Volatilome Analysis for Differentiating Terroir Expression: A Case Study of Three Wineries in a Limestone-Rich, Warm-Climate Region
Abstract
1. Introduction
2. Results and Discussion
2.1. Oenological Parameters
2.2. Major Volatile Compounds and Polyols
2.3. Minor Volatile Compounds—Screening of Chemical Families for Best Differentiation of Terroir Effects
2.4. Selection of a Set of Volatile Compounds and Chemical Families for a Better and Easier Differentiation Among Terroirs
3. Materials and Methods
3.1. Location of Terroirs and Wineries
3.2. Grape Variety and Wine Sampling
3.3. Oenological Parameter Analysis
3.4. Quantification of Major and Minor Volatile Compounds
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Organisation Internationale de la Vigne et du Vin (OIV). Definition of Vitivinicultural Terroir; OIV: Paris, France, 2010; Available online: https://www.oiv.int/public/medias/379/viti-2010-1-en.pdf (accessed on 5 April 2025).
- Van Leeuwen, C.; Seguin, G. The Concept of Terroir in Viticulture. J. Wine Res. 2006, 17, 1–10. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Friant, P.; Chone, X.; Tregoat, O.; Koundouras, S.; Dubourdieu, D. Influence of Climate, Soil, and Cultivar on Terroir. Am. J. Enol. Vitic. 2004, 55, 207–217. [Google Scholar] [CrossRef]
- Belda, I.; Zarraonaindia, I.; Perisin, M.; Palacios, A.; Acedo, A. From Vineyard Soil to Wine Fermentation: Microbiome Approximations to Explain the “terroir” Concept. Front. Microbiol. 2017, 8, 821. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Collins, T.S.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Associations Among Wine Grape Microbiome, Metabolome, and Fermentation Behavior Suggest Microbial Contribution to Regional Wine Characteristics. mBio 2016, 7, 101128. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial Biogeography of Wine Grapes is Conditioned by Cultivar, Vintage, and Climate. Proc. Natl. Acad. Sci. USA 2014, 111, e139–e148. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, E.C.; Mina, M.; Valanides, N.; Taliadorou, A.M.; Koundouras, S.; D’Onofrio, C.; Bellincontro, A.; Mencarelli, F.; Barbayiannis, N.; Fotopoulos, V.; et al. The effect of terroir on volatilome fingerprinting and qualitative attributes of non-irrigated grapes reveals differences on glycosylated aroma compounds. J. Sci. Food Agric. 2024, 104, 3489–3499. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C.; Barbe, J.C.; Darriet, P.; Geffroy, O.; Gomès, E.; Guillaumie, S.; Helwi, P.; Laboyrie, J.; Lytra, G.; Le Menn, N. Recent advancements in understanding the terroir effect on aromas in grapes and wines. OENO One 2020, 54, 683–698. [Google Scholar] [CrossRef]
- Crook, A.A.; Zamora-Olivares, D.; Bhinderwala, F.; Woods, J.; Winkler, M.; Rivera, S.; Shannon, C.E.; Wagner, H.R.; Zhuang, D.L.; Lynch, J.E.; et al. Combination of two analytical techniques improves wine classification by Vineyard, Region, and vintage. Food Chem. 2021, 357, 129531. [Google Scholar] [CrossRef] [PubMed]
- Zoecklein, B.W.; Gump, B.H. Practical methods of evaluating grape quality and quality potential. In Managing Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing: Cambridge, UK, 2022; pp. 135–185. [Google Scholar] [CrossRef]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.J.; Gilbert, J.A. The soil microbiome influences grapevine-associated microbiota. mBio 2015, 6, e02527-14. [Google Scholar] [CrossRef] [PubMed]
- Franco, G.C.; Leiva, J.; Nand, S.; Lee, D.M.; Hajkowski, M.; Dick, K.; Withers, B.; Soto, L.; Mingoa, B.-R.; Acholonu, M.; et al. Soil Microbial Communities and Wine Terroir: Research Gaps and Data Needs. Foods 2024, 13, 2475. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 2012, 15, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; El-Khoury, M.; Lucas, P.; Bely, M.; Russo, P.; Spano, G.; Capozzi, V. Autochthonous starter cultures and indigenous grape variety for regional wine production. J. Appl. Microbiol. 2015, 118, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, A.; Brillante, L. Terroir analysis and its complexity. OENO One 2022, 56, 5448. [Google Scholar] [CrossRef]
- Kamilari, E.; Mina, M.; Karallis, C.; Tsaltas, D. Metataxonomic Analysis of Grape Microbiota During Wine Fermentation Reveals the Distinction of Cyprus Regional terroirs. Front. Microbiol. 2021, 12, 726483. [Google Scholar] [CrossRef] [PubMed]
