Potential of Boronic Acid Derivatization and Activity in Agrochemical Discovery
Abstract
1. Introduction
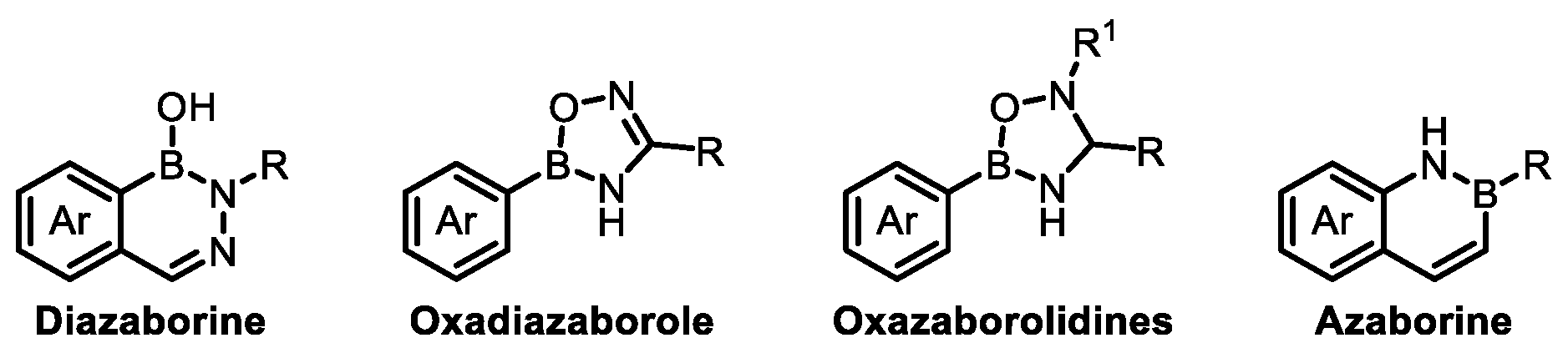
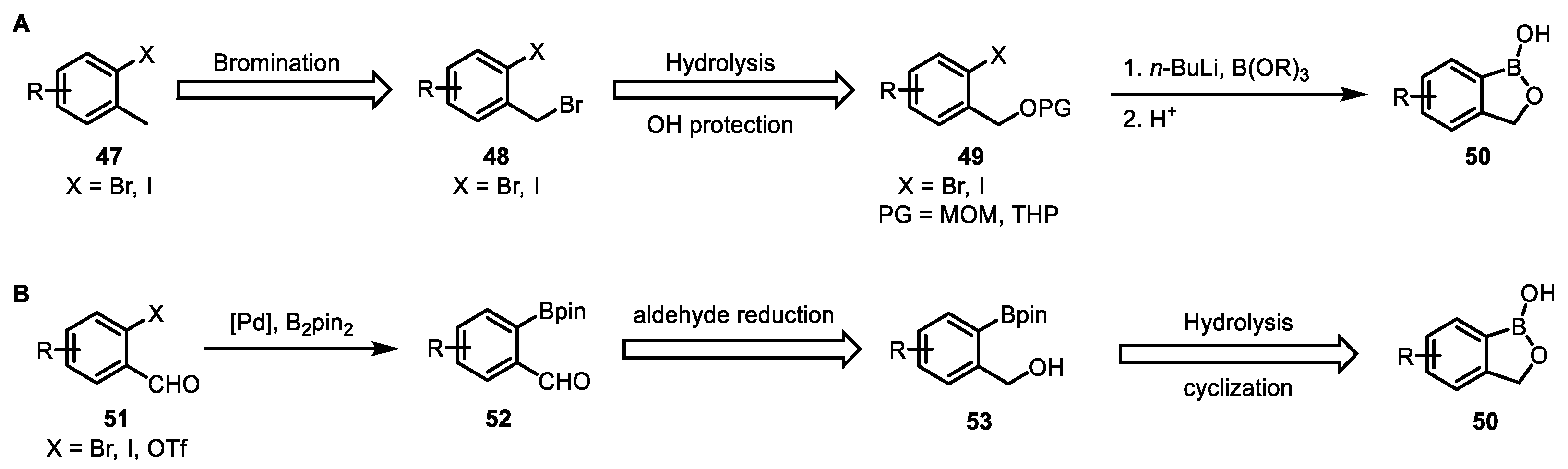
2. Boron-Containing Building Blocks and Targets
3. Boron-Containing Compounds in Agrochemical
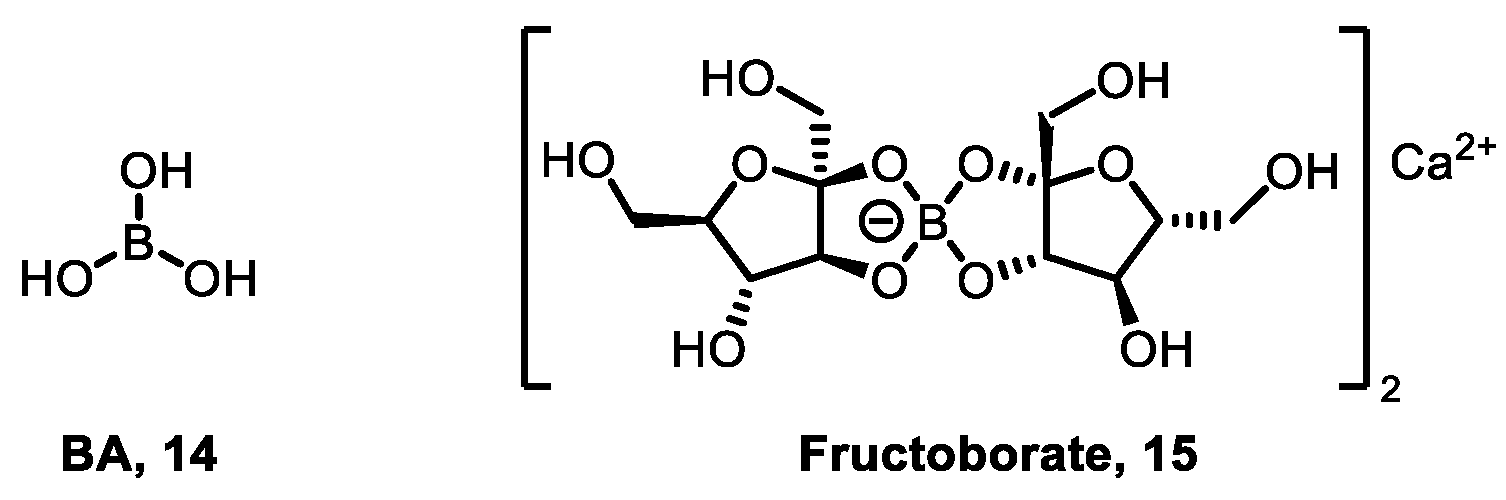
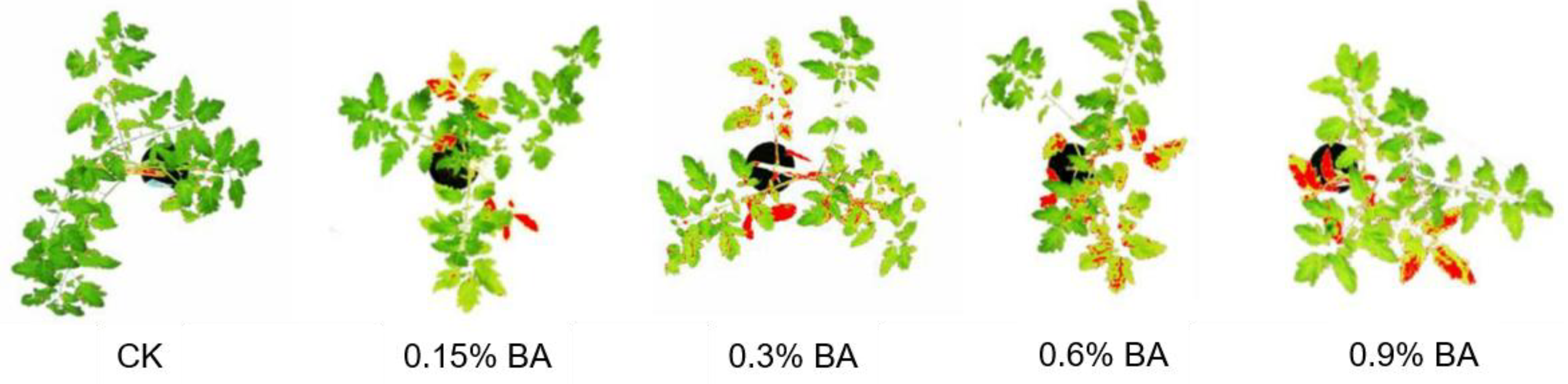
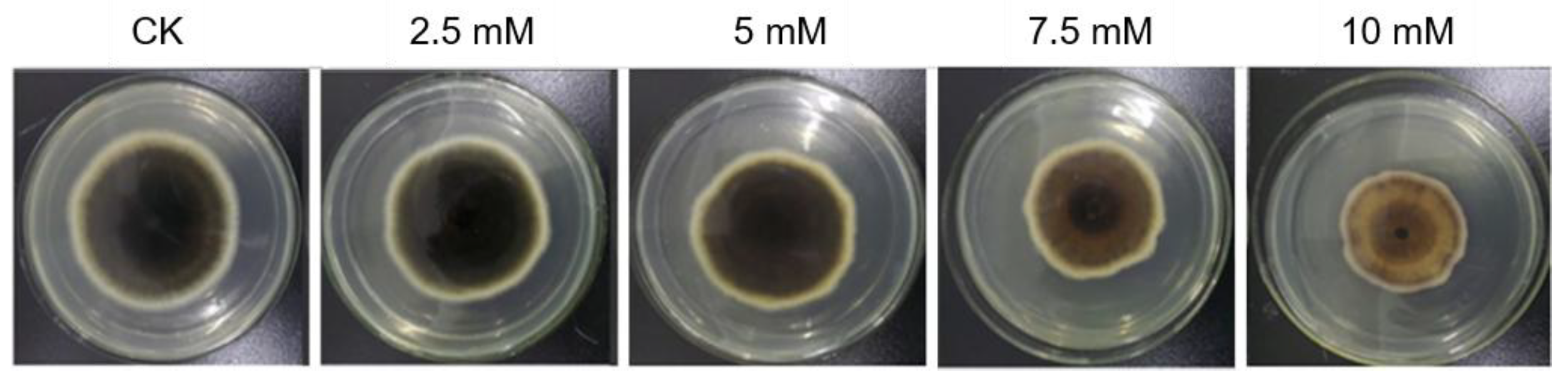
3.1. Boric Acid as Fungicides and Growth Regulators
3.2. Organic Boron Compounds
4. Current Challenges in Boron-Containing Agrochemicals
4.1. Stability
4.2. Synthesis Cost
4.3. Environmental Safety
5. Conclusion and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almássy, J.; Csernoch, L.; Nánási, P.P. Safety Concerns of Diamide Insecticides. Toxicol. Sci. 2019, 171, 281. [Google Scholar] [CrossRef] [PubMed]
- Balba, H. Review of strobilurin fungicide chemicals. J. Environ. Sci. Health B. 2007, 42, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. Agbiotech: A growing threat down on the farm. Science 2007, 316, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Hauvermale, A.L.; Steber, C.M. GA signaling is essential for the embryo-to-seedling transition during Arabidopsis seed germination, a ghost story. Plant Signal. Behav. 2020, 15, e1705028. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Ning, Y.L.; Rao, B.Q. Comprehensive Overview of β-Methoxyacrylate Derivatives as Cytochrome Inhibitors for Novel Pesticide Discovery. J. Agric. Food Chem. 2022, 70, 15615–15630. [Google Scholar] [CrossRef] [PubMed]
- Musso, L.; Fabbrini, A.; Dallavalle, S. Natural Compound-Derived Cytochrome bc1 Complex Inhibitors as Antifungal Agents. Molecules 2020, 25, 4582. [Google Scholar] [CrossRef] [PubMed]
- Stukenbrock, E.; Gurr, S. Address the growing urgency of fungal disease in crops. Nature 2023, 617, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Grams, R.J.; Santos, W.L.; Scorei, I.R.; Abad-Garcia, A.; Rosenblum, C.A.; Bita, A.; Cerecetto, H.; Vinas, C.; Soriano-Ursua, M.A. The Rise of Boron-Containing Compounds: Advancements in Synthesis, Medicinal Chemistry, and Emerging Pharmacology. Chem. Rev. 2024, 124, 2441–2511. [Google Scholar] [CrossRef] [PubMed]
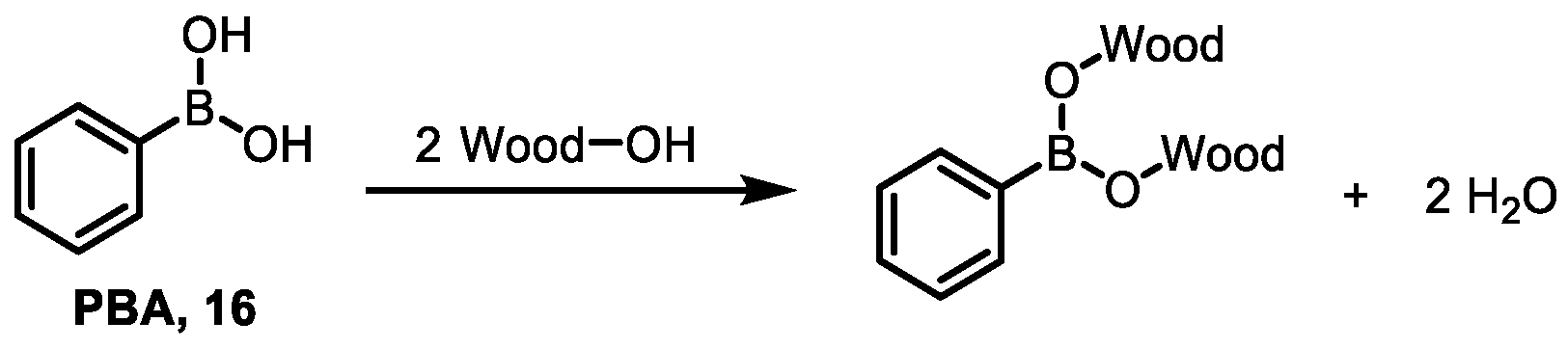
- Yalinkiliç, M.K.; Yoshimura, T.; Takahashi, M. Enhancement of the biological resistance of wood by phenylboronic acid treatment. J. Wood Sci. 1998, 44, 152–157. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Smoum, R.; Al-Quntar, A.A.; Abu Ali, H.; Pergament, I.; Srebnik, M. Natural occurrence of boron-containing compounds in plants, algae and microorganisms. Plant Sci. 2002, 163, 931–942. [Google Scholar] [CrossRef]
- Camacho-Cristobal, J.J.; Rexach, J.; Gonzalez-Fontes, A. Boron in plants: Deficiency and toxicity. J. Integr. Plant Biol. 2008, 50, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.L.; Siqueira, J.A.; Batista-Silva, W.; Cardoso, F.B.; Nunes-Nesi, A.; Araujo, W.L. Boron: More Than an Essential Element for Land Plants? Front. Plant Sci. 2020, 11, 610307. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, M.A.; Abreu, I.; Bell, R.W.; Bienert, M.D.; Brown, P.H.; Dell, B.; Fujiwara, T.; Goldbach, H.E.; Lehto, T.; Mock, H.P.; et al. Boron: An essential element for vascular plants. New Phytol. 2020, 226, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- FRAC Classification of Fungicides. Available online: https://www.frac.info/media/42keb12k/frac-moa-poster-2024.pdf (accessed on 16 July 2025).
- Diaz, D.B.; Yudin, A.K. The versatility of boron in biological target engagement. Nat. Chem. 2017, 9, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Lennox, A.J.; Lloyd-Jones, G.C. Selection of boron reagents for Suzuki-Miyaura coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed]
- Hajinasiri, R. Arylboronic acids in organic synthesis. J. Mol. Struct. 2025, 1336, 142029. [Google Scholar] [CrossRef]
