Electron and Hole Doping Effects on the Magnetic Properties and Band Gap Energy of Ba2FeMoO6 and Sr2FeMoO6
Abstract
1. Introduction
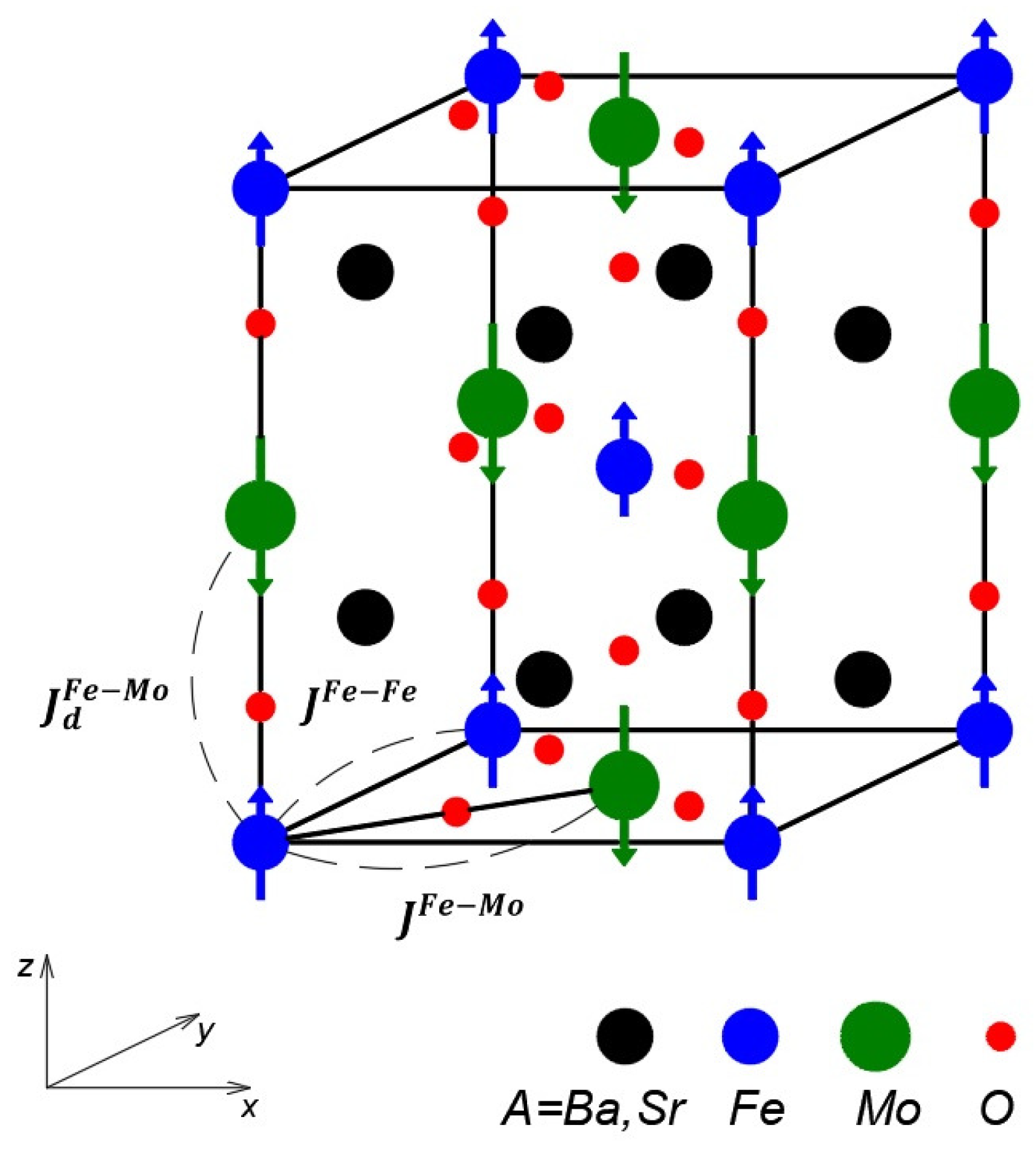
2. Model and Method
3. Numerical Results and Discussion
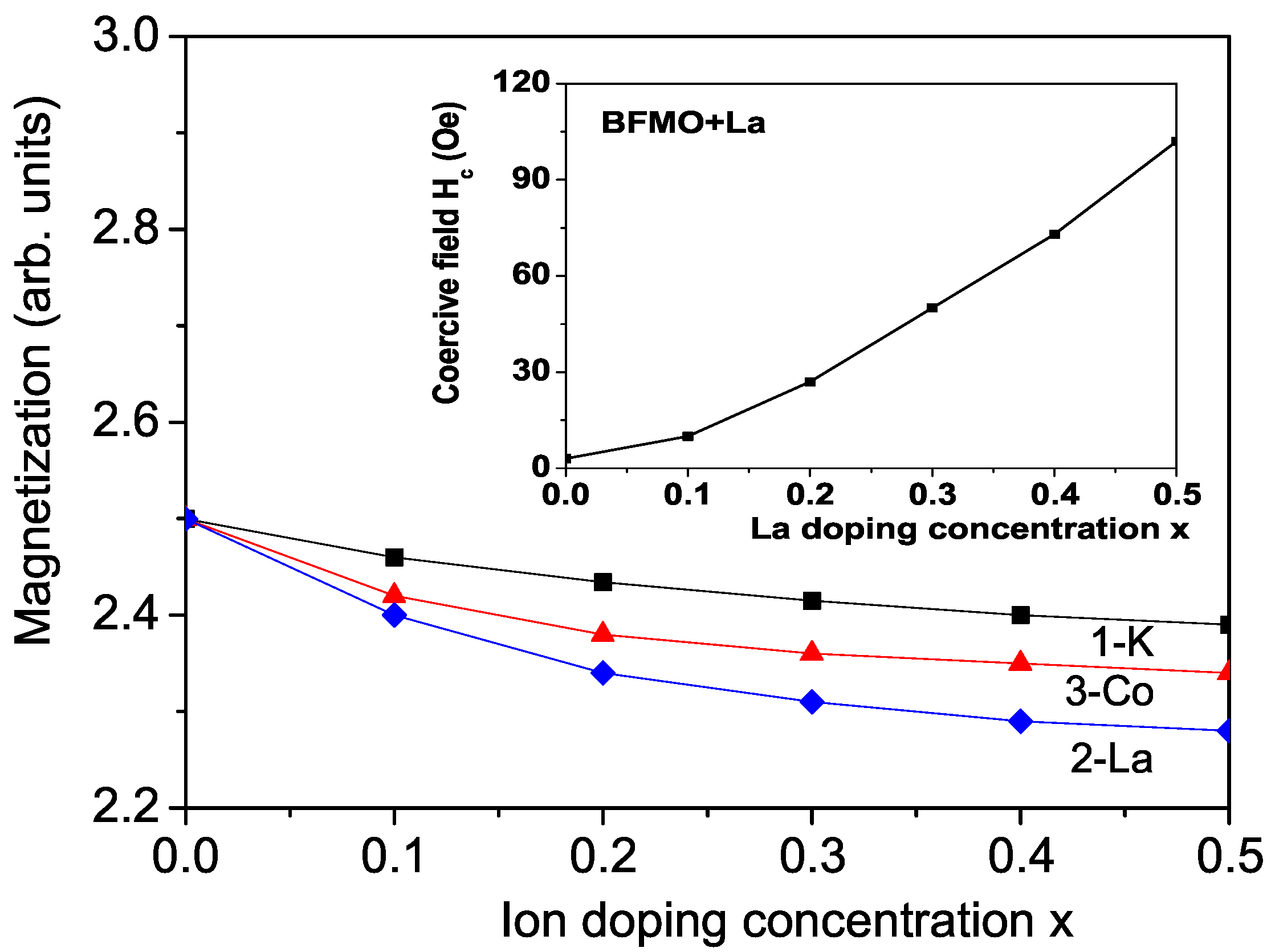
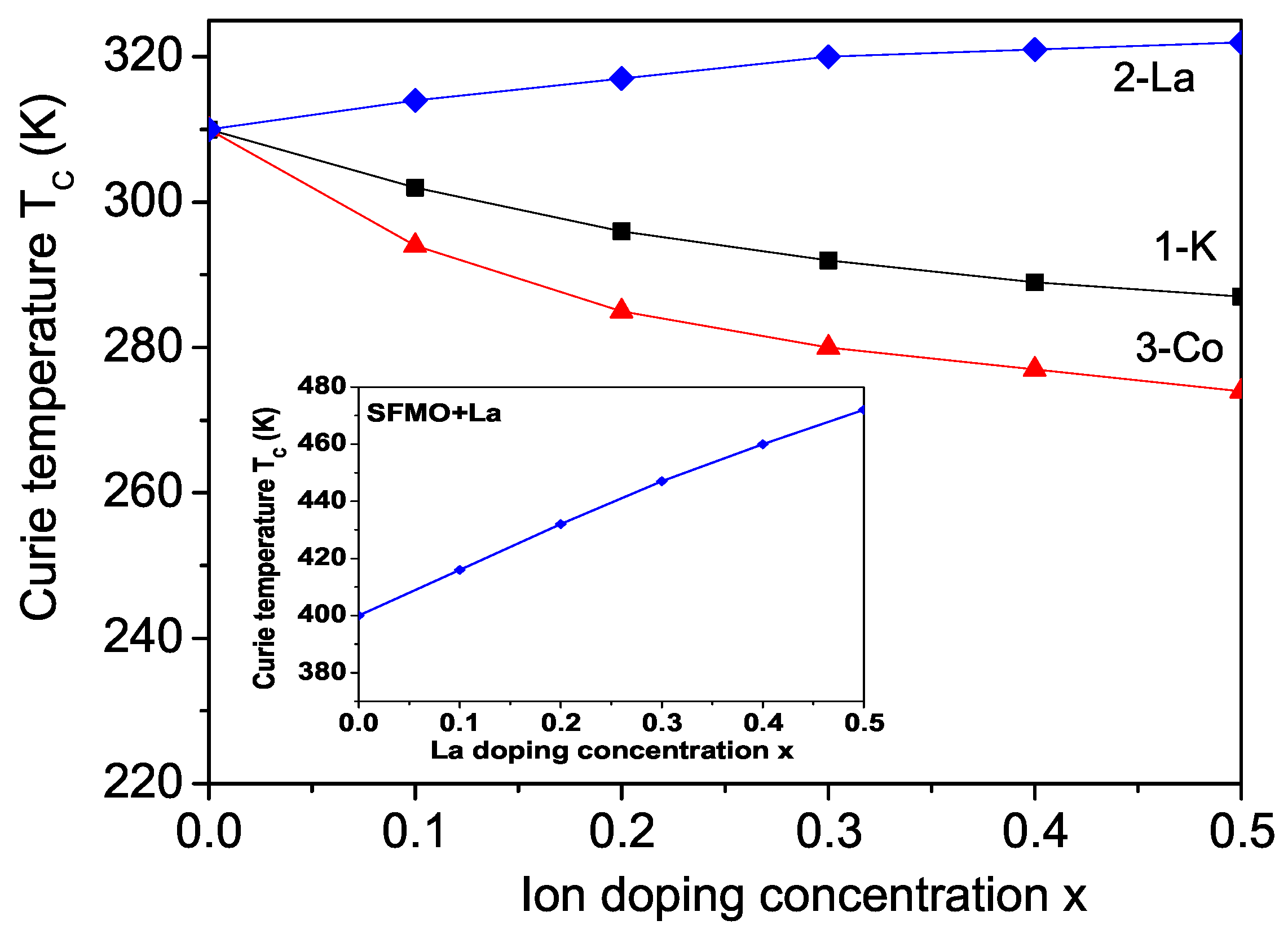
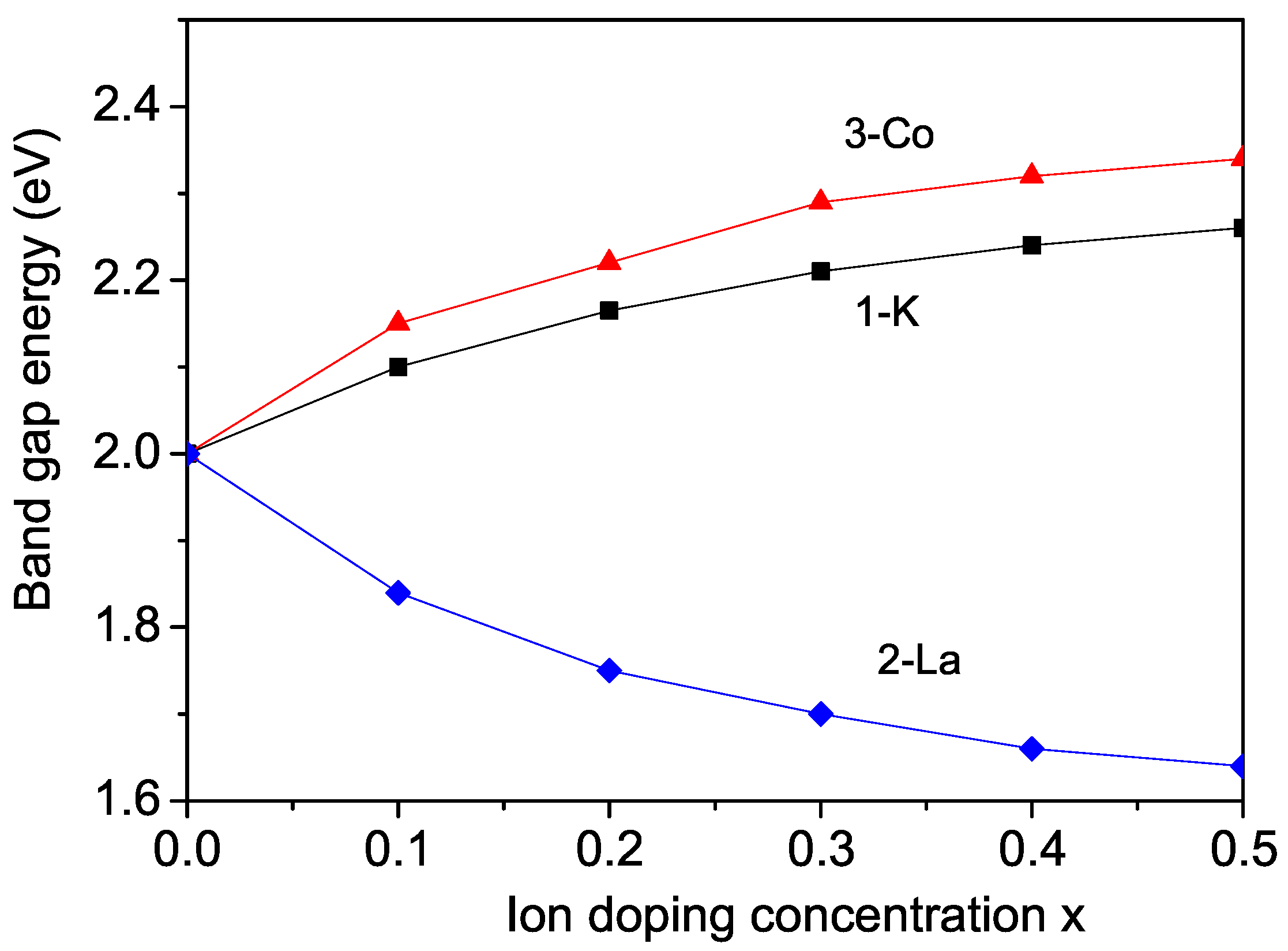
3.1. Properties of K-Doped BFMO and SFMO at the Ba(Sr) Site
3.2. Properties of La-Doped BFMO and SFMO at the Ba(Sr) Site
3.3. Properties of Co-Doped BFMO and SFMO at the Fe Site
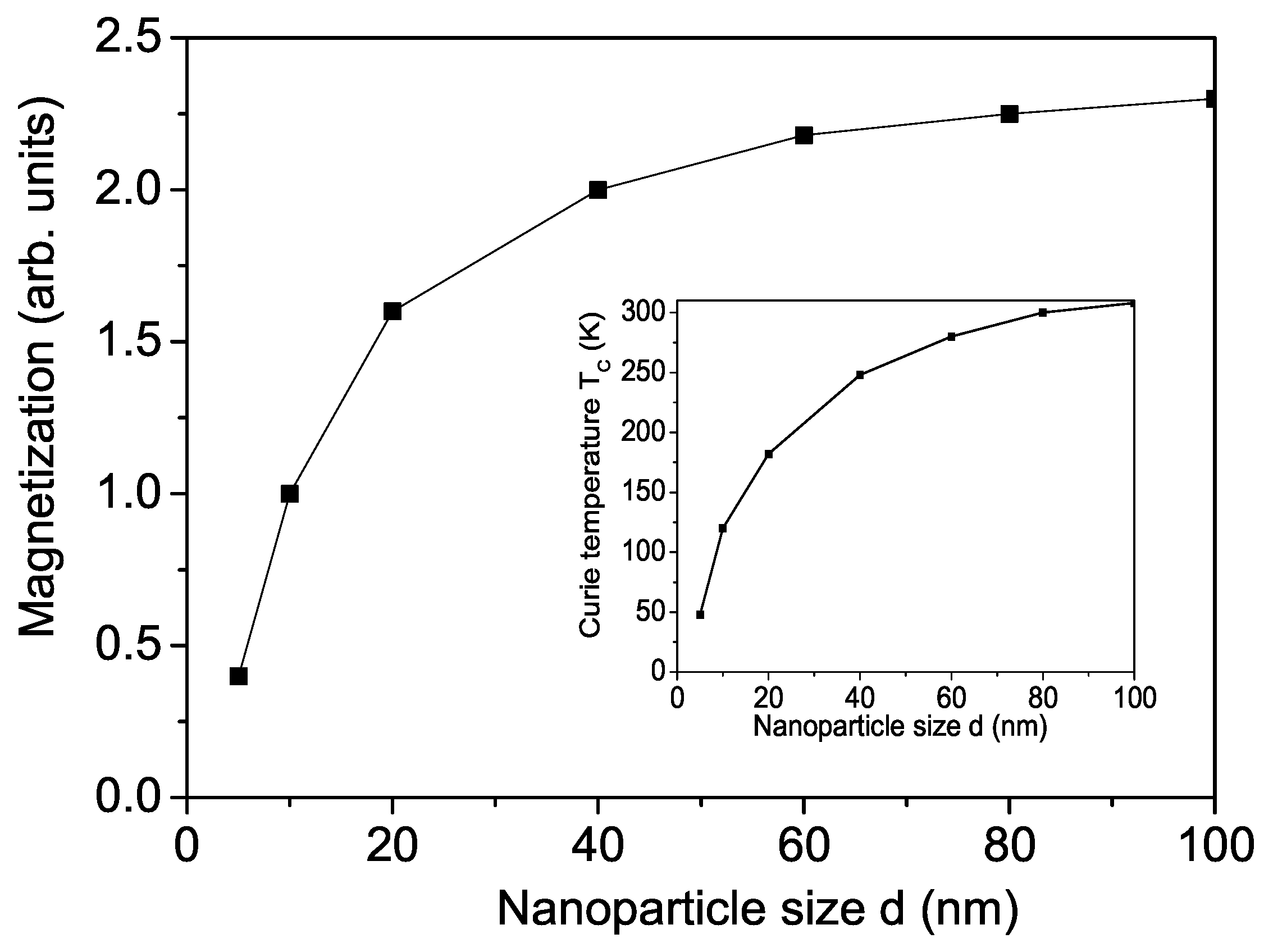
3.4. Properties of BFMO and SFMO Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.H.; Wi, S.C.; Yoon, S.; Suh, L.J.; Kang, J.-S.; Han, S.W.; Kim, K.H.; Sekiyama, A.; Kasai, S.; Suga, S.; et al. Photoemission and X-ray Absorption Spectroscopy Study of Magnetoresistive Double Perovskite Oxides. J. Korean Phys. Soc. 2003, 43, 416. [Google Scholar][Green Version]
- Liu, R.; Wang, Z.; Liu, Y.; Yang, T.; Yang, D. Magneto-elastic coupling behavior of the double perovskite Ba2FeMoO6. J. Phys. Condens. Matter 2019, 31, 015801. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.S.; Hussain, I.; Koo, B.H. Reversible magnetocaloric response in Sr2FeMoO6 double pervoskite. Mater. Lett. 2016, 181, 56. [Google Scholar] [CrossRef]
- Sahnoun, O.; Bouhani-Benziane, H.; Sahnoun, M.; Driz, M. Magnetic and thermoelectric properties of ordered double perovskite Ba2FeMoO6. J. Alloys Comp. 2017, 714, 704–708. [Google Scholar] [CrossRef]
- Chmaissem, O.; Dabrowski, B.; Kolesnik, S.; Short, S.; Jorgensen, J.D. Nuclear and magnetic structural properties of Ba2FeMoO6. Phys. Rev. B 2008, 71, 174421. [Google Scholar] [CrossRef]
- Retuerto, M.; Alonso, J.A.; Martínez-Lope, M.J.; Martínez, J.L.; García-Hernández, M. Record saturation magnetization, Curie temperature, and magnetoresistance in double perovskite synthesized by wet-chemistry techniques. Appl. Phys. Lett. 2004, 85, 266. [Google Scholar] [CrossRef]
