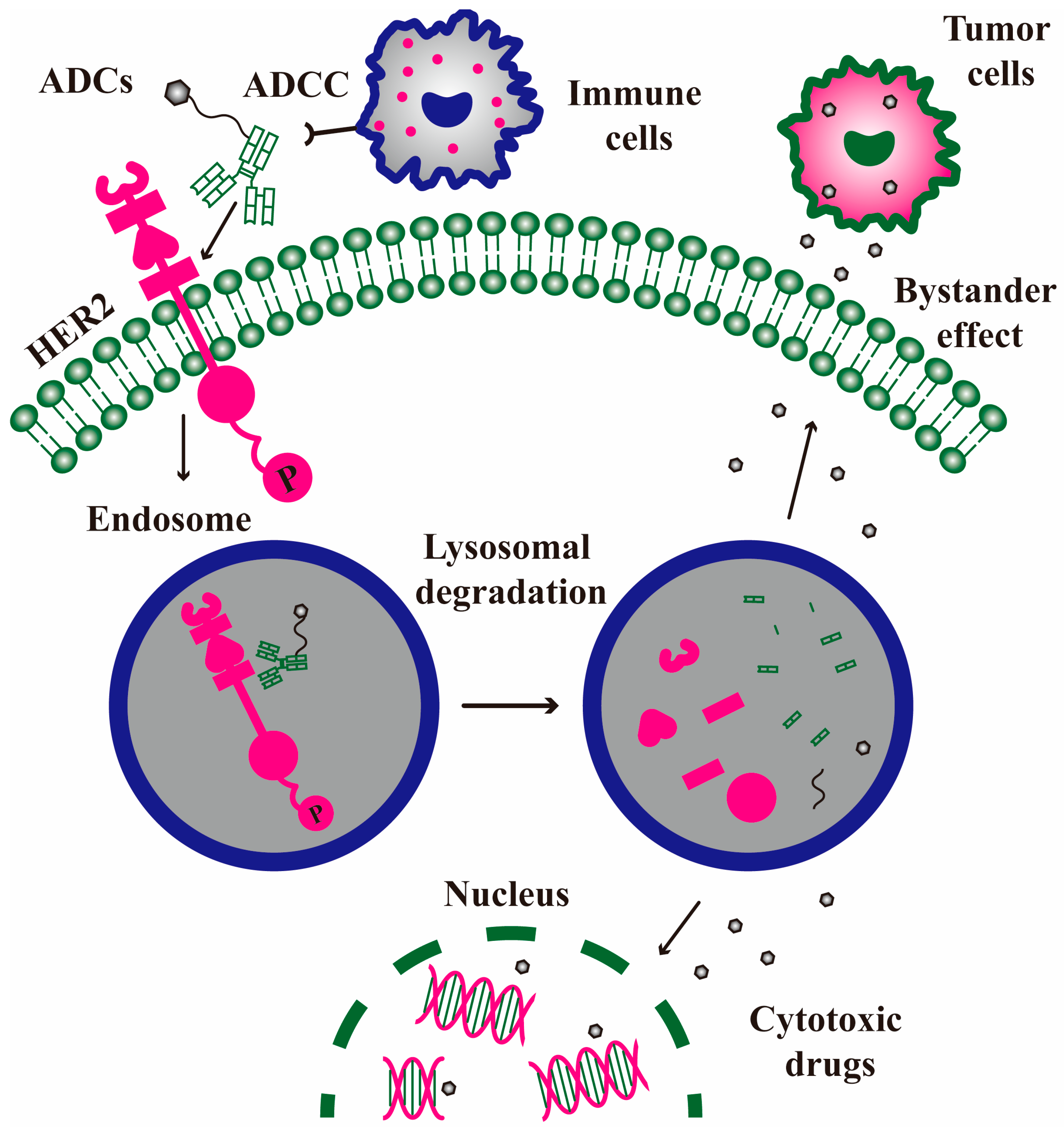
5. ADC Drugs
5.1. Trastuzumab Emtansine
5.2. Trastuzumab Deruxtecan
5.3. Other ADC Drugs and Bystander Effect
| ADC Drugs | Phase | Components | Bystander Effect | Refs. |
|---|---|---|---|---|
| Trastuzumab emtansine (T-DM1) | Approved | Trastuzumab + thioether linker +tubulin inhibitor DM1 | × | [77,78,79,80] |
| Trastuzumab deruxtecan (T-DXd) | Approved | Trastuzumab + tetrapeptide linker +topoisomerase inhibitor DXd | √ | [81,82,83,84] |
| RC48 | Phase III (NCT04400695) | Hertuzumab + valine-citrulline linker +tubulin inhibitor MMAE | √ | [86] |
| A166 | Phase II (NCT05346328) | Trastuzumab + valine-citrulline linker +tubulin inhibitor Duo-5 | √ | [87] |
| SHR-A1811 | Phase III (NCT06057610) | Trastuzumab + tetrapeptide linker +topoisomerase inhibitor SHR9265 | √ | [88] |
6. BsAb Drugs
6.1. BsAb
6.2. BsADC
| BsAb Drugs | Targets | Components | Phase | Ref. |
|---|---|---|---|---|
| Anbenitamab | HER2 ECD II/IV | BsAb | Phase III (NCT06747338) | [90] |
| Zanidatamab | HER2 ECD II/IV | BsAb | Phase III (NCT06435429) | [91] |
| TQB2930 | HER2 ECD II/IV | BsAb | Phase II (NCT06202261) | - |
| JSKN003 | HER2 ECD II/IV | Anbenitamab + gly-gly-phe-gly linker +topoisomerase inhibitor | Phase III (NCT06846437) | [92] |
| ZW49 | HER2 ECD II/IV | Zanidatamab + disulfide bond linker +tubulin inhibitor auristatin | Phase I (NCT03821233) | - |
| HER2/HER3 BsADC | HER2 HER3 | BsAb + maleimidocaproyl/valine-citrulline/ PABA linker + tubulin inhibitor MMAE | Preclinical study | [93] |
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADCC | Antibody-dependent cell-mediated cytotoxicity |
| ADCs | Antibody-drug conjugates |
| BsAbs | Bispecific antibody |
| CDK | Cyclin-dependent kinase |
| CSCO | Chinese Society of Clinical Oncology |
| DAR | Drug-antibody ratio |
| Duo-5 | Duostatin-5 |
| EGFR | Epidermal growth factor receptor |
| ER | Estrogen receptor |
| ECD | Extracellular domain |
| FDA | Food and Drug Administration |
| HB-EGF | Heparin-binding epidermal growth factor-like growth factor |
| HER | Human epidermal growth factor receptor |
| IDFS | Invasive disease-free survival |
| IGF-1R | Insulin-like growth factor 1 receptor |
| MAbs | Monoclonal antibodies |
| MMAE | Monomethyl auristatin E |
| NCCN | National Comprehensive Cancer Network |
| NRG | Neuregulin |
| OS | Overall survival |
| PR | Progesterone receptor |
| PFS | Progression-free survival |
| RTK | Tyrosine kinase receptor |
| TKIs | Tyrosine kinase inhibitors |
| TNBC | Triple negative breast cancer |
| TGF-α | Transforming growth factor-α |
| T-DXd | Trastuzumab deruxtecan |
| T-DM1 | Trastuzumab emtansine |
References
- Abubakar, M.; Klein, A.; Fan, S.; Lawrence, S.; Mutreja, K.; Henry, J.E.; Pfeiffer, R.M.; Duggan, M.A.; Gierach, G.L. Host, reproductive, and lifestyle factors in relation to quantitative histologic metrics of the normal breast. Breast Cancer Res. 2023, 25, 97. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global patterns and trends in breast cancer incidence and mortality across 185 countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Qiu, Y.; Long, Z.; Jin, Z. Epidemiology of breast cancer in Chinese women from 1990 to 2021: A systematic analysis and comparison with the global burden. BMC Cancer 2025, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xia, C.; Wang, Y.; Qi, Y.; Qi, X.; Zhao, J.; Ye, S.; Lei, Z.; Yue, J.; Huang, H.; et al. Landscape of young breast cancer under 35 years in China over the past decades: A multicentre retrospective cohort study (YBCC-Catts study). EClinicalMedicine 2023, 64, 102243. [Google Scholar] [CrossRef]
