Technical Lignins Antibacterial Effects Against Environmental Mastitis Pathogens Across Various Levels of Bedding Cleanliness In Vitro
Abstract
1. Introduction
2. Results
2.1. MIC and MBC Tests
2.2. Antimicrobial Activity of CND in Bedding Substrates
3. Discussion
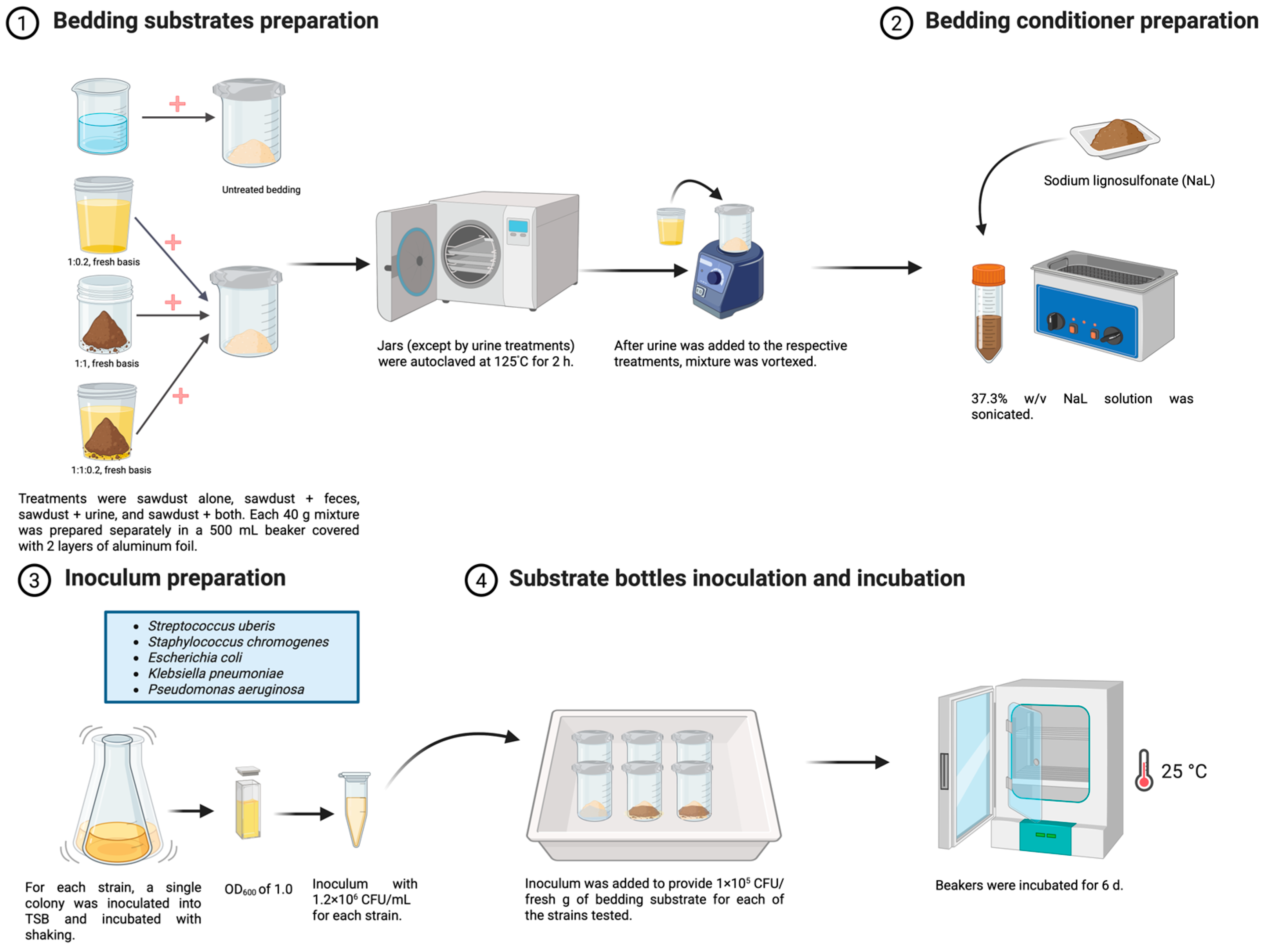
4. Materials and Methods
4.1. Minimum Inhibitory Concentration (MIC) and Maximum Bactericidal Concentration (MBC) Tests
4.1.1. Bacterial Inoculum Preparation
4.1.2. Bedding Conditioners (CND)
4.1.3. Antimicrobial Assays
4.2. Antimicrobial Activity of CND in Bedding Substrates
4.2.1. Bedding Substrates
4.2.2. Bedding Conditioners (CND)
4.2.3. Inoculum Preparation
4.2.4. Antimicrobial Activity Assay
4.2.5. Sampling and pH Analysis
4.2.6. Component Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production Effects Related to Mastitis and Mastitis Economics in Dairy Cattle Herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Schrick, F.N.; Hockett, M.E.; Saxton, A.M.; Lewis, M.J.; Dowlen, H.H.; Oliver, S.P. Influence of Subclinical Mastitis During Early Lactation on Reproductive Parameters. J. Dairy Sci. 2001, 84, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Singh, N.K.; Bhadwal, M.S. Relationship of Somatic Cell Count and Mastitis: An Overview. Asian Australas. J. Anim. Sci. 2011, 24, 429–438. [Google Scholar] [CrossRef]
- Malek Dos Reis, C.B.; Barreiro, J.R.; Mestieri, L.; Porcionato, M.A.D.F.; Dos Santos, M.V. Effect of Somatic Cell Count and Mastitis Pathogens on Milk Composition in Gyr Cows. BMC Vet. Res. 2013, 9, 67. [Google Scholar] [CrossRef]
- Eckersall, P.D.; Young, F.J.; McComb, C.; Hogarth, C.J.; Safi, S.; Fitzpatrick, J.L.; Nolan, A.M.; Weber, A.; McDonald, T. Acute Phase Proteins in Serum and Milk from Dairy Cows with Clinical Mastitis. Vet. Rec. 2001, 148, 35–41. [Google Scholar] [CrossRef]
- Jones, M.; Bailey, T. Understanding the Basics of Mastitis; Virginia Tech: Blacksburg, VA, USA, 2009. [Google Scholar]