- Fontcuberta, J.; Rubi, D.; Frontera, C.; García-Munoz, J.L.; Wojcik, M.; Jedryka, E.; Nadolski, S.; Izquierdo, M.; Avila, J.; Asensio, M.C. Ferromagnetic coupling strength and electron-doping effects in double perovskites. J. Magn. Magn. Mater. 2005, 290–291, 974. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Ehsani, M.H.; Ji, P.; Zhang, Y.; Piao, H.G. Influence of electron and hole doping on structural, Magnetic, and magnetocaloric properties of Ba2FeMoO6 double perovskite. J. Magn. Magn. Mater. 2024, 609, 172459. [Google Scholar] [CrossRef]
- Habib, A.H.; Tomy, C.V.; Nigam, A.K.; Bahadur, D. Structural, transport and magnetic properties of Sr2-xNdxFeMoO6 (0 < x < 1). Physica B 2005, 362, 108. [Google Scholar] [CrossRef]
- Hussain, I.; Khan, S.N.; Rao, T.N.; Kumar, A.; Koo, S.H. Structural, magnetic and magnetocaloric properties of double perovskites Ba2-xLaxFeMoO6. Solid State Sci. 2019, 97, 105991. [Google Scholar] [CrossRef]
- Kim, J.; Sung, J.G.; Yang, H.M.; Lee, B.W. Effects of carrier doping on Curie temperature in double perovskite Ba2FeMoO6. J. Magn. Magn. Mater. 2005, 290–291, 1009. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, G.Y.; Xu, Z.F.; Feng, X.M.; Rao, G.H. Structural and magnetic properties of rare-earth doped (Sr2-xSmx)FeMoO6 compounds. Physica B 2010, 405, 1428. [Google Scholar] [CrossRef]
- Navarro, J.; Frontera, C.; Balcells, L.; Martínez, B.; Foncuberta, J. Raising the Curie temperature in Sr2FeMoO6 double perovskites by electron doping. Phys. Rev. B 2001, 64, 092411. [Google Scholar] [CrossRef]
- Navarro, J.; Nogue, J.; Muñoz, J.S.; Fontcuberta, J. Antisites and electron-doping effects on the magnetic transition of Sr2FeMoO6 double perovskite. Phys. Rev. B 2003, 67, 174416. [Google Scholar] [CrossRef]
- Yang, K.M.; Lee, W.Y.; Han, H.; Lee, B.; Kim, C.-S. Enhancement of Curie temperature in double perovskites Ba2-xLaxFeMoO6. J. Appl. Phys. 2003, 93, 6987. [Google Scholar] [CrossRef]
- Zhang, Q.; Rao, G.H.; Xiao, Y.G.; Dong, H.Z.; Liu, G.Y.; Zhang, Y.; Liang, J.K. Crystal structure, magnetic and electrical-transport properties of rare-earth-doped Sr2FeMoO6. Physica B 2006, 381, 233. [Google Scholar] [CrossRef]
