Revealing Serotonin Derivatives in Safflower Seed Meal as Potential Anti-Ulcerative Colitis Drugs: In Vitro and Computational Evidence
Abstract
1. Introduction
2. Results
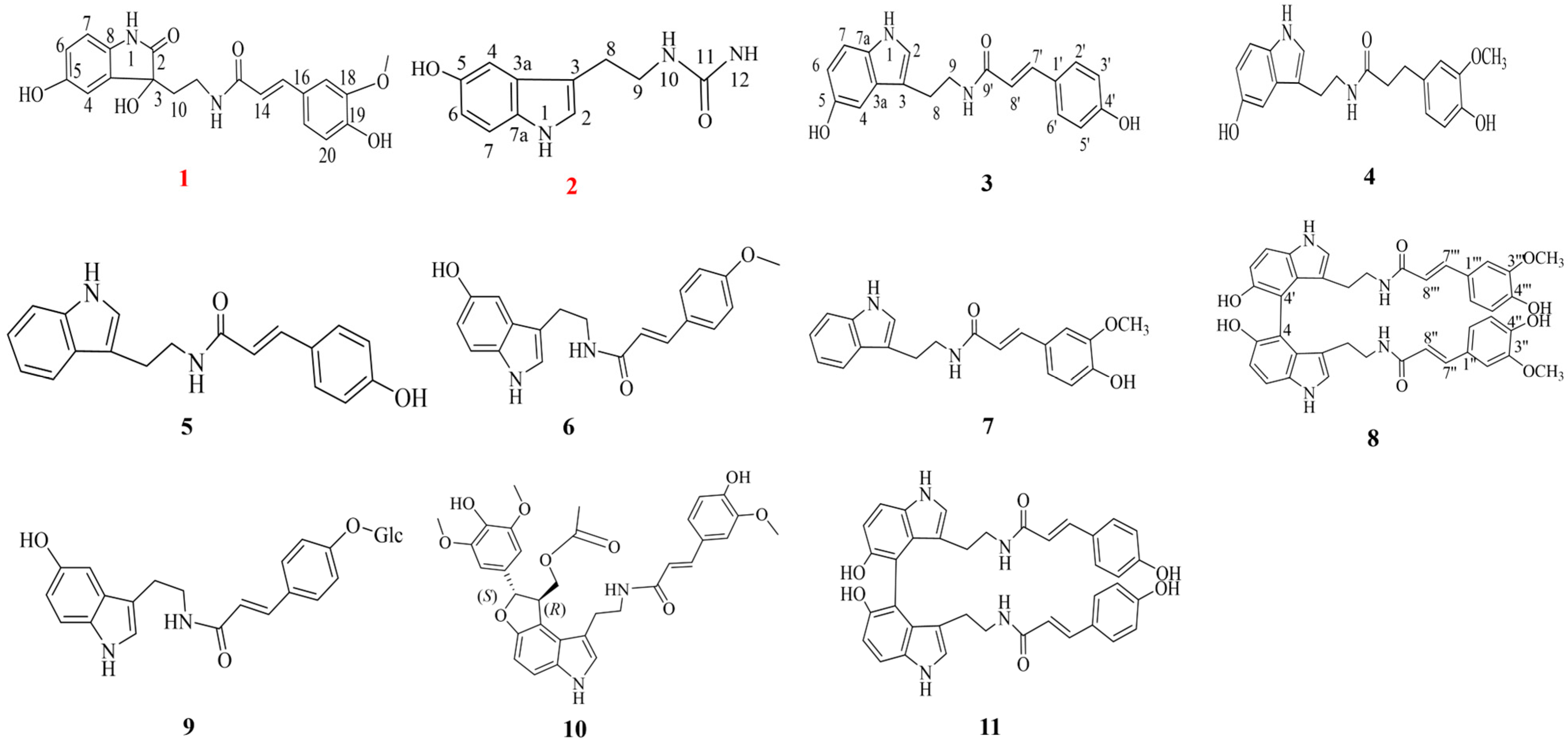
2.1. Structural Characterization of Serotonin Derivatives
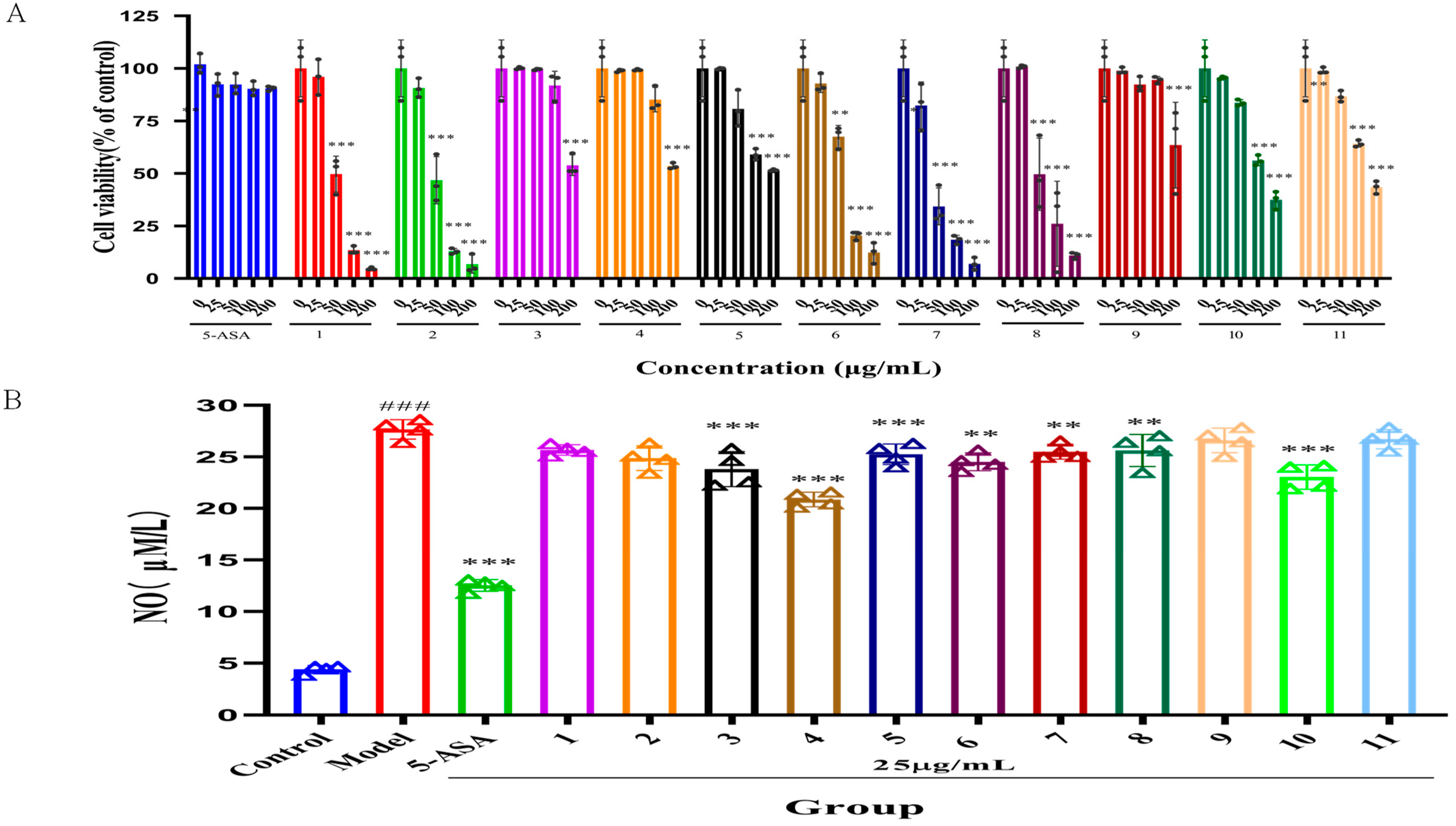
2.2. Effect of Serotonin Derivatives on Nitric Oxide (NO) Level
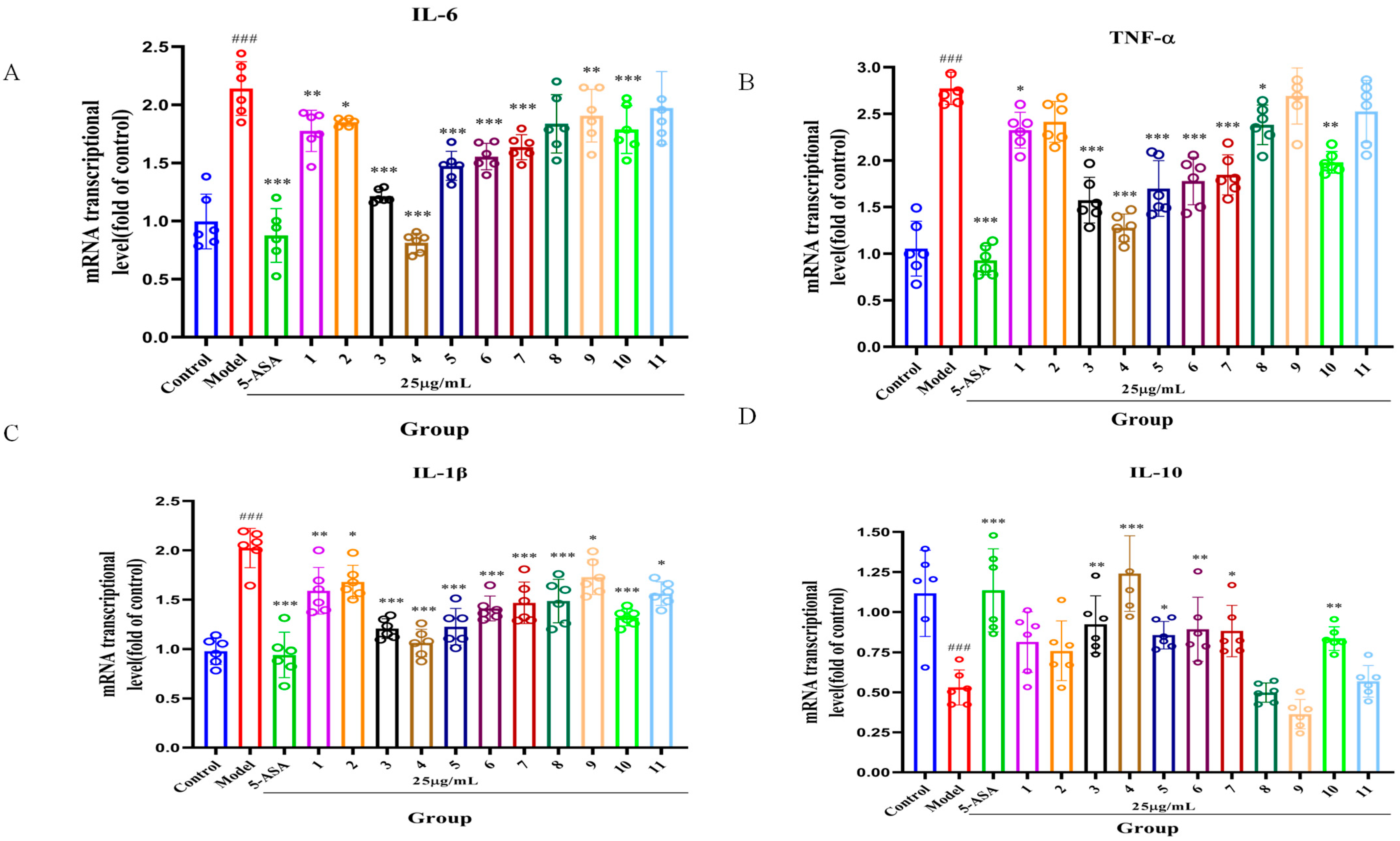
2.3. Effect of Serotonin Derivatives on Inflammatory Factors
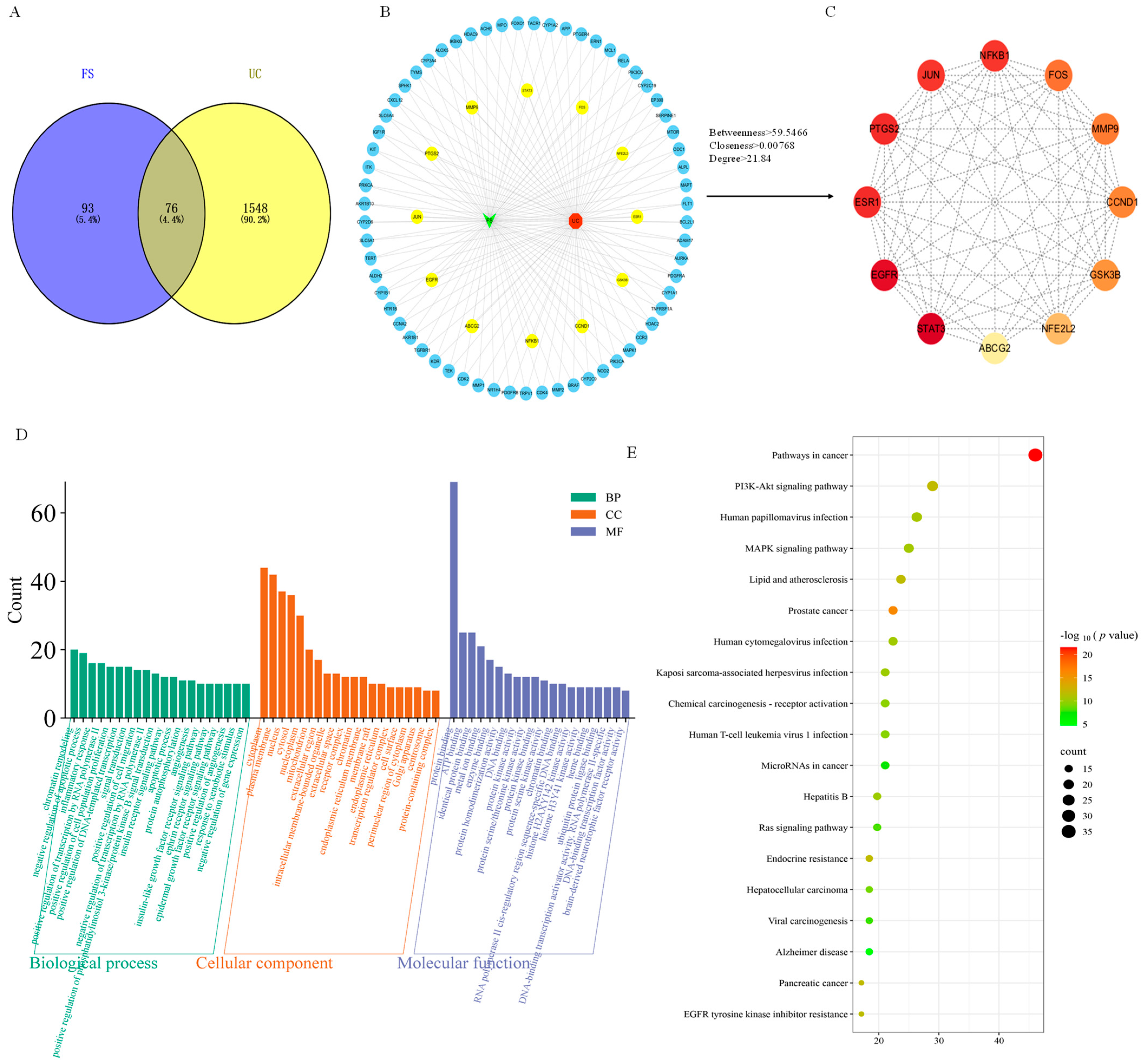
2.4. Construction of Target Database
2.5. PPI Network
2.6. GO and KEGG Enrichment Analysis
2.7. Molecular Dynamics Simulation Results
2.8. Formatting of Mathematical Components
3. Materials and Methods
3.1. Plant Materials and Reagents
3.2. Extraction and Purification
3.3. Cell Culture
3.4. Anti-Inflammatory Effect of Serotonin Derivatives In Vivo
3.5. Compound Targets, Ulcerative Colitis Target Acquisition and Intersecting Genes
3.6. Screening of Core Targets and Construction of Compound–Target Networks
3.7. GO and KEGG Enrichment Assay Experiments
3.8. Molecular Docking
3.9. Molecular Dynamics Simulation
3.10. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wangchuk, P.; Yeshi, K.; Loukas, A. Ulcerative colitis: Clinical biomarkers, therapeutic targets, and emerging treatments. Trends Pharmacol. Sci. 2024, 45, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mao, T.; Lu, X.; Wang, M.; Yun, Y.; Jia, Z.; Shi, L.; Jiang, H.; Li, J.; Shi, R. A potential therapeutic approach for ulcerative colitis: Targeted regulation of macrophage polarization through phytochemicals. Front. Immunol. 2023, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gros, B.; Kaplan, G.G. Ulcerative Colitis in Adults. JAMA 2023, 330, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Vermeire, S. Combination biologic therapy for ulcerative colitis. Lancet Gastroenterol. Hepatol. 2023, 8, 288–290. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef]