- Isobe, N.; Iwamoto, C.; Kubota, H.; Yoshimura, Y. Relationship Between the Somatic Cell Count in Milk and Reproductive Function in Peripartum Dairy Cows. J. Reprod. Dev. 2014, 60, 433–437. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.M.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.M.; Puglia, D. Valorization of Acid Isolated High Yield Lignin Nanoparticles as Innovative Antioxidant/Antimicrobial Organic Materials. ACS Sustain. Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Hillerton, J.E.; Berry, E.A. The Management and Treatment of Environmental Streptococcal Mastitis. Vet. Clin. N. Am. Food Anim. Pract. 2003, 19, 157–169. [Google Scholar] [CrossRef]
- Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M. Biorefinery Review: Wide-Reaching Products Through Kraft Lignin. BioResources 2019, 14, 7543–7581. [Google Scholar]
- Hogan, J.S.; Bogacz, V.L.; Thompson, L.M.; Romig, S.; Schoenberger, P.S.; Weiss, W.P.; Smith, K.L. Bacterial Counts Associated with Sawdust and Recycled Manure Bedding Treated with Commercial Conditioners. J. Dairy Sci. 1999, 82, 1690–1695. [Google Scholar] [CrossRef]
- Olde Riekerink, R.G.M.; Barkema, H.W.; Kelton, D.F.; Scholl, D.T. Incidence Rate of Clinical Mastitis on Canadian Dairy Farms. J. Dairy Sci. 2008, 91, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.S.; Wolf, S.L.; Petersson-Wolfe, C.S. Bacterial Counts in Organic Materials Used as Free-Stall Bedding Following Treatment with a Commercial Conditioner. J. Dairy Sci. 2007, 90, 1058–1062. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Shelford, J.A.; Tucker, C.B.; Weary, D.M.; Von Keyserlingk, M.A.G. Bacterial Populations on Teat Ends of Dairy Cows Housed in Free Stalls and Bedded with Either Sand or Sawdust. J. Dairy Sci. 2004, 87, 1694–1701. [Google Scholar] [CrossRef]
- Rowbotham, R.F. Associations Between Dairy Cow Bedding and Milk Quality. Ph.D. Thesis, University of Wisconsin-Madison, Madison, WI, USA, 2015. [Google Scholar]
- USDA. Dairy Cattle Management Practices in the United States; USDA–APHIS–VS–CEAH–NAHMS: Fort Collins, CO, USA, 2016.