- Sanchez, D.; Alonso, J.A.; García-Hernandez, M.; Martínez-Lope, M.J.; Casais, M.T.; Martínez, J.L.; Fernandez-Díaz, M.T. Electron and hole doping effects in Sr2FeMoO6 double perovskites. J. Magn. Magn. Mater. 2004, 272–276, 1732. [Google Scholar] [CrossRef]
- Ramdane, O.; Labidi, M.; Masrour, R.; Labidi, S.; Ellouze, M.; Rehamnia, R. Study of Structural, Electronic, and Magnetic Properties of Cubic and Tetragonal Ba2FeMoO6. J. Supercond. Nov. Magn. 2023, 36, 373. [Google Scholar] [CrossRef]
- Meetei, O.N.; Erten, O.; Mukherjee, A.; Randeria, M.; Trivedi, N.; Woodward, P. Theory of half-metallic double perovskites. I. Double exchange mechanism. Phys. Rev. B 2013, 87, 165104. [Google Scholar] [CrossRef]
- Erten, O.; Meetei, O.N.; Mukherjee, A.; Randeria, M.; Trivedi, N.; Woodward, P. Theory of half-metallic double perovskites. II. Effective spin Hamiltonian and disorder effects. Phys. Rev. B 2013, 87, 165105. [Google Scholar] [CrossRef]
- Sanyal, P.; Halder, A.; Si, L.; Wallerberger, M.; Held, K.; Saha-Dasgupta, T. Magnetism in Sr2CrMoO6: A combined ab initio and model study. Phys. Rev. B 2016, 94, 035132. [Google Scholar] [CrossRef]
- Lu, R.; Wu, H.; Qian, Y.; Kan, E.; Liu, Y.; Tan, W.; Xiao, C.; Deng, K. The effect of biaxial mechanical strain on the physical properties of double perovskite Sr2FeMoO6: A theoretical study. Solid State Commun. 2014, 191, 70. [Google Scholar] [CrossRef]
- Wu, H.; Ma, Y.; Qian, Y.; Kan, E.; Lu, R.; Liu, Y.; Tan, W.; Xiao, C.; Deng, K. The effect of oxygen vacancy on the half-metallic nature of double perovskite Sr2FeMoO6: A theoretical study. Solid State Commun. 2014, 177, 57–60. [Google Scholar] [CrossRef]
- Petrone, P.; Aligia, A.A. Effective three-band model for double perovskites. Phys. Rev. B 2002, 66, 104418. [Google Scholar] [CrossRef]
- Sarma, D.D.; Mahadevan, P.; Saha-Dasgupta, T.; Ray, S.; Kumar, A. Electronic Structure of Sr2FeMoO6. Phys. Rev. Lett. 2000, 85, 2549. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Kumar, A.; Khan, S.N.; Brojabasi, P.; Koo, S.H. Effect of B-site vanadium (V) doping on the structural, magnetic and magnetocaloric properties of Ba2FeMo1-xVxO6 perovskite. Solid State Commun. 2020, 310, 113861. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Ehsani, M.H. Viability and antibacterial properties of Ba2-xAgxFeMoO6 (x=0.0,0.05) double perovskite oxides. Heliyon 2024, 10, e38869. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.L.; McMillan, P.F. Raman scattering study and electrical properties characterization of elpasolite perovskites Ln2BB’O6 (Ln = La; Sm…Gd and B;B’=Ni, Co, Mn). J. Solid State Chem. 2004, 177, 2323. [Google Scholar] [CrossRef]
- Islam, S.A.U.; Ikram, M. Structural stability improvement, Williamson Hall analysis and band-gap tailoring through A-site Sr doping in rare earth based double perovskite La2NiMnO6. Rare Met. 2019, 38, 6703. [Google Scholar] [CrossRef]
- Kalanda, N.; Yarmolich, M.; Burko, A.; Temirov, A.; Kislyuk, A.; Demyanov, S.; Lenz, K.; Lindner, J.; Kim, D.-H. Superparamagnetism and ferrimagnetism in the Sr2FeMoO6 nanoscale powder. Ceram. Intern. 2022, 48, 23931. [Google Scholar] [CrossRef]
- Suominen, T.; Raittila, J.; Salminen, T.; Schlesier, K.; Linden, J.; Paturi, P. Magnetic properties of fine SFMO particles: Superparamagnetism. J. Magn. Magn. Mater. 2007, 309, 278. [Google Scholar] [CrossRef]
- Suchaneck, G.; Kalanda, N.; Yarmolich, M.; Artiukh, E.; Gerlach, G.; Sobolev, N.A. Magnetization of Magnetically Inhomogeneous Sr2FeMoO6-d Nanoparticles. Electron. Mater. 2022, 3, 82–92. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.P.; Lu, W.J.; Ang, R.; Zhang, S.L.; Zhu, K.B.; Song, W.H.; Dai, J.M. Size dependence of electronic and magnetic properties of double-perovskite Sr2FeMoO6. Solid State Commun. 2008, 145, 98. [Google Scholar] [CrossRef]
- Naushahi, N.A.; Angervoa, I.; Saloaroa, M.; Schulmana, A.; Huhtinena, H.; Paturi, P. Increased Curie temperature and magnetoresistive response by modifying Fe/Mo ratio in Sr2FeMoO6 thin films. J. Magn. Magn. Mater. 2022, 564, 169990. [Google Scholar] [CrossRef]
- Najar, F.A.; Sultan, K. From halfmetallicity to dielectric tensor: Atomistic study of phase stability and optical spectrum of Sr2FeMoO6 double perovskite and its potential applications. J. Phys. Chem. Solids 2022, 171, 111039. [Google Scholar] [CrossRef]