- Tarko, T.; Duda, A. Volatilomics of Fruit Wines. Molecules 2024, 29, 2457. [Google Scholar] [CrossRef] [PubMed]
- Deis, L.; Martínez, L.; da Costa, B.S.; Vilanova, M. The Influence of Climatic Conditions Associated with Altitude on the Volatile Composition of Cabernet Sauvignon Wines from Argentina, Spain and Portugal. Horticulturae 2024, 10, 870. [Google Scholar] [CrossRef]
- Di Gennaro, S.F.; Matese, A.; Manfrini, L.; Primicerio, J. The Influence of Soil Properties on Volatile Organic Compounds in Grapes. Compr. Rev. Food Sci. Food Saf. 2022, 21, 157–169. [Google Scholar] [CrossRef]
- Chen, Y.; Lei, X.; Wu, Q.; Qin, Y.; Song, Y.; Liu, Y. Oenological Suitability of Chinese Indigenous Saccharomyces cerevisiae in Chardonnay Wine: The Observation of Grape Maturity and Vintage. Food Biosci. 2024, 56, 104904. [Google Scholar] [CrossRef]
- González, M.L.; Chimeno, S.V.; Sturm, M.E.; Becerra, L.M.; Lerena, M.C.; Rojo, M.C.; Combina, M.; Mercado, L.A. Populations of Saccharomyces cerevisiae in Vineyards: Biodiversity and Persistence Associated with Terroir. Fermentation 2023, 9, 292. [Google Scholar] [CrossRef]
- Padilla, B.; García-Fernández, D.; González, B.; Izidoro, I.; Esteve-Zarzoso, B.; Beltran, G.; Mas, A. Yeast Biodiversity from DOQ Priorat Uninoculated Fermentations. Front. Microbiol. 2016, 7, 930. [Google Scholar] [CrossRef] [PubMed]
- Argentero, A.; Caillé, S.; Nolleau, V.; Godet, T.; Mouls, L.; Rigou, P. Exploring the aromatic typicity of blended red wines from geographically close sub-regions in AOC Corbières: A sensory and chemical approach. OENO One 2024, 58, 7802. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Moio, L. Influence of Yeast Strain on Odor-Active Compounds in Fiano Wine. Appl. Sci. 2021, 11, 7767. [Google Scholar] [CrossRef]
- Gobbi, A.; Acedo, A.; Imam, N.; Santini, R.G.; Ortiz-Álvarez, R.; Ellegaard-Jensen, L.; Belda, I.; Hansen, L.H. A global microbiome survey of vineyard soils highlights the microbial dimension of viticultural terroirs. Commun Biol. 2022, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C.; Barbe, J.-C.; Darriet, P.; Destrac-Irvine, A.; Gowdy, M.; Lytra, G.; Marchal, A.; Marchand, S.; Plantevin, M.; Poitou, X.; et al. Aromatic maturity is a cornerstone of terroir expression in red wine: This article is published in cooperation with Terclim 2022 (XIVth International Terroir Congress and 2nd ClimWine Symposium), 3–8 July 2022, Bordeaux, France. OENO One 2022, 56, 5441. [Google Scholar] [CrossRef]
- Liu, X.; Lou, Y.; Li, Y.; Zhao, Y.; Laaksonen, O.; Li, P.; Zhang, J.; Battino, M.; Yang, B.; Gu, Q. Aroma characteristics of volatile compounds brought by variations in microbes in winemaking. Food Chem. 2023, 420, 136075. [Google Scholar] [CrossRef] [PubMed]
- Della Vedova, G.; Capozzi, V.; Fiore, A.; Masi, P.; Romano, A. Influence of Volcanic Soils on Wine Aroma: Terroir-Driven Microbial Interactions. Sci. Total Environ. 2019, 678, 93–102. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine Aroma and Terroir: Application of Untargeted Profiling. Foods 2021, 10, 1239. [Google Scholar] [CrossRef]
- Palladino, G.; Nanetti, E.; Scicchitano, D.; Cinte, N.; Foresto, L.; Cozzi, A.; González-Vara, A.; Interino, N.; Fiori, J.; Turroni, S.; et al. Zonation of the Vitis vinifera microbiome in Vino Nobile di Montepulciano PDO production area. Commun. Biol. 2024, 7, 1626. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Edwards, P.J.B.; Gardner, R.C.; Villas-Boas, S.G. A Review on the Contribution of Metabolomics to Wine Research. Food Chem. 2014, 161, 424–432. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Morrison-Whittle, P.; Goddard, M.R. From vineyard to winery: A source map of microbial diversity driving wine fermentation. Environ. Microbiol. 2018, 20, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Espinosa, J.M.; Muñoz-Castells, R.; Moreno-García, J.; García-Martínez, T.; Mauricio, J.C.; Moreno, J. Analytical Differentiation of Wines from Three Terroirs Located in a Warm Wine-growing Area Based on Their Volatilome. Molecules 2025, 30, 238. [Google Scholar] [CrossRef] [PubMed]
- Protected Denomination of Origin Montilla-Moriles. Pliego de Condiciones. Available online: https://www.montillamoriles.es/Documents/PC_DO_Montilla_Moriles%20modificado.pdf (accessed on 6 February 2025).