- Fisher, R.S. The use of boric acid in dermatologic practice. AMA Arch. Derm. 1956, 73, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.S.; Denny, W.A.; Dos Santos, J.L. Boron in drug design: Recent advances in the development of new therapeutic agents. Eur. J. Med. Chem. 2019, 179, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Plescia, J.; Moitessier, N. Design and discovery of boronic acid drugs. Eur. J. Med. Chem. 2020, 195, 112270. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Gao, P.; Sun, L.; Kang, D.; Kongsted, J.; Poongavanam, V.; Zhan, P.; Liu, X. Recent developments in the medicinal chemistry of single boron atom-containing compounds. Acta Pharm. Sin. B. 2021, 11, 3035–3059. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Adil Shareef, M.; Das, S.; Nandwana, N.K.; Das, Y.; Saito, M.; Weiss, L.M. Boron-Containing heterocycles as promising pharmacological agents. Bioorg. Med. Chem. 2022, 63, 116748. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Nandwana, N.K.; Das, S.; Nandwana, V.; Shareef, M.A.; Das, Y.; Saito, M.; Weiss, L.M.; Almaguel, F.; Hosmane, N.S.; et al. Boron Chemicals in Drug Discovery and Development: Synthesis and Medicinal Perspective. Molecules 2022, 27, 2615. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.C.; Farrell, A.T.; Sridhara, R.; Pazdur, R. United States Food and Drug Administration approval summary: Bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin. Cancer Res. 2006, 12, 2955–2960. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Ixazomib: First Global Approval. Drugs 2016, 76, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Dudley, M.N.; Lomovskaya, O. Meropenem-Vaborbactam Resistance Selection, Resistance Prevention, and Molecular Mechanisms in Mutants of KPC-Producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2017, 61, e0169417. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Tavaborole: First global approval. Drugs 2014, 74, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Akama, T.; Baker, S.J.; Zhang, Y.K.; Hernandez, V.; Zhou, H.; Sanders, V.; Freund, Y.; Kimura, R.; Maples, K.R.; Plattner, J.J. Discovery and structure-activity study of a novel benzoxaborole anti-inflammatory agent (AN2728) for the potential topical treatment of psoriasis and atopic dermatitis. Bioorg. Med. Chem. Lett. 2009, 19, 2129–2132. [Google Scholar] [CrossRef] [PubMed]
- Messner, K.; Vuong, B.; Tranmer, G.K. The Boron Advantage: The Evolution and Diversification of Boron’s Applications in Medicinal Chemistry. Pharmaceuticals 2022, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- Groll, M.; Berkers, C.R.; Ploegh, H.L.; Ovaa, H. Crystal structure of the boronic acid-based proteasome inhibitor bortezomib in complex with the yeast 20S proteasome. Structure 2006, 14, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Rock, F.L.; Mao, W.; Yaremchuk, A.; Tukalo, M.; Crepin, T.; Zhou, H.; Zhang, Y.K.; Hernandez, V.; Akama, T.; Baker, S.J.; et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science 2007, 316, 1759–1761. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.P.; Windsor, I.W.; Forest, K.T.; Raines, R.T. Stilbene Boronic Acids Form a Covalent Bond with Human Transthyretin and Inhibit Its Aggregation. J. Med. Chem. 2017, 60, 7820–7834. [Google Scholar] [CrossRef] [PubMed]
- Smoum, R.; Rubinstein, A.; Dembitsky, V.M.; Srebnik, M. Boron containing compounds as protease inhibitors. Chem. Rev. 2012, 112, 4156–4220. [Google Scholar] [CrossRef] [PubMed]
- Adams, J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell 2004, 5, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Arkwright, R.; Pham, T.M.; Zonder, J.A.; Dou, Q.P. The preclinical discovery and development of bortezomib for the treatment of mantle cell lymphoma. Expert Opin. Drug Discov. 2017, 12, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; Ghazarian, R.N.; Ou, M.; Luderer, M.J.; Kusdono, H.D.; Azab, A.K. Spotlight on ixazomib: Potential in the treatment of multiple myeloma. Drug Des. Devel. Ther. 2016, 10, 217–226. [Google Scholar] [PubMed]
- Collins, G.A.; Goldberg, A.L. The Logic of the 26S Proteasome. Cell 2017, 169, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.; Henneberg, F.; Mata, R.A.; Tittmann, K.; Schneider, T.R.; Stark, H.; Bourenkov, G.; Chari, A. The inhibition mechanism of human 20S proteasomes enables next-generation inhibitor design. Science 2016, 353, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M.; Mushtaq, S. Activity of biapenem (RPX2003) combined with the boronate beta-lactamase inhibitor RPX7009 against carbapenem-resistant Enterobacteriaceae. J. Antimicrob. Chemother. 2013, 68, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. FDA Approves New Antibacterial Drug. 2017. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=209776 (accessed on 16 July 2025).
