Preparation, Physicochemical Properties and Stability of Anthocyanin Nanoliposomes Before and After Double-Layer Modification Using Synanthrin and Pea Protein Isolate
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Preparation Conditions for ACN-NLs via Single-Factor Experiments
2.1.1. Effect of Mass Ratio of Soybean Lecithin to Cholesterol on Preparation of ACN-NLs
2.1.2. Effect of Mass Ratio of Soybean Lecithin to Anhydrous Ethanol on Preparation of ACN-NLs
2.1.3. Influence of Drug-to-Lipid Ratio on Preparation of ACN-NLs
2.2. Results of Orthogonal Experiment for Preparation of ACN-NLs
2.3. Effects of pH and PPI Concentration on the Preparation of PPI-ACN-NLs
2.3.1. Effect of pH Value on the Preparation of PPI-ACN-NLs
2.3.2. Effect of PPI Concentration on the Preparation of PPI-ACN-NLs
2.4. Effect of SY Concentration on the Preparation of SY-PPI-ACN-NLs
2.5. Analysis of Appearance, Particle Size and Zeta Potential Results of ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs
2.6. ABTS+ Free Radical Scavenging Capacity of ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs
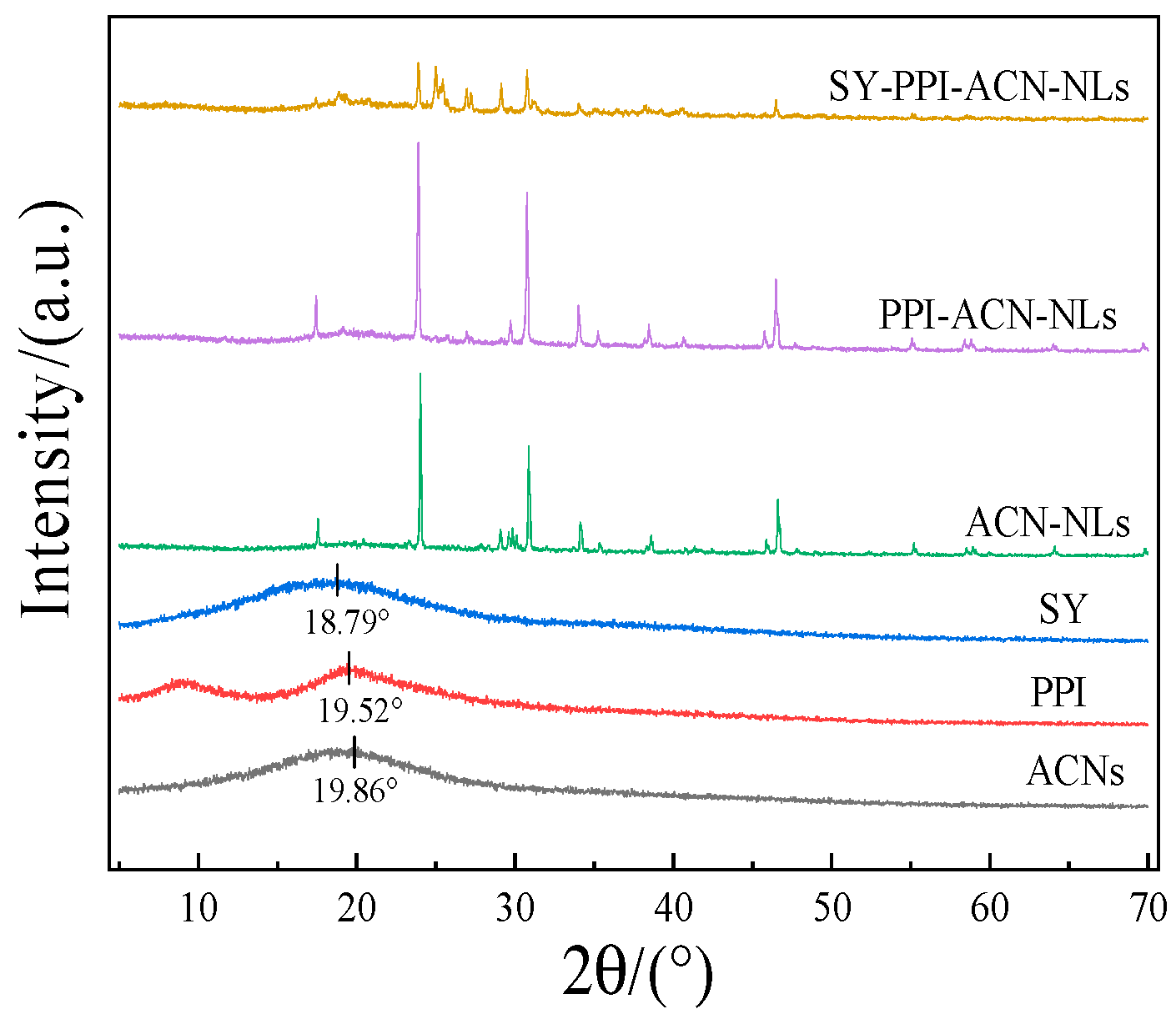
2.7. X-Ray Diffraction (XRD) Analysis by ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs
2.8. In Vitro Digestive Analysis of ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs
2.9. Analysis of Stability of ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs at Different Temperatures
2.10. Analysis of Storage ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs of Liposomes Under Light
2.11. Analysis of Storage Stability Results of ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs in Beverage Model
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Production of ACN-NLs
3.2.2. Process Optimization of ACN-NLs via Reverse Evaporation Method
3.2.3. Orthogonal Test of ACN-NLs
3.2.4. Preparation of PPI and SY Reserve Solution
3.2.5. Preparation of Anthocyanin Nanoliposomes Modified by PPI
3.2.6. Preparation of Bilayer Modified Anthocyanin Nanoliposomes with SY and PPI
3.3. Characterization of ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs
3.3.1. Encapsulation Efficiency and Release Rate of ACN-NLs, PPI-ACN-NLs, and SY-PPI-ACN-NLs
3.3.2. Determination of Particle Size and Zeta Potential for ACN-NLs, PPI-ACN-NLs, and SY-PPI-ACN-NLs
3.3.3. Determination of Color Difference for ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs
3.3.4. Determination of ABTS Free Radical Scavenging Efficiency in ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs
3.3.5. X-Ray Diffraction (XRD) Analysis of ACN-NLs, PPI-ACN-NLs, and SY-PPI-ACN-NLs
3.3.6. Determination of Malondialdehyde (MDA) Content
3.4. In Vitro Digestion Study of ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs
3.5. Study of Storage Stability of ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs
3.5.1. Stability Experiment of ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs at Different Temperatures
