The Role of Adenosine A1 and A2a Receptors in Cerebral Blood Vessel Reactivity of Sprague Dawley Rats Exposed to Hyperbaric Oxygenation
Abstract
1. Introduction
2. Results
2.1. General Data
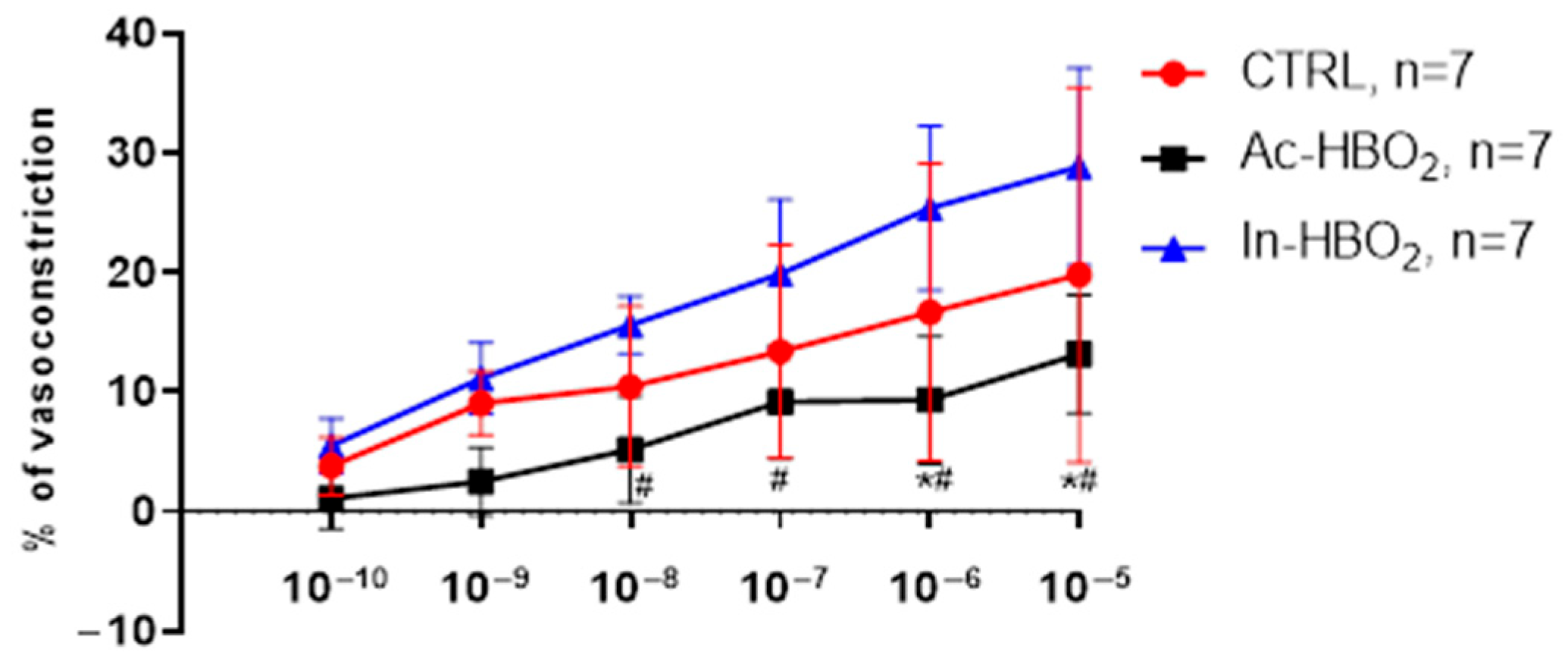
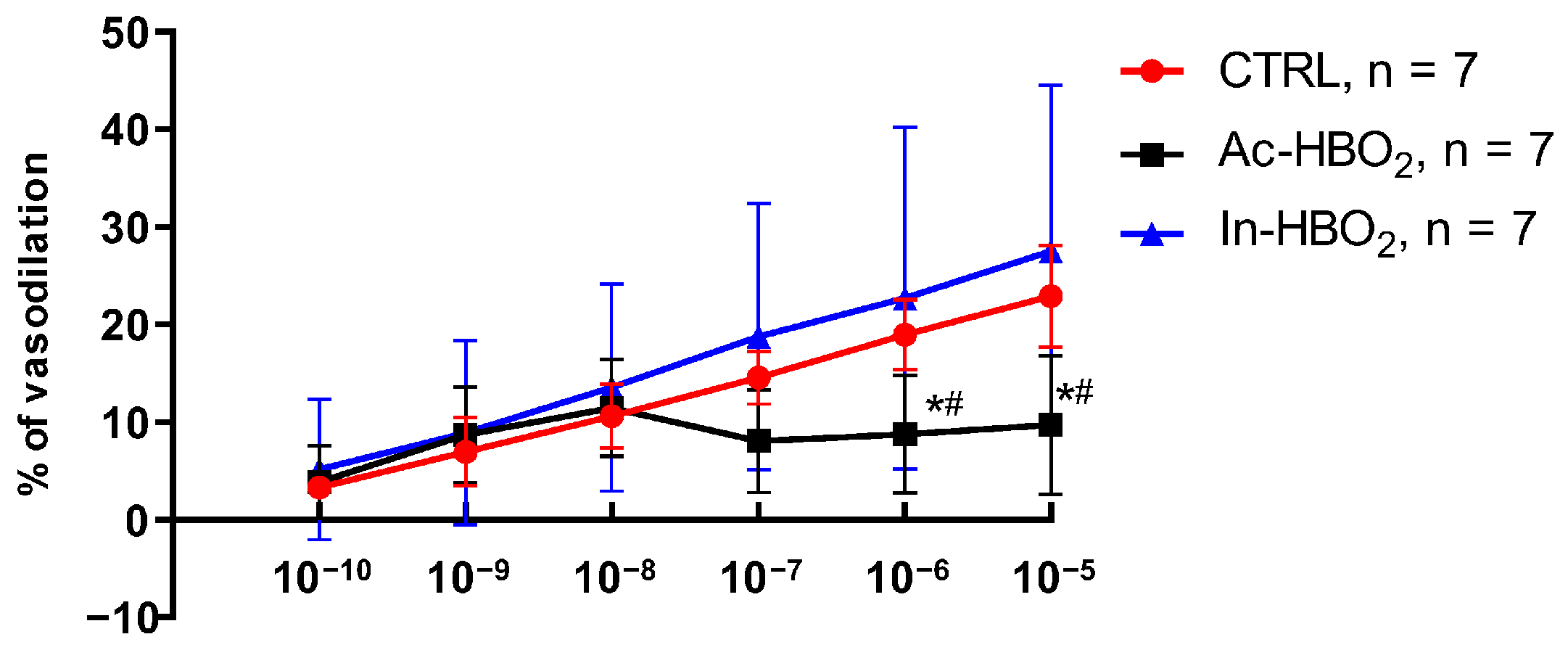
2.2. Dose–Response Effects of A1R and A2aR Selective Agonists
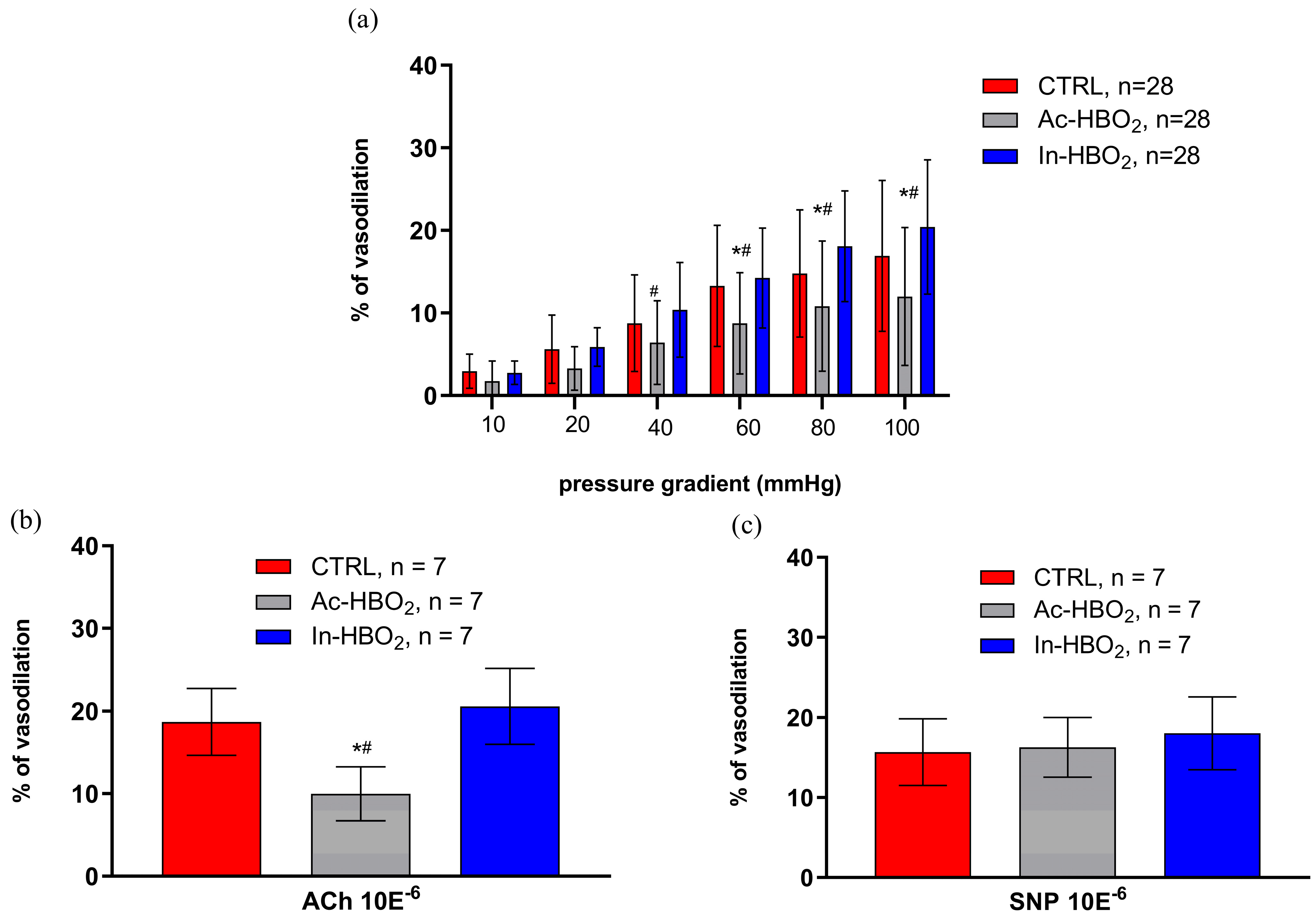
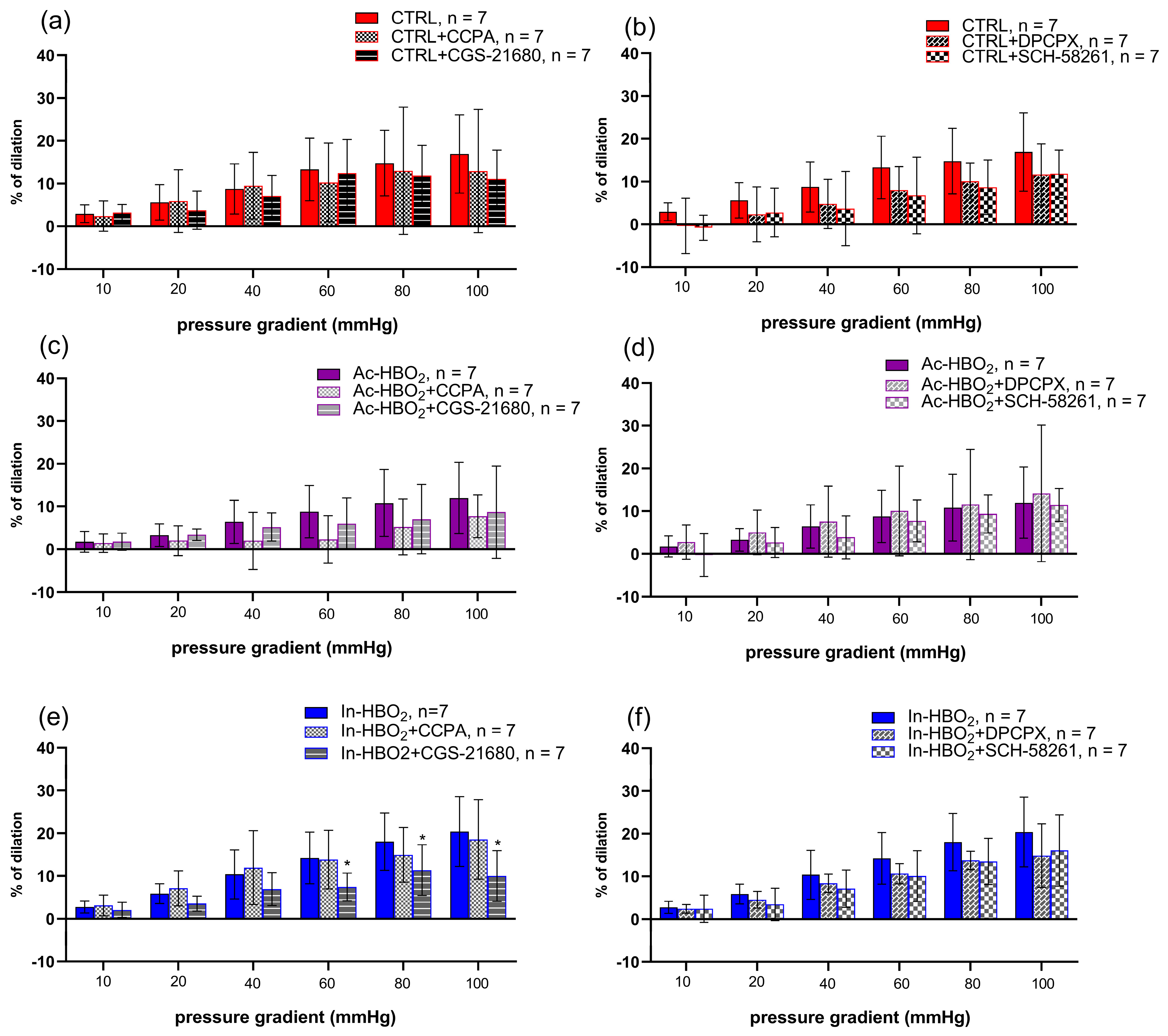
2.3. Flow-Induced Dilation (FID) and the Use of Agonists and Antagonists of Adenosine A1 and A2a Receptors
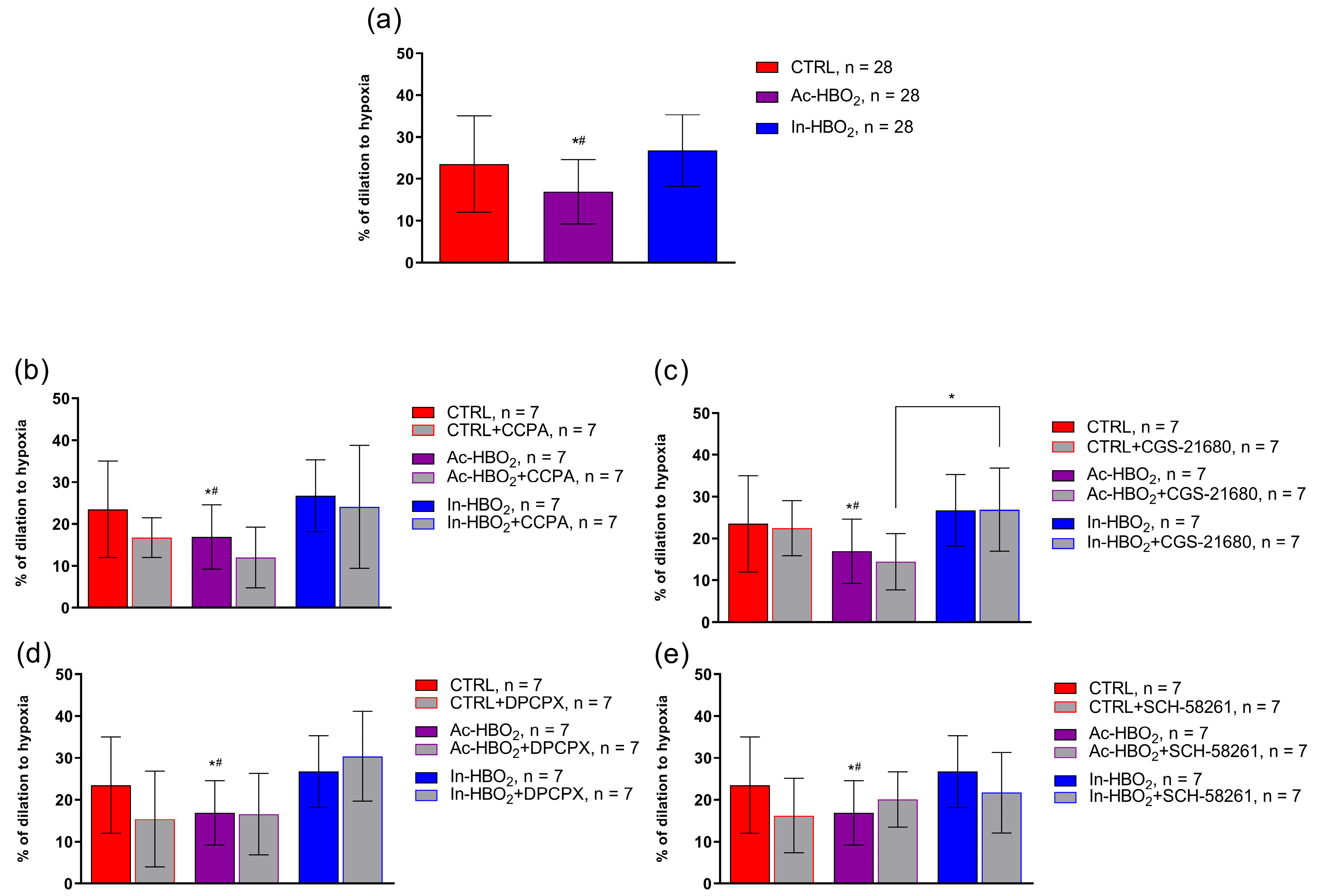
2.4. The Role of AR1R and AR2aR in Hypoxia-Induced Vasodilation in the MCA of Rats Treated with HBO2
2.5. Gene Expression of Adenosine A1 and A2a Receptors
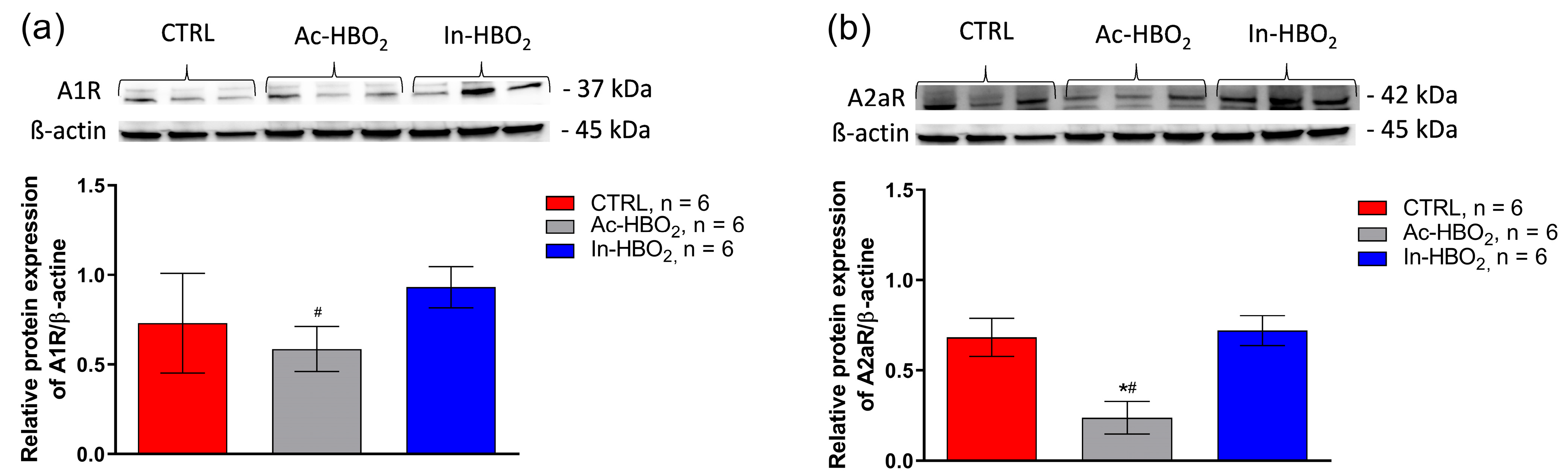
2.6. Protein Expression of Adenosine A1 and A2a Receptors
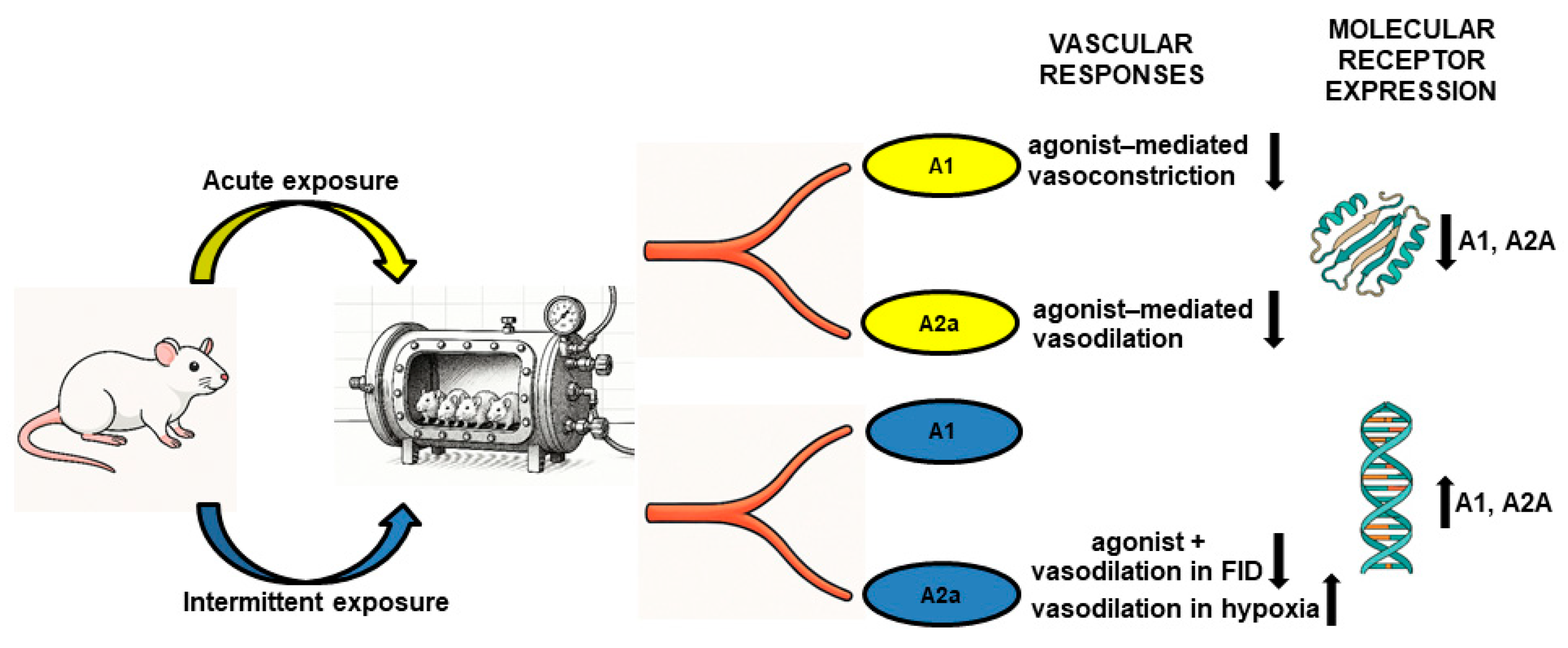
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Experimental Groups and Protocols for Exposure to HBO2
4.3. Preparation of Isolated MCAs
4.4. Determination of the Dose–Response of Adenosine A1 and A2a Receptor Agonists
4.5. FID and Hypoxia-Induced Dilation (HID) in Isolated MCAs
4.6. Acetylcholine-Induced Dilation, Endothelium-Independent Dilation, and Maximum Diameter Measurements
4.7. mRNA Expression Experiments
4.8. Protein Levels of Adenosine A1 and A2a Receptors
4.9. Statistical Analysis
5. Conclusions
Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | acetylcholine |
| Ac-HBO2 | acute hyperbaric oxygenation |
| A1R | adenosine A1 receptor |
| A2a | adenosine A2a receptor |
| ADA | adenosine deaminase |
| AMP | adenosine monophosphate |
| ARs | adenosine receptors |
| ATP | adenosine triphosphate |
| ANG-(1-7) | angiotensin-(1-7) |
| BK | big potassium channels |
| COX-2 | cyclooxygenase-2 |
| CTRL | control group |
| DMT | Danish Myo Technology |
| eNOS | endothelial nitric oxide synthase |
| EDHF | endothelium-derived hyperpolarizing factor |
| EET | epoxyeicosatrienoic acid |
| FID | flow-induced dilation |
| HO-1 | heme oxygenase-1 |
| H2O2 | hydrogen peroxide |
| HBO2 | hyperbaric oxygenation |
