Structural Characterization and Anti-Ultraviolet Radiation Damage Activity of Polysaccharides from Helianthus annuus (Sunflower) Receptacles
Abstract
1. Introduction
2. Results and Discussion
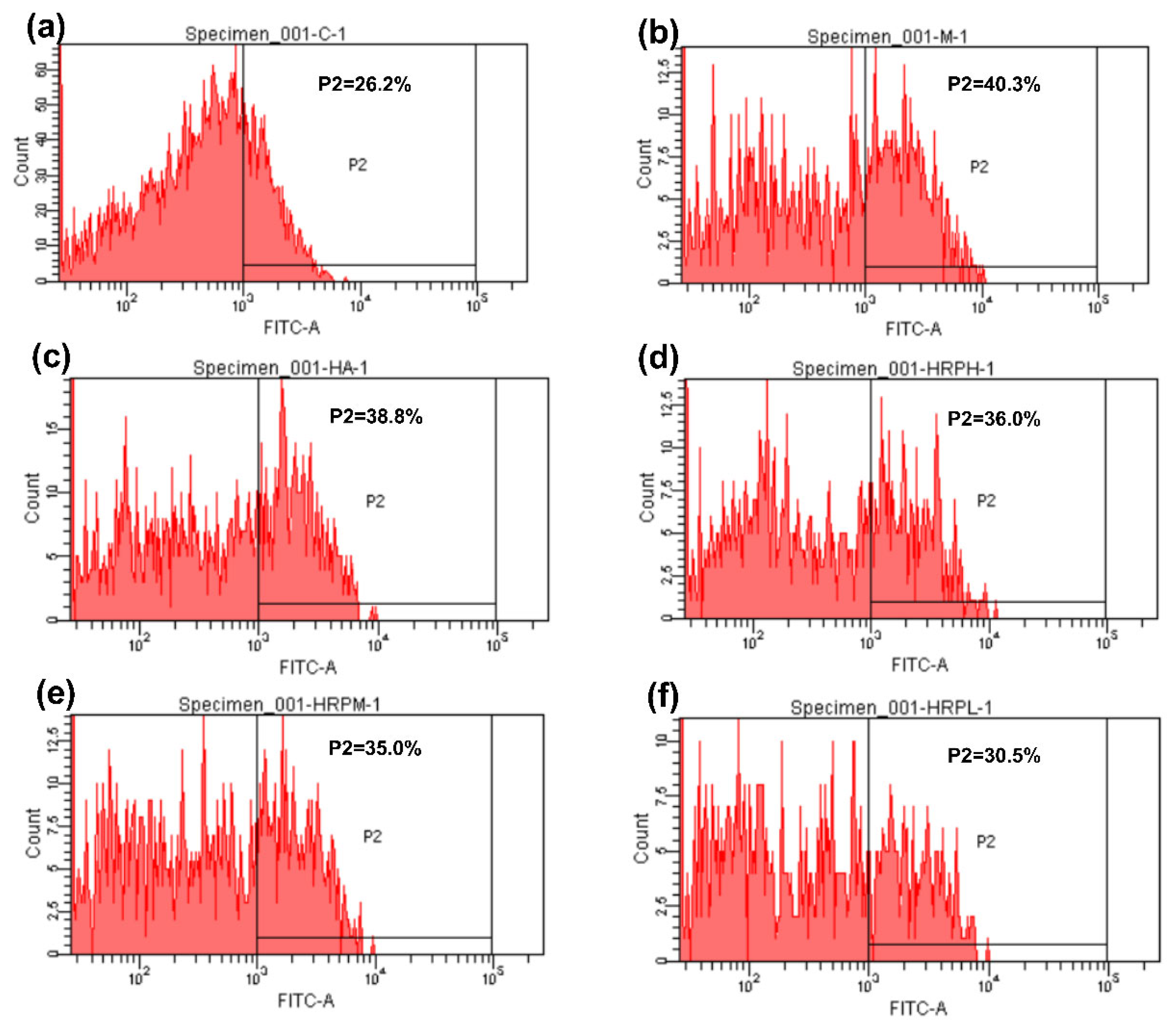
2.1. The Purity Test Results of HRP-1 and the HRP-1 Content in HRTP
2.2. Structural Characterization Results of HRP-1
2.2.1. Determination Results of the Molecular Weight of HRP-1
2.2.2. Monosaccharide Composition of HRP-1
2.2.3. Methylation Analysis Resoults of HRP-1
2.2.4. Fourier Transform Infrared (FT-IR) Analysis of HRP-1
2.2.5. Nuclear Magnetic Resonance (NMR) Analysis of HRP-1
2.2.6. Characterization of the Primary Structure of HRP-1
2.2.7. Scanning Electron Microscopy (SEM) Analysis Results of HRP-1
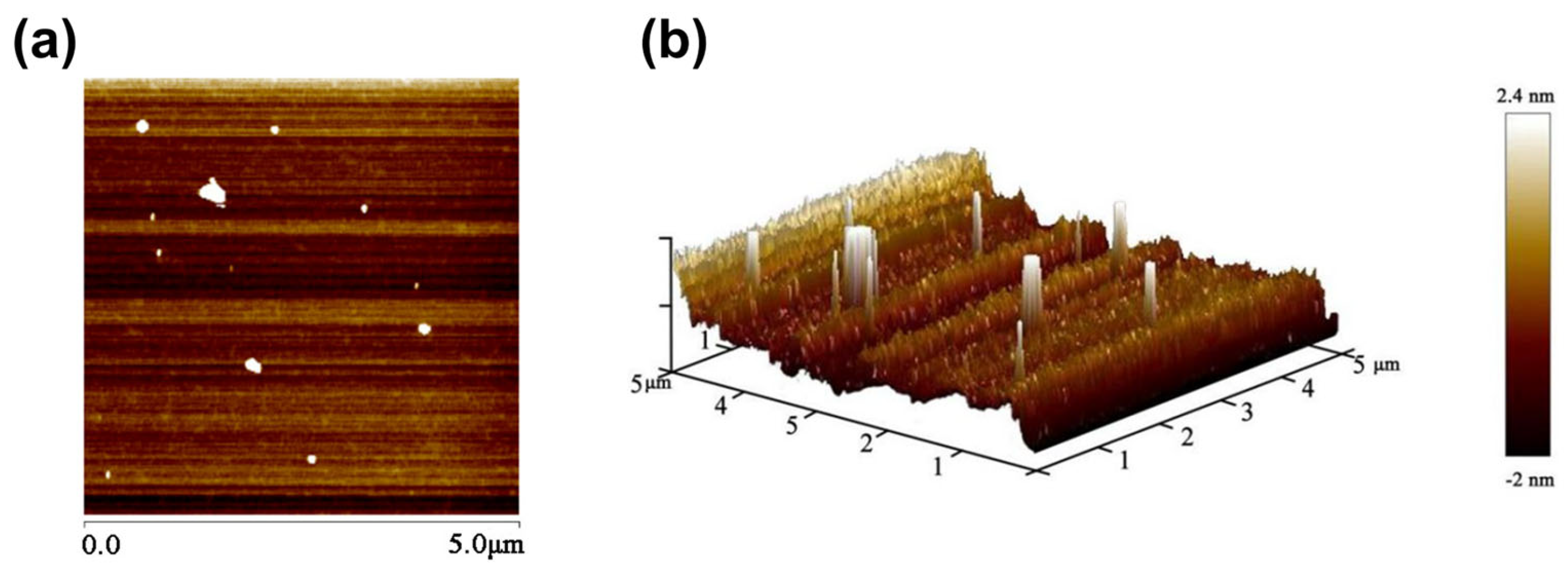
2.2.8. Atomic Force Microscopy (AFM) Analysis Results of HRP-1
2.3. Protective Effect of HRTP Against UV-Induced Damage
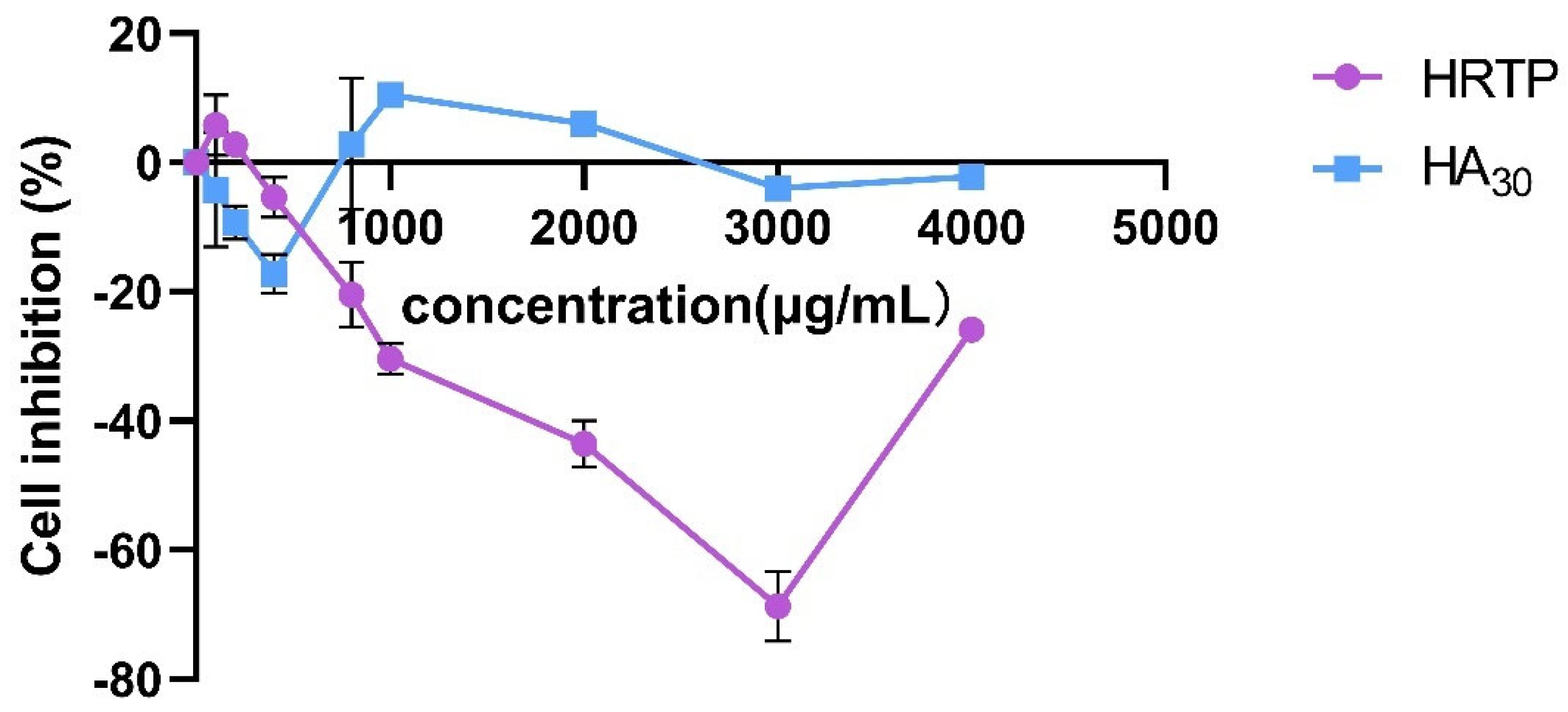
2.3.1. Cytotoxicity Resoults
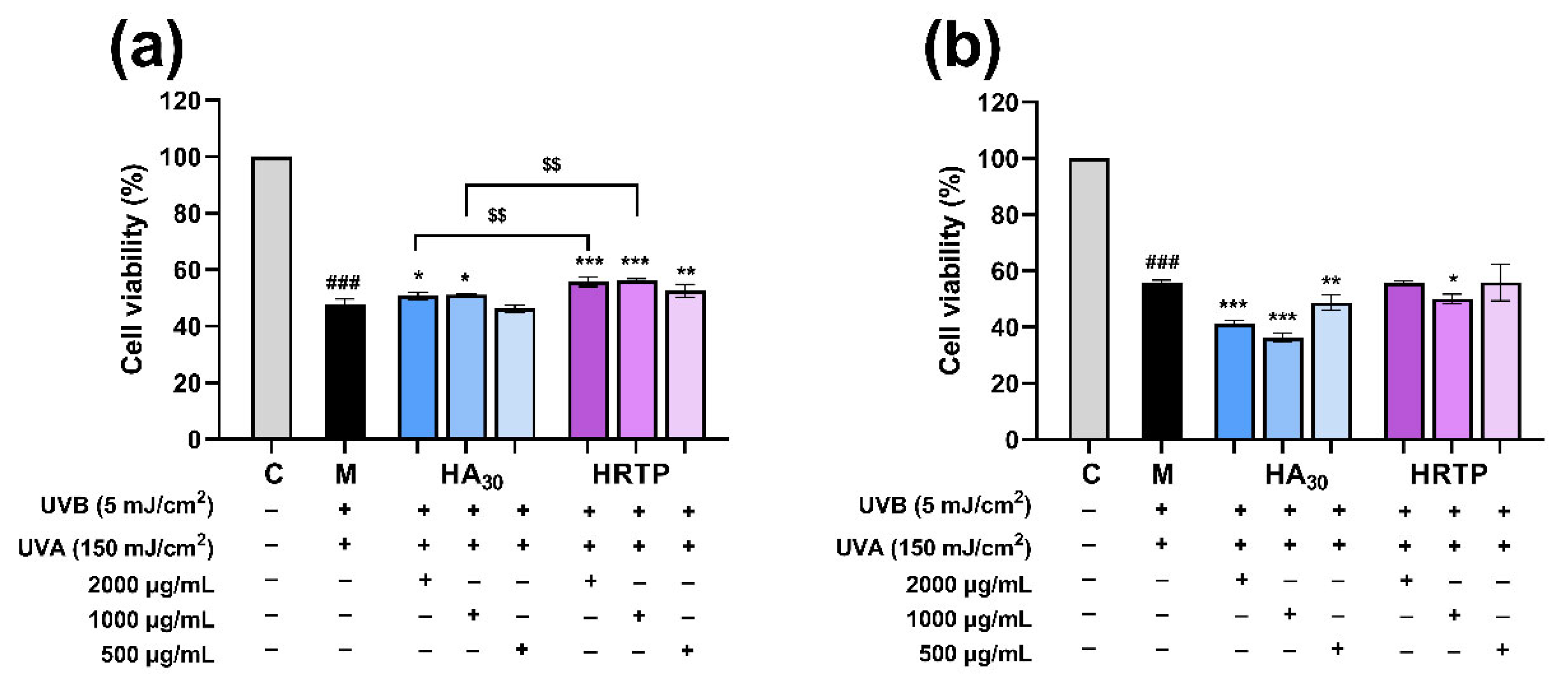
2.3.2. The Preventive and Reparative Effects of HRTP on UV-Induced Damage in HaCaT Cells
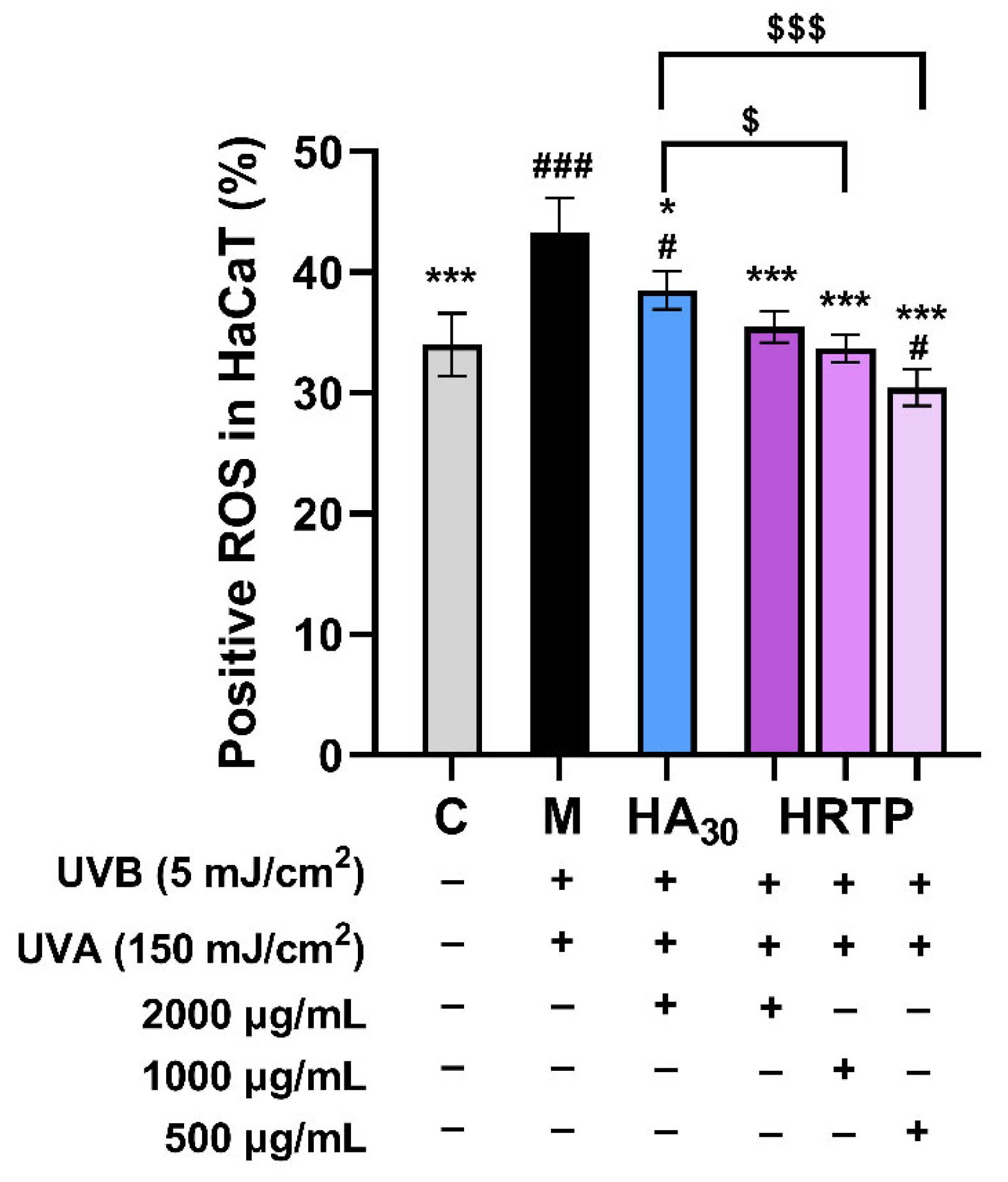
2.3.3. Levels of Inflammatory Factors, MMPs, and ROS
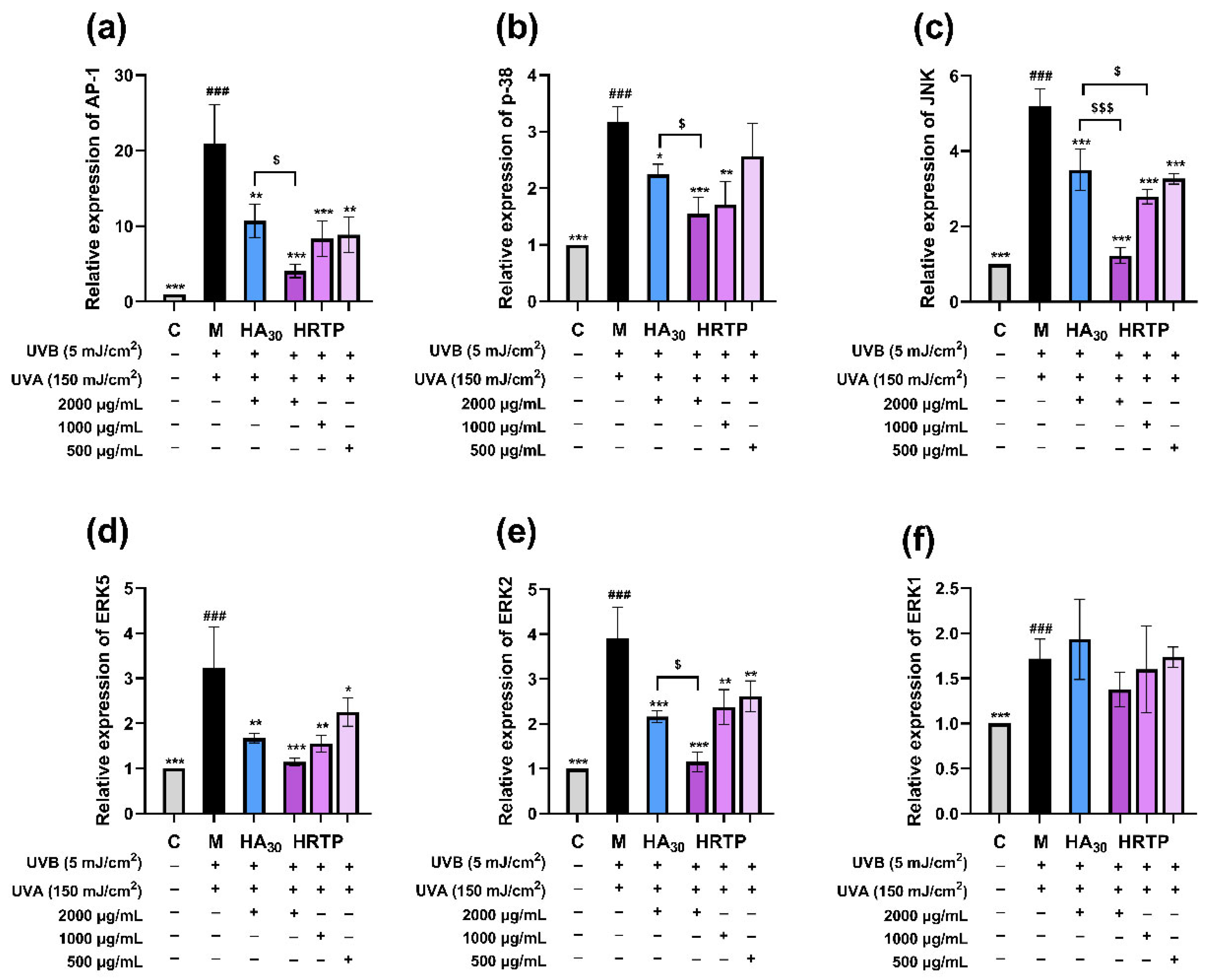
2.3.4. RT-qPCR Results
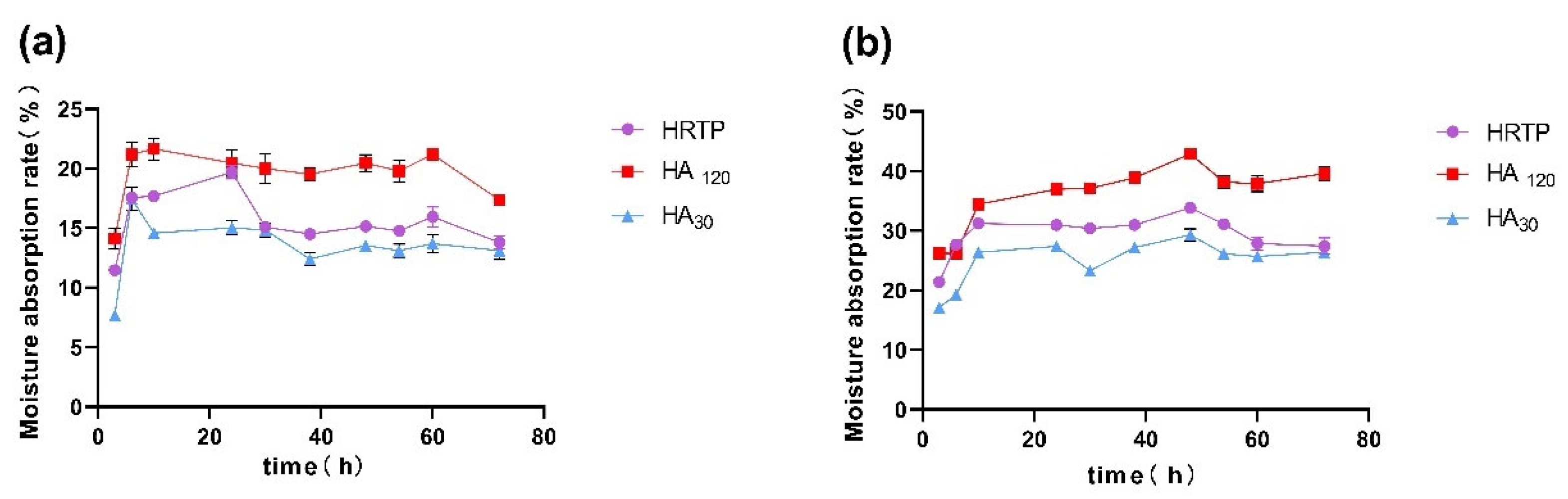
2.4. Hygroscopicity Test Results
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of HRTP and HRP-1
3.3. Purity Test and Determination of the HRP-1 Content in HRTP
3.3.1. Purity Test of HRP-1
3.3.2. Determination of HRP-1 in HRTP
3.4. Structural Characterization of HRP-1
3.4.1. Determination of the Molecular Weight of HRP-1
3.4.2. Monosaccharide Composition Analysis of HRP-1
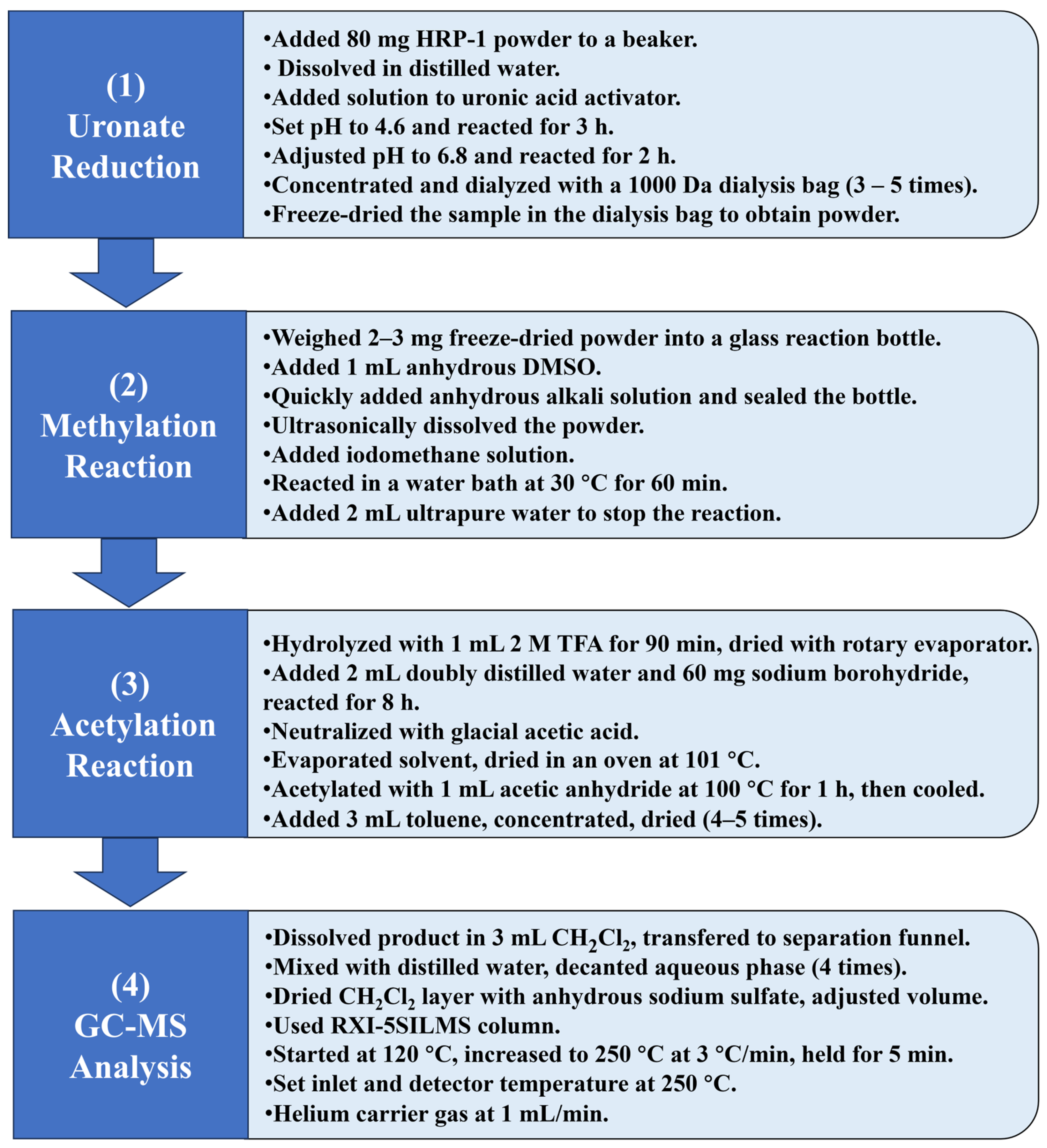
3.4.3. Methylation Analysis of HRP-1
3.4.4. FT-IR Analysis of HRP-1
3.4.5. NMR Analysis of HRP-1
3.4.6. SEM Analysis of HRP-1
3.4.7. AFM Analysis of HRP-1
3.5. Study on the Protective Effect of HRTP Against UV-Induced Damage
3.5.1. Cell Culture
3.5.2. Cytotoxicity
3.5.3. Ultraviolet Radiation (UVR) Procedure
- (1)
- UVR for Assessing the Preventive Effect
- (2)
- UVR for Assessing the Reparative Effect
3.5.4. ELISA
3.5.5. Determination of ROS Levels