- Feng, S.; Li, J.; Yan, A.; Zhu, X.; Zhang, L.; Tang, D.; Liu, L. Application of Gene Editing in Triple-Negative Breast Cancer Research. Cell Biochem. Funct. 2025, 43, e70044. [Google Scholar] [CrossRef]
- Luz, P.; Ramos, S.; Oliveira, M.J.; Costa, J.G.; Saraiva, N.; Fernandes, A.S. Interaction between redox regulation, immune activation, and response to treatment in HER2+ breast cancer. Redox Biol. 2025, 82, 103609. [Google Scholar] [CrossRef]
- Zakaria, N.H.; Hashad, D.; Saied, M.H.; Hegazy, N.; Elkayal, A.; Tayae, E. Genetic mutations in HER2-positive breast cancer: Possible association with response to trastuzumab therapy. Hum. Genom. 2023, 17, 43. [Google Scholar] [CrossRef]
- Li, Y.W.; Dai, L.J.; Wu, X.R.; Zhao, S.; Xu, Y.Z.; Jin, X.; Xiao, Y.; Wang, Y.; Lin, C.J.; Zhou, Y.F.; et al. Molecular Characterization and Classification of HER2-Positive Breast Cancer Inform Tailored Therapeutic Strategies. Cancer Res. 2024, 84, 3669–3683. [Google Scholar] [CrossRef]
- Ullrich, A.; Coussens, L.; Hayflick, J.S.; Dull, T.J.; Gray, A.; Tam, A.W.; Lee, J.; Yarden, Y.; Libermann, T.A.; Schlessinger, J.; et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 1984, 309, 418–425. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ikawa, S.; Akiyama, T.; Semba, K.; Nomura, N.; Miyajima, N.; Saito, T.; Toyoshima, K. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature 1986, 319, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Fukushige, S.; Matsubara, K.; Yoshida, M.; Sasaki, M.; Suzuki, T.; Semba, K.; Toyoshima, K.; Yamamoto, T. Localization of a novel v-erbB-related gene, c-erbB-2, on human chromosome 17 and its amplification in a gastric cancer cell line. Mol. Cell Biol. 1986, 6, 955–958. [Google Scholar] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yanai, Y.; Kosaka, T.; Nakamura, K.; Aimono, E.; Matsumoto, K.; Morita, S.; Mikami, S.; Nishihara, H.; Oya, M. CDK12 and HER2 coamplification in two urothelial carcinomas with rapid and aggressive clinical progression. Cancer Sci. 2020, 111, 4652–4655. [Google Scholar] [CrossRef]
- Bai, X.; Sun, P.; Wang, X.; Long, C.; Liao, S.; Dang, S.; Zhuang, S.; Du, Y.; Zhang, X.; Li, N.; et al. Structure and dynamics of the EGFR/HER2 heterodimer. Cell Discov. 2023, 9, 18. [Google Scholar] [CrossRef]
- Shetty, S.R.; Kar, T.; Das, A. Epidermal growth factor receptor mutations in breast Cancer: Therapeutic challenges and way forward. Bioorg. Chem. 2025, 154, 108037. [Google Scholar] [CrossRef]
- Moody, T.W.; Ramos-Alvarez, I.; Jensen, R.T. Bombesin, endothelin, neurotensin and pituitary adenylate cyclase activating polypeptide cause tyrosine phosphorylation of receptor tyrosine kinases. Peptides 2021, 137, 170480. [Google Scholar] [CrossRef]
- Al-Salihi, M.; Bornikoel, A.; Zhuang, Y.; Stachura, P.; Scheller, J.; Lang, K.S.; Lang, P.A. The role of ADAM17 during liver damage. Biol. Chem. 2021, 402, 1115–1128. [Google Scholar] [CrossRef]
- Ferrer-Soler, L.; Vazquez-Martin, A.; Brunet, J.; Menendez, J.A.; De Llorens, R.; Colomer, R. An update of the mechanisms of resistance to EGFR-tyrosine kinase inhibitors in breast cancer: Gefitinib (Iressa) -induced changes in the expression and nucleo-cytoplasmic trafficking of HER-ligands (Review). Int. J. Mol. Med. 2007, 20, 3–10. [Google Scholar] [CrossRef]
- Parker, M.I.; Nikonova, A.S.; Sun, D.; Golemis, E.A. Proliferative signaling by ERBB proteins and RAF/MEK/ERK effectors in polycystic kidney disease. Cell Signal. 2020, 67, 109497. [Google Scholar] [CrossRef]
- Garrett, J.T.; Tendler, S.; Feroz, W.; Kilroy, M.K.; Yu, H. Emerging importance of HER3 in tumorigenesis and cancer therapy. Nat. Rev. Clin. Oncol. 2025, 22, 348–370. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.; Gong, M.; Zhuang, Z.; Wang, Y.; Mu, L.; Wang, T.; Pan, J.; Liu, Y.; Xu, J.; et al. Gab2 Ablation Reverses the Stemness of HER2-Overexpressing Breast Cancer Cells. Cell Physiol. Biochem. 2018, 50, 52–65. [Google Scholar] [CrossRef]