3.5.2. Storage Stability of ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs Under Light
3.5.3. Storage Stability of ACN-NLs, PPI-ACN-NLs and SY-PPI-ACN-NLs in the Food Simulation System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Cheng, H.; Chen, J.; Ye, X.; Liu, Y. Changes in characteristics of Chinese bayberry (Myrica rubra) during different growth stages. J. Food Sci. Technol. 2019, 56, 654–662. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Lu, S. Effects of different drying methods on phenolic components and in vitro hypoglycemic activities of pulp extracts from two Chinese bayberry (Myrica rubra Sieb. et Zucchi.) cultivars. Food Sci. Hum. Well. 2022, 11, 366–373. [Google Scholar] [CrossRef]
- Huang, F.; Zhao, R.; Xia, M. Impact of Cyanidin-3-Glucoside on gut microbiota and relationship with metabolism and inflammation in high fat-high sucrose diet-induced insulin resistant mice. Microorganisms 2020, 8, 1238. [Google Scholar] [CrossRef]
- He, J.; Ye, S.; Correia, P. Dietary polyglycosylated anthocyanins: The smart option? A comprehensive review on their health benefits and technological applications. Trends Food Sci. Technol. 2022, 121, 3096–3128. [Google Scholar] [CrossRef]
- Fornabaio, S.; Zi, B.L.; Maserati, M. Determination of cyanidin 3-glucoside in rat brain, liver and kidneys by UPLC/MS-MS and its application to a short-term pharmacokinetic study. Sci. Rep. 2016, 6, 22815. [Google Scholar] [CrossRef]
- Tor, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Song, J.W.; Liu, Y.S.; Guo, Y.R. Nanoliposomes double loaded with curcumin and tetrandrine: Preparation, characterization, hepatotoxicity and anti-tumor effects. Int. J. Mol. Sci. 2022, 23, 6858. [Google Scholar] [CrossRef]
- Li, R.; Deng, L.; Cai, Z. Liposomes coated with thiolate chitosan as drug carriers of curcumin. Mater. Sci. Eng. 2017, 80, 156–164. [Google Scholar] [CrossRef]
- Sharif, N.; Chowhounds, S.; Jafari, S.M. Nano/microencapsulation of anthocyanins: A systematic review and meta-analysis. Food Res. Int. 2020, 132, 109077. [Google Scholar] [CrossRef]
- Shishir, M.I.; Karim, N.; Xie, J. Colonic delivery of pelargonidin-3-O-glucoside using pectin-chitosan-nanoliposome: Transport mechanism and bioactivity retention. Int. J. Biol. Macromol. 2020, 159, 341–355. [Google Scholar] [CrossRef]
- Tan, C.; Wang, J.; Sun, B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: Recent advances. Biotechnol. Adv. 2021, 48, 107727. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zan, L.; Li, X.; Cai, Y.; Hao, G. Modification of liposomes: Preparation, purpose, methods and application in food. Int. J. Food Sci. Technol. 2024, 59, 3523–3536. [Google Scholar] [CrossRef]
- Tan, C.; Feng, B.; Zhang, X. Biopolymer-coated liposomes by electrostatic adsorption of chitosan as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar] [CrossRef]