- Zhang, L.L.; Tian, K.; Tang, Z.H.; Chen, X.J.; Bian, Z.X.; Wang, Y.T.; Lu, J.J. Phytochemistry and Pharmacology of Carthamus tinctorius L. Am. J. Chin. Med. 2016, 44, 197–226. [Google Scholar] [CrossRef]
- Delshad, E.; Yousefi, M.; Sasannezhad, P.; Rakhshandeh, H.; Ayati, Z. Medical uses of Carthamus tinctorius L. (Safflower): A comprehensive review from Traditional Medicine to Modern Medicine. Electron. Physician 2018, 10, 6672–6681. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, C.; Ge, P.; Liu, Y.; Zafar, M.M.; Hu, B.; Zhang, T.; Luo, Z.; Lu, S.; Zhou, Q.; et al. Genetic diversity, clinical uses, and phytochemical and pharmacological properties of safflower (Carthamus tinctorius L.): An important medicinal plant. Front. Pharmacol. 2024, 15, 1–15. [Google Scholar] [CrossRef]
- Adamska, I.; Biernacka, P. Bioactive Substances in Safflower Flowers and Their Applicability in Medicine and Health-Promoting Foods. Int. J. Food Sci. 2021, 2021, 6657639. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, N.; Li, W.; Ding, C.; Ma, T.; Bai, B.; Wang, H.; Suo, Y.; Wang, X.; Ding, C. Preparative Separation of N-Feruloyl Serotonin and N-(p-Coumaroyl) Serotonin from Safflower Seed Meal Using High-Speed Counter-Current Chromatography. J. Chromatogr. Sci. 2015, 53, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, J.A.; Rosas-Ulloa, P.; Ulloa-Rangel, B.E. Physicochemical and functional properties of a protein isolate produced from safflower (Carthamus tinctorius L.) meal by ultrafiltration. J. Sci. Food Agric. 