IrO2-Decorated Titania Nanotubes as Oxygen Evolution Anodes
Abstract
1. Introduction
2. Results
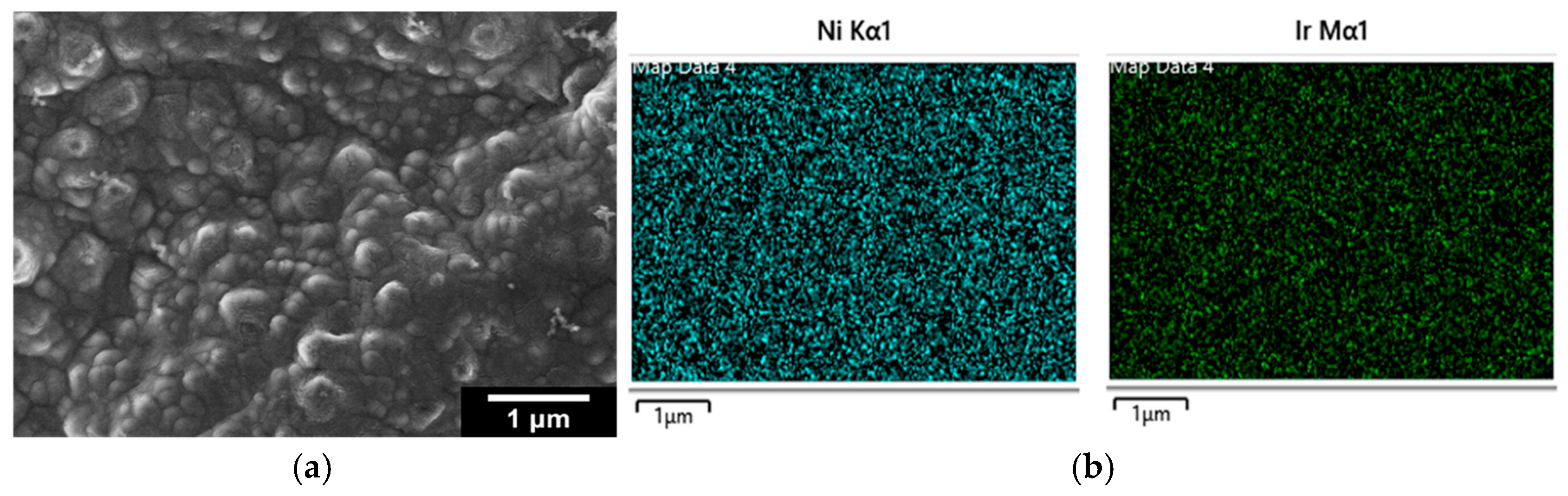
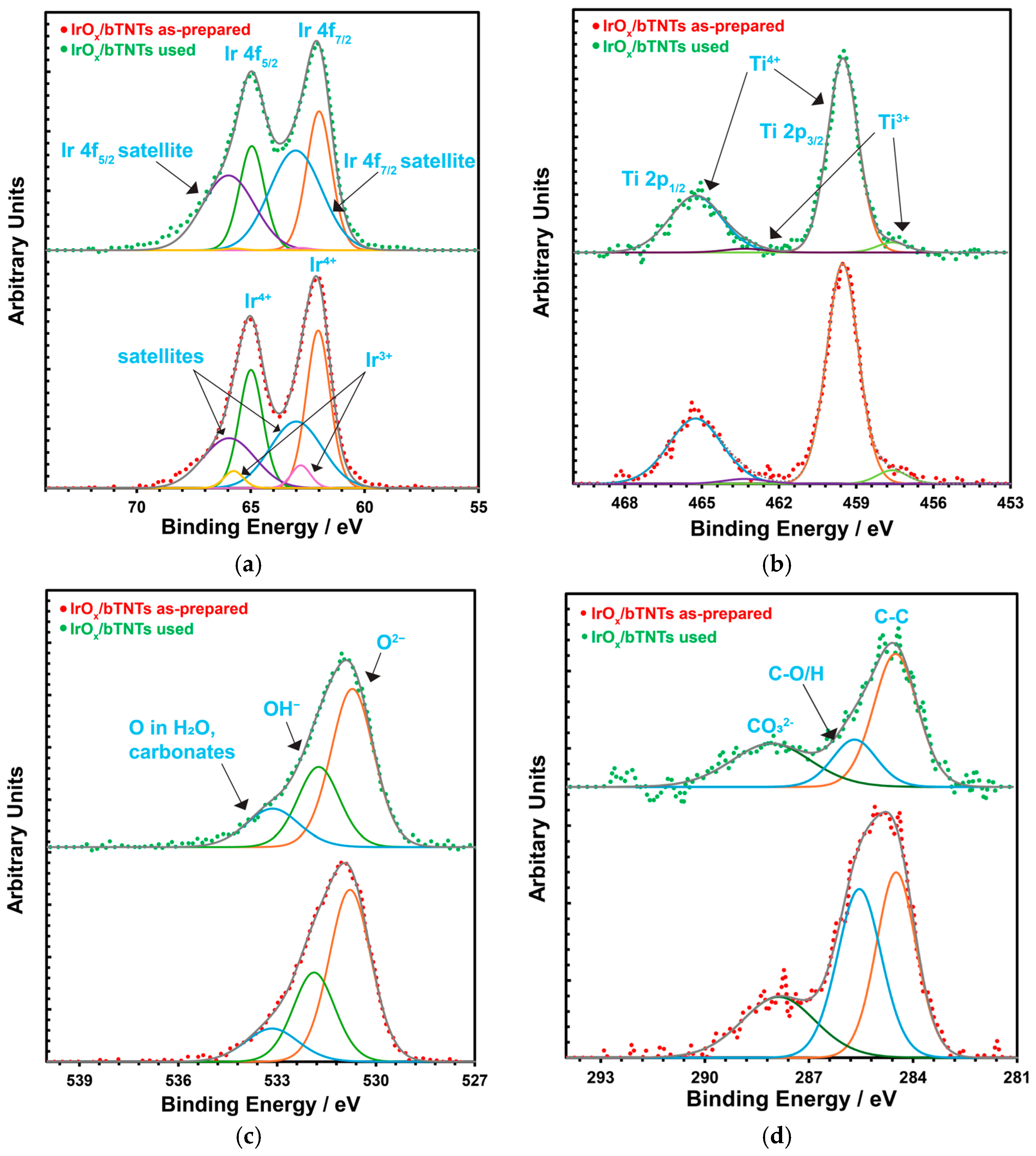
2.1. Microscopic (SEM) and Spectroscopic (EDS, ICP-MS, XPS) Analysis
2.2. Electrochemical Characterization
2.2.1. Surface Electrochemistry of the Catalysts
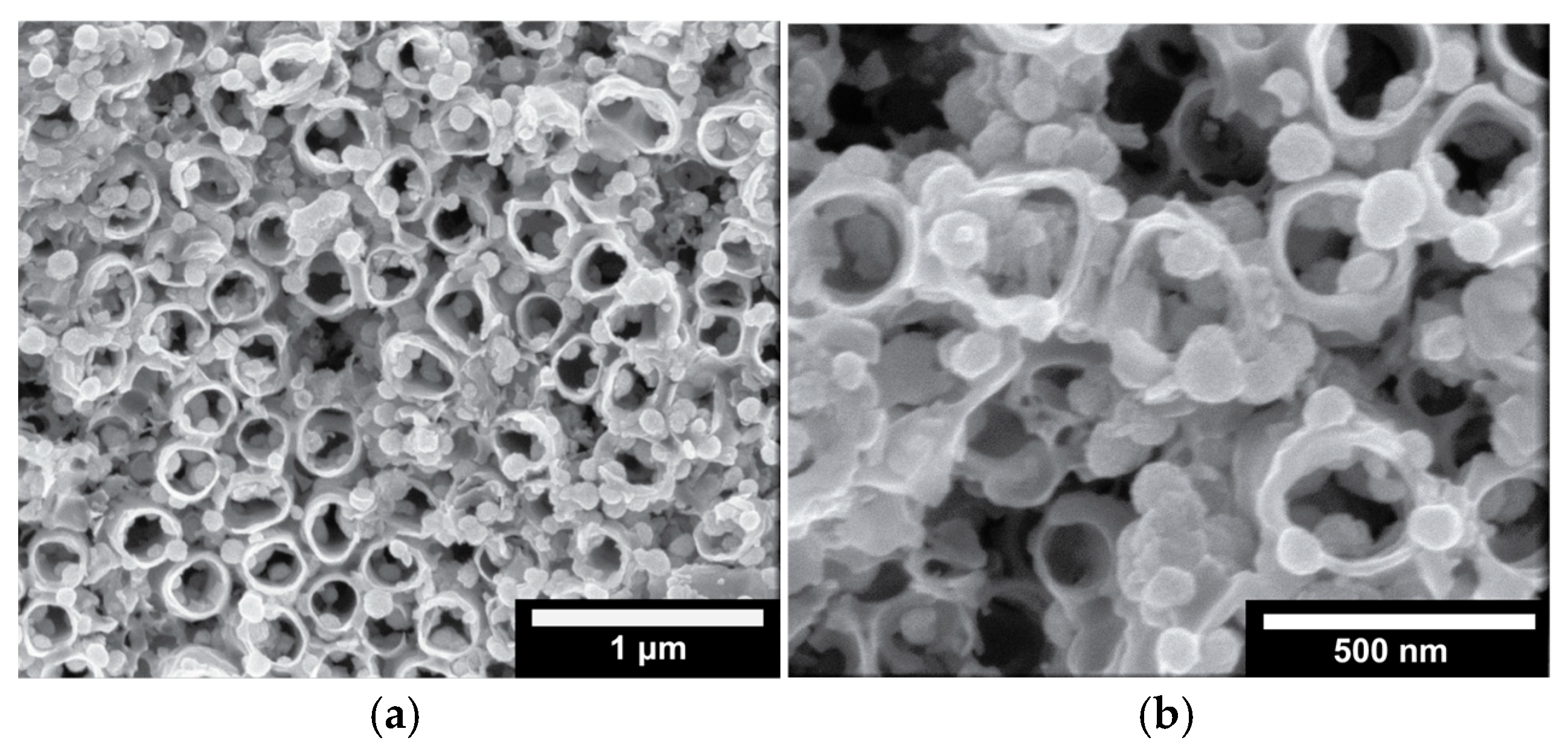
- 0.0 VRHE to +0.6 VRHE, corresponding to the potential range between the onset of hydrogen evolution and the end of Ir double layer potential region [76,77], for the electrochemical dissolution of any surface uncovered/unreacted Ni and the formation of an Ir(Ni) mixed core–skin structure [65] in the samples that contain Ni, until only the peaks attributed to the adsorption and desorption of an under-potentially deposited hydrogen (UPD-H) layer on metallic Ir could be clearly recorded, as depicted indicatively in Figure 4 for the case of open-structure Ir/bTNTs. The electrochemical surface area (ECSA) of metallic Ir is related to the charge associated with the adsorption of a H monolayer on Ir; the former could be calculated from the voltammograms of Figure 4 (by integration of the anodic/H-desorption peak between −0.05 and 0.30 V) as 21.8, 49.4, and 239 cm2Ir cm−2 for the close-packed Ir(Ni)/TNTs, the close-packed Ir(Ni)/bTNTs, and the open-structure Ir/bTNTs, respectively [15,64,78], in line with the increase in IrO2 coating roughness/particle dispersion depicted in Figure 2, Figure 1c, and Figure 1d, respectively, that correspond to these three different electrode types.
- 0.0 VRHE to +1.5 VRHE, for the electrochemical anodization of metallic Ir to different oxidation states (IV, V) in order to form stable, porous 3D-IrOx, which also extends to the interior of the material [79,80,81]. In Figure 5, the electrochemistry due to anodically generated IrOx appears above +1.0 VRHE [67,82,83]. From the CVs, the charge corresponding to Ir oxides, which is representative of the electroactive surface area available for OER [84,85,86], was calculated (by integration of the anodic/IrOx formation peaks between 0.30 and 1.40 V) as 16.6, 26, and 80 mC cm−2 for the close-packed IrOx(Ni)/TNT and IrOx(Ni)/bTNTs, as well as the open-structure IrOx/bTNTs, respectively (again, in line with coating roughness/particle dispersion shown in the SEM micrographs of Figure 1 and Figure 2). Taking into account the mass loading of Ir (see Section 2.1 above), the mass specific electroactive area/oxide charge of the IrOx/bTNT electrode can thus be estimated as 267 C gIr−1, a value that compares favorably with those of commercial IrO2 powder electrodes (100–200 C gIr−1 [19,85]) and IrO2 supported on TiO2 powder electrodes (54–125 C gIr−1 [19]).