- Haddadi, M.; Boustani, S.; Jafari, S.M. Pea proteins as emerging biopolymers for the emulsification and encapsulation of food bioactives. Food Hydrocoll. 2022, 126, 107474. [Google Scholar] [CrossRef]
- Zhi, Z.; Yan, L.; Li, H.; Dewettinck, K.; der Meeren, P.V.; Liu, R.; Van Bockstaele, F. A combined approach for modifying pea protein isolate to greatly improve its solubility and emulsifying stability. Food Chem. 2022, 380, 131832. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can, A.; Tyler, R.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Dickinson, E. Interfacial structure and stability of food emulsions as affected by protein—polysaccharide interactions. Soft Matter 2008, 4, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Qi, B.; Han, L.; Wang, D.; Zhang, S.; Jiang, L.; Xie, F.; Li, Y. Study on the gel properties, interactions, and pH stability of pea protein isolate emulsion gels as influenced by inulin. LWT 2021, 137, 110421. [Google Scholar] [CrossRef]
- Song, X.; Cao, L.; Cong, S. Characterization of endogenous nanoparticles from roasted chicken breasts. J. Agric. Food Chem. 2018, 66, 6543–6551. [Google Scholar] [CrossRef]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 4048. [Google Scholar] [CrossRef]
- Wang, R.; Ma, C.; Yan, H.; Zhao, H.; Wang, P.; Zhang, S.; Ju, J.; Yu, S.; Yin, Z. Lecithin/Cholesterol/Tween 80 Liposomes for Co-Encapsulation of Vitamin C and Xanthoxylin. ACS Appl. Nano Mater. 2024, 7, 5982–5995. [Google Scholar] [CrossRef]
- Gouda, A.; Sakr, O.S.; Nasr, M.; Sammour, O. Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102174. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Tripathi, P.; Pandey, S.; Chaurasia, D.; Patil, P. Tailored Non-ionic Surfactant Vesicles of Cyclosporine for the Treatment of Psoriasis: Formulation, Ex-Vivo and In-Vivo Investigation-Application of Box-Behnken Design. Adv. Mater. Sci. Technol. 2023, 5, 2. [Google Scholar] [CrossRef]
- Kruijff, C.; Cullis, P.R.; Radda, G.K. Outside-inside distributions and sizes of mixed phosphatidylcholine-cholesterol vesicles. Biochim. Et Biophys. Acta. 1976, 436, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.S.; Akhavan, N.S.; Pour, S. Cynara officinalis var. Korean Kitami polyphenol extract decreases pro-inflammatory markers in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages by reducing Akt phosphorylation. J. Ethnopharmacol. 2021, 270, 113734. [Google Scholar] [CrossRef]
- Lakshmi, S.S.; Vijayakumar, M.R.; Dewangan, H.K. Lipid Based Aqueous Core Nanocapsules (ACNs) for Encapsulating Hydrophillic Vinorelbine Bitartrate: Preparation, Optimization, Characterization and In vitro Safety Assessment for Intravenous Administration. Curr. Drug Deliv. 2018, 15, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yuan, D.; Wang, Q.; Hu, Z.; Wu, Y.; Cai, J.; Huang, Q.; Li, S.; Liu, G. Maillard-reacted whey protein isolates enhance thermal stability of anthocyanins over a wide pH range. J. Agric. Food Chem. 2018, 66, 9556–9564. [Google Scholar] [CrossRef]