| HHP | hyperoxic-hypoxic paradox |
| HID | hypoxia-induced dilation |
| HIF-1α | hypoxia-inducible factor 1 alpha |
| iNOS | inducible nitric oxide synthase |
| In-HBO2 | intermittent hyperbaric oxygenation |
| MCA | middle cerebral artery |
| PGI2 | prostaglandin I 2 |
| PKC | protein kinase C |
| ROS | reactive oxygen species |
| SNP | sodium nitroprusside |
| SD rats | Sprague Dawley rats |
References
- Drenjancevic, I.; Kibel, A. Restoring vascular function with hyperbaric oxygen treatment: Recovery mechanisms. J. Vasc. Res. 2014, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Drenjancević-Perić, I.; Gros, M.; Kibel, A. Influence of hyperbaric oxygen on blood vessel reactivity: Concept of changes in conducted vasomotor response. Coll. Antropol. 2009, 33, 681–685. [Google Scholar] [PubMed]
- Mihaljević, Z.; Matić, A.; Stupin, A.; Rašić, L.; Jukić, I.; Drenjančević, I. Acute Hyperbaric Oxygenation, Contrary to Intermittent Hyperbaric Oxygenation, Adversely Affects Vasorelaxation in Healthy Sprague-Dawley Rats due to Increased Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, 7406027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kibel, A.; Cavka, A.; Cosic, A.; Falck, J.R.; Drenjancevic, I. Effects of hyperbaric oxygenation on vascular reactivity to angiotensin II and angiotensin-(1-7) in rats. Undersea Hyperb. Med. 2012, 39, 1053–1066. [Google Scholar] [PubMed]
- Kibel, A.; Novak, S.; Cosic, A.; Mihaljevic, Z.; Falck, J.R.; Drenjancevic, I. Hyperbaric oxygenation modulates vascular reactivity to angiotensin-(1-7) in diabetic rats: Potential role of epoxyeicosatrienoic acids. Diabetes Vasc. Dis. Res. 2015, 12, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Mihaljević, Z.; Matić, A.; Stupin, A.; Frkanec, R.; Tavčar, B.; Kelava, V.; Tartaro Bujak, I.; Kolobarić, N.; Kibel, A.; Drenjančević, I. Arachidonic Acid Metabolites of CYP450 Enzymes and HIF-1α Modulate Endothelium-Dependent Vasorelaxation in Sprague-Dawley Rats under Acute and Intermittent Hyperbaric Oxygenation. Int. J. Mol. Sci. 2020, 21, 6353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carroll, M.A.; Doumad, A.B.; Li, J.; Cheng, M.K.; Falck, J.R.; McGiff, J.C. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am. J. Physiol. Renal. Physiol. 2006, 291, F155–F161. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.K.; Doumad, A.B.; Jiang, H.; Falck, J.R.; McGiff, J.C.; Carroll, M.A. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br. J. Pharmacol. 2004, 141, 441–448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruzzese, L.; Rostain, J.C.; Née, L.; Condo, J.; Mottola, G.; Adjriou, N.; Mercier, L.; Berge-Lefranc, J.L.; Fromonot, J.; Kipson, N.; et al. Effect of hyperoxic and hyperbaric conditions on the adenosinergic pathway and CD26 expression in rat. J. Appl. Physiol. 2015, 119, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Maille, B.; Lalevée, N.; Marlinge, M.; Vahdat, J.; Mottola, G.; Degioanni, C.; De Maria, L.; Klein, V.; Thuny, F.; Franceschi, F.; et al. Adenosine and Adenosine Receptors: Advances in Atrial Fibrillation. Biomedicines 2022, 10, 2963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef] [PubMed]
- By, Y.; Jacquin, L.; Franceschi, F.; Durand-Gorde, J.M.; Condo, J.; Michelet, P.; Guieu, R.; Ruf, J. Fall in oxygen tension of culture medium stimulates the adenosinergic signalling of a human T cell line. Purinergic Signal 2012, 8, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.M.; Minter, L.M.; Osborne, B.A.; Granowitz, E.V. Hyperbaric oxygen inhibits stimulus-induced proinflammatory cytokine synthesis by human blood-derived monocyte-macrophages. Clin. Exp. Immunol. 2003, 134, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Matic, A.; Jukic, I.; Stupin, A.; Baric, L.; Mihaljevic, Z.; Unfirer, S.; Tartaro Bujak, I.; Mihaljevic, B.; Lombard, J.H.; Drenjancevic, I. High salt intake shifts the mechanisms of flow-induced dilation in the middle cerebral arteries of Sprague-Dawley rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H718–H730. [Google Scholar] [CrossRef] [PubMed]
