Mechanistic Study of Oil Adsorption Behavior and CO2 Displacement Mechanism Under Different pH Conditions
Abstract
1. Introduction
2. Results and Discussion
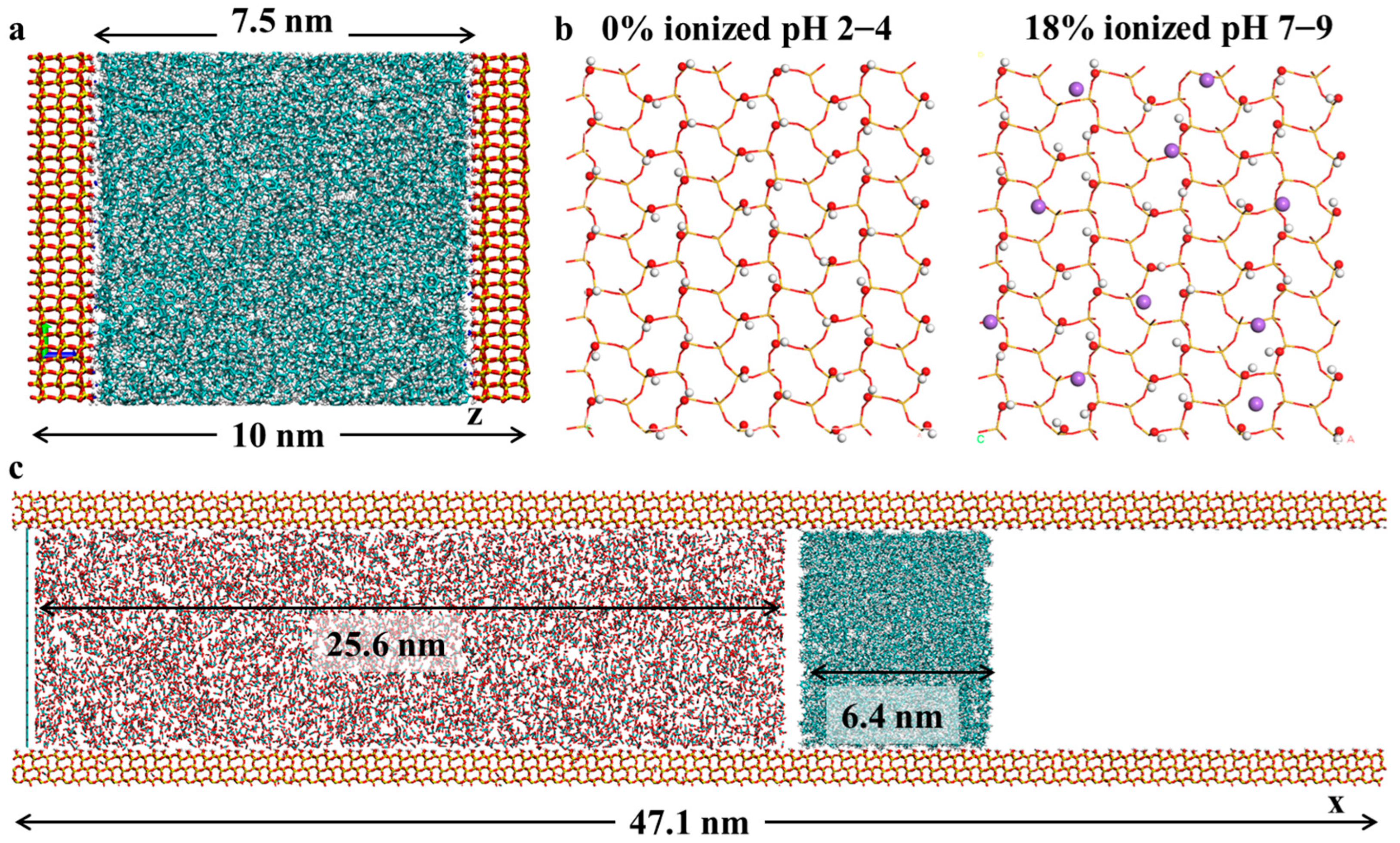
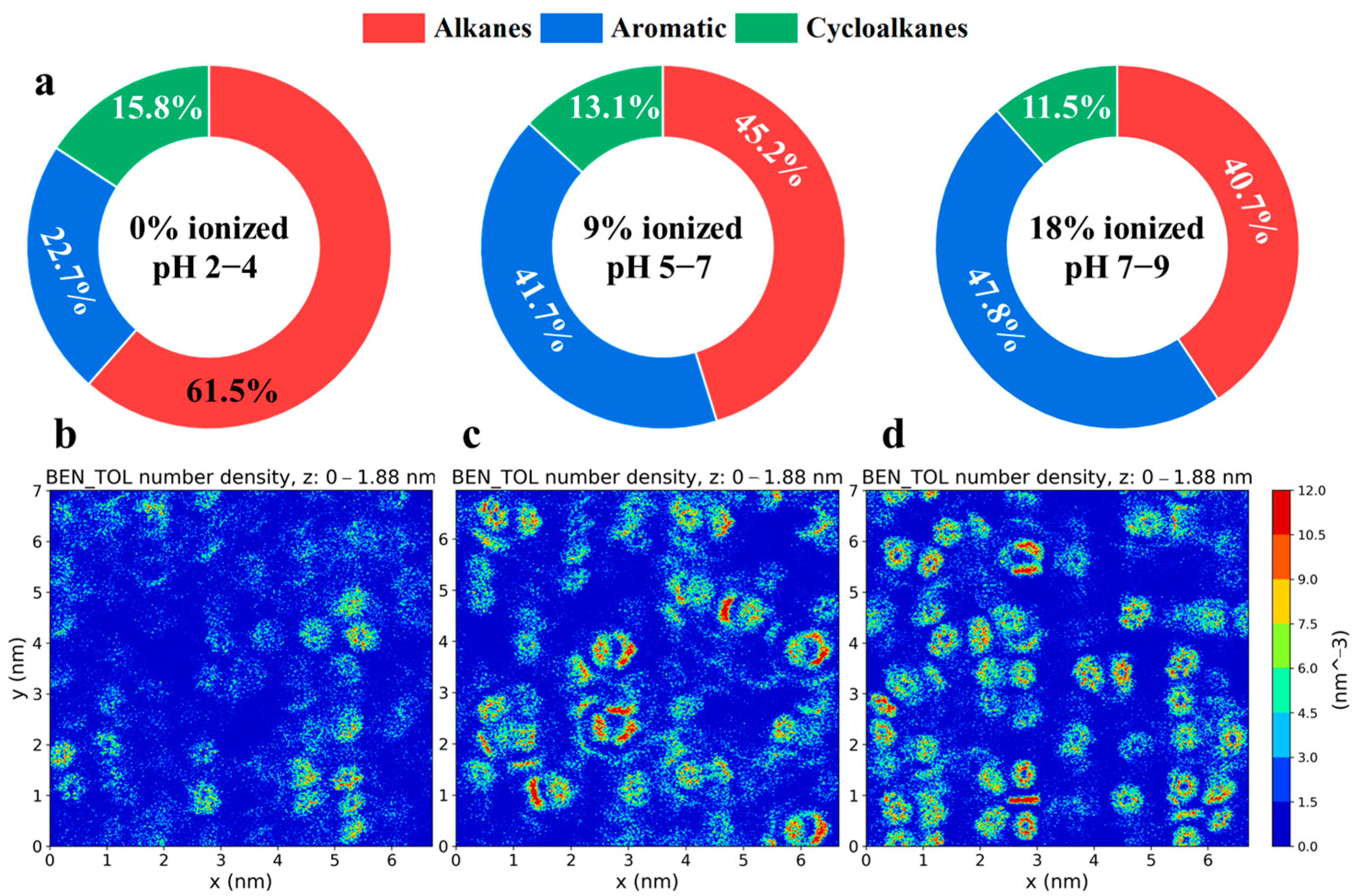
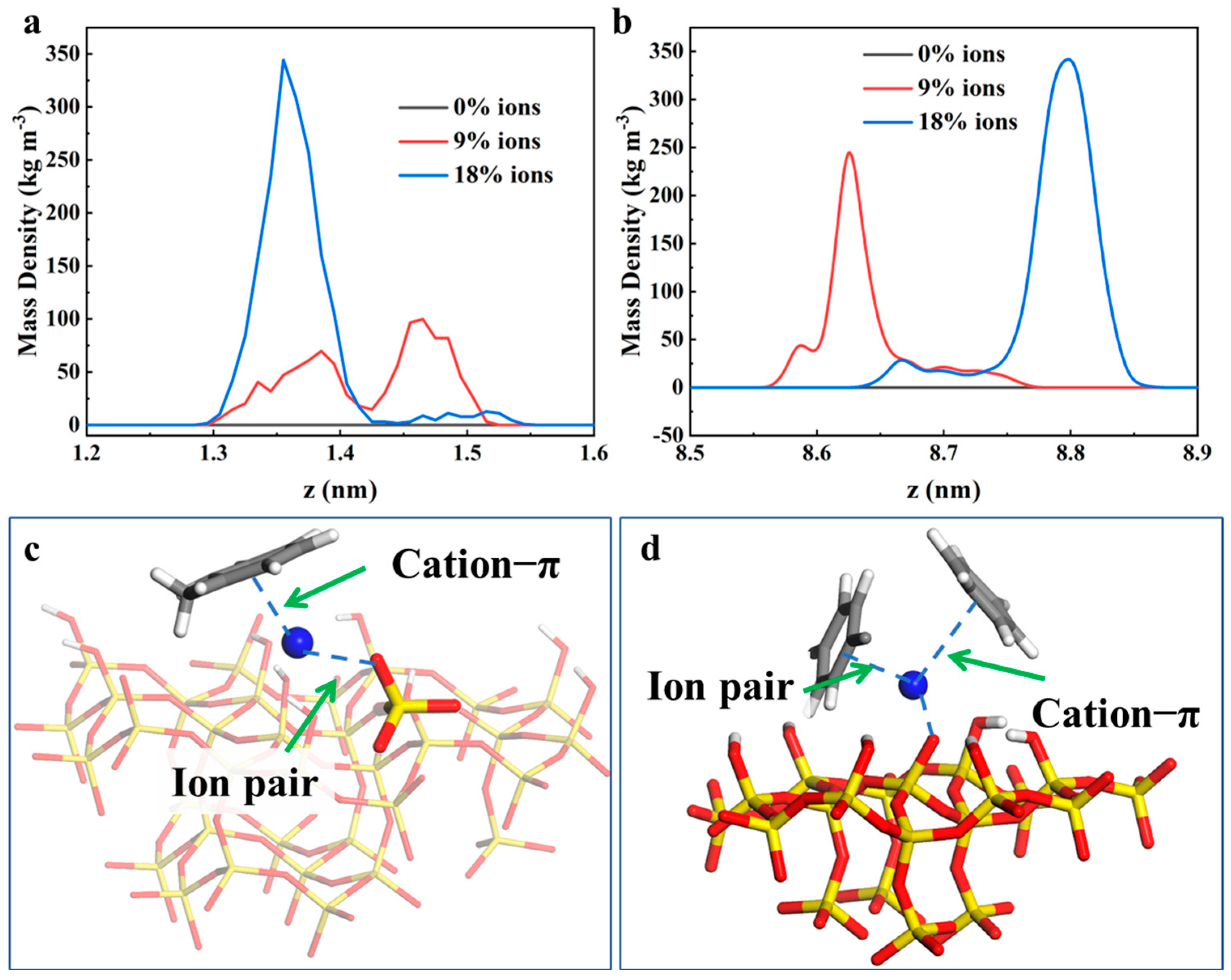
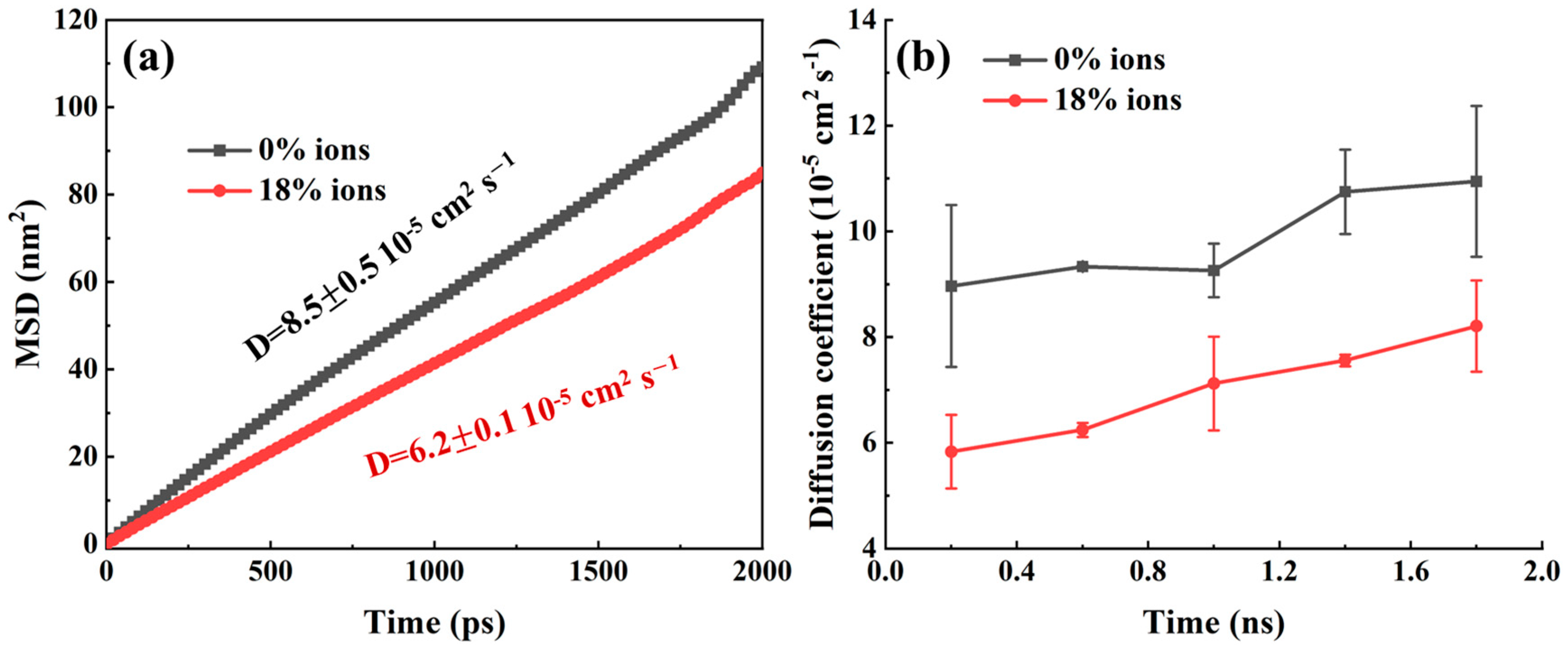
2.1. Distribution of Crude Oil Within Silica Slits
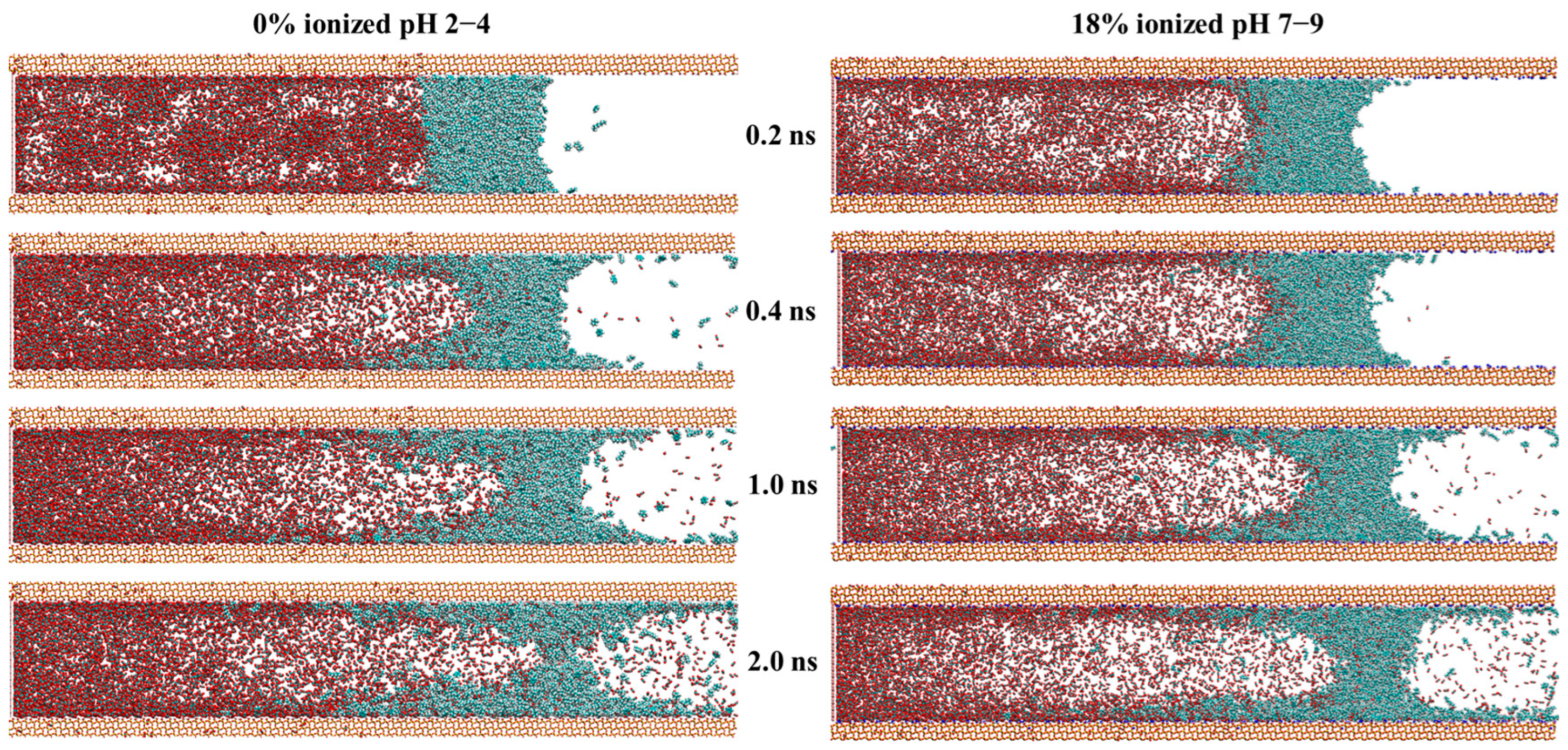
2.2. CO2 Displacement Process Under Different pH Conditions
3. Methodology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Yao, X.; Feng, Q.; Javadpour, F.; Yang, Y.; Xue, Q.; Li, X. Molecular insights into carbon dioxide enhanced multi-component shale gas recovery and its sequestration in realistic kerogen. Chem. Eng. J. 2021, 425, 130292. [Google Scholar] [CrossRef]
- Liu, Y.; Shang, Y.; Yan, Z.; Li, H.; Wang, Z.; Li, Z.; Liu, Z. Pim1 kinase provides protection against high glucose-induced stress and apoptosis in cultured dorsal root ganglion neurons. Neurosci. Res. 2021, 169, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, C.R.; Haghshenas, B.; Ghanizadeh, A.; Qanbari, F.; Williams-Kovacs, J.D.; Riazi, N.; Debuhr, C.; Deglint, H.J. Nanopores to megafractures: Current challenges and methods for shale gas reservoir and hydraulic fracture characterization. J. Nat. Gas Sci. Eng. 2016, 31, 612–657. [Google Scholar] [CrossRef]