3.5.6. Quantitative Real-Time PCR (RT-qPCR)
3.6. Study of Hygroscopicity
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gude, V.G.; Martinez-Guerra, E. Green Chemistry with Process Intensification for Sustainable Biodiesel Production. Environ. Chem. Lett. 2018, 16, 327–341. [Google Scholar] [CrossRef]
- Ebrahimian, E.; Denayer, J.F.M.; Aghbashlo, M.; Tabatabaei, M.; Karimi, K. Biomethane and Biodiesel Production from Sunflower Crop: A Biorefinery Perspective. Renew Energy 2022, 200, 1352–1361. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Ighalo, J.O.; Adaobi Igwegbe, C.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; Pashalidis, I.; Kalderis, D. Sunflower-Biomass Derived Adsorbents for Toxic/Heavy Metals Removal from (Waste) Water. J. Mol. Liq. 2021, 342, 117540. [Google Scholar] [CrossRef]
- Nazemi, S.; Mohmmadkhani, S.; Yeganeh, J. Sunflower Wasted Biomass as a Remarkable Adsorbent for Removal of Heavy Metals from Waste Waters. Int. J. Health Stud. 2016, 2, 26. [Google Scholar]
- Li, L.; Huang, T.; Lan, C.; Ding, H.; Yan, C.; Dou, Y. Protective Effect of Polysaccharide from Sophora japonica L. Flower Buds against UVB Radiation in a Human Keratinocyte Cell Line (HaCaT Cells). J. Photochem. Photobiol. B 2019, 191, 135–142. [Google Scholar] [CrossRef]
- Ye, Y.; Ji, D.; You, L.; Zhou, L.; Zhao, Z.; Brennan, C. Structural Properties and Protective Effect of Sargassum fusiforme Polysaccharides against Ultraviolet B Radiation in Hairless Kun Ming Mice. J. Funct. Foods 2018, 43, 8–16. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Peng, L.; Jiang, N.; Liu, Q.; Zhang, E.; Liang, B.; Li, R.; Zhu, H. Lycium barbarum Polysaccharide Protects Human Keratinocytes against UVB-Induced Photo-Damage. Free Radic. Res. 2017, 51, 200–210. [Google Scholar] [CrossRef]
- Conway, A.J.; Gonsior, M.; Clark, C.; Heyes, A.; Mitchelmore, C.L. Acute Toxicity of the UV Filter Oxybenzone to the Coral Galaxea fascicularis. Sci. Total Environ. 2021, 796, 148666. [Google Scholar] [CrossRef]
- Miller, I.B.; Pawlowski, S.; Kellermann, M.Y.; Petersen-Thiery, M.; Moeller, M.; Nietzer, S.; Schupp, P.J. Toxic Effects of UV Filters from Sunscreens on Coral Reefs Revisited: Regulatory Aspects for “Reef Safe” Products. Environ. Sci. Eur. 2021, 33, 74. [Google Scholar] [CrossRef]