- Cosic, A.; Jukic, I.; Stupin, A.; Mihalj, M.; Mihaljevic, Z.; Novak, S.; Vukovic, R.; Drenjancevic, I. Attenuated flow-induced dilatation of middle cerebral arteries is related to increased vascular oxidative stress in rats on a short-term high salt diet. J. Physiol. 2016, 594, 4917–4931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Unfirer, S.; Mihalj, M.; Novak, S.; Kibel, A.; Cavka, A.; Mijalevic, Z.; Gros, M.; Brizic, I.; Budimir, D.; Cosic, A.; et al. Hyperbaric oxygenation affects the mechanisms of acetylcholine-induced relaxation in diabetic rats. Undersea Hyperb. Med. 2016, 43, 787–803. [Google Scholar] [PubMed]
- Unfirer, S.; Drenjančević, I. The mechanisms of vascular reactivity to ACh and serotonin are modulated by hyperbaric oxygen treatment in cerebral resistance arteries of diabetic rats (Pohl U, Sperandio M, editors). J. Vasc. Res. 2011, 48 (Suppl. S1), 276. [Google Scholar] [CrossRef]
- Unfirer, S.; Falck, J.R.; Drenjancevic, I. Cytochrome P450-epoxygenase metabolites play role in vasodilation of middle cerebral arteries in response to reduced pO2 in healthy and diabetic rats that underwent hyperbaric oxygenation. In Proceedings of the International Union of Physiological Sciences, Birmingham, UK, 21–26 July 2013. Abstract Book 950p. [Google Scholar]
- Wang, Y.; Yang, J.N.; Arner, A.; Boels, P.J.; Fredholm, B.B. Adenosine A(1) receptors and vascular reactivity. Acta Physiol. 2010, 199, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Bryan, P.T.; Marshall, J.M. Adenosine receptor subtypes and vasodilatation in rat skeletal muscle during systemic hypoxia: A role for A1 receptors. J. Physiol. 1999, 514 Pt 1, 151–162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coney, A.M.; Marshall, J.M. Role of adenosine and its receptors in the vasodilatation induced in the cerebral cortex of the rat by systemic hypoxia. J. Physiol. 1998, 509 Pt 2, 507–518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ngai, A.C.; Coyne, E.F.; Meno, J.R.; West, G.A.; Winn, H.R. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2329–H2335. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.A. Role of the adenosine(2A) receptor-epoxyeicosatrienoic acid pathway in the development of salt-sensitive hypertension. Prostaglandins Other Lipid Mediat. 