- Dordzie, G.; Balhoff, M. A review of chemical methods and testing techniques for enhanced oil recovery in shale reservoirs. Fuel 2025, 394, 135060. [Google Scholar] [CrossRef]
- Li, L.; Su, Y.; Hao, Y.; Zhan, S.; Lv, Y.; Zhao, Q.; Wang, H. A comparative study of CO2 and N2 huff-n-puff EOR performance in shale oil production. J. Pet. Sci. Eng. 2019, 181, 106174. [Google Scholar] [CrossRef]
- Song, C.; Yang, D. Experimental and numerical evaluation of CO2 huff-n-puff processes in Bakken formation. Fuel 2017, 190, 145–162. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Li, D.; Fu, J.; Su, Y.; Lv, Y. Experimental investigation of shale oil recovery from Qianjiang core samples by the CO2 huff-n-puff EOR method. RSC Adv. 2019, 9, 28857–28869. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Zhang, Y.; Ding, B.; Yan, Y.; Zhang, J. Static and dynamic behavior of CO2 enhanced oil recovery in nanoslits: Effects of mineral type and oil components. Int. J. Heat Mass Transf. 2020, 153, 119583. [Google Scholar] [CrossRef]
- Falk, K.; Coasne, B.; Pellenq, R.; Ulm, F.-J.; Bocquet, L. Subcontinuum mass transport of condensed hydrocarbons in nanoporous media. Nat. Commun. 2015, 6, 6949. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Bocquet, L.; Coasne, B. Activated desorption at heterogeneous interfaces and long-time kinetics of hydrocarbon recovery from nanoporous media. Nat. Commun. 2016, 7, 11890. [Google Scholar] [CrossRef] [PubMed]