- Hecker, S.J.; Reddy, K.R.; Totrov, M.; Hirst, G.C.; Lomovskaya, O.; Griffith, D.C.; King, P.; Tsivkovski, R.; Sun, D.; Sabet, M.; et al. Discovery of a Cyclic Boronic Acid beta-Lactamase Inhibitor (RPX7009) with Utility vs Class A Serine Carbapenemases. J. Med. Chem. 2015, 58, 3682–3692. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Kirschner, R.A.; Geyer, A.; Klebe, G. Conceptional Design of Self-Assembling Bisubstrate-like Inhibitors of Protein Kinase A Resulting in a Boronic Acid Glutamate Linkage. ACS Omega 2019, 4, 775–784. [Google Scholar] [CrossRef]
- Ciaravino, V.; Plattner, J.; Chanda, S. An assessment of the genetic toxicology of novel boron-containing therapeutic agents. Environ. Mol. Mutagen. 2013, 54, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Tomsho, J.W.; Benkovic, S.J. The unique chemistry of benzoxaboroles: Current and emerging applications in biotechnology and therapeutic treatments. Bioorg. Med. Chem. 2014, 22, 4462–4473. [Google Scholar] [CrossRef] [PubMed]
- Jinna, S.; Finch, J. Spotlight on tavaborole for the treatment of onychomycosis. Drug Des. Devel. Ther. 2015, 9, 6185–6190. [Google Scholar] [PubMed]
- Benkovic, S.J.; Baker, S.J.; Alley, M.R.; Woo, Y.H.; Zhang, Y.K.; Akama, T.; Mao, W.; Baboval, J.; Rajagopalan, P.T.; Wall, M.; et al. Identification of borinic esters as inhibitors of bacterial cell growth and bacterial methyltransferases, CcrM and MenH. J. Med. Chem. 2005, 48, 7468–7476. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.J.; Zhang, Y.K.; Akama, T.; Lau, A.; Zhou, H.; Hernandez, V.; Mao, W.; Alley, M.R.; Sanders, V.; Plattner, J.J. Discovery of a new boron-containing antifungal agent, 5-fluoro-1,3-dihydro-1-hydroxy-2,1- benzoxaborole (AN2690), for the potential treatment of onychomycosis. J. Med. Chem. 2006, 49, 4447–4450. [Google Scholar] [CrossRef] [PubMed]
- Markinson, B.; Ghannoum, M.; Winter, T.; Rycerz, A.; Rock, F.; Gupta, A.K. Examining the Benefits of the Boron-Based Mechanism of Action and Physicochemical Properties of Tavaborole in the Treatment of Onychomycosis. J. Am. Podiatr. Med. Assoc. 2018, 108, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.S.; Grau, M.; Mavis, C.; Hailfinger, S.; Wolf, A.; Madle, H.; Deeb, G.; Dorken, B.; Thome, M.; Lenz, P.; et al. MCL1 is deregulated in subgroups of diffuse large B-cell lymphoma. Leukemia 2013, 27, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Cal, P.M.; Vicente, J.B.; Pires, E.; Coelho, A.V.; Veiros, L.F.; Cordeiro, C.; Gois, P.M. Iminoboronates: A new strategy for reversible protein modification. J. Am. Chem. Soc. 2012, 134, 10299–10305. [Google Scholar] [CrossRef] [PubMed]
