Reinforcing Urea–Formaldehyde Resins with Low-Cost, Mechanically Derived Nanocellulose: A Sustainable Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. Nanocellulose Characterization
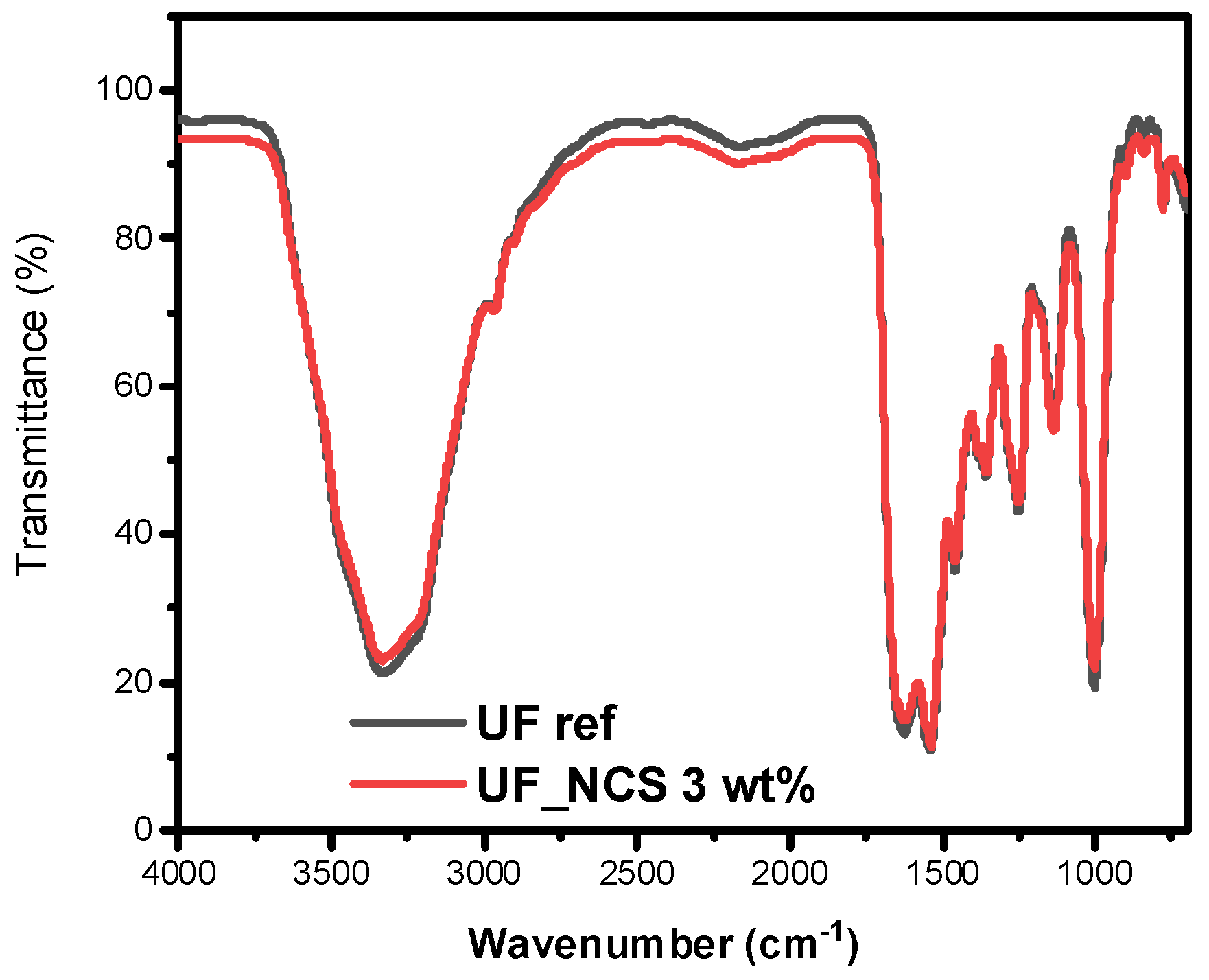
2.1.1. FT-IR Spectroscopy
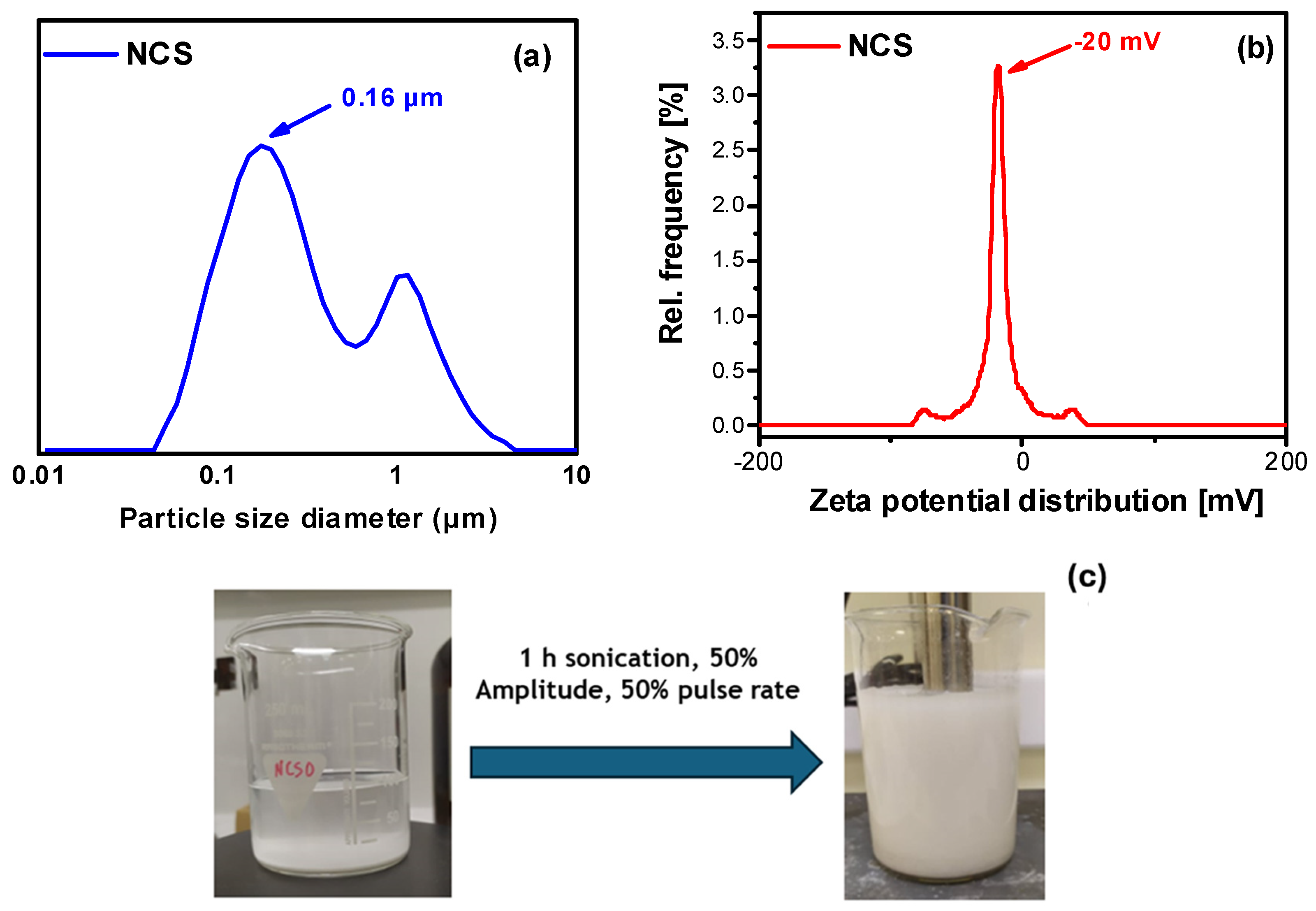
2.1.2. Particle Size Measurements and Colloidal Stability
2.1.3. Cellulose Crystallinity
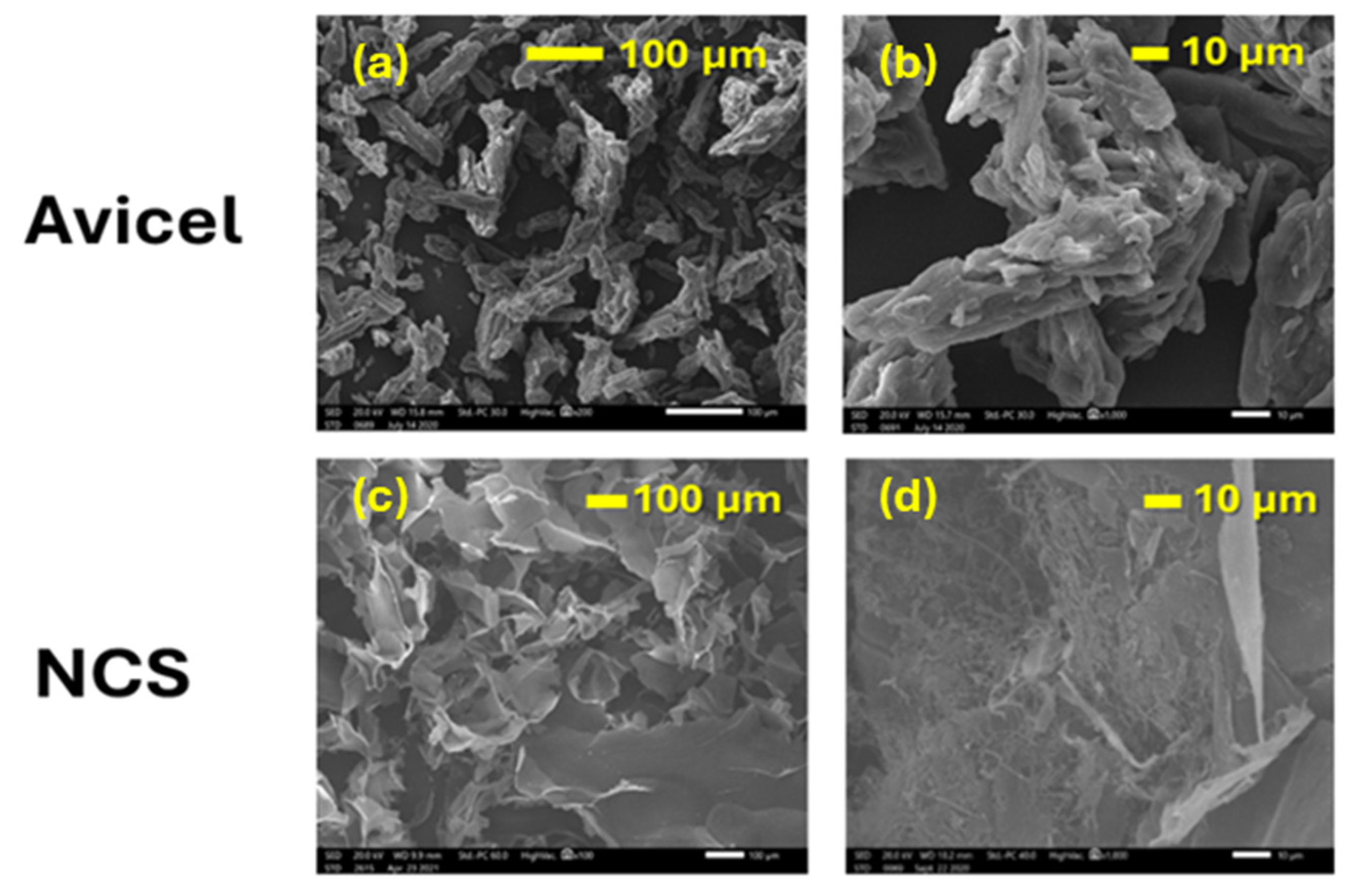
2.1.4. Morphologies of Parent and Ultrasonicated Cellulose Micro-/Nanoparticles
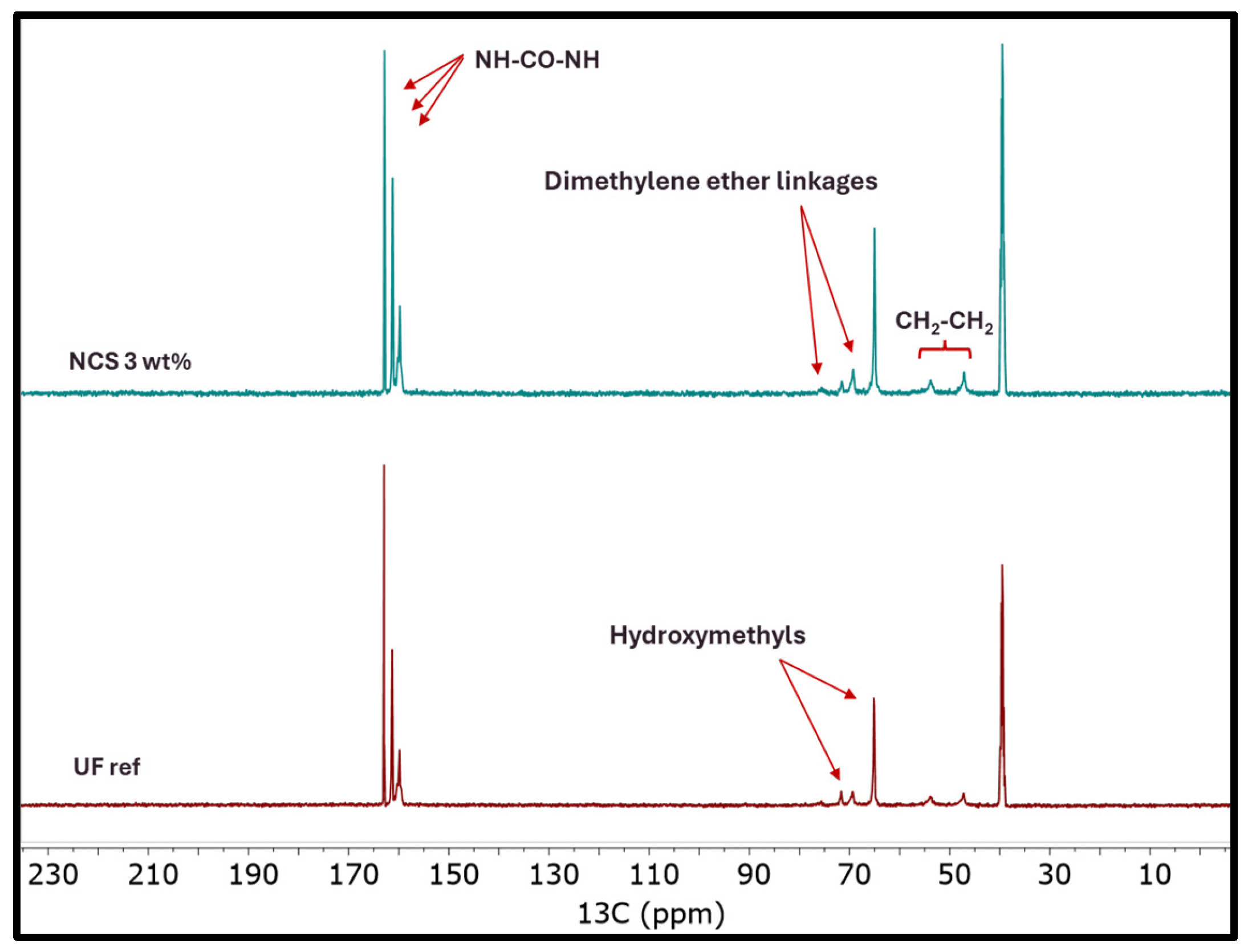
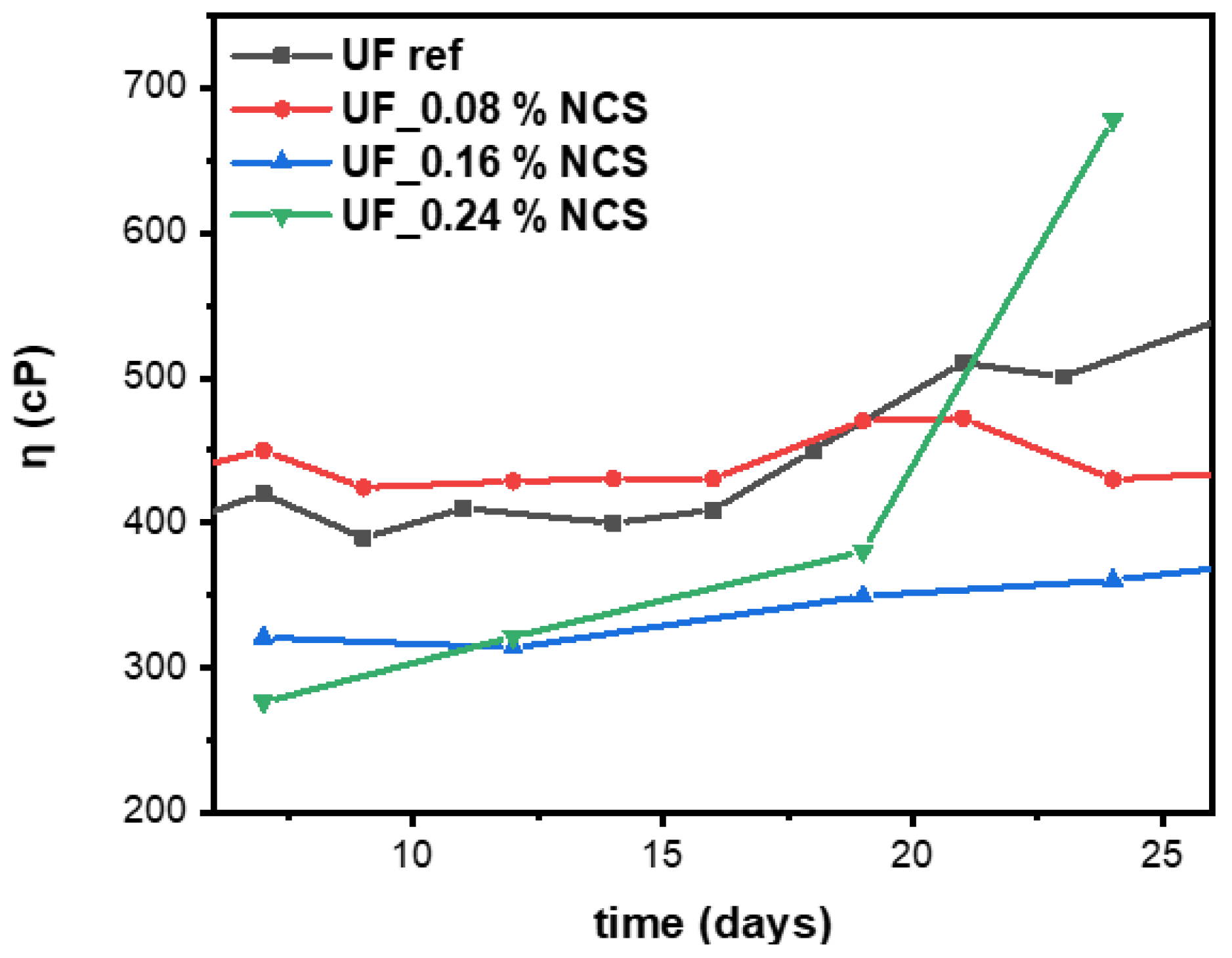
2.2. Nanocomposite UF Resin Characterization
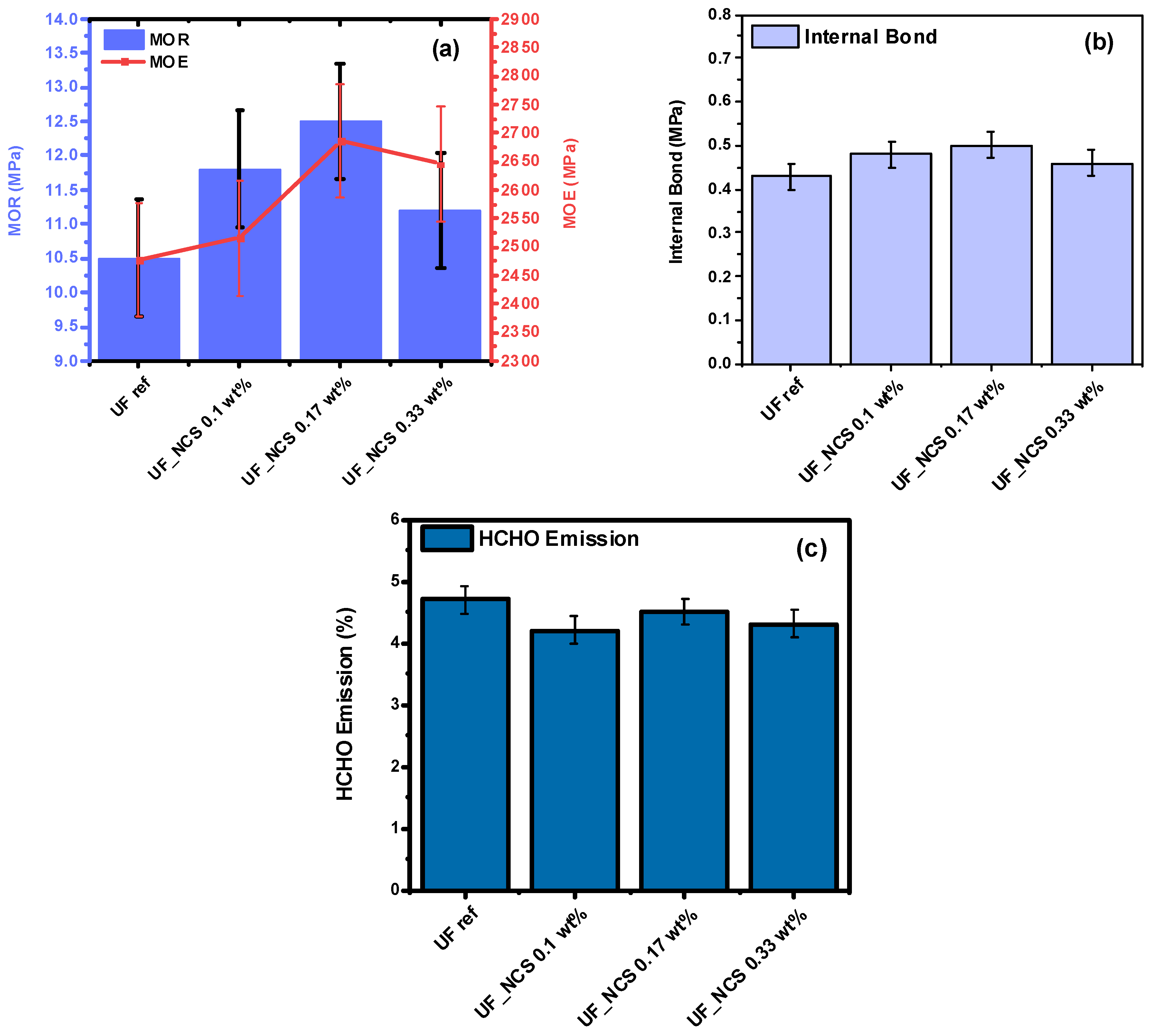
2.3. Particle Boards’ Properties
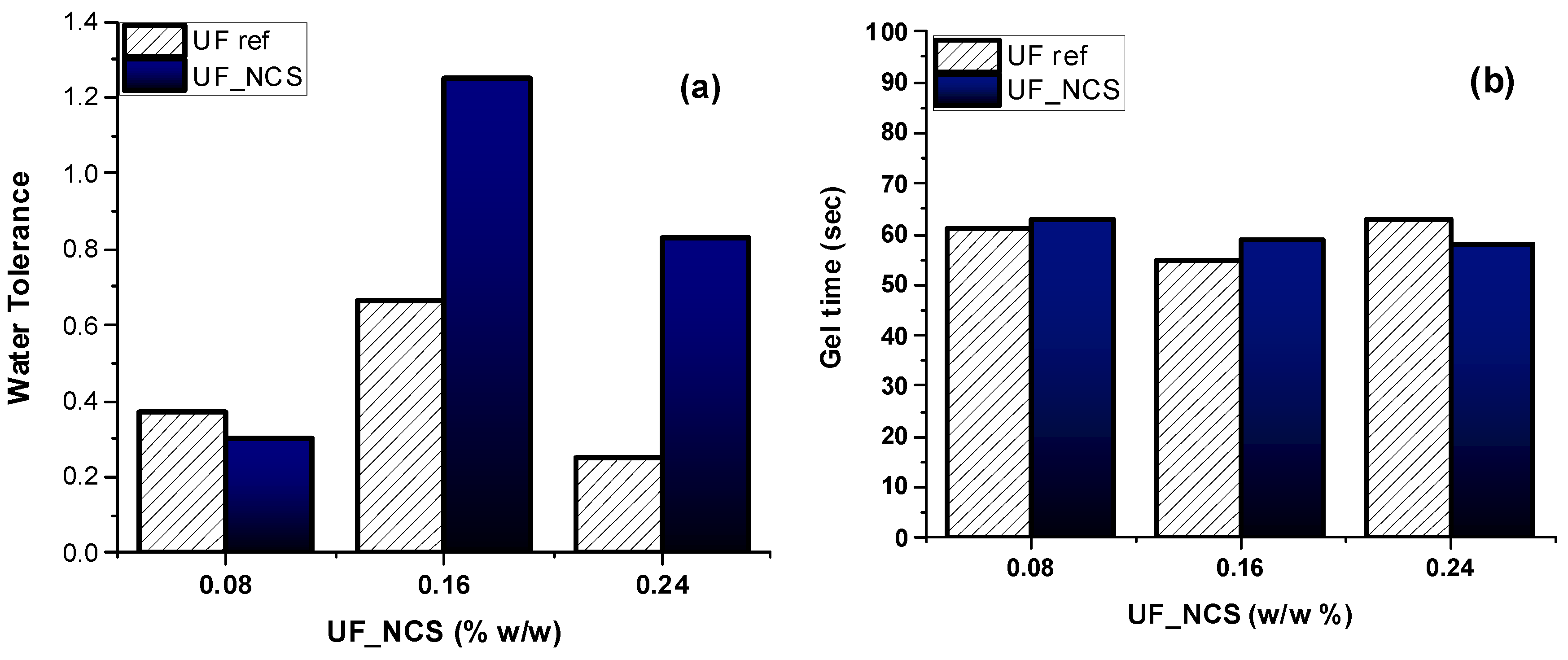
2.4. Effects of Nanocellulose Addition Protocol on Particle Boards’ Properties
3. Materials and Methods
3.1. Materials
3.2. Nanocellulose Production and Characterization
3.2.1. High-Intensity Ultrasonication
3.2.2. Dynamic Laser Scattering (DLS)
3.2.3. X-Ray Diffraction (XRD)
3.2.4. Fourier Transform Infrared Spectroscopy (FT-IR)
3.2.5. Scanning Electron Microscopy (SEM)
3.3. Synthesis and Characterization of Neat and Nanocomposite UF Resins
3.3.1. Synthesis of Reference and Nanocomposite UF Resins
3.3.2. Physicochemical Characterization of the Liquid UF Resins
3.4. Particle Board (PB) Production and Testing
3.4.1. Mechanical Testing