- Alcalá-Jiménez, M.T.; García-Martínez, T.; Mauricio, J.C.; Moreno, J.; Peinado, R.A. Influence of Terroir on Microbial Diversity and Wine Volatilome. Appl. Sci. 2025, 15, 3237. [Google Scholar] [CrossRef]
- Rigou, P.; Mekoue, J.; Sieczkowski, N.; Doco, T.; Vernhet, A. Impact of industrial yeast derivative products on the modification of wine aroma compounds and sensorial profile. A review. Food Chem. 2021, 358, 129760. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Peinado, R.A. Biological aging. In Enological Chemistry, 1st ed.; Moreno, J., Peinado, R., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 405–415. [Google Scholar] [CrossRef]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary aroma: Influence of wine microorganisms in their aroma profile. Foods 2021, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, R.; Roldán-Romero, Y.; Moreno, J.; Puig-Pujol, A.; Mauricio, J.C.; García-Martínez, T. Use of a flor yeast strain for the second fermentation of sparkling wines: Effect of endogenous CO2 over-pressure on the volatilome. Food Chem. 2020, 308, 125555. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castells, R.; Moreno, J.; García-Martínez, T.; Mauricio, J.C.; Moreno-García, J. Chemometric Differentiation of White Wines from a Low-Aromatic Grape Obtained by Spontaneous Fermentation Enriched with Non-Saccharomyces, or with a High-Glutathione-Producing Saccharomyces Yeast. Fermentation 2023, 9, 1023. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research progress of wine aroma components: A critical review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.L.; Shi, Y.; Jiang, R.; Yang, Q.; Wang, Y.Q.; Liu, P.T.; Duan, G.; Yan, G.L. Effects of adding unsaturated fatty acids on fatty acid composition of Saccharomyces cerevisiae and major volatile compounds in wine. S. Afr. J. Enol. Vitic. 2015, 36, 285–295. [Google Scholar] [CrossRef]
- Darıcı, M.; Cabaroglu, T. Chemical and Sensory Characterization of Kalecik Karası Wines Produced from Two Different Regions in Turkey Using Chemometrics. J. Food Process. Preserv. 2022, 46, e16278. [Google Scholar] [CrossRef]
- Parker, M.; Capone, D.L.; Francis, I.L.; Herderich, M.J. Aroma Precursors in Grapes and Wine: Flavor Release During Wine Production and Consumption. J. Agric. Food Chem. 2018, 66, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Bento-Silva, A.; Duarte, N.; Santos, M.; Costa, C.P.; Vaz Patto, M.C.; Rocha, S.M.; Bronze, M.R. Comprehensive Two-Dimensional Gas Chromatography as a Powerful Strategy for the Exploration of Broas Volatile Composition. Molecules 2022, 27, 2728. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jin, G.J.; Wang, X.J.; Kong, C.L.; Liu, J.; Tao, Y.S. Chemical Profiles and Aroma Contribution of Terpene Compounds in Meili (Vitis vinifera L.) Grape and Wine. Food Chem. 2019, 284, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Phajon, Y.; Tan, H.; Liu, B.; Zhang, Y.; Ju, Y.; Shen, T.; Xu, M.; Fang, Y. Effect of Terroir on Phenolic Content and Aroma Properties of Grapes and Wines. Foods 2025, 14, 1409. [Google Scholar] [CrossRef] [PubMed]
- Alberico, G.; Capece, A.; Mauriello, G.; Pietrafesa, R.; Siesto, G.; Garde-Cerdán, T.; Maresca, D.; Romano, R.; Romano, P. Influence of Microencapsulation on Fermentative Behavior of Hanseniaspora osmophila in Wine Mixed Starter Fermentation. Fermentation 2021, 7, 112. [Google Scholar] [CrossRef]
- Urvieta, R.; Jones, G.; Buscema, F.; Bottini, R.; Fontana, A. Terroir and vintage discrimination of Malbec wines based on phenolic composition across multiple sites in Mendoza, Argentina. Sci. Rep. 2021, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lei, X.; Jiang, J.; Qin, Y.; Jiang, L.; Liu, Y.-L. Microbial Diversity on Grape Epidermis and Wine Volatile Aroma in Spontaneous Fermentation Comprehensively Driven by Geography, Subregion, and Variety. Int. J. Food Microbiol. 2023, 404, 110315. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Stepp, J.R. Beyond yields: Climate change effects on specialty crop quality and agroecological management. Elementa 2016, 4, 000092. [Google Scholar] [CrossRef]
- Tian, B.; Dhami, M.; Moukarzel, R.; Zhang, J.; On, S.; Wimalasiri, P.; Dias-Araujo, L.; Maxwell, D. Extending the Influence of Terroir through the Spontaneous Fermentation of Pinot Noir in the Vineyard: A Case Study of Greystone Vineyard Fermentation. J. Agricult. Food Chem. 2025, 73, 8531–8542. [Google Scholar] [CrossRef] [PubMed]
- Palma-López, J.; Sánchez-Rodríguez, A.R.; del Campillo, M.C.; León-Gutiérrez, J.M.; Ramírez-Pérez, P. Influence of soil properties on grape and must quality in the Montilla—Moriles protected designation of origin (southern Spain). Catena 2024, 241, 108041. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Wine and Must Analysis. Available online: https://www.oiv.int/standards/compendium-of-international-methods-of-wine-and-must-analysis (accessed on 8 September 2024).