2.2.2. Oxygen Evolution Reaction
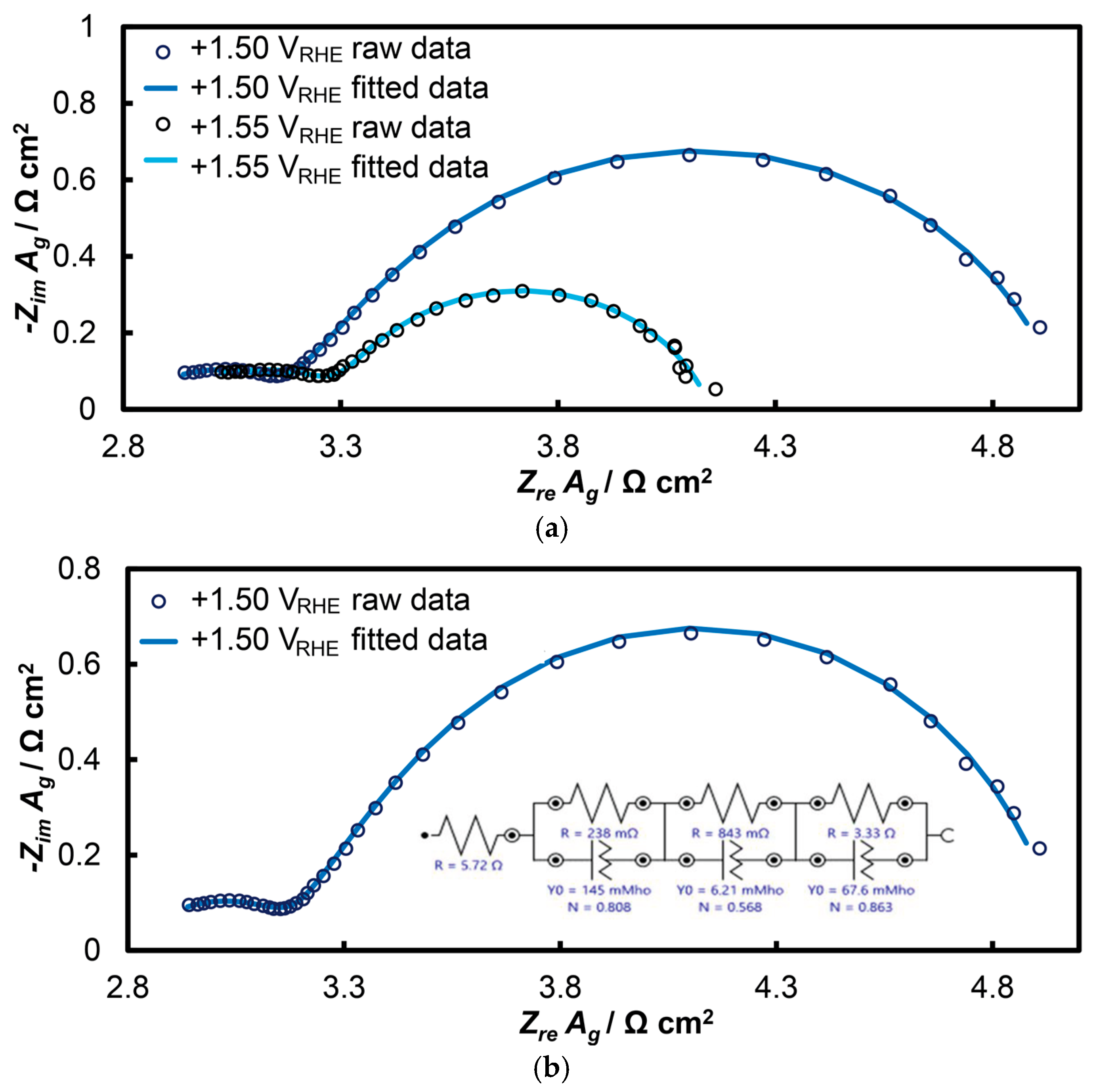
2.2.3. Electrochemical Impedance Spectroscopy (EIS)
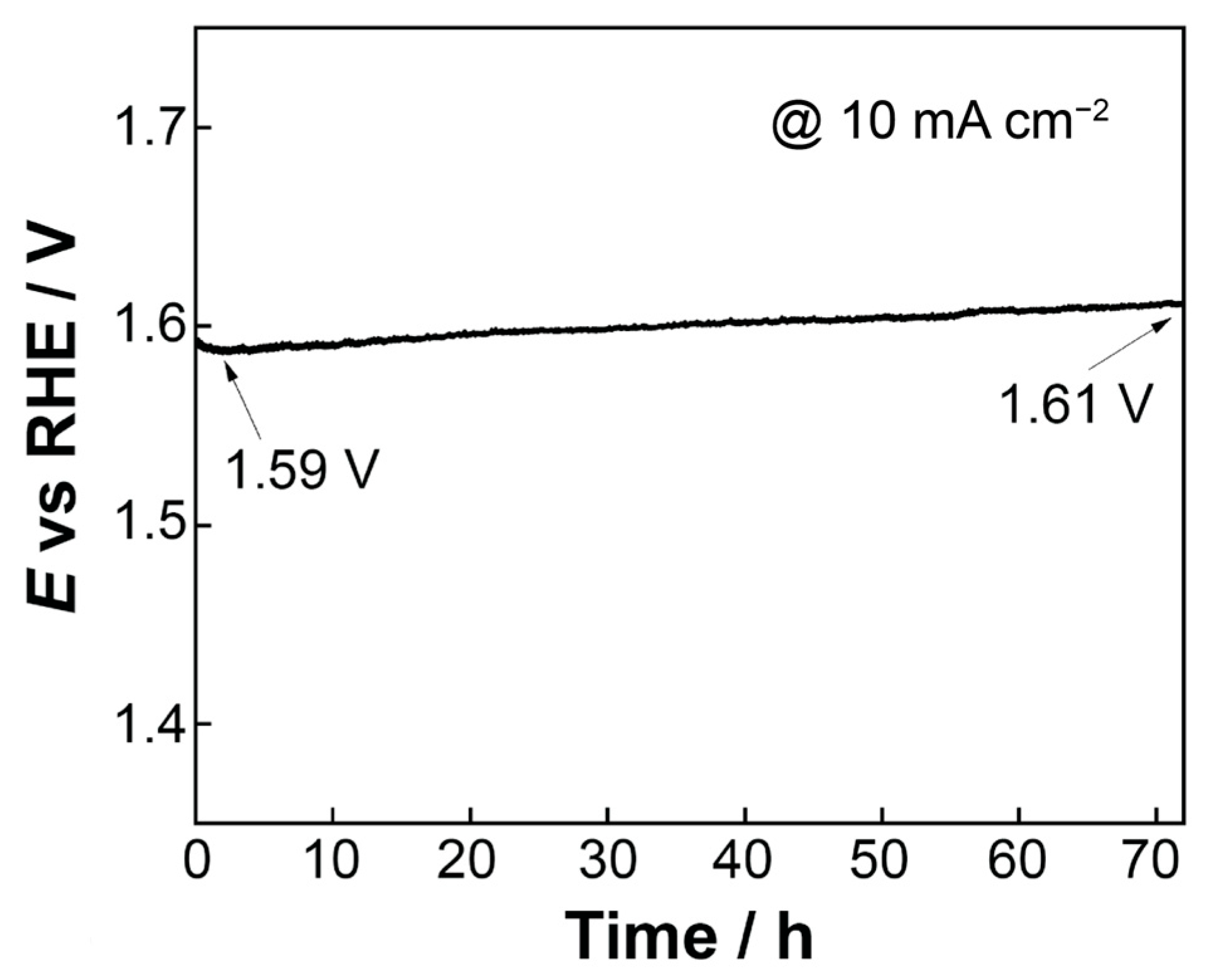
2.2.4. Stability Testing
3. Discussion
3.1. Catalytic Electrode Morphology
3.2. Catalytic Electrode Performance
4. Materials and Methods
4.1. Preparation of IrO2 Catalysts Supported on TNTs and bTNTs
4.1.1. Preparation of Open-Structure and Close-Packed TNTs and bTNTs Substates
4.1.2. Preparation of IrOx(Ni)/TNTs and bTNTs
4.2. Electrochemical Setup and Procedures
4.3. Microscopic and Spectroscopic Characterization
5. Conclusions
- Electrodeposition of sacrificial Ni on conducting bTNTs and its subsequent galvanic replacement by Ir resulted (depending on substrate type) in Ir particles (<100 nm) for open-structure bTNTs or in larger aggregates for close-packed, bTNTs; in the case of open-structure bTNTs, these particles were highly dispersed (some residing inside the nanotubes), thus increasing the electroactive area while at the same time retaining an open electrode structure.