2011, 91, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.H.J.; Alexander, B.M.; Hess, B.W.; Bottger, J.D.; Hixon, D.L.; Van Kirk, E.A.; Nett, T.M.; Moss, G.E. Dietary supplementation with safflower seeds differing in fatty acid composition differentially influences serum concentrations of prostaglandin F metabolite in postpartum beef cows. Reprod. Nutr. Dev. 2005, 45, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Peng, C.; Zeng, D.; Yang, Z.; Wang, X.; Zhang, S.; Bai, Y.; Kuang, L.; Guo, L.; Qin, Y.; et al. Electrospinning is a potential way for preparing edible plant protein-based nanofibers with porous surface using safflower seed meal. Food Hydrocoll. 2024, 146, 109201. [Google Scholar] [CrossRef]
- Bhat, R.; Reddy, K.R. Challenges and issues concerning mycotoxins contamination in oil seeds and their edible oils: Updates from last decade. Food Chem. 2017, 215, 425–437. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Yang, M.L.; Wang, S.M. Study on the correlation between soil microbial diversity and ambient environmental factors influencing the safflower distribution in Xinjiang. J. Basic Microbiol. 2020, 60, 517–531. [Google Scholar] [CrossRef]
- Xia, C.; Tong, X. Moschamine-Related Indole Alkaloids. In The Alkaloids; Xia, C., Tong, X., Eds.; Yunnan University: Kunming, China, 2018; pp. 139–189. [Google Scholar]
- Qi, M.; Chu, S.; Wang, W.; Fu, X.; Jiang, C.; Zhang, L.; Ali, M.H.; Lu, Y.; Jia, M.; Ubul, D.; et al. Safflower polysaccharide ameliorates acute ulcerative colitis by regulating STAT3/NF-κB signaling pathways and repairing intestinal barrier function. Biomed. Pharmacother. 2024, 174, 116553. [Google Scholar] [CrossRef]