- Carstensen, L.; Beil, S.; Börnick, H.; Stolte, S. Structure-Related Endocrine-Disrupting Potential of Environmental Transformation Products of Benzophenone-Type UV Filters: A Review. J Hazard. Mater. 2022, 430, 128495. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Garrido-Galand, S.; Taladrid, D.; Moreno-Arribas, M.V.; Villamiel, M.; Montilla, A. Use of Natural Low-methoxyl Pectin from Sunflower By-products for the Formulation of Low-sucrose Strawberry Jams. J. Sci. Food Agric. 2022, 102, 5957–5964. [Google Scholar] [CrossRef]
- Peng, X.; Yang, G.; Shi, Y.; Zhou, Y.; Zhang, M.; Li, S. Box–Behnken Design Based Statistical Modeling for the Extraction and Physicochemical Properties of Pectin from Sunflower Heads and the Comparison with Commercial Low-Methoxyl Pectin. Sci. Rep. 2020, 10, 3595. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Hua, X.; Liu, J.; Wang, M.; Liu, Y.; Yang, R.; Cao, Y. Extraction of Sunflower Head Pectin with Superfine Grinding Pretreatment. Food Chem. 2020, 320, 126631. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, S.; Manickam, L.P.; Murugesan, V.; Muthukumaran, C.; Pugazhendhi, A. Pectin Extraction from Helianthus annuus (Sunflower) Heads Using RSM and ANN Modelling by a Genetic Algorithm Approach. Int. J. Biol. Macromol. 2019, 124, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Han, L.; Liu, X.; Dai, H.; Liu, C.; Yan, M.; Li, W.; Han, W.; Li, X.; Huang, S.; et al. Extraction, Radical Scavenging Activities, and Chemical Composition Identification of Flavonoids from Sunflower (Helianthus annuus L.) Receptacles. Molecules 2021, 26, 403. [Google Scholar] [CrossRef]
- Chen, M.; Liu, C.; Shen, Y.; Zou, J.; Zhang, Z.; Wan, Y.; Yang, L.; Jiang, S.; Qian, D.; Duan, J. A Powerful HPLC-ELSD Method for Simultaneous Determination of Fecal Bile Acids in T2DM Rats Interfered by Sanhuang Xiexin Tang. J. Chromatogr. Sci 2021, 59, 871–876. [Google Scholar] [CrossRef]
- Frosi, I.; Balduzzi, A.; Moretto, G.; Colombo, R.; Papetti, A. Towards Valorization of Food-Waste-Derived Pectin: Recent Advances on Their Characterization and Application. Molecules 2023, 28, 6390. [Google Scholar] [CrossRef]