- Zadoks, R.N.; Middleton, J.R.; McDougall, S.; Katholm, J.; Schukken, Y.H. Molecular Epidemiology of Mastitis Pathogens of Dairy Cattle and Comparative Relevance to Humans. J. Mammary Gland. Biol. Neoplasia 2011, 16, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Sampimon, O.C.; Sol, J.; Kock, P.A. An outbreak of Klebsiella pneumoniae mastitis. Tijdschr. Diergeneeskd. 2006, 131, 2–4. [Google Scholar] [PubMed]
- Proietto, R.L.; Hinckley, L.S.; Fox, L.K.; Andrew, S.M. Evaluation of a Clay-Based Acidic Bedding Conditioner for Dairy Cattle Bedding. J. Dairy Sci. 2013, 96, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Clanton, C.J.; Endres, M.I.; Bey, R.F.; Farnsworth, R.J.; Janni, K.A.; Schmidt, D.R. Dolomitic Limestone Bedding Effects on Microbial Counts and Cow Comfort. Appl. Eng. Agric. 2005, 21, 1073–1077. [Google Scholar] [CrossRef]
- Reyes, D.C.; Annis, S.L.; Rivera, S.A.; Leon-Tinoco, A.Y.; Wu, C.; Perkins, L.B.; Perry, J.J.; Ma, Z.X.; Knight, C.W.; Castillo, M.S.; et al. In Vitro Screening of Technical Lignins to Determine Their Potential as Hay Preservatives. J. Dairy Sci. 2020, 103, 6114–6134. [Google Scholar]
- Leon-Tinoco, A.Y.; Annis, S.L.; Almeida, S.T.; Guimarães, B.C.; Killerby, M.; Zhang, J.; Wu, C.; Perkins, L.B.; Ma, Z.; Jeong, K.C.; et al. Evaluating the Potential of Lignosulfonates and Chitosans as Alfalfa Hay Preservatives Using In Vitro Techniques. J. Anim. Sci. 2022, 100, skac154. [Google Scholar] [CrossRef]
- Durmaz, S.; Erisir, E.; Yildiz, U.C.; Kurtulus, O.C. Using Kraft Black Liquor as a Wood Preservative. Procedia Soc. Behav. Sci. 2015, 195, 2177–2180. [Google Scholar] [CrossRef]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-Adding to Cellulosic Ethanol: Lignin Polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Pang, B.; Yan, J.; Yao, L.; Liu, H.; Guan, J.; Wang, H.; Liu, H. Preparation and Characterization of Antibacterial Paper Coated with Sodium Lignosulfonate Stabilized ZnO Nanoparticles. RSC Adv. 2016, 6, 9753–9759. [Google Scholar] [CrossRef]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and Antioxidant Activities of Lignin from Residue of Corn Stover to Ethanol Production. Ind. Crops Prod. 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Kim, S.; Fernandes, M.M.; Matamá, T.; Loureiro, A.; Gomes, A.C.; Cavaco-Paulo, A. Chitosan–Lignosulfonates Sono-Chemically Prepared Nanoparticles: Characterisation and Potential Applications. Colloids Surf. B Biointerfaces 2013, 103, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Kumar, A. Deciphering the Role of Sodium Lignosulfonate Against Candida Spp. as Persuasive Anticandidal Agent. Int. J. Biol. Macromol. 2018, 107, 1212–1219. [Google Scholar] [CrossRef]
- Wang, G.; Pang, T.; Xia, Y.; Liu, X.; Li, S.; Parvez, A.M.; Kong, F.; Si, C. Subdivision of Bamboo Kraft Lignin by One-Step Ethanol Fractionation to Enhance Its Water-Solubility and Antibacterial Performance. Int. J. Biol. Macromol. 2019, 133, 156–164. [Google Scholar] [CrossRef]
- Zemek, J.; Košíková, B.; Augustín, J.; Joniak, D. Antibiotic Properties of Lignin Components. Folia Microbiol. 1979, 24, 483–486. [Google Scholar] [CrossRef]
- Barber, M.S.; McConnell, V.S.; DeCaux, B.S. Antimicrobial Intermediates of the General Phenylpropanoid and Lignin Specific Pathways. Phytochemistry 2000, 54, 53–56. [Google Scholar] [CrossRef]