3.4.2. Particle Board Morphology
3.4.3. Differential Scanning Calorimetry (DSC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antunes, A.; Paiva, N.; Ferra, J.; Martins, J.; Carvalho, L.; Barros-Timmons, A.; Magalhães, F.D. Highly Flexible Glycol-Urea-Formaldehyde Resins. Eur. Polym. J. 2018, 105, 167–176. [Google Scholar] [CrossRef]
- Kawalerczyk, J.; Walkiewicz, J.; Dziurka, D.; Mirski, R.; Brózdowski, J. APTES-Modified Nanocellulose as the Formaldehyde Scavenger for UF Adhesive-Bonded Particleboard and Strawboard. Polymers 2022, 14, 5037. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Park, B.-D. Crystalline Lamellar Structure of Thermosetting Urea-Formaldehyde Resins at a Low Molar Ratio. Macromolecules 2021, 54, 2366–2375. [Google Scholar] [CrossRef]
- Myers, G.E. How Mole Ratio of Uf Resin Affects Formaldehyde Emission and Other Properties: A Literature Critique. For. Products J. 1984, 34, 35–41. [Google Scholar]
- Pizzi, A.; Lipschitz, L.; Valenzuela, J. Theory and Practice of the Preparation of Low Formaldehyde Emission Uf Adhesives. Holzforschung 1994, 48, 254–261. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Park, B.-D. Direct Measurement of Surface Adhesion between Thin Films of Nanocellulose and Urea–Formaldehyde Resin Adhesives. Cellulose 2021, 28, 8459–8481. [Google Scholar] [CrossRef]