- Deng, R.; Wang, F.; Wang, L.; Xiong, L.; Shen, X.; Song, H. Advances in Plant Polysaccharides as Antiaging Agents: Effects and Signaling Mechanisms. J. Agric. Food Chem. 2023, 71, 7175–7191. [Google Scholar] [CrossRef]
- Hu, J.; Yao, W.; Chang, S.; You, L.; Zhao, M.; Chi-Keung Cheung, P.; Hileuskaya, K. Structural Characterization and Anti-Photoaging Activity of a Polysaccharide from Sargassum fusiforme. Food Res. Int. 2022, 157, 111267. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, X.; Ma, J.; Zhang, M.; Wu, H. Structural Elucidation of a Novel Pectin-Polysaccharide from the Petal of Saussurea laniceps and the Mechanism of Its Anti-HBV Activity. Carbohydr. Polym. 2019, 223, 115077. [Google Scholar] [CrossRef]
- Pappas, C.S.; Malovikova, A.; Hromadkova, Z.; Tarantilis, P.A.; Ebringerova, A.; Polissiou, M.G. Determination of the Degree of Esterification of Pectinates with Decyl and Benzyl Ester Groups by Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS) and Curve-Fitting Deconvolution Method. Carbohydr. Polym. 2004, 56, 465–469. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Huo, D.; Cao, C.; Li, Y.; Liang, Y.; Li, B.; Li, L. Preliminary Characterization, Antioxidant and α-Glucosidase Inhibitory Activities of Polysaccharides from Mallotus Furetianus. Carbohydr. Polym. 2019, 215, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Chetouani, A.; Follain, N.; Marais, S.; Rihouey, C.; Elkolli, M.; Bounekhel, M.; Benachour, D.; Le Cerf, D. Physicochemical Properties and Biological Activities of Novel Blend Films Using Oxidized Pectin/Chitosan. Int. J. Biol. Macromol. 2017, 97, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Liu, Y.; Wu, D.; Cai, P.; Pan, Y. Structural Characterization and Immunomodulatory Activity of a Water Soluble Polysaccharide Isolated from Botrychium ternatum. Carbohydr. Polym. 2017, 171, 136–142. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, H.; Nie, C.; Zhao, T.; Xia, Y.; Ai, L. Structural Characteristics of Tamarind Seed Polysaccharides Treated by High-Pressure Homogenization and Their Effects on Physicochemical Properties of Corn Starch. Carbohydr. Polym. 2021, 262, 117661. [Google Scholar] [CrossRef]
