In Search of New Drugs: Elucidating the Activity of Structurally Similar Potential Antibiotics Using Molecular Modelling
Abstract
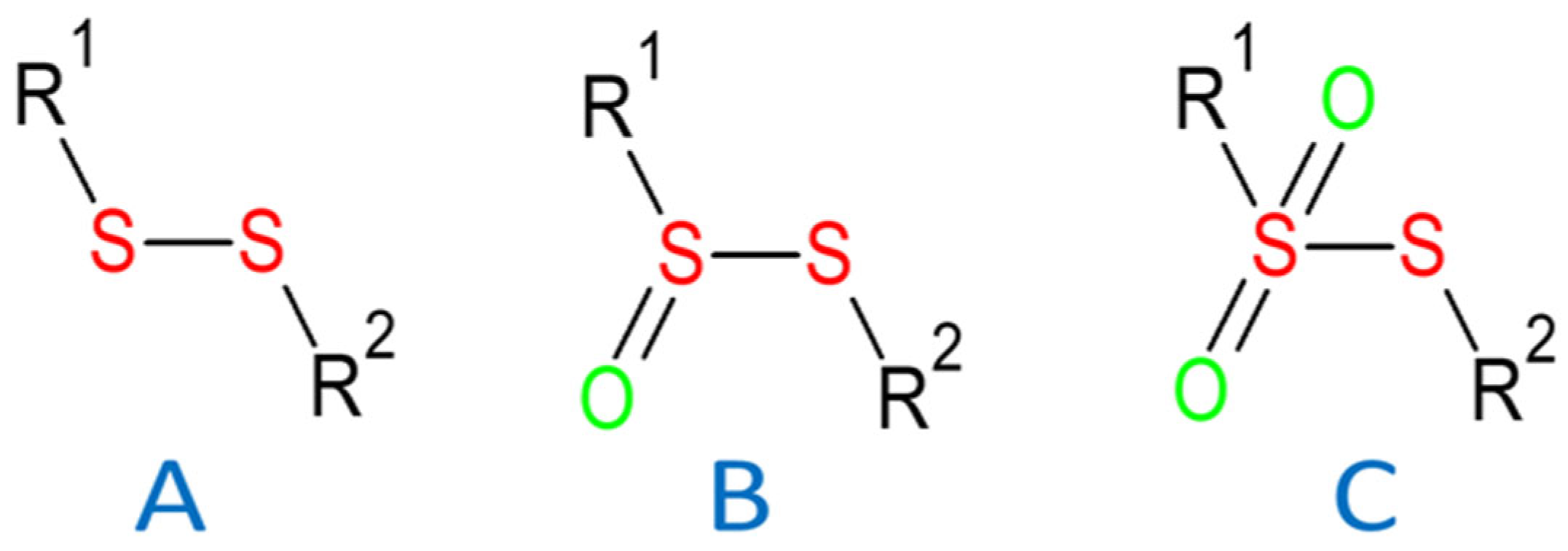
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis and Purification
3.2. Antimicrobial Activity
3.2.1. Bacterial Strains and Tested Compounds Solutions
3.2.2. Disk Diffusion Method
3.2.3. Statistical Analysis
3.3. Theoretical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compound | NBO Orbitals–Gas phase, % | NBO Orbitals–Water (PCM), % | ||
|---|---|---|---|---|
| S(O) | S | S(O) | S | |
| 1 | 50.14 | 49.86 | 50.03 | 49.97 |
| 2 | 49.79 | 50.21 | 50.20 | 49.80 |
| 3 | 49.30 | 50.70 | 49.59 | 50.41 |
References
- Schurer, M.; Patel, R.; van Keep, M.; Horgan, J.; Matthijsse, S.; Madin-Warburton, M. Recent Advances in Addressing the Market Failure of New Antimicrobials: Learnings from NICE’s Subscription-Style Payment Model. Front. Med. Technol. 2023, 5, 1010247. [Google Scholar] [CrossRef]
- Ai, T.; Yao, S.; Yu, Y.; Peng, K.; Jin, L.; Zhu, X.; Zhou, H.; Huang, J.; Sun, J.; Zhu, L. Transformation Process and Phytotoxicity of Sulfamethoxazole and N4-Acetyl-Sulfamethoxazole in Rice. Sci. Total Environ. 2024, 918, 170857. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.; Reinhold, D. Metabolism of Sulfamethoxazole by the Model Plant Arabidopsis Thaliana. Environ. Sci. Technol. 2019, 53, 4901–4911. [Google Scholar] [CrossRef]
- Blume, L.; Long, T.E.; Turos, E. Applications and Opportunities in Using Disulfides, Thiosulfinates, and Thiosulfonates as Antibacterials. Int. J. Mol. Sci. 2023, 24, 8659. [Google Scholar] [CrossRef] [PubMed]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]
- Seo, K.I.; Moon, Y.H.; Choi, S.U.; Park, K.H. Antibacterial Activity of S-Methyl Methanethiosulfinate and S-Methyl 2-Propene-1-Thiosulfinate from Chinese Chive toward Escherichia Coli O157:H7. Biosci. Biotechnol. Biochem. 2001, 65, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.S.K.H.; Alakolanga, A.G.A.W.; Amarasinghe, N.R.; Adikaram, N.K.B.; Jayasinghe, L.; Fujimoto, Y. Antiviral Activities of Some Traditional Medicinal Plants of Sri Lanka. Curr. Tradit. Med. 2023, 9, 25–38. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Sanches-Silva, A.; Daglia, M.; Nabavi, S.F.; Jafari, N.J.; Izadi, M.; Ajami, M.; Nabavi, S.M. Antifungal and Antibacterial Activities of Allicin: A Review. Trends Food Sci. Technol. 2016, 52, 49–56. [Google Scholar] [CrossRef]
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of Quinolone Action and Resistance: Where Do We Stand? J. Med. Microbiol. 2017, 66, 551–559. [Google Scholar] [CrossRef]
- Floss, H.G.; Yu, T.-W. RifamycinMode of Action, Resistance, and Biosynthesis. Chem. Rev. 2005, 105, 621–632. [Google Scholar] [CrossRef]