- Tserkovnikov, Y.A. Decoupling of chains of equations for two-time Green’s functions. Theor. Math. Phys. 1971, 7, 511. [Google Scholar] [CrossRef]
- Kerrai, H.; Zaim, A.; Kerouad, M. Magnetic and magnetocaloric effect of Ba2FeReO6 double perovskite: Ab-initio and Monte Carlo studies. Vacuum 2024, 222, 113090. [Google Scholar] [CrossRef]
- Hu, Y.C.; Han, H.X.; Hu, H.J.; Zhang, K.L.; Wang, H.Y.; Jiang, Y.J.; Ma, H.; Lu, Q.F. Potassium doping effects on the structure and magnetic properties of Sr2FeMoO2. J. Alloys Compd. 2012, 526, 1–5. [Google Scholar] [CrossRef]
- Yoshida, K.; Fujii, Y.; Shimizu, H. Reduction of Curie temperature and saturation magnetization in Ba2-xNaxFeMoO6. J. Appl. Phys. 2005, 98, 103901. [Google Scholar] [CrossRef]
- Zhu, X.F.; Li, Q.F.; Chen, L.F. First-principles study of electron doping and disorder effects on electronic and magnetic properties in Sr2-xNdxFeMoO6 double perovskites. Solid State Commun. 2007, 144, 230. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Panthi, D.; Niu, B.; Lei, L.; Yuan, Z.; Du, Y.; Li, Y.; Chen, F.; He, T. Electron doping of Sr2FeMoO6-δ as high performance anode materials for solid oxide fuel cells. J. Mater. Chem. A 2019, 7, 733. [Google Scholar] [CrossRef]
- Muthuselvam, I.P.; Poddar, A.; Bhowmik, R.N. Study of disorder effects in La substituted Ca2FeMoO6 ferrimagnet using magnetic and transport measurements. J. All. Comp. 2009, 486, 536. [Google Scholar] [CrossRef]
- Sanchez, D.; Alonso, J.A.; García-Hernandez, M.; Martínez-Lope, M.J.; Martínez, J.L.; Mellergard, A. Origin of neutron magnetic scattering in antisite-disordered Sr2FeMoO6 double perovskites. Phys. Rev. B 2002, 65, 104426. [Google Scholar] [CrossRef]
- Chang, H.; García-Hernández, M.; Retuerto, M.; Alonso, J.A. Co-doped Sr2FeMoO6 double perovskites: A plausible scenario for phase segregation. Phys. Rev. B 2006, 73, 104417. [Google Scholar] [CrossRef]
- Frontera, C.; Rubı, D.; Navarro, J.; Garcıia-Munoz, J.L.; Ritter, C.; Fontcuberta, J. Mechanism for Curie temperature variation in LaxSr2-xFeMoO6 and CaxSr2-xFeMoO6. Physica B 2004, 350, E285. [Google Scholar] [CrossRef]