- Li, F.; Feng, K.-L.; Yang, J.-C.; He, Y.-S.; Guo, H.; Wang, S.-P.; Gan, R.-Y.; Wu, D.-T. Polysaccharides from Dandelion (Taraxacum mongolicum) Leaves: Insights into Innovative Drying Techniques on Their Structural Characteristics and Biological Activities. Int. J. Biol. Macromol. 2021, 167, 995–1005. [Google Scholar] [CrossRef]
- Zhao, Z.; Dai, H.; Wu, X.; Chang, H.; Gao, X.; Liu, M.; Tu, P. Characterization of a Pectic Polysaccharide from the Fruit of Ziziphus jujuba. Chem. Nat. Compd. 2007, 43, 374–376. [Google Scholar] [CrossRef]
- Li, J.; Ai, L.; Yang, Q.; Liu, Y.; Shan, L. Isolation and Structural Characterization of a Polysaccharide from Fruits of Zizyphus jujuba Cv. Junzao. Int. J. Biol. Macromol. 2013, 55, 83–87. [Google Scholar] [CrossRef]
- Ezzati, S.; Ayaseh, A.; Ghanbarzadeh, B.; Heshmati, M.K. Pectin from Sunflower By-Product: Optimization of Ultrasound-Assisted Extraction, Characterization, and Functional Analysis. Int. J. Biol. Macromol. 2020, 165, 776–786. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, D.; Garg, Y.; Mahmood, S.; Chopra, S.; Bhatia, A. Marine-Derived Polysaccharides and Their Therapeutic Potential in Wound Healing Application—A Review. Int. J. Biol. Macromol. 2023, 253, 127331. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.; Cui, W.; Zhou, F.; Zhang, S.; Wang, X.; Gao, P.; Wei, K.; Zhu, R. Pine Pollen Polysaccharides Promote Cell Proliferation and Accelerate Wound Healing by Activating the JAK2-STAT3 Signaling Pathway. Int. J. Biol. Macromol. 2022, 210, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Fourtanier, A.; Moyala, D.; Seite, S. UVA Filters in Sun-Protection Products: Regulatory and Biological Aspects. Photochem. Photobiol. Sci. 2012, 11, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, B.M.; Freitas, F.; Lima, J.C.; Silva, J.C.; Reis, M.A.M. Photoprotective Effect of the Fucose-Containing Polysaccharide FucoPol. Carbohydr. Polym. 2021, 259, 117761. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Bai, D.; Cai, C.; Li, J.; Yan, C.; Zhang, S.; Wu, Z.; Hao, J.; Yu, G. Photoprotective Effect of Astragalus membranaceus Polysaccharide on UVA-Induced Damage in HaCaT Cells. PLoS ONE 2020, 15, e0235515. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wang, W.; Zhang, Y.; Du, F.; Zhang, S.; Li, Z.; Deng, J.; Li, J. Photoprotective Effects of Dendrobium nobile Lindl. Polysaccharides against UVB-Induced Oxidative Stress and Apoptosis in HaCaT Cells. Int. J. Mol. Sci. 2023, 24, 6120. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Y.; Pu, Y.; Mao, S.; Nie, Y.; Liu, Y.; Jiang, X. Dendrobium Officinale Kimura & Migo Polysaccharide and Its Multilayer Emulsion Protect Skin Photoaging. J. Ethnopharmacol 2024, 318, 116974. [Google Scholar]
- Hirano, T. IL-6 in Inflammation, Autoimmunity and Cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- de Moura, J.P.; de Moura Fernandes, E.P.; Lustoza Rodrigues, T.C.M.; Messias Monteiro, A.F.; de Sousa, N.F.; Dos Santos, A.M.F.; Scotti, M.T.; Scotti, L. Targets Involved in Skin Aging and Photoaging and their Possible Inhibitors: A Mini-review. Curr. Drug Targets 2023, 24, 797–815. [Google Scholar] [CrossRef]
- Fang, M.; Lee, H.-M.; Oh, S.; Zheng, S.; Bellere, A.D.; Kim, M.; Choi, J.; Kim, M.; Yu, D.; Yi, T.-H. Rosa davurica Inhibits Skin Photoaging via Regulating MAPK/AP-1, NF-ΚB, and Nrf2/HO-1 Signaling in UVB-Irradiated HaCaTs. Photochem. Photobiol. Sci. 2022, 21, 2217–2230. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Xie, F.; Yaseen, A.; Chen, B.; Zhang, G.L.; Wang, M.K.; Shen, X.F.; Li, F. A Polysaccharide TKP-2–1 from Tamarindus indica L.: Purification, Structural Characterization and Immunomodulating Activity. J. Funct. Foods 2021, 78, 104384. [Google Scholar] [CrossRef]
- Niu, L.; Wu, Y.; Liu, H.; Wang, Q.; Li, M.; Jia, Q. The Structural Characterization of a Novel Water-Soluble Polysaccharide from Edible Mushroom Leucopaxillus giganteus and Its Antitumor Activity on H22 Tumor-Bearing Mice. Chem. Biodivers. 2021, 18, e2001010. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, M.; Liu, Z.; Cheng, L.; Li, M.; Li, C. Purification, Structural Characterization, and Antitumor Activity of a Polysaccharide from Perilla Seeds. Int. J. Mol. Sci. 2023, 24, 15904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, P.; Zhao, H.; Qiu, J.; Regenstein, J.M.; Yang, X. Isolation, Purification, Structure and Antioxidant Activity of Polysaccharide from Pinecones of Pinus koraiensis. Carbohydr. Polym. 