- Beyer, D.; Pepper, K. The Streptogramin Antibiotics: Update on Their Mechanism of Action. Expert Opin. Investig. Drugs 1998, 7, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Sköld, O. Sulfonamide Resistance: Mechanisms and Trends. Drug Resist. Updat. 2000, 3, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Markley, J.L.; Wencewicz, T.A. Tetracycline-Inactivating Enzymes. Front. Microbiol. 2018, 9, 1058. [Google Scholar] [CrossRef] [PubMed]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular Mechanisms of Antibiotic Resistance Revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef]
- Kumari, A.; Mathur, G.; Sharma, G. Antimicrobial Peptides in Tuberculosis: Insights into the Immunomodulatory Mechanisms. Chem. Biol. Lett. 2025, 12, 1253. [Google Scholar] [CrossRef]
- Basabe-Tuero, L.; Ayala, L.; Espinosa, I.; Machín, Y.; Coto, L.; Duarte, C.; Piloto, S.; Morales, A.; Rodrigo, O.; Diago, D.; et al. Oreochromicin-2 Shows Antimicrobial and Immunostimulant Effect against Respiratory Pathogens in Pigs. Res. Vet. Sci. 2025, 184, 105523. [Google Scholar] [CrossRef]
- Stevens, M.T.; Hawkins, P.M.E.; Wang, T.; Payne, R.J.; Britton, W.J. Analogue of the Natural Product Ecumicin Causes Sustained Growth Inhibition of Mycobacterium tuberculosis under Multiple Growth Conditions. Tuberculosis 2025, 151, 102594. [Google Scholar] [CrossRef]
- León Madrazo, A.; Quintana Owen, P.; Pérez Mendoza, G.; Segura Campos, M.R. Chia Derived Peptides Affecting Bacterial Membrane and DNA: Insights from Staphylococcus Aureus and Escherichia Coli Studies. Plant Foods Hum. Nutr. 2024, 80, 22. [Google Scholar] [CrossRef]
- Dogheim, G.M.; Alazhary, N.N.; Elbadry, O.A.; Amralla, M.T. Biosynthesized Silver Nanoparticles as an Environmental-Friendly Antibacterial Nanosystem against Methicillin-Resistant Staphylococcus Aureus. Inorg. Chem. Commun. 2025, 173, 113809. [Google Scholar] [CrossRef]
- Ghadimi, N.; Asadpour, L.; Mokhtary, M. Enhanced Antimicrobial, Anti-Biofilm, and Efflux Pump Inhibitory Effects of Ursolic Acid-Conjugated Magnetic Nanoparticles against Clinical Isolates of Multidrug-Resistant Pseudomonas aeruginosa. Microb. Pathog. 2025, 199, 107241. [Google Scholar] [CrossRef]
- Si, Z.; Sun, Y.; Tan, C.; Ooi, Y.J.; Li, M.; Raju, C.; Shubi, J.; Gan, Y.-H.; Zhu, Y.; Li, P.; et al. A Cationic Main-Chain Poly(Carbonate-Imidazolium) Potent against Mycobacterium abscessus and Other Resistant Bacteria in Mice. Biomaterials 2025, 316, 123003. [Google Scholar] [CrossRef] [PubMed]
- Nabawy, A.; Chattopadhyay, A.N.; Makabenta, J.M.V.; Hassan, M.A.; Yang, J.; Park, J.; Jiang, M.; Jeon, T.; Im, J.; Rotello, V.M. Cationic Conjugated Polymers with Tunable Hydrophobicity for Efficient Treatment of Multidrug-Resistant Wound Biofilm Infections. Biomaterials 2025, 316, 123015. [Google Scholar] [CrossRef] [PubMed]