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology. In The Chemistry of Wines, Stabilization and Treatments; John Wiley: Chichester, UK, 2000. [Google Scholar]
- Martínez-García, R.; Mauricio, J.C.; García-Martínez, T.; Peinado, R.A.; Moreno, J. Towards a Better Understanding of the Evolution of Odour-Active Compounds and the Aroma Perception of Sparkling Wines During Ageing. Food Chem. 2021, 357, 129784. [Google Scholar] [CrossRef] [PubMed]
- Palenzuela, M.d.V.; López de Lerma, N.; Sánchez-Suárez, F.; Martínez-García, R.; Peinado, R.A.; Rosal, A. Aroma Composition of Wines Produced from Grapes Treated with Organic Amendments. Appl. Sci. 2023, 13, 8001. [Google Scholar] [CrossRef]
- MetaboAnalyst Web Platform. Available online: https://www.metaboanalyst.ca (accessed on 30 April 2025).
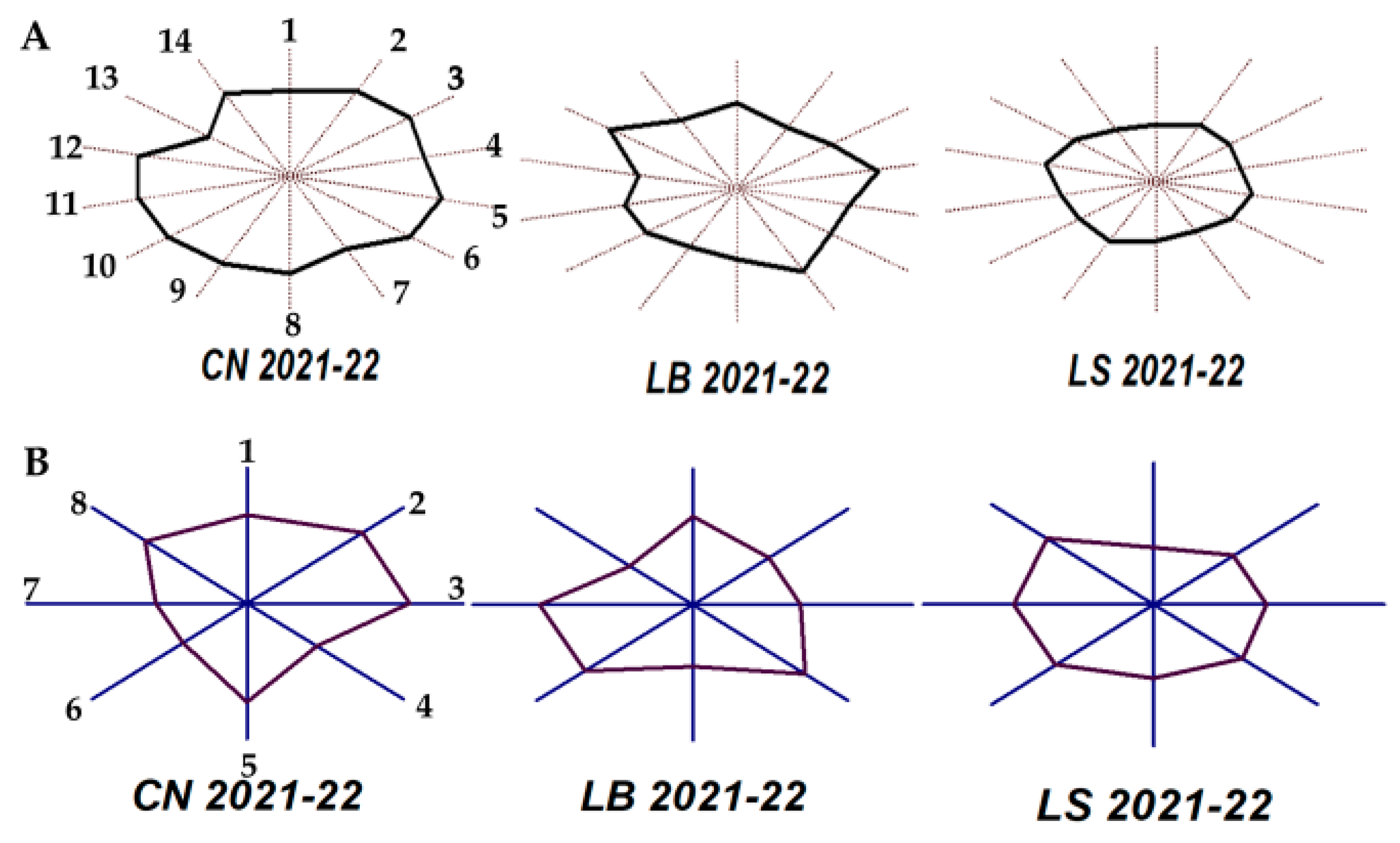

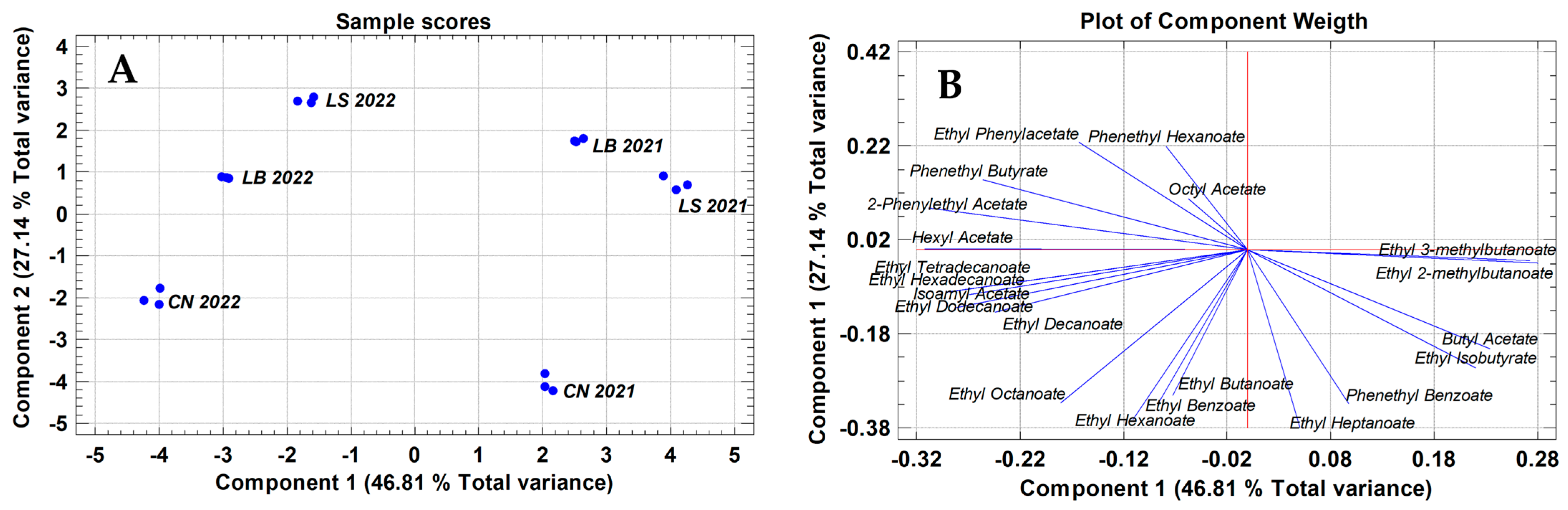
| Parameters | CN2021 | CN2022 | LS2021 | LS2022 | LB2021 | LB2022 | HGs |
|---|---|---|---|---|---|---|---|
| Ethanol (% V/V) | 14.0 ± 0.0 a | 14.5 ± 0.0 b | 15.0 ± 0.1 c | 14.5 ± 0.0 b | 15.0 ± 0.1 c | 15.0 ± 0.1 c | 3 |
| pH | 3.43 ± 0.01 d | 3.40 ± 0.00 c | 3.40 ± 0.02 c | 3.24 ± 0.01 a | 3.35 ± 0.01 b | 3.47 ± 0.01 e | 5 |
| Volatile acidity (g L−1) | 0.39 ± 0.00 f | 0.36 ± 0.00 e | 0.33 ± 0.00 d | 0.24 ± 0.00 b | 0.26 ± 0.02 c | 0.18 ± 0.00 a | 6 |
| Total acidity (g L−1) | 4.31 ± 0.00 b | 5.55 ± 0.00 f | 4.24 ± 0.00 a | 4.95 ± 0.00 e | 4.77 ± 0.03 d | 4.39 ± 0.00 c | 6 |
| Malic acid (g L−1) | 0.27 ± 0.00 b | 0.53 ± 0.03 c | 0.01 ± 0.00 a | 0.90 ± 0.01 d | 0.98 ± 0.02 e | 0.01 ± 0.00 a | 5 |
| Lactic acid (g L−1) | 0.52 ± 0.01 c | 0.54 ± 0.03 c | 0.65 ± 0.02 d | 0.39 ± 0.01 b | 0.04 ± 0.00 a | 0.37 ± 0.01 b | 4 |
| Density (g L−1) | 986 ± 0 b | 987 ± 0 c | 985 ± 0 a | 986 ± 0 b | 985 ± 0 a | 985 ± 0 a | 3 |
| Reducing sugars (g L−1) | 0.96 ± 0.00 c | 1.20 ± 0.00 d | 1.1 ± 0.1 c,d | 0.96 ± 0.00 c | 0.8 ± 0.1 b | 0.70 ± 0.00 a | 4 |
| TPI | 8.75 ± 0.03 e | 10.23 ± 0.02 f | 7.57 ± 0.01 a | 8.44 ± 0.02 c | 8.52 ± 0.05 d | 8.33 ± 0.02 b | 6 |
| Absorbance 420 nm | 0.1938 ± 0.0003 e | 0.2083 ± 0.003 f | 0.079 ± 0.02 a | 0.1127 ± 0.0003 b | 0.1566 ± 0.0006 c | 0.1685 ± 0.0004 d | 6 |