- Labrèche, C.; Cook, D.P.; Abou-Hamad, J.; Pascoal, J.; Pryce, B.R.; Al-Zahrani, K.N.; Sabourin, L.A. Periostin gene expression in neu-positive breast cancer cells is regulated by a FGFR signaling cross talk with TGFβ/PI3K/AKT pathways. Breast Cancer Res. 2021, 23, 107. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; San, M.; Zhang, J.; Yang, A.; Xie, W.; Chen, Y.; Lu, X.; Zhang, Y.; Zhao, M.; Feng, X.; et al. Oxytocin receptor induces mammary tumorigenesis through prolactin/p-STAT5 pathway. Cell Death Dis. 2021, 12, 588. [Google Scholar] [CrossRef] [PubMed]
- Fromm, J.A.; Johnson, S.A.; Johnson, D.L. Epidermal growth factor receptor 1 (EGFR1) and its variant EGFRvIII regulate TATA-binding protein expression through distinct pathways. Mol. Cell Biol. 2008, 28, 6483–6495. [Google Scholar] [CrossRef] [PubMed]
- Elster, N.; Collins, D.M.; Toomey, S.; Crown, J.; Eustace, A.J.; Hennessy, B.T. HER2-family signalling mechanisms, clinical implications and targeting in breast cancer. Breast Cancer Res. Treat. 2015, 149, 5–15. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Z.; Qiu, N.; Ling, L.; Jia, X.; Song, Y.; Li, H.; Li, J.; Lyu, H.; Liu, H.; et al. Disruption of FOXO3a-miRNA feedback inhibition of IGF2/IGF-1R/IRS1 signaling confers Herceptin resistance in HER2-positive breast cancer. Nat. Commun. 2021, 12, 2699. [Google Scholar] [CrossRef]
- Maldonado, H.; Dreger, M.; Bedgood, L.D.; Kyriakou, T.; Wolanska, K.I.; Rigby, M.E.; Marotta, V.E.; Webster, J.M.; Wang, J.; Rusilowicz-Jones, E.V.; et al. A trafficking regulatory subnetwork governs αVβ6 integrin-HER2 cross-talk to control breast cancer invasion and drug resistance. Sci. Adv. 2024, 10, eadk9944. [Google Scholar] [CrossRef]
- Elshazly, A.M.; Gewirtz, D.A. An overview of resistance to Human epidermal growth factor receptor 2 (Her2) targeted therapies in breast cancer. Cancer Drug Resist. 2022, 5, 472–486. [Google Scholar] [CrossRef]
- Paul, S.; Konig, M.F.; Pardoll, D.M.; Bettegowda, C.; Papadopoulos, N.; Wright, K.M.; Gabelli, S.B.; Ho, M.; van Elsas, A.; Zhou, S. Cancer therapy with antibodies. Nat. Rev. Cancer 2024, 24, 399–426. [Google Scholar] [CrossRef]
- Zhao, J.; Mohan, N.; Nussinov, R.; Ma, B.; Wu, W.J. Trastuzumab Blocks the Receiver Function of HER2 Leading to the Population Shifts of HER2-Containing Homodimers and Heterodimers. Antibodies 2021, 10, 7. [Google Scholar] [CrossRef]
- Gampenrieder, S.P.; Castagnaviz, V.; Rinnerthaler, G.; Greil, R. Treatment Landscape for Patients with HER2-Positive Metastatic Breast Cancer: A Review on Emerging Treatment Options. Cancer Manag. Res. 2020, 12, 10615–10629. [Google Scholar] [CrossRef]
- Du, R.; Cao, C.; Fan, D.; Li, G.; Pu, S.; Xu, X.; Liu, M.; Shi, G.; Wu, Y.; Hao, Q.; et al. NK cell immunopotentiators-loaded nanoliposomes enhance ADCC effect for targeted therapy against HER2-positive breast cancer. Cell Commun. Signal 2025, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Minckwitz, G.V.; Procter, M.; Azambuja, E.D.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. APHINITY Steering Committee and Investigators. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Cai, R.G.; Song, G.H.; Li, C.J.; Zhang, B.H.; Kang, X.Y.; Li, H.P.; Xu, B.H. QL1701 investigators. QL1701 (a proposed trastuzumab biosimilar) versus reference trastuzumab plus docetaxel as first-line therapy for HER2-positive metastatic breast cancer: A multicenter, randomized, double-blinded, parallel-controlled, phase III equivalence trial. ESMO Open 2024, 9, 103682. [Google Scholar]