2012, 98, 39–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eltzschig, H.K.; Faigle, M.; Knapp, S.; Karhausen, J.; Ibla, J.; Rosenberger, P.; Odegard, K.C.; Laussen, P.C.; Thompson, L.F.; Colgan, S.P. Endothelial catabolism of extracellular adenosine during hypoxia: The role of surface adenosine deaminase and CD26. Blood 2006, 108, 1602–1610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Görlach, A. Control of adenosine transport by hypoxia. Circ. Res. 2005, 97, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, L.; Fromonot, J.; By, Y.; Durand-Gorde, J.M.; Condo, J.; Kipson, N.; Guieu, R.; Fenouillet, E.; Ruf, J. NF-κB enhances hypoxia-driven T-cell immunosuppression via upregulation of adenosine A(2A) receptors. Cell Signal. 2014, 26, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Kunduri, S.; Dick, G.; Nayeem, M.; Mustafa, S. Adenosine A1 receptor signaling inhibits BK channels through a PKCα-dependent mechanism in mouse aortic smooth muscle. Physiol. Rep. 2013, 1, e00037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharifi-Sanjani, M.; Zhou, X.; Asano, S.; Tilley, S.; Ledent, C.; Teng, B.; Dick, G.M.; Mustafa, S.J. Interactions between A(2A) adenosine receptors, hydrogen peroxide, and KATP channels in coronary reactive hyperemia. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1294–H1301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DiChiara, T.J.; Reinhart, P.H. Redox modulation of hslo Ca2+-activated K+ channels. J. Neurosci. 1997, 17, 4942–4955. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, X.D.; Daggett, H.; Hanner, M.; Garcia, M.L.; McManus, O.B.; Brot, N.; Weissbach, H.; Heinemann, S.H.; Hoshi, T. Oxidative regulation of large conductance calcium-activated potassium channels. J. Gen. Physiol. 2001, 117, 253–274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carroll, M.A.; Balazy, M.; Margiotta, P.; Huang, D.D.; Falck, J.R.; McGiff, J.C. Cytochrome P-450-dependent HETEs: Profile of biological activity and stimulation by vasoactive peptides. Am. J. Physiol. 1996, 271 Pt 2, R863–R869. [Google Scholar] [CrossRef]