- Reyes, D.C.; Ma, Z.; Romero, J.J. The Antimicrobial Properties of Technical Lignins and Their Derivatives—A Review. Polymers 2024, 16, 2181. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; De Jong, E.; Guran, B.; Abächerli, A. Co-Ordination Network for Lignin—Standardisation, Production and Applications Adapted to Market Requirements (EUROLIGNIN). Ind. Crops Prod. 2004, 20, 121–129. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Beales, N. Adaptation of Microorganisms to Cold Temperatures, Weak Acid Preservatives, Low pH, and Osmotic Stress: A Review. Compr. Rev. Food Sci. Food Saf. 2004, 3, 1–20. [Google Scholar] [CrossRef] [PubMed]
- O’May, G.A.; Reynolds, N.; Macfarlane, G.T. Effect of pH on an in Vitro Model of Gastric Microbiota in Enteral Nutrition Patients. Appl. Environ. Microbiol. 2005, 71, 4777–4783. [Google Scholar] [CrossRef]
- Freu, G.; Fusar Poli, S.; Monistero, V.; Biscarini, F.; Rota, N.; Hossain, D.; Gusmara, C.; Musa, L.; Gioia, G.; Leso, L.; et al. Effect of a Lime-Based Bedding Conditioner on Physical-Chemical Characteristics and Microbiological Counts of Recycled Manure Solids. Front. Vet. Sci. 2024, 11, 1408798. [Google Scholar] [CrossRef]
- Kuechle, B.; Godden, S.; Bey, R. Effect of Two Bedding Conditioners on Bacteria Counts and pH in Shavings, Digested Manure Solids and Recycled Sand Bedding. Am. Assoc. Bov. Pract. Conf. Proc. 2008, 223. [Google Scholar] [CrossRef]
- Sommer, S.G.; Hutchings, N.J. Ammonia Emission from Field Applied Manure and Its Reduction—Invited Paper. Eur. J. Agron. 2001, 15, 1–15. [Google Scholar] [CrossRef]
- Larney, F.J.; Hao, X. A Review of Composting as a Management Alternative for Beef Cattle Feedlot Manure in Southern Alberta, Canada. Bioresour. Technol. 2007, 98, 3221–3227. [Google Scholar] [CrossRef]
- Battelli, M.; Rapetti, L.; Rota Graziosi, A.; Colombini, S.; Crovetto, G.M.; Galassi, G. Use of Undigested NDF for Estimation of Diet Digestibility in Growing Pigs. Animals 2020, 10, 2007. [Google Scholar] [CrossRef]
- Duniere, L.; Frayssinet, B.; Achard, C.; Chevaux, E.; Plateau, J. Conditioner Application Improves Bedding Quality and Bacterial Composition with Potential Beneficial Impacts for Dairy Cow’s Health. Microbiol. Spectr. 2024, 12, e04263-23. [Google Scholar] [CrossRef]
- Fréchette, A.; Généreux, M.; Fecteau, G.; Côté, C.; Dufour, S. Ability of Unsterilized Recycled Manure Solids Bedding to Support Growth of Klebsiella pneumoniae and Escherichia coli. Can. J. Vet. Res. 2024, 88, 12–18. [Google Scholar]
- Patel, K.; Godden, S.M.; Royster, E.; Crooker, B.A.; Timmerman, J.; Fox, L. Relationships Among Bedding Materials, Bedding Bacteria Counts, Udder Hygiene, Milk Quality, and Udder Health in US Dairy Herds. J. Dairy Sci. 2019, 102, 10213–10234. [Google Scholar] [CrossRef]
- Ahn, G.C.; Jang, S.S.; Lee, K.Y.; Baek, Y.C.; Oh, Y.K.; Park, K.K. Characteristics of Sawdust, Wood Shavings and Their Mixture from Different Pine Species as Bedding Materials for Hanwoo Cattle. Asian-Australas. J. Anim. Sci. 2020, 33, 856–865. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Spigno, G.; Labidi, J. Antioxidant and Biocide Behaviour of Lignin Fractions from Apple Tree Pruning Residues. Ind. Crops Prod. 2017, 104, 242–252. [Google Scholar] [CrossRef]
- Ruwoldt, J. A Critical Review of the Physicochemical Properties of Lignosulfonates: Chemical Structure and Behavior in Aqueous Solution, at Surfaces and Interfaces. Surfaces 2020, 3, 622–648. [Google Scholar] [CrossRef]
- Reyes, D.C.; Rivera, S.A.; Ma, Z.X.; Dubuc, H.M.; Leon-Tinoco, A.Y.; Lichtenwalner, A.B.; Romero, J.J. Mitigating Environmental Mastitis Microbes with the Novel Use of Paper Mill Byproducts. J. Dairy Sci. 2019, 102, 182–183. [Google Scholar]
- 21 CFR § 573.600; Food and Drugs Lignin Sulfonates. U.S. Government Publishing Office: Washington, DC, USA, 1976.