- For the semiconducting TNTs, an increase in the charge of electrodeposited Ni was necessary to rectify their original low electrical conductivity and resulting in the filling of the nanotubes for Ni deposits to act also as a current collector. The eventual formation of a continuous Ir(Ni) film on the surface following galvanic replacement, resulted in a significant decrease in the electroactive surface area.
- The open-structure IrOx(Ni)/bTNTs (more precisely, IrOx/bTNTs since no Ni has been detected after the galvanic replacement/metal exhange process), exhibited an enhanced activity towards the OER. This can be attributed to the higher surface area of the support, higher Ir dispersion and catalytic activity (due to IrO2-bTNT interactions) as well as less pore clogging during O2 evolution. An overpotential of η = 240 mV at 10 mA cm−2 and a mass-specific current density of 258 mA mgIr−1 at η = 300 mV has been recorded, rendering them comparable or better than similar electrodes reported in the literature (in the 30–140 mA mgIr−1 range at η = 300 mV and in the η = 240–360 mV range at 10 mA cm−2 [54,58,92,93]). Furthermore, the optimized electrodes, when tested for prolonged periods of time under OER conditions, were characterized by good short-term (72 h) stability.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNTs | Titania Nanotubes |
| bTNTS | Titania Black Nanotubes |
| CV | Cyclic Voltammogram/Voltammetry |
| LSV | Linear Sweep Voltammogram/Voltammetry |
| EIS | Electrochemical Impedance Spectroscopy |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| SEM | Scanning Electron Microscopy |
| EDS | Energy Dispersive Spectroscopy |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Duby, P. The History of Progress in Dimensionally Stable Anodes. JOM 1993, 45, 41–43. [Google Scholar] [CrossRef]
- Pavlović, M.G.; Dekanski, A. On the Use of Platinized and Activated Titanium Anodes in Some Electrodeposition Processes. J. Solid. State Electrochem. 1997, 1, 208–214. [Google Scholar] [CrossRef]
- Zhang, W.; Ghali, E.; Houlachi, G. Review of Oxide Coated Catalytic Titanium Anodes Performance for Metal Electrowinning. Hydrometallurgy 2017, 169, 456–467. [Google Scholar] [CrossRef]
- Choi, H.; Kim, O.H.; Kim, M.; Choe, H.; Cho, Y.H.; Sung, Y.E. Next-Generation Polymer-Electrolyte-Membrane Fuel Cells Using Titanium Foam as Gas Diffusion Layer. ACS Appl. Mater. Interfaces 2014, 6, 7665–7671. [Google Scholar] [CrossRef] [PubMed]
- Yasutake, M.; Kawachino, D.; Noda, Z.; Matsuda, J.; Lyth, S.M.; Ito, K.; Hayashi, A.; Sasaki, K. Catalyst-Integrated Gas Diffusion Electrodes for Polymer Electrolyte Membrane Water Electrolysis: Porous Titanium Sheets with Nanostructured TiO2 Surfaces Decorated with Ir Electrocatalysts. J. Electrochem. Soc. 2020, 167, 124523. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating Metal–Organic Frameworks for Catalyzing Acidic Oxygen Evolution for Proton Exchange Membrane Water Electrolysis. SusMat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Jung, W.C.; Zhou, W.; Shao, Z. Electrochemical Water Splitting: Bridging the Gaps Between Fundamental Research and Industrial Applications. Energy Environ. Mater. 2023, 6, e12441. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.-P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and Hydrogen Evolution Reactions on Ru, RuO2, Ir, and IrO2 Thin Film Electrodes in Acidic and Alkaline Electrolytes: A Comparative Study on Activity and Stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Alia, S.M.; Rasimick, B.; Ngo, C.; Neyerlin, K.C.; Kocha, S.S.; Pylypenko, S.; Xu, H.; Pivovar, B.S. Activity and Durability of Iridium Nanoparticles in the Oxygen Evolution Reaction. J. Electrochem. Soc. 2016, 163, F3105–F3112. [Google Scholar] [CrossRef]
- Liang, Q.; Brocks, G.; Bieberle-Hütter, A. Oxygen Evolution Reaction (OER) Mechanism under Alkaline and Acidic Conditions. J. Physics Energy 2021, 3, 026001. [Google Scholar] [CrossRef]
- Ledendecker, M.; Geiger, S.; Hengge, K.; Lim, J.; Cherevko, S.; Mingers, A.M.; Göhl, D.; Fortunato, G.V.; Jalalpoor, D.; Schüth, F.; et al. Towards Maximized Utilization of Iridium for the Acidic Oxygen Evolution Reaction. Nano Res. 2019, 12, 2275–2280. [Google Scholar] [CrossRef]
- Oh, H.S.; Nong, H.N.; Reier, T.; Bergmann, A.; Gliech, M.; Ferreira De Araújo, J.; Willinger, E.; Schlögl, R.; Teschner, D.; Strasser, P. Electrochemical Catalyst-Support Effects and Their Stabilizing Role for IrOx Nanoparticle Catalysts during the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2016, 138, 12552–12563. [Google Scholar] [CrossRef]
- Reier, T.; Teschner, D.; Lunkenbein, T.; Bergmann, A.; Selve, S.; Kraehnert, R.; Schlögl, R.; Strasser, P. Electrocatalytic Oxygen Evolution on Iridium Oxide: Uncovering Catalyst-Substrate Interactions and Active Iridium Oxide Species. J. Electrochem. Soc. 2014, 161, F876–F882. [Google Scholar] [CrossRef]
- Yu, S.; Xie, Z.; Li, K.; Ding, L.; Wang, W.; Yang, G.; Zhang, F.Y. Morphology Engineering of Iridium Electrodes via Modifying Titanium Substrates with Controllable Pillar Structures for Highly Efficient Oxygen Evolution Reaction. Electrochim. Acta 2022, 405, 139797. [Google Scholar] [CrossRef]
- Touni, A.; Grammenos, O.A.; Banti, A.; Karfaridis, D.; Prochaska, C.; Lambropoulou, D.; Pavlidou, E.; Sotiropoulos, S. Iridium Oxide-Nickel-Coated Titanium Anodes for the Oxygen Evolution Reaction. Electrochim. Acta 2021, 390, 138866. [Google Scholar] [CrossRef]