- Koller, A.; Toth, P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J. Vasc. Res. 2012, 49, 375–389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ku, H.C.; Chen, W.P.; Su, M.J. DPP4 deficiency exerts protective effect against H2O2 induced oxidative stress in isolated cardiomyocytes. PLoS ONE 2013, 8, e54518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moro, P.J.; Quilici, J.; Giorgi, R.; Cuisset, T.; By, Y.; Boussuges, A.; Jammes, Y.; Bonnet, J.L.; Ruf, J.; Fenouillet, E.; et al. Mononuclear cell adenosine deaminase and CD26/dipeptidylpeptidase-IV activities are sensitive markers of reperfusion during percutaneous transluminal angioplasty. Int. J. Cardiol. 2013, 166, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Karmouty-Quintana, H.; Le, T.T.; Chen, N.Y.; Weng, T.; Luo, F.; Molina, J.; Moorthy, B.; Blackburn, M.R. Adenosine promotes vascular barrier function in hyperoxic lung injury. Physiol. Rep. 2014, 2, e12155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melani, A.; Corti, F.; Cellai, L.; Vannucchi, M.G.; Pedata, F. Low doses of the selective adenosine A2A receptor agonist CGS21680 are protective in a rat model of transient cerebral ischemia. Brain Res. 2014, 1551, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Bryan, R.M., Jr.; Marrelli, S.P.; Steenberg, M.L.; Schildmeyer, L.A.; Johnson, T.D. Effects of luminal shear stress on cerebral arteries and arterioles. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2011–H2022. [Google Scholar] [CrossRef]
- Toth, P.; Rozsa, B.; Springo, Z.; Doczi, T.; Koller, A. Isolated human and rat cerebral arteries constrict to increases in flow: Role of 20-HETE and TP receptors. J. Cereb. Blood Flow. Metab. 2011, 31, 2096–2105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, Y.S.; Bogert, L.W.; Immink, R.V.; Harms, M.P.; Colier, W.N.; van Lieshout, J.J. Effects of aging on the cerebrovascular orthostatic response. Neurobiol. Aging 2011, 32, 344–353. [Google Scholar] [CrossRef]