- Stebbing, J.; Baranau, Y.; Baryash, V.; Manikhas, A.; Moiseyenko, V.; Dzagnidze, G.; Zhavrid, E.; Boliukh, D.; Stroyakovskii, D.; Pikiel, J.; et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: A randomised, double-blind, active-controlled, phase 3 equivalence trial. Lancet Oncol. 2017, 18, 917–928. [Google Scholar] [CrossRef]
- Li, R.K.; Tokunaga, E.; Adamchuk, H.; Vladimirov, V.; Yanez, E.; Lee, K.S.; Bondarenko, I.; Vana, A.; Hilton, F.; Ishikawa, T.; et al. Long-Term Safety and Effectiveness of PF-05280014 (a Trastuzumab Biosimilar) Treatment in Patients with HER2-Positive Metastatic Breast Cancer: Updated Results of a Randomized, Double-Blind Study. BioDrugs 2022, 36, 55–69. [Google Scholar] [CrossRef]
- Bischoff, H.; O’Connor, N.K.; Kim, J.; Popescu, B.V.; Bigot, C.; Pradhan, S.; Chakraborty, R.; Jaison, L.; Majeed, F.; Park, L.S.; et al. Comparative Preclinical Evaluation of Tuznue Versus Referent Herceptin: A Registered Trastuzumab Biosimilar. Drugs R D 2025, 25, 67–77. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Kong, L.; Huang, S.; He, L.; Liu, P.; Mo, S.; Lu, X.; Lin, X.; Xiao, Y.; et al. Inhibition of DPAGT1 suppresses HER2 shedding and trastuzumab resistance in human breast cancer. J. Clin. Invest. 2023, 133, e164428. [Google Scholar] [CrossRef]
- Yoon, J.; Oh, D.Y. HER2-targeted therapies beyond breast cancer—An update. Nat. Rev. Clin. Oncol. 2024, 21, 675–700. [Google Scholar] [CrossRef]
- Mekhamer, A.M.; Saied, M.H.; Elneily, D.A.E.; El-Fayoumi, T.A.H.; Hashad, D.I. Targeted Sequencing of HER2-Positive Breast Cancer Mutations Revealed a Potential Association between PIK3CA and Trastuzumab Resistance. Asian Pac. J. Cancer Prev. 2024, 25, 4051–4059. [Google Scholar] [CrossRef]
- Noyan, S.; Gür Dedeoğlu, B. miR-770-5p-induced cellular switch to sensitize trastuzumab resistant breast cancer cells targeting HER2/EGFR/IGF1R bidirectional crosstalk. Turk. J. Biol. 2024, 48, 153–162. [Google Scholar] [CrossRef]
- Geng, W.; Thomas, H.; Chen, Z.; Yan, Z.; Zhang, P.; Zhang, M.; Huang, W.; Ren, X.; Wang, Z.; Ding, K.; et al. Mechanisms of acquired resistance to HER2-Positive breast cancer therapies induced by HER3: A comprehensive review. Eur. J. Pharmacol. 2024, 977, 176725. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.R.; Mitra, A.; Chakraborty, A.; Tawate, M.; Sahu, S.; Rakshit, S.; Gaikwad, S.; Dhotre, G.; Damle, A.; Banerjee, S. Clinical Dose Preparation of [177Lu]Lu-DOTA-Pertuzumab Using Medium Specific Activity [177Lu]LuCl3 for Radioimmunotherapy of Breast and Epithelial Ovarian Cancers, with HER2 Receptor Overexpression. Cancer Biother. Radiopharm. 2022, 37, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Starosławska, E.; de la Haba-Rodriguez, J.; Im, S.A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Cortés, J.; Kim, S.B.; Im, S.A.; Hegg, R.; Im, Y.H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef]
- Tan, A.R.; Im, S.A.; Mattar, A.; Colomer, R.; Stroyakovskii, D.; Nowecki, Z.; De Laurentiis, M.; Pierga, J.Y.; Jung, K.H.; Schem, C.; et al. Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): A randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2021, 22, 85–97. [Google Scholar] [CrossRef]
- Xu, D.; Wu, J.; Yu, J.; Yang, Y.; Wen, X.; Yang, J.; Wei, H.; Xu, X.; Li, Y.; Yang, L.; et al. A historical controlled study of domestic trastuzumab and pertuzumab in combination with docetaxel for the neoadjuvant treatment of early HER2-positive breast cancer. Front. Oncol. 2024, 14, 1281643. [Google Scholar] [CrossRef]