- Khovental, P.; Kopanichuk, I.; Vishnyakov, A. Molecular simulation of quartz wetting in crude oil/brine system at reservoir conditions using a novel protocol for contact angle calculation. Colloids Surf. A Physicochem. Eng. Asp. 2025, 708, 135978. [Google Scholar] [CrossRef]
- Li, B.; Sui, H.; Wang, D.; Wang, Y.; Zhang, F.; Yao, J. Molecular insights into CO2 enhanced oil recovery and CO2 storage in quartz nanopores. Geoenergy Sci. Eng. 2025, 246, 213640. [Google Scholar] [CrossRef]
- Nie, H.; Liu, Q.; Li, P.; Li, P.; Ding, J.; Sun, C.; Zhai, C.; Zhao, J.; Jin, Z.; Dang, W. Quartz types, formation mechanism, and its effect on shale oil and gas enrichment: A review. Earth-Sci. Rev. 2025, 261, 105011. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, J.; Kong, D.; Zhou, J.; Wang, X.; Wei, B. Solvent-responsive switching wettability of superoleophobic/superhydrophilic quartz sand filter medium facilitates rapidly oil/water separation and demulsification. Colloids Surf. A Physicochem. Eng. Asp. 2024, 697, 134418. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, Y. Effects of mineral type on competitive adsorption and miscibility of CO2-oil system in tight reservoirs: A molecular dynamics study. J. Mol. Liq. 2025, 423, 127032. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, J.; Jia, Y.; Liu, W.; Xiao, C.; Guo, X.; Cheng, Q. The critical role of temperature on the CO2 replacement characteristics within inorganic minerals in shale oil reservoirs. J. Mol. Liq. 2025, 421, 126865. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; Yang, Z.; Yue, W.; Zhang, R.; Qi, J.; Lu, H. pH-regulated superhydrophobic quartz sands for controllable oil-water separation. J. Environ. Chem. Eng. 2023, 11, 110818. [Google Scholar] [CrossRef]
- Hong, Q.; Pang, Z.; Liu, X.; Wang, B.; Liu, D.; Liao, H.; Wang, L. Quantitative macro and micro analysis on enhanced oil recovery (EOR) mechanisms of multi-component composite steam flooding (MCCSF) based on image recognition algorithm. Geoenergy Sci. Eng. 2025, 249, 213766. [Google Scholar] [CrossRef]
- Zhang, A.; Lu, Y. Confined transport property of multi-polar mixed shale oil components induced by interfacial effects within kerogen nanopores. Fuel 2025, 393, 134985. [Google Scholar] [CrossRef]
- Wang, L.; Zou, R.; Yuan, Y.; Zhang, Y.; Zou, R.; Huang, L.; Liu, Y.; Meng, Z.; Chen, H. Dynamics of impure CO2 and composite oil in mineral nanopores: Implications for shale oil recovery and gas storage performance. Chem. Eng. J. 2024, 500, 157421. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Z.; Yuan, S. Insights into the pH-dependent interactions of sulfadiazine antibiotic with soil particle models. Sci. Total Environ. 2024, 917, 170537. [Google Scholar] [CrossRef] [PubMed]