- Novello, C.R.; Marques, L.C.; Pires, M.E.; Kutschenco, A.P.; Nakamura, C.V.; Nocchi, S.; Sarragiotto, M.H.; Mello, J.C.P. Bioactive Indole Alkaloids from Croton echioides. J. Braz. Chem. Soc. 2016, 27, 2203–2209. [Google Scholar]
- Liu, R.H.; Luo, H.; Li, Y.L.; Yang, M.; Li, H.L.; Shen, Y.H.; Zhang, C.; Su, J.; Zhang, W.D. Three New Alkaloids from the Traditional Chinese Medicine ChanSu. Helv. Chim. Acta 2007, 90, 2427–2431. [Google Scholar] [CrossRef]
- Takahashi, T.; Miyazawa, M. Potent α-glucosidase inhibitors from safflower (Carthamus tinctorius L.) seed. Phytother. Res. 2012, 26, 722–726. [Google Scholar] [CrossRef]
- Takahashi, T.; Miyazawa, M. Serotonin derivatives as inhibitors of β-secretase (BACE 1). Pharmazie 2011, 66, 301–305. [Google Scholar] [PubMed]
- Takahashi, T.; Miyazawa, M. Synthesis and structure–activity relationships of serotonin derivatives effect on α-glucosidase inhibition. Med. Chem. Res. 2011, 21, 1762–1770. [Google Scholar] [CrossRef]
- Zhang, H.L.; Nagatsu, A.; Watanabe, T.; Sakakibara, J.; Okuyama, H. Antioxidative Compounds Isolated from Safflower (Carthamus tinctorius L.) Oil Cake. Chem. Pharm. Bull. 1997, 45, 1910–1914. [Google Scholar] [CrossRef]
- Peng, X.R.; Wang, X.; Dong, J.R.; Qin, X.J.; Li, Z.R.; Yang, H.; Zhou, L.; Qiu, M.H. Rare Hybrid Dimers with Anti-Acetylcholinesterase Activities from a Safflower (Carthamus tinctorius L.) Seed Oil Cake. J. Agric. Food Chem. 2017, 65, 9453–9459. [Google Scholar] [CrossRef]
- Vargas-Maya, N.I.; Padilla-Vaca, F.; Romero-González, O.E.; Rosales-Castillo, E.A.S.; Rangel-Serrano, Á.; Arias-Negrete, S.; Franco, B. Refinement of the Griess method for measuring nitrite in biological samples. J. Microbiol. Methods 2021, 187, 106260. [Google Scholar] [CrossRef]