2021, 251, 117078. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, K.; Chen, X.; Zhou, M.; Chen, Y.; Ye, X.; Zhou, F.; Ding, Z.; Zhu, B. A Berberine-Loaded Bletilla striata Polysaccharide Hydrogel as a New Medical Dressing for Diabetic Wound Healing. Int. J. Mol. Sci. 2023, 24, 16286. [Google Scholar] [CrossRef]
- Huang, X.; Li, T.; Li, S. Encapsulation of Vitexin-Rhamnoside Based on Zein/Pectin Nanoparticles Improved Its Stability and Bioavailability. Curr. Res. Food Sci. 2023, 6, 100419. [Google Scholar] [CrossRef]
- Mo, X.; Chen, X.; Pan, X.; Lu, Y.; Pan, G.; Xie, J.; Pan, Z.; Li, L.; Tian, H.; Li, Y. Protective Effect of Helianthus Annuus Seed Byproduct Extract on Ultraviolet Radiation-induced Injury in Skin Cells. Photochem Photobiol 2024, 100, 756–771. [Google Scholar] [CrossRef]
- Shi, C.; Fang, Y.; Liu, Z.; Wang, Y.; Shen, L.; Zhao, L. Effect of Moisture Sorption and Lactose Type on Tablet Quality: A Hygroscopicity Study between Lactose Powder and Tablets. Mol. Pharm. 2025, 22, 544–557. [Google Scholar] [CrossRef]

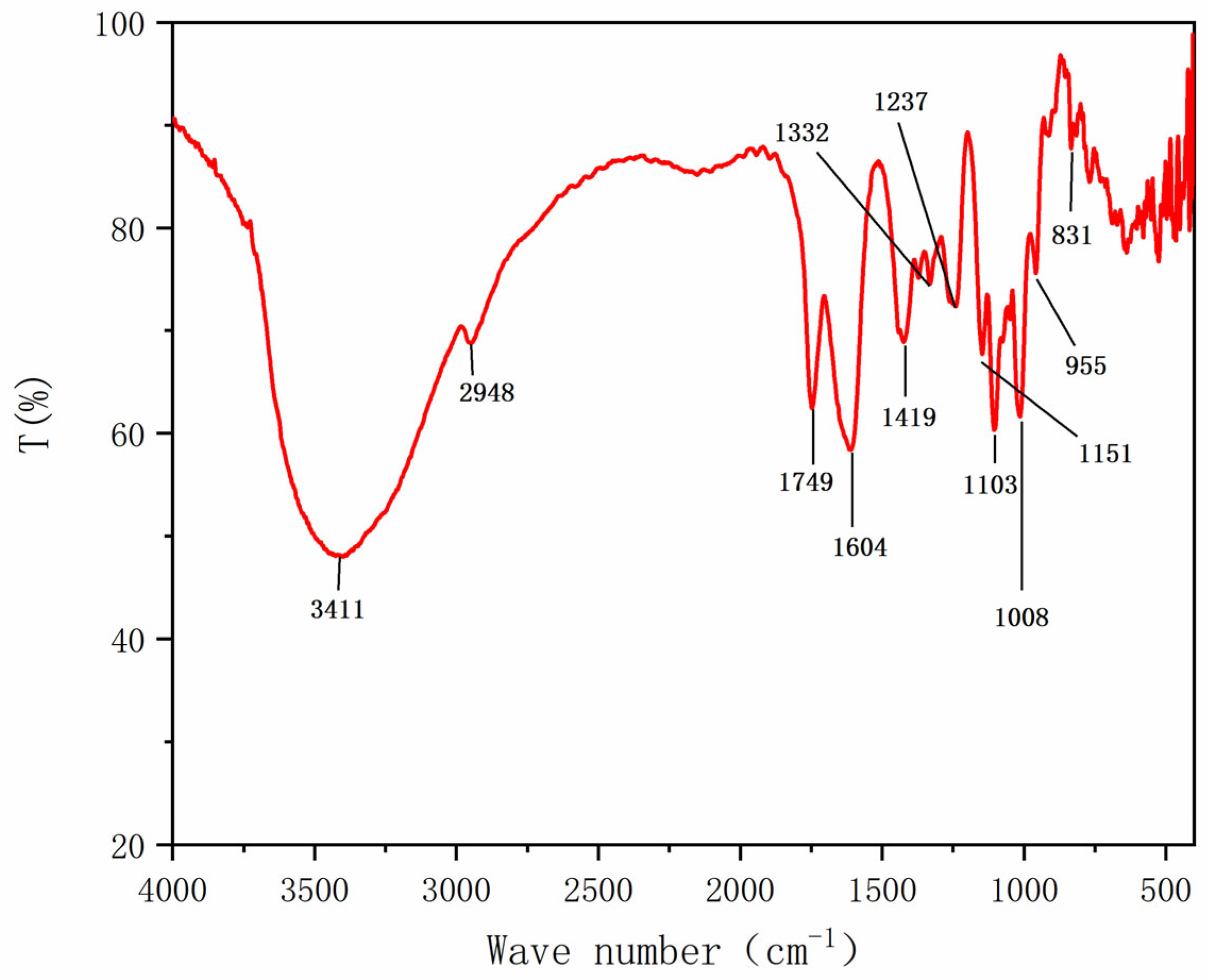
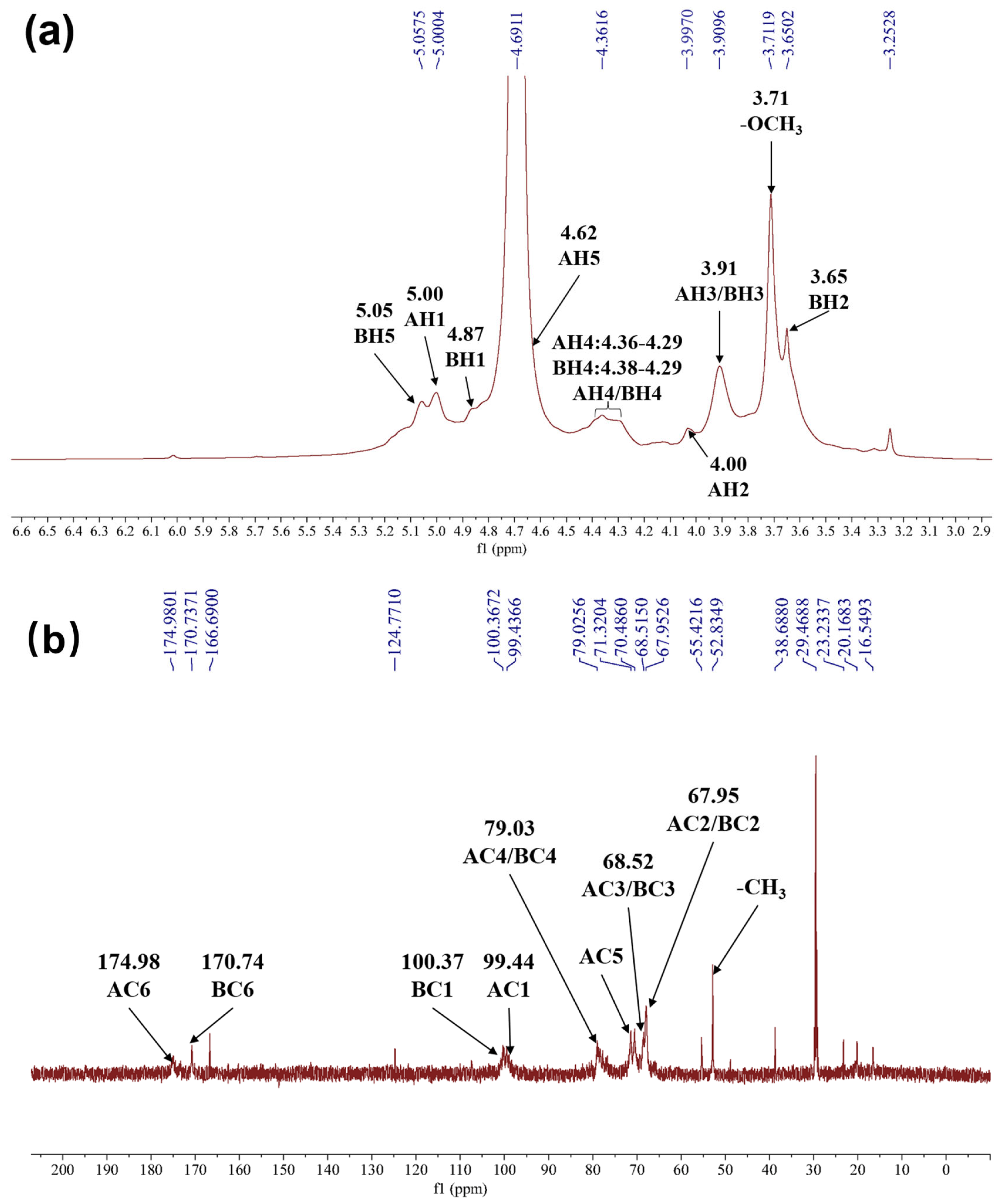
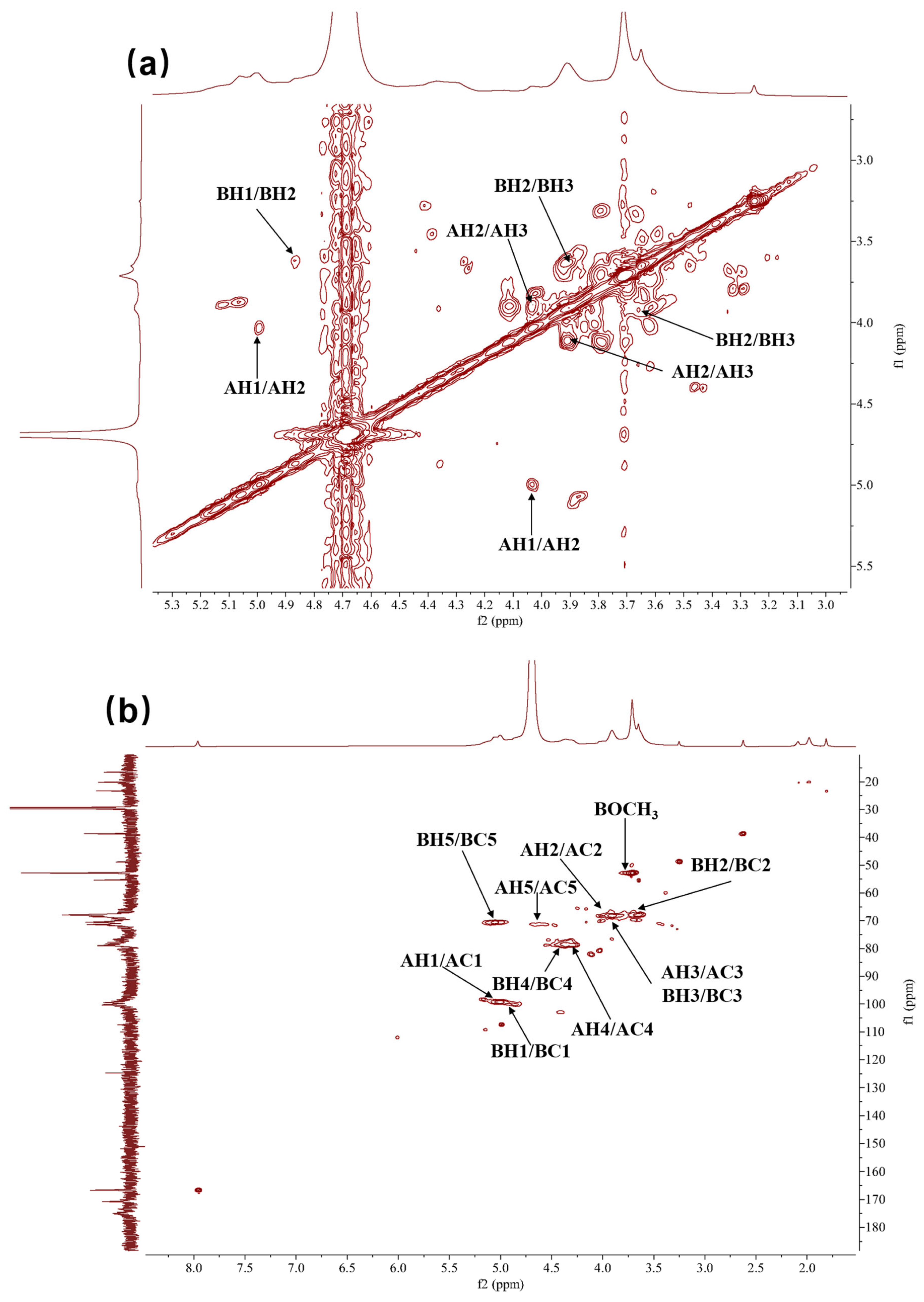


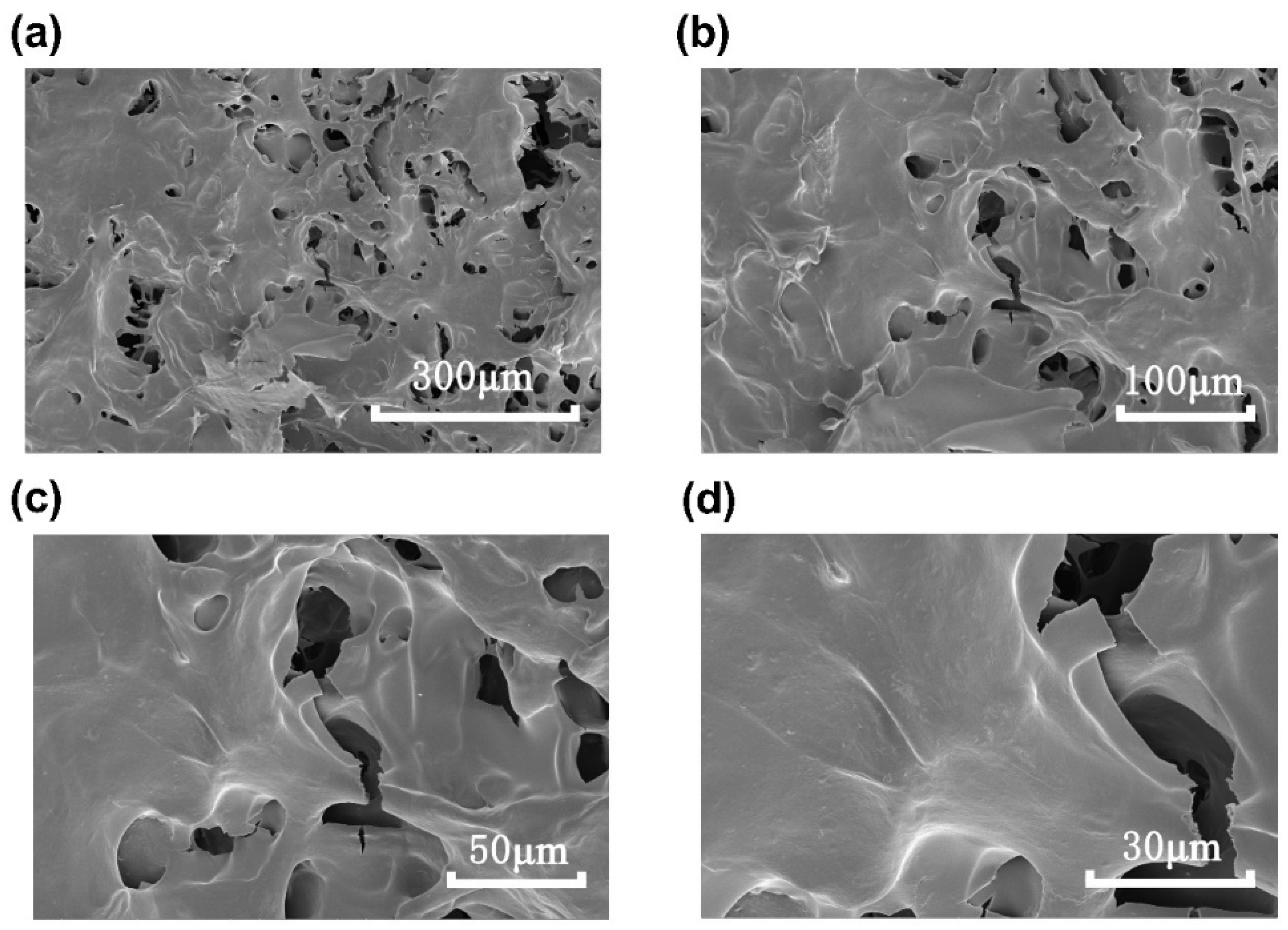

| RT (min) | Methylated Sugar | Mass Fragments (m/z) | Molar Ratio (%) | Type of Linkage |
|---|---|---|---|---|
| 17.520 | 2,3,4,6-Me4-Galp | 45, 71, 87, 101, 117, 129, 145, 161, 205 | 1.8 | Galp-(1→ |
| 20.780 | 2,3,6-Me3-Galp | 45, 87, 99, 101, 113, 117, 129, 131, 161, 173, 233 | 92.8 | →4)-Galp-(1→ |
| 21.381 | 2,4,6-Me3-Galp | 45, 87, 99, 101, 117, 129, 161, 173, 233 | 1.4 | →3)-Galp-(1→ |
| 24.849 | 3,6-Me2Galp | 45, 87, 99, 113, 129, 173, 189, 233 | 1.5 | →2,4)-Galp-(1→ |
| 27.988 | 2,4-Me2-Galp | 45, 87, 117, 129, 159, 189, 233 | 2.5 | →3,6)-Galp-(1→ |
| Position | δC, Type | δH | 1H-1H COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| →4)-α-D-GalA-(1→ | |||||
| 1 | 99.44, CH | 5.00 | 4.00 | 4.36, 3.91, 4.00 | |
| 2 | 67.95, CH | 4.00 | 5.00, 3.91 | ||
| 3 | 68.52, CH | 3.91 | 4.00 | ||
| 4 | 79.03, CH | 4.36–4.29 | |||