- Cong, Z.; Yan, Z.; Xiao, X.; Liu, L.; Luo, Z.; Zou, J.; Chen, M.; Wu, Y.; Zhou, M.; Liu, R. Peptide-Mimicking Poly(2-Oxazoline) Displaying Potent Antibacterial and Antibiofilm Activities against Multidrug-Resistant Gram-Positive Pathogenic Bacteria. J. Mater. Sci. Technol. 2025, 214, 233–244. [Google Scholar] [CrossRef]
- Shakir, M.; Ali, A.; Lakshmi, S.; Garg, M.; Abdulhameed Almuqdadi, H.T.; Irfan, I.; Kamthan, M.; Joshi, M.C.; Javed, S.; Rawat, D.S.; et al. Synthesis and Mechanistic Studies of 4-Aminoquinoline-Isatin Molecular Hybrids and Schiff’s Bases as Promising Antimicrobial Agents. Eur. J. Med. Chem. 2025, 283, 117127. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, J.; Wang, B.-L.; Yang, X.-Y.; Zhang, Y. Discovery, Synthesis, and Antibacterial Activity of Novel Myrtucommulone Analogs as Inhibitors of DNA Gyrase and Topoisomerase IV. Eur. J. Med. Chem. 2025, 283, 117138. [Google Scholar] [CrossRef]
- Lu, X.; Xu, X.; Ding, Y.; Gong, X.; Ming, L.; Dai, X.; Gu, C.; Wang, J.; Zhao, J.; Gao, M.; et al. Discovery and Optimization of Tetrahydroacridine Derivatives as a Novel Class of Antibiotics against Multidrug-Resistant Gram-Positive Pathogens by Targeting Type I Signal Peptidase and Disrupting Bacterial Membrane. Eur. J. Med. Chem. 2025, 283, 117101. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Hussein, N.N.; Sulaiman, G.M.; Khan, R.A.; Mohammed, H.A. Piperacillin-Loaded Amine Functionalized Mesoporous Silica Nanoparticles: A New Frontier in Combating Multidrug-Resistant Pathogenic Bacteria through Value-Added Piperacillin Antibiotic. J. Drug Deliv. Sci. Technol. 2025, 105, 106580. [Google Scholar] [CrossRef]
- Fesendouz, S.A.; Hamishehkar, H.; Alizadeh, E.; Rahbarghazi, R.; Akbarzadeh, A.; Yousefi, S.; Milani, M. Bactericidal Activity and Biofilm Eradication of Pseudomonas Aeruginosa by Liposome-Encapsulated Piperacillin/Tazobactam. BioNanoScience 2024, 15, 87. [Google Scholar] [CrossRef]
- Liao, M.; Gong, H.; Ge, T.; Shen, K.; Campana, M.; McBain, A.J.; Wu, C.; Hu, X.; Lu, J.R. Probing Antimicrobial Synergy by Novel Lipopeptides Paired with Antibiotics. J. Colloid Interface Sci. 2025, 681, 82–94. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Yan, P.; Ouyang, X.; Ba, Z.; Wang, Y.; Yang, T.; Yu, Z.; Ren, B.; Zhong, C.; et al. The Novel β-Hairpin Antimicrobial Peptide D-G(RF)3 Demonstrates Exceptional Antibacterial Efficacy. Eur. J. Med. Chem. 2025, 283, 117149. [Google Scholar] [CrossRef]
- Ghilardi, A.F.; Yaaghubi, E.; Ferreira, R.B.; Law, M.E.; Yang, Y.; Davis, B.J.; Schilson, C.M.; Ghiviriga, I.; Roitberg, A.E.; Law, B.K.; et al. Anticancer Agents Derived from Cyclic Thiosulfonates: Structure-Reactivity and Structure-Activity Relationships. ChemMedChem 2022, 17, e202200165. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Lim, B.; Cheng, Y.; Pham, A.-T.; Maynard, J.; Moreau, D.; Poblador-Bahamonde, A.I.; Sakai, N.; Matile, S. Cyclic Thiosulfonates for Thiol-Mediated Uptake: Cascade Exchangers, Transporters, Inhibitors. JACS Au 2022, 2, 839–852. [Google Scholar] [CrossRef]