- Ren, J.; Wu, H.; Lu, Z.; Qin, Q.; Jiao, X.; Meng, G.; Liu, W.; Li, G. pH-driven preparation of pea protein isolate-curcumin nanoparticles effectively enhances antitumor activity. Int. J. Biol. Macromolecules 2024, 256, 128383. [Google Scholar] [CrossRef]
- Emkani, M.; Moundanga, S.; Oliete, B.; Saurel, R. Protein composition and nutritional aspects of pea protein fractions obtained by a modified isoelectric precipitation method using fermentation. Front. Nutr. 2023, 10, 1284413. [Google Scholar] [CrossRef]
- Rezazadeh, L.; Hosseini, S.F.; Nikkhah, M. Biopolymer-coated nanoliposomes as carriers of rainbow trout skin-derived antioxidant peptides. Food Chem. 2017, 234, 220–229. [Google Scholar] [CrossRef]
- Šeremet, D.; Vugrinec, K.; Petrović, P.; Butorac, A.; Kuzmić, S.; Cebin, A.V.; Mandura, A.; Lovrić, M.; Pjanović, R.; Komes, D. Formulation and characterization of liposomal encapsulated systems of bioactive ingredients from traditional plant mountain germander (Teucrium montanum L.) for incorporation into coffee drinks. Food Chem. 2022, 370, 113734. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllopoulou, E.; Pippa, N.; Demetzos, C. Protein-liposome interactions: The impact of surface charge and fluidisation effect on protein binding. Liposome Res. 2023, 33, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Kashcooli, Y.; Park, K.; Bose, A.; Greenfield, M.; Bothun, G.D. Patchy Layersomes Formed by Layer-by-Layer Coating of Liposomes with Strong Biopolyelectrolytes. Biomacromolecules 2016, 17, 3838–3844. [Google Scholar] [CrossRef]
- Lai, W.-F.; Wong, W.-T.; Rogach, A.L. Molecular Design of Layer-by-Layer Functionalized Liposomes for Oral Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 43341–43351. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, K.; Ebisawa, A.; Tsuchiya, K.; Sakai, K.; Sakai, H. Effect of lipid composition on the characteristics of liposomes prepared using the polyol dilution method. Colloids Surf. 2024, 699, 134609. [Google Scholar] [CrossRef]
- Huang, R.; Song, H.; Wang, X. Fatty acids-modified liposomes for encapsulation of bioactive peptides: Fabrication, characterization, storage stability and in vitro release. Food Chem. 2024, 440, 138139. [Google Scholar] [CrossRef]
- Emami, S.; Azadmard-Damirchi, S.; Peighambardoust, S.H.; Valizadeh, H.; Hesari, J. Liposomes as carrier vehicles for functional compounds in food sector. J. Exp. Nanosci. 2016, 11, 737–759. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Yin, Y. Design and characterization of Glypican-3 targeted liposomes with cantharidin encapsulation for hepatocellular carcinoma treatment. J. Drug Deliv. Sci. Technol. 2024, 99, 105934. [Google Scholar] [CrossRef]