- Kariman, A.; Marshall, A.T. Improving the Stability of DSA Electrodes by the Addition of TiO2 Nanoparticles. J. Electrochem. Soc. 2019, 166, E248–E251. [Google Scholar] [CrossRef]
- Oakton, E.; Lebedev, D.; Povia, M.; Abbott, D.F.; Fabbri, E.; Fedorov, A.; Nachtegaal, M.; Copéret, C.; Schmidt, T.J. IrO2-TiO2: A High-Surface-Area, Active, and Stable Electrocatalyst for the Oxygen Evolution Reaction. ACS Catal. 2017, 7, 2346–2352. [Google Scholar] [CrossRef]
- Reier, T.; Weidinger, I.; Hildebrandt, P.; Kraehnert, R.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction on Iridium Oxide Model Film Catalysts: Influence of Oxide Type and Catalyst Substrate Interactions. ECS Trans. 2013, 58, 39–51. [Google Scholar] [CrossRef]
- Banti, A.; Zafeiridou, C.; Charalampakis, M.; Spyridou, O.N.; Georgieva, J.S.; Binas, V.D.; Mitrousi, E.; Sotiropoulos, S. IrO2 Oxygen Evolution Catalysts Prepared by an Optimized Photodeposition Process on TiO2 Substrates. Molecules 2024, 29, 2392. [Google Scholar] [CrossRef]
- Bagheri, S.; Muhd Julkapli, N.; Bee Abd Hamid, S. Titanium Dioxide as a Catalyst Support in Heterogeneous Catalysis. Sci. World J. 2014, 2014, 727496. [Google Scholar] [CrossRef]
- Hamad, S.; Catlow, C.R.A.; Woodley, S.M.; Lago, S.; Mejías, J.A. Structure and Stability of Small TiO2 Nanoparticles. J. Phys. Chem. B 2005, 109, 15741–15748. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Liu, X.; Gu, F.; Jiang, H.; Shao, W.; Zhang, L.; He, Y. Synthesis and Optical Properties of TiO2 Nanoparticles. Mater. Lett. 2007, 61, 79–83. [Google Scholar] [CrossRef]
- Viana, M.M.; Soares, V.F.; Mohallem, N.D.S. Synthesis and Characterization of TiO2 Nanoparticles. Ceram. Int. 2010, 36, 2047–2053. [Google Scholar] [CrossRef]
- Jitputti, J.; Suzuki, Y.; Yoshikawa, S. Synthesis of TiO2 Nanowires and Their Photocatalytic Activity for Hydrogen Evolution. Catal. Commun. 2008, 9, 1265–1271. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-Treated TiO2 Nanowire Arrays for Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Chuangchote, S.; Jitputti, J.; Sagawa, T.; Yoshikawa, S. Photocatalytic Activity for Hydrogen Evolution of Electrospun TiO2 Nanofibers. ACS Appl. Mater. Interfaces 2009, 1, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Guan, R.; Xie, M.; Dong, P.; Yang, X.; Zhang, J. Advances in Electrospun TiO2 Nanofibers: Design, Construction, and Applications. Chem. Eng. J. 2022, 431, 134343. [Google Scholar] [CrossRef]
- Chen, F.; Fang, P.; Liu, Z.; Gao, Y.; Liu, Y.; Dai, Y.; Luo, H.; Feng, J. Dimensionality-Dependent Photocatalytic Activity of TiO2-Based Nanostructures: Nanosheets with a Superior Catalytic Property. J. Mater. Sci. 2013, 48, 5171–5179. [Google Scholar] [CrossRef]
- Yu, J.; Fan, J.; Lv, K. Anatase TiO2 Nanosheets with Exposed (001) Facets: Improved Photoelectric Conversion Efficiency in Dye-Sensitized Solar Cells. Nanoscale 2010, 2, 2144–2149. [Google Scholar] [CrossRef]
- Fleischer, C.; Chatzitakis, A.; Norby, T. Intrinsic Photoelectrocatalytic Activity in Oriented, Photonic TiO2 Nanotubes. Mater. Sci. Semicond. Process. 2018, 88, 186–191. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 Nanotubes: Self-Organized Electrochemical Formation, Properties and Applications. Curr. Opin. Solid. State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A Review on Highly Ordered, Vertically Oriented TiO2 Nanotube Arrays: Fabrication, Material Properties, and Solar Energy Applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Smith, Y.R.; Ray, R.S.; Carlson, K.; Sarma, B.; Misra, M. Self-Ordered Titanium Dioxide Nanotube Arrays: Anodic Synthesis and Their Photo/Electro-Catalytic Applications. Materials 2013, 6, 2892–2957. [Google Scholar] [CrossRef]
- Tighineanu, A.; Albu, S.P.; Schmuki, P. Conductivity of Anodic TiO2 Nanotubes: Influence of Annealing Conditions. Phys. Status Solidi-Rapid Res. Lett. 2014, 8, 158–162. [Google Scholar] [CrossRef]
- Nah, Y.C.; Paramasivam, I.; Schmuki, P. Doped TiO2 and TiO2 Nanotubes: Synthesis and Applications. ChemPhysChem 2010, 11, 2698–2713. [Google Scholar] [CrossRef]
- Lu, X.; Wang, G.; Zhai, T.; Yu, M.; Gan, J.; Tong, Y.; Li, Y. Hydrogenated TiO2 Nanotube Arrays for Supercapacitors. Nano Lett. 2012, 12, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of Nature and Location of Defects on Bandgap Narrowing in Black TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef]
- Li, S.; Qiu, J.; Ling, M.; Peng, F.; Wood, B.; Zhang, S. Photoelectrochemical Characterization of Hydrogenated TiO2 Nanotubes as Photoanodes for Sensing Applications. ACS Appl. Mater. Interfaces 2013, 5, 11129–11135. [Google Scholar] [CrossRef]
- Malik, H.; Barrera, K.; Mohanty, S.; Carlson, K. Enhancing Electrochemical Properties of TiO2 Nanotubes via Engineered Defect Laden Crystal Structures. Mater. Lett. 2020, 273, 127956. [Google Scholar] [CrossRef]
- Wu, H.; Xu, C.; Xu, J.; Lu, L.; Fan, Z.; Chen, X.; Song, Y.; Li, D. Enhanced Supercapacitance in Anodic TiO2 Nanotube Films by Hydrogen Plasma Treatment. Nanotechnology 2013, 24, 455401. [Google Scholar] [CrossRef] [PubMed]
- Siuzdak, K.; Szkoda, M.; Lisowska-Oleksiak, A.; Karczewski, J.; Ryl, J. Highly Stable Organic-Inorganic Junction Composed of Hydrogenated Titania Nanotubes Infiltrated by a Conducting Polymer. RSC Adv. 2016, 6, 33101–33110. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Xia, Z.; Xing, J. Microwave-Assisted Preparation of Self-Doped TiO2 Nanotube Arrays for Enhanced Photoelectrochemical Water Splitting. J. Mater. Chem. A Mater. 2015, 3, 699–705. [Google Scholar] [CrossRef]