| Major Volatiles | CAS | CN2021 | CN2022 | LS2021 | LS2022 | LB2021 | LB2022 | HGs |
|---|---|---|---|---|---|---|---|---|
| Carbonyl Compounds (3) | ||||||||
| Acetaldehyde | 75-07-0 | 246 ± 16 d | 104 ± 4 a | 211 ± 6 c | 168 ± 8 b | 241 ± 1 d | 99 ± 5 a | 4 |
| 1.1-Diethoxyethane | 105-57-7 | 2.4 ± 0.2 d | 0 a | 0.81 ± 0.04 b | 0 a | 1.19 ± 0.04 c | 0 a | 4 |
| Acetoin | 513-86-0 | 122 ± 12 c | 50 ± 3 b | 56 ± 2 b | 46 ± 4 b | 50 ± 4 b | 33 ± 1 a | 3 |
| Alcohols (6) | ||||||||
| 2-Phenylethanol | 60-12-8 | 22 ± 2 a | 46 ± 2 b | 64 ± 2 d,e | 68 ± 3 e | 59 ± 1 c | 63 ± 3 c,d | 5 |
| Methanol | 67-56-1 | 103 ± 7 b,c | 87 ± 7 a | 92 ± 8 a,b | 89 ± 5 a | 105 ± 9 c | 84 ± 5 a | 3 |
| 1-Propanol | 71-23-8 | 28 ± 2 c | 51 ± 2 e | 22.8 ± 0.8 b | 32 ± 1 d | 18.3 ± 0.6 a | 20.9 ± 0.2 b | 5 |
| Isobutanol | 78-83-1 | 37 ± 3 d | 41.5 ± 0.6 e | 23.9 ± 0.6 c | 18.4 ± 0.5 a | 17.6 ± 0.7 a | 21.1 ± 0.4 b | 5 |
| 2-Methyl-1-Butanol | 137-32-6 | 30 ± 1 a | 50 ± 1 c | 51.2 ± 0.5 c | 34.4 ± 0.7 b | 49.9 ± 0.9 c | 50.1 ± 0.3 c | 3 |
| 3-Methyl-1-Butanol | 123-51-3 | 193 ± 9 a | 247 ± 4 b | 284 ± 6 d | 264 ± 4 c | 249 ± 2 b | 268 ± 3 c | 4 |
| Esters (3) | ||||||||
| Ethyl Acetate | 141-78-6 | 61 ± 1 d | 66.1 ± 0.8 e | 42 ± 1 b | 62.3 ± 0.2 d | 26.5 ± 0.9 a | 46.2 ± 0.6 c | 5 |
| Ethyl Lactate | 97-64-3 | 61 ± 4 d | 14 ± 1 b | 64 ± 3 d | 11.8 ± 0.6 a,b | 22.8 ± 0.4 c | 10.7 ± 0.8 a | 4 |
| Diethyl Succinate | 123-25-1 | 10.5 ± 0.5 d | 6.4 ± 0.4 b | 17.9 ± 0.9 e | 8 ± 0.5 c | 6.4 ± 0.3 b | 5.2 ± 0.2 a | 5 |
| Polyols (3) | ||||||||
| 2.3-Butanediol (levo) | 24347-58-8 | 626 ± 59 a | 1435 ± 56 b | 676 ± 48 a | 1426 ± 102 b | 629 ± 45 a | 648 ± 62 a | 2 |
| 2.3-Butanediol (meso) | 5341-95-7 | 265 ± 13 b | 473 ± 19 c | 228 ± 15 a | 457 ± 32 c | 205 ± 13 a | 229 ± 11 a | 3 |
| Glycerol | 56-81-5 | 8563 ± 830 a | 16,108 ± 965 c | 9106 ± 809 a | 18,225 ± 1319 d | 8071 ± 235 a | 11,298 ± 1127 b | 4 |
| Compounds | CAS | CN2021 | CN2022 | LB2021 | LB2022 | LS2021 | LS2022 | HGs |
|---|---|---|---|---|---|---|---|---|
| Acetates (6) | ||||||||
| Butyl Acetate | 123-86-4 | 3.45 ± 0.05 c | 1.6 ± 0.2 a | 2.1 ± 0.1 b | 2.14 ± 0.01 b | 3.2 ± 0.4 c | 1.5 ± 0.2 a | 3 |