- Zilbeyaz, K.; Oztekin, A.; Kutluana, E.G. Design and Synthesis of Garlic-Related Unsymmetrical Thiosulfonates as Potential Alzheimer’s Disease Therapeutics: In Vitro and in Silico Study. Bioorg. Med. Chem. 2021, 40, 116194. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.A.; Marchand, M.E.; Langler, R.F. Improving upon the in Vitro Biological Activity of Antithrombotic Disulfides. Blood Coagul. Fibrinolysis 2004, 15, 447. [Google Scholar] [CrossRef] [PubMed]
- Men’shchikova, E.B.; Zenkov, N.K.; Kozhin, P.M.; Chechushkov, A.V.; Kovner, A.V.; Khrapova, M.V.; Kandalintseva, N.V.; Martinovich, G.G. Synthetic Phenolic Antioxidant TS-13 Suppresses the Growth of Lewis Lung Carcinoma and Potentiates Oncolytic Effect of Doxorubicin. Bull. Exp. Biol. Med. 2019, 166, 646–650. [Google Scholar] [CrossRef]
- Smith, M.; Hunter, R.; Stellenboom, N.; Kusza, D.A.; Parker, M.I.; Hammouda, A.N.H.; Jackson, G.; Kaschula, C.H. The Cytotoxicity of Garlic-Related Disulphides and Thiosulfonates in WHCO1 Oesophageal Cancer Cells Is Dependent on S-Thiolation and Not Production of ROS. Biochim. Biophys. Acta BBA—Gen. Subj. 2016, 1860, 1439–1449. [Google Scholar] [CrossRef]
- Griffiths, R.; Wong, W.W.-L.; Fletcher, S.P.; Penn, L.Z.; Langler, R.F. Novel Disulfides with Antitumour Efficacy and Specificity. Aust. J. Chem. 2005, 58, 128–136. [Google Scholar] [CrossRef]
- Müller, A.; Eller, J.; Albrecht, F.; Prochnow, P.; Kuhlmann, K.; Bandow, J.E.; Slusarenko, A.J.; Leichert, L.I.O. Allicin Induces Thiol Stress in Bacteria through S-Allylmercapto Modification of Protein Cysteines. J. Biol. Chem. 2016, 291, 11477–11490. [Google Scholar] [CrossRef]
- Chan, A.N.; Wever, W.J.; Massolo, E.; Allen, S.E.; Li, B. Reducing Holomycin Thiosulfonate to Its Disulfide with Thiols. Chem. Res. Toxicol. 2019, 32, 400–404. [Google Scholar] [CrossRef]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO, Version 3.1; Gaussian Inc.: Pittsburgh, PA, USA, 2003.
- Kupka, T.; Dziuk, B.; Ejsmont, K.; Makieieva, N.; Fizer, L.; Monka, N.; Konechna, R.; Stadnytska, N.; Vasyliuk, S.; Lubenets, V. Impact of Crystal and Molecular Structure of Three Novel Thiosulfonate Crystals on Their Vibrational and NMR Parameters. J. Mol. Struct. 2024, 1313, 138642. [Google Scholar] [CrossRef]
- Lubenets, V.; Karpenko, O.; Ponomarenko, M.; Zahoriy, G.; Krychkovska, A.; Novikov, V. Development of New Antimicrobial Compositions of Thiosulfonate Structure. Chem. Chem. Technol. 2013, 7, 119–124. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, G.E.; Scuseria, M.A.; Robb, J.R.; Cheeseman, G.; Scalmani, V.; Barone, G.A.; Petersson, H.; Nakatsuji, X.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019.