- Emami, F.S.; Puddu, V.; Berry, R.J.; Varshney, V.; Patwardhan, S.V.; Perry, C.C.; Heinz, H. Force Field and a Surface Model Database for Silica to Simulate Interfacial Properties in Atomic Resolution. Chem. Mater. 2014, 26, 2647–2658. [Google Scholar] [CrossRef]
- Kirch, A.; Celaschi, Y.M.; de Almeida, J.M.; Miranda, C.R. Brine–Oil Interfacial Tension Modeling: Assessment of Machine Learning Techniques Combined with Molecular Dynamics. ACS Appl. Mater. Interfaces 2020, 12, 15837–15843. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Ji, D.; Wen, Y.; Luo, H.; Zhao, Y.; Li, Y. Promising combination of CO2 enhanced oil recovery and CO2 sequestration in calcite nanoslits: Insights from molecular dynamics simulations. J. Mol. Liq. 2023, 391, 123243. [Google Scholar] [CrossRef]
- Lu, N.; Dong, X.; Wang, H.; Liu, H.; Chen, Z.; Li, Y.; Zeng, D. Hybrid CO2 thermal system for post-steam heavy oil recovery: Insights from microscopic visualization experiments and molecular dynamics simulations. Energy Geosci. 2025, 6, 100394. [Google Scholar] [CrossRef]
- Wang, R.; Peng, F.; Song, K.; Feng, G.; Guo, Z. Molecular dynamics study of interfacial properties in CO2 enhanced oil recovery. Fluid Phase Equilibria 2018, 467, 25–32. [Google Scholar] [CrossRef]