- Gebremedhin, D.; Weinberger, B.; Lourim, D.; Harder, D.R. Adenosine can mediate its actions through generation of reactive oxygen species. J. Cereb. Blood Flow. Metab. 2010, 30, 1777–1790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- You, J.; Golding, E.M.; Bryan, R.M., Jr. Arachidonic acid metabolites, hydrogen peroxide, and EDHF in cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1077–H1083. [Google Scholar] [CrossRef]
- Hadanny, A.; Efrati, S. The Hyperoxic-Hypoxic Paradox. Biomolecules 2020, 10, 958. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rocco, M.; D’Itri, L.; De Bels, D.; Corazza, F.; Balestra, C. The “normobaric oxygen paradox”: A new tool for the anesthetist? Minerva Anestesiol. 2014, 80, 366–372. [Google Scholar] [PubMed]
- Khayat, M.T.; Nayeem, M.A. The Role of Adenosine A2A Receptor, CYP450s, and PPARs in the Regulation of Vascular Tone. Biomed. Res. Int. 2017, 2017, 1720920. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waypa, G.B.; Smith, K.A.; Schumacker, P.T. O2 sensing, mitochondria and ROS signaling: The fog is lifting. Mol. Aspects. Med. 2016, 47–48, 76–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Unfirer, S.; Kibel, A.; Drenjancevic-Peric, I. The effect of hyperbaric oxygen therapy on blood vessel function in diabetes mellitus. Med. Hypotheses 2008, 71, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Drenjančević, I.; Jukić, I.; Đambić, V.; Stupin, A.; Kozina, N.; Matić, A.; Šušnjara, P.; Kibel, A.; Biljan, D.; Mihaljević, Z. Variability in flow-induced vasodilation mechanisms in cerebral arteries: The impact of different hyperbaric oxygen protocols. Med. Gas. Res. 2025, 15, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, A.; Conti, A.; Dionisotti, S.; Casati, C.; Camaioni, E.; Cristalli, G.; Ongini, E. Pharmacology of the highly selective A1 adenosine receptor agonist 2-chloro-N6-cyclopentyladenosine. Arzneimittelforschung 1994, 44, 1305–1312. [Google Scholar] [PubMed]