- Moritomo, Y.; Xu, S.; Akimoto, T.; Machida, A.; Hamada, N.; Ohoyama, K.; Nishibori, E.; Takata, M.; Sakata, M. Electron doping effects in conducting Sr2FeMoO6. Phys. Rev. B 2000, 62, 14224. [Google Scholar] [CrossRef]
- Wojcik, M.; Jedryka, E.; Nadolski, S.; Navarro, J.; Rubi, G.; Fontcuberta, J. NMR evidence for selective enhancement of Mo magnetic moment by electron doping in Sr2-xLaxFeMoO6. Phys. Rev. B 2004, 69, 100407(R). [Google Scholar] [CrossRef]
- Kahoul, A.; Azizi, A.; Colis, S.; Stoeffler, D.; Moubah, R.; Schmerber, G.; Leuvrey, C.; Dinia, A. Effect of La doping on the properties of Sr2-xLaxFeMoO6 double perovskite. J. Appl. Phys. 2008, 104, 123903. [Google Scholar] [CrossRef]
- Aguilar, B.; Soto, T.E.; Medina, J.T.T.; Navarro, O. Curie temperature enhancement in the double perovskite Sr2-xLaxFeMoO6 system: An experimental study. Phys. B 2019, 556, 108. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Ehsani, M.H. Influence of rare earth Sm3+doping on structural and magnetic properties of Ba2FeMoO6 double perovskite. Results Phys. 2025, 73, 108254. [Google Scholar] [CrossRef]
- Azad, A.; Eriksson, S.-G.; Khan, A.A.; Eriksson, A.; Tseggai, M. Electron Doping Effect on Structural and Magnetic Phase Transitions in Sr2-xNdxFeMoO6 Double Perovskites. J. Solid State Chem. 2006, 179, 1303. [Google Scholar] [CrossRef]
- Rubi, D.; Frontera, C.; Nogues, J.; Fontcuberta, J. Enhanced ferromagnetic interactions in electron doped NdxSr2-xFeMoO6 double perovskites. J. Phys. Condens. Matter 2004, 16, 3173. [Google Scholar] [CrossRef]
- Guzman, E.J.; Estrada, F.; Aguilar, B.; Navarro, O.; Avignon, M. Increase of Curie temperature with La doping in the double perovskite Sr2-yLayFeMoO6 within an electronic correlation approach. Rev. Mex. Fis. 2018, 64, 145. [Google Scholar] [CrossRef]
- Hoffmann, M.; Antonov, V.N.; Bekenov, L.V.; Kokko, K.; Hergert, W.; Ernst, A. Variation of magnetic properties of Sr2FeMoO6 due to oxygen vacancies. J. Phys. Cond. Matter 2018, 30, 305801. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Murtaza, G.; Zelai, T.; Nazir, G.; Alkhaldi, H.; Albalawi, H.; Kattan, N.A.; Irfan, M.; Mahmood, Q.; Mahmoud, Z. Study of structural, electronic, magnetic, and optical properties of A2FeMnO6 (A = Ba, La) double perovskites, experimental and DFT analysis. Coll. Surf. A 2023, 664, 131145. [Google Scholar] [CrossRef]