| Isoamyl Acetate | 123-92-2 | 1893 ± 165 c | 2501 ± 162 d | 708 ± 5 b | 3509 ± 114 e | 387 ± 24 a | 1763 ± 49 c | 5 |
| Hexyl Acetate | 142-92-7 | 21 ± 1 b | 98 ± 4 d | 7.7 ± 0.1 a | 70.4 ± 0.1 c | 2.6 ± 0.7 a | 76 ± 6 c | 4 |
| Octyl Acetate | 112-14-1 | 2.74 ± 0.08 a | 2.9 ± 0.1 a,b | 3.2 ± 0.3 a,b | 4.9 ± 0.2 d | 3.6 ± 0.9 b | 2.76 ± 0.04 a | 3 |
| Ethyl-phenyl Acetate | 101-97-3 | 2.3 ± 0.1 a | 1.8 ± 0.1 a | 3.41 ± 0.03 a | 96 ± 5 d | 9.5 ± 0.6 b | 81 ±4 c | 4 |
| 2-Phenyl-ethyl Acetate | 103-45-7 | 940 ± 32 a | 3603 ± 110 b | 1088 ± 77 a | 3573 ± 177 b | 872 ± 79 a | 3374 ± 224 b | 2 |
| Ethyl Esters (12) | ||||||||
| Ethyl Isobutyrate | 97-62-1 | 93 ± 8 d | 11.2 ± 0.9 b | 12.7 ± 0.9 b | 14 ± 4 b | 79.6 ± 0.4 c | 0 a | 4 |
| Ethyl Butyrate | 105-54-4 | 116.17 ± 0.09 d | 100 ± 5 c | 62 ± 6 a | 115 ± 6 d | 85 ± 4 b | 60 ± 2 a | 4 |
| Ethyl 2-methyl-butyrate | 7452-79-1 | 9.8 ± 0.7 c | 0 a | 8.0 ± 0.5 b | 0 a | 24.3 ± 0.9 d | 0 a | 4 |
| Ethyl 3-methyl-butyrate | 108-64-5 | 16 ± 1 c | 0 a | 12.2 ± 0.6 b | 0 a | 44 ± 2 d | 0 a | 4 |
| Ethyl Hexanoate | 123-66-0 | 601 ± 36 d | 422 ± 25 c | 14 ± 1 a | 213 ± 11 b | 2.6 ± 0.7 a | 184 ± 7 b | 4 |
| Ethyl Heptanoate | 106-30-9 | 0.86 ± 0.05 d | 0.30 ± 0.01 c | 0.13 ± 0.00 b | 0 a | 0 a | 0 a | 4 |
| Ethyl Octanoate | 106-32-1 | 590 ± 28 d | 772 ± 55 e | 0 a | 277 ± 10 c | 94 ± 9 b | 262 ± 18 c | 5 |
| Ethyl Decanoate | 110-38-3 | 443 ± 11 b | 1988 ± 104 d | 45 ± 1 a | 847 ± 20 c | 58 ± 5 a | 811 ± 12 c | 4 |
| Ethyl Benzoate | 93-89-0 | 1.5 ± 0.1 c | 2.71 ± 0.09 d | 0 a | 0 a | 0.78 ± 0.09 b | 0 a | 4 |
| Ethyl Dodecanoate | 106-33-2 | 20 ± 2 a | 555 ± 42 d | 10.9 ± 0.3 a | 161 ± 10 c | 10.2 ± 0.7 a | 59 ± 4 b | 4 |
| Ethyl Tetradecanoate | 124-06-1 | 10.8 ± 0.5 b | 18 ± 2 c | 6.2 ± 0.3 a | 19.2 ± 0.3 d | 6.0 ± 0.3 a | 12 ± 1 b | 4 |
| Ethyl Hexadecanoate | 628-97-7 | 23 ± 2 c | 61 ± 1 d | 14 ± 1 b | 60 ± 1 d | 6.2 ± 0.3 a | 24 ± 1 c | 4 |
| Other Esters (3) | ||||||||
| Phenethyl Butyrate | 103-52-6 | 0 a | 2.12 ± 0.01 c | 0 a | 2.0 ± 0.1 c | 1 ± 0.1 b | 2.5 ± 0.1 d | 4 |
| Phenethyl Hexanoate | 6290-37-5 | 0 a | 0 a | 0 a | 0 a | 0 a | 0.44 ± 0.02 b | 2 |