- Werker, A.G. The Effect of pH on Microbial Activity and Community Structure in the Biological Removal of Resin Acids from Wastewater. Ph.D. Thesis, The University of British Columbia, Kelowna, BC, Canada, 1998. [Google Scholar]
- Núñez-Flores, R.; Giménez, B.; Fernández-Martín, F.; López-Caballero, M.E.; Montero, M.P.; Gómez-Guillén, M.C. Role of Lignosulphonate in Properties of Fish Gelatin Films. Food Hydrocoll. 2012, 27, 60–71. [Google Scholar] [CrossRef]
- Principles and Practice of Disinfection, Preservation and Sterilisation; Russell, A.D., Hugo, W.B., Eds.; Blackwell: Oxford, UK, 1982; ISBN 978-0-632-00547-5. [Google Scholar]
- Garrido, M.N.; Skjervheim, M.; Oppegaard, H.; Sørum, H. Acidified Litter Benefits the Intestinal Flora Balance of Broiler Chickens. Appl. Environ. Microbiol. 2004, 70, 5208–5213. [Google Scholar] [CrossRef]
- Dizhbite, T. Characterization of the Radical Scavenging Activity of Lignins-Natural Antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef]
- Chen, X.; Lan, W.; Xie, J. Natural Phenolic Compounds: Antimicrobial Properties, Antimicrobial Mechanisms, and Potential Utilization in the Preservation of Aquatic Products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef]
- Tomani, P. THE Lignoboost Process. Cellul. Chem. Technol. 2010, 44, 53. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Wu, L. Effect of Chlorogenic Acid on Antioxidant Activity of Flos Lonicerae Extracts. J. Zhejiang Univ.-Sci. B 2007, 8, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Plank, D.W.; Szpylka, J.; Sapirstein, H.; Woollard, D.; Zapf, C.M.; Lee, V.; Chen, C.Y.O.; Liu, R.H.; Tsao, R.; Düsterloh, A.; et al. Determination of antioxidant activity in foods and beverages by reaction with 2,2′-diphenyl-1-picrylhydrazyl (DPPH): Collaborative study First Action 2012.04. J. AOAC Int. 2012, 95, 1562–1569. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. 69th Meeting. In Compendium of Food Additive Specifications; Food and Agriculture Organization: Rome, Italy, 2008. [Google Scholar]
- Beliciu, C.M.; Sauer, A.; Moraru, C.I. The Effect of Commercial Sterilization Regimens on Micellar Casein Concentrates. J. Dairy Sci. 2012, 95, 5510–5526. [Google Scholar] [CrossRef] [PubMed]
- Piyasena, P.; Mohareb, E.; McKellar, R.C. Inactivation of Microbes Using Ultrasound: A Review. Int. J. Food Microbiol. 2003, 87, 207–216. [Google Scholar] [CrossRef]
- Ma, Z.; Kim, D.; Adesogan, A.T.; Ko, S.; Galvao, K.; Jeong, K.C. Chitosan Microparticles Exert Broad-Spectrum Antimicrobial Activity Against Antibiotic-Resistant Micro-Organisms Without Increasing Resistance. ACS Appl. Mater. Interfaces 2016, 8, 10700–10709. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Church, D.C. The Ruminant Animal: Digestive Physiology and Nutrition; Waveland Press: Prospective Heights, IL, USA, 1993. [Google Scholar]