- Akcay, G.; Belmonte, M.A.; Aquila, B.; Chuaqui, C.; Hird, A.W.; Lamb, M.L.; Rawlins, P.B.; Su, N.; Tentarelli, S.; Grimster, N.P.; et al. Inhibition of Mcl-1 through covalent modification of a noncatalytic lysine side chain. Nat. Chem. Biol. 2016, 12, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, E.; Kerff, F.; Terrak, M.; Ayala, J.A.; Charlier, P. The penicillin-binding proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Zervosen, A.; Herman, R.; Kerff, F.; Herman, A.; Bouillez, A.; Prati, F.; Pratt, R.F.; Frere, J.M.; Joris, B.; Luxen, A.; et al. Unexpected tricovalent binding mode of boronic acids within the active site of a penicillin-binding protein. J. Am. Chem. Soc. 2011, 133, 10839–10848. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.; Frank, D.; Kettner, C.A.; Agard, D.A. Structural analysis of specificity: Alpha-lytic protease complexes with analogues of reaction intermediates. Biochemistry 1989, 28, 7600–7609. [Google Scholar] [CrossRef] [PubMed]
- Wlodawer, A.; Vondrasek, J. Inhibitors of HIV-1 protease: A major success of structure-assisted drug design. Annu. Rev. Biophys. Biomol. Struct. 1998, 27, 249–284. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, E.; Schiffer, C.A. Resilience to resistance of HIV-1 protease inhibitors: Profile of darunavir. AIDS Rev. 2008, 10, 131–142. [Google Scholar] [PubMed]
- Nalam, M.N.; Ali, A.; Reddy, G.S.; Cao, H.; Anjum, S.G.; Altman, M.D.; Yilmaz, N.K.; Tidor, B.; Rana, T.M.; Schiffer, C.A. Substrate envelope-designed potent HIV-1 protease inhibitors to avoid drug resistance. Chem. Biol. 2013, 20, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Kincaid, J.F.; Cho, W.; Walters, D.E.; Krishnan, K.; Hussain, K.A.; Koo, Y.; Cho, H.; Rudall, C.; Holland, L.; et al. Potent HIV protease inhibitors incorporating high-affinity P2-ligands and (R)-(hydroxyethylamino)sulfonamide isostere. Bioorg. Med. Chem. Lett. 1998, 8, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Windsor, I.W.; Palte, M.J.; Lukesh, J.C., 3rd; Gold, B.; Forest, K.T.; Raines, R.T. Sub-picomolar Inhibition of HIV-1 Protease with a Boronic Acid. J. Am. Chem. Soc. 2018, 140, 14015–14018. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.C.; Yu, J.L.; Dai, Q.Q.; Li, G.; Li, G.B. Targeting Metalloenzymes by Boron-Containing Metal-Binding Pharmacophores. J. Med. Chem. 2021, 64, 17706–17727. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.A.; Eberhard, S.; Albersheim, P.; Darvill, A.G. Requirement of borate cross-linking of cell wall hamnogalacturonan II for growth. Science 2001, 294, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.A.; Islam, M.M.; Islam, A.T.M.S.; Hassan, S.M.E.; Hasan, M.M. In vitro and in vivo efficacy of some chemicals against common scab (Streptomyces scabies) of potato. Arch. Phytopathol. Plant Prot. 2022, 55, 886–899. [Google Scholar] [CrossRef]
- Cale, N.L.; Walker, P.L.; Sankar, S.; Robertson, S.M.; Wilkins, O.; Belmonte, M.F. Global mRNA profiling reveals the effect of boron as a crop protection tool against Sclerotinia sclerotiorum. AoB Plants 2024, 16, plae056. [Google Scholar] [CrossRef] [PubMed]
- Martinko, K.; Ivankovic, S.; Lazarevic, B.; Dermic, E.; Dermic, D. Control of Early Blight Fungus (Alternaria alternata) in Tomato by Boric and Phenylboronic Acid. Antibiotics 2022, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Perveen, K.; Alwathnani, H.A. Antifungal Activity of Methanol, Acetone and Diethyl Ether Extracts of Cyanobacteria Against Plant Pathogenic Fungi. Asian J. Chem. 2013, 25, 7531–7534. [Google Scholar] [CrossRef]