| Phenethyl Benzoate | 94-47-3 | 156 ± 9 b | 3.16 ± 0.08 a | 3.20 ± 0.02 a | 3.3 ± 0.2 a | 3.2 ± 0.3 a | 3.1 ± 0.2 a | 3 |
| Higher Alcohols (5) | ||||||||
| Hexanol | 111-27-3 | 885 ± 41 a | 1016 ± 98 a,b | 1564 ± 141 c | 1644 ± 59 c | 1055 ± 57 b | 1548 ± 30 c | 3 |
| 2-Ethyl-1-Hexanol | 104-76-7 | 453 ± 34 c | 32 ± 4 a | 45 ± 3 a,b | 51 ± 1 ab | 59 ± 2 b | 28 ± 8 a | 3 |
| Furanmethanol | 98-00-0 | 0 a | 2.87 ± 0.04 b | 6.8 ± 0.4 c | 6.7 ± 0.4 c | 3.50 ± 0.08 b | 2.5 ± 0.1 a,b | 3 |
| Octanol | 111-87-5 | 97 ± 3 c | 0 a | 0 a | 0 a | 0 a | 88 ± 4 b | 3 |
| Dodecanol | 112-53-8 | 7.9 ± 0.6 b | 10.9 ± 0.6 c | 6.0 ± 0.3 a | 10.30 ± 0.01 c | 8.2 ± 0.2 b | 9 ± 2 b | 3 |
| Phenols (2) | ||||||||
| 4-Ethyl Guaiacol | 2785-89-9 | 319 ± 13 c | 0 a | 0 a | 0 a | 219 ± 7 b | 0 a | 2 |
| 2-Methoxy-4-Vinyl-phenol | 7786-61-0 | 0 a | 357 ± 5 e | 39 ± 2 b | 120 ± 11 d | 0 a | 50 ± 4 c | 5 |
| Lactones (3) | ||||||||
| γ-Butyrolactone | 96-48-0 | 11,924 ± 596 a | 12,738 ± 1015 a,b | 15,723 ± 1392 d | 14,002 ± 174 b,c | 13,610 ± 795 a,b,c | 14,559 ± 1185 c,d | 4 |
| γ-Nonalactone | 104-61-0 | 15.2 ± 0.7 b,c | 22.8 ± 0.8 e | 13.46 ± 0.01 a,b | 16 ± 2 cd | 11.3 ± 0.2 a | 18 ± 2 d | 5 |
| β-Damascenone | 23726-93-4 | 13.0 ± 0.4 b | 63 ± 3 d | 4.9 ± 0.3 a | 27.3 ± 0.3 c | 5.7 ± 0.3 a | 25.0 ± 0.4 c | 4 |
| Carbonyl Compounds (8) | ||||||||
| Hexanal | 66-25-1 | 3.6 ± 0.2 a | 3.4 ± 0.5 a | 4.9 ± 0.4 b | 5.9 ± 0.9 b,c | 6.1 ± 0.9 c | 3.4 ± 0.5 a | 3 |
| Furfural | 98-01-1 | 419 ± 28 a | 438 ± 9 a | 876 ± 36 c | 434 ± 12 a | 758 ± 109 b | 403 ± 21 a | 3 |
| Benzaldehyde | 100-52-7 | 0 a | 0.001 ± 0.000 a | 2.3 ± 0.3 b | 0 a | 3.0 ± 0.6 c | 6.0 ± 0.1 d | 4 |
| Octanal | 124-13-0 | 0 a | 2.0 ± 0.2 d | 1 ± 0.1 b | 2.32 ± 0.06 e | 1.3 ± 0.1 c | 2.2 ± 0.1 e | 5 |
| Decanal | 112-31-2 | 5.6 ± 0.2 a | 9.8 ± 0.9 b | 6.6 ± 0.6 a | 10 ± 1 b | 7 ± 2 a | 11.6 ± 0.8 b | 2 |
| (E)-2-Nonenal | 18829-56-6 | 6.4 ± 0.4 c | 0 a | 4.3 ± 0.3 b | 0 a | 4.3 ± 0.5 b | 0 a | 3 |
| Phenylacetaldehyde | 122-78-1 | 0 a | 0 a | 0 a | 0 a | 9.4 ± 0.9 b | 0 a | 2 |
| 3-Heptanone | 106-35-4 | 1.6 ± 0.3 c | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0 a | 0.4 ± 0.1 b | 3 |
| Terpenes and Derivatives (7) | ||||||||