- Jagatai, M.; Hosseini, F.; Tehrani, E. Cinnamaldehyde loaded chitosan/tripolyphosphate nanoassemblies: Fabrication, characterization, and in vitro evaluation of antioxidant activity. J. Food Process. Preserv. 2019, 43, e13972. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Wang, X. Preparation of blueberry anthocyanin liposomes and changes of vesicle properties, physicochemical properties, in vitro release, and antioxidant activity before and after chitosan modification. Food Sci. Nutr. 2022, 10, 75–87. [Google Scholar] [CrossRef]
- Zhou, M.; Han, Y.; McClements, J. Co-encapsulation of anthocyanin and cinnamaldehyde in nanoparticle-filled carrageenan films: Fabrication, characterization, and packaging applications. Food Hydrocoll. 2024, 149, 109609. [Google Scholar] [CrossRef]
- Sun, Y.; Chi, J.; Ye, X.; Wang, S.; Liang, J.; Yue, P.; Xiao, H.; Gao, X. Nanoliposomes as delivery system for anthocyanins: Physicochemical characterization, cellular uptake, and antioxidant properties. LWT 2021, 139, 110554. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, J.; Bina, Y. Oral polysaccharide-coated liposome-modified double-layered nanoparticles containing anthocyanins: Preparation, characterization, biocompatibility and evaluation of lipid-lowering activity in vitro. Food Chem. 2024, 439, 138166. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, Q.; Liu, Y.; Cai, Z. Enhancing the resistance of anthocyanins to environmental stress by constructing ovalbumin-propylene glycol alginate nanocarriers with novel configurations. Food Hydrocoll. 2021, 118, 106668. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Chen, K.; Jin, M.; Vu, S.H.; Jung, S.; He, N.; Zheng, Z.; Lee, M.-S. Application of chitosan/alginate nanoparticles in oral drug delivery systems: Prospects and challenges. Drug Deliv. 2022, 29, 1142–1149. [Google Scholar] [CrossRef]
- Tang, C.; Tan, B.; Sun, X. Elucidation of interaction between whey proteins and proanthocyanidins and its protective effects on proanthocyanidins during in vitro digestion and storage. Molecules 2021, 26, 5468. [Google Scholar] [CrossRef]
- Shi, S.; Lv, M.; Jin, L.; Qin, G.; Gao, Y.; Ji, J.; Hao, L. Antioxidant properties of anthocyanin revealed through the hydrogen atom transfer: Combined effects of temperature and pH. Mol. Phys. 2021, 119, e1936246. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Costa Lima, S.A.; Reis, S. Application of pH responsive fucoidan/chitosan nanoparticles to improve oral quercetin delivery. Molecules 2019, 24, 346. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernandez, M.D.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Aniya; Zhang, L.; Li, Y.; Fu, X. Nanoliposome modified with inulin and pea protein isolate improves the thermal stability and slows the release of anthocyanin during simulated in vitro digestion and in hot cocoa beverage. Foods 2025, 14, 731. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, X.; Fan, X. Whey protein isolate coated liposomes as novel carrier systems for astaxanthin. Eur. J. Lipid Sci. Technol. 2020, 122, 1900325. [Google Scholar] [CrossRef]