- Song, G.; Zhang, W. Comparative studies on the room-temperature ferrielectric and ferrimagnetic Ni3TeO6-type A2FeMoO6 compounds (A = Sc, Lu). Sci. Rep. 2016, 6, 20133. [Google Scholar] [CrossRef] [PubMed]
- Rajender, G.; Markandeya, Y.; Bale, S.; Suresh, K.; Bhikshamaiah, G. Effect of Al on Structure, Magnetic, Resistivity, and Magnetoresistance Studies of Ba2FeMoO6. J. Supercond. Novel Magn. 2020, 33, 2101. [Google Scholar] [CrossRef]
- Qi, L.; Qing, H.; Hang-Dong, W.; Jing-Hu, Y.; Jian-Hua, D.; Ming-Hu, F. The element substitution effect of Co in Sr2Fe1-xCoxMoO6 system. Acta Phys. Sin. 2006, 55, 6113. [Google Scholar] [CrossRef]
- Ritter, C.; Blasco, J.; Morellon, L.; De Teresa, J.M.; Garcia, J.; Ibarra, M.R. The influence of doping on the magnetic and structural properties of the double perovskite SrFeMoO6. J. Magn. Magn. Mater. 2001, 226–230, 1070. [Google Scholar] [CrossRef]
- Hemery, E.K.; Williams, G.V.M.; Trodahl, H.J. The effects of Al substitution on the magnetic and electronic properties of Sr2Fe1-xAlxMoO6. Phys. B 2007, 394, 74. [Google Scholar] [CrossRef]
- Feng, X.M.; Rao, G.H.; Liu, G.Y.; Yang, H.F.; Liu, W.F.; Ouyang, Z.W.; Yang, L.T.; Liu, Z.X.; Yu, R.C.; Jin, C.Q.; et al. Structure and magnetoresistance of the double perovskite Sr2FeMoO6 doped at the Fe site. J. Phys. Cond. Matter 2002, 14, 12503. [Google Scholar] [CrossRef]
- Lue, M.F.; Wang, J.P.; Liu, J.F.; Song, W.; Hao, X.F.; Zhou, D.F.; Liu, X.J.; Wu, Z.J.; Meng, J. Structural and magnetic properties of Sr2Fe1-xGaxMoO6 (0 < x < 0.25). J. Alloys Comp. 2007, 428, 214. [Google Scholar] [CrossRef]
- Moritomo, Y.; Kusuya, H.; Machida, A.; Nishibori, E.; Takata, M.; Sakata, M.; Nakamura, A. Fe-Site Substitution Effects on Conductive Ferromagnet Sr2FeMoO6. J. Phys. Soc. Jpn. 2001, 70, 3182. [Google Scholar] [CrossRef]