| (DL)-Limonene | 138-86-3 | 0 a | 5217 ± 213 b | 0 a | 28 ± 3 a | 0 a | 5348 ± 55 b | 2 |
| (E)-Geranyl Acetone | 3796-70-1 | 1.2 ± 0.1 a | 1.6 ± 0.1 b | 1.3 ± 0.2 a | 1.16 ± 0.08 a | 1.76 ± 0.03 b | 2.3 ± 0.1 c | 3 |
| Geranyl Acetate | 105-87-3 | 3.08 ± 0.06 a | 5.8 ± 0.3 b | 7.9 ± 0.4 c | 38.7 ± 0.5 f | 8.9 ± 0.5 d | 35.5 ± 0.5 e | 6 |
| (E)-Methyldihydrojasmonate | 2630-39-9 | 0.7 ± 0.2 a | 2.3 ± 0.2 c | 1.7 ± 0.5 b | 1.3 ± 0.1 b | 0.8 ± 0.1 a | 1.4 ± 0.2 b | 3 |
| (Z)-Geranyl Acetone | 3879-26-3 | 1.93 ± 0.05 a,b | 1.98 ± 0.03 a,b | 1.90 ± 0.08 a | 1.88 ± 0.08 a | 1.83 ± 0.04 a | 1.89 ± 0.04 a,b | 2 |
| (Z)-Citral | 106-26-3 | 0 a | 0 a | 22.9 ± 0.5 b | 0 a | 0 a | 0 a | 2 |
| (Z)-Nerolidol | 7212-44-4 | 0 a | 0.95 ± 0.05 c | 0.30 ± 0.02 b | 0.98 ± 0.03 c | 0 a | 0 a | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes-Espinosa, J.M.; Muñoz-Castells, R.; Moreno-García, J.; García-Martínez, T.; Mauricio, J.C.; Moreno, J. Volatilome Analysis for Differentiating Terroir Expression: A Case Study of Three Wineries in a Limestone-Rich, Warm-Climate Region. Molecules 2025, 30, 2982. https://doi.org/10.3390/molecules30142982
Fuentes-Espinosa JM, Muñoz-Castells R, Moreno-García J, García-Martínez T, Mauricio JC, Moreno J. Volatilome Analysis for Differentiating Terroir Expression: A Case Study of Three Wineries in a Limestone-Rich, Warm-Climate Region. Molecules. 2025; 30(14):2982. https://doi.org/10.3390/molecules30142982
Chicago/Turabian StyleFuentes-Espinosa, José Miguel, Raquel Muñoz-Castells, Jaime Moreno-García, Teresa García-Martínez, Juan Carlos Mauricio, and Juan Moreno. 2025. "Volatilome Analysis for Differentiating Terroir Expression: A Case Study of Three Wineries in a Limestone-Rich, Warm-Climate Region" Molecules 30, no. 14: 2982. https://doi.org/10.3390/molecules30142982
APA StyleFuentes-Espinosa, J. M., Muñoz-Castells, R., Moreno-García, J., García-Martínez, T., Mauricio, J. C., & Moreno, J. (2025). Volatilome Analysis for Differentiating Terroir Expression: A Case Study of Three Wineries in a Limestone-Rich, Warm-Climate Region. Molecules, 30(14), 2982. https://doi.org/10.3390/molecules30142982