- Chan Hum, P.; Sung Woo, H.; Su Hui, S.; Jae Sue, C.; Jin Pyeong, J.; Takako, Y. N-feruloylserotonin inhibits lipopolysaccharide-induced inflammation via SIRT1 -stimulated FOXO1 and NF-κB signaling pathways in RAW 264.7 cells. Cell. Mol. Biol. 2023, 69, 109–115. [Google Scholar] [CrossRef]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef]
- Wagner, E.M. Monitoring Gene Expression: Quantitative Real-Time RT-PCR. In Lipoproteins and Cardiovascular Disease; Springer: Berlin/Heidelberg, Germany, 2013; pp. 19–45. [Google Scholar]
- Johnston, S.; Gallaher, Z.; Czaja, K. Exogenous reference gene normalization for real-time reverse transcription-polymerase chain reaction analysis under dynamic endogenous transcription. Neural Regen. Res. 2012, 7, 1064–1072. [Google Scholar]
- Singhal, G.; Singh, P.; Bhagyawant, S.S.; Srivastava, N. Safflower Crop: Comprehensive Review with International and National Status. World J. Pharm. Pharm. Sci. 2009, 10, 1–16. [Google Scholar]
- Chen, Y.; Xiang, Q.; Peng, F.; Gao, S.; Yu, L.; Tang, Y.; Yang, Z.; Pu, W.; Xie, X.; Peng, C. The mechanism of action of safflower total flavonoids in the treatment of endometritis caused by incomplete abortion based on network pharmacology and 16S rDNA sequencing. J. Ethnopharmacol. 2023, 315, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Luo, Z.; Zhou, Y.; Zhang, L.; Wu, Y.; Ding, L.; Shi, Y. Uncovering the pharmacological mechanism of Carthamus tinctorius L. on cardiovascular disease by a systems pharmacology approach. Biomed. Pharmacother. 2019, 117, 109094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Q.; Mu, Y.-P.; Xu, Y.; Chen, J.