- Monopoli, A.; Casati, C.; Lozza, G.; Forlani, A.; Ongini, E. Cardiovascular pharmacology of the A2A adenosine receptor antagonist, SCH 58261, in the rat. J. Pharmacol. Exp. Ther. 1998, 285, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Monopoli, A.; Gamba, M.; Borea, P.A.; Ongini, E. Effects of selective A1 and A2 adenosine receptor agonists on cardiovascular tissues. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1993, 348, 108–112. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Di Paola, R.; Esposito, E.; Mazzon, E.; Paterniti, I.; Melani, A.; Bramanti, P.; Pedata, F.; Cuzzocrea, S. CGS 21680, an agonist of the adenosine (A2A) receptor, decreases acute lung inflammation. Eur. J. Pharmacol. 2011, 668, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| CTRL | Ac-HBO2 | In-HBO2 | |
|---|---|---|---|
| Body mass [g] | 372 ± 53 | 350 ± 44 | 349 ± 60 |
| Diameter of MCAs at ∆0 mmHg [μm] | 133 ± 23 | 123 ± 26 | 125 ± 23 |
| Max. diameter of MCAs (Ca2+ free) [μm] | 207 ± 19 | 190 ± 11 | 207 ± 14 |
| Active tone (%) | 43 ± 8 | 40 ± 6 | 42 ± 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đambić, V.; Mihaljević, Z.; Drenjančević, I.; Jukić, I.; Šušnjara, P.; Kibel, A. The Role of Adenosine A1 and A2a Receptors in Cerebral Blood Vessel Reactivity of Sprague Dawley Rats Exposed to Hyperbaric Oxygenation. Molecules 2025, 30, 2918. https://doi.org/10.3390/molecules30142918
Đambić V, Mihaljević Z, Drenjančević I, Jukić I, Šušnjara P, Kibel A. The Role of Adenosine A1 and A2a Receptors in Cerebral Blood Vessel Reactivity of Sprague Dawley Rats Exposed to Hyperbaric Oxygenation. Molecules. 2025; 30(14):2918. https://doi.org/10.3390/molecules30142918
Chicago/Turabian StyleĐambić, Vedran, Zrinka Mihaljević, Ines Drenjančević, Ivana Jukić, Petar Šušnjara, and Aleksandar Kibel. 2025. "The Role of Adenosine A1 and A2a Receptors in Cerebral Blood Vessel Reactivity of Sprague Dawley Rats Exposed to Hyperbaric Oxygenation" Molecules 30, no. 14: 2918. https://doi.org/10.3390/molecules30142918
APA StyleĐambić, V., Mihaljević, Z., Drenjančević, I., Jukić, I., Šušnjara, P., & Kibel, A. (2025). The Role of Adenosine A1 and A2a Receptors in Cerebral Blood Vessel Reactivity of Sprague Dawley Rats Exposed to Hyperbaric Oxygenation. Molecules, 30(14), 2918. https://doi.org/10.3390/molecules30142918