- Weatherburn, M.W. Phenol-Hypochlorite Reaction for Determination of Ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
| Bacteria 1 | Trt 2 | ||||
|---|---|---|---|---|---|
| NaL | WKL | AKL | MgL | ||
| S. chromogenes KCJ4610 | MIC | 5 ± 0 | 30 ± 0 | 2.5 ± 0 | 10 ± 0 |
| MBC | 7.5 ± 0 | 60 ± 0 | 5 ± 0 | 12.5 ± 0 | |
| S. chromogenes KCJ4679 | MIC | 6.25 ± 2.5 | >60 | 16.6 ± 11.5 | 25 ± 5.77 |
| MBC | 8.75 ± 0.6 | - | 50 ± 17.3 | 30 ± 4.8 | |
| S. uberis BAA-854 | MIC | 10 ± 0 | >60 | 2.5 ± 0 | 15 ± 0 |
| MBC | 15 ± 0 | >60 | 2.5 ± 0 | 40 ± 0 | |
| S. uberis KCJ145 | MIC | 5.8 ± 1.4 | >60 | 10 ± 0 | 23.3 ± 5.77 |
| MBC | 5.8 ± 1.4 | - | >60 | 30 ± 0 | |
| P. aeruginosa KCJ4633 | MIC | 35 ± 0 | 50 ± 0 | 50 ± 0 | 60 ± 0 |
| MBC | >60 | >60 | >60 | >60 | |
| P. aeruginosa KCJ4686 | MIC | 16.6 ± 5.77 | >60 | >60 | 26.6 ± 11.5 |
| MBC | 20 ± 0 | - | - | 40 ± 0 | |
| E. coli 10.0371 | MIC | 60 ± 0 | 60 ± 0 | >60 | 30 ± 0 |
| MBC | 60 ± 0 | >60 | >60 | 30 ± 0 | |
| E. coli KCJ3819 | MIC | 27.5 ± 5.0 | >60 | >60 | 20.0 ± 0 |
| MBC | 30 ± 8.2 | - | - | 56.7 ± 5.77 | |
| K. pneumoniae KCJ4749 | MIC | 15 ± 0 | n.d | 50 ± 0 | 20 ± 0 |
| MBC | 20 ± 0 | >60 | >60 | 20 ± 0 | |
| K. pneumoniae KCJ4740 | MIC | 8.33 ± 2.88 | n.d | 2.5 ± 0 | 10 ± 0 |
| MBC | 40 ± 0 | 56.7 ± 1.25 | 4.37 ± 1.25 | 10 ± 0 | |
| Item | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| CND | CLN | DAY | CND × CLN | CND × DAY | CLN × DAY | CND × CLN × DAY | |
| Bedding chemical composition | |||||||
| DM, % | 0.936 | <0.001 | 0.662 | 0.859 | 0.520 | 0.137 | 0.254 |
| OM, % of DM | <0.001 | <0.001 | 0.649 | 0.007 | 0.032 | 0.560 | 0.548 |
| WSC, % of DM | <0.001 | <0.001 | 0.007 | 0.658 | 0.860 | 0.011 | 0.739 |
| N, % of DM | 0.816 | <0.001 | 0.102 | 0.762 | 0.201 | 0.639 | 0.659 |
| NH3-N, % of total N | 0.002 | 0.004 | 0.271 | 0.892 | 0.944 | 0.362 | 0.779 |
| NDF, % of DM | 0.002 | <0.001 | 0.008 | 0.699 | 0.269 | 0.499 | 0.915 |
| pH | 0.102 | <0.001 | 0.002 | 0.003 | 0.002 | <0.001 | 0.005 |
| Pathogen counts (log CFU/fresh g) | |||||||
| Escherichia coli 10.0371 | <0.001 | <0.001 | <0.001 | <0.001 | 0.228 | <0.001 | 0.959 |
| Streptococcus uberis BAA-854 | <0.001 | <0.001 | <0.001 | 0.165 | 0.005 | <0.001 | 0.179 |
| Staphylococcus chromogenes, KCJ4610 | <0.001 | <0.001 | <0.001 | 0.197 | 0.002 | <0.001 | 0.082 |
| Pseudomonas aeruginosa, KCJ4633 | <0.001 | <0.001 | <0.001 | 0.549 | 0.114 | <0.001 | 0.471 |
| Klebsiella pneumoniae, KCJ4749 | <0.001 | <0.001 | <0.001 | <0.001 | 0.686 | <0.001 | 0.987 |
| Item | Bedding Conditioner | SEM | |
|---|---|---|---|
| CON | NaL-O | ||
| Pathogen counts (log CFU/fresh g of substrate) | |||