-M.; Liu, P.; Liu, W. Research Progress in Chinese Medicine Preparations for Promoting Blood Circulation and Removing Blood Stasis for Cirrhotic Patients with Portal Vein Thrombosis Following Splenectomy. Chin. J. Integr. Med. 2020, 28, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Kim, E.K.; Nam, Y.S.; Kim, J.H.; Ko, W.K.; Lee, J.M.; Lee, C.H.; Jang, J.B.; Lee, K.S.; Kwon, I.K. Safflower Seed Extract Inhibits Osteoclast Differentiation by Suppression of the p38 Mitogen-activated Protein Kinase and IκB Kinase Activity. Phytother. Res. 2012, 26, 1648–1655. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Wang, R.; Xian, B.; Ren, C.; Liu, Q.; Wu, Q.; Pei, J. Integrated metabolomics and transcriptome analysis on flavonoid biosynthesis in safflower (Carthamus tinctorius L.) under MeJA treatment. BMC Plant Biol. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Sato, H.; Kawagishi, H.; Nishimura, T.; Yoneyama, S.; Yoshimoto, Y.; Sakamura, S.; Furusaki, A.; Katsuragi, S.; Matsumoto, T. Serotobenine, a Novel Phenolic Amide from Safflower Seeds (Carthamus tinctorius L.). Agric. Biol. Chem. 1985, 49, 2969–2974. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Kazemivash, N. Phytochemistry, Pharmacology and Medicinal Properties of Carthamus tinctorius L. Chin. J. Integr. Med. 2013, 19, 153–159. [Google Scholar]
- Nakase, H.; Sato, N.; Mizuno, N.; Ikawa, Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun. Rev. 2022, 21, 1–8. [Google Scholar] [CrossRef]
- El Mahdy, R.N.; Nader, M.A.; Helal, M.G.; Abu-Risha, S.E.; Abdelmageed, M.E. Eicosapentaenoic acid mitigates ulcerative colitis-induced by acetic acid through modulation of NF-κB and TGF-β/EGFR signaling pathways. Life Sci. 2023, 327, 121820. [Google Scholar] [CrossRef]