- Barone, V.; Cossi, M.; Tomasi, J. Geometry Optimization of Molecular Structures in Solution by the Polarizable Continuum Model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results Obtained with the Correlation Energy Density Functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Kupka, T.; Stachów, M.; Nieradka, M.; Stobiński, L. DFT Calculation of Structures and NMR Chemical Shifts of Simple Models of Small Diameter Zigzag Single Wall Carbon Nanotubes (SWCNTs). Magn. Reson. Chem. 2011, 49, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Chełmecka, E.; Pasterny, K.; Kupka, T.; Stobiński, L. Density Functional Theory Studies of OH-Modified Open-Ended Single-Wall Zigzag Carbon Nanotubes (SWCNTs). J. Mol. Struct. THEOCHEM 2010, 948, 93–98. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent Molecular Orbital Methods. XXIII. A Polarization-type Basis Set for Second-row Elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]





| Compound | NBO Charges–Gas Phase | NBO Charges–Water (PCM) | ||||||
|---|---|---|---|---|---|---|---|---|
| S(O2) | O1 | O2 | Δ(S-2O) | S(O2) | O1 | O2 | Δ(S-2O) | |
| 1 | 1.990 | −0.903 | −0.892 | 0.195 | 1.914 | −0.935 | −0.939 | 0.040 |
| 2 | 1.986 | −0.895 | −0.893 | 0.198 | 1.909 | −0.927 | −0.933 | 0.049 |
| 3 | 2.031 | −0.898 | −0.890 | 0.243 | 1.909 | −0.928 | −0.932 | 0.049 |
| Compound | NBO Orbitals–Gas Phase, % | NBO Orbitals–Water (PCM), % | ||||||
| S(O2) | O1 | S(O2) | O2 | S(O2) | O1 | S(O2) | O2 | |
| 1 | 35.91 | 64.09 | 36.25 | 63.75 | 35.74 | 64.26 | 35.95 | 64.05 |
| 2 | 35.94 | 64.06 | 36.25 | 63.75 | 35.78 | 64.22 | 36.00 | 64.00 |
| 3 | 36.00 | 64.00 | 36.25 | 63.75 | 35.81 | 64.19 | 36.01 | 63.99 |
| Compound | NBO Charges–Gas Phase | NBO Charges–Water (PCM) | ||||
|---|---|---|---|---|---|---|
| S(O) | O | Δ(S-O) | S(O) | O | Δ(S-O) | |
| 1 | 1.097 | −0.906 | 0.191 | 1.100 | −0.959 | 0.141 |
| 2 | 1.095 | −0.900 | 0.195 | 1.109 | −0.965 | 0.144 |
| 3 | 1.108 | −0.897 | 0.211 | 1.103 | −0.957 | 0.146 |
| Compound | NBO Orbitals–Gas Phase, % | NBO Orbitals–Water (PCM), % | ||||
| S(O) | O | S(O) | O | |||
| 1 | 36.49 | 63.51 | 36.08 | 63.92 | ||
| 2 | 36.54 | 63.46 | 36.14 | 63.86 | ||
| 3 | 36.56 | 63.44 | 36.17 | 63.83 | ||
| Compound | Gas phase | Water (PCM) | ||
|---|---|---|---|---|
| E | E + ZPE | E | E + ZPE | |
| 1 | −30.5566 | −27.8834 | −31.4194 | −28.7205 |
| 2 | −34.7697 | −31.6691 | −33.9137 | −31.0096 |
| 3 | −35.1643 | −32.3023 | −34.2955 | −31.4583 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makieieva, N.; Kupka, T.; Lodowski, P.; Balwierz, R.; Kasperkiewicz, K.; Byrski, A.; Konechna, R.; Lubenets, V. In Search of New Drugs: Elucidating the Activity of Structurally Similar Potential Antibiotics Using Molecular Modelling. Molecules 2025, 30, 2920. https://doi.org/10.3390/molecules30142920
Makieieva N, Kupka T, Lodowski P, Balwierz R, Kasperkiewicz K, Byrski A, Konechna R, Lubenets V. In Search of New Drugs: Elucidating the Activity of Structurally Similar Potential Antibiotics Using Molecular Modelling. Molecules. 2025; 30(14):2920. https://doi.org/10.3390/molecules30142920
Chicago/Turabian StyleMakieieva, Natalina, Teobald Kupka, Piotr Lodowski, Radosław Balwierz, Katarzyna Kasperkiewicz, Adam Byrski, Roksolana Konechna, and Vira Lubenets. 2025. "In Search of New Drugs: Elucidating the Activity of Structurally Similar Potential Antibiotics Using Molecular Modelling" Molecules 30, no. 14: 2920. https://doi.org/10.3390/molecules30142920
APA StyleMakieieva, N., Kupka, T., Lodowski, P., Balwierz, R., Kasperkiewicz, K., Byrski, A., Konechna, R., & Lubenets, V. (2025). In Search of New Drugs: Elucidating the Activity of Structurally Similar Potential Antibiotics Using Molecular Modelling. Molecules, 30(14), 2920. https://doi.org/10.3390/molecules30142920