- Zuo, W.; Wang, Z.; Qian, J.; Ma, X.; Niu, Z.; Ou, J.; Mo, Q.; Sun, J.; Li, X.; Wang, Q.; et al. QL1209 (pertuzumab biosimilar) versus reference pertuzumab plus trastuzumab and docetaxel in neoadjuvant treatment for HER2-positive, ER/PR-negative, early or locally advanced breast cancer: A multicenter, randomized, double-blinded, parallel-controlled, phase III equivalence trial. Br. J. Cancer 2024, 131, 668–675. [Google Scholar]
- Yang, J.; Lin, L.; Long, Q.; Zhang, Q.; Sun, G.; Zhou, L.; Wang, Q.; Zhu, J.; Li, F.; Hu, W. HLX11, a Proposed Pertuzumab Biosimilar: Pharmacokinetics, Immunogenicity, and Safety Profiles Compared to Three Reference Biologic Products (US-, EU-, and CN-Approved Pertuzumab) Administered to Healthy Male Subjects. BioDrugs 2022, 36, 393–409. [Google Scholar] [CrossRef]
- Alasmari, M.M. A Review of Margetuximab-Based Therapies in Patients with HER2-Positive Metastatic Breast Cancer. Cancers 2022, 15, 38. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Cortés, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escrivá-de-Romaní, S.; et al. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 573–584. [Google Scholar] [CrossRef]
- Dickerson, H.; Diab, A.; Al Musaimi, O. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Cancer: Current Use and Future Prospects. Int. J. Mol. Sci. 2024, 25, 10008. [Google Scholar] [CrossRef] [PubMed]
- Wahdan-Alaswad, R.; Liu, B.; Thor, A.D. Targeted lapatinib anti-HER2/ErbB2 therapy resistance in breast cancer: Opportunities to overcome a difficult problem. Cancer Drug Resist. 2020, 3, 179–198. [Google Scholar] [CrossRef] [PubMed]
- Neamati, D.; Khedri, A.; Aberomand, M.; Hemmati, A.A.; Mohammadzadeh, M.; Akbari Baghbani, K.; Mohammadzadeh, G. Metformin synergistically increases the anticancer effects of lapatinib through induction of apoptosis and modulation of Akt/AMPK pathway in SK-BR3 breast cancer cell line. Iran J. Basic Med. Sci. 2021, 24, 1529–1537. [Google Scholar] [PubMed]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef]
- Robidoux, A.; Tang, G.; Rastogi, P.; Geyer, C.E., Jr.; Azar, C.A.; Atkins, J.N.; Fehrenbacher, L.; Bear, H.D.; Baez-Diaz, L.; Sarwar, S.; et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): An open-label, randomised phase 3 trial. Lancet Oncol. 2013, 14, 1183–1192. [Google Scholar] [CrossRef]
- Ho, C.Y.; Wei, C.Y.; Zhao, R.W.; Ye, Y.L.; Huang, H.C.; Lee, J.C.; Cheng, F.J.; Huang, W.C. Artemisia argyi extracts overcome lapatinib resistance via enhancing TMPRSS2 activation in HER2-positive breast cancer. Environ. Toxicol. 2024, 39, 3389–3399. [Google Scholar] [CrossRef]
- Tanaka, Y. Development of High-Throughput Quantitative Imaging Mass Spectrometry for Analysis of Drug Distribution in Tissues. J. Mass Spectrom. 2025, 60, e5135. [Google Scholar] [CrossRef]
- Rabindran, S.K.; Discafani, C.M.; Rosfjord, E.C.; Baxter, M.; Floyd, M.B.; Golas, J.; Hallett, W.A.; Johnson, B.D.; Nilakantan, R.; Overbeek, E.; et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004, 64, 3958–3965. [Google Scholar] [CrossRef]
- Guo, L.; Shao, W.; Zhou, C.; Yang, H.; Yang, L.; Cai, Q.; Wang, J.; Shi, Y.; Huang, L.; Zhang, J. Neratinib for HER2-positive breast cancer with an overlooked option. Mol. Med. 2023, 29, 134. [Google Scholar] [CrossRef]
- Martin, M.; Holmes, F.A.; Ejlertsen, B.; Delaloge, S.; Moy, B.; Iwata, H.; von Minckwitz, G.; Chia, S.K.L.; Mansi, J.; Barrios, C.H.; et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1688–1700. [Google Scholar] [CrossRef]