- Liu, F.; Gao, X.; Du, J.; Lin, L.; Hou, D.; Luo, J.; Zhao, J. Microscopic mechanism of enhancing shale oil recovery through CO2 flooding- insights from molecular dynamics simulations. J. Mol. Liq. 2024, 410, 125593. [Google Scholar] [CrossRef]
- Tang, X.; Xiao, S.; Lei, Q.; Yuan, L.; Peng, B.; He, L.; Luo, J.; Pei, Y. Molecular Dynamics Simulation of Surfactant Flooding Driven Oil-Detachment in Nano-Silica Channels. J. Phys. Chem. B 2019, 123, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Yuan, S. Molecular Dynamics Simulation of the Oil Detachment Process within Silica Nanopores. J. Phys. Chem. C 2016, 120, 2667–2674. [Google Scholar] [CrossRef]
- Xiong, Y.; Cao, T.; Chen, Q.; Li, Z.; Yang, Y.; Xu, S.; Yuan, S.; Sjöblom, J.; Xu, Z. Adsorption of a Polyaromatic Compound on Silica Surfaces from Organic Solvents Studied by Molecular Dynamics Simulation and AFM Imaging. J. Phys. Chem. C 2017, 121, 5020–5028. [Google Scholar] [CrossRef]
- Patwardhan, S.V.; Emami, F.S.; Berry, R.J.; Jones, S.E.; Naik, R.R.; Deschaume, O.; Heinz, H.; Perry, C.C. Chemistry of Aqueous Silica Nanoparticle Surfaces and the Mechanism of Selective Peptide Adsorption. J. Am. Chem. Soc. 2012, 134, 6244–6256. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sengupta, A. A Molecular Simulation Study on Selective Adsorption of CO2 from an Industrial Ternary Gas Mixture inside Porous Silica and Kaolinite Adsorbents. Energy Fuels 2025, 39, 8540–8566. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Guo, Z.; Feng, G. Molecular Insights into Effects of Methanol Additive on CO2 Enhanced Oil Recovery in the Dead-End Nanopore. ACS Appl. Energy Mater. 2024, 7, 10251–10258. [Google Scholar] [CrossRef]
- Liu, B.; Liu, W.; Pan, Z.; Yu, L.; Xie, Z.; Lv, G.; Zhao, P.; Chen, D.; Fang, W. Supercritical CO2 Breaking Through a Water Bridge and Enhancing Shale Oil Recovery: A Molecular Dynamics Simulation Study. Energy Fuels 2022, 36, 7558–7568. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, J.; Yu, X.; Zhu, X.; Zhang, N.; Yuan, S.; Wang, Z. Molecular Mechanisms of Humic Acid in Inhibiting Silica Scaling during Membrane Distillation. Environ. Sci. Technol. 2025, 59, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Kunieda, M.; Nakaoka, K.; Liang, Y.; Miranda, C.R.; Ueda, A.; Takahashi, S.; Okabe, H.; Matsuoka, T. Self-Accumulation of Aromatics at the Oil−Water Interface through Weak Hydrogen Bonding. J. Am. Chem. Soc. 2010, 132, 18281–18286. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Zhang, Y.; Liu, J.; Ding, B.; Yan, Y.; Zhang, J. Molecular insight into the miscible mechanism of CO2/C10 in bulk phase and nanoslits. Int. J. Heat Mass Transf. 2019, 141, 643–650. [Google Scholar] [CrossRef]