| 5 | 71.32, CH | 4.62 | 4.00, 3.91 | ||
| 6 | 174.98, C | ||||
| →4)-α-D-GalA(6-OCH3)-(1→ | |||||
| 1 | 100.37, CH | 4.87 | 3.65 | 3.65, 3.91, 4.38, 5.05 | |
| 2 | 67.95, CH | 3.65 | 4.87, 3.91 | ||
| 3 | 68.52, CH | 3.91 | 3.65 | ||
| 4 | 79.03, CH | 4.38–4.29 | |||
| 5 | 70.49, CH | 5.05 | 170.74 | ||
| 6 | 170.74, C | ||||
| OCH3 | 52.83, OCH3 | 3.71 | 170.74 | 5.05, 4.87, 4.38, 3.91 |
| Gene | Direction | Primer Pair Sequence (5′ → 3′) |
|---|---|---|
| GAPDH | F | AATCAAGTGGGGCGATGCTG |
| R | GCAAATGAGCCCCAGCCTTC | |
| AP-1 | F | TCTGGGAAGTGAGTTCGCCT |
| R | ATGCCTCCCGCACTCTTACT | |
| p38 | F | GATTTTGGACTGGCTCGGCA |
| R | CAGTCAACAGCTCGGCCATT | |
| JNK | F | CCAGTCAGGCAAGGGATTTGTTAT |
| R | TCTTTGGTGGTGGAGCTTCTG | |
| ERK5 | F | AGCTATCTAAGTCACAGGTGGAGG |
| R | CAAAGCCAACACCGTAGCCA | |
| ERK2 | F | CGCCGAAGCACCATTCAAGT |
| R | CAGCGCCTCCCTTGCTAGA | |
| ERK1 | F | CAACACCACCTGCGACCTTA |
| R | TGGACTTGGTATAGCCCTTGGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wei, Z.; Mo, X.; Lu, Y.; Pan, G.; Pan, Z.; Li, Y.; Tian, H.; Pan, X. Structural Characterization and Anti-Ultraviolet Radiation Damage Activity of Polysaccharides from Helianthus annuus (Sunflower) Receptacles. Molecules 2025, 30, 2943. https://doi.org/10.3390/molecules30142943
Chen X, Wei Z, Mo X, Lu Y, Pan G, Pan Z, Li Y, Tian H, Pan X. Structural Characterization and Anti-Ultraviolet Radiation Damage Activity of Polysaccharides from Helianthus annuus (Sunflower) Receptacles. Molecules. 2025; 30(14):2943. https://doi.org/10.3390/molecules30142943
Chicago/Turabian StyleChen, Xiaochun, Zhiying Wei, Xiaoying Mo, Yantong Lu, Guangjuan Pan, Zhenzhen Pan, Yaohua Li, Hui Tian, and Xiaojiao Pan. 2025. "Structural Characterization and Anti-Ultraviolet Radiation Damage Activity of Polysaccharides from Helianthus annuus (Sunflower) Receptacles" Molecules 30, no. 14: 2943. https://doi.org/10.3390/molecules30142943
APA StyleChen, X., Wei, Z., Mo, X., Lu, Y., Pan, G., Pan, Z., Li, Y., Tian, H., & Pan, X. (2025). Structural Characterization and Anti-Ultraviolet Radiation Damage Activity of Polysaccharides from Helianthus annuus (Sunflower) Receptacles. Molecules, 30(14), 2943. https://doi.org/10.3390/molecules30142943