- Dorieh, A.; Selakjani, P.P.; Shahavi, M.H.; Pizzi, A.; Movahed, S.G.; Pour, M.F.; Aghaei, R. International Journal of Adhesion and Adhesives Recent Developments in the Performance of Micro/Nanoparticle-Modified Urea-Formaldehyde Resins Used as Wood-Based Composite Binders: A Review. Int. J. Adhes. Adhes. 2022, 114, 103106. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Malletzidou, L.; Patsiaoura, D.; Magaziotis, A.; Psochia, E.; Terzopoulou, Z.; Chrissafis, K.; Markessini, C.; Papadopoulou, E.; Bikiaris, D.N. Synthesis and Characterization of Unsaturated Succinic Acid Biobased Polyester Resins. Appl. Sci. 2021, 11, 896. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Tang, J.; Zhang, J.; Shang, Q.; Hu, Y.; Wang, H.; Wu, Q.; Zhou, Y.; Lei, W.; et al. High-Performance Biobased Unsaturated Polyester Nanocomposites with Very Low Loadings of Graphene. Polymers 2018, 10, 1288. [Google Scholar] [CrossRef]
- Pizzi, A.; Papadopoulos, A.N.; Policardi, F. Wood Composites and Their Polymer Binders. Polymers 2020, 12, 1115. [Google Scholar] [CrossRef]
- Toriz, G.; Arvidsson, R.; Westin, M.; Gatenholm, P. Novel Cellulose Ester–Poly(Furfuryl Alcohol)–Flax Fiber Biocomposites. J. Appl. Polym. Sci. 2003, 88, 337–345. [Google Scholar]