- Li, C.; Gong, L.; Jiang, Y.; Huo, X.; Huang, L.; Lei, H.; Gu, Y.; Wang, D.; Guo, D.; Deng, Y. Sanguisorba officinalis ethyl acetate extract attenuates ulcerative colitis through inhibiting PI3K-AKT/NF-κB/STAT3 pathway uncovered by single-cell RNA sequencing. Phytomedicine 2023, 120, 155052. [Google Scholar] [CrossRef]
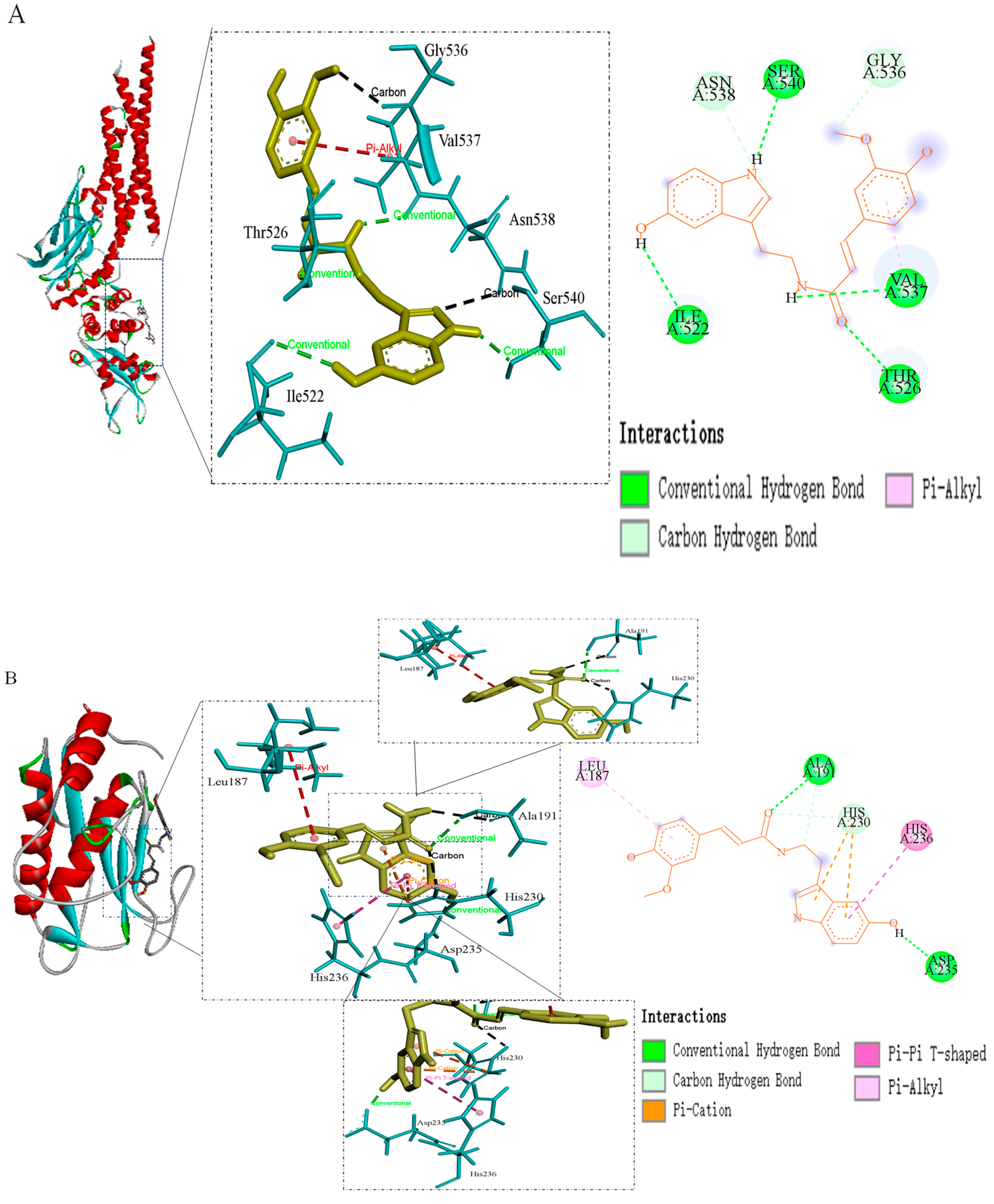
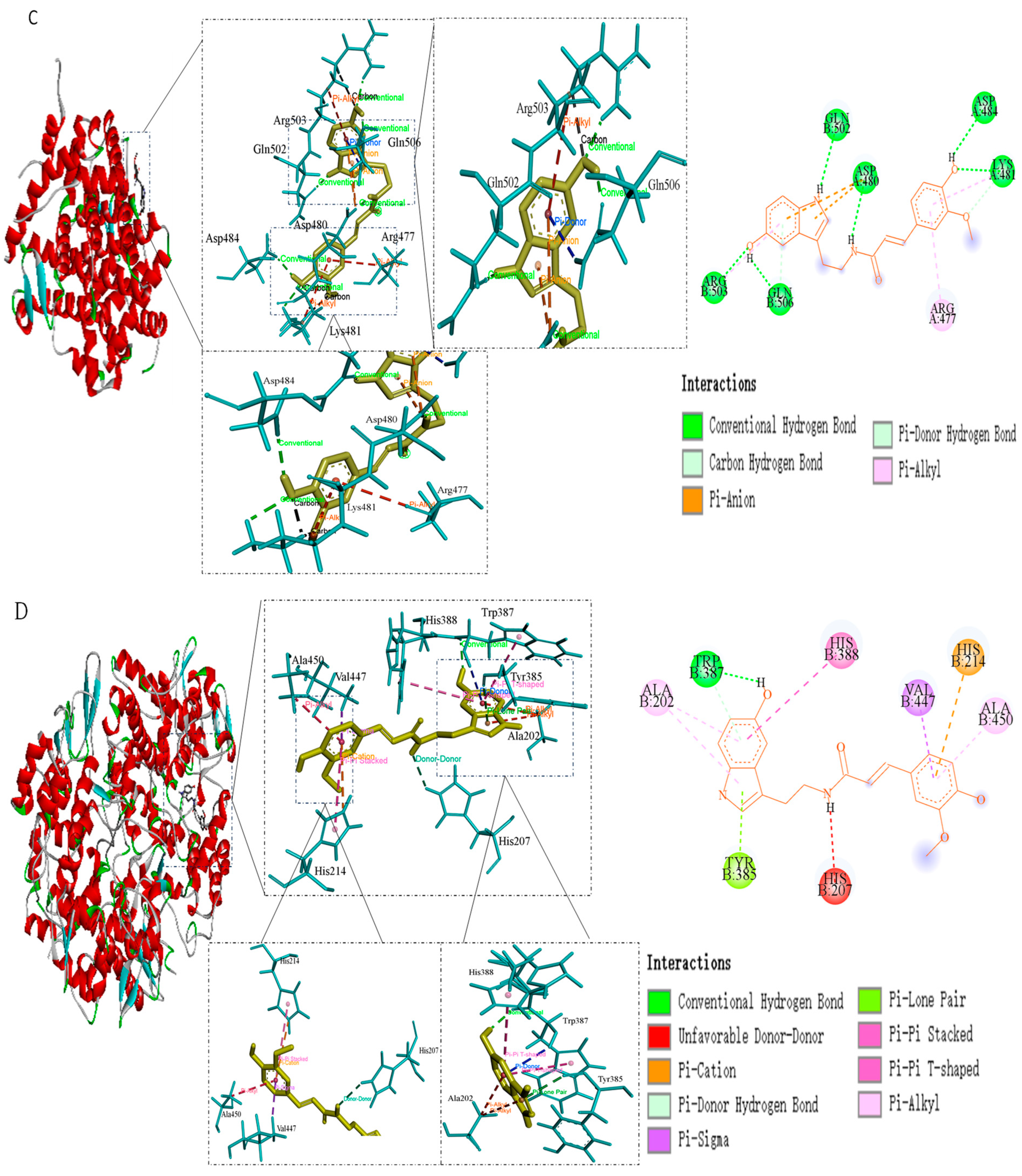
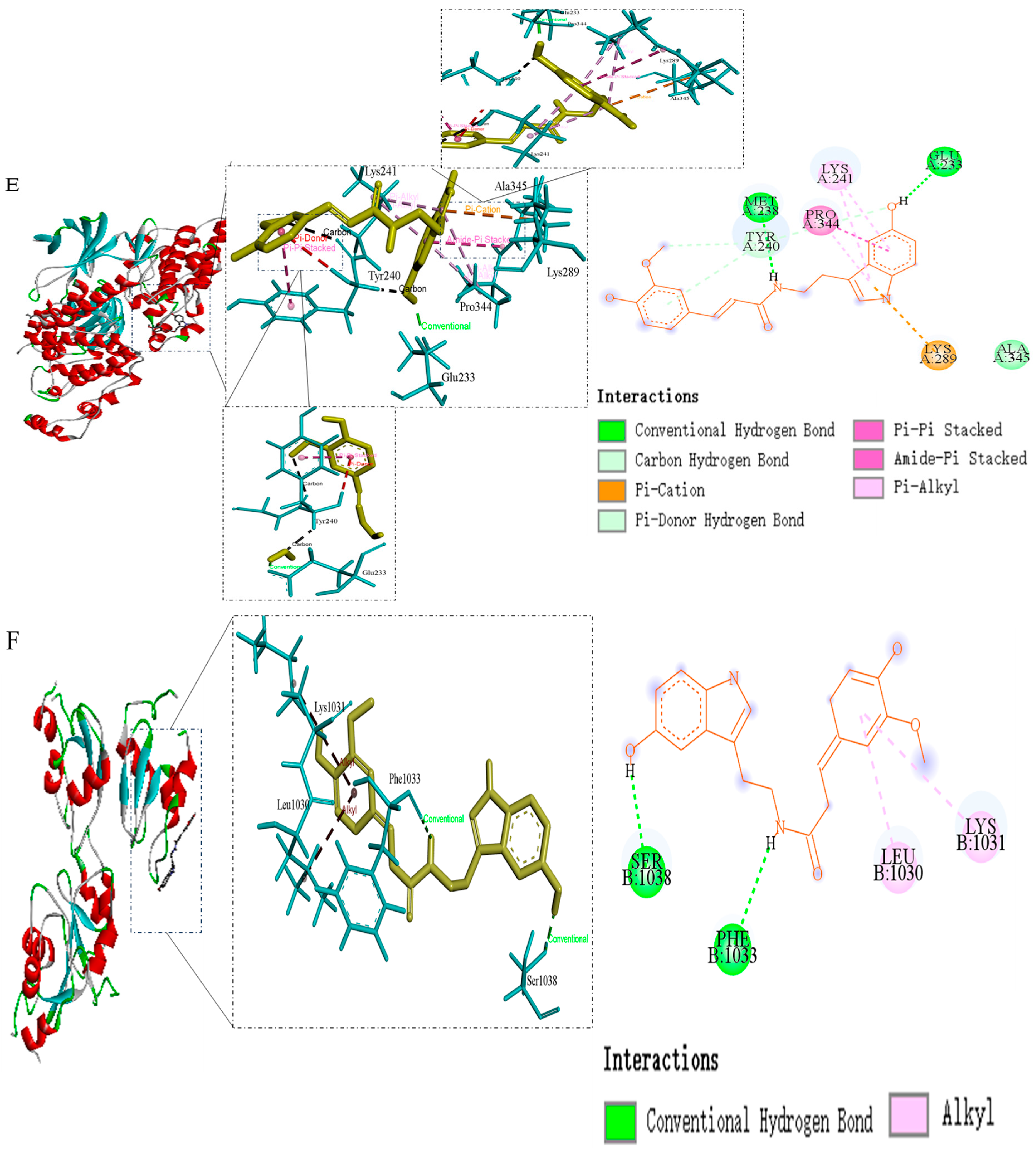

| NO. | Name | Uniprot ID | Inference Score | Betweenness Centrality | Closeness Centrality | Degree |
|---|---|---|---|---|---|---|
| 1 | STAT3 | P40763 | 66.06 | 357.4028265 | 0.0107527 | 55 |
| 2 | EGFR | P00533 | 29.04 | 413.4285767 | 0.0104167 | 53 |
| 3 | ESR1 | P03372 | 27.54 | 360.2069451 | 0.010101 | 50 |
| 4 | PTGS2 | P35354 | 75.77 | 454.1053214 | 0.010101 | 50 |
| 5 | NFKB1 | P19838 | 58.21 | 259.5625728 | 0.010101 | 49 |
| 6 | JUN | P05412 | 49.01 | 260.1712984 | 0.009901 | 49 |
| 7 | FOS | P01100 | 40.92 | 350.4217096 | 0.0093458 | 43 |
| 8 | MMP9 | P14780 | 53.2 | 95.65275435 | 0.0092593 | 42 |
| 9 | CCND1 | P24385 | 44.95 | 92.37872806 | 0.0092593 | 41 |
| 10 | GSK3B | P49841 | 52.59 | 120.5941294 | 0.0091743 | 39 |
| 11 | MTOR | P42345 | 56.1 | 52.77919749 | 0.0088496 | 38 |
| 12 | BCL2L1 | Q07817 | 38.14 | 31.33862389 | 0.0086957 | 35 |
| Acceptor | Bond Energy (kcal/mol) | Amino Acid Residue |
|---|---|---|
| STAT3 | −3.61 | Gly-536, Val-537, Asn-538, Ser-540, Thr-526, Ile-522 |
| EGFR | −5.19 | Leu-187, His-236, Ala-191, His-230, ASP-235 |
| ESR1 | −5.17 | Asp-484, Gln-502, Lys-481, Asp-480, Arg-477, Gln-506, Arg-503 |
| PTGS2 | −4.65 | Ala-202, Ala-450, Tyr-385, Trp-387, His-207, His-388, His-214 |
| JUN | −4.83 | Ala-345, Lys-241, Tyr-240, Pro-344, Lys-289, Glu-233, Met-238 |