- Wehbe, M.; Malhotra, A.; Anantha, M.; Roosendaal, J.; Leung, A.W.; Plackett, D.; Edwards, K.; Gilabert-Oriol, R.; Bally, M.B. A simple passive equilibration method for loading carboplatin into pre-formed liposomes incubated with ethanol as a temperature dependent permeability enhancer. J. Control. Release 2017, 252, 50–61. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery: Design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Ye, A. Environmental stress stability of microencapsules based on liposomes decorated with chitosan and sodium alginate. Food Chem. 2016, 196, 396–404. [Google Scholar] [CrossRef]
- Gorja Nović, S.; Komes, D.; Pastor, F.T. Antioxidant capacity of teas and herbal infusions: Polarographic assessment. J. Agric. Food Chem. 2012, 60, 9573–9580. [Google Scholar] [CrossRef]
- Chen, Y.K.; Guan, R.; Huang, H. Antioxidative effect of bilberry anthocyanin liposomes on Caco-2 cells. J. Food Sci. Technol. 2023, 23, 56–63. [Google Scholar] [CrossRef]
- Xue, M.L.; Mohammadi, A.; Ghasemi-Pours, F. Characterization of pH sensitive sago starch films enriched with anthocyanin-rich torch ginger extract. Int. J. Biol. Macromol. 2020, 164, 4603–4612. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Zeeb, B.; Thongkaew, C.; Weiss, J. Theoretical and practical considerations in electrostatic deposition of charged polymers. J. Appl. Polym. Sci. 2014, 131, 40099. [Google Scholar] [CrossRef]
- Qiang, M.; Pang, X.; Ma, D.; Ma, C.; Liu, F. Effect of membrane surface modification using chitosan hydrochloride and lactoferrin on the properties of astaxanthin-loaded liposomes. Molecules 2020, 25, 610. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, M.; Pei, Y. Enhancing stability of liposomes using high molecular weight chitosan to promote antioxidative stress effects and lipid-lowering activity of encapsulated lutein in vivo and in vitro. Int. J. Biol. Macromol. 2023, 253, 126564. [Google Scholar] [CrossRef] [PubMed]
- Kozik, V.; Pentak, D.; Paździor, M.; Zięba, A.; Bąk, A. From design to study of liposome-driven drug release part 1: Impact of temperature and pH on environment. Int. J. Mol. Sci. 2023, 24, 11686. [Google Scholar] [CrossRef] [PubMed]
- Uzun, D.E.; Ceyhan, T.; Tomas, M.; Capanoglu, E. Recent advances in improving anthocyanin stability in black carrots. Crit. Rev. Food. Sci. Nutr. 2025, 1–23. [Google Scholar] [CrossRef]
- Gonçalves, R.F.S.; Vicente, A.A.; Pinheiro, A.C. Incorporation of curcumin-loaded lipid-based nanodelivery systems into food: Release behavior in food simulants and a case study of application in a beverage. Food Chem. 2023, 405, 134740. [Google Scholar] [CrossRef]


| Mass Ratio of Soybean Lecithin to Cholesterol (g/g) | Encapsulation Efficiency (%) | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| 1:1 | 35.05 ± 0.22 | 198.67 ± 047 | −12.20 ± 0.23 |
| 3:1 | 42.36 ± 0.33 | 138.77 ± 0.31 | −24.8 ± 0.18 |
| 5:1 | 50.62 ± 0.43 | 128.70 ± 0.26 | −38.23 ± 0.20 |
| 7:1 | 39.80 ± 0.25 | 114.17 ± 0.33 | −35.97 ± 0.17 |