- El Oudiani, A.; Msahli, S.; Sakli, F. In-Depth Study of Agave Fiber Structure Using Fourier Transform Infrared Spectroscopy. Carbohydr. Polym. 2017, 164, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Turki, A.; El Oudiani, A.; Msahli, S.; Sakli, F. Infrared Spectra for Alfa Fibers Treated with Thymol. J. Glycobiol. 2022, 7, 268–275. [Google Scholar] [CrossRef]
- Yang, X.; Han, F.; Xu, C.; Jiang, S.; Huang, L.; Liu, L.; Xia, Z. Effects of Preparation Methods on the Morphology and Properties of Nanocellulose (NC) Extracted from Corn Husk. Ind. Crop. Prod. 2017, 109, 241–247. [Google Scholar] [CrossRef]
- Margellou, A.G.; Psochia, E.A.; Torofias, S.A.; Pappa, C.P.; Triantafyllidis, K.S. Isolation of Highly Crystalline Cellulose via Combined Pretreatment/Fractionation and Extraction Procedures within a Biorefinery Concept. AcsSustain. Resour. Manag. 2024, 1, 1432–1443. [Google Scholar] [CrossRef]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Cellulose and Protein Nanofibrils: Singular Biobased Nanostructures for the Design of Sustainable Advanced Materials. Front. Bioeng. Biotechnol. 2022, 10, 1059097. [Google Scholar] [CrossRef]
- Psochia, E.A.; Delliere, P.; Sanchez, R.; Triantafyllidis, K.S.; Guigo, N. Sustainable Alliance between Nanocellulose and Biobased Poly Ur Uryl Alcohol. Ind. Crops Prod. 2024, 212, 118346. [Google Scholar]
- Inamuddin; Thomas, S.; Mishra, R.K.; Asiri, A.M. Sustainable Polymer Composites and Nanocomposites; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Veigel, S.; Rathke, J.; Weigl, M.; Gindl-Altmutter, W. Particle Board and Oriented Strand Board Prepared with Nanocellulose-Reinforced Adhesive. J. Ofnanomaterials 2012. [Google Scholar] [CrossRef]