- Saura, C.; Oliveira, M.; Feng, Y.H.; Dai, M.S.; Chen, S.W.; Hurvitz, S.A.; Kim, S.B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.; Braga, S.; Zotto, V.D.; Finnie, J.; DiPrimeo, D.; Cooke, B.; Bischof, G.F.; Wong, A.; Di Palma, J.A. Preclinical and clinical evaluation through serial colonoscopic evaluation of neratinib-induced diarrhea in HER2-positive breast cancer-A pilot study. Physiol. Rep. 2024, 12, e70008. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Ruiz-Borrego, M.; Marx, G.; Chien, A.J.; Rugo, H.S.; Brufsky, A.; Thirlwell, M.; Trudeau, M.; Bose, R.; García-Sáenz, J.A.; et al. Final findings from the CONTROL trial: Strategies to reduce the incidence and severity of neratinib-associated diarrhea in patients with HER2-positive early-stage breast cancer. Breast 2023, 67, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Moulder, S.L.; Borges, V.F.; Baetz, T.; Mcspadden, T.; Fernetich, G.; Murthy, R.K.; Chavira, R.; Guthrie, K.; Barrett, E.; Chia, S.K. Phase I Study of ONT-380, a HER2 Inhibitor, in Patients with HER2+-Advanced Solid Tumors, with an Expansion Cohort in HER2+ Metastatic Breast Cancer (MBC). Clin. Cancer Res. 2017, 23, 3529–3536. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- Sun, K.; Wang, X.; Zhang, H.; Lin, G.; Jiang, R. Management and Mechanisms of Diarrhea Induced by Tyrosine Kinase Inhibitors in Human Epidermal Growth Factor Receptor-2-Positive Breast Cancer. Cancer Control 2024, 31, 10732748241278039. [Google Scholar] [CrossRef]
- Li, X.; Yang, C.; Wan, H.; Zhang, G.; Feng, J.; Zhang, L.; Chen, X.; Zhong, D.; Lou, L.; Tao, W.; et al. Discovery and development of pyrotinib: A novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur. J. Pharm. Sci. 2017, 110, 51–61. [Google Scholar] [CrossRef]
- Xu, B.; Yan, M.; Ma, F.; Hu, X.; Feng, J.; Ouyang, Q.; Tong, Z.; Li, H.; Zhang, Q.; Sun, T.; et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Berton Giachetti, P.P.M.; Curigliano, G. Pyrotinib in combination with first-line trastuzumab and docetaxel for patients with HER2-positive metastatic breast cancer: A new therapeutic option? Nat. Rev. Clin. Oncol. 2024, 21, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Xia, Y.; Zhu, Y.; Yang, Y.; Lin, Q.; Liu, Q.; Yang, W.; Ling, L.; Zhong, J.; Duan, Z.; et al. Preclinical study and phase 2 trial of neoadjuvant pyrotinib combined with chemotherapy in luminal/HER2-low breast cancer: PILHLE-001 study. Cell Rep. Med. 2024, 5, 101807. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Huang, C.S.; Hurvitz, S.; Yeh, D.C.; Shao, Z.; Im, S.A.; Jung, K.H.; Shen, K.; Ro, J.; Jassem, J.; et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1): An open-label, randomised, phase 3 trial. Lancet Oncol. 2016, 17, 357–366. [Google Scholar] [CrossRef]
- Kalous, O.; Conklin, D.; Desai, A.J.; O’Brien, N.A.; Ginther, C.; Anderson, L.; Cohen, D.J.; Britten, C.D.; Taylor, I.; Christensen, J.G.; et al. Dacomitinib (PF-00299804), an irreversible Pan-HER inhibitor, inhibits proliferation of HER2-amplified breast cancer cell lines resistant to trastuzumab and lapatinib. Mol. Cancer Ther. 2012, 11, 1978–1987. [Google Scholar] [CrossRef]
- Larose, É.A.; Hua, X.; Yu, S.; Pillai, A.T.; Yi, Z.; Yu, H. Antibody-drug conjugates in breast cancer treatment: Resistance mechanisms and the role of therapeutic sequencing. Cancer Drug Resist. 2025, 8, 11. [Google Scholar] [CrossRef]
- Najdi, T.; Awad, L.; Chartouni, A.; Soueidy, C.; Kourie, H. Navigating antibody-drug conjugates (ADCs): From metastatic to early breast cancer treatment strategies. Investig. New Drugs 2025. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Geyer, C.E., Jr.; Untch, M.; Huang, C.S.; Mano, M.S.; Mamounas, E.P.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; Fischer, H.H.; et al. Survival with Trastuzumab Emtansine in Residual HER2-Positive Breast Cancer. N. Engl. J. Med. 2025, 392, 249–257. [Google Scholar] [CrossRef]
- Kang, S.; Kim, S.B. Toxicities and management strategies of emerging antibody-drug conjugates in breast cancer. Ther. Adv. Med. Oncol. 2025, 17, 17588359251324889. [Google Scholar] [CrossRef]