- Chen, T.; He, S.; Li, S.; Xiao, H.; Song, H.; Meng, L.; Shi, X. Intracellular and mitochondrial proteomic analysis reveals antifungal mechanisms of borate on mango black spot pathogen Alternaria alternata. Plant Pathol. 2024, 73, 69–89. [Google Scholar] [CrossRef]
- Dong, X.; Wang, M.; Ling, N.; Shen, Q.R.; Guo, S.W. Effects of iron and boron combinations on the suppression of wilt in banana. Sci. Rep. 2016, 6, 38944. [Google Scholar] [CrossRef] [PubMed]
- Kabdrakhmanova, S.K.; Kabdrakhmanova, A.K.; Shaimardan, E.; Akatan, K.; Beisebekov, M.M.; Selenova, B.S.; Aubakirova, R.A.; Maussumbayeva, A.; Thomas, S.; Seilkhanov, T.M. Growth Stimulating and Fungicidal Properties of Succinic Acid Complexes with Silver, Copper and Boron Ions During Pre-Sowing Treatment of Soybean Seeds. Eng. Sci. 2023, 26, 973. [Google Scholar] [CrossRef]
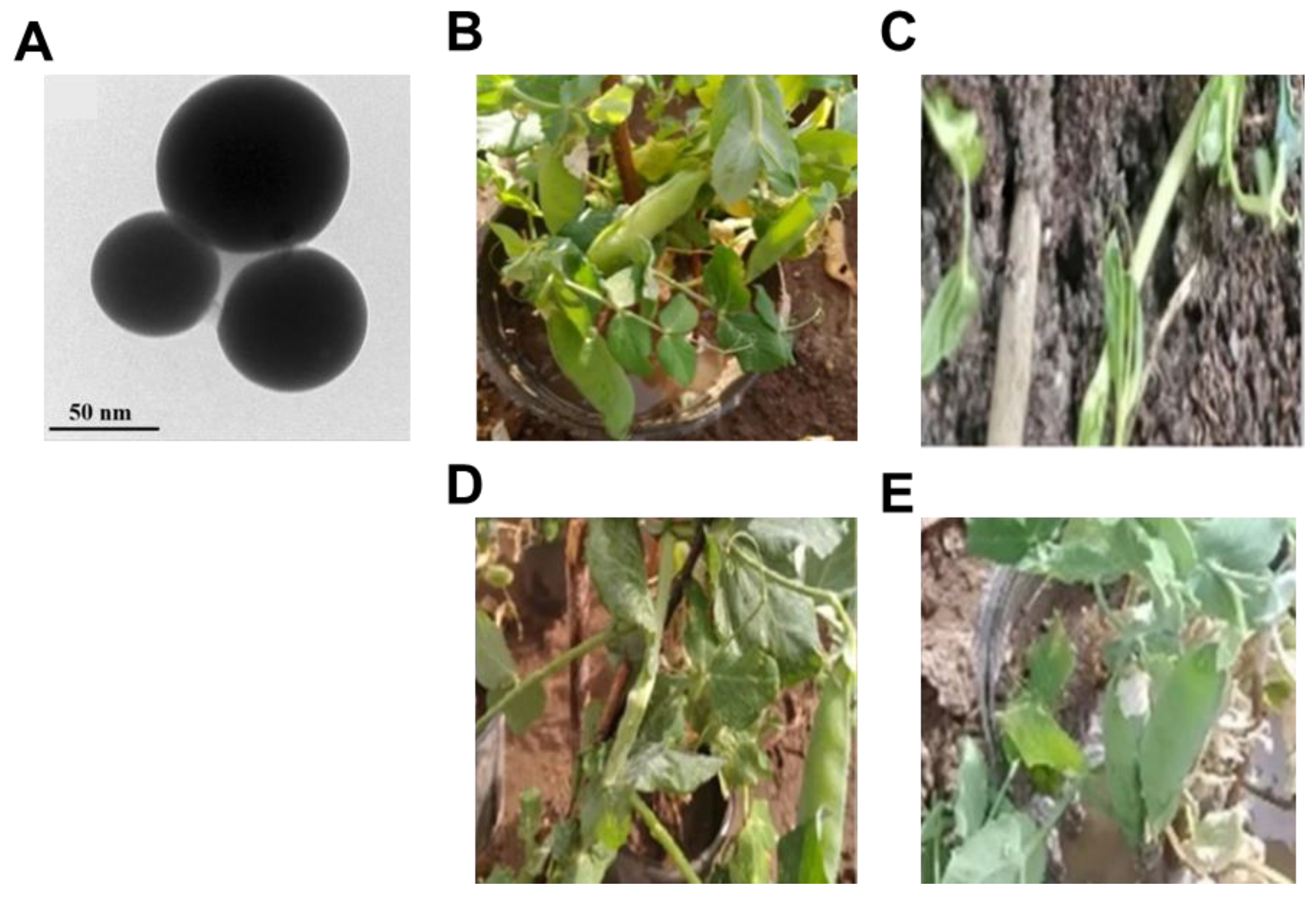
- Elkhodary, B.H.; Attia, M.S.; El-Sayyad, G.S.; Salem, M.S. Effectiveness of bimetallic ZnO-B2O3 nanoparticles produced by Streptomyces gancidicus as prospective antifungal agents and therapeutic nutrients to enhance pea plant immunity against damping off-causing Pythium irregulare: In vivo and in vitro investigations. Biomass Conv. Biorefin. 2023, 15, 2363–2386. [Google Scholar]
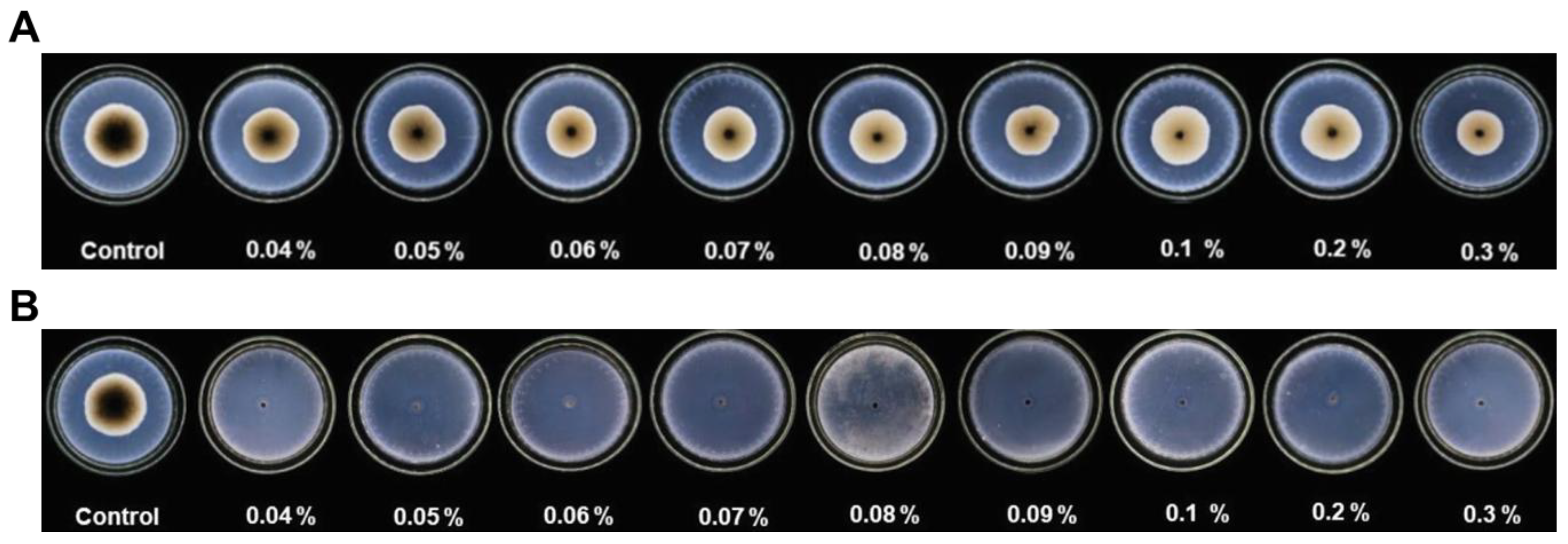
- Martinko, K.; Ivankovic, S.; Dermic, E.; Dermic, D. In vitro antifungal effect of phenylboronic and boric acid on Alternaria alternata. Arh. Hig. Rada. Toksikol. 2022, 73, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Martinko, K.; Ivankovic, S.; Dermic, E.; Dermic, D. Phenylboronic acid as a novel agent for controlling plant pathogenic bacteria. Pest. Manag. Sci. 2022, 78, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
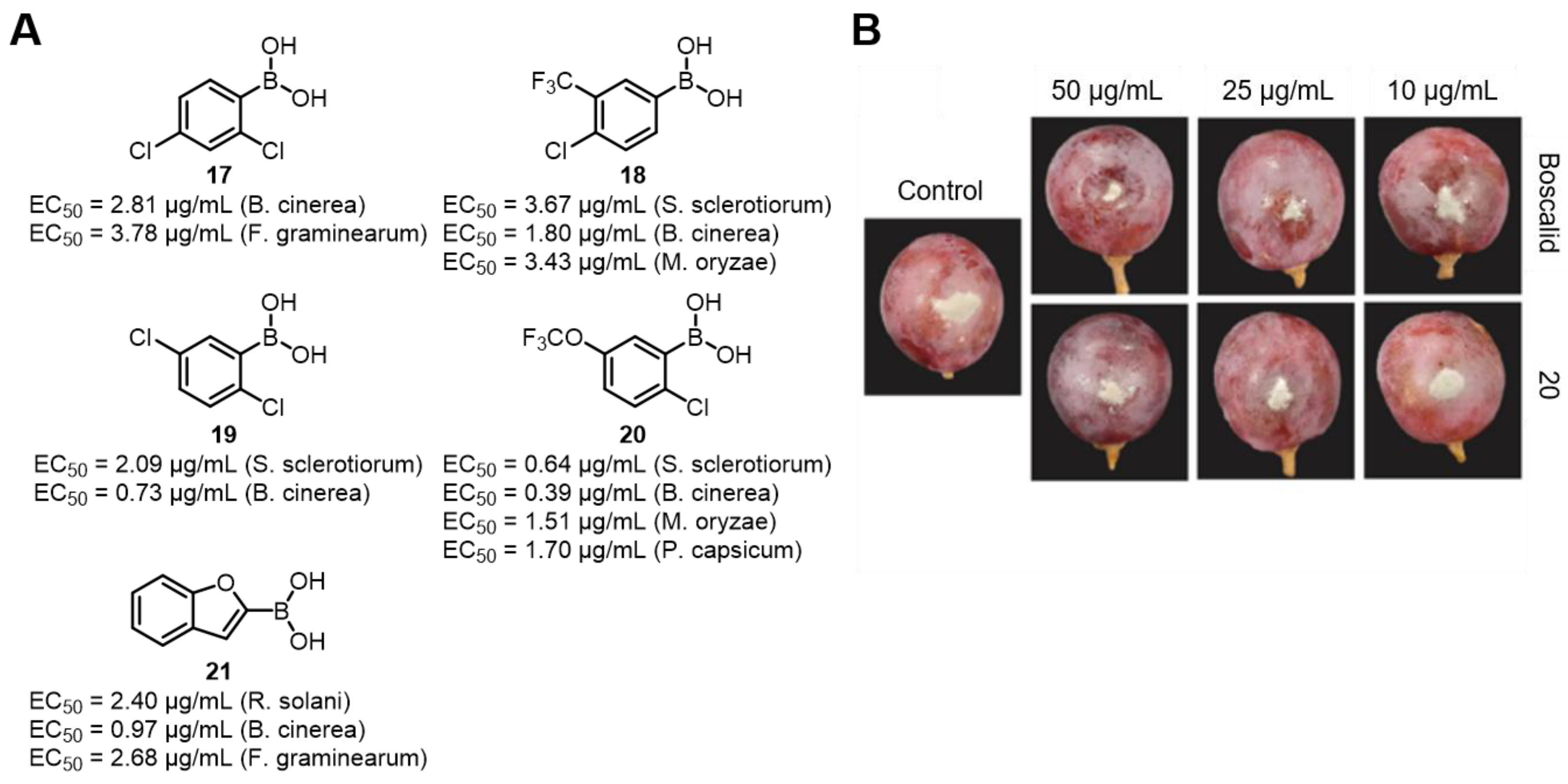
- Du, S.S.; Luo, X.F.; An, J.X.; Zhang, Z.J.; Zhang, S.Y.; Wang, Y.R.; Ding, Y.Y.; Jiang, W.Q.; Zhang, B.Q.; Ma, Y.; et al. Exploring boron applications in modern agriculture: Antifungal activities and mechanisms of phenylboronic acid derivatives. Pest. Manag. Sci. 2023, 79, 2748–2761. [Google Scholar] [CrossRef] [PubMed]
- Dowlut, M.; Hall, D.G. An improved class of sugar-binding boronic acids, soluble and capable of complexing glycosides in neutral water. J. Am. Chem. Soc. 2006, 128, 4226–4227. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Huang, D.-C.; Guo, D.; Deng, F.; Sha, Q.; Zhang, M.-Z.; Zhang, W.-H.; Gu, Y.-C. Synthesis, fungicidal activity and molecular docking studies of tavaborole derivatives. Adv. Agrochem. 2023, 2, 185–195. [Google Scholar] [CrossRef]