- Mahrdt, E.; Pinkl, S.; Schmidberger, C.; Van Herwijnen, H.W.G.; Veigel, S.; Gindl-Altmutter, W. Effect of Addition of Microfibrillated Cellulose to Urea-Formaldehyde on Selected Adhesive Characteristics and Distribution in Particle Board. Cellulose 2015, 23, 571–580. [Google Scholar] [CrossRef]
- Karagiannidis, E.; Markessini, C.; Athanassiadou, E. Micro-Fibrillated Cellulose in Adhesive Systems for the Production of Wood-Based Panels. Molecules 2020, 25, 4846. [Google Scholar] [CrossRef]
- Kojima, Y.; Kato, N.; Ota, K.; Kobori, H.; Suzuki, S.; Aoki, K.; Ito, H. Cellulose Nanofiber as Complete Natural Binder for Particleboard. For. Prod. J. 2018, 68, 203–210. [Google Scholar] [CrossRef]
- Iglesias, M.C.; McMichael, P.S.; Asafu-Adjaye, O.; Via, B.K.; Peresin, M.S. Interfacial Interactions between Urea Formaldehyde and Cellulose Nanofibrils (CNFs) of Varying Chemical Composition and Their Impact on Particle Board (PB) Manufacture. Cellulose 2021, 28, 7969–7979. [Google Scholar] [CrossRef]
- Hansted, F.A.S.; Hansted, A.L.S.; Padilla, E.R.D.; Caraschi, J.C.; Goveia, D.; De Campos, C.I. The Use of Nanocellulose in the Production of Medium Density Particleboard Panels and the Modification of Its Physical Properties. BioResources 2019, 14, 5071–5079. [Google Scholar] [CrossRef]