- Doi, T.; Shitara, K.; Naito, Y.; Shimomura, A.; Fujiwara, Y.; Yonemori, K.; Shimizu, C.; Shimoi, T.; Kuboki, Y.; Matsubara, N.; et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet Oncol. 2017, 18, 1512–1522. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Cortés, J.; Hurvitz, S.A.; Im, S.A.; Iwata, H.; Curigliano, G.; Kim, S.B.; Chiu, J.W.Y.; Pedrini, J.L.; Li, W.; Yonemori, K.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer: Long-term survival analysis of the DESTINY-Breast03 trial. Nat. Med. 2024, 30, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Lu, R.; Wu, X.; Liu, Q.; Zha, M.; Li, H.; Yuan, Y.; Han, Z.; Cai, D.; Huang, X.; et al. Efficacy and safety of RC48-ADC in HER2-positive and HER2-low metastatic breast cancer: A multicenter, real-world study. Front. Oncol. 2024, 14, 1435485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, R.; Gao, S.; Li, W.; Chen, Y.; Meng, Y.; Liu, C.; Jin, W.; Wu, J.; Wang, Y.; et al. Phase I study of A166, an antibody-drug conjugate in advanced HER2-expressing solid tumours. NPJ Breast Cancer 2023, 9, 28. [Google Scholar] [CrossRef]
- Ma, D.; Dai, L.J.; Wu, X.R.; Liu, C.L.; Zhao, S.; Zhang, H.; Chen, L.; Xiao, Y.; Li, M.; Zhao, Y.Z.; et al. Spatial determinants of antibody-drug conjugate SHR-A1811 efficacy in neoadjuvant treatment for HER2-positive breast cancer. Cancer Cell 2025, 43, 1061–1075. [Google Scholar] [CrossRef]
- Dengl, S.; Mayer, K.; Bormann, F.; Duerr, H.; Hoffmann, E.; Nussbaum, B.; Tischler, M.; Wagner, M.; Kuglstatter, A.; Leibrock, L.; et al. Format chain exchange (FORCE) for high-throughput generation of bispecific antibodies in combinatorial binder-format matrices. Nat. Commun. 2020, 11, 4974. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, D.; Cai, L.; Yao, H.; Yan, M.; Wang, X.; Shen, W.; Du, Y.; Pang, H.; Lai, X.; et al. First-in-human HER2-targeted Bispecific Antibody KN026 for the Treatment of Patients with HER2-positive Metastatic Breast Cancer: Results from a Phase I Study. Clin. Cancer Res. 2022, 28, 618–628. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Beeram, M.; Hamilton, E.; Oh, D.Y.; Hanna, D.L.; Kang, Y.K.; Elimova, E.; Chaves, J.; Goodwin, R.; Lee, J.; et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: A phase 1, dose-escalation and expansion study. Lancet Oncol. 2022, 23, 1558–1570. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y. Technical, preclinical, and clinical developments of Fc-glycan-specific antibody-drug conjugates. RSC Med. Chem. 2024, 16, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.F.; Li, X.; Han, L.; Wang, L.; Liu, J.J.; Yue, Y.L.; Chen, J.; Ke, Y.; Jiang, H.; Xie, Y.Q.; et al. A novel bispecific antibody drug conjugate targeting HER2 and HER3 with potent therapeutic efficacy against breast cancer. Acta Pharmacol. Sin. 2024, 45, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- de Goeij, B.E.; Vink, T.; Ten Napel, H.; Breij, E.C.; Satijn, D.; Wubbolts, R.; Miao, D.; Parren, P.W. Efficient Payload Delivery by a Bispecific Antibody-Drug Conjugate Targeting HER2 and CD63. Mol. Cancer Ther. 2016, 15, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Andreev, J.; Thambi, N.; Perez Bay, A.E.; Delfino, F.; Martin, J.; Kelly, M.P.; Kirshner, J.R.; Rafique, A.; Kunz, A.; Nittoli, T.; et al. Bispecific Antibodies and Antibody-Drug Conjugates (ADCs) Bridging HER2 and Prolactin Receptor Improve Efficacy of HER2 ADCs. Mol. Cancer Ther. 2017, 16, 681–693. [Google Scholar] [CrossRef]
- DeVay, R.M.; Delaria, K.; Zhu, G.; Holz, C.; Foletti, D.; Sutton, J.; Bolton, G.; Dushin, R.; Bee, C.; Pons, J.; et al. Improved Lysosomal Trafficking Can Modulate the Potency of Antibody Drug Conjugates. Bioconjug. Chem. 2017, 28, 1102–1114. [Google Scholar] [CrossRef]