| Staphylococcus chromogenes KCJ4610 | 0.094 | ||
| d 0 | 3.38 A, y | 2.41 B, z | |
| d 2 | 3.50 A, y | 2.75 B, y | |
| d 6 | 3.95 A, x | 3.52 B, x | |
| Streptococcus uberis BAA-854 | 0.154 | ||
| d 0 | 3.02 A, y | 1.72 B, z | |
| d 2 | 3.19 A, y | 2.11 B, y | |
| d 6 | 3.69 A, x | 3.19 B, x | |
| Chemical properties | |||
| OM 2 (% of DM) | 0.227 | ||
| d 0 | 98.1 A | 97.7 B, x | |
| d 6 | 98.4 A | 97.3 B, y | |
| Item | Bedding Cleanliness | SEM | |||
|---|---|---|---|---|---|
| SD | SDU | SDF | SDFU | ||
| Pathogen counts (log CFU/fresh g of substrate) | |||||
| Escherichia coli 10.0371 | 0.032 | ||||
| CON | 4.07 B, x | 3.88 C, x | 5.09 A, x | 5.05 A, x | |
| NaL-O | 3.76 C, y | 3.48 D, y | 4.98 A, y | 4.89 B, y | |
| Klebsiella pneumoniae KCJ4749 | 0.018 | ||||
| CON | 4.20 B, x | 4.01 C, x | 5.13 A, x | 5.10 A, x | |
| NaL-O | 3.94 B, y | 3.68 C, y | 5.01 A, y | 4.99 A, y | |
| Chemical properties | |||||
| OM 3 (% of DM) | 0.27 | ||||
| CON | 98.8 A | 98.9 A | 98.0 B, x | 97.3 C, x | |
| NaL-O | 98.9 A | 98.3 B | 96.6 C, y | 96.3 C, y | |
| Item | Incubation Days | SEM | ||
|---|---|---|---|---|
| 0 | 2 | 6 | ||
| pH | 0.103 | |||
| SD | ||||
| NaL-O | 4.11 | 4.10 | 4.03 | |
| CON | 3.87 | 3.85 | 3.78 | |
| SDU | ||||
| NaL-O | 4.58 A | 4.49 B | 4.42 B | |
| CON | 4.50 A | 4.41 B | 4.32 B | |
| SDF | ||||
| NaL-O | 5.03 B | 5.11 AB | 5.19 A, n | |
| CON | 5.14 C | 5.27 B | 5.54 A, m | |
| SDFU | ||||
| NaL-O | 5.03 B, y | 5.19 A, y | 5.23 A, y | |
| CON | 5.49 C, x | 5.62 B, x | 6.12 A, x | |
| Item | Bedding Cleanliness | SEM | |||
|---|---|---|---|---|---|
| SD | SDU | SDF | SDFU | ||
| Pathogen counts (log CFU/fresh g) | |||||
| Escherichia coli 10.0371 | 0.033 | ||||
| d 0 | 3.88 C, y | 3.62 D, y | 4.37 A, y | 4.26 B, y | |
| d 2 | 3.79 C, z | 3.64 D, y | 4.38 A, y | 4.28 B, y | |
| d 6 | 4.06 B, x | 3.79 C, x | 6.36 A, x | 6.37 A, x | |
| Klebsiella pneumoniae KCJ4749 | 0.019 | ||||
| d 0 | 4.01 B, y | 3.77 C, y | 4.41 A, y | 4.36 A, y | |
| d 2 | 4.00 B, y | 3.81 C, y | 4.41 A, y | 4.38 A, y | |
| d 6 | 4.19 B, x | 3.95 C, x | 6.39 A, x | 6.40 A, x | |
| Streptococcus uberis BAA-854 | 0.197 | ||||
| d 0 | 2.10 B, y | 2.31 B, y | 3.04 A, y | 2.05 B, y | |
| d 2 | 2.24 B, y | 2.72 B, xy | 3.22 A, xy | 2.45 B, y | |
| d 6 | 2.81 C, x | 3.13 BC, x | 3.59 B, x | 4.23 A, x | |
| Staphylococcus chromogenes KCJ4610 | 0.119 | ||||
| d 0 | 2.95 B, y | 2.49 C, z | 3.26 A, y | 2.86 B, y | |
| d 2 | 3.09 BC, y | 3.00 C, y | 3.33 A, y | 3.07 BC, y | |
| d 6 | 3.53 BC, x | 3.34 C, x | 3.69 B, x | 4.37 A, x | |
| Pseudomonas aeruginosa KCJ4633 | 0.244 | ||||
| d 0 | 2.06 B | 0.56 C, z | 2.70 A, y | 1.87 B, z | |
| d 2 | 2.13 BC | 1.71 C, y | 2.72 A, y | 2.36 AB, y | |
| d 6 | 2.42 B | 2.70 B, x | 3.42 A, x | 3.89 A, x | |