- Huang, D.C.; He, Z.; Guo, D.; Deng, F.; Bian, Q.; Zhang, H.; Ali, A.S.; Zhang, M.Z.; Zhang, W.H.; Gu, Y.C. Discovery of Novel Benzoxaborole-Containing Streptochlorin Derivatives as Potential Antifungal Agents. J. Agric. Food Chem. 2023, 71, 6226–6235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Duan, C.B.; Xu, H.L.; Zhao, X.Y.; Huang, D.C.; Jin, B.; Sha, Q.; Miu, S.; Bian, Q.; Guo, D.L.; et al. Dual-Target Inhibitors horizontal line Discovery of Novel Diphenyl-(Thio)ether-Containing Benzoxaborole Derivatives as Potential Antifungal and Herbicidal Agents. J. Agric. Food Chem. 2025, 73, 4497–4506. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Liu, C.; Zhou, Y.; Zhang, Y.K.; McGregor, C.; Steere, L.; Frederick, B.H.; Liu, C.T.; Whitesell, L.; Cowen, L.E. Inhibiting Protein Prenylation with Benzoxaboroles to Target Fungal Plant Pathogens. ACS Chem. Biol. 2020, 15, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Steere, L.; McGregor, C.; Frederick, B.H.; Pastoor, T.; Zhou, Y.; Liu, C.T.; Cai, Y.; Zhou, H.; Xu, M.; et al. Exploring boron applications in modern agriculture: A structure-activity relationship study of a novel series of multi-substitution benzoxaboroles for identification of potential fungicides. Bioorg Med. Chem. Lett. 2021, 43, 128089. [Google Scholar] [CrossRef] [PubMed]
- Mogg, T.D.; Pollitt, C.C.; Willmore, J.P.; Thompson, H. Efficacy of Avermectin B1 Given Orally against Equine Intestinal Strongyles and Onchocera Microfilaria. Aust. Vet. J. 1990, 67, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Sasanelli, N.; Toderas, I.; Veronico, P.; Iurcu-Straistaru, E.; Rusu, S.; Melillo, M.T.; Caboni, P. Abamectin Efficacy on the Potato Cyst Nematode Globodera pallida. Plants 2020, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Cen, Y.K.; Yi, Y.L.; Zhang, L.L.; Xue, Y.P.; Zheng, Y.G. Avermectins and Their Derivatives: Recent Advances in Biosynthesis and Application. J. Agric. Food. Chem. 2025, 73, 1757–1774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhong, B.; Yang, S.M.; Pan, L.K.; Yu, S.W.; Li, Z.J.; Li, S.C.; Su, B.; Meng, X.B. Synthesis and biological evaluation of thiabendazole derivatives as anti-angiogenesis and vascular disrupting agents. Bioorgan. Med. Chem. 2015, 23, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Budetic, M.; Kopf, D.; Dandic, A.; Samardzic, M. Review of Characteristics and Analytical Methods for Determination of Thiabendazole. Molecules 2023, 28, 3926. [Google Scholar] [CrossRef] [PubMed]
- Hayes, H.L.D.; Wei, R.; Assante, M.; Geogheghan, K.J.; Jin, N.; Tomasi, S.; Noonan, G.; Leach, A.G.; Lloyd-Jones, G.C. Protodeboronation of (Hetero)Arylboronic Esters: Direct versus Prehydrolytic Pathways and Self-/Auto-Catalysis. J. Am. Chem. Soc. 2021, 143, 14814–14826. [Google Scholar] [CrossRef] [PubMed]
- Zarzeczanska, D.; Adamczyk-Wozniak, A.; Kulpa, A.; Ossowski, T.; Sporzynski, A. Fluorinated Boronic Acids: Acidity and Hydrolytic Stability of Fluorinated Phenylboronic Acids. Eur. J. Inorg. Chem. 2017, 2017, 4493–4498. [Google Scholar] [CrossRef]
- Adamczyk-Wozniak, A.; Borys, K.M.; Sporzynski, A. Recent developments in the chemistry and biological applications of benzoxaboroles. Chem. Rev. 2015, 115, 5224–5247. [Google Scholar] [CrossRef] [PubMed]