- Khoury, R.; Saleh, K.; Khalife, N.; Saleh, M.; Chahine, C.; Ibrahim, R.; Lecesne, A. Mechanisms of Resistance to Antibody-Drug Conjugates. Int. J. Mol. Sci. 2023, 24, 9674. [Google Scholar] [CrossRef]
- Abelman, R.O.; Wu, B.; Barnes, H.; Medford, A.; Norden, B.; Putur, A.; Bitman, E.; Thant, W.; Liu, T.; Weipert, C.; et al. TOP1 Mutations and Cross-Resistance to Antibody-Drug Conjugates in Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2025, 31, 1966–1974. [Google Scholar] [CrossRef]
- Herbener, P.; Schönfeld, K.; König, M.; Germer, M.; Przyborski, J.M.; Bernöster, K.; Schüttrumpf, J. Functional relevance of in vivo half antibody exchange of an IgG4 therapeutic antibody-drug conjugate. PLoS ONE 2018, 13, e0195823. [Google Scholar] [CrossRef]
- Srikanthan, M.A.; Humbert, O.; Haworth, K.G.; Ironside, C.; Rajawat, Y.S.; Blazar, B.R.; Palchaudhuri, R.; Boitano, A.E.; Cooke, M.P.; Scadden, D.T.; et al. Effective Multi-lineage Engraftment in a Mouse Model of Fanconi Anemia Using Non-genotoxic Antibody-Based Conditioning. Mol. Ther. Methods Clin. Dev. 2020, 17, 455–464. [Google Scholar] [CrossRef]
- Yu, J.; Fang, T.; Yun, C.; Liu, X.; Cai, X. Antibody-Drug Conjugates Targeting the Human Epidermal Growth Factor Receptor Family in Cancers. Front. Mol. Biosci. 2022, 9, 847835. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Shao, T.; Wang, Y.; Chen, T.; Chen, Y.; Chen, Y.L.; Wang, Q.; Yu, X.; Yu, J.; He, K.; et al. PEGylation of Dipeptide Linker Improves Therapeutic Index and Pharmacokinetics of Antibody-Drug Conjugates. Bioconjug. Chem. 2025, 36, 179–189. [Google Scholar] [CrossRef]
- Ashman, N.; Bargh, J.D.; Spring, D.R. Non-internalising antibody-drug conjugates. Chem. Soc. Rev. 2022, 51, 9182–9202. [Google Scholar] [CrossRef]
- Garrett, J.T.; Olivares, M.G.; Rinehart, C.; Granja-Ingram, N.D.; Sánchez, V.; Chakrabarty, A.; Dave, B.; Cook, R.S.; Pao, W.; McKinely, E.; et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc. Natl. Acad. Sci. USA 2011, 108, 5021–5026. [Google Scholar] [CrossRef]
- Vanni, S.; Caputo, T.M.; Cusano, A.M.; De Vita, A.; Cusano, A.; Cocchi, C.; Mulè, C.; Principe, S.; Liverani, C.; Celetti, G.; et al. Engineered anti-HER2 drug delivery nanosystems for the treatment of breast cancer. Nanoscale 2025, 17, 9436–9457. [Google Scholar] [CrossRef]
- Liu, F.; Mao, K.; Chen, H.; Cong, X.; Tan, H.; Xin, Y.; Wang, X.; Ke, J.; Song, Y.; Yang, Y.G.; et al. Enhancing the Safety and Efficacy of Trastuzumab Emtansine (T-DM1) Through Nano-Delivery System in Breast Cancer Therapy. Small 2024, 20, e2400977. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, X.; Fu, R.; Fang, Y.; Zhang, C.; Ma, B.; Ding, Y.; Shi, C.; Zhou, Q. Recent Research Advances in HER2-Positive Breast Cancer Concerning Targeted Therapy Drugs. Molecules 2025, 30, 3026. https://doi.org/10.3390/molecules30143026
Li J, Li X, Fu R, Fang Y, Zhang C, Ma B, Ding Y, Shi C, Zhou Q. Recent Research Advances in HER2-Positive Breast Cancer Concerning Targeted Therapy Drugs. Molecules. 2025; 30(14):3026. https://doi.org/10.3390/molecules30143026
Chicago/Turabian StyleLi, Junmin, Xue Li, Ruixin Fu, Yakun Fang, Chunmei Zhang, Bingbing Ma, Yanan Ding, Chuanxin Shi, and Qingfeng Zhou. 2025. "Recent Research Advances in HER2-Positive Breast Cancer Concerning Targeted Therapy Drugs" Molecules 30, no. 14: 3026. https://doi.org/10.3390/molecules30143026
APA StyleLi, J., Li, X., Fu, R., Fang, Y., Zhang, C., Ma, B., Ding, Y., Shi, C., & Zhou, Q. (2025). Recent Research Advances in HER2-Positive Breast Cancer Concerning Targeted Therapy Drugs. Molecules, 30(14), 3026. https://doi.org/10.3390/molecules30143026