- Júnior, R.R.M.; Cardoso, G.V.; Ferreira, E.S.; Costa, H.L. Surface characterization, mechanical and abrasion resistance of nanocellulose-reinforced wood panels. Surf. Topogr. Metrol. Prop. 2020, 8, 025011. [Google Scholar] [CrossRef]
- Amoroso, L.; Muratore, G.; Ortenzi, M.A.; Gazzotti, S.; Limbo, S.; Piergiovanni, L. Fast Production of Cellulose Nanocrystals by Hydrolytic-Oxidative Microwave-Assisted Treatment. Polymers 2020, 12, 68. [Google Scholar] [CrossRef]
- Zinge, C.; Kandasubramanian, B. Nanocellulose Based Biodegradable Polymers. Eur. Polym. J. 2020, 133, 109758. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, Q.; Liu, J.; Sun, J.; Zhu, Q.; Chen, H. Processing Nanocellulose to Bulk Materials: A Review. Cellulose 2019, 26, 7585–7617. [Google Scholar] [CrossRef]
- Indran, S.; Raj, R.E.; Sreenivasan, V.S. Characterization of New Natural Cellulosic Fiber from Cissus Quadrangularis Root. Carbohydr. Polym. 2014, 110, 423–429. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile Extraction of Thermally Stable Cellulose Nanocrystals with a High Yield of 93% through Hydrochloric Acid Hydrolysis under Hydrothermal Conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Kountouras, S.; Nikolaidou, Z.; Chrissafis, K.; Michailof, C.; Kalogiannis, K.; Lappas, A.A. Urea-Formaldehyde (UF) Resins Prepared by Means of the Aqueous Phase of the Catalytic Pyrolysis of European Beech Wood. COST Action FP1105. Holzforschung 2016, 70, 1139–1145. [Google Scholar] [CrossRef]