- Gillis, E.P.; Burke, M.D. A simple and modular strategy for small molecule synthesis: Iterative Suzuki-Miyaura coupling of B-protected haloboronic acid building blocks. J. Am. Chem. Soc. 2007, 129, 6716–6717. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Cognetta, A.B., 3rd; Niphakis, M.J.; He, Z.; Zajdlik, A.; St Denis, J.D.; Scully, C.C.; Cravatt, B.F.; Yudin, A.K. Facile synthesis of borofragments and their evaluation in activity-based protein profiling. Chem. Commun. 2015, 51, 3608–3611. [Google Scholar] [CrossRef] [PubMed]
- Llona-Minguez, S.; Hoglund, A.; Jacques, S.A.; Johansson, L.; Calderon-Montano, J.M.; Claesson, M.; Loseva, O.; Valerie, N.C.K.; Lundback, T.; Piedrafita, J.; et al. Discovery of the First Potent and Selective Inhibitors of Human dCTP Pyrophosphatase 1. J. Med. Chem. 2016, 59, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Blair, D.J.; Chitti, S.; Trobe, M.; Kostyra, D.M.; Haley, H.M.S.; Hansen, R.L.; Ballmer, S.G.; Woods, T.J.; Wang, W.; Mubayi, V.; et al. Automated iterative Csp(3)-C bond formation. Nature 2022, 604, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.M. The Future Role of Pesticides in US Agriculture, Committee on the Future Role of Pesticides in US Agriculture, Board of Agriculture and Natural Resources and Board on Environmental Studies and Toxicology, Commission on Life Sciences. J. Agric. Sci. 2002, 137, 373–375. [Google Scholar] [CrossRef]
- Yasukawa, T.; Kuremoto, T.; Miyamura, H.; Kobayashi, S. Asymmetric Arylation of Imines Catalyzed by Heterogeneous Chiral Rhodium Nanoparticles. Org. Lett. 2016, 18, 2716–2718. [Google Scholar] [CrossRef] [PubMed]
- Gorad, S.S.; Ghorai, P. Organocatalytic Desymmetric Double Aza-Michael Addition Cascade: Enantioselective Synthesis of Fused Morpholines. Org. Lett. 2024, 26, 5571–5576. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, T.; Murata, M.; Miyaura, N. Palladium(0)-Catalyzed Cross-Coupling Reaction of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters. J. Org. Chem. 1995, 60, 7508–7510. [Google Scholar] [CrossRef]
- Morgan, A.B.; Jurs, J.L.; Tour, J.M. Synthesis, flame-retardancy testing, and preliminary mechanism studies of nonhalogenated aromatic boronic acids: A new class of condensed-phase polymer flame-retardant additives for acrylonitrile-butadiene-styrene and polycarbonate. J. Appl. Polym. Sci. 2000, 76, 1257–1268. [Google Scholar] [CrossRef]
- Yamamoto, E.; Izumi, K.; Horita, Y.; Ito, H. Anomalous Reactivity of Silylborane: Transition-Metal-Free Boryl Substitution of Aryl, Alkenyl, and Alkyl Halides with Silylborane/Alkoxy Base Systems. J. Am. Chem. Soc. 2012, 134, 19997–20000. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Yang, H.J.; Fu, H. Visible-Light Photoredox Borylation of Aryl Halides and Subsequent Aerobic Oxidative Hydroxylation. Org. Lett. 2016, 18, 5248–5251. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cheung, M.S.; Lin, Z.Y.; Li, P.F. Metal-free borylation of electron-rich aryl (pseudo)halides under continuous-flow photolytic conditions. Org. Chem. Front. 2016, 3, 875–879. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Xu, Q.; Li, H. Synthesis of Benzoxaboroles by ortho-Oxalkylation of Arylboronic Acids with Aldehydes/Ketones in the Presence of Bronsted Acids. Org. Lett. 2021, 23, 1986–1990. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Frederiksen, M.; Sharma, A.K. Toxicity of boric acid, borax and other boron containing compounds: A review. Regul. Toxicol. Pharmacol. 2021, 121, 104873. [Google Scholar] [CrossRef] [PubMed]