| 9:1 | 23.22 ± 0.41 | 103.30 ± 0.25 | −32.37 ± 0.24 |
| Mass Ratio of Soybean Lecithin to Anhydrous Ethanol (g/mL) | Encapsulation Efficiency (%) | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| 1:100 | 47.41 ± 0.21 | 127.43 ± 0.23 | −35.10 ± 0.24 |
| 2:100 | 48.35 ± 0.23 | 125.00 ± 0.15 | −35.40 ± 0.10 |
| 3:100 | 51.59 ± 0.34 | 120.03 ± 0.33 | −33.77 ± 0.21 |
| 4:100 | 48.77 ± 0.42 | 122.73 ± 0.28 | −32.37 ± 0.34 |
| 5:100 | 42.91 ± 0.13 | 131.73 ± 0.31 | −38.13 ± 0.27 |
| Drug-to-Lipid Ratio (g/g) | Encapsulation Efficiency (%) | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| 1:10 | 36.42 ± 0.45 | 81.46 ± 0.54 | −34.10 ± 0.42 |
| 2:10 | 48.29 ± 0.25 | 135.30 ± 0.48 | −34.43 ± 0.44 |
| 3:10 | 52.59 ± 0.34 | 134.13 ± 0.76 | −32.40 ± 0.75 |
| 4:10 | 42.02 ± 0.48 | 150.43 ± 0.34 | −26.77 ± 0.23 |
| 5:10 | 38.48 ± 0.15 | 262.73 ± 0.39 | −22.63 ± 0.33 |
| pH Value | Encapsulation Efficiency (%) | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| 1 | 70.41 ± 0.17 | 244.43 ± 0.67 | −12.40 ± 0.25 |
| 2 | 72.67 ± 0.22 | 174.73 ± 1.21 | −29.63 ± 0.31 |
| 3 | 76.26 ± 0.14 | 159.50 ± 1.23 | −34.06 ± 0.21 |
| 4 | 68.24 ± 0.15 | 157.40 ± 0.93 | −33.31 ± 0.19 |
| 5 | 64.93 ± 0.18 | 152.80 ± 0.57 | −23.36 ± 0.24 |
| Pea Protein Isolate Concentration (mg/mL) | Encapsulation Efficiency (%) | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| 4 | 74.43 ± 0.34 | 185.40 ± 0.27 | −30.46 ± 0.54 |
| 6 | 78.20 ± 0.25 | 191.30 ± 034 | −29.40 ± 0.48 |
| 8 | 83.80 ± 0.43 | 213.20 ± 0.41 | −27.46 ± 0.69 |
| 10 | 83.40 ± 0.19 | 215.20 ± 0.23 | −19.30 ± 0.21 |
| 12 | 83.10 ± 0.35 | 219.60 ± 0.18 | −14.60 ± 0.12 |
| Synanthrin Concentration (mg/mL) | Encapsulation Efficiency (%) | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| 4 | 84.33 ± 0.38 | 224.66 ± 0.83 | −16.76 ± 0.21 |
| 6 | 87.59 ± 0.44 | 241.00 ± 1.31 | −16.79 ± 0.26 |
| 8 | 90.38 ± 0.24 | 246.60 ± 0.24 | −16.93 ± 0.31 |
| 10 | 87.76 ± 0.22 | 251.16 ± 1.33 | −16.32 ± 0.18 |
| 12 | 86.74 ± 0.50 | 263.40 ± 0.75 | −17.16 ± 0.22 |
| Sample | Encapsulation Efficiency (%) | Particle Size (nm) | Zeta Potential (mV) |
|---|---|---|---|
| ACN-NLs | 52.59 ± 0.24 | 134.60 ± 0.76 | −32.40 ± 0.75 |
| PPI-ACN-NLs | 83.80 ± 0.43 | 213.20 ± 0.41 | −27.46 ± 0.69 |
| SY-PPI-ACN-NLs | 90.38 ± 0.24 | 246.60 ± 0.24 | −16.93 ± 0.31 |
| Sample | Zero Order | First-Order | |||||
|---|---|---|---|---|---|---|---|
| R2 | K | t1/2(day) | R2 | K | t1/2 | ||
| 5 °C | ACN-NLs | 0.78 | 0.52 | 1.33 | 0.99 | 0.09 | 7.57 |
| PPI-ACN-NLs | 0.71 | 0.45 | 1.54 | 0.92 | 0.09 | 8.06 | |
| SY-PPI-ACN-NLs | 0.68 | 0.34 | 2.02 | 0.89 | 0.07 | 9.00 | |
| 25 °C | ACN-NLs | 0.78 | 0.88 | 0.79 | 0.95 | 0.11 | 6.36 |
| PPI-ACN-NLs | 0.79 | 0.73 | 0.95 | 0.96 | 0.10 | 6.75 | |
| SY-PPI-ACN-NLs | 0.68 | 0.58 | 1.2 | 0.89 | 0.09 | 7.30 | |
| 45 °C | ACN-NLs | 0.80 | 1.49 | 0.47 | 0.94 | 0.13 | 5.51 |
| PPI-ACN-NLs | 0.76 | 1.25 | 0.55 | 0.94 | 0.12 | 5.74 | |
| SY-PPI-ACN-NLs | 0.82 | 1.04 | 0.67 | 0.97 | 0.11 | 6.04 | |