| Chemical properties | |||||
| WSC (% of DM) | 0.283 | ||||
| d 0 | 0.82 B | 1.43 A | 1.74 A, y | 1.94 A, y | |
| d 6 | 0.76 C | 1.32 B | 2.75 A, x | 2.61 A, x | |
| Microorganism Name (Genus, Species, Strain Name) | Source |
|---|---|
| Streptococcus uberis BAA-854 | ATCC |
| Streptococcus uberis KCJ145 | University of Florida |
| Staphylococcus chromogenes KCJ4610 | University of Florida |
| Staphylococcus chromogenes KCJ4679 | University of Florida |
| Escherichia coli 10.0371 | Penn State University |
| Escherichia coli KCJ3819 | University of Florida |
| Klebsiella pneumoniae KCJ4749 | University of Florida |
| Klebsiella pneumoniae KCJ4740 | University of Florida |
| Pseudmonas aeruginosa KCJ4633 | University of Florida |
| Pseudmonas aeruginosa KCJ4686 | University of Florida |
| Lignin 1 | Total Soluble Phenolics 2 | ORAC 3 (Mmol Trolox Equivalent (TE)/g DM) | DPPH Scavenging Effect 4 | WSC 5 | Ash 6 | Magnesium 7 | Sodium | Sulfur |
|---|---|---|---|---|---|---|---|---|
| (mg/g DM) | % of DM | |||||||
| AKL | 219.1 | 10.53 | −4.8 | 18.05 | 19.1 | 0.02 | 6.86 | 4.80 |
| WKL | 322.2 | 728.1 | 74.0 | 0.11 | 0.26 | 0.01 | 0.04 | 1.51 |
| NaL | 184.3 | 12.1 | 14.2 | 22.8 | 33.9 | 0.05 | 12.8 | 8.01 |
| MgL | 142.5 | 10.1 | 10.5 | 15.7 | 13.6 | 6.21 | 0.04 | 8.25 |
| Pooled SD | 9.14 | 34.08 | 12.7 | 0.45 | 0.27 | 0.034 | 0.084 | 0.142 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oppong, G.M.; Reyes, D.C.; Ma, Z.; Rivera, S.A.; Killerby, M.A.; Zamudio, D.; Lichtenwalner, A.B.; Romero, J.J. Technical Lignins Antibacterial Effects Against Environmental Mastitis Pathogens Across Various Levels of Bedding Cleanliness In Vitro. Molecules 2025, 30, 2904. https://doi.org/10.3390/molecules30142904
Oppong GM, Reyes DC, Ma Z, Rivera SA, Killerby MA, Zamudio D, Lichtenwalner AB, Romero JJ. Technical Lignins Antibacterial Effects Against Environmental Mastitis Pathogens Across Various Levels of Bedding Cleanliness In Vitro. Molecules. 2025; 30(14):2904. https://doi.org/10.3390/molecules30142904
Chicago/Turabian StyleOppong, Godloves M., Diana C. Reyes, Zhengxin Ma, Santiago A. Rivera, Marjorie A. Killerby, Diego Zamudio, Anne B. Lichtenwalner, and Juan J. Romero. 2025. "Technical Lignins Antibacterial Effects Against Environmental Mastitis Pathogens Across Various Levels of Bedding Cleanliness In Vitro" Molecules 30, no. 14: 2904. https://doi.org/10.3390/molecules30142904
APA StyleOppong, G. M., Reyes, D. C., Ma, Z., Rivera, S. A., Killerby, M. A., Zamudio, D., Lichtenwalner, A. B., & Romero, J. J. (2025). Technical Lignins Antibacterial Effects Against Environmental Mastitis Pathogens Across Various Levels of Bedding Cleanliness In Vitro. Molecules, 30(14), 2904. https://doi.org/10.3390/molecules30142904