- Moslemi, A.; Koohi, M.Z.; Behzad, T.; Pizzi, A. Addition of Cellulose Nanofibers Extracted from Rice Straw to Urea Formaldehyde Resin; Effect on the Adhesive Characteristics and Medium Density Fiberboard Properties. Int. J. Adhes. Adhes. 2020, 99, 102582. [Google Scholar] [CrossRef]
- Zhang, H.; She, Y.; Song, S.P.; Pu, J.W. Modified Nanocrystalline Cellulose Used for Improving Formaldehyde Emission and Bonding Strength of Urea Formaldehyde Resin Adhesive. Key Eng. Mater. 2013, 562–565, 846–851. [Google Scholar] [CrossRef]
- Dunky, M. Urea-Formaldehyde Adhesive Resins for Wood. Int. J. Adhes. Adhesives. 1998, 18, 95–107. [Google Scholar]
- Moutousidis, D.; Karidi, K.; Athanassiadou, E.; Stylianou, E.; Giannakis, N.; Koutinas, A. Sustainable Chemistry for the Environment Reinforcement of Urea Formaldehyde Resins with Pectins Derived from Orange Peel Residues for the Production of Wood-Based Panels. Sustain. Chem. Environ. 2023, 4, 100037. [Google Scholar] [CrossRef]
- Kawalerczyk, J.; Dziurka, D.; Dukarska, D.; Woźniak, M.; Walkiewicz, J.; Mirski, R. The effect of urea-formaldehyde adhesive modification with diisocyanate-functionalized nanocellulose on the properties of particleboard. Int. J. Adhes. Adhes. 2024, 135, 103850. [Google Scholar] [CrossRef]