| 65 °C | ACN-NLs | 0.88 | 2.09 | 0.33 | 0.99 | 0.14 | 5.02 |
| PPI-ACN-NLs | 0.79 | 1.46 | 0.48 | 0.95 | 0.13 | 5.51 | |
| SY-PPI-ACN-NLs | 0.83 | 1.31 | 0.53 | 0.97 | 0.97 | 5.66 | |
| Sample | Zero Order | First-Order | |||||
|---|---|---|---|---|---|---|---|
| R2 | K | t1/2(day) | R2 | K | t1/2 | ||
| Illuminated | ACN-NLs | 0.86 | 1.24 | 0.56 | 0.99 | 0.12 | 5.75 |
| PPI-ACN-NLs | 0.79 | 0.95 | 0.73 | 0.96 | 0.11 | 6.22 | |
| SY-PPI-ACN-NLs | 0.75 | 0.79 | 0.87 | 0.92 | 0.11 | 6.56 | |
| Without light | ACN-NLs | 0.78 | 0.88 | 0.79 | 0.95 | 0.11 | 6.36 |
| PPI-ACN-NLs | 0.80 | 0.73 | 0.95 | 0.98 | 0.10 | 6.75 | |
| SY-PPI-ACN-NLs | 0.69 | 0.58 | 1.20 | 0.89 | 0.09 | 7.29 | |
| Sample | Zero Order | First-Order | |||||
|---|---|---|---|---|---|---|---|
| R2 | K | t1/2(day) | R2 | K | t1/2 | ||
| 10% C2H5OH | ACN-NLs | 0.92 | 1.29 | 0.76 | 0.99 | 0.12 | 5.68 |
| PPI-ACN-NLs | 0.97 | 0.77 | 0.89 | 0.98 | 0.10 | 6.93 | |
| SY-PPI-ACN-NLs | 0.94 | 0.48 | 1.45 | 0.94 | 0.09 | 7.87 | |
| 20% C2H5OH | ACN-NLs | 0.96 | 1.31 | 0.53 | 0.96 | 0.12 | 5.77 |
| PPI-ACN-NLs | 0.98 | 0.78 | 0.88 | 0.98 | 0.11 | 6.33 | |
| SY-PPI-ACN-NLs | 0.91 | 0.47 | 1.46 | 0.99 | 0.09 | 7.79 | |
| 50% C2H5OH | ACN-NLs | 0.99 | 1.53 | 0.45 | 0.96 | 0.13 | 5.45 |
| PPI-ACN-NLs | 0.96 | 1.02 | 0.68 | 0.98 | 0.11 | 6.08 | |
| SY-PPI-ACN-NLs | 0.93 | 0.61 | 1.13 | 0.99 | 0.09 | 7.14 | |
| 3% CH3COOH | ACN-NLs | 0.97 | 1.59 | 0.43 | 0.98 | 0.13 | 5.37 |
| PPI-ACN-NLs | 0.961 | 1.24 | 0.55 | 0.98 | 0.12 | 5.72 | |
| SY-PPI-ACN-NLs | 0.89 | 0.88 | 0.78 | 0.99 | 0.11 | 6.35 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Aniya; Xing, S.; Li, J.; Liu, Y.; Li, C.; Zhu, J.; Li, Y.; Fu, X. Preparation, Physicochemical Properties and Stability of Anthocyanin Nanoliposomes Before and After Double-Layer Modification Using Synanthrin and Pea Protein Isolate. Molecules 2025, 30, 2892. https://doi.org/10.3390/molecules30142892
Zhang L, Aniya, Xing S, Li J, Liu Y, Li C, Zhu J, Li Y, Fu X. Preparation, Physicochemical Properties and Stability of Anthocyanin Nanoliposomes Before and After Double-Layer Modification Using Synanthrin and Pea Protein Isolate. Molecules. 2025; 30(14):2892. https://doi.org/10.3390/molecules30142892
Chicago/Turabian StyleZhang, Lianlian, Aniya, Shengping Xing, Jing Li, Ying Liu, Chaozhi Li, Jianhang Zhu, Yan Li, and Xiaoji Fu. 2025. "Preparation, Physicochemical Properties and Stability of Anthocyanin Nanoliposomes Before and After Double-Layer Modification Using Synanthrin and Pea Protein Isolate" Molecules 30, no. 14: 2892. https://doi.org/10.3390/molecules30142892
APA StyleZhang, L., Aniya, Xing, S., Li, J., Liu, Y., Li, C., Zhu, J., Li, Y., & Fu, X. (2025). Preparation, Physicochemical Properties and Stability of Anthocyanin Nanoliposomes Before and After Double-Layer Modification Using Synanthrin and Pea Protein Isolate. Molecules, 30(14), 2892. https://doi.org/10.3390/molecules30142892