- Andronic, L.; Enesca, A. Black TiO2 Synthesis by Chemical Reduction Methods for Photocatalysis Applications. Front Chem 2020, 8, 565489. [Google Scholar] [CrossRef]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 Nanotube Arrays for Photoelectrochemical Water Splitting. J. Mater. Chem. A Mater. 2013, 1, 5766–5774. [Google Scholar] [CrossRef]
- Liu, X.; Carvalho, P.; Getz, M.N.; Norby, T.; Chatzitakis, A. Black Anatase TiO2 Nanotubes with Tunable Orientation for High Performance Supercapacitors. J. Phys. Chem. C 2019, 123, 21931–21940. [Google Scholar] [CrossRef]
- Touni, A.; Liu, X.; Kang, X.; Papoulia, C.; Pavlidou, E.; Lambropoulou, D.; Tsampas, M.N.; Chatzitakis, A.; Sotiropoulos, S. Methanol Oxidation at Platinum Coated Black Titania Nanotubes and Titanium Felt Electrodes. Molecules 2022, 27, 6382. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Song, Y.; Lu, L.; Cheng, C.; Liu, D.; Fang, X.; Chen, X.; Zhu, X.; Li, D. Electrochemically Hydrogenated TiO2 Nanotubes with Improved Photoelectrochemical Water Splitting Performance. Nanoscale Res. Lett. 2013, 8, 391. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Barea, E.M.; Bisquert, J.; Mor, G.K.; Shankar, K.; Grimes, C.A. High Carrier Density and Capacitance in TiO2 Nanotube Arrays Induced by Electrochemical Doping. J. Am. Chem. Soc. 2008, 130, 11312–11316. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y. Electrochemically Self-Doped TiO2 Nanotube Arrays for Supercapacitors. J. Phys. Chem. C 2014, 118, 5626–5636. [Google Scholar] [CrossRef]
- Zhang, Z.; Hedhili, M.N.; Zhu, H.; Wang, P. Electrochemical Reduction Induced Self-Doping of Ti3+ for Efficient Water Splitting Performance on TiO2 Based Photoelectrodes. Phys. Chem. Chem. Phys. 2013, 15, 15637–15644. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, H.B.; Zhiani, M.A. Novel Ir–Ru-Based Nanoparticle Supported on Ordered Electrochemically Synthesized TiO2-Nanotube as a Highly Active and Stable Oxygen Evolution Reaction Catalyst for Water Splitting in Acidic Media. Int. J. Hydrogen Energy 2023, 48, 33042–33061. [Google Scholar] [CrossRef]
- Genova-Koleva, R.V.; Alcaide, F.; Álvarez, G.; Cabot, P.L.; Grande, H.J.; Martínez-Huerta, M.V.; Miguel, O. Supporting IrO2 and IrRuOx Nanoparticles on TiO2 and Nb-Doped TiO2 Nanotubes as Electrocatalysts for the Oxygen Evolution Reaction. J. Energy Chem. 2019, 34, 227–239. [Google Scholar] [CrossRef]
- Lu, Z.X.; Shi, Y.; Yan, C.F.; Guo, C.Q.; Wang, Z. Da Investigation on IrO2 Supported on Hydrogenated TiO2 Nanotube Array as OER Electro-Catalyst for Water Electrolysis. Int. J. Hydrogen Energy 2017, 42, 3572–3578. [Google Scholar] [CrossRef]
- Bele, M.; Jovanovič, P.; Marinko, Ž.; Drev, S.; Simon Šelih, V.; Kovač, J.; Gaberšček, M.; Koderman Podboršek, G.; Dražić, G.; Hodnik, N.; et al. Increasing the Oxygen Evolution Reaction Performance of Nanotubular Titanium Oxynitride Supported Ir Nanoparticles by Strong Metal-Support Interaction. ACS Catal. 2020, 10, 13688–13700. [Google Scholar] [CrossRef]
- Koderman Podborsek, G.; Suhadolnik, L.; Loncar, A.; Bele, M.; Hrnjic, A.; Marinko, Z.; Kovac, J.; Kokalj, A.; Gasparic, L.; Surca, A.K.; et al. Iridium Stabilizes Ceramic Titanium Oxynitride Support for Oxygen Evolution Reaction. ACS Catal. 2022, 12, 15135–15145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hwang, I.; Tomanec, O.; Fehn, D.; Mazare, A.; Zboril, R.; Meyer, K.; Schmuki, P. Advanced Photocatalysts: Pinning Single Atom Co-Catalysts on Titania Nanotubes. Adv. Funct. Mater. 2021, 31, 2102843. [Google Scholar] [CrossRef]
- Schlicht, S.; Büttner, P.; Bachmann, J. Highly Active Ir/TiO2 Electrodes for the Oxygen Evolution Reaction Using Atomic Layer Deposition on Ordered Porous Substrates. ACS Appl. Energy Mater. 2019, 2, 2344–2349. [Google Scholar] [CrossRef]
- Lačnjevac, U.; Vasilić, R.; Dobrota, A.; Đurđić, S.; Tomanec, O.; Zbořil, R.; Mohajernia, S.; Nguyen, N.T.; Skorodumova, N.; Manojlović, D.; et al. High-Performance Hydrogen Evolution Electrocatalysis Using Proton-Intercalated TiO2 nanotube Arrays as Interactive Supports for Ir Nanoparticles. J. Mater. Chem. A Mater. 2020, 8, 22773–22790. [Google Scholar] [CrossRef]
- Touni, A.; Liu, X.; Kang, X.; Carvalho, P.A.; Diplas, S.; Both, K.G.; Sotiropoulos, S.; Chatzitakis, A. Galvanic Deposition of Pt Nanoparticles on Black TiO2 Nanotubes for Hydrogen Evolving Cathodes. ChemSusChem 2021, 14, 4993–5003. [Google Scholar] [CrossRef]
- Hou, X.; Li, Y.; Zhang, H.; Lund, P.D.; Kwan, J.; Tsang, S.C.E. Black Titanium Oxide: Synthesis, Modification, Characterization, Physiochemical Properties, and Emerging Applications for Energy Conversion and Storage, and Environmental Sustainability. Chem. Soc. Rev. 2024, 53, 10660–10708. [Google Scholar] [CrossRef] [PubMed]
- Papaderakis, A.; Mintsouli, I.; Georgieva, J.; Sotiropoulos, S. Electrocatalysts Prepared by Galvanic Replacement. Catalysts 2017, 7, 80. [Google Scholar] [CrossRef]
- Touni, A.; Papaderakis, A.; Karfaridis, D.; Vourlias, G.; Sotiropoulos, S. Oxygen Evolution Reaction at IrO2/Ir(Ni) Film Electrodes Prepared by Galvanic Replacement and Anodization: Effect of Precursor Ni Film Thickness. Molecules 2019, 24, 2095. [Google Scholar] [CrossRef]