- Yan, Y.; Li, C.; Dong, Z.; Fang, T.; Sun, B.; Zhang, J. Enhanced oil recovery mechanism of CO2 water-alternating-gas injection in silica nanochannel. Fuel 2017, 190, 253–259. [Google Scholar] [CrossRef]
- Dong, T.; Harris, N.B. Pore Size Distribution and Morphology in the Horn River Shale, Middle and Upper Devonian, Northeastern British Columbia, Canada. Electron Microsc. Shale Hydrocarb. Reserv. 2013, 102, 67–79. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Guo, Y.; Chen, Y.; Yuan, S. Mechanistic Study of Oil Adsorption Behavior and CO2 Displacement Mechanism Under Different pH Conditions. Molecules 2025, 30, 2999. https://doi.org/10.3390/molecules30142999
Song X, Guo Y, Chen Y, Yuan S. Mechanistic Study of Oil Adsorption Behavior and CO2 Displacement Mechanism Under Different pH Conditions. Molecules. 2025; 30(14):2999. https://doi.org/10.3390/molecules30142999
Chicago/Turabian StyleSong, Xinwang, Yang Guo, Yanchang Chen, and Shiling Yuan. 2025. "Mechanistic Study of Oil Adsorption Behavior and CO2 Displacement Mechanism Under Different pH Conditions" Molecules 30, no. 14: 2999. https://doi.org/10.3390/molecules30142999
APA StyleSong, X., Guo, Y., Chen, Y., & Yuan, S. (2025). Mechanistic Study of Oil Adsorption Behavior and CO2 Displacement Mechanism Under Different pH Conditions. Molecules, 30(14), 2999. https://doi.org/10.3390/molecules30142999