- Brdar-Jokanovic, M. Boron Toxicity and Deficiency in Agricultural Plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology, P. NTP Toxicology and Carcinogenesis Studies of Boric Acid (CAS No. 10043-35-3) in B6C3F1 Mice (Feed Studies). Natl. Toxicol. Program Tech. Rep. Ser. 1987, 324, 1–126. [Google Scholar]
- Loewengart, G. Toxicity of boron to rainbow trout: A weight-of-the-evidence assessment. Environ. Toxicol. Chem. 2001, 20, 796–803. [Google Scholar] [CrossRef] [PubMed]
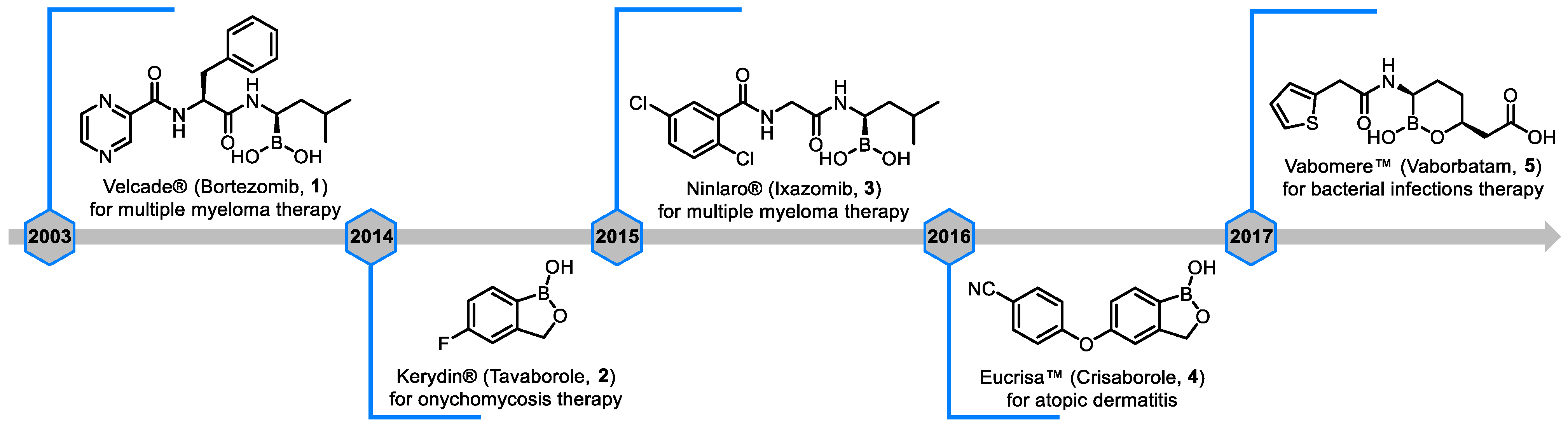

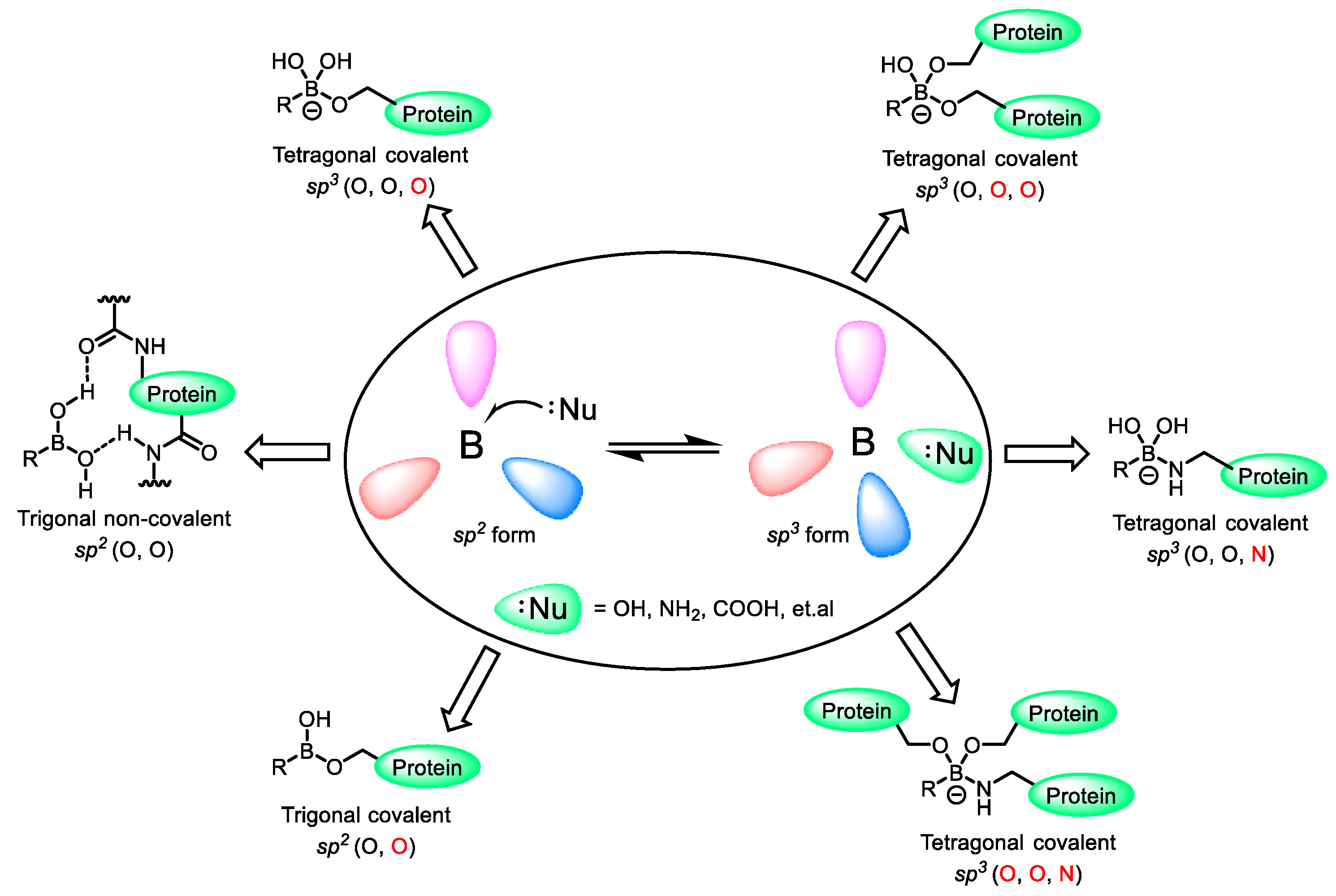
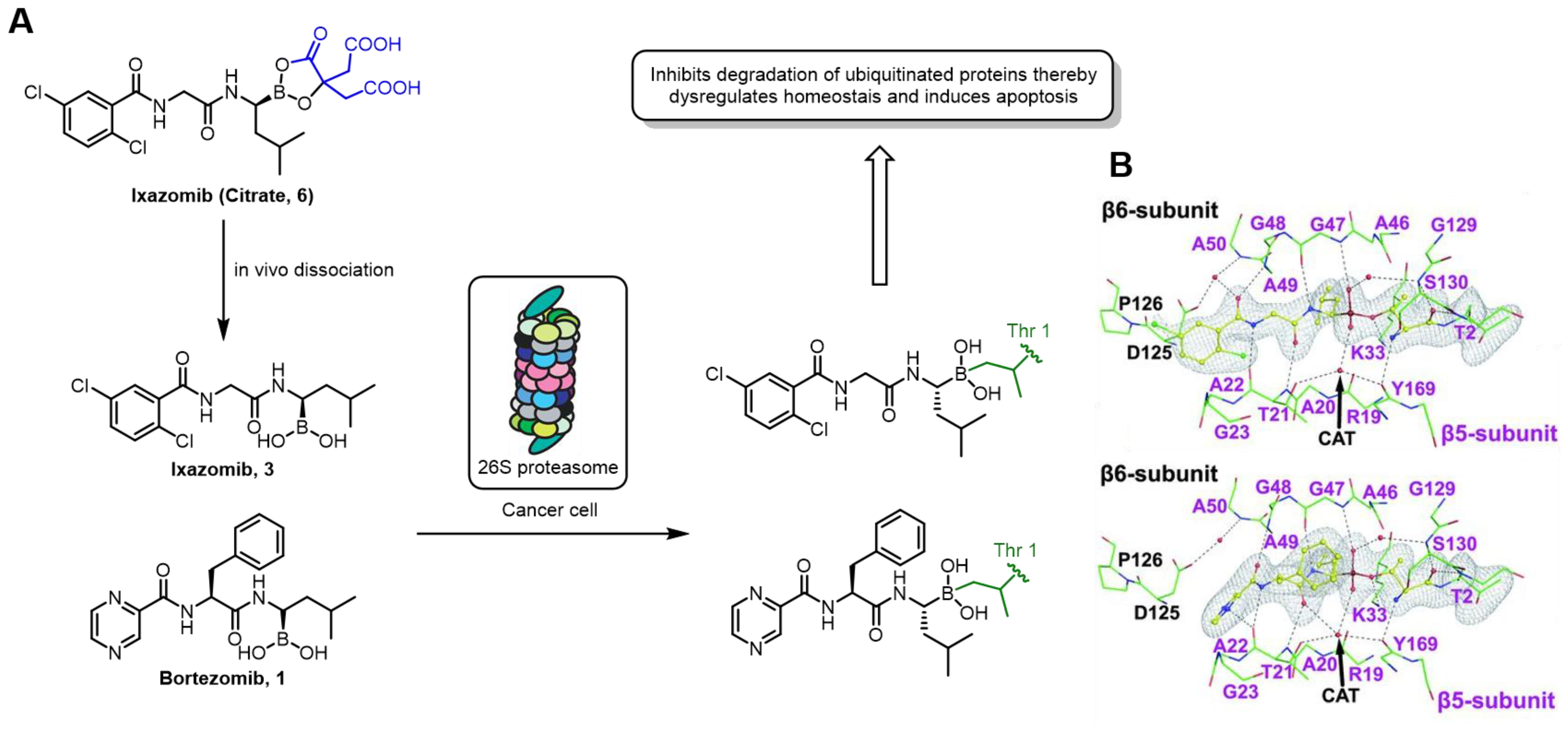
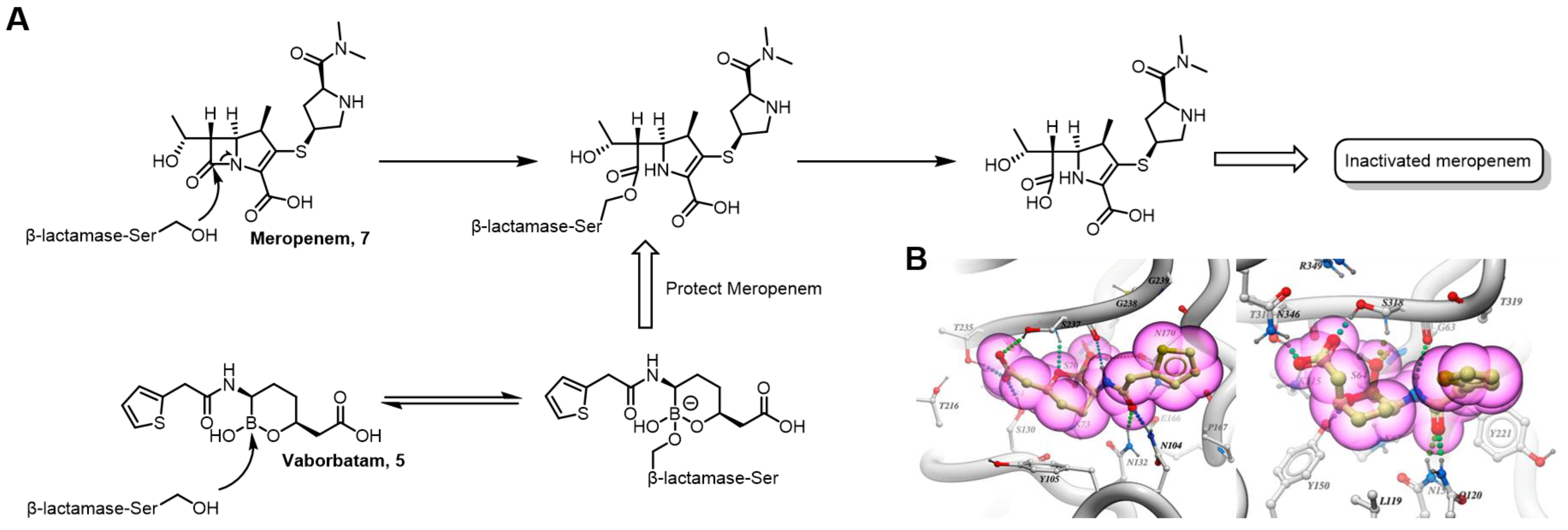
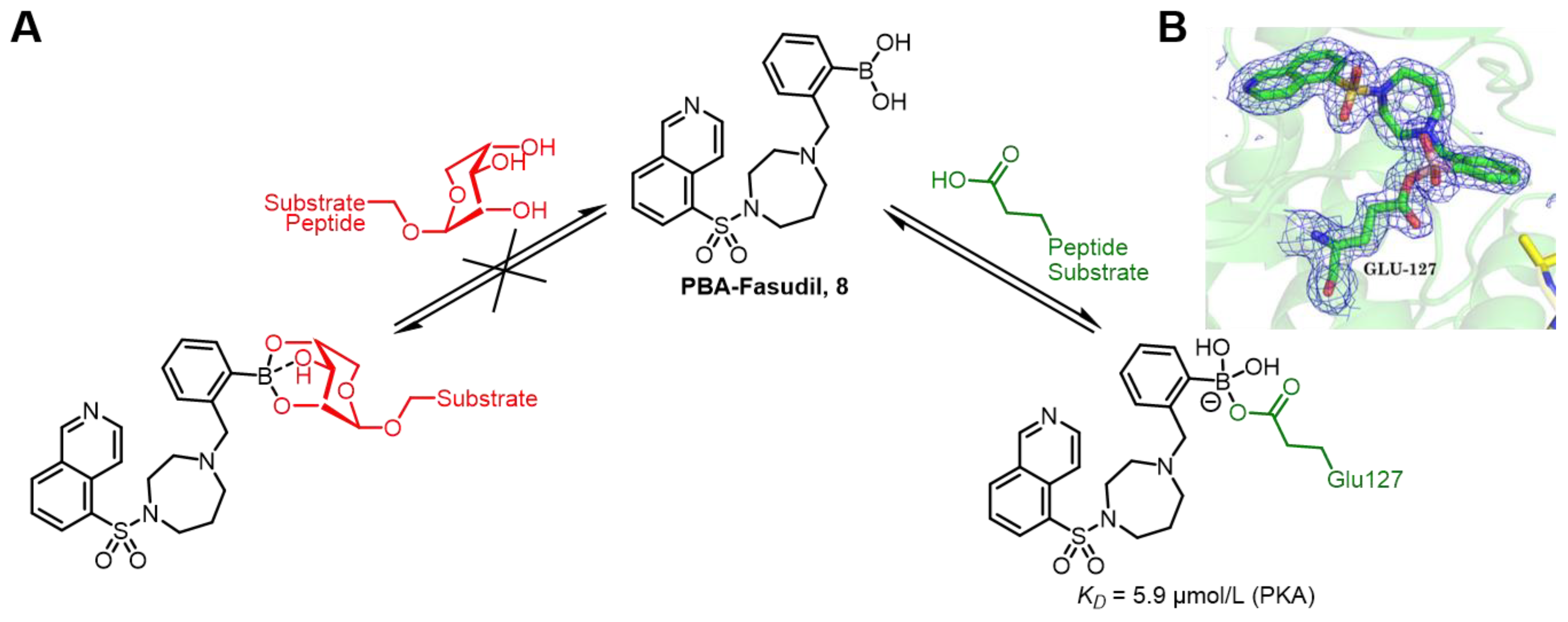

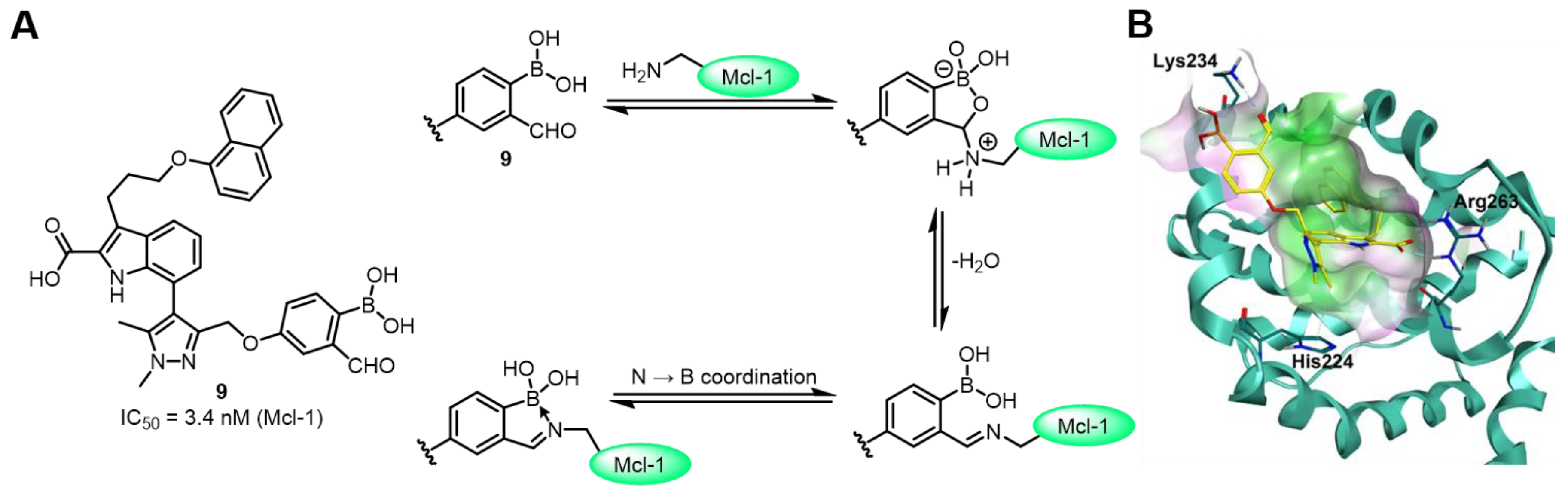
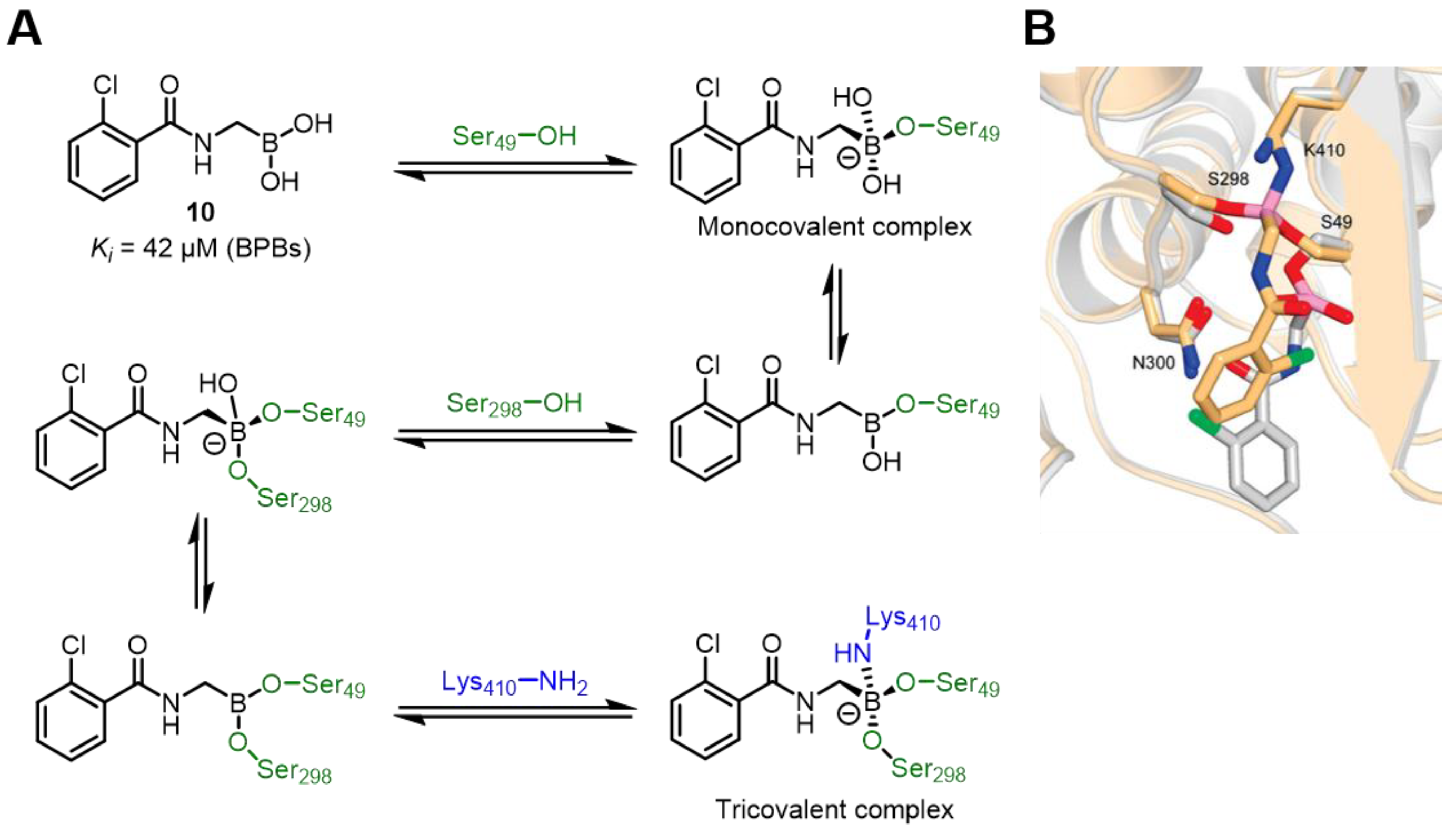


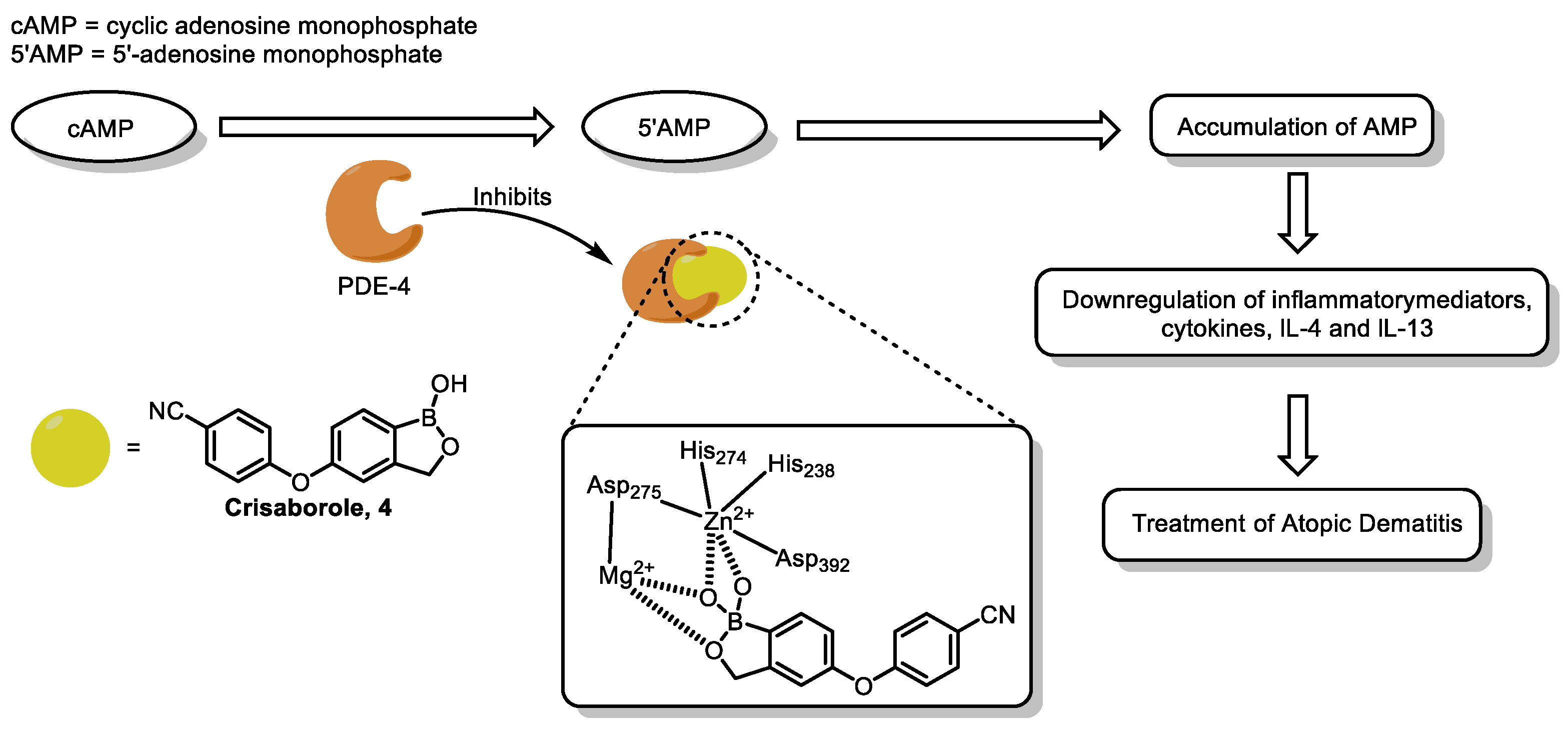



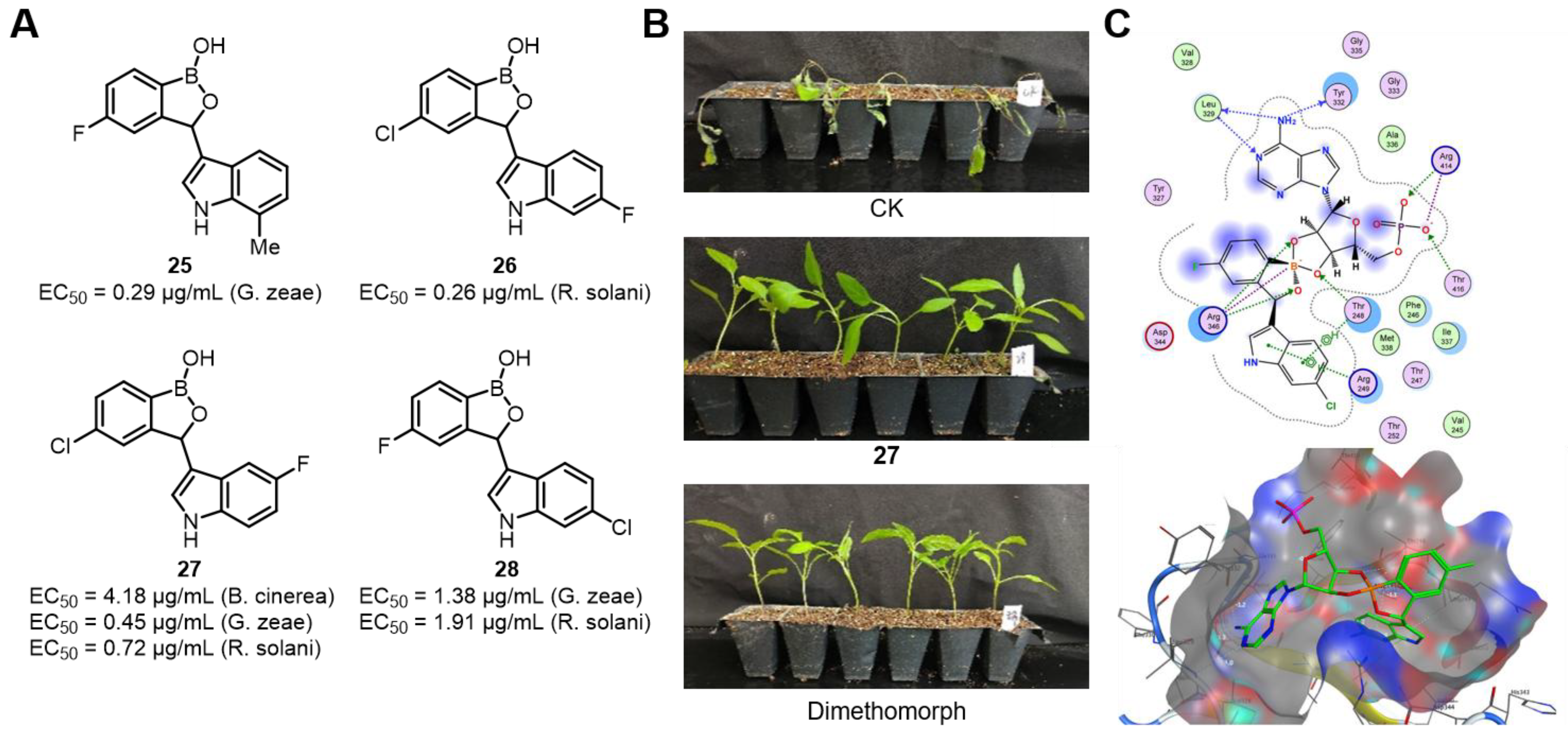


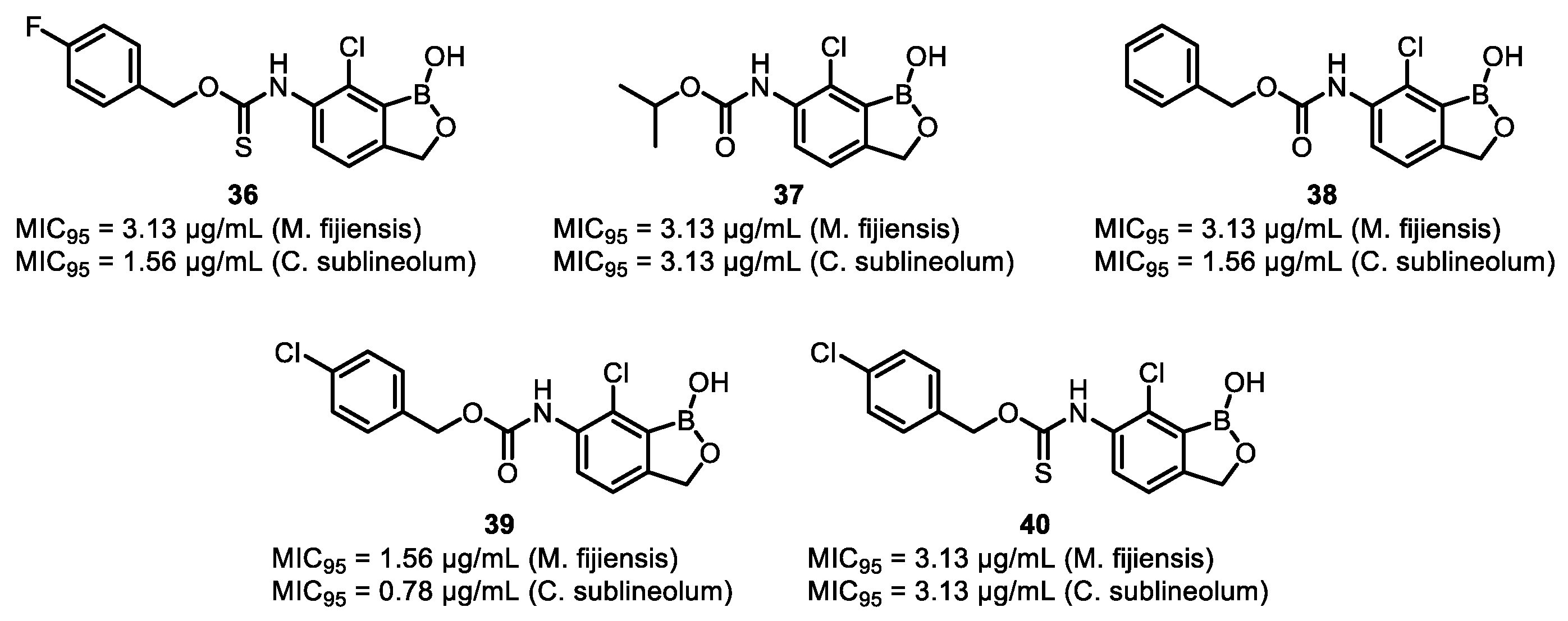
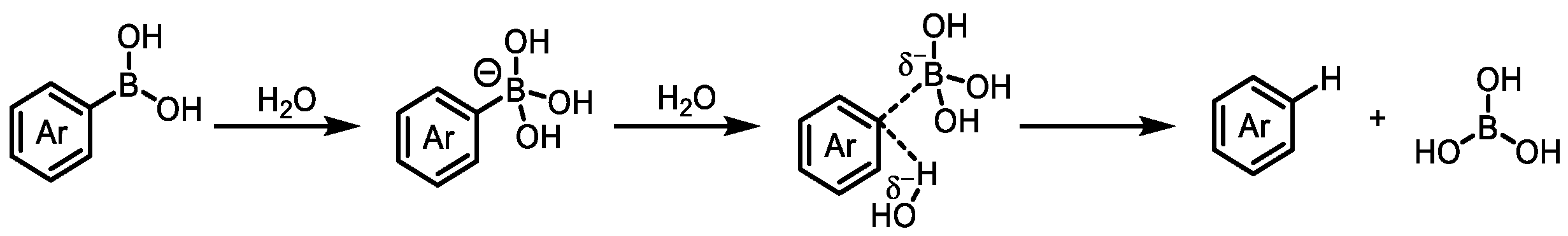
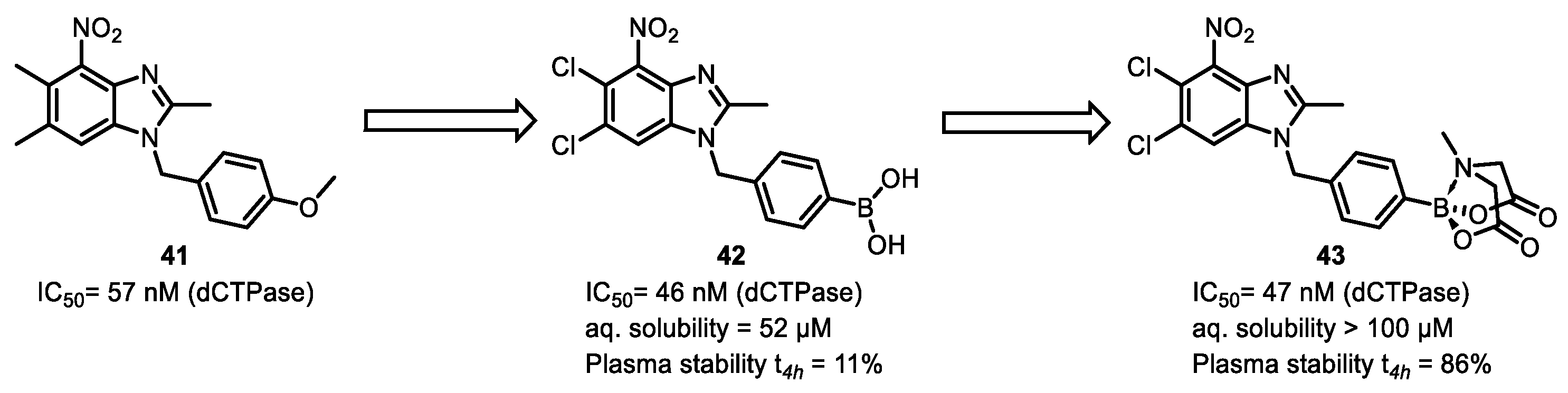


| Field | Compound Type | Protein Target | Mechanism and Solved Problems |
|---|---|---|---|
| Medicine | Peptide Boronic Acid | Proteasome; β-lactamase; PBPs | Treats multiple myeloma; Treats bacterial infections; Resolves bacterial drug resistance |
| Benzoxaborole | LeuRS; PDE-4 | Treats onychomycosis; Treats atopic dermatitis | |
| Phenylboronic Acid Derivatives | PKA; Mcl-1; HIV-1 | Uncovers the covalent bonding mechanism of boronic acid and Glu; Addresses non-covalent Mcl-1 inhibitor off-target effects; Improves Darunavir’s HIV-1 protease targeting efficiency | |
| Agriculture | Boric Acid | NPR3 | Induces plant resistance |
| Borates | POD; PPO | Enhances plant disease resistance | |
| Phenylboronic Acid Derivatives | POD; PPO | Disrupts cell membrane integrity Affects redox balance Interferes with amino acid metabolism | |
| Benzoxaborole | LeuRS; NtPPO; GGTase I | Develops novel antifungal agents and addresses fungicide resistance issues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, L.; Wu, J.; Zuo, Y.; Gao, W.; Feng, J.; Zhang, Z. Potential of Boronic Acid Derivatization and Activity in Agrochemical Discovery. Molecules 2025, 30, 3018. https://doi.org/10.3390/molecules30143018
Ji L, Wu J, Zuo Y, Gao W, Feng J, Zhang Z. Potential of Boronic Acid Derivatization and Activity in Agrochemical Discovery. Molecules. 2025; 30(14):3018. https://doi.org/10.3390/molecules30143018
Chicago/Turabian StyleJi, Liangshuo, Jianxin Wu, Yachen Zuo, Wenqiang Gao, Jiyao Feng, and Zhenhua Zhang. 2025. "Potential of Boronic Acid Derivatization and Activity in Agrochemical Discovery" Molecules 30, no. 14: 3018. https://doi.org/10.3390/molecules30143018
APA StyleJi, L., Wu, J., Zuo, Y., Gao, W., Feng, J., & Zhang, Z. (2025). Potential of Boronic Acid Derivatization and Activity in Agrochemical Discovery. Molecules, 30(14), 3018. https://doi.org/10.3390/molecules30143018