- Papaderakis, A.; Pliatsikas, N.; Patsalas, P.; Tsiplakides, D.; Balomenou, S.; Touni, A.; Sotiropoulos, S. Hydrogen Evolution at Ir-Ni Bimetallic Deposits Prepared by Galvanic Replacement. J. Electroanal. Chem. 2018, 808, 21–27. [Google Scholar] [CrossRef]
- Papaderakis, A.; Pliatsikas, N.; Prochaska, C.; Vourlias, G.; Patsalas, P.; Tsiplakides, D.; Balomenou, S.; Sotiropoulos, S. Oxygen Evolution at IrO2 Shell-Ir-Ni Core Electrodes Prepared by Galvanic Replacement. J. Phys. Chem. C 2016, 120, 19995–20005. [Google Scholar] [CrossRef]
- Papaderakis, A.; Pliatsikas, N.; Prochaska, C.; Papazisi, K.M.; Balomenou, S.P.; Tsiplakides, D.; Patsalas, P.; Sotiropoulos, S. Ternary Pt-Ru-Ni Catalytic Layers for Methanol Electrooxidation Prepared by Electrodeposition and Galvanic Replacement. Front. Chem. 2014, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Papaderakis, A.; Matouli, I.; Spyridou, O.N.; Grammenos, A.O.; Banti, A.; Touni, A.; Pliatsikas, N.; Patsalas, P.; Sotiropoulos, S. Ternary IrO2-Pt-Ni Deposits Prepared by Galvanic Replacement as Bifunctional Oxygen Catalysts. J. Electroanal. Chem. 2020, 877, 114499. [Google Scholar] [CrossRef]
- Getz, M.N.; Chatzitakis, A.; Liu, X.; Carvalho, P.A.; Bjørheim, T.S.; Norby, T. Voids in Walls of Mesoporous TiO2 Anatase Nanotubes by Controlled Formation and Annihilation of Protonated Titanium Vacancies. Mater. Chem. Phys. 2020, 239, 121953. [Google Scholar] [CrossRef]
- Yan, S.; Chen, Y.; Wang, Z.; Han, A.; Shan, Z.; Yang, X.; Zhu, X. Essential Distinction between One-Step Anodization and Two-Step Anodization of Ti. Mater. Res. Bull. 2017, 95, 444–450. [Google Scholar] [CrossRef]
- Zeng, H.; Li, C.; Dan, Y.; Lu, Y.; Sun, W.; Zhang, S.; Song, Y. A Comparative Study of Two-Step Anodization with One-Step Anodization at Constant Voltage. Nanotechnology 2023, 34, 065603. [Google Scholar] [CrossRef]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 Nanotubes, Nanochannels and Mesosponge: Self-Organized Formation and Applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Valota, A.; LeClere, D.J.; Skeldon, P.; Curioni, M.; Hashimoto, T.; Berger, S.; Kunze, J.; Schmuki, P.; Thompson, G.E. Influence of Water Content on Nanotubular Anodic Titania Formed in Fluoride/Glycerol Electrolytes. Electrochim. Acta 2009, 54, 4321–4327. [Google Scholar] [CrossRef]
- Chatzitakis, A.; Papaderakis, A.; Karanasios, N.; Georgieva, J.; Pavlidou, E.; Litsardakis, G.; Poulios, I.; Sotiropoulos, S. Comparison of the Photoelectrochemical Performance of Particulate and Nanotube TiO2 Photoanodes. Catal. Today 2017, 280, 14–20. [Google Scholar] [CrossRef]
- Pfeifer, V.; Jones, T.E.; Velasco Vélez, J.J.; Massué, C.; Arrigo, R.; Teschner, D.; Girgsdies, F.; Scherzer, M.; Greiner, M.T.; Allan, J.; et al. The Electronic Structure of Iridium and Its Oxides. Surf. Interface Anal. 2016, 48, 261–273. [Google Scholar] [CrossRef]
- Freakley, S.J.; Ruiz-Esquius, J.; Morgan, D.J. The X-Ray Photoelectron Spectra of Ir, IrO2 and IrCl3 Revisited. Surf. Interface Anal. 2017, 49, 794–799. [Google Scholar] [CrossRef]
- Tegou, A.; Armyanov, S.; Valova, E.; Steenhaut, O.; Hubin, A.; Kokkinidis, G.; Sotiropoulos, S. Mixed Platinum-Gold Electrocatalysts for Borohydride Oxidation Prepared by the Galvanic Replacement of Nickel Deposits. J. Electroanal. Chem. 2009, 634, 104–110. [Google Scholar] [CrossRef]
- Mozota, J.; Conway, B.E. Modification of Apparent Electrocatalysis for Anodic Chlorine Evolution on Electrochemically Conditioned Oxide Films at Iridium Anodes. J. Electrochem. Soc. 1981, 128, 2142–2149. [Google Scholar] [CrossRef]
- Mozota, J.; Conway, B.E. Surface and Bulk Processes at Oxidized Iridium Electrodes—I. Monolayer Stage and Transition to Reversible Multilayer Oxide Film Behaviour. Electrochim. Acta 1983, 28, 1–8. [Google Scholar] [CrossRef]
- Birss, V.; Myers, R.; Angerstein-Kozlowska, H.; Conway, B.E. Electron Microscopy Study of Formation of Thick Oxide Films on Ir and Ru Electrodes. J. Electrochem. Soc. 1984, 131, 1502–1510. [Google Scholar] [CrossRef]
- Michell, D.; Rand, D.A.J.; Woods, R. Analysis of the Anodic Oxygen Layer on Iridium by X-Ray Emission, Electron Diffraction and Electron Microscopy. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 117–126. [Google Scholar] [CrossRef]
- Conway, B.E.; Mozota, J. Surface and Bulk Processes at Oxidized Iridium Electrodes—II. Conductivity-Switched Behaviour of Thick Oxide Films. Electrochim. Acta 1983, 28, 9–16. [Google Scholar] [CrossRef]
- Juodkazyte, J.; Šebeka, B.; Valsiunas, I.; Juodkazis, K. Iridium Anodic Oxidation to Ir(III) and Ir(IV) Hydrous Oxides. Electroanalysis 2005, 17, 947–952. [Google Scholar] [CrossRef]
- Kötz, R.; Neff, H.; Stucki, S. Anodic Iridium Oxide Films: XPS-Studies of Oxidation State Changes and O2-Evolution. J. Electrochem. Soc. 1984, 131, 72–77. [Google Scholar] [CrossRef]
- Spöri, C.; Briois, P.; Nong, H.N.; Reier, T.; Billard, A.; Kühl, S.; Teschner, D.; Strasser, P. Experimental Activity Descriptors for Iridium-Based Catalysts for the Electrochemical Oxygen Evolution Reaction (OER). ACS Catal. 2019, 9, 6653–6663. [Google Scholar] [CrossRef]
- Papaderakis, A.; Tsiplakides, D.; Balomenou, S.; Sotiropoulos, S. Electrochemical Impedance Studies of IrO2 Catalysts for Oxygen Evolution. J. Electroanal. Chem. 2015, 757, 216–224. [Google Scholar] [CrossRef]