- Markandeya, Y.; Reddy, Y.S.; Bale, S.; Bhikshamaiah, G. Synthesis, characterization, conductivity, magnetic and magnetoresistance studies of Sr2Fe1-xInxMoO6 (x = 0.0 and 0.2) double perovskite. J. Mater. Sci. Mater. Electr. 2018, 29, 6711. [Google Scholar] [CrossRef]
- Zhang, Q.; Rao, G.H.; Feng, K.M.; Liu, G.; Xiao, Y.; Zhang, Y.; Liang, J.K. Influence of V substitution for Fe on the transport and magnetic properties of Sr2FeMoO6. Solid State Commun. 2008, 133, 223. [Google Scholar] [CrossRef]
- Sriti, F.; Maignan, A.; Martin, C.; Raveau, S. Influence of Fe-Site Substitutions upon Intragrain and Intergrain Magnetoresistance in the Double-Perovskite Ba2FeMoO6. Chem. Mater. 2001, 13, 1746. [Google Scholar] [CrossRef]
- Mahmood, Q. Study of half metallic ferromagnetism, Curie temperature, and thermoelectric aspects of double perovskite oxides Ba2XMoO6 X=Cr,Mn,Fe,Co) for spintronic applications. Mater. Sci. Semicond. Proc. 2025, 193, 109519. [Google Scholar] [CrossRef]
- Wang, W.; Feng, W.; Yuan, J.; Pang, N.; Zhao, X.; Li, M.; Bao, Z.; Zhu, K.; Odkhuu, D. Tunable magnetic properties of double perovskite La2Fe2-xCoxO6. Physica B 2018, 540, 33. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolov, A.T.; Apostolova, I.N.; Wesselinowa, J.M. Electron and Hole Doping Effects on the Magnetic Properties and Band Gap Energy of Ba2FeMoO6 and Sr2FeMoO6. Molecules 2025, 30, 2987. https://doi.org/10.3390/molecules30142987
Apostolov AT, Apostolova IN, Wesselinowa JM. Electron and Hole Doping Effects on the Magnetic Properties and Band Gap Energy of Ba2FeMoO6 and Sr2FeMoO6. Molecules. 2025; 30(14):2987. https://doi.org/10.3390/molecules30142987
Chicago/Turabian StyleApostolov, Angel T., Iliana N. Apostolova, and Julia M. Wesselinowa. 2025. "Electron and Hole Doping Effects on the Magnetic Properties and Band Gap Energy of Ba2FeMoO6 and Sr2FeMoO6" Molecules 30, no. 14: 2987. https://doi.org/10.3390/molecules30142987
APA StyleApostolov, A. T., Apostolova, I. N., & Wesselinowa, J. M. (2025). Electron and Hole Doping Effects on the Magnetic Properties and Band Gap Energy of Ba2FeMoO6 and Sr2FeMoO6. Molecules, 30(14), 2987. https://doi.org/10.3390/molecules30142987