| NF-κB1 | −4.35 | Leu-1030, Lys-1031, Phe-1033, Ser-1038 |
| Genomics | Pre-Primer (5′-3′) | Posterior Primers (3′-5′) |
|---|---|---|
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| IL-1β | GCAACTGTTCCTGAACTCAACTT | ATCTTTTGGGGTCCGTCAACT |
| TNF-α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| IL-10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| GAPDH | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ali, M.H.; Jiang, C.; Fan, F.; Zhu, F.; Lu, Y.; Jia, M.; Yin, H.; Wei, J.; Wu, D.; et al. Revealing Serotonin Derivatives in Safflower Seed Meal as Potential Anti-Ulcerative Colitis Drugs: In Vitro and Computational Evidence. Molecules 2025, 30, 2886. https://doi.org/10.3390/molecules30132886
Zhang L, Ali MH, Jiang C, Fan F, Zhu F, Lu Y, Jia M, Yin H, Wei J, Wu D, et al. Revealing Serotonin Derivatives in Safflower Seed Meal as Potential Anti-Ulcerative Colitis Drugs: In Vitro and Computational Evidence. Molecules. 2025; 30(13):2886. https://doi.org/10.3390/molecules30132886
Chicago/Turabian StyleZhang, Liang, Md Hasan Ali, Chao Jiang, Furong Fan, Furong Zhu, Yating Lu, Mengwei Jia, Haipeng Yin, Jianwang Wei, Dongsen Wu, and et al. 2025. "Revealing Serotonin Derivatives in Safflower Seed Meal as Potential Anti-Ulcerative Colitis Drugs: In Vitro and Computational Evidence" Molecules 30, no. 13: 2886. https://doi.org/10.3390/molecules30132886
APA StyleZhang, L., Ali, M. H., Jiang, C., Fan, F., Zhu, F., Lu, Y., Jia, M., Yin, H., Wei, J., Wu, D., Chu, S., & Liu, M. (2025). Revealing Serotonin Derivatives in Safflower Seed Meal as Potential Anti-Ulcerative Colitis Drugs: In Vitro and Computational Evidence. Molecules, 30(13), 2886. https://doi.org/10.3390/molecules30132886