- Reier, T.; Pawolek, Z.; Cherevko, S.; Bruns, M.; Jones, T.; Teschner, D.; Selve, S.; Bergmann, A.; Nong, H.N.; Schlögl, R.; et al. Molecular Insight in Structure and Activity of Highly Efficient, Low-Ir Ir-Ni Oxide Catalysts for Electrochemical Water Splitting (OER). J. Am. Chem. Soc. 2015, 137, 13031–13040. [Google Scholar] [CrossRef]
- Hu, J.M.; Zhang, J.Q.; Cao, C.N. Oxygen Evolution Reaction on IrO2-Based DSA® Type Electrodes: Kinetics Analysis of Tafel Lines and EIS. Int. J. Hydrogen Energy 2004, 29, 791–797. [Google Scholar] [CrossRef]
- De Oliveira-Sousa, A.; Da Silva, M.A.S.; Machado, S.A.S.; Avaca, L.A.; De Lima-Neto, P. Influence of the Preparation Method on the Morphological and Electrochemical Properties of Ti/IrO2-Coated Electrodes. Electrochim. Acta 2000, 45, 4467–4473. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical Note: Concerning the Conversion of the Constant Phase Element Parameter Y 0 into a Capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy─A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Lončar, A.; Escalera-López, D.; Cherevko, S.; Hodnik, N. Inter-Relationships between Oxygen Evolution and Iridium Dissolution Mechanisms. Angew. Chem.-Int. Ed. 2022, 61, e202114437. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhang, L.; Chen, M. Characteristics of Anodic TiO2 Nanotube Arrays Mediated IrO2 Active Anode in the Oxygen Evolution Reaction. Int. J. Electrochem. Sci. 2022, 17, 220461. [Google Scholar] [CrossRef]
- Shi, Y.; Lu, Z.; Guo, L.; Wang, Z.; Guo, C.; Tan, H.; Yan, C. Fabrication of IrO2 Decorated Vertical Aligned Self-Doped TiO2 Nanotube Arrays for Oxygen Evolution in Water Electrolysis. Int. J. Hydrogen Energy 2018, 43, 9133–9143. [Google Scholar] [CrossRef]
- Suhadolnik, L.; Bele, M.; Čekada, M.; Jovanovič, P.; Maselj, N.; Lončar, A.; Dražić, G.; Šala, M.; Hodnik, N.; Kovač, J.; et al. Nanotubular TiOxNy-Supported Ir Single Atoms and Clusters as Thin-Film Electrocatalysts for Oxygen Evolution in Acid Media. Chem. Mater. 2023, 35, 2612–2623. [Google Scholar] [CrossRef] [PubMed]
- Chatzitakis, A.; Grandcolas, M.; Xu, K.; Mei, S.; Yang, J.; Jensen, I.J.T.; Simon, C.; Norby, T. Assessing the Photoelectrochemical Properties of C, N, F Codoped TiO2 Nanotubes of Different Lengths. Catal. Today 2017, 287, 161–168. [Google Scholar] [CrossRef]
- Liu, X.; Risbakk, S.; Almeida Carvalho, P.; Yang, M.; Hoff Backe, P.; Bjørås, M.; Norby, T.; Chatzitakis, A. Immobilization of FeFe-Hydrogenase on Black TiO2 Nanotubes as Biocathodes for the Hydrogen Evolution Reaction. Electrochem. Commun. 2022, 135, 107221. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, J.; Zheng, L.; Tsang, C.K.; Li, H.; Shu, S.; Cheng, H.; Li, Y.Y. Selective Electrodeposition of Ni into the Intertubular Voids of Anodic TiO2 Nanotubes for Improved Photocatalytic Properties. J. Mater. Res. 2013, 28, 405–410. [Google Scholar] [CrossRef]
- Macak, J.M.; Gong, B.G.; Hueppe, M.; Schmuki, P. Filling of TiO2 Nanotubes by Self-Doping and Electrodeposition. Adv. Mater. 2007, 19, 3027–3031. [Google Scholar] [CrossRef]
- Xu, B.; He, Y.; Zhang, Y.; Ma, Z.; Zhang, Y.; Song, W. In Situ Growth of Tunable Gold Nanoparticles by Titania Nanotubes Templated Electrodeposition for Improving Osteogenesis through Modulating Macrophages Polarization. ACS Appl. Mater. Interfaces 2022, 14, 50520–50533. [Google Scholar] [CrossRef]




| Parameters | +1.50 VRHE | +1.55 VRHE | |
|---|---|---|---|
| Rsolution (Rsol) | rs/Ω cm2 | 2.800 | 2.714 |
| Rnanotubes (Rt) | rt/Ω cm2 | 0.117 | 0.290 |
| CPEnanotubes (Qt) | yt/S sn cm−2 | 0.296 | 0.001 |
| nt | 0.808 | 0.635 | |
| Rpores (Rp) | rp/Ω cm2 | 0.413 | 0.298 |
| CPEpores (Qp) | yp/S sn cm−2 | 0.013 | 0.012 |
| np | 0.568 | 0.643 | |
| Rchargetransfer (Rct) | rct/Ω cm2 | 1.632 | 0.851 |
| CPEdouble layer (Qdl) | ydl/S sn cm−2 | 0.138 | 0.144 |
| ndl | 0.863 | 0.792 | |
| χ2 | 0.00019 1 | 0.00033 1 | |
| Cdl/mF cm−2 | 108.8 | 83.1 | |
| RctCdl/Ω F (s) | 0.178 | 0.071 | |
| Catalyst | Support | η @ 10 mA cm−2 (mV) | Jgeom @ 300 mV (mA cm−2) | Jmass @ 300 mV (A gIr−1) | Ref. |
|---|---|---|---|---|---|
| IrOx | bTNTs | 240 | 70 | 258 | This work |
| IrO2 | TNTs | 360 | 3.9 | – | [92] |
| IrO2 | Self-doped TNTs | – | – | 116 | [93] |
| IrO2 | Hydrogenated TNTs | – | 0.68 | 29.5 | [54] |
| Ir | TNTs | 240 | 23 | 143 | [58] |
| Ir | TiOxNy NTs | – | – | 286 | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touni, A.; Mitrousi, E.; Carvalho, P.; Nikopoulou, M.; Pavlidou, E.; Lambropoulou, D.A.; Sotiropoulos, S. IrO2-Decorated Titania Nanotubes as Oxygen Evolution Anodes. Molecules 2025, 30, 2921. https://doi.org/10.3390/molecules30142921
Touni A, Mitrousi E, Carvalho P, Nikopoulou M, Pavlidou E, Lambropoulou DA, Sotiropoulos S. IrO2-Decorated Titania Nanotubes as Oxygen Evolution Anodes. Molecules. 2025; 30(14):2921. https://doi.org/10.3390/molecules30142921
Chicago/Turabian StyleTouni, Aikaterini, Effrosyni Mitrousi, Patricia Carvalho, Maria Nikopoulou, Eleni Pavlidou, Dimitra A. Lambropoulou, and Sotiris Sotiropoulos. 2025. "IrO2-Decorated Titania Nanotubes as Oxygen Evolution Anodes" Molecules 30, no. 14: 2921. https://doi.org/10.3390/molecules30142921
APA StyleTouni, A., Mitrousi, E., Carvalho, P., Nikopoulou, M., Pavlidou, E., Lambropoulou, D. A., & Sotiropoulos, S. (2025). IrO2-Decorated Titania Nanotubes as Oxygen Evolution Anodes. Molecules, 30(14), 2921. https://doi.org/10.3390/molecules30142921