- Kong, X.; Wei, Z.; Xia, S.; Jia, B.; Gan, L.; Han, S. The characterizations of nanofluid type urea formaldehyde resins. Int. J. Adhes. Adhes. 2023, 126, 103451. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psochia, E.A.; Karagiannidis, E.; Athanasiadou, E.; Triantafyllidis, K.S. Reinforcing Urea–Formaldehyde Resins with Low-Cost, Mechanically Derived Nanocellulose: A Sustainable Approach. Molecules 2025, 30, 2911. https://doi.org/10.3390/molecules30142911
Psochia EA, Karagiannidis E, Athanasiadou E, Triantafyllidis KS. Reinforcing Urea–Formaldehyde Resins with Low-Cost, Mechanically Derived Nanocellulose: A Sustainable Approach. Molecules. 2025; 30(14):2911. https://doi.org/10.3390/molecules30142911
Chicago/Turabian StylePsochia, Eleni A., Emmanouil Karagiannidis, Eleftheria Athanasiadou, and Konstantinos S. Triantafyllidis. 2025. "Reinforcing Urea–Formaldehyde Resins with Low-Cost, Mechanically Derived Nanocellulose: A Sustainable Approach" Molecules 30, no. 14: 2911. https://doi.org/10.3390/molecules30142911
APA StylePsochia, E. A., Karagiannidis, E., Athanasiadou, E., & Triantafyllidis, K. S. (2025). Reinforcing Urea–Formaldehyde Resins with Low-Cost, Mechanically Derived Nanocellulose: A Sustainable Approach. Molecules, 30(14), 2911. https://doi.org/10.3390/molecules30